Abstract
Cannabis sativa is one of the first medicinal plants used by humans. Its medical use remains controversial because it is a psychotropic drug whose use has been banned. Recently, however, some countries have approved its use, including for recreational and medical purposes, and have allowed the scientific study of its compounds. Cannabis is characterized by the production of special types of natural products called phytocannabinoids that are synthesized exclusively by this genus. Phytocannabinoids and endocannabinoids are chemically different, but both pharmacologically modulate CB1, CB2, GRP55, GRP119 and TRPV1 receptor activities, involving activities such as memory, sleep, mood, appetite and motor regulation, pain sensation, neuroinflammation, neurogenesis and apoptosis. Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are phytocannabinoids with greater pharmacological potential, including anti-inflammatory, neuroprotective and anticonvulsant activities. Cannabidiol is showing promising results for the treatment of COVID-19, due to its capability of acting on the unleashed cytokine storm, on the proteins necessary for both virus entry and replication and on the neurological consequences of patients who have been infected by the virus. Here, we summarize the latest knowledge regarding the advantages of using cannabinoids in the treatment of COVID-19.
1. Introduction
The use of cannabis products has dramatically increased in recent times, due to the lack of response to standard treatments, for example to anxiety, chronical inflammation problems and seizures, which puts patients’ lives at risk. The hemp plant has been used for centuries in medicinal and recreational products such as hashish or marijuana. Cannabis use for medicinal purposes is legal in some countries, including Germany, Austria, Canada, Spain, Finland, Israel, Italy, Holland, Portugal and some states of the United States of America [1]. In Latin American countries, its use is permitted in Uruguay, Colombia and Argentina [2]. In Chile, the sale of cannabis-based medicines was authorized after the modification of a restricting law in 2015. For these reasons, it is expected that by 2025 the global medical cannabis market will reach $43 billion [3]. The positive effects of the medicinal use of marijuana in conditions such as chronic pain, multiple sclerosis, nausea, vomiting, terminal diseases and epilepsy have been reported [1,2]. Several studies suggest that cannabidiol (CBD) may be useful in the treatment of patients with COVID-19 by inhibiting the expression of the protein critical for SARS-CoV2 virus replication and by binding as an agonist to the cannabinoid receptor 2 (CB-2), thus reducing inflammation and lung damage [4,5,6,7].
To date, seven strains of human-infecting coronaviruses have been identified [8]. The currently available treatment is supportive, as vaccination prevents the severe illness and death of patients, but its efficacy in controlling virus spread and preventing future deaths is not clear yet [9]. For these reasons, it has become a global public health emergency, making it extremely important to discover new alternatives for effective treatment.
Despite the few studies that exist on the antiviral activity of cannabinoids, some research has been conducted that demonstrates their high anti-inflammatory and immunosuppressive power [2,10]. Thus, by inhibiting both the coronavirus replication processes and the inflammation provoked, there is the possibility of developing an effective therapeutic strategy [6,7]. The aim of this article is to review the therapeutic applications of cannabis derivatives and their applications for the treatment of COVID-19, based on the mechanisms through which they exert their pharmacological activity.
2. Endocannabinoids
The endocannabinoid system is a neuromodulatory network involved in many cognitive and physiological processes, such as in anti-convulsant, anti-inflammatory, anti-depressant and anti-tumorigenic actions, as well as exerting analgesic, hyperphagic, hypophagic, neuroprotective and antiemetic activities [11] and appetite regulation [12]. The endocannabinoid system is composed of endogenous cannabinoids (chemical derivatives of arachidonic acid), cannabinoid receptors and enzymes that produce and degrade endocannabinoids [13,14].
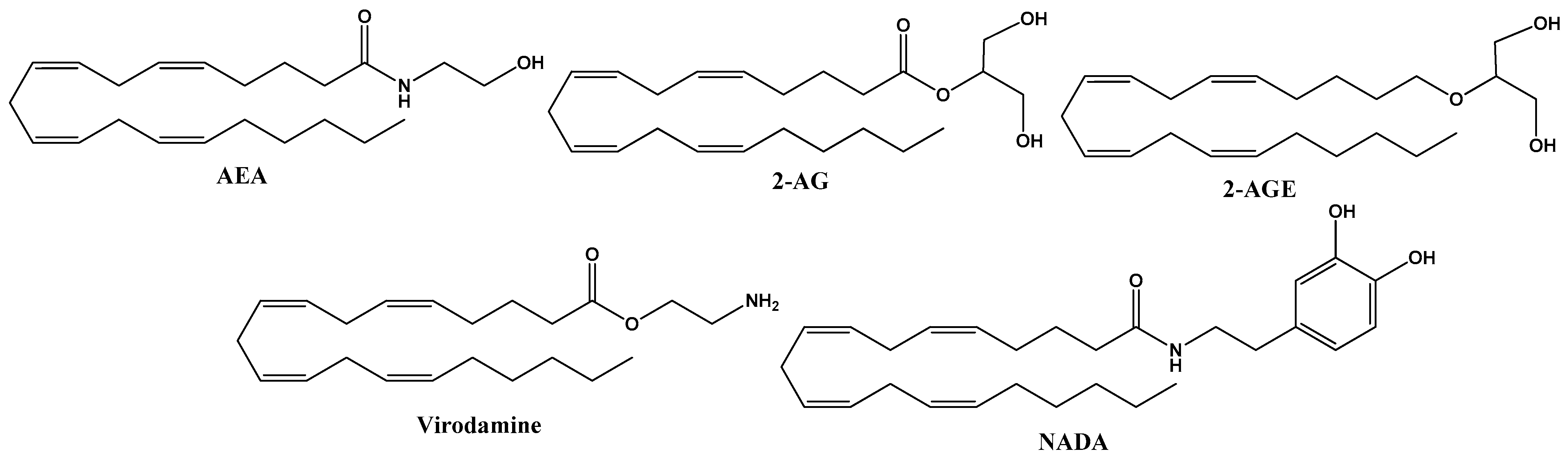
These natural molecules are derived from membrane phospholipids, which include arachidonic acid as endogenous cannabinoids present in the organism of all animals. They are synthesized in neurons on demand and released into the synaptic space, where they activate membrane receptors, acting in the vicinity of the site where they are released, regulating synaptic transmission. The principal endocannabinoids are anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), but they also include 2-arachidonoyl-glycerol (2-AGE), O-arachidonoyl-ethanolamine (virodamine) and N-arachidonoyl-dopamine (NADA) [11]. The structures of endocannabinoids are given in Figure 1.
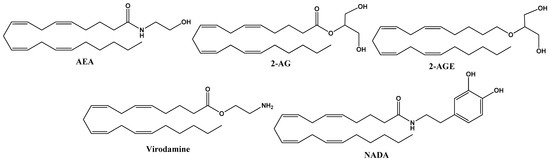
Figure 1.
Chemical structure of endocannabinoid derivates from arachidonic acid, including anandamide (AEA), 2–arachidonoyl glycerol (2–AG), 2-arachidonoyl–glycerol (2–AGE), O-arachidonoyl-ethanolamine (virodamine) and N-arachidonoyl-dopamine (NADA).
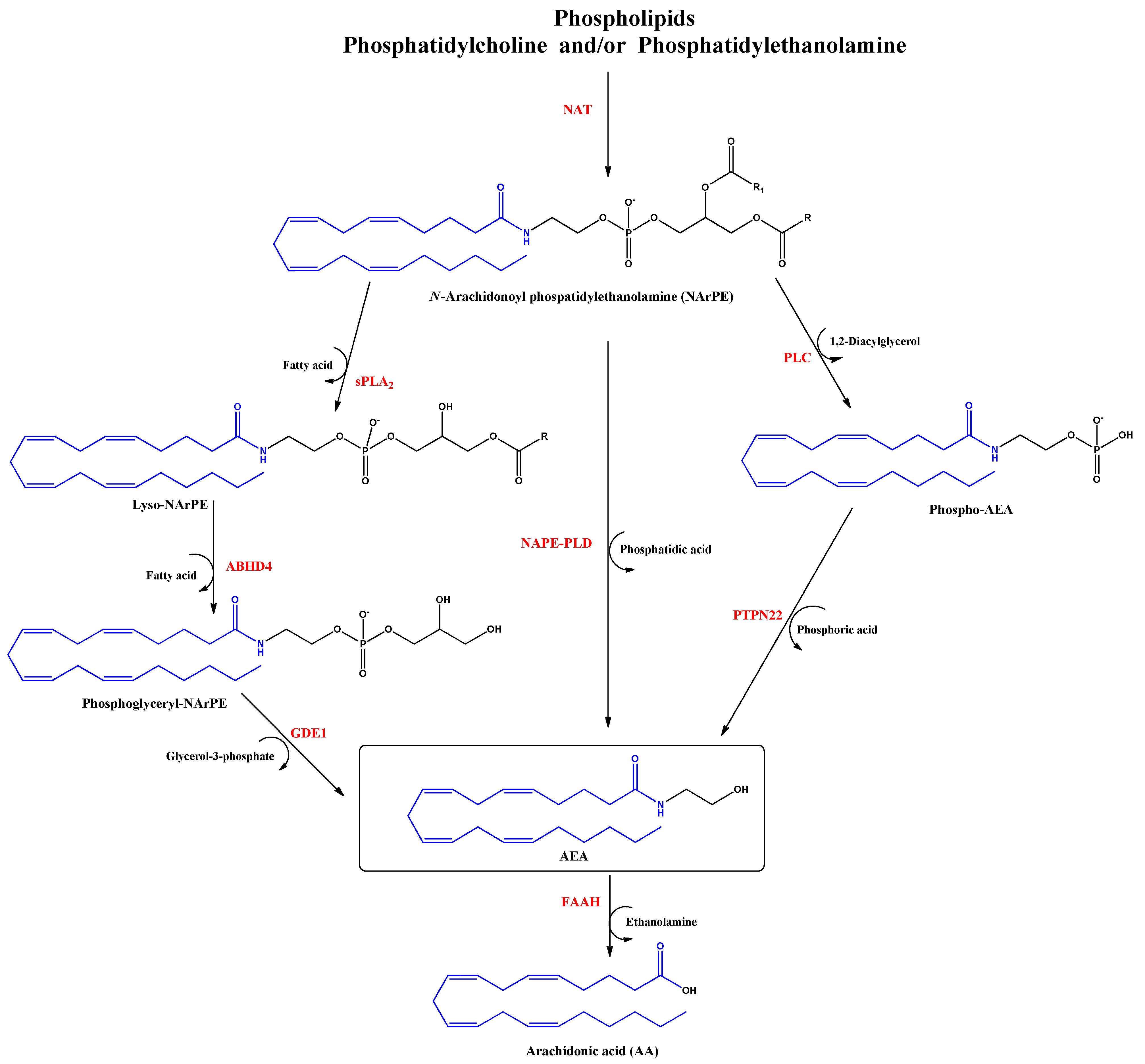
Anandamide (AEA) was the first discovered endocannabinoid from pig brain in 1992 [15]. Anandamide is produced from N-arachidonoyl phosphatidylethanolamine, as shown in Figure 2. It behaves as a full or partial agonist of the cannabinoid receptor 1 (CB1) as well as a partial CB2 agonist. It is present in the CNS, spleen, heart, testis, uterus and vascular endothelium of humans [16].
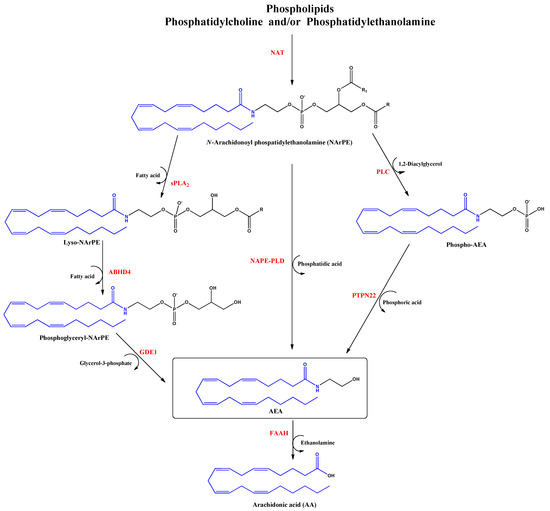
Figure 2.
Biosynthesis and hydrolysis of N–Arachidonoylethanolamine (AEA) pathway. Abbreviations in red mean the enzymes: NAT: N–acyltransferase; sPLA2: soluble phospholipase A2; PLC: phospholipase C; ABHD4: α/β–hydrolase domain 4; GDE1: glycerophosphodiesterase; NAPE–PLD: NAPE–specific phospholipase D; PTPN22: non–receptor protein tyrosine phosphatase 22; FAAH: fatty acid amide hydrolase.
In patients with autism spectrum disorder, the plasma concentrations of AEA are significantly lower compared to control children, suggesting that AEA signaling participates in the pathophysiology of autism, evaluated in 116 children around 3 to 12 years old [17]. The pharmacological increase in AEA levels results from the inhibition of fatty acid amide hydrolase (FAAH), which is responsible for AEA degradation to arachidonic acid (AA) and ethanolamine. Elevated levels of AEA may aid the treatment of post-traumatic stress disorder [18]. For example, in a double-blind, placebo-controlled study, the inhibition of FAAH (PF-04457845, 4 mg/once daily, over 10 days, n = 16) increased 10-fold in baseline anandamide concentration, with an improvement of fear extinction memories in the patients and attenuation of the anxiogenic effects of stress [19]. Further, increased AEA concentrations caused by the inhibition of FAAH could promote learning during psychotherapeutic interventions [20]. On the other hand, AEA production appears to be deficient in patients with hypertrophic scars suggesting a correlation between the systemic and local skin endocannabinoid systems during human wound healing [21].
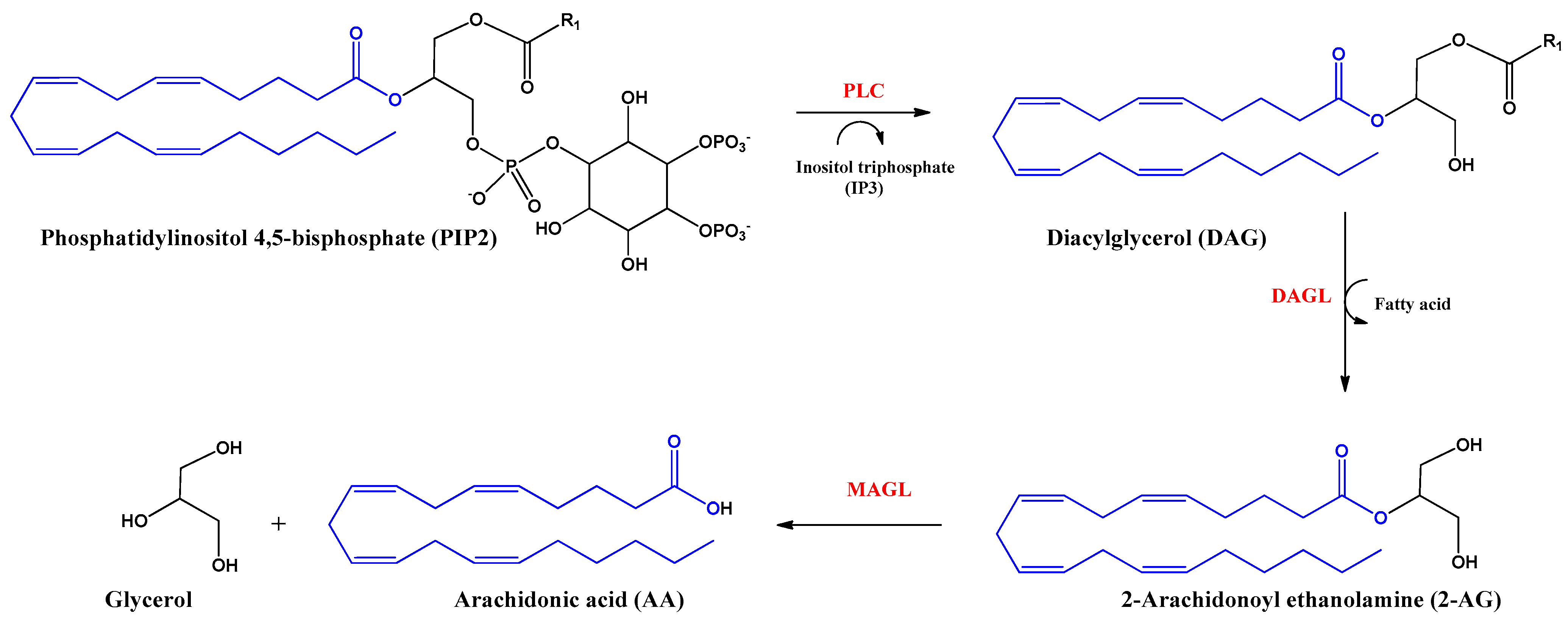
2-Arachidonoyl glycerol (2-AG) was the second endocannabinoid to be discovered. It is the most abundant endogenous cannabinoid in the CNS. It is an ester derived from arachidonic acid and glycerol, produced by the enzyme diacylglycerol lipase alpha and beta (DAGL). The inhibition of the actions of this endocannabinoid is mediated by hydrolysis with the enzyme monoacylglycerol lipase (MAGL) producing arachidonic acid (AA) and glycerol (Figure 3). 2-AG behaves as a full agonist of the CB1 and CB2 receptors. However, it also binds to other receptors with cannabimimetic cellular activity, such as GRP55 and TRPV1 receptors [16,22].
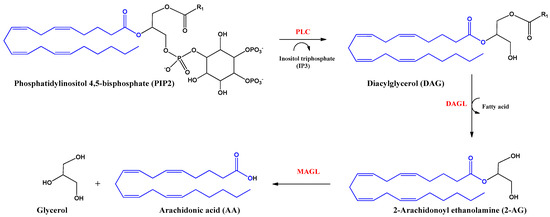
Figure 3.
Biosynthesis and hydrolysis of 2–Arachidonoyl ethanolamine (2–AG) and arachidonic acid (AA). Abbreviations in red mean the enzymes: PLC: Phospholipase C–β; DAGL: Diacylglycerol lipase; MAGL: monoacylglycerol lipase.
Alternatively to the aforementioned hydrolysis routes, AEA and 2AG can be oxidized by cyclooxygenase-2 (COX-2), different lipoxygenases or cytochrome P450 [23]. 2-AG hydrolysis inhibition could be an anti-inflammatory strategy to increase 2-AG levels and decrease eicosanoids levels. Human leukocytes use AA and unsaturated fatty acids to biosynthesize 2-AG and other monoacylglycerols (MAGs), which might decrease inflammation processes [24].
3. Cannabinoid Receptors
In 1990, the first cannabinoid-specific receptor (CB1) was identified and cloned, followed by a second receptor (CB2). Cannabinoid receptor 1 and CB2 differ in their signal transduction and in their distribution in different tissues. The identification of these receptors led to a better understanding of phytocannabinoid and endocannabinoid functions and to the creation of synthetic cannabinoids [25]. Cannabinoid binding to their receptors results in distinct effects depending on which signaling pathway is activated. Signaling pathway activation depends on the agonist structure and the monomeric or heteromeric cannabinoid receptor states [26]. Furthermore, CB1 and CB2 receptors differentially regulate TNF-α-induced apoptosis in HT22 hippocampal cells, CB1 being a promoter of signaling and neuroprotection [27]. Once endocannabinoids have been released by the presynaptic neuron, the cannabinoid receptors are activated, inhibiting the release of neurotransmitters, such as glutamate [28]. This action of cannabinoids confers to them important physiological roles as regulators of synaptic activity.
CB1 receptors are principally expressed in the neurons of the cortex, spinal cord and peripheral nervous system as well as in peripheral organs and tissues. These receptors are expressed in brain areas responsible for memory processing, movement, and pain modulation. Their activation results in different effects on pain sensation as well as on sleep, memory, appetite, mood, and motor regulation [29,30].
CB2 receptors are expressed by immune cells, such as leukocytes, in the spleen, tonsils and in the peripheral nervous system. The functions of CB2 receptors include the modulation of the immune system and inflammation by the release of cytokines. Since selective CB2 receptor agonists do not cause psychotropic effects, these are increasingly investigated for the therapeutic applications of cannabinoids, such as analgesics, anti-inflammatories and anti-neoplastics [29,30].
The GRP55 receptor is a putative cannabinoid receptor 3 (CB3). Experimental evidence links the GPR55 receptor to pathological conditions, providing a therapeutic target in cancer, neurodegenerative and metabolic disorders as well as in the regulation of microglia-mediated neuroinflammation [31]. The receptor has been found in the adrenal glands, spleen, intestine and CNS. The expression of the active receptor has been detected in bone cells [32,33]. GRP55 receptor activation occurs through phospholipase C (PLC)-β pathways. Consequently, there is an increase in cytosolic calcium concentration with the further stimulation of GTPases (Rac1, RhoA and Cdc42) and mitogen- or stress-activated protein kinases (MAPKs, ERK and p38) and NFAT, NFKB, CREB and ATF2 transcription factor activation [34,35,36]. GRP119 cannabinoid receptors are localized in humans predominantly in the pancreatic tissue and in the enteroendocrine L cells of the gastrointestinal tract. Their activation elevates plasma insulin concentrations in mice and decreases food intake and weight in rats. It has been proposed as the endogenous receptor for oleoyl ethanolamide [37].
TRPV1 cannabinoid receptors are vanilloid receptors and their activation promotes programmed death (apoptosis) [38] by augmenting intracellular calcium concentration, ceasing mitochondrial membrane potential, cytochrome C release and caspase 3 and 9 activation [39].
4. Phytocannabinoids
The genus Cannabis comprises the plants Cannabis sativa, Cannabis indica, Cannabis americana and Cannabis ruderalis. C. sativa grows in tropical and subtropical regions as well as temperate climate zones, e.g., in Europe [1,2]. The original plant has been known for 10,000 years in practically all cultures since the discovery of agriculture. Countries such as China, India, Turkestan and Arabia have used its compounds for medicinal purposes to treat diseases such as malaria, beriberi, constipation, rheumatic pains, headaches, female ailments and other conditions [11]. In the 19th century, its widespread use as a stimulant, sedative and analgesic became popular [30]. In 1860, the first committee of physicians in the USA was created to systematically study its use and properties. In 1964, delta-9 tetrahydrocannabinol (Δ9-THC), the main substance responsible for the psychoactive and pharmacological properties of marijuana extracts, was isolated.
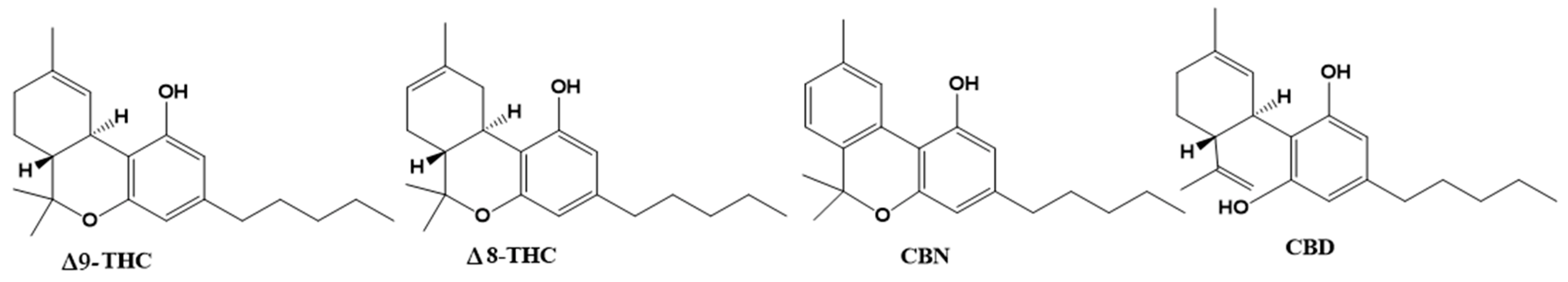
Cannabis plants contain over 500 bioactive compounds in their 18 different species, including over 100 different naturally occurring phytocannabinoids. Phytocannabinoids are produced in glandular hairs or trichomes. While they are found on most aerial surfaces of the plant, phytocannabinoids are primarily located in the bracts and flowers of the plant. [30] Phytocannabinoids are compounds of terpene phenolic structure with 21 carbon atoms. The main phytocannabinoids are Δ9-tetrahydrocannabinol (THC), Δ8-tetrahydrocannabinol (Δ8-THC), cannabidiol (CBD), cannabinol (CBN), and CBD being the known active compounds of pharmacological plant activity (Figure 4).
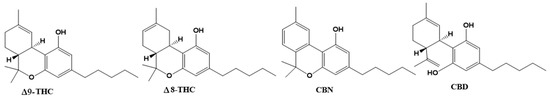
Figure 4.
Chemical structures of the phytocannabinoids Δ9–tetrahydrocannabinol (Δ9-THC), Δ8–THC, cannabinol (CBN) and cannabidiol (CBD).
Despite THC displaying the highest psychoactive potential, its pharmacological properties have been proven in clinical and preclinical assays as appetite stimulation, the suppression of chemotherapy-induced nausea and vomiting and the inhibition of pain, spasticity, neuroprotective, anti-inflammatory and antitumor effects [40,41,42].
Δ8-THC and CBD are found in some plant varieties and always in low concentrations. CBD also exerts psychoactive properties, and its action on the CB2 receptor in splenocytes and thymocytes causes a decrease in the transcription of the gene for interleukin-2 (IL-2) [43]. CBD has shown anxiolytic, anticonvulsant and antipsychotic effects. Further antioxidant, anti-inflammatory, antiemetic, antitumor and neuroprotective properties of CBD have been reported in preclinical studies. Cannabinol has analgesic properties with fewer psychoactive effects than Δ9-TCH. The activity of commercial formulations based on cannabinoids is summarized in Table 1.

Table 1.
Commercial formulations of phytocannabinoids with therapeutic properties.
5. Clinical Evidence for Therapeutic Efficacies of Cannabinoids
Cannabinoids are mostly used as anxiolytic, relaxing and anti-inflammatory natural agents with adjuvant properties for the treatment of epilepsy, schizophrenia, multiple sclerosis, depression or chronic pain [38,46,50,51,52,53,54,55]. In the case of epilepsy, it has been shown that the brain tissue of these patients shows an overexpression of proinflammatory cytokine IL-1β and IL-6 genes together with nuclear transcription factor kappa B (NFKB) [56]. Cannabinol can inhibit the G protein-coupled orphan receptor (GRP55) and decrease NFKB signaling, the latter probably by binding to nuclear PPAR-g receptors, thus reducing the expression of proinflammatory enzymes such as iNOS (nitric oxide synthase) and COX-2 (cyclooxygenase type 2) and metalloprotease and proinflammatory cytokine production [10,43,53,57,58].
There are numerous clinical trials proving the pharmacological efficacy of CBD, which are summarized in Table 2.

Table 2.
Pharmacological efficacy of cannabinoid products tested in clinical trials.
Another issue of great importance is the recently discovered interaction between first generation antiepileptic drugs (carbamazepine, oxcarbazepine, phenytoin, phenobarbital and primidone) and the decrease in the concentration of some drugs used for COVID-19 treatment (atazanavir and remdesivir, darunavir/cobicistat and lopinavir/ritonavir). Therefore, in these cases, the use of CBD to treat both conditions can be an alternative that can change the results obtained [64].
6. Cannabis and COVID-19: In Vitro Studies
As of 24 October 2022, there have been 624,235,272 confirmed cases of COVID-19, including 6,555,270 deaths, reported to the WHO, and a total of 12,814,704,622 doses of vaccine have been applied [65].
The seriousness of SARS-CoV2-caused illness lies in its capability of inducing in the infected person a rapid and intense immune response that courses in an uncontrolled manner, unleashing a storm of cytokines resulting in a variety of symptoms, including severe morbidity and mortality. This cytokine storm is produced by the organism attempting to defend itself against viral invasion, thus stimulating the production of macrophages, interleukins, chemokines and proinflammatory mediators [66]. The endocannabinoid system has the role of acting as a key regulator of the immune system, as it possesses the capacity of immunosuppression and repressing cytokine cascade, inhibiting immune cell proliferation and antibody production [7,67,68,69,70].
The angiotensin-converting enzyme 2 (ACE 2) has been pointed out as the principal receptor for the interaction of SARS-CoV 2 with human cells, which is mainly expressed in type I and II pneumocytes, making these cells are more susceptible of being infected by the virus. ACE 2 receptors are also expressed by lung AT2, liver cholangiocytes, colon colonocytes, esophageal keratinocytes, ileum EC, rectum EC, stomach epithelial cells and kidney proximal tubules [70]. CD169+ macrophages residing in the spleen and lymph node tissues can be infected [71,72,73,74,75]. Alveolar macrophages do not only protect the lungs from pathogens, but also participate in enhancing specific T-cell responses, repairing damaged tissue and recruiting neutrophils leading to increased inflammatory cell traction [76,77,78]. The hyperactivation of lung macrophages and proinflammatory monocyte-derived macrophages (MDM) occurs in the small airways. The transcriptomic analysis of plasma and bronchoalveolar lavage fluids from SARS-CoV2 patients has demonstrated large amounts of proinflammatory cytokines, chemokines and soluble inflammatory mediators, including TNF-α, IL-6, IL-1β, IL-2R, IL-8, inducible protein (IP)-10, C-reactive protein and D-dimer, culminating in a cytokine storm [79]. In the inflammation process, the overexpression of genes that express chemokines was observed, which are critical for recruiting neutrophils (CXCL17) and monocytes (CCL2, CCL7) in the lungs [80]. In SARS-CoV2 and MERS-CoV, a rapid and specific memory CD8 T-cell response is required to protect against infection [24,25]. However, severe SARS-CoV-2 infection results in CD4 and CD8 T-cell lymphopenia and a decrease in INF-γ-producing T cells [81,82].
7. Indispensable Proteins for the Development of Infection and CBD
The structural characteristics of SARS-CoV2 indicate that there are host cell proteins that are indispensable for the progress of viral infection. Once inside the lung cells, it binds to the host cell membrane receptor: angiotensin-converting enzyme 2 (ACE 2), via the transmembrane spike glycoprotein (structural glycoprotein of the virus) and induces its entry through membrane endocytosis. This glycoprotein forms homotrimers that protrude from the surface of SARS-CoV2. The subunits of the SARS-CoV2 spike glycoprotein trimer consist of an S1 subunit that binds to host cell ACE2 to initiate infection, an S2 subunit that mediates the fusion of the virus with host cells and a transmembrane domain [83]. While ACE2 is the receptor for viral entry, the transmembrane enzyme transmembrane serine protease 2 (TMPRSS2) primes viral spike proteins for SARS-CoV2 entry into host cells. Its function is given by proteolysis and the consequent activation of the spike protein by cleaving it into two covalently linked peptides: the S1 (ACE2 receptor-binding terminal) and S2 (viral cell membrane fusion-mediated terminal) sites or subunits, leading to conformational changes for the fusion of the virus with the host membrane and the virus entering of the cytoplasm [84,85,86]. The S1 subunit is primarily responsible for host virus range determination and cell tropism [71,87].
Then, ORF1, one of the nonstructural viral proteins, stimulates virus replication and RNA synthesis, while the nucleocapsid phosphoprotein (structural viral protein) packages the viral genome, forming a helical nucleocapsid with an RNA chaperone function. The surface glycoproteins evoke virion assembly and morphogenesis, producing virus particles and releasing them by exocytosis [66,88,89]. Any interference with the expression of one of these proteins may disrupt the transmission cycle of SARS-CoV2 [6].
Erukainure et al. demonstrated that C. sativa compounds have strong binding affinities to the initiation and termination codons of ORF1, the surface glycoprotein, the envelope protein and nucleocapsid phosphoprotein mRNAs of the complete SARS-CoV2 genome isolated from KwaZulu-Natal, South Africa [90]. This highlights the utility of phytocannabinoids in slowing down virus replication, translation, assembly, and release. The nature of these binding affinities could be due to their chemical properties. The cannabinoid compounds present in their structure free hydroxyl group and aromatic rings that enable them to interact with purines, pyrimidines or the phosphate terminus of the mRNA sequence of the SARS-CoV2 genome through hydrogen bonds or intermolecular interaction.
Purines and pyrimidines, as part of nucleotides, are capable of undergoing nucleophilic or electrophilic reactions, specifically the formation of hydrogen bonds between the oxygen at carbon 2 of the purines in the SARS-CoV2 mRNA genome [91]. The series of electrostatic interactions between the pyrimidines and the natural product could be due to the free amine bound to carbons 2 and 6 of guanine and adenine, respectively. Similarly, the polar region of phytocannabinoids could electrostatically interact with the phosphate terminal of SARS-CoV2 mRNA [92,93]. CBD inhibits SARS-CoV2 replication with an IC50 of 8 µM. In view of that, CBD is at least as potent as the antiviral drugs Remdesivir (RDV) and Lopinavir, which are employed for COVID-19 treatment [94,95,96,97].
CBD effectively eradicates viral mRNA expression in host cells. SARS-CoV2-induced gene expression reduction connected to chromatin modification and transcription was reversed. Gene expression reduction of mRNA spike, membrane, envelope and nucleocapsid protein-coding mRNAs was observed following 24 h of CBD treatment. It also suggested that CBD acts to prevent viral protein translation and the associated cellular changes [98]. As underlying mechanisms, CBD may suppress viral infection and promote viral RNA degradation through the induction of the interferon signaling pathway. CBD may also reduce the viral titer to allow the normal host activation of the interferon pathway, which is suppressed upon infection by SARS-CoV2 [99,100].
CBD is metabolized to 7-carboxy-cannabidiol (7-COOH-CBD) and 7-hydroxy-cannabidiol (7-OH-CBD) in the liver and intestine. CBD and 7-OH-CBD inhibit SARS-CoV2 replication in A549-ACE2 cells (EC50 of 3.6 μM) [98]. The levels of these cannabinoids in healthy patients taking CBD (Epidiolex ®) show a maximum concentration (Cmax) of CBD in blood plasma in the nM range, while 7-OH-CBD has a Cmax at μM concentrations. These results suggest that CBD itself is not present at sufficient concentrations for the effective inhibition of the SARS-CoV2 transmission cycle in humans. In contrast, the plasma concentrations of its metabolite 7-OH-CBD, whose Cmax is augmented following co-administration of CBD with a high-fat meal, are sufficient for the potential blockade of human SARS-CoV2 infection [101].
The crystal structure of the SARS-CoV2 spike protein (C-terminal domain) in complex with the ACE2 receptor (human) was solved in 2020, giving the opportunity to understand the interaction between the virus and the human receptor [102]. This knowledge gives opportunities to create selective ligands targeting the viral S1 protein and preventing human cells from SARS-CoV2 infection [5]. In this sense, cannabidiolic acid (CBDA) and cannabigerolic acid (CBGA) showed high binding affinity to recombinant S1 [103].
On the other hand, in silico analysis suggests that CBD binds to the S1 virus membrane protein by interactions with the residues Q189, M165 and E16, causing an inhibitory effect with an IC50 of 7.91 µM [104]. On the other hand, ACE2 expression in the epithelium is high in males, older people and smokers, evidencing the risk of the population to the expression of the ACE2 receptor [105]. Downregulation of ACE2 levels due to CBD actions could be a strategy to decrease susceptibility for virus infection. CBD decreases the expression of ACE2 and TMPRSS2 in oral, lung and intestinal epithelia, which could reduce the burden of the virus [71,106,107]. A study conducted in different tissues (EpiAirway TM, EpiAirwayFT TM, EpiOral TM, EpiIntestinal TM) with a level of ACE2 and TMPRSS2 overexpression identified the significant effects of cannabis in decreasing ACE2 and TMPRSS2 expression levels [107]. This information suggests that phytocannabinoids may prevent SARS-CoV2 infection.
To determine the mechanisms by which the expression downregulation of ACE2 and TMPRSS2 takes place, the mRNA levels of the genes miR-200c-3p and let-7a-5p encoding ACE2 and TMPRSS2 were measured. qRT-PCR showed that CBD modulated ACE2 and TMPRSS2 expression levels through transcriptional and post-transcriptional mechanisms. A possible mechanism by which they obtain these effects is through the activation of the AKT pathway participating in the post-transcriptional regulation of ACE2 and TMPRSS2 expression. CBD significatively decreased p65 (p-p65, Ser536) and phosphorylated AKT1/2/3 (p-AKT1/2/3) levels [108].
On the other hand, CBD is capable of significantly inhibiting the human hepatic microsomal metabolism of Remdesivir (RDV), the first drug for viral therapy to treat SARS-CoV2/MERS-CoV infection, extending the half-life of RVD through the remarkable inhibitory activity of human liver carboxylesterase-1 (CES1) and CYP3A4 microsomal metabolism. These findings suggest that CBD could be an adjuvant to RDV in the treatment of SARS-CoV2 infection [109].
8. Cytokine Storm and the Anti-Inflammatory Activity of CBD
Viral proteins and RNA from the virus genome accumulate, forming virons in the endoplasmic reticulum and the Golgi apparatus and are then transported in vesicles to the extracellular space [104]. In this process, proinflammatory M1 macrophages and helper T cells secrete interleukins, which produce inflammation within lung cells. Airway epithelial cells are known to respond to the effects of cannabinoids, which are dependent or independent of CB2 receptor activation [110]. In this inflammatory stage, CB2 receptors activated by CBD inhibited inflammatory processes such as macrophage migration to the lungs. This may be due to cannabinoid CB2 receptor overexpression in immune cells, e.g., CD8 lymphocytes, CD4 lymphocytes, monocytes and neutrophils in the spleen, thymus, kidney, lung, liver, nasal epithelium, and brain [111]. Furthermore, the stimulation of this receptor decreased the release of proinflammatory cytokines IL-1, IL-6, IL-8, IL-12 and TNF-α, by suppressing TNFα, IFNγ and IL-6 and IL-8 production as well as the transcription of COX-2 [4,69,108,112]. Moreover, CB2 agonists augmented IL-10 expression with anti-inflammatory characteristics [113]. Subsequently, CBD is capable of regulating lung cell inflammation and respiratory stress [114].
Polymorphonuclear leukocytes (PMN) produce and release cytokines and chemokines as the first line of defense against pathogen microorganisms; however, they often cause tissue damage. Cosentino et al. demonstrated that an extract standardized to 5% CBD and low THC content (<0.2%) effectively inhibited cell migration and oxidative metabolism in human PMN [115]. Another in vitro study demonstrated the effect of the extracts of inflorescences of the Arbel variety of C. sativa (with high CBD content) on the pulmonary epithelial inflammation that develops in the severe phase of the disease. The extract has significant activity in reducing cytokine secretion levels that characterize the cytokine storm (IL-6 and IL-8) in pulmonary epithelial cells, with IC50 values of 3.45 µg/mL and 3.49 µg/mL, respectively. In an experimental asthma rat model, CBD diminished airway inflammation and IL-4, IL-5, IL-13, IL-6, IL-7 and TNF-α serum levels, which contribute to fibrosis and asthma pathophysiologies [116,117]. In addition, CBD reduced the secretion of IL-1 and IFNγ by T cells [118]. Kovalchuk et al. analyzed several cannabinoid extracts, showing that six of them diminished Toll-like receptor 2 (TLR2), NFKB2, CCL2 (known as MCP-1) and WNT-2 and WNT-5a expression levels. All these proteins are involved in lung disease, ARDS, expression of further proinflammatory genes and fibrosis [115].
9. Cannabis and COVID-19: In Vivo Studies
Currently, apart from supportive measures for COVID-19 patients, there is no definitive cure for ARDS. This demonstrates the urgent necessity for effective therapeutics to treat this complex condition. An interesting in vivo study was performed by Khoadadadj et al., in which they reproduced the histopathological and viral features of ARDS associated with SARS-CoV2 infection using a synthetic high molecular weight analog of double-stranded RNA, Poly(I:C) polycytidylic acid Poly(I:C). CBD treatment resulted in the reduced presence of lymphocytes, neutrophils and monocytes and the levels of proinflammatory cytokines (e.g., IL-6, IFNγ and TNFα) [119]. In parallel, by using intranasal administration of the synthetic analog Poly (I:C) in a mouse model, another research group demonstrated that CBD can also improve ARDS symptoms through a significant increase in both blood and lung tissue apelin expression, a peptide with an important role in the central and peripheral regulation of immunity and the central nervous, metabolic and cardiovascular systems [120].
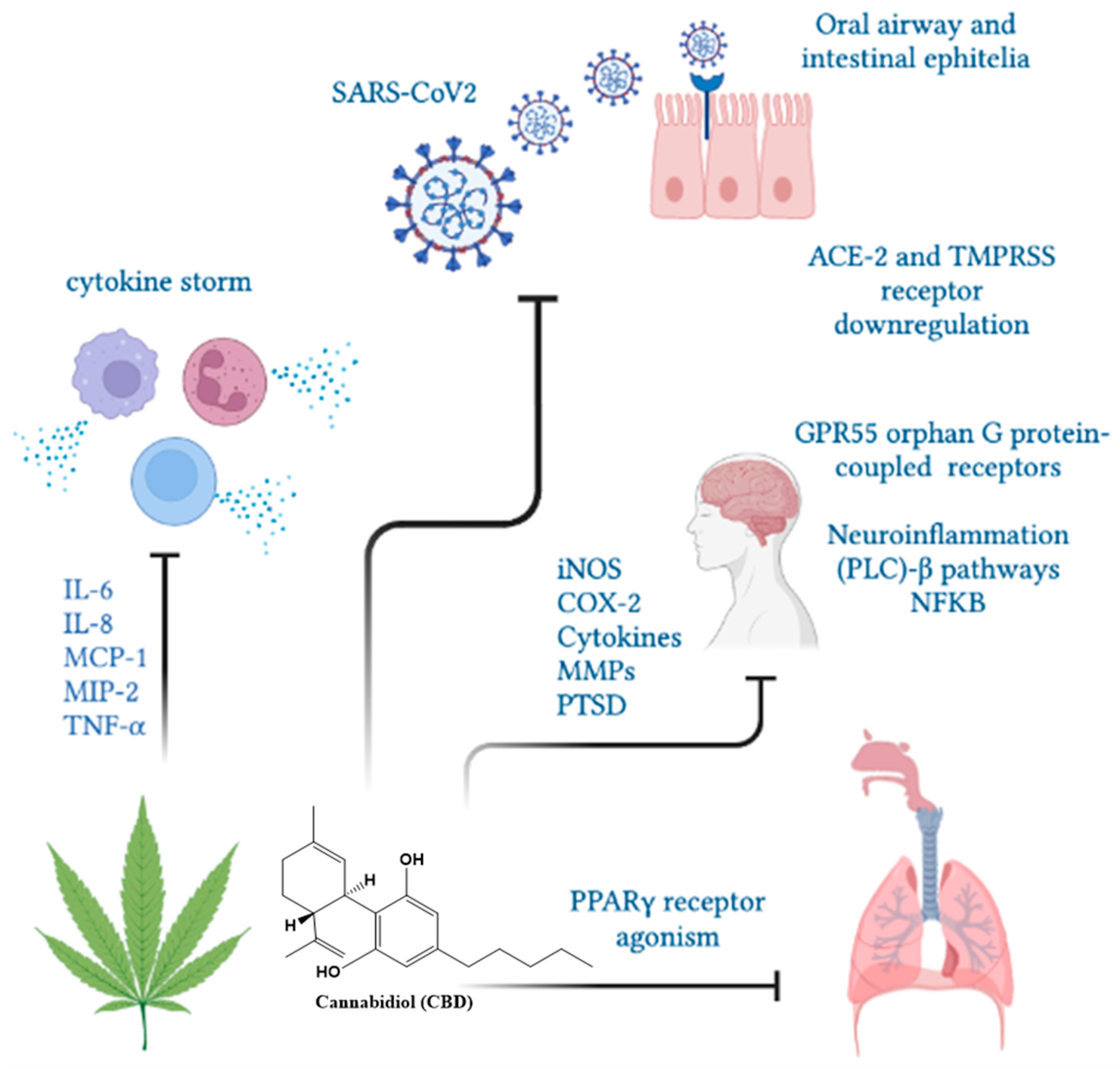
Nuclear PPARγ receptors are overexpressed in alveolar macrophages and responsible for cytokine hypersecretion resulting in the amelioration of tissue damage. CBD and other cannabinoids modulate PPARγ receptor activity, reducing pulmonary inflammation and promoting lung recovery after viral infections [121]. CBD, as a PPARγ receptor agonist, may show direct antiviral activity by regulating fibroblast/myofibroblast activation, potentially limiting the onset of late-onset pulmonary fibrosis in cured COVID-19 patients [122]. In addition, the intraperitoneal administration of CBD improved lung function and reduced inflammation in experimental acute lung injury (ALI) [123,124], pulmonary hypertension [125], hypoxic/ischemic brain injury-induced lung injury [126] and asthma [116,117]. A clinical trial demonstrated that 300 mg/day of oral-administered CBD diminished or prevented the deterioration from mild/moderate to severe/critical clinical status as determined by the COVID-19 scale or the natural course of evolution of typical clinical symptoms [127]. In humans, the use of cannabinoids prevented the induction of proinflammatory CD16 monocyte and IP-10 production, showing anti-inflammatory effects [55]. Moreover, cannabis use decreased CD4 and CD8 T cell numbers as well as the inflammatory IL-10, IL-12 and TNF-α production compared to non-cannabis users [72]. An overview of CBD functions in SARS-CoV2 infection is presented in Figure 5.
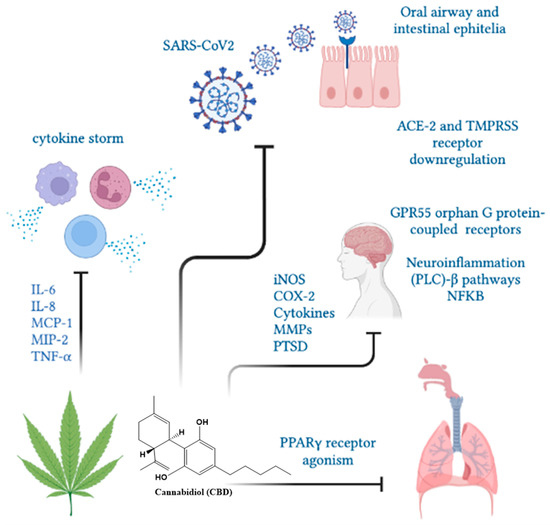
Figure 5.
The CBD functions in SARS-CoV2 infection. Cannabinol could limit the severity of SARS-CoV2 infection by decreasing ACE-2 receptors, cytokine mitigation and lung inflammation and fibrosis through PPARγ receptors.
10. Cannabidiol and the Psychological Effects of COVID
The COVID-19 pandemic resulted in devastating mortality and morbidity. Billions of lives were altered in a socioeconomic and health scale, including respiratory, cardiovascular and mental health consequences such as anxiety, depression and substance use after recovery [128]. In the U.S., 45% of affected adults showed mental health problems because of stress from the virus, job loss or imposed shelter-in-place [129]. Phytocannabinoids such as CBD could be an alternative therapy for panic disorders, anxiety, post-traumatic disorder (PTSD) and/or depressive disorders [51,130].
Within the endocannabinoid system, the highest density of CB1 receptors is distributed in key areas for stress and emotion regulation such as the prefrontal cortex (PFC), the hippocampus and the amygdala [131]. Since the development of the COVID-19 pandemics, many people have presented high levels of stress, fear and anxiety as the result of an anormal CB1 receptor expression in the amygdala, nucleus accumbens (reward system) and in the PFC, the latter being associated with increased levels of aggressive behavior [132,133]. Chronic stress conditions are characterized by low levels of the endocannabinoid anandamide; such effects are apparently more pronounced in women than in men [134]. During the global COVID-19 pandemic, women have shown higher rates of anxiety and depression than men, due to an increased domestic overload, domestic violence and impoverishment [135,136].
CBD reduced euphoria and depressive and psychotic symptoms [137] as well as reducing anxiety and stress-induced reactions in healthy volunteers [138,139,140]. It showed beneficial effects in patients with naïve social anxiety disorder [141,142], post-traumatic stress disorder [143], psychiatric symptoms [144] or at high risk for psychosis [145,146]. Therefore, the anxiolytic and antinociceptive properties of CBD make this compound an important adjuvant treatment for improving the well-being and life quality of COVID-19 patients. Cannabinol may even be applied after recovery from COVID-19 infection to alleviate post-traumatic stress symptoms.
In a randomized clinical trial, 300 mg of CBD (150 mg twice a day) was administered to healthcare professionals caring for COVID-19 patients. The results showed that CBD reduced burnout and emotional exhaustion symptoms [147]. Another study revealed that CBD may also benefit wakefulness by increasing the levels of dopamine in the lateral hypothalamus or dorsal raphe nuclei, which are responsible for wakefulness, suggesting that CBD may be used for sleep disorder therapy [148], as one of the symptoms that appear after contracting COVID. As discussed in this work, cannabinoids, especially CBD, can efficiently ameliorate or attenuate this type of pathology.
11. Conclusions
Cannabinoids, especially CBD, appear to be promising in the treatment of COVID-19, as an adjuvant of current antiviral drugs, reducing lung inflammation by decreasing chemokines and cytokines secreted by the cells of the immune system or mediating in the CNS reducing morbidity as fear, anxiety, stress, sleep disorders. However, more research and clinical studies are necessary, especially to establish the effects of their long-term use. In any case, many countries are allowing the use of medical cannabis and this plant, which has been used since ancient times, could be a natural therapeutic alternative for COVID-19 infected patients, but there is still a long way to go for its acceptance and use in routine clinical practice.
Author Contributions
Conceptualization, C.P. investigation, R.P., T.G., C.V. and V.B.; writing—review and editing, H.U. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
Authors thank the financial support given by the São Paulo Research Foundation grant (FAPESP project No. 2018/07366-4), awarded to H.U. and a FAPESP (Brazil)-Conicyt (Chile) grant awarded to H.U. and C.P. (201808426-0). H.U. also gratefully acknowledges support from the National Council for Scientific and Technological Development (CNPq Project No. CNPq (406396/2021-3)) and USP-PIPAE (Excellence Projects promoted by the University of São Paulo).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
- Plancarte-Sánchez, R.; Mansilla-Olivares, A.; De los Reyes-Pacheco, V.A.; Meneses-González, F. Aplicaciones terapéuticas por acción de los cannabinoides. Gac. Médica México 2019, 155, 307–318. [Google Scholar]
- Covarrubias-Torres, N. Uso medicinal de la Marihuana. Anest. En México 2019, 31, 49–58. [Google Scholar]
- Álvarez, L.G.; Gomar, J.J.; García-Portilla, M.P.; Bobes, J. Consumo de cannabis y alteraciones cognitivas en esquizofrenia y primeros episodios psicóticos. Adicciones 2019, 31, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1462. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- El Biali, M.; Broers, B.; Besson, M.; Demeules, J. Cannabinoids and COVID-19. Med. Cannabis Cannabinoids 2020, 3, 111–115. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharm. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encycl. Virol. 2021, 2, 428–440. [Google Scholar] [CrossRef]
- Lucaciu, O.; Aghiorghiesei, O.; Petrescu, N.B.; Mirica, I.C.; Benea, H.R.C.; Apostu, D. In quest of a new therapeutic approach in COVID-19: The endocannabinoid system. Drug Metab. Rev. 2021, 53, 478–490. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Galzerano Guida, J.; Orellana Navone, C.; Pérez, R.; Coitiño González, A.; Velázquez Ramos, P. Medicinal Cannabis as a therapeutic resource: Preliminary study. Rev. Med. Del Urug. 2019, 35, 113–137, 289–297. [Google Scholar]
- Kirkham, T. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Berrendero, F. Endogenous cannabinoid and opioid systems and their role in nicotine addiction. Curr. Drug Targets 2010, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Holm, L.; Nätt, D.; Augier, G.; Stensson, N.; Vecchiarelli, H.A.; Balsevich, G.; Aukema, R.J. Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Mol. Psychiatry 2020, 25, 993–1005. [Google Scholar] [CrossRef]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Morena, M.; Haataja, R.; Hammar, V.; Augier, G.; Hill, M.N.; Heilig, M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: A randomized, controlled experimental medicine trial. Biol. Psychiatry 2020, 87, 538–547. [Google Scholar] [CrossRef]
- Spohrs, J.; Ulrich, M.; Grön, G.; Prost, M.; Plener, P.L.; Fegert, J.M.; Bindila, L.; Abler, B. Fear extinction learning and anandamide: An fMRI study in healthy humans. Transl. Psychiatry 2021, 11, 161. [Google Scholar] [CrossRef]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 11134. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols activate TRPV1-a link between phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.G. Editor From Cannabis to the Endogenous Cannabinoid System: New Perspectives in Neuroinflammation. Annals of the Royal Academy of Doctors of Spain. 2018, 3, 379–393. Available online: https://wwwpublicacionesradees/indexphp/arade/article/view/93/72 (accessed on 10 September 2022).
- Turcotte, C.; Archambault, A.S.; Dumais, É.; Martin, C.; Blanchet, M.R.; Bissonnette, E.; Ohashi, N.; Yamamoto, K.; Itoh, T.; Laviolette, M. Endocannabinoid hydrolysis inhibition unmasks that unsaturated fatty acids induce a robust biosynthesis of 2-arachidonoyl-glycerol and its congeners in human myeloid leukocytes. FASEB J. 2020, 34, 4253–4265. [Google Scholar] [CrossRef] [PubMed]
- Prospéro García, O.E.; Ruiz Contreras, A.E.; Cortés Morelos, J.; Herrera Solís, A.; Méndez Díaz, M. Marihuana: Legalization and Medical Care. Rev. De La Fac. De Med. 2019, 62, 6–23. [Google Scholar] [CrossRef]
- Raïch, I.; Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Reyes-Resina, I.; Nadal, X.; Ferreiro-Vera, C.; de Medina, V.S.; Majellaro, M.; Sotelo, E.; et al. Similarities and differences upon binding of naturally occurring Δ(9)-tetrahydrocannabinol-derivatives to cannabinoid CB(1) and CB(2) receptors. Pharmacol. Res. 2021, 174, 105970. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Cannabinoid CB(1) and CB(2) receptors differentially regulate TNF-α-induced apoptosis and LPA(1)-mediated pro-survival signaling in HT22 hippocampal cells. Life Sci. 2021, 276, 119407. [Google Scholar] [CrossRef]
- Köfalvi, A.; Moreno, E.; Cordomí, A.; Cai, N.S.; Fernández-Dueñas, V.; Ferreira, S.G.; Guixà-González, R.; Sánchez-Soto, M.; Yano, H.; Casadó-Anguera, V.; et al. Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol. 2020, 18, 9. [Google Scholar] [CrossRef]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef]
- Malik, A.; Fatehi, K.S.; Menon, N.N.; Chaturvedi, P. Review of Medicinal Use of Cannabis Derivatives and the Societal Impact of Legalization. Indian J. Palliat. Care 2020, 26, 369–380. [Google Scholar] [CrossRef]
- Ceni, C.; Benko, M.J.; Mohamed, K.A.; Poli, G.; Di Stefano, M.; Tuccinardi, T.; Digiacomo, M.; Valoti, M.; Laprairie, R.B.; Macchia, M.; et al. Novel Potent and Selective Agonists of the GPR55 Receptor Based on the 3-Benzylquinolin-2(1H)-One Scaffold. Pharmaceuticals 2022, 15, 768. [Google Scholar] [CrossRef]
- Johns, D.; Behm, D.; Walker, D.; Ao, Z.; Shapland, E.; Daniels, D.; Riddick, M.; Dowell, S.; Staton, P.; Green, P. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007, 152, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Balenga, N.A.; Ford, L.A.; Ross, R.A.; Waldhoer, M.; Irving, A.J. The GPR55 ligand L-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009, 23, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Balenga, N.A.; Schröder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 2010, 160, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Brown, A. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef]
- Reddy, D.S.; Golub, V.M. The pharmacological basis of cannabis therapy for epilepsy. J. Pharmacol. Exp. Ther. 2016, 357, 45–55. [Google Scholar] [CrossRef]
- Maccarrone, M.; Lorenzon, T.; Bari, M.; Melino, G.; Finazzi-Agro, A. Anandamide induces apoptosis in human cells via vanilloid receptors: Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000, 275, 31938–31945. [Google Scholar] [CrossRef]
- Brown, J.D. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug–drug interactions. J. Clin. Med. 2020, 9, 919. [Google Scholar] [CrossRef]
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef]
- Mead, A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef]
- Suero-García, C.; Martín-Banderas, L.; Holgado, M. Efecto neuroprotector de los cannabinoides en las enfermedades neurodegenerativas. Ars Pharm. 2015, 56, 77–87. [Google Scholar] [CrossRef][Green Version]
- U.S. Food & Drug Administration Fel. Marinol® Data Sheet or Summary of Product Characteristics 06/12/2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 10 September 2022).
- Gloss, D. An overview of products and bias in research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F.; Müller-Vahl, K. Medicinal uses of marijuana and cannabinoids. Crit. Rev. Plant Sci. 2016, 35, 378–405. [Google Scholar] [CrossRef]
- Gover, J. Sativex 2.7 mg/2.5 mg Oral Spray Solution. GW Pharma Ltd. 2018. Available online: https://cima.aemps.es/cima/dochtml/ft/72544/FT_72544.html (accessed on 25 July 2021).
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging use of epidiolex (cannabidiol) in epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N. Peruvian patients use cannabidiol for refractory epilepsy: Apropos of the evidence-based indication of marijuana-derived products. J. Neuro-Psychiatry 2019, 82, 101–103. [Google Scholar]
- Antonio, L.; De Los Milagros, P. Marihuana Medicinal en el Tratamiento de la Epilepsia. Rev. Médica Hosp. Hipólito Unanue Tacna 2018, 11, 38–42. [Google Scholar]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Devinsky, O.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Laux, L.; Wright, S. Cannabidiol (CBD) Reduces Convulsive Seizure Frequency in Dravet Syndrome: Results of a Multi-Center, Randomized, Controlled Trial (GWPCARE1)(CT. 001); AAN Enterprises: Barcelona, España, 2017. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Rizzo, M.D.; Crawford, R.B.; Henriquez, J.E.; Aldhamen, Y.A.; Gulick, P.; Amalfitano, A.; Kaminski, N.E. HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-γ-inducible protein 10 levels compared with nonusing HIV patients. Aids 2018, 32, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vázquez, O.; Rojas, A.T.; Fleury, A. Neuroinflammation and epilepsy. TIP J. Spec. Chem. Biol. Sci. 2016, 19, 24–31. [Google Scholar]
- Fernández-Ruiz, J.; García, C.; Sagredo, O.; Gómez-Ruiz, M.; de Lago, E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin. Ther. Targets 2010, 14, 387–404. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; Group, G.P.A.S. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Bebin, E.M.; Cutter, G.; DeWolfe, J.; Dure, L.S.; Gaston, T.E.; Kankirawatana, P.; Liu, Y.; Singh, R.; Standaert, D.G. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018, 87, 131–136. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Główka, A.K.; Mikulska, A.A.; Główka, F.K. Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients. Int. J. Mol. Sci. 2021, 22, 9582. [Google Scholar] [CrossRef]
- OMS. Panel de Control de la Enfermedad por Coronavirus (COVID-19) [En Línea]. 2022. Available online: https://covid19.who.int/ (accessed on 18 May 2022).
- Costiniuk, C.T.; Jenabian, M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020, 53, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.D.; Henriquez, J.E.; Blevins, L.K.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Targeting Cannabinoid Receptor 2 on Peripheral Leukocytes to Attenuate Inflammatory Mechanisms Implicated in HIV-Associated Neurocognitive Disorder. J. Neuroimmune Pharmacol. 2020, 15, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Argenziano, M.; Di Paola, A.; Punzo, F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020, 21, 3809. [Google Scholar] [CrossRef]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A., Jr.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Manuzak, J.A.; Gott, T.M.; Kirkwood, J.S.; Coronado, E.; Hensley-McBain, T.; Miller, C.; Cheu, R.K.; Collier, A.C.; Funderburg, N.T.; Martin, J.N.; et al. Heavy Cannabis Use Associated With Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1872–1882. [Google Scholar] [CrossRef]
- Park, M.D. Macrophages: A Trojan horse in COVID-19? Nat. Rev. Immunol. 2020, 20, 351. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Hasenberg, M.; Stegemann-Koniszewski, S.; Gunzer, M. Cellular immune reactions in the lung. Immunol. Rev. 2013, 251, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.A.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217, e20200678. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e882. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e1003. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e116. [Google Scholar] [CrossRef]
- Schneider, W.M.; Luna, J.M.; Hoffmann, H.H.; Sánchez-Rivera, F.J.; Leal, A.A.; Ashbrook, A.W.; Le Pen, J.; Ricardo-Lax, I.; Michailidis, E.; Peace, A.; et al. Genome-Scale Identification of SARS-CoV-2 and Pan-coronavirus Host Factor Networks. Cell 2021, 184, 120–132.e114. [Google Scholar] [CrossRef]
- Wang, R.; Simoneau, C.R.; Kulsuptrakul, J.; Bouhaddou, M.; Travisano, K.A.; Hayashi, J.M.; Carlson-Stevermer, J.; Zengel, J.R.; Richards, C.M.; Fozouni, P.; et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 2021, 184, 106–119.e114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Tavakoli-Yaraki, M.; Salimi, V. Opioids/cannabinoids as a potential therapeutic approach in COVID-19 patients. Expert Rev. Respir. Med. 2020, 14, 965–967. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Muhammad, A.; Abarshi, M.M.; Amaku, J.F.; Katsayal, S.B.; Nde, A.L. Targeting of Protein's Messenger RNA for Viral Replication, Assembly and Release in SARS-CoV-2 Using Whole Genomic Data From South Africa: Therapeutic Potentials of Cannabis Sativa L. Front. Pharmacol. 2021, 12, 736511. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V. Purines, Pyrimidines and Nucleotides and the Chemistry of Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Attia, Y.A.; Alagawany, M.M.; Farag, M.R.; Alkhatib, F.M.; Khafaga, A.F.; Abdel-Moneim, A.E.; Asiry, K.A.; Mesalam, N.M.; Shafi, M.E.; Al-Harthi, M.A.; et al. Phytogenic Products and Phytochemicals as a Candidate Strategy to Improve Tolerance to Coronavirus. Front. Vet. Sci. 2020, 7, 573159. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Kaur, R.; Bhardwaj, A.; Bhardwaj, V.; Ohri, P.; Sharma, A.; Ahmad, A.; Bhardwaj, R.; Ahmad, P. Herbal immune-boosters: Substantial warriors of pandemic COVID-19 battle. Phytomed. Int. J. Phytother. Phytopharm. 2021, 85, 153361. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Ozaki, M.; Otsuka, C.; Vecchione, A.; Arai, T.; Kitagawa, T.; Ofusa, K.; Yabumoto, M.; Hirotsu, T.; et al. COVID-19 Drug Discovery Using Intensive Approaches. Int. J. Mol. Sci. 2020, 21, 2839. [Google Scholar] [CrossRef]
- Peng, M. Outbreak of COVID-19: An emerging global pandemic threat. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 129, 110499. [Google Scholar] [CrossRef]
- Tu, Y.F.; Chien, C.S.; Yarmishyn, A.A.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Lai, W.Y.; Yang, D.M.; Chou, S.J.; Yang, Y.P.; et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef]
- Weisberg, E.; Sattler, M.; Yang, P.L.; Parent, A.; Gray, N.; Griffin, J.D. Current therapies under investigation for COVID-19: Potential COVID-19 treatments. Can. J. Physiol. Pharmacol. 2020, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Ohtsuki, T.; Chen, S.N.; Friesen, J.B.; Drayman, N.; Mohamed, A.; Dann, C.; et al. Cannabidiol Inhibits SARS-CoV-2 Replication and Promotes the Host Innate Immune Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Kochs, G.; Weber, F. The interferon response circuit: Induction and suppression by pathogenic viruses. Virology 2006, 344, 119–130. [Google Scholar] [CrossRef]
- Food and Drug Administration. Application Number 210365Orig1s000. In Clinical Pharmacology and Biopharmaceutical Reviews; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Waghray, A.; Heimberg, G.; Sikkema, L.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smilie, C.; et al. Integrated single-cell atlas analyses reveal associations of age, gender, and smoking with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlight inflammatory programs in putative target cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Kicman, A.; Schlicker, E. Opportunities, Challenges and Pitfalls of Using Cannabidiol as an Adjuvant Drug in COVID-19. Int. J. Mol. Sci. 2021, 22, 1986. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020, 12, 22425–22444. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Fiselier, A.; Kovalchuk, I.; Kovalchuk, O. New AKT-dependent mechanisms of anti-COVID-19 action of high-CBD Cannabis sativa extracts. Cell Death Discov. 2022, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Vartak, R.; Patki, M.; Patel, K. Cannabidiol Inhibits In Vitro Human Liver Microsomal Metabolism of Remdesivir: A Promising Adjuvant for COVID-19 Treatment. Cannabis Cannabinoid Res. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.; Montes, C.; Harui, A.; Beedanagari, S.R.; Kiertscher, S.; Stripecke, R.; Hossepian, D.; Kitchen, C.; Kern, R.; Belperio, J.; et al. Clarifying CB2 receptor-dependent and independent effects of THC on human lung epithelial cells. Toxicol. Appl. Pharmacol. 2008, 231, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Janecki, M.; Graczyk, M.; Lewandowska, A.A.; Pawlak, Ł. Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 4170. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Gertsch, J. Editorial: Lung macrophages high on cannabinoids: Jamming PAMs and taming TAMs? J. Leukoc. Biol. 2016, 99, 518–520. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol–recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Wang, B.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, S.; Kovalchuk, I.; Kovalchuk, O. Fighting the storm: Could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? Aging 2021, 13, 1571–1590. [Google Scholar] [CrossRef]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Dal-Pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediat. Inflamm. 2015, 2015, 538670. [Google Scholar] [CrossRef]
- Kaplan, B.L.; Springs, A.E.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharm. 2008, 76, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Shin, E.; Jarrahi, A.; Costigliola, V.; Kumar, P.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; et al. A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. J. Cannabis. Res. 2021, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Milam, J.E.; Keshamouni, V.G.; Phan, S.H.; Hu, B.; Gangireddy, S.R.; Hogaboam, C.M.; Standiford, T.J.; Thannickal, V.J.; Reddy, R.C. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L891–L901. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef]
- Arruza, L.; Pazos, M.R.; Mohammed, N.; Escribano, N.; Lafuente, H.; Santos, M.; Alvarez-Díaz, F.J.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr. Res. 2017, 82, 79–86. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Pacheco, J.C.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.L.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. 2021, 7, 658–669. [Google Scholar] [CrossRef]
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Krizinger, A.K.A.; Hamel, L.; Brodie, M. KFF Health Follow-up Survey—Early April 2020: The Impact of Coronavirus on Life in the United States. Available online: https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-early-april-2020/ (accessed on 10 August 2022).
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharm. 2007, 152, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, C.S.; Sim-Selley, L.J. Basic neuroanatomy and neuropharmacology of cannabinoids. Int. Rev. Psychiatry 2009, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.; Núñez, E.; Benito, C.; Tolón, R.M.; Romero, J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. Behav. 2005, 81, 239–247. [Google Scholar] [CrossRef]
- Neumeister, A.; Normandin, M.D.; Pietrzak, R.H.; Piomelli, D.; Zheng, M.Q.; Gujarro-Anton, A.; Potenza, M.N.; Bailey, C.R.; Lin, S.F.; Najafzadeh, S.; et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol. Psychiatry 2013, 18, 1034–1040. [Google Scholar] [CrossRef]
- Thibaut, F.; van Wijngaarden-Cremers, P.J.M. Women’s Mental Health in the Time of COVID-19 Pandemic. Front. Glob. Women's Health 2020, 1, 588372. [Google Scholar] [CrossRef]
- Yan, S.; Xu, R.; Stratton, T.D.; Kavcic, V.; Luo, D.; Hou, F.; Bi, F.; Jiao, R.; Song, K.; Jiang, Y. Sex differences and psychological stress: Responses to the COVID-19 pandemic in China. BMC Public Health 2021, 21, 79. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.A.; Greenwood, L.M.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [Google Scholar] [CrossRef]
- Crippa, J.A.; Zuardi, A.W.; Garrido, G.E.; Wichert-Ana, L.; Guarnieri, R.; Ferrari, L.; Azevedo-Marques, P.M.; Hallak, J.E.; McGuire, P.K.; Filho Busatto, G. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 2004, 29, 417–426. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; de Oliveira, D.C.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in anxiety and sleep: A large case series. Perm. J. 2019, 23, 18–41. [Google Scholar] [CrossRef]
- Appiah-Kusi, E.; Petros, N.; Wilson, R.; Colizzi, M.; Bossong, M.G.; Valmaggia, L.; Mondelli, V.; McGuire, P.; Bhattacharyya, S. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology 2020, 237, 1121–1130. [Google Scholar] [CrossRef]
- Wilson, R.; Bossong, M.G.; Appiah-Kusi, E.; Petros, N.; Brammer, M.; Perez, J.; Allen, P.; McGuire, P.; Bhattacharyya, S. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl. Psychiatry 2019, 9, 203. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; de Lima Osório, F.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; Pacheco, J.C.; et al. Efficacy and Safety of Cannabidiol Plus Standard Care vs. Standard Care Alone for the Treatment of Emotional Exhaustion and Burnout Among Frontline Health Care Workers During the COVID-19 Pandemic: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2120603. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Millán-Aldaco, D.; Palomero-Rivero, M.; Mechoulam, R.; Drucker-Colín, R. The nonpsychoactive Cannabis constituent cannabidiol is a wake-inducing agent. Behav. Neurosci. 2008, 122, 1378–1382. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).