Network Meta-Analysis of Bevacizumab Gamma Versus Competing Interventions for Treating Neovascular Age-Related Macular Degeneration in the United Kingdom
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods: Systematic Literature Review
2.2. Materials and Methods: Network Meta-Analysis
3. Results
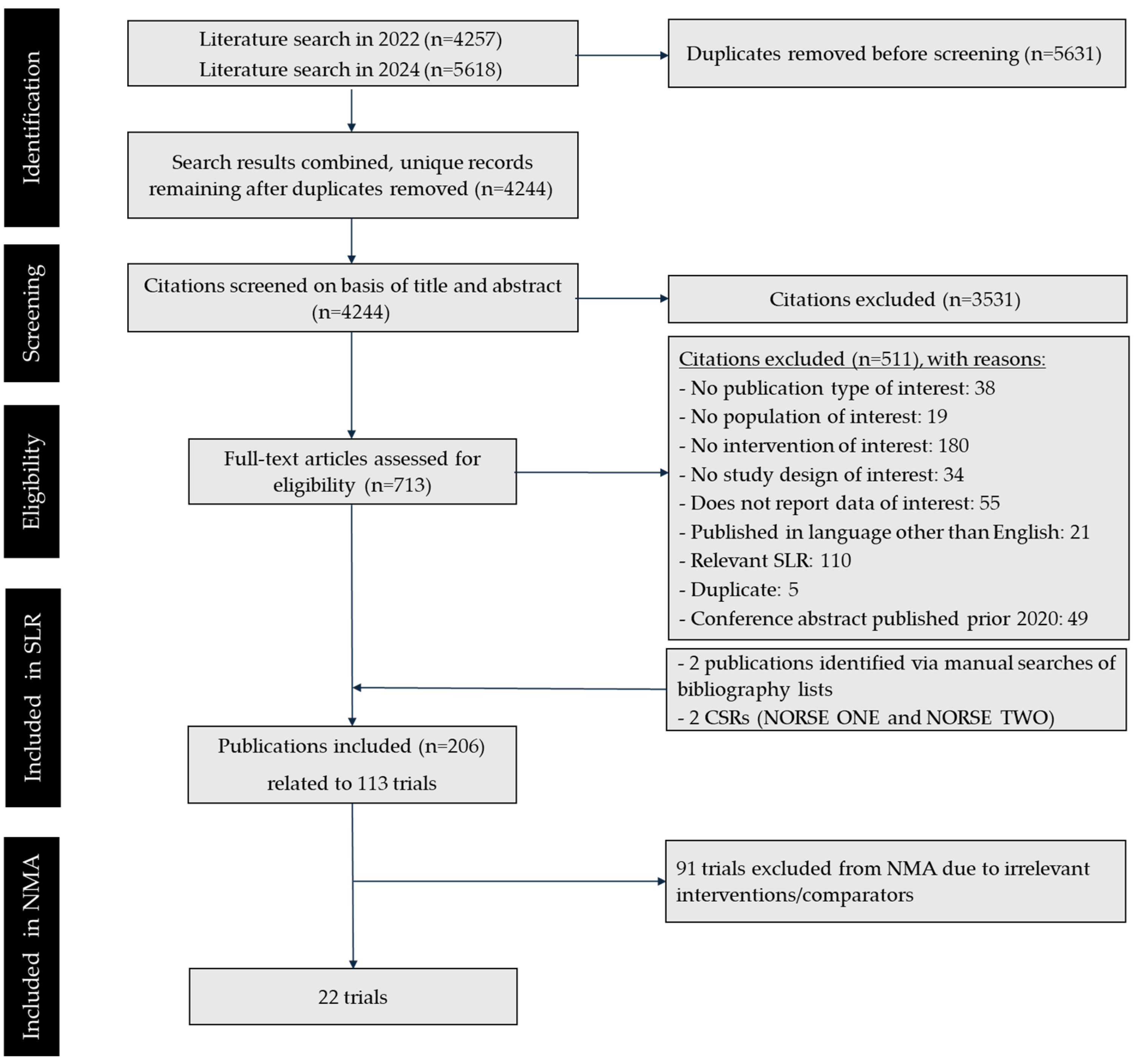
3.1. Results: Systematic Literature Review
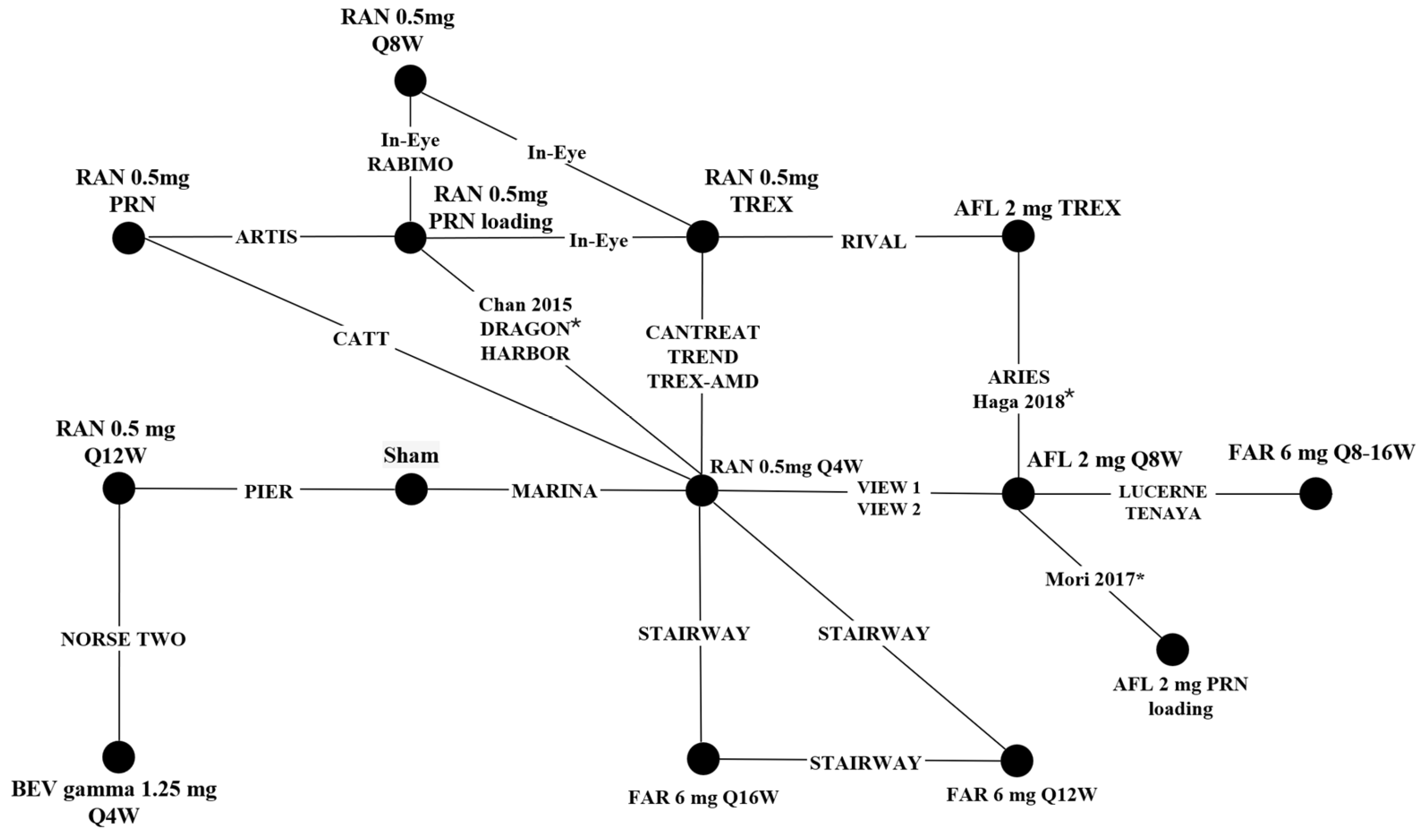
3.2. Results: Network Meta-Analysis
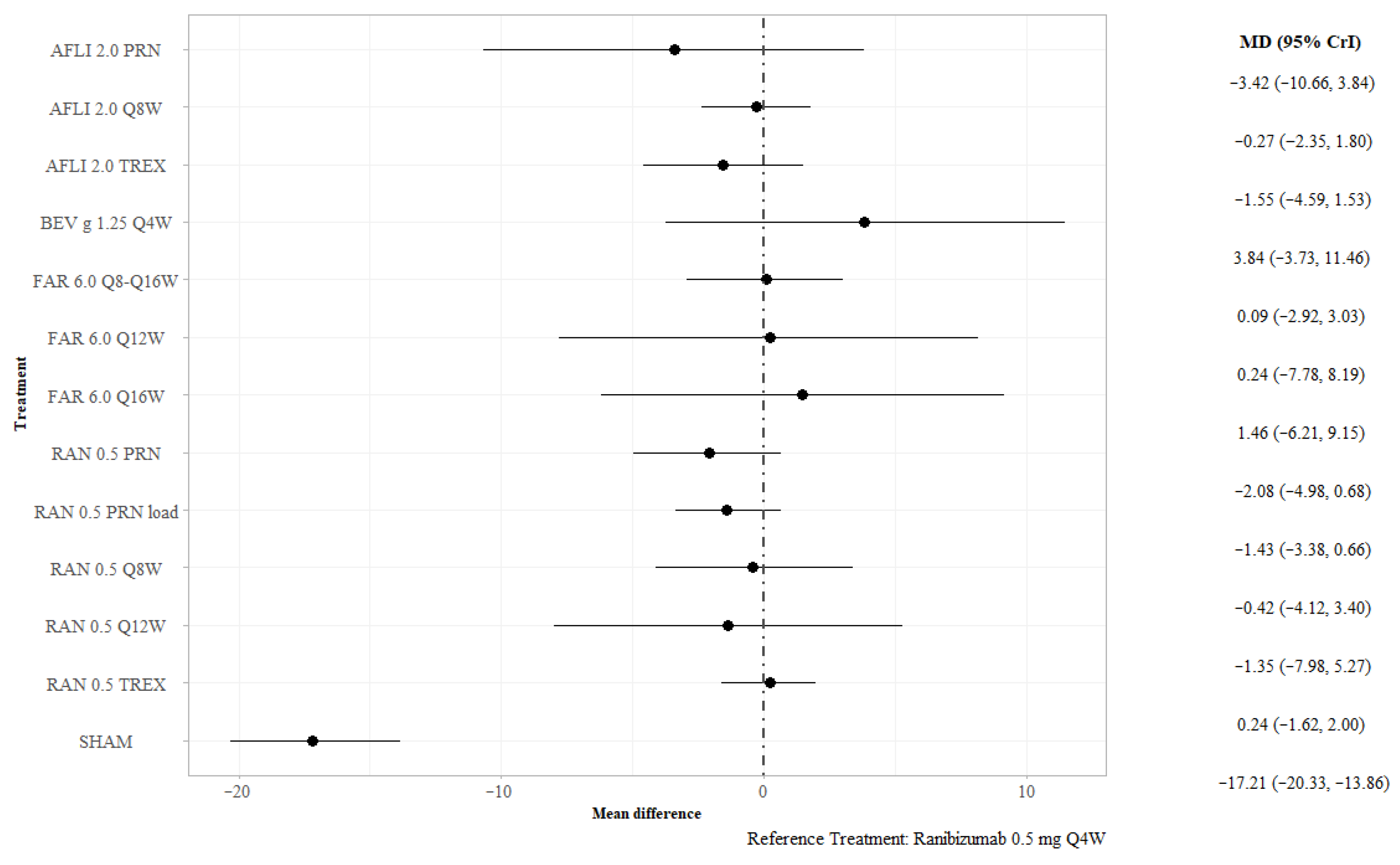
3.2.1. BCVA at 12 Months
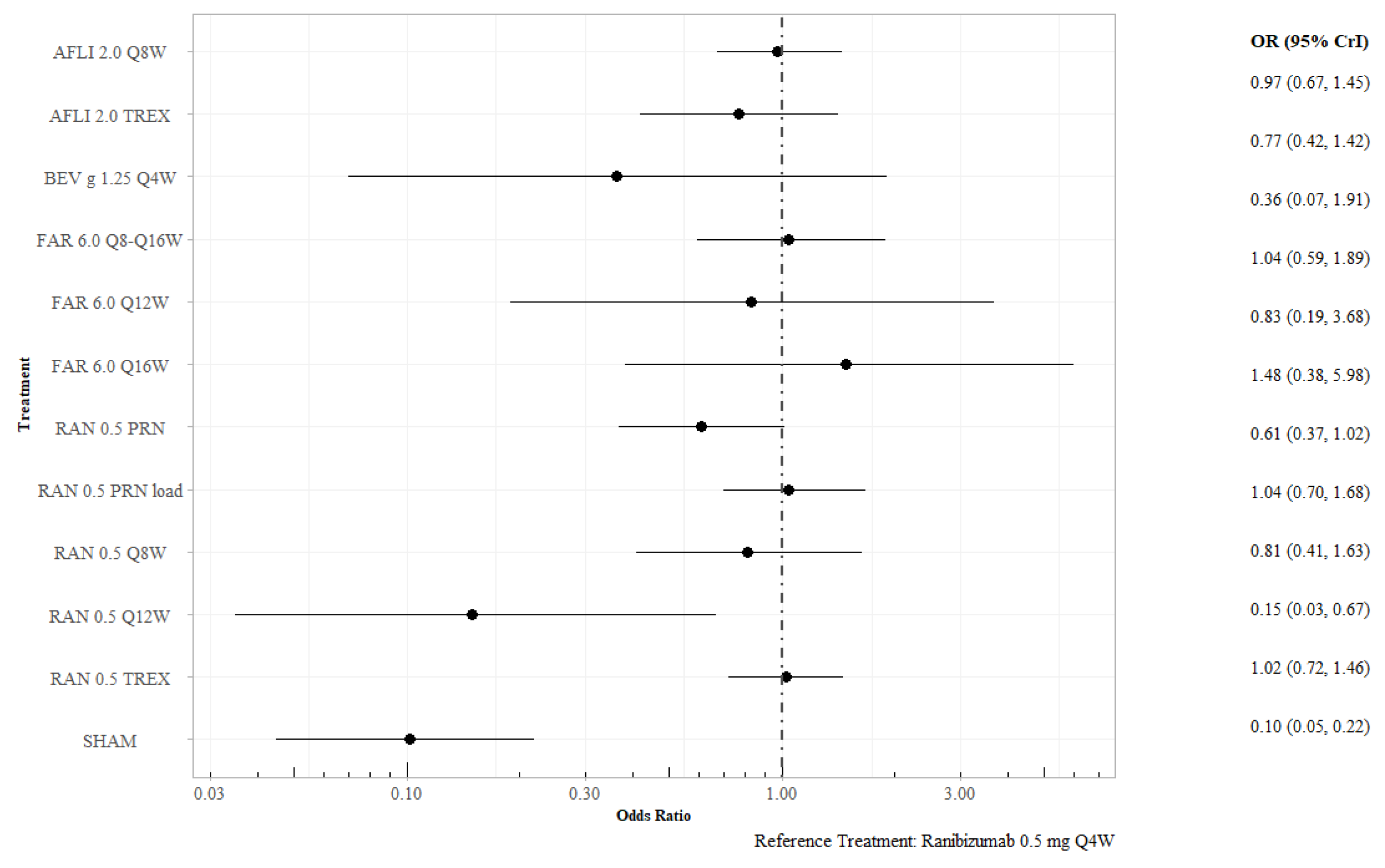
3.2.2. Proportion of Patients Gaining at Least 15 Letters at 12 Months
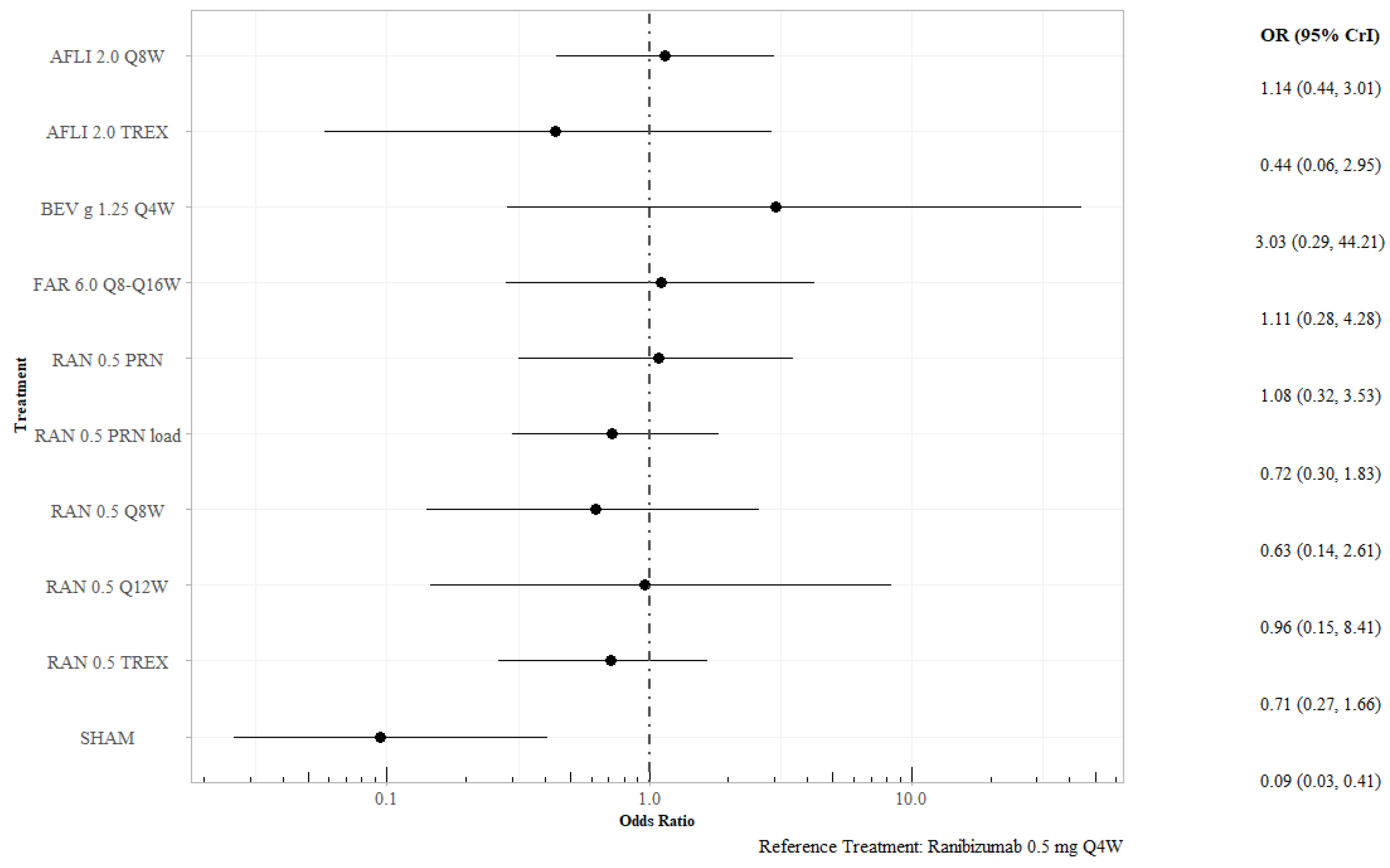
3.2.3. Proportion of Patients Losing Fewer than 15 Letters at 12 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFL | aflibercept |
| Anti-VEGF | anti-vascular endothelial growth factor |
| BCVA | best-corrected visual acuity |
| BEV | bevacizumab |
| BMI | body mass index |
| CFB | change from baseline |
| CNV | choroidal neovascularization |
| CrI | credible interval |
| DIC | deviance information criterion |
| FAR | faricimab |
| MAIC | matching-adjusted indirect comparison |
| MeSH | Medical Subject Headings |
| nAMD | neovascular age-related macular degeneration |
| NMA | network meta-analysis |
| OR | odds ratio |
| PICOS | population, intervention, comparison, outcome measures, and study design |
| PRN | pro re nata |
| Q4W | every four weeks |
| Q8W | every eight weeks |
| Q12W | every twelve weeks |
| Q16W | every sixteen weeks |
| RAN | ranibizumab |
| RCT | randomized controlled trials |
| SD | standard deviation |
| SLR | systematic literature review |
| SUCRA | surface under the cumulative |
| TREX | treat and extend |
| UK | United Kingdom |
References
- Colijn, J.; Buitendijk, G.; Prokofyeva, E.; Alves, D.; Cachulo, M.; Khawaja, A.; Cougnard-Gregoire, A.; Merle, B.; Korb, C.; Erke, M.; et al. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Hua, R. Clinical effectiveness of ranibizumab and conbercept for neovascular age-related macular degeneration: A meta-analysis. Drug Des. Dev. Ther. 2018, 12, 3625–3633. [Google Scholar] [CrossRef]
- Owen, C.; Jarrar, Z.; Wormald, R.; Cook, D.; Fletcher, A.; Rudnicka, A. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.; Dennis, N.; Freitas, R.; Quenéchdu, A.; Clemens, A.; Karcher, H.; Souied, E. Comparative efficacy of brolucizumab in the treatment of neovascular age-related macular degeneration: A systematic literature review and network meta-analysis. Adv. Ther. 2022, 39, 3425–3448. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, J.; Wang, J.; Feng, Z.; Yao, L.; Zhang, X. Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: Systematic review and Bayesian network meta-analysis. Ther. Adv. Chronic Dis. 2020, 11, 2040622320953349. [Google Scholar] [CrossRef]
- Tricco, A.; Thomas, S.M.; Lillie, E.; Veroniki, A.A.; Hamid, J.S.; Pham, B.; Lee, T.; Agarwal, A.; Sharpe, J.P.; Scott, A.; et al. Anti-vascular endothelial growth factor therapy for age-related macular degeneration: A systematic review and network meta-analysis. Syst. Rev. 2021, 10, 315. [Google Scholar] [CrossRef]
- Augsburger, M.; Sarra, G.; Imesch, P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: A comparative study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1889–1895. [Google Scholar] [CrossRef]
- Rahhal, F.; Hu, A.; Humayun, M.; George, M.; Javid, C.; Brown, J.J.; Pitcher, J.; Dagnon, T.; Kissner, J. ONS-5010 (bevacizumab-vikg) safety and efficacy in subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2025, 56, 178–189. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Ades, A.E. A chain of evidence with mixed comparisons: Models for multi-parameter synthesis and consistency of evidence. Stat. Med. 2003, 22, 2995–3016. [Google Scholar] [CrossRef]
- Caldwell, D.; Ades, A.; Higgins, J. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 2005, 331, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Haga, A.; Kawaji, T.; Ideta, R.; Inomata, Y.; Tanihara, H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018, 96, e393–e398. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.; Egger, A.; Chang, L.; Wolf, S.; Song, Y.; Zhang, J.; Dong, F.; Xu, X.; Weisberger, A. Two different treatment regimens of ranibizumab 0.5 mg for neovascular age-related macular degeneration with or without polypoidal choroidal vasculopathy in Chinese patients: Results from the Phase IV, randomized, DRAGON study. Acta Opthalmol. 2021, 99, e336–e345. [Google Scholar] [CrossRef]
- Phillippo, D. multinma: Bayesian Network Meta-Analysis of Individual and Aggregate Data; 2024. [Google Scholar] [CrossRef]
- Mitchell, P.; Holz, F.G.; Hykin, P.; Midena, E.; Souied, E.; Allmeier, H.; Lambrou, G.; Schmelter, T.; Wolf, S. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: The ARIES study: A randomized clinical trial. Retina 2022, 41, 1911–1920. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Y.; Wang, L.; Ye, X.; Zhao, J.; Shen, M.; Zhang, Q.; Xu, D.; Qin, G.; Zhang, W.; et al. One-year outcomes of 1 dose versus 3 loading doses followed by pro re nata regimen using ranibizumab for neovascular age-related macular degeneration: The ARTIS Trial. J. Opthalmol. 2019, 2019, 7530458. [Google Scholar] [CrossRef]
- Kertes, P.J.; Galic, I.J.; Greve, M.; Williams, R.G.; Rampakakis, E.; Scarino, A.; Sheidow, T. Canadian Treat-and-Extend Analysis Trial with Ranibizumab in patients with neovascular age-related macular disease: One-year results of the randomized Canadian Treat-and-Extend Analysis Trial with Ranibizumab Study. Opthalmology 2019, 126, 841–848. [Google Scholar] [CrossRef]
- Martin, D.; Maguire, M.; Ying, G.; Grunwald, J.; Fine, S.; Jaffe, G. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Chan, C.K.; Abraham, P.; Sarraf, D.; Nuthi, A.; Lin, S.; McCannel, C. Earlier therapeutic effects associated with high dose (2.0 mg) ranibizumab for treatment of vascularized pigmnet epithelial detachment in age-related macular degeneration. Eye 2015, 29, 80–87. [Google Scholar] [CrossRef]
- Busbee, B.G.; Ho, A.; Brown, D.; Heier, J.; Suñer, I.; Li, Z.; Rubio, R.; Lai, P. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Opthalmology 2013, 120, 1046–1056. [Google Scholar] [CrossRef]
- López Gálvez, M.; Arias Barquet, L.; Figueroa, M.S.; García-Layana, A.; Ruiz Moreno, J.M. Bimonthly, treat-and-extend and as-needed ranibizumab in naïve neovascular age-related macular degeneration patients: 12-month outcomes of a randomized study. Acta Ophthalmol. 2020, 98, e820–e829. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.; Heier, J.; Boyer, D.; Kaiser, P.; Chung, C.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Mori, R.; Tanaka, K.; Haruyama, M.; Kawamura, A.; Furuya, K.; Yuzawa, M. Comparison of pro re nata versus bimonthly injection of intravitreal aflibercept for typical neovascular age-related macular degeneration. Opthalmologica 2017, 238, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Safety and efficacy results of ONS-5010, an opthalmic bevacizumab. In Proceedings of the Asia-Pacific Vitreo-retina Society Meeting 2021, Virtual, 11–12 December 2021. [Google Scholar]
- Regillo, C.D.; Brown, D.; Abraham, P.; Yue, H.; Ianchulev, T.; Schneider, S.; Shams, N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am. J. Opthalmol. 2008, 145, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Feltgen, N.; Bertelmann, T.; Bretag, M.; Pfeiffer, S.; Hilgers, R.; Callizo, J.; Goldammer, L.; Bemme, S.; Hoerauf, H. Efficacy and safety of a fixed bimonthly ranibizumab treatment regimen in eyes with neovascular age-related macular degeneration: Results from the RABIMO trial. Arch. Clin. Exp. Opthalmol. 2017, 255, 923–934. [Google Scholar] [CrossRef]
- Gillies, M.C.; Hunyor, A.P.; Arnold, J.J.; Guymer, R.H.; Wolf, S.; Ng, P.; Pecheur, F.L.; McAllister, I.L. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: A randomized clinical trial. JAMA Opthalmol. 2019, 137, 372–379. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: The STAIRWAY phase 2 randomized clinical trial. JAMA Opthalmol. 2020, 138, 964–972. [Google Scholar] [CrossRef]
- Silva, R.; Berta, A.; Larsen, M.; Macfadden, W.; Feller, C.; Monés, J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: Results with ranibizumab from the TREND Study. Opthalmology 2018, 125, 57–65. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Croft, D.E.; Brown, D.M.; Wang, R.; Payne, J.F.; Clark, L.; Abdelfattah, N.S.; Sadda, S.R. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Opthalmology 2015, 122, 2514–2522. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.; Chong, V.; Korobelnik, J.; Kaiser, P.; Nguyen, Q.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Opthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Heloterä, H.; Siintamo, L.; Kivinen, N.; Abrahamsson, N.; Aaltonen, V.; Kaarniranta, K. Analysis of prognostic and predictive factors in neovascular age-related macular degeneration Kuopio cohort. Acta Opthalmol. 2024, 102, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B.; Muñoz, B.; Solomon, S.; West, S.K. Racial differences in the prevalence of age-related macular degeneration: The Salisbury Eye Evaluation (SEE) Project. Arch. Opthalmol. 2008, 126, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Gadhvi, K.; Mensah, E. What effect does ethnicity have on the response to ranibizumab in the treatment of wet age-related macular degeneration? Opthalmologica 2018, 240, 157–162. [Google Scholar] [CrossRef]
- Steinle, N.; Du, W.; Gibson, A.; Saroj, N. Outcomes by baseline choroidal neovascularization features in age-related macular degeneration: A post-hoc analysis of the VIEW studies. Opthamol. Retin. 2021, 5, 141–150. [Google Scholar]
- The Royal College of Ophthalmologists. National Ophthalmology Database Audit: The Second Report of Age-Related Macular Degeneration Audit (AMD). Available online: https://nodaudit.org.uk/sites/default/files/2024-03/NOD%20AMD%20Audit%20Full%20Annual%20Report%202024_0.pdf (accessed on 9 October 2025).
- Zhao, X.-Y.; Meng, L.-H.; Liu, S.-Z.; Chen, Y.-X. Efficacy and safety of different agents, dosages and strategies of anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: A network meta-analysis of randomized controlled trials. Acta Ophthalmol. 2021, 99, e1041–e1050. [Google Scholar] [CrossRef]
- Investigators, I.S.; Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Wordsworth, S.; Reeves, B.C. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 2012, 119, 1399–1411. [Google Scholar] [CrossRef]
- Singh, R.P.; Avery, R.L.; Barakat, M.R.; Kim, J.E.; Kiss, S. Evidence-based use of bevacizumab in the management of neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2024, 55, 156–162. [Google Scholar] [CrossRef]
| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adult (≥18 years old) patients with nAMD | Any divergent population, such as: Patients with other diseases Mixed populations of patients (e.g., all AMD) without stratified outcomes data for the population of interest Pediatric populations |
| Interventions | Bevacizumab Faricimab Aflibercept Conbercept Ranibizumab Brolucizumab Pegaptanib Biosimilar formulations of the above interventions of interest All potential therapeutic regimens were evaluated: Fixed interval: injections given according to a pre-specified schedule (e.g., Q4W, Q8W, Q12W, Q16W) PRN (pro re nata): injections given as needed, defined a priori in the protocol of the specific study PRN and extend (pro re nata and extend): PRN with the opportunity to extend the interval between assessments TREX (treat-and-extend): treatment with the flexibility to adjust the timing between injections (e.g., +2-week adjustment, −2-week adjustment) | Any other intervention not listed in the Inclusion column |
| Comparators | Any other interventions of interest Placebo/sham Standard of care/observation Any other treatment that provides the necessary link(s) to conduct an indirect comparison (e.g., to create a connected network of treatments for analyses) | Any other comparator not listed in the Inclusion column |
| Outcomes | Efficacy, e.g., BCVA (mean change from baseline, proportion of patients who achieve threshold change) Visual acuity Snellen equivalent Central foveal thickness Safety, e.g., Proportion of patients who experienced any AE Proportion of patients who experienced an ocular AE Proportion of patients who experienced a serious AE Proportion of patients who discontinued treatment due to AE | Any outcomes not listed in the Inclusion column |
| Study design | RCTs Existing published SLRs/meta-analyses of RCT data were included for manual bibliography checks | Any other study design not listed in the Inclusion column |
| Time | No date restriction for full-text articles; 2020–2024 for conference abstracts | Conference abstracts published before 2020 |
| Language | English language publications | Publications in languages other than English |
| Study/Reference | Treatment | Total N | Age, Mean (SD) | Proportion White Pts | CNV Type, % |
|---|---|---|---|---|---|
| ARIES [16] | AFLI 2.0 mg TREX | 106 | 75.5 (9) | NR | NR |
| AFLI 2.0 mg Q8W TREX | 104 | 76.6 (8.7) | NR | NR | |
| ARTIS [17] | RAN 0.5 mg PRN | 45 | 69.7 (8.6) | NR | NR |
| RAN 0.5 mg PRN loading | 49 | 70 (8.8) | NR | NR | |
| CANTREAT [18] | RAN 0.5 mg TREX | 287 | 78.9 (7.7) | 95.5 | NR |
| RAN 0.5 mg Q4W | 293 | 78.7 (8) | 93.2 | NR | |
| CATT [19] | RAN 0.5 mg Q4W | 301 | 79.2 (7.4) | 98.7 | NR |
| RAN 0.5 mg PRN | 298 | 78.4 (7.8) | 99.3 | NR | |
| Chan 2015 [20] | RAN 0.5 mg Q4W | 6 | 82 (6.2) | NR | NR |
| RAN 0.5 mg PRN loading | 7 | 84 (6.0) | NR | NR | |
| DRAGON [14] | RAN 0.5 mg Q4W | 167 | 65.6 (8.43) | 0 | PC 18; MC 9; OC 65.3; Other 7.8 |
| RAN 0.5 mg PRN loading | 166 | 66.8 (8.31) | 0 | PC 17.5; MC 7.8; OC 66.9; Other 10.2 | |
| HARBOR [21] | RAN 0.5 mg Q4W | 275 | 78.8 (8.4) | 964. | PC 15.3; MC 46.2; OC 38.5; |
| RAN 0.5 mg PRN loading | 275 | 78.5 (8.3) | 97.5 | PC 17.1; MC 46.5; OC 36.4; | |
| Haga 2018 [13] | AFLI 2.0 mg TREX | 21 | 75.5 (6.7) | 0 | NR |
| AFLI 2.0 mg Q8W | 20 | 78.5 (8.1) | 0 | NR | |
| In-Eye [22] | RAN 0.5 mg Q8W | 92 | 76.9 (7.4) | NR | NR |
| RAN 0.5 mg TREX | 88 | 77.8 (7.4) | NR | NR | |
| RAN 0.5 mg PRN loading | 90 | 77.5 (8.2) | NR | NR | |
| LUCERNE [23] | FAR 6.0 mg Q8-Q16W | 331 | 74.8 (8.4) | 84 | PC 2; MC 9; OC 52; Cl 30 |
| AFLI 2.0 mg Q8W | 327 | 76.1 (8.6) | 83 | PC 5; MC 9; OC 43; Cl 33 | |
| MARINA [24] | RAN 0.5 mg Q4W | 240 | 77 (8) | 96.7 | PC 0; MC 37.9; OC 62.1; Other 0 |
| SHAM | 238 | 77 (7) | 97.1 | PC 0; MC 36.6; OC 63.4; Other 0 | |
| Mori 2017 [25] | AFLI 2.0 mg PRN | 30 | 76.5 (NR) | NR | NR |
| AFLI 2.0 mg Q8W | 28 | 72.8 (NR) | NR | NR | |
| NORSE TWO [26] | BEV gamma 1.25 mg Q4W | 113 | 78.8 (8.3) | 97.3 | NR |
| RAN 0.5 mg Q12W | 115 | 79.1 (8.5) | 98.3 | NR | |
| PIER [27] | SHAM | 63 | 77.8 (7.1) | 93.7 | PC 22.2; MC 46; OC 31.7; Other 0 |
| RAN 0.5 mg Q12W | 61 | 78.8 (7.9) | 91.8 | PC 21.3; MC 29.5; OC 49.2; Other 0 | |
| RABIMO [28] | RAN 0.5 mg Q8W | 20 | 79 * | NR | NR |
| RAN 0.5 mg PRN loading | 20 | 81 * | NR | NR | |
| RIVAL [29] | RAN 0.5 mg TREX | 142 | 76.6 (8.5) | 93 | PC 15; MC/OC 84; Other 1 |
| AFLI 2.0 mg TREX | 139 | 78.7 (7.5) | 93.5 | PC 19; MC/OC 80; Other 1 | |
| STAIRWAY [30] | RAN 0.5 mg Q4W | 16 | 77.3 (10.3) | 100 | Cl/OC 37.5; Cl 12.5; OC 50 |
| FAR 6.0 mg Q12W | 24 | 80.3 (7.2) | 95.8 | Cl/OC 37.5; Cl 0; OC 62.5 | |
| FAR 6.0 mg Q16W | 31 | 77.7 (8.4) | 96.8 | Cl/OC 29; Cl 6.5; OC 64.5 | |
| TENAYA [23] | FAR 6.0 mg Q8-Q16W | 334 | 75.9 (8.6) | 91 | PC 5; MC 10; OC 53; Cl 25 |
| AFLI 2.0 mg Q8W | 337 | 76.7 (8.8) | 90 | PC 6; MC 9; OC 52; Cl 22 | |
| TREND [31] | RAN 0.5 mg TREX | 323 | 75.3 (8.61) | 91.6 | PC 8.4; MC 10.2; OC 42.7; Cl 26.6; Other 12.1 |
| RAN 0.5 mg Q4W | 327 | 75.2 (8.13) | 92 | PC 7; MC 3.4; OC 52.3; Cl 26.9; Other 10.5 | |
| TREX-AMD [32] | RAN 0.5 mg Q4W | 20 | NR | NR | NR |
| RAN 0.5 mg TREX | 40 | NR | NR | NR | |
| VIEW 1 [33] | RAN 0.5 mg Q4W | 304 | 78.2 (7.6) | 97.4 | PC 27; MC 33.2; OC 37.8; |
| AFLI 2.0 mg Q8W | 301 | 77.9 (8.4) | 95.3 | PC 23.6; MC 36.5; OC 39.2; | |
| VIEW 2 [33] | RAN 0.5 mg Q4W | 291 | 73 (9) | 73.2 | PC 24.1; MC 35.7; OC 39.9; |
| AFLI 2.0 mg Q8W | 306 | 73.8 (8.6) | 70.9 | PC 28.8; MC 34.6; OC 35.9; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzi, M.; Ebohon, S.; Kissner, J.; Comiskey, J.; Paap, M.; Bouchet, C.; Garnham, A.; Wissinger, E. Network Meta-Analysis of Bevacizumab Gamma Versus Competing Interventions for Treating Neovascular Age-Related Macular Degeneration in the United Kingdom. J. Mark. Access Health Policy 2025, 13, 58. https://doi.org/10.3390/jmahp13040058
Lorenzi M, Ebohon S, Kissner J, Comiskey J, Paap M, Bouchet C, Garnham A, Wissinger E. Network Meta-Analysis of Bevacizumab Gamma Versus Competing Interventions for Treating Neovascular Age-Related Macular Degeneration in the United Kingdom. Journal of Market Access & Health Policy. 2025; 13(4):58. https://doi.org/10.3390/jmahp13040058
Chicago/Turabian StyleLorenzi, Maria, Stephen Ebohon, Jennifer Kissner, Jedd Comiskey, Mayke Paap, Christine Bouchet, Andy Garnham, and Erika Wissinger. 2025. "Network Meta-Analysis of Bevacizumab Gamma Versus Competing Interventions for Treating Neovascular Age-Related Macular Degeneration in the United Kingdom" Journal of Market Access & Health Policy 13, no. 4: 58. https://doi.org/10.3390/jmahp13040058
APA StyleLorenzi, M., Ebohon, S., Kissner, J., Comiskey, J., Paap, M., Bouchet, C., Garnham, A., & Wissinger, E. (2025). Network Meta-Analysis of Bevacizumab Gamma Versus Competing Interventions for Treating Neovascular Age-Related Macular Degeneration in the United Kingdom. Journal of Market Access & Health Policy, 13(4), 58. https://doi.org/10.3390/jmahp13040058






