The Role of Medical Societies and the Relevance of Clinical Perspective in the Evolving EU HTA Process: Insights Generated at the 2023 Fall Convention and Survey of the European Access Academy
Abstract
1. Introduction
What is the role of PICO in HTA?
2. Methods
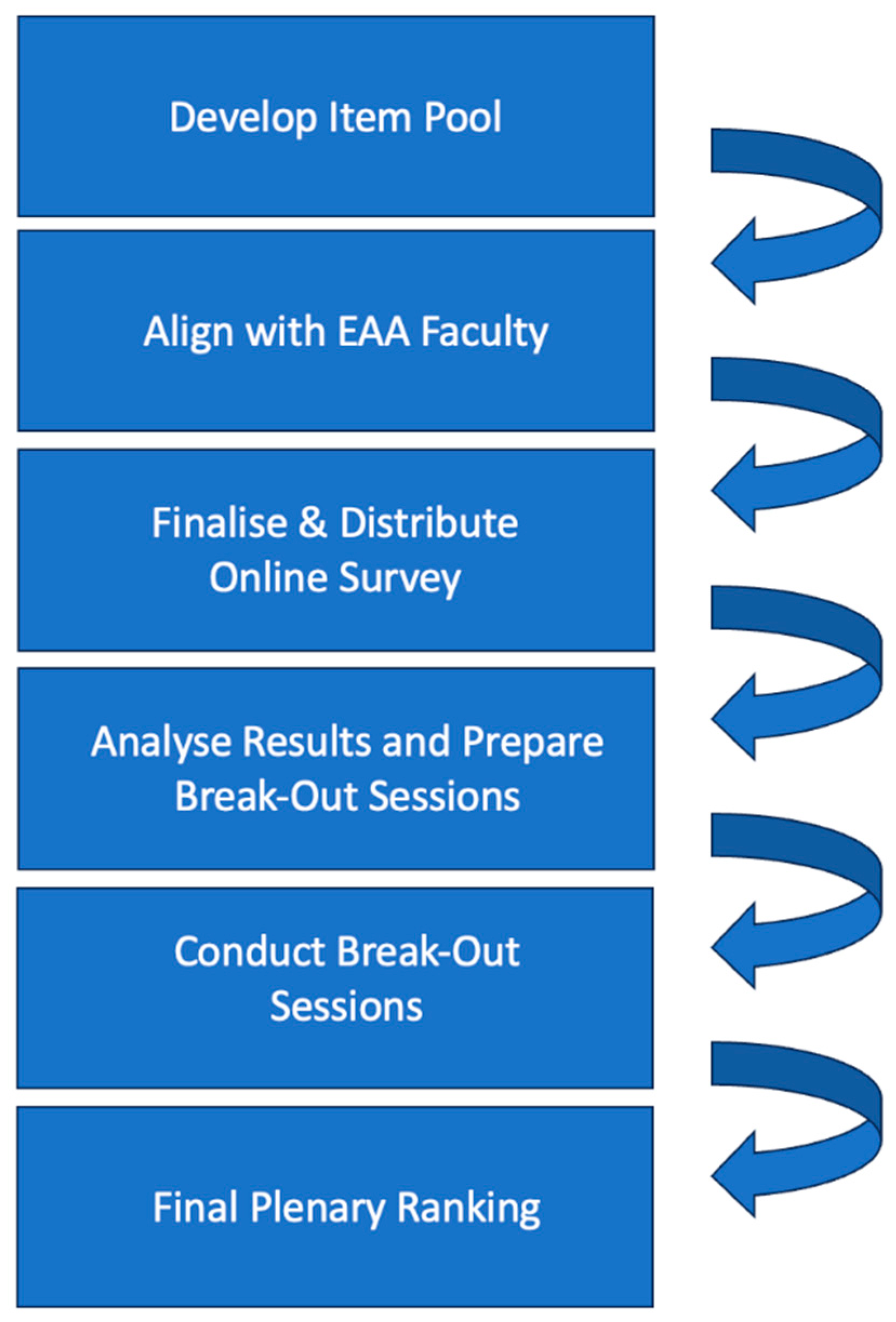
2.1. Generation of Input for Discussions through a Pre-Convention Survey
2.2. Preparation of Break-Out Sessions during the EAA Convention
- Medical Societies’ Role in EU HTA (WG 1, Medical Societies);
- Role of clinical guidelines in informing EU HTA scoping and assessment outcomes (WG 2, Clinical Guidelines);
- Interface of the European Society for Medical Oncology–Magnitude of Clinical Benefit Scale (ESMO-MCBS) and HTA (WG 3, Interface with ESMO-MCBS);
- Approaching ‘best-available evidence’ (BAE) for EU HTA (WG 4, Approaching BAE).
What is ESMO-MCBS and what is it used for?
2.3. Procedural Approach of the Break-Out Sessions
2.4. Plenary Session and Ranking
2.5. Data Handling and Analysis
3. Results
3.1. Outcomes of the Pre-Convention Survey
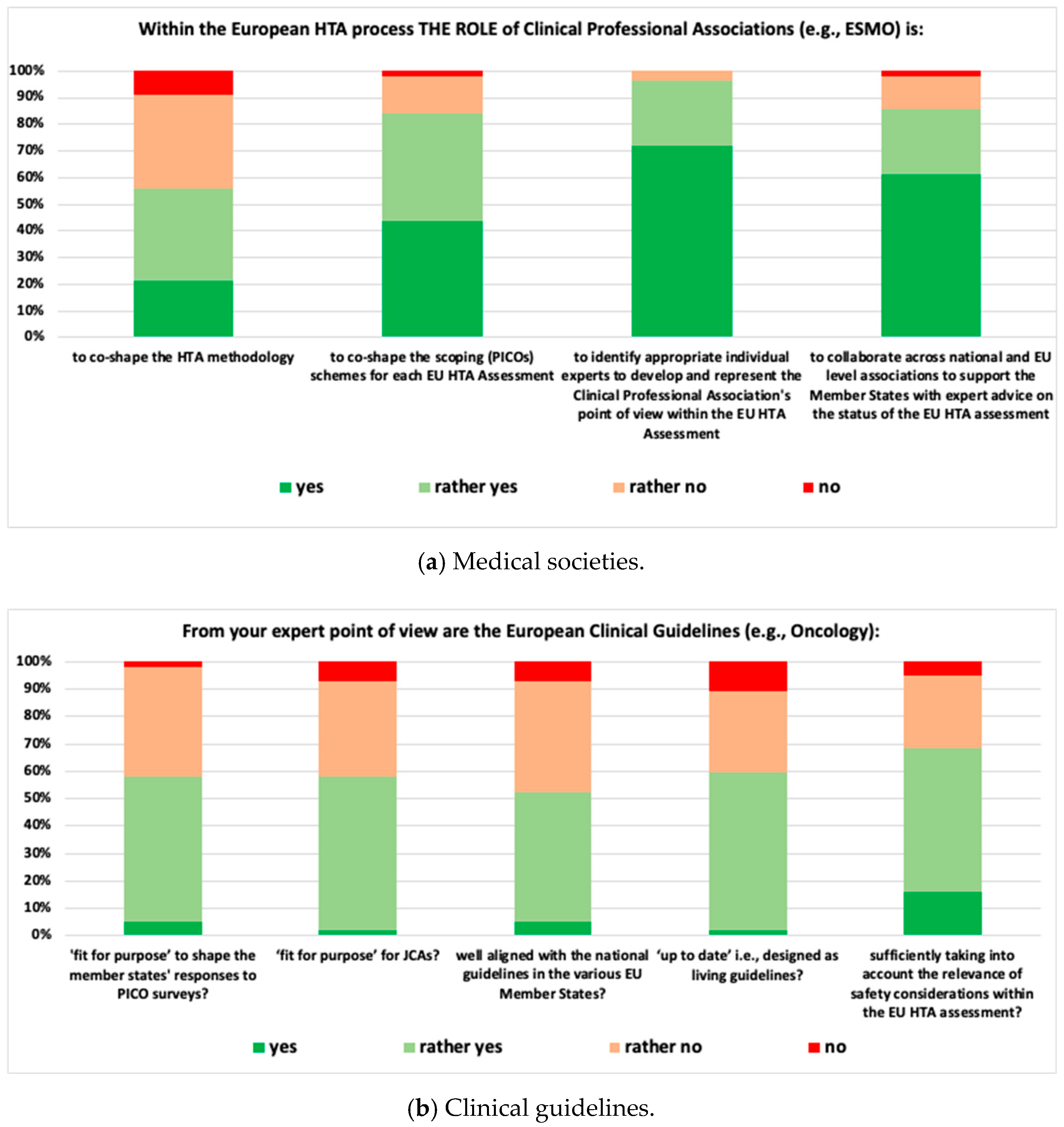
- Medical Societies: 96.5% of respondents suggested that medical societies should be involved in identifying experts to represent the clinical perspective within the EU HTA Assessment, and 56.1% suggested that medical societies co-shape evidence generation methodology.
- Clinical Guidelines: 68.4% of respondents indicated that clinical guidelines sufficiently take into account the particular clinical context when discussing acceptable safety concerns and 52.6% considered clinical guidelines well aligned with national guidelines.
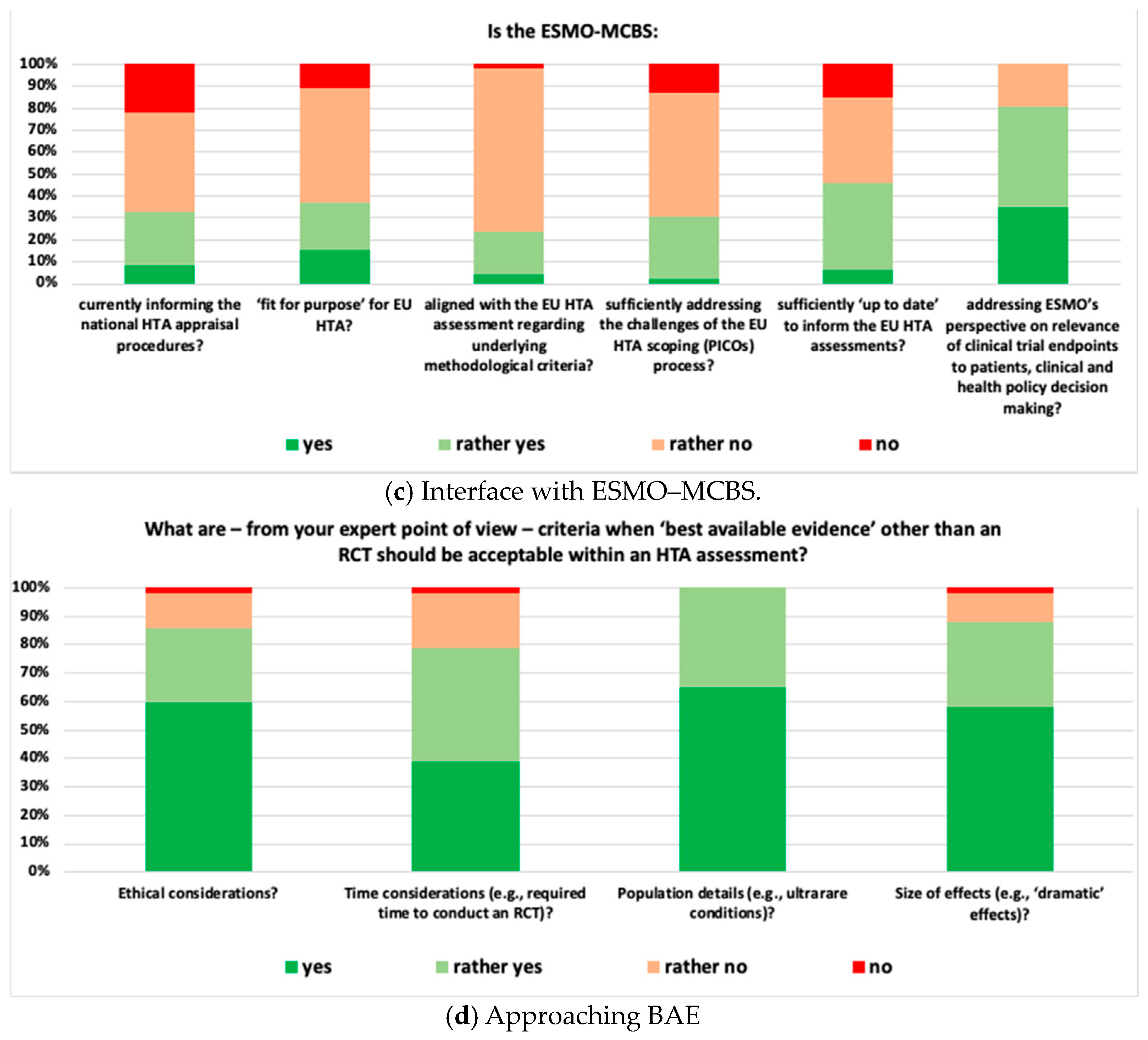
- Interface with ESMO-MCBS: 80.4% of respondents agreed that the ESMO-MCBS Scorecards adequately address ESMO’s perspective on the relevance of clinical trial endpoints, and 23.9% considered the ESMO-MCBS well aligned with the methodological criteria of the EU HTA assessment.
- Approaching BAE: 78.8% of respondents suggested that, when dealing with high unmet medical need situations, both time considerations and type of evidence should be taken into account to determine when best-available evidence other than an RCT could be acceptable in an HTA assessment. All respondents considered population characteristics (e.g., ultra-rare conditions) as very relevant criteria.
3.2. Insights from the Break-Out Sessions
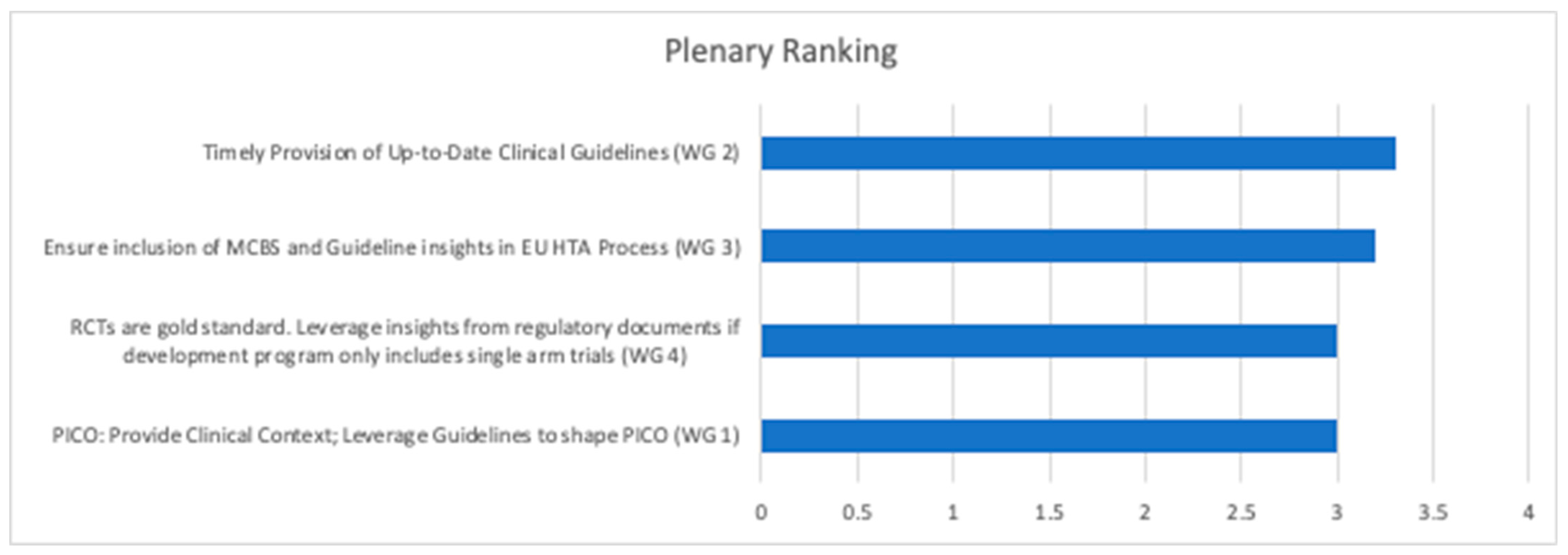
- WG 1: Within the break-out session, there was a clear consensus that medical societies have an important role to play in EU HTA. However, neither medical societies in general nor the European haematology/oncology societies were perceived as ready to take on that role. Heterogeneity across medical societies, e.g., in terms of organisational structure and resourcing, level of external activities, like involvement in policy shaping and national recommendations, was considered high. Fragmentation into the various subdisciplines, in some cases, lack of an umbrella organisation, limited alignment between national and EU-level societies, lack of established and/or standardised processes to provide timely input into HTA processes, and lack of sufficient and appropriate (i.e., not generating a conflict of interest) funding (the HTA work comes ‘on top of routine clinical work’) were highlighted as major reasons for medical societies not being ready for EU HTA. An overview of the four most relevant contributions of medical societies in the EU HTA process is as agreed in this break-out session provided in Table 1.
- WG 2: The general perception within the group was that clinical guidelines are highly relevant for EU HTA but are not yet ‘fit for purpose’ of adequately informing EU HTA. Heterogeneity, e.g., between national treatment guidelines and recommendations on patient management and standard of care for a particular clinical context, lack of timely updates in line with changes in clinical practice, partially limited scope (e.g., not including guidance on biomarker testing) and frequent lack of alignment between European and national level guidelines, particularly when no reference treatment exists, were mentioned as the rationale why clinical guidelines were not perceived ‘fit for purpose’ for EU HTA. Also, break-out participants indicated that so far, guidelines were aimed at ‘bedside’ rather than societal decision-making. To be more useful for EU-level HTA assessment, the process and scope of clinical guidelines may need to be adjusted in order to cover not only adequate PICO choice but also the main aspects of evidence generation for a given clinical condition. Suggested next steps and activities for clinical guidelines are displayed in Table 1.
- WG 3: The ESMO-MCBS was perceived as highly relevant but not designed to inform the evolving EU HTA process. Elaboration of the respective MCBS scorecard(s) as well as the underlying scientific rationale occurs once marketing authorisation has been granted—well after the target delivery date of the EU HTA Assessment Report (i.e., ~40 days after granting of marketing authorisation). Moreover, the methodology for the generation of MCBS scorecards and EU HTA methodology differ, and detailed discussion is needed to develop common methodological concepts and define the scope and relevance of the MCBS in the EU HTA process. The scope of EU HTA is broader when compared to the scope of the MCBS; e.g., it reflects by definition a wide variety of treatment standards across the EU. Also, ESMO-MCBS scorecards usually focus on the results of one particular trial rather than reflecting the totality of available evidence. Alignment of a specific MCBS scorecard and the related clinical guidelines was considered key to appropriately informing the EU HTA process. The top priority actions suggested by the WG are displayed in Table 1.
- WG 4: The WG agreed (i) that RCTs constitute the ‘gold standard’ for clinical evidence generation, (ii) that evidence other than that derived from RCTs should nevertheless be considered in some specific situations, and (iii) that the concept of ‘totality of evidence’ should be leveraged in the EU HTA assessment. Additional sources of evidence such as single-arm trials, real-world data, post hoc analyses in pre-specified subgroups of patients, indirect treatment comparisons, and systematic reviews were discussed. In particular, when considering a ‘multiplicity’ of European PICO schemes, evidence beyond the primary clinical trial data needs to be taken into account. Contextualization of evidence, considering the unmet medical need, disease characteristics, and size of the eligible patient population, were mentioned as relevant aspects to consider in the EU HTA assessment. Timeline challenges with the EU HTA process were mentioned as a key obstacle in taking an integrative approach to the available evidence. Additionally, clinical data cuts were raised as a challenging topic, i.e., data available for the EU HTA assessment might still be immature, resulting in a potential need for follow-up assessments once more mature data are available. Suggested priority actions are shown in Table 1.
3.3. Ranking Obtained in the Final Plenary Session
4. Discussion
Limitations and Further Research Agenda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Health Technology Assessment: Commission Welcomes the Adoption of New Rules to Improve Access to Innovative Technologies. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_6771 (accessed on 15 June 2023).
- European Parliament; Council of the European Union. REGULATION (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on Health Technology Assessment and Amending Directive 2011/24/EU. Off. J. Eur. Union 2021, 458, 1–32. [Google Scholar]
- Julian, E.; Gianfrate, F.; Sola-Morales, O.; Mol, P.; Bergmann, J.F.; Salmonson, T.; Hebborn, A.; Grande, M.; Ruof, J. How Can a Joint European Health Technology Assessment Provide an ‘Additional Benefit’ over the Current Standard of National Assessments?: Insights Generated from a Multi-Stakeholder Survey in Hematology/Oncology. Health Econ. Rev. 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- van Haesendonck, L.; Ruof, J.; Desmet, T.; van Dyck, W.; Simoens, S.; Huys, I.; Giuliani, R.; Toumi, M.; Dierks, C.; Dierks, J.; et al. The Role of Stakeholder Involvement in the Evolving EU HTA Process: Insights Generated through the European Access Academy’s Multi-Stakeholder Pre-Convention Questionnaire. J. Mark. Access Health Policy 2023, 11, 2217543. [Google Scholar] [CrossRef] [PubMed]
- Julian, E.; Pavlovic, M.; Sola-Morales, O.; Gianfrate, F.; Toumi, M.; Bucher, H.C.; Dierks, C.; Greiner, W.; Mol, P.; Bergmann, J.F.; et al. Shaping a Research Agenda to Ensure a Successful European Health Technology Assessment: Insights Generated during the Inaugural Convention of the European Access Academy. Health Econ. Rev. 2022, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Member State Coordination Group on HTA (HTACG). Available online: https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment/member-state-coordination-group-hta-htacg_en (accessed on 23 June 2023).
- European Commission. Health Technology Assessment Stakeholder Network—List of Members. Available online: https://health.ec.europa.eu/system/files/2023-07/hta_htar_list-org.pdf (accessed on 19 June 2023).
- eTendering. Tender Reference Number CHAFEA/LUX/2020/OP/0013. Calls for Tenders from the European Institutions. Available online: https://etendering.ted.europa.eu/cft/cft-display.html?cftId=7416 (accessed on 15 June 2023).
- European Commission. EUnetHTA21 Service Contract. Available online: https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment/eunethta21-service-contract_en (accessed on 19 January 2024).
- European Access Academy. Home Page. Available online: https://www.euaac.org/ (accessed on 15 June 2023).
- Pichon-Riviere, A.; Soto, N.; Augustovski, F.; Garcia-Marti, S.; Sampietro-Colom, L. Involvement of Relevant Stakeholders in Health Technology Assessment Development. In Proceedings of the 2nd Latin-American Forum on Health Technology Assessment Policies, Lima, Peru, 24–25 April 2017; pp. 1–13. [Google Scholar]
- Wale, J.L.; Thomas, S.; Hamerlijnck, D.; Hollander, R. Patients and Public Are Important Stakeholders in Health Technology Assessment but the Level of Involvement Is Low—A Call to Action. Res. Involv. Engagem. 2021, 7, 1. [Google Scholar] [CrossRef]
- Lips, P.; Timmers, L.; Bal, R.; Delnoij, D. Involvement of Patients and Medical Professionals in the Assessment of Relative Effectiveness: A Need for Closer Cooperation. Value Health 2022, 25, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency; EUnetHTA. Priority Topics for European Collaboration between Regulators and Health Technology Assessment Bodies: Development of a Joint Work Plan (2021–2023) between EMA and European HTA Bodies Facilitated through EUnetHTA21. 2022, pp. 1–9. Available online: https://www.ema.europa.eu/system/files/documents/work-programme/development_of_a_joint_work_plan_between_ema_and_eunethta21_-_revision_june_2023_en.pdf (accessed on 29 January 2024).
- EUnetHTA 21. D7.2—Guidance on Patient & Healthcare Professional Involvement. 2023, pp. 1–41. Available online: https://www.eunethta.eu/wp-content/uploads/2023/04/EUnetHTA-21-D7.2-Guidance-for-involvement-of-patient-and-clinical-expert-in-JSC-and-JCA-v1.0.pdf (accessed on 29 January 2024).
- EUnetHTA 21. D7.1.1—Practical Guideline for Interaction between Health Technology Developer and Hta Bodies. 2023, pp. 1–20. Available online: https://www.eunethta.eu/wp-content/uploads/2023/02/EUnetHTA-21-D7.1.1-Guidance-for-the-interaction-between-HTD-and-HTAb-v1.0.pdf (accessed on 29 January 2024).
- EUnetHTA 21. Practical Guideline D4.2 SCOPING PROCESS Version 1.0. 2022, pp. 1–25. Available online: https://www.eunethta.eu/wp-content/uploads/2023/10/EUnetHTA-21-D4.2-practical-guideline-on-scoping-process.pdf (accessed on 29 January 2024).
- Cherny, N.I.; Sullivan, R.; Dafni, U.; Kerst, J.M.; Sobrero, A.; Zielinski, C.; de Vries, E.G.E.; Piccart, M.J. A Standardised, Generic, Validated Approach to Stratify the Magnitude of Clinical Benefit That Can Be Anticipated from Anti-Cancer Therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Human Medicines Highlights 2023; European Medicines Agency: Amsterdam, The Netherlands, 2024; pp. 1–15. [Google Scholar]
- European Commission. Europe’s Beating Cancer Plan. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=COM%3A2021%3A44%3AFIN (accessed on 15 June 2023).
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuis, F.; Ruof, J.; van den Ham, H.; Gianfrate, F.; Strammiello, V.; Berntgen, M.; Pavlovic, M.; Mol, P.; Wasem, J.; van Dyck, W.; et al. Evaluating Progress towards EU HTA: Insights Generated from the European Access Academy’s Multi-Stakeholder Questionnaire. 2023; submitted for publication. [Google Scholar]
- European Commission. EU Health Policy Platform. Available online: https://webgate.ec.europa.eu/hpf/ (accessed on 15 June 2023).
- European Access Academy. In Proceedings of the EAA Convention Proceedings: Haemato-/Oncology-A Pace Maker for EU HTA, Barcelona, Spain, 18 October 2023; pp. 1–32.
- Kiesewetter, B.; Dafni, U.; de Vries, E.G.E.; Barriuso, J.; Curigliano, G.; González-Calle, V.; Galotti, M.; Gyawali, B.; Huntly, B.J.P.; Jäger, U.; et al. ESMO-Magnitude of Clinical Benefit Scale for Haematological Malignancies (ESMO-MCBS:H) Version 1.0. Ann. Oncol. 2023, 34, 734–771. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale Version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- European Society for Medical Oncology. ESMO-Magnitude of Clinical Benefit Scale: ESMO-MCBS Factsheet; European Society for Medical Oncology: Lugano, Switzerland, 2015; pp. 1–32. [Google Scholar]
- Slido. Home Page. Available online: https://www.slido.com (accessed on 15 June 2023).
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence Based Medicine: What It Is and What It Isn’t. BMJ 1996, 312, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, U.; Ottersen, T.; Cyr, P.-R.; Chalkidou, K. Evidence-Informed Deliberative Processes for HTA Around the Globe: Exploring the Next Frontiers of HTA and Best Practices: Comment on “Use of Evidence-Informed Deliberative Processes by Health Technology Assessment Agencies Around the Globe” Commentary. Int. J. Health Policy Manag. 2021, 10, 232–236. [Google Scholar] [CrossRef] [PubMed]
- European Access Academy. Transparency an Expertise – The Guiding Principles in Managing Conflict of Interest. In Proceedings of the EAA Convention Proceedings: Midterms & Status of the Preparation Phase of the EU HTA Regulation, Utrecht, The Netherlands, 20–21 April 2023; pp. 1–20. [Google Scholar]
- Atkins, B.; Briffa, T.; Connell, C.; Buttery, A.K.; Jennings, G.L.R. Improving Prioritization Processes for Clinical Practice Guidelines: New Methods and an Evaluation from the National Heart Foundation of Australia. Health Res. Policy Syst. 2023, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- National Academies. Standards for Developing Trustworthy Clinical Practice Guidelines. Available online: https://www.nationalacademies.org/our-work/standards-for-developing-trustworthy-clinical-practice-guidelines (accessed on 26 January 2024).
- Cardwell, K.; Quigley, J.; Clyne, B.; Tyner, B.; Carrigan, M.; Smith, S.; Ryan, M.; O’Neill, M. Processes for Updating Guidelines: Protocol for a Systematic Review. HRB Open Res. 2021, 4, 116. [Google Scholar] [CrossRef]
- Vernooij, R.W.M.; Sanabria, A.J.; Solà, I.; Alonso-Coello, P.; Martínez García, L. Guidance for Updating Clinical Practice Guidelines: A Systematic Review of Methodological Handbooks. Implement. Sci. 2014, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft; Deutsche Krebshilfe; AWMF). In S3-Leitlinie Früherkennung, Diagnose, Therapie Und Nachsorge Des Mammakarzinoms; AWMF: Berlin, Germany, 2021; pp. 1–467.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies: Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Kolberg-Liedtke, C.; Albert, U.S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.U.; Budach, W.; Dall, P.; Ditsch, N.; Fallenberg, E.M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2023. Breast Care 2023, 18, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Ayala de la Peña, F.; Antolín Novoa, S.; Gavilá Gregori, J.; González Cortijo, L.; Henao Carrasco, F.; Martínez Martínez, M.T.; Morales Estévez, C.; Stradella, A.; Vidal Losada, M.J.; Ciruelos, E. SEOM-GEICAM-SOLTI Clinical Guidelines for Early-Stage Breast Cancer (2022). Clin. Transl. Oncol. 2023, 25, 2647–2664. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Health Union. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/promoting-our-european-way-life/european-health-union_en (accessed on 26 January 2024).
- Eichler, H.G.; Pignatti, F.; Schwarzer-Daum, B.; Hidalgo-Simon, A.; Eichler, I.; Arlett, P.; Humphreys, A.; Vamvakas, S.; Brun, N.; Rasi, G. Randomized Controlled Trials Versus Real World Evidence: Neither Magic Nor Myth. Clin. Pharmacol. Ther. 2021, 109, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, R. Design and Analysis of Clinical Trials for Small Rare Disease Populations. J. Rare Dis. Res. Treat. 2016, 1, 53–60. [Google Scholar] [CrossRef]
- Venetis, C.; d’Hooghe, T.; Barnhart, K.T.; Bossuyt, P.M.M.; Mol, B.W.J. Methodologic Considerations in Randomized Clinical Trials in Reproductive Medicine. Fertil. Steril. 2020, 113, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Hämatologie und med. Onkologie. Frühe Nutzenbewertung. Available online: https://www.dgho.de/publikationen/stellungnahmen/fruehe-nutzenbewertung (accessed on 15 June 2023).

| Priority | Medical Societies | Clinical Guidelines | Interface with ESMO-MCBS | Approaching BAE |
|---|---|---|---|---|
| 1 | PICO: Provide Clinical Context; Leverage Guidelines to shape PICO | Timely provision of Up-to-Date Clinical Guidelines | Ensure inclusion of MCBS and Guideline insights in EU HTA process | RCTs are gold standard. Leverage insights from regulatory documents if development program only includes single arm trials |
| 2 | Identification of experts to provide input into EU HTA | Ensure comprehensive Clinical Guidelines (diagnostics; treatment pathways, toxicity) | Include MCBS comparators in EU HTA PICO | Totality of evidence included feed-back from patient organizations should be used |
| 3 | PICO: Harmonization of clinical perspective; alignment of EU and national perspective | Integrate and reflect European and national treatment standards in guidelines | Developers to consider leveraging MCBS to inform design of confirmatory trials | Disease Context is critical (e.g., unmet need; ultrarare conditions; poor prognosis etc.) |
| 4 | Conflict of Interest (CoI): Contribute to pragmatic and effective CoI management | Adjust purpose of clinical guidelines to serve both HTA and bedside decision making | Consider MCBS tool for national HTA appraisals | Clear guidance re confirmatory follow-up data generation required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julian, E.; Solà-Morales, O.; Garcia, M.J.; Brinkhuis, F.; Pavlovic, M.; Martín-Saborido, C.; Doeswijk, R.; Giuliani, R.; Willemsen, A.; Goettsch, W.; et al. The Role of Medical Societies and the Relevance of Clinical Perspective in the Evolving EU HTA Process: Insights Generated at the 2023 Fall Convention and Survey of the European Access Academy. J. Mark. Access Health Policy 2024, 12, 128-143. https://doi.org/10.3390/jmahp12030011
Julian E, Solà-Morales O, Garcia MJ, Brinkhuis F, Pavlovic M, Martín-Saborido C, Doeswijk R, Giuliani R, Willemsen A, Goettsch W, et al. The Role of Medical Societies and the Relevance of Clinical Perspective in the Evolving EU HTA Process: Insights Generated at the 2023 Fall Convention and Survey of the European Access Academy. Journal of Market Access & Health Policy. 2024; 12(3):128-143. https://doi.org/10.3390/jmahp12030011
Chicago/Turabian StyleJulian, Elaine, Oriol Solà-Morales, Maria João Garcia, Francine Brinkhuis, Mira Pavlovic, Carlos Martín-Saborido, Robin Doeswijk, Rosa Giuliani, Anne Willemsen, Wim Goettsch, and et al. 2024. "The Role of Medical Societies and the Relevance of Clinical Perspective in the Evolving EU HTA Process: Insights Generated at the 2023 Fall Convention and Survey of the European Access Academy" Journal of Market Access & Health Policy 12, no. 3: 128-143. https://doi.org/10.3390/jmahp12030011
APA StyleJulian, E., Solà-Morales, O., Garcia, M. J., Brinkhuis, F., Pavlovic, M., Martín-Saborido, C., Doeswijk, R., Giuliani, R., Willemsen, A., Goettsch, W., Wörmann, B., Dafni, U., Bucher, H. C., Pérez-Valderrama, B., Bernardini, R., Gianfrate, F., Uyl-de Groot, C. A., & Ruof, J. (2024). The Role of Medical Societies and the Relevance of Clinical Perspective in the Evolving EU HTA Process: Insights Generated at the 2023 Fall Convention and Survey of the European Access Academy. Journal of Market Access & Health Policy, 12(3), 128-143. https://doi.org/10.3390/jmahp12030011







