Cost-Effectiveness of Electrical Stimulation Therapy in the Treatment of Chronic Wounds: A Systematic Review, Meta-Analysis and Economic Analysis
Abstract
1. Introduction
2. Materials and Methods
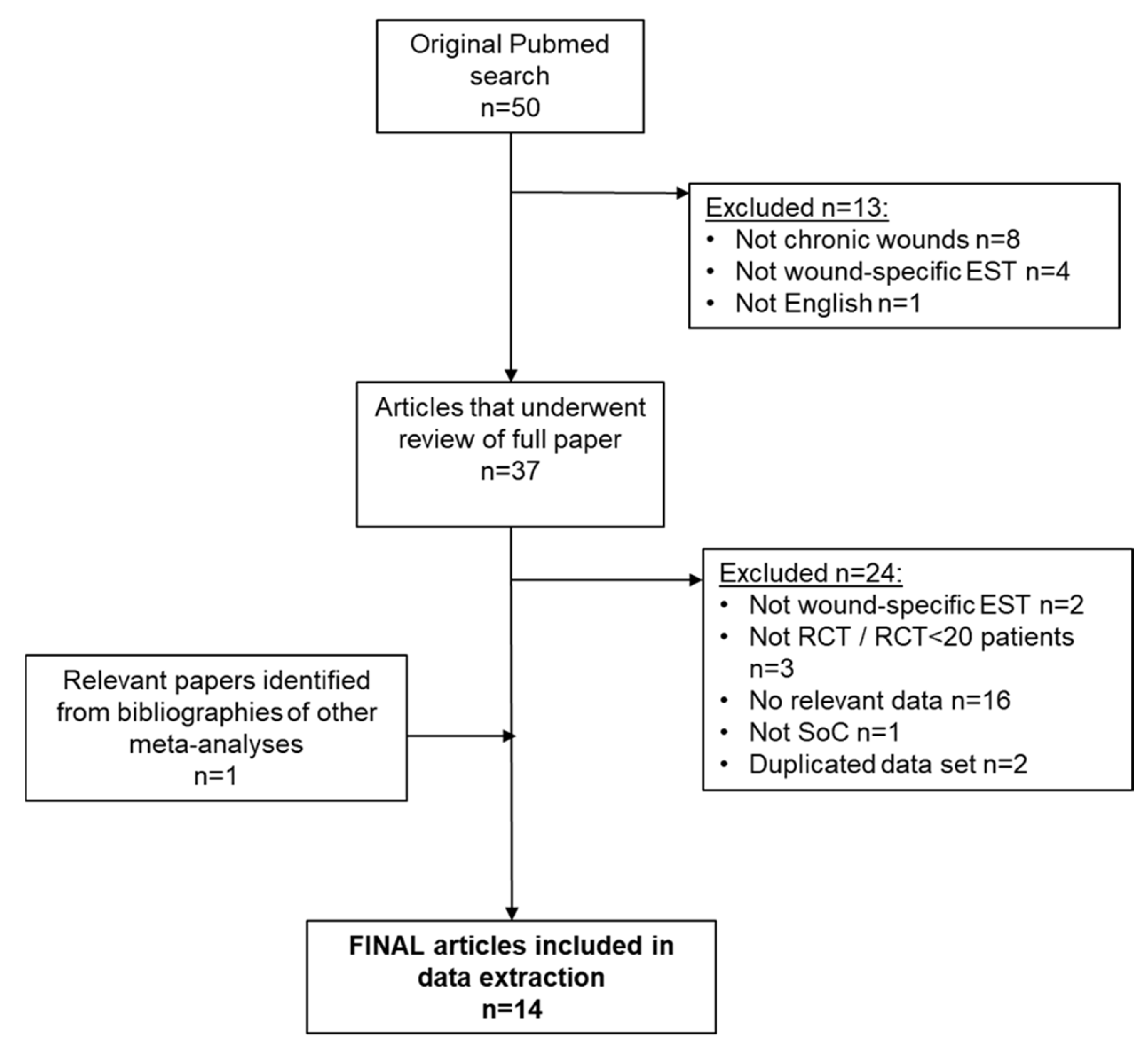
2.1. Systematic Review and Meta-Analysis
2.2. Study Eligibility
2.3. Data Extraction
2.4. Analysis of Study Quality
2.5. Statistical Analysis
2.6. Subgroup Analysis
2.7. Sensitivity Analysis
2.8. Cost Effectiveness Analysis
3. Results
3.1. Systematic Review
3.1.1. Proportion of Chronic Wounds Healed
3.1.2. Time to Healing for Chronic Wounds
3.2. Cost Effectiveness of EST
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| DFU | Diabetic foot ulcer |
| EST | Electrical Stimulation Therapy |
| GP | General practitioner |
| HCP | Healthcare professional |
| NHS | National Health Service |
| OR | Odds ratio |
| PU | Pressure ulcer |
| RCT | Randomised controlled trial |
| SoC | Standard of care |
| VLU | Venous leg ulcer |
References
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef]
- NHS Confederation. Autumn Budget 2024: What You Need to Know. Available online: https://www.nhsconfed.org/publications/autumn-budget-2024-what-you-need-know (accessed on 12 June 2025).
- Avendaño-Coy, J.; López-Muñoz, P.; Serrano-Muñoz, D.; Comino-Suárez, N.; Avendaño-López, C.; Martin-Espinosa, N. Electrical microcurrent stimulation therapy for wound healing: A meta-analysis of randomized clinical trials. J. Tissue Viability 2022, 31, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.-Y.; Liu, W.-H.; Li, G.-S. Electric Stimulation as an Effective Adjunctive Therapy for Diabetic Foot Ulcer: A Meta-analysis of Randomized Controlled Trials. Adv. Skin Wound Care 2020, 33, 608–612. [Google Scholar] [CrossRef]
- Lan, X.; Huang, Z.; Zheng, Y.; Huang, Z.; Tang, Y.; Zhou, T.; Wang, C.; Ma, Y.; Li, D. Electrical stimulation as an adjunctive therapy for diabetic ulcers: A systematic review and meta-analysis. Int. Wound J. 2024, 21, e70104. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Harvey, L.A.; Glinsky, J.V.; Nier, L.; Lavrencic, L.; Kifley, A.; Cameron, I.D. Electrical stimulation for treating pressure ulcers. Cochrane Database Syst. Rev. 2020, 1, CD012196. [Google Scholar] [CrossRef] [PubMed]
- Szołtys-Brzezowska, B.; Bańkowska, A.; Piejko, L.; Zarzeczny, R.; Nawrat-Szołtysik, A.; Kloth, L.C.; Polak, A. Electrical Stimulation in the Treatment of Pressure Injuries: A Systematic Review of Clinical Trials. Adv. Skin Wound Care 2023, 36, 292–302. [Google Scholar] [CrossRef]
- Borges, D.; Pires, R.; Ferreira, J.; Dias-Neto, M. The effect of wound electrical stimulation in venous leg ulcer healing—A systematic review. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 1070–1079.e1. [Google Scholar] [CrossRef]
- Ofstead, C.L.; Buro, B.L.; Hopkins, K.M.; Eiland, J.E. The impact of continuous electrical microcurrent on acute and hard-to-heal wounds: A systematic review. J. Wound Care 2020, 29 (Suppl. S7), S6–S15. [Google Scholar] [CrossRef]
- Kawasaki, L.; Mushahwar, V.K.; Ho, C.; Dukelow, S.P.; Chan, L.L.H.; Chan, K.M. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: A systematic review. Wound Repair Regen. 2014, 22, 161–173. [Google Scholar] [CrossRef]
- Guest, J.F.; Singh, H.; Rana, K.; Vowden, P. Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: Results of an RCT. J. Wound Care 2018, 27, 230–243. [Google Scholar] [CrossRef]
- Jünger, M.; Arnold, A.; Zuder, D.; Stahl, H.; Heising, S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse®): A prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008, 16, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; Greaves, T. Clinical outcomes and cost-effectiveness of an externally applied electroceutical device in managing venous leg ulcers in clinical practice in the UK. J. Wound Care 2015, 24, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.P.; Guest, J.F. Modelling the cost-utility of bio-electric stimulation therapy compared to standard care in the treatment of elderly patients with chronic non-healing wounds in the UK. Curr. Med. Res. Opin. 2007, 23, 871–883. [Google Scholar] [CrossRef]
- Mittmann, N.; Chan, B.C.; Craven, B.C.; Isogai, P.K.; Houghton, P. Evaluation of the cost-effectiveness of electrical stimulation therapy for pressure ulcers in spinal cord injury. Arch. Phys. Med. Rehabil. 2011, 92, 866–872. [Google Scholar] [CrossRef]
- Ghatak, P.D.; Schlanger, R.; Ganesh, K.; Lambert, L.; Gordillo, G.M.; Martinsek, P.; Roy, S. A Wireless Electroceutical Dressing Lowers Cost of Negative Pressure Wound Therapy. Adv. Wound Care 2015, 4, 302–311. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; The Cochrane Collaboration: London, UK, 2019; Available online: https://handbook.cochrane.org (accessed on 25 April 2025).
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef]
- Kent Academic Repositor Unit. Costs Analysis for 2023/24, Unit Costs of Health and Social Care 2024 Manual. Available online: https://kar.kent.ac.uk/109563/ (accessed on 12 June 2025).
- New Research Finds UK Nurses Carry out 180 Wound Dressing Changes a Year—On Each Chronic Wound Patient. Wounds UK, August 2018. Available online: https://wounds-uk.com/must-reads/new-research-finds-uk-nurses-carry-out-180-wound-dressing-changes-a-year-on-each-chronic-wound-patient/ (accessed on 12 June 2025).
- Tuson, R.; Metry, A.; Harding, K. Cost-effectiveness analysis of the geko™ device (an NMES technology) in managing venous leg ulcers in UK healthcare settings. Int. Wound J. 2024, 21, e70048. [Google Scholar] [CrossRef]
- Avendaño-Coy, J.; Martín-Espinosa, N.M.; Ladriñán-Maestro, A.; Gómez-Soriano, J.; Suárez-Miranda, M.I.; López-Muñoz, P. Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 10045. [Google Scholar] [CrossRef]
- Elio, C.; Fontani, V.; Rinaldi, S.; Gasbarro, V. REAC-induced endogenous bioelectric currents in the treatment of venous ulcers: A three-arm randomized controlled prospective study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 109–113. [Google Scholar] [CrossRef]
- Polak, A.; Kloth, L.C.; Blaszczak, E.; Taradaj, J.; Nawrat-Szoltysik, A.; Ickowicz, T.; Hordynska, E.; Franek, A.; Kucio, C. The Efficacy of Pressure Ulcer Treatment with Cathodal and Cathodal-Anodal High-Voltage Monophasic Pulsed Current: A Prospective, Randomized, Controlled Clinical Trial. Phys. Ther. 2017, 97, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Kloth, L.C.; Blaszczak, E.; Taradaj, J.; Nawrat-Szoltysik, A.; Walczak, A.; Bialek, L.; Paczula, M.; Franek, A.; Kucio, C. Evaluation of the Healing Progress of Pressure Ulcers Treated with Cathodal High-Voltage Monophasic Pulsed Current: Results of a Prospective, Double-blind, Randomized Clinical Trial. Adv. Skin Wound Care 2016, 29, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Taradaj, J.; Nawrat-Szoltysik, A.; Stania, M.; Dolibog, P.; Blaszczak, E.; Zarzeczny, R.; Juras, G.; Franek, A.; Kucio, C. Reduction of pressure ulcer size with high-voltage pulsed current and high-frequency ultrasound: A randomised trial. J. Wound Care 2016, 25, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.E.; Campbell, K.E.; Fraser, C.H.; Harris, C.; Keast, D.H.; Potter, P.J.; Hayes, K.C.; Woodbury, M.G. Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch. Phys. Med. Rehabil. 2010, 91, 669–678. [Google Scholar] [CrossRef]
- Adunsky, A.; Ohry, A. Decubitus direct current treatment (DDCT) of pressure ulcers: Results of a randomized double-blinded placebo controlled study. Arch. Gerontol. Geriatr. 2005, 41, 261–269. [Google Scholar] [CrossRef]
- Peters, E.J.; Lavery, L.A.; Armstrong, D.G.; Fleischli, J.G. Electric stimulation as an adjunct to heal diabetic foot ulcers: A randomized clinical trial. Arch. Phys. Med. Rehabil. 2001, 82, 721–725. [Google Scholar] [CrossRef]
- Baker, L.L.; Rubayi, S.; Villar, F.; Demuth, S.K. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Repair Regen. 1996, 4, 21–28. [Google Scholar] [CrossRef]
- Jercinovic, A.; Karba, R.; Vodovnik, L.; Stefanovska, A.; Kroselj, P.; Turk, R.; Dzidic, I.; Benko, H.; Savrin, R. Low frequency pulsed current and pressure ulcer healing. IEEE Trans. Rehabil. Eng. 1994, 2, 225–233. [Google Scholar] [CrossRef]
- Lundeberg, T.C.; Eriksson, S.V.; Malm, M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann. Plast. Surg. 1992, 29, 328–331. [Google Scholar] [CrossRef]
- Ovens, L. Application of Accel-Heal® for patients with chronic venous leg ulcers: An evaluation in a community UK NHS trust. Wounds UK 2019, 15, 78–84. [Google Scholar]
- Moon, M.; Hawes, L.; Hazell, D. Use of electrical stimulation therapy to reduce pain associated with hard-to-heal wounds and reduce reliance on pharmacological analgesics: A case series. J. Wound Care 2025, 34, 2–9. [Google Scholar]
- Kurz, P.; Danner, G.; Lembelembe, J.; Nair, H.K.R.; Martin, R. Activation of healing and reduction of pain by single-use automated microcurrent electrical stimulation therapy in patients with hard-to-heal wounds. Int. Wound J. 2023, 20, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Du, X.; Yin, L.; Liu, H. Effect of electrical stimulation on patients with diabetes-related ulcers: A systematic review and meta-analysis. BMC Endocr. Disord. 2022, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ruan, Y.; Ma, Y.; Ge, L.; Han, L. Effectiveness and safety of electrical stimulation for treating pressure ulcers: A systematic review and meta-analysis. Int. J. Nurs. Pract. 2023, 29, e13041. [Google Scholar] [CrossRef] [PubMed]
- Girgis, B.; Carvalho, D.; Duarte, J.A. The effect of high-voltage monophasic pulsed current on diabetic ulcers and their potential pathophysiologic factors: A systematic review and meta-analysis. Wound Repair Regen. 2023, 31, 171–186. [Google Scholar] [CrossRef]
- Girgis, B.; Duarte, J.A. High Voltage Monophasic Pulsed Current (HVMPC) for stage II–IV pressure ulcer healing. A systematic review and meta-analysis. J. Tissue Viability 2018, 27, 274–284. [Google Scholar] [CrossRef]
- Khouri, C.; Kotzki, S.; Roustit, M.; Blaise, S.; Gueyffier, F.; Cracowski, J.L. Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: An effect size meta-analysis. Wound Repair Regen. 2017, 25, 883–891. [Google Scholar] [CrossRef]
- Lala, D.; Spaulding, S.J.; Burke, S.M.; Houghton, P.E. Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: A systematic review and meta-analysis. Int. Wound J. 2016, 13, 1214–1226. [Google Scholar] [CrossRef]
- Liu, L.; Moody, J.; Gall, A. A Quantitative, Pooled Analysis and Systematic Review of Controlled Trials on the Impact of Electrical Stimulation Settings and Placement on Pressure Ulcer Healing Rates in Persons with Spinal Cord Injuries. Ostomy Wound Manag. 2016, 62, 16–34. [Google Scholar]
- Barnes, R.; Shahin, Y.; Gohil, R.; Chetter, I. Electrical stimulation vs. standard care for chronic ulcer healing: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Investig. 2014, 44, 429–440. [Google Scholar] [CrossRef]
- Gardner, S.E.; Frantz, R.A.; Schmidt, F.L. Effect of electrical stimulation on chronic wound healing: A meta-analysis. Wound Repair Regen. 1999, 7, 495–503. [Google Scholar] [CrossRef]
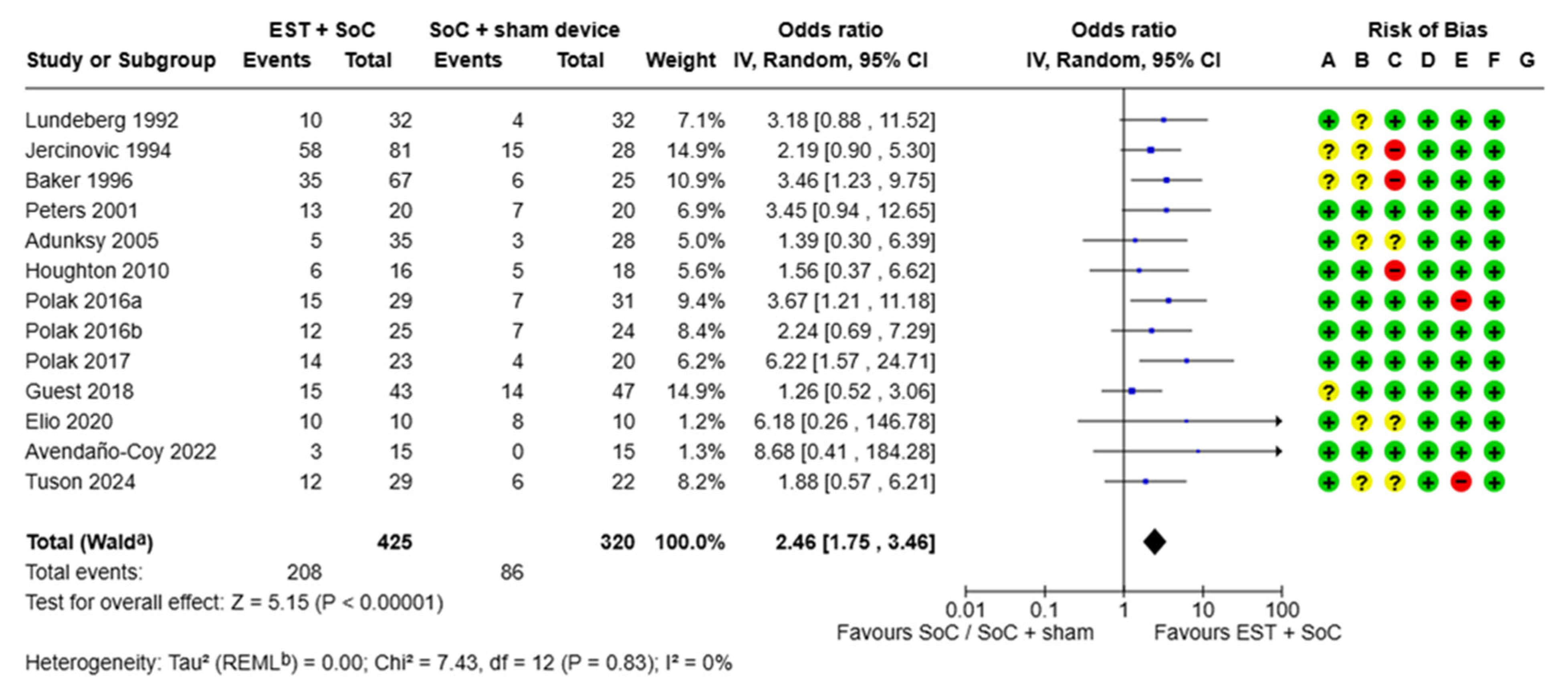
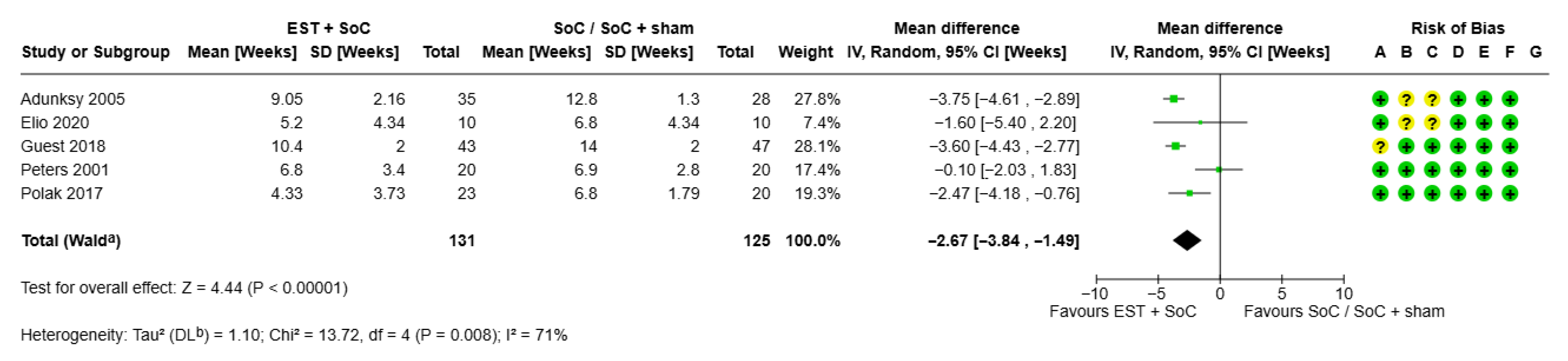
| Paper | Wound Type | N Patients | Study Duration | Experimental/Control Arm | Proportion of Wounds Healed, % | Mean (SD) Time to Healing, Weeks | Cost Analysis |
|---|---|---|---|---|---|---|---|
| Tuson et al. (2024) [23] | VLU | 51 | 16 weeks | NMES + SoC | 42% | 25.3 | N |
| SoC only | 27% | 37.6 | |||||
| Avendano-Coy (2022) [24] | PU (nursing care setting) | 30 | 25 days (3.5 weeks) | Microcurrent EST + SoC | 20% | NR | N |
| Sham device + SoC | 0% | NR | |||||
| Elio (2020) [25] | VLU | 30 | 8 weeks | REAC + SoC | 100% | 5.2 (4.3) | N |
| SoC only | 80% | 6.8 (4.3) | |||||
| Guest (2018) [12] | VLU | 90 | 8, 16 and 24 weeks | Microcurrent EST + SoC | 34% | 10.4 (2.0) | Y |
| Sham device + SoC | 30% | 14.0 (2.0) | |||||
| Polak (2017) [26] | PU (nursing care) | 63 | 12 weeks | HVPC + SoC | 60.9% | 4.3 (3.7) | N |
| Sham device + SoC | 20.0% | 6.8 (1.8) | |||||
| Polak (2016a) [27] | PU (nursing care) | 77 | 6 weeks | HVPC + SoC | 51.7% | NR | N |
| Sham device + SoC | 22.6 | NR | |||||
| Polak (2016b) [28] | PU (nursing care) | 49 | 6 weeks | HVPC + SoC | 48.0% | NR | N |
| Sham device + SoC | 29.2% | NR | |||||
| Houghton (2010) [29] | PU (spinal cord injury) | 34 | 12 weeks | HVPC + SoC | 37.5% | NR | N |
| Sham device + SoC | 27.8% | NR | |||||
| Junger (2008) [13] | VLU | 39 | 20 weeks | 300 μA EST + SoC | NR | NR | Y |
| Sham device + SoC | NR | NR | |||||
| Adunsky (2005) [30] | PU | 63 | 8–12 weeks | DC EST | 14.0% | 9.1 (2.2) | N |
| Sham device | 10.7% | 12.8 (1.3) | |||||
| Peters (2001) [31] | DFU | 40 | 12 weeks | PC (Micro-Z) + SoC | 65.0% | 6.8 (3.4) | N |
| Sham device + SoC | 35.0% | 6.9 (2.8) | |||||
| Baker (1996) [32] | PU (spinal cord injury) | 80 * | 4 weeks | EST (biphasic pulsed current) | 52.2% | NR | N |
| Sham device | 24.0% | NR | |||||
| Jercinovich (1994) [33] | PU (spinal cord injury) | 73 ** | 52 weeks | EST (NMES type) + SoC | 71.6% | NR | N |
| SoC | 53.6% | NR | |||||
| Lundeberg (1992) [34] | DFU | 64 | 12 weeks | EST (AC) | 31.3% | NR | N |
| Sham device | 12.5% | NR |
| SoC | EST + SoC | EST Incremental | ||
|---|---|---|---|---|
| Cohort size, patients treated | 100 | 100 | ||
| Time period modelled, weeks | 12 | 12 | ||
| Patients healed, % * | 26.9 | 48.9 | ||
| Time to heal, weeks * | 10.93 | 8.26 | ||
| Cost of SoC, GBP per week ^ | 403.69 | 403.69 | ||
| Cost of EST † | 0 | GBP 240.00 | ||
| Number of nursing visits per week | 2.5 | 2.5 | ||
| Time/visit, min | 18 | 18 | ||
| Resource and cost differences between arms based on treating 100 patients over a 12-week period | ||||
| Weeks of treatment | Wounds that healed | 293.74 | 403.91 | |
| Wounds not healed | 877.50 | 613.20 | ||
| Total | 1171.24 | 1017.11 | −154.13 | |
| Weeks free of ulcer | Total | 28.76 | 182.89 | 154.13 |
| Costs, GBP | Cost of SoC | GBP 472,825 | GBP 410,599 | |
| Cost of EST | 0 | GBP 24,000 | ||
| Total | GBP 472,825 | GBP 434,599 | −GBP 38,226 | |
| Resources | Treatment weeks | 1171.24 | 1017.11 | |
| Number of visits | 2928 | 2543 | −385 | |
| Time required for visits, hours | 878 | 763 | −116 | |
| Reduced Time to Healing as a Result of Adding EST to SoC * | Additional % Healed with Addition of EST to SoC ^ | |||
|---|---|---|---|---|
| 22% | 11% | 5.5% | 0% | |
| 2.67 weeks faster | GBP 38,226 savings | GBP 33,464 savings | GBP 31,088 savings | GBP 28,712 savings |
| 1.49 weeks faster | GBP 25,424 savings | GBP 15,409 savings | GBP 10,414 savings | GBP 5418 savings |
| 3.94 weeks faster | GBP 50,919 savings | GBP 51,365 savings | GBP 51,587 savings | GBP 51,809 savings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.M.; Posnett, J.; Woodmansey, E.J. Cost-Effectiveness of Electrical Stimulation Therapy in the Treatment of Chronic Wounds: A Systematic Review, Meta-Analysis and Economic Analysis. J. Mark. Access Health Policy 2025, 13, 59. https://doi.org/10.3390/jmahp13040059
Smith JM, Posnett J, Woodmansey EJ. Cost-Effectiveness of Electrical Stimulation Therapy in the Treatment of Chronic Wounds: A Systematic Review, Meta-Analysis and Economic Analysis. Journal of Market Access & Health Policy. 2025; 13(4):59. https://doi.org/10.3390/jmahp13040059
Chicago/Turabian StyleSmith, Jennifer M., John Posnett, and Emma J. Woodmansey. 2025. "Cost-Effectiveness of Electrical Stimulation Therapy in the Treatment of Chronic Wounds: A Systematic Review, Meta-Analysis and Economic Analysis" Journal of Market Access & Health Policy 13, no. 4: 59. https://doi.org/10.3390/jmahp13040059
APA StyleSmith, J. M., Posnett, J., & Woodmansey, E. J. (2025). Cost-Effectiveness of Electrical Stimulation Therapy in the Treatment of Chronic Wounds: A Systematic Review, Meta-Analysis and Economic Analysis. Journal of Market Access & Health Policy, 13(4), 59. https://doi.org/10.3390/jmahp13040059







