Abstract
Objective: Currently there are no disease-specific approved therapies for non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH); however, several treatments are under development. This study aimed to estimate the cost-effectiveness of hypothetical innovative therapies compared with lifestyle intervention alone and combined with pioglitazone, and assess the health economic consequences of their future availability for patients. Methods: A Markov cohort model was developed, considering fourteen disease health states and one absorbing state representing death. Transition probabilities, costs, utilities, and treatment efficacy were based on published data and assumptions. Four treatment strategies were considered, including two existing therapies (lifestyle intervention, small molecule treatment) and two hypothetical interventions (biological and curative therapy). The analysis was performed from the US third-party payer perspective. Results: The curative treatment with the assumed efficacy of 70% of patients cured and assumed price of $500,000 was the only cost-effective option. Although it incurred higher costs (a difference of $188,771 vs. lifestyle intervention and $197,702 vs. small molecule), it generated more QALYs (a difference of 1.58 and 1.38 QALYs, respectively), resulting in an ICER below the willingness-to-pay threshold of $150,000 per QALY. The sensitivity analyses showed that the results were robust to variations in model parameters. Conclusions: This study highlighted the potential benefits of therapies aimed at curing a disease rather than stopping its progression. Nonetheless, each of the analyzed therapies could be cost-effective compared with lifestyle intervention at a relatively high price.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) comprises a spectrum of hepatic conditions closely associated with metabolic syndrome, including non-alcoholic fatty liver (NAFL) or hepatic steatosis, and non-alcoholic steatohepatitis (NASH) or steatohepatitis [1]. Patients classified with NAFLD can progress or regress between different fibrosis stages (F0 to F2) of NASH or NAFL and vice versa. NAFL generally follows a benign non-progressive clinical course, and NASH may progress to cirrhosis (F4), decompensated cirrhosis (DC), and hepatocellular carcinoma (HCC) (Figure 1).
NAFLD poses a major public health concern with a rising prevalence, becoming a leading cause of worldwide liver disease [2]. The global prevalence of NAFLD is estimated to be 30.05% among the adult population, with the highest prevalence in Latin America (44.37%), followed by Middle East and North Africa (MENA) (36.53%) [3]. The estimated prevalence of NASH among biopsied NAFLD patients is 59.10% worldwide [3]. This indicates that the overall prevalence of NASH ranges between 1.50% and 6.45% worldwide [4,5]. NASH is the second leading indicator of chronic liver diseases for liver transplantation (LT) in the United States following alcohol-related disease (38%), accounting for 28% of patients. In Europe, it represents 8.4% of annual LTs [4,5]. Metabolic comorbidities associated with NAFL and NASH include obesity (51.34%), type 2 diabetes (22.51%), hyperlipidemia (69.16%), hypertension (39.34%), and metabolic syndrome (42.54%), based on the provided global statistical review [3]. The liver-specific and overall global mortality rates in patients with NASH were 11.77 and 25.56 per 1000 person-years, respectively, in 2016 [3].
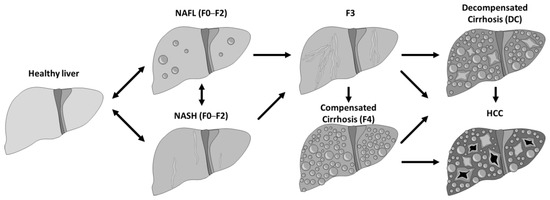
Figure 1.
Natural progression of the NASH/NAFL disease.
There are currently no disease-specific approved therapies for NAFL or NASH, however there are constantly new developments regarding the identification of this disease [6]. Lifestyle intervention remains the standard of care, with limited evidence of reducing liver fibrosis. In contrast, pioglitazone or vitamin E, used as add-on therapy to the standard of care, have shown potential in reversing steatohepatitis and improving liver fibrosis [7]. The pathophysiology of NASH is complicated and poorly understood, which has hindered progress in finding effective treatments. Given the multifactorial nature of the disease, there is ongoing interest in exploring combination therapies and diverse modes of action. In May 2019, the first series of studies focusing on cell-based therapy was launched to evaluate safety in NASH F3 and F4 patients at different dosages [8], building upon interim results from a phase I/II trial involving 19 patients with acute liver failure [9]. Over the course of nearly three years, studies on cell-based therapies and liver treatments have significantly increased. The summary of currently ongoing trials holds promise for the development of more efficient treatments [10,11].
Given the large number of patients with NAFL and NASH worldwide, medical costs related to these conditions are enormous, in both affluent and developing countries [12]. The financing of potential treatments for NAFL and NASH currently represents an area of uncertainty, considering the increasing number of pipeline agents advancing to late-phase clinical trials. In addition to demonstrating clinical efficacy, the evidence of long-term cost-effectiveness in a budget-constrained world is becoming increasingly critical. Previous attempts have been made to build models assessing the cost-effectiveness of treatments for NAFL and NASH [13,14,15]. However, these models did not incorporate the detailed interplay between NAFL and NASH, which can significantly influence the results. Furthermore, there was no differentiation across various fibrosis stages in the reviewed models.
The objective of this study was to estimate the cost-effectiveness of hypothetical innovative therapies compared with lifestyle intervention alone, as well as in combination with a small molecule (pioglitazone). Additionally, the study aimed to identify the key factors that drive cost-effectiveness and investigate economically justifiable prices. Therefore, this paper aims to focus on developing a more comprehensive model without becoming overly complex.
In our investigations, we wanted to understand the potential of different therapies for NAFL and NASH from the perspective of their economic evaluation. Thus, we used lifestyle intervention as a baseline and compared it with three other treatments (small molecule, biological, and curative). Each intervention had an assumed mode of action and efficacy.
2. Methods
We performed an economic evaluation from the US third-party payer perspective, following the recommendations of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) for economic evaluations [16].
In the base case scenario, it was assumed that all modeled patients were at the NAFL F0 health stage, which is the stage with the mildest symptoms of the disease. To ensure the robustness of our study, we conducted several calculations with the various initial disease stages of the cohort. This numerical experiment enabled us to assess the influence of starting conditions on the cohort’s behavior, particularly in cases where our model allowed for complete regression from NASH. The key endpoints in the clinical trials of NASH patients, as recommended by the US Food and Drugs Administration (FDA) [17], were NASH resolution and slowing down fibrosis progression. Therefore, the model utilized these endpoints to inform the regression from NASH to NAFL and the transition of fibrosis.
The analyses were conducted over a lifetime horizon, with a 1-year cycle length. This cycle length aligns effectively with the available data, and furthermore, the same cycle length has been employed in previously published models. Four different treatment strategies were considered, including two real ones and two theoretical ones. The first two strategies were lifestyle intervention and small molecule treatment, which are currently available in the market. The small molecule treatment was slowing down progression in early stages of fibrosis (F0–F2). The third strategy was a hypothetical biological treatment that aimed to prevent disease progression in the late stages of fibrosis (F3 and F4). The fourth strategy was a theoretical curative therapy that was assumed to cure the disease and return subjects from F4 stage to the initial stage of fibrosis (F0).
A Markov cohort model was constructed, consisting of fourteen health states and one absorbing state that represented death:
- NAFL F0
- NAFL F1
- NAFL F2
- NASH F0
- NASH F1
- NASH F2
- F3 as advanced fibrosis
- 1st year with F4 as compensated cirrhosis
- Next year(s) with F4
- DC
- HCC
- Liver transplant after DC
- Liver transplant after HCC
- Post-liver transplant (PLT)
The model structure was inspired by the NASH model developed by the Institute for Clinical and Economic Review (ICER US) [13] and presented by Tapper et al. [18], which included comprehensive fibrosis and advanced complications-related health states defined according to the fibrosis stage. In addition, it included steatosis-specific health states defined according to the NAFLD activity score (NAS).
The graphical structure of the model is presented in Figure 2. The cohort could progress and regress in the model between the stages of NAFL and NASH in different stages of fibrosis. Patients with NAFL or NASH and early stages of fibrosis (F0–F2) progressed to advanced fibrosis (F3) or compensated cirrhosis (F4). The latter fibrosis stages were not differentiated between NAFL and NASH as the severity of cirrhosis was considered to fully drive the associated outcomes. Patients with advanced fibrosis (F3) and compensated cirrhosis (CC) could transition to DC or HCC. The model’s mortality depends on the disease state, patient age, and sex. Furthermore, three types of mortality were allowed in the model:
- Cardiovascular death (CV death),
- Liver-related death,
- Other-cause mortality.
To illustrate all the potential influences of the explored treatment strategies, each transition marked with a thin black arrow represents the baseline lifestyle intervention pathway. Additionally, the impact of each particular treatment is shown by pattern-coded arrows that represent:
- Small molecule treatment with thick “zebra” line represents regressing towards NAFL from NASH into corresponding fibrosis stages and reducing fibrosis progression within NAFL or NASH.
- Biological therapy with thick checkered arrows shows a reduction of progression into DC and HCC from F3 and F4 states.
- Curative therapy with the thick grey arrow shows the transition probability into the initial stage of NAFL.
After treatment with curative therapy, subjects had a high probability of achieving full recovery and returning to the NAFL F0 stage. However, the possibility of relapse in these individuals was considered in the model.
Patients diagnosed with DC and HCC were considered eligible for LT in the model. It was assumed that individuals receiving LT would transition directly into the PLT health state, without accounting for potential disease relapse.
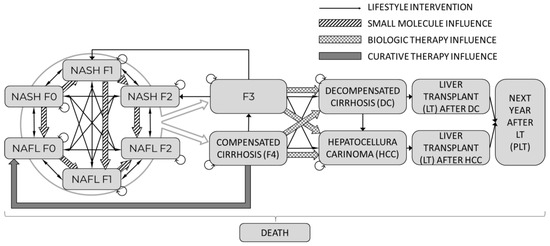
Figure 2.
Flow chart of NAFL/NASH model.
For each strategy, the following outcomes were evaluated: total life years (LY) and quality-adjusted life years (QALY); percentage of patients reaching advanced complications (DC, HCC, and LT); cause-specific mortality (liver-related mortality, fatal cardiovascular events (CVE), and other-cause mortality); and average cumulative costs (treatment acquisition costs, direct medical costs, and total costs). Then, the incremental cost-effectiveness ratio (ICER), expressed as total costs per QALY gained, was computed to compare the baseline and lifestyle intervention with other investigated types of treatments.
The general summary of model inputs is presented below. Additionally, all the values used in the model are available in Appendix A, Table A1.
3. Inputs
The model incorporated published data on the natural course of the disease, including probabilities sourced from Singh 2015 [19], Younossi et al., 2019 [12], and Tapper 2016 [18]. The costs and health-state utilities were based on the data provided by Younossi 2016. The efficacy of the treatments was informed by studies conducted by Tapper 2016 [18] and Zhang 2015 [20]. By utilizing these published data sources, the model aimed to provide a comprehensive and accurate representation of the disease and its potential treatment outcomes.
The baseline characteristics of the NAFL patients without advanced fibrosis were obtained from the NASH Clinical Research Network Study, which focused on patients with non-alcoholic fatty liver disease activity score (NAS) ≤ 4 [21]. The NAS score represented the sum of scores for steatosis, lobular inflammation, and ballooning, ranging from 0 to 8. Typically, subjects with a score equal to or above 4 were considered as having experienced NAFLD. The initial cohort was assumed to enter the model at an average age of 47.7, with females comprising 55.8% of the cohort
Transition probabilities across fibrosis stages originated from the study in which liver biopsies were conducted with a minimum one-year interval, reporting the annual fibrosis progression rates separately for patients with NAFL and NASH [19]. These transitions were recalculated using the same methodology as shown in the evidence report published by the ICER US [13]. The transition probability between NAFL and NASH was calculated based on Tapper, E.B. et al. [18]. Each transition from NAFL to NASH, or the opposite, was multiplied by the internal transition across fibrosis stages within NAFL/NASH. Transition probabilities to and across advanced complications and liver-related death were sourced from the evidence mentioned by the ICER US [13]. While developing the transition matrix, it was noticed that in the results from Singh, S. et al. [19], no NAFL subjects progressed from the F1 fibrosis stage into cirrhosis. However, there was one progression into cirrhosis from stage NAFL F0. Hence, the transition probability to cirrhosis from F0 was used also for F1 to mitigate this incoherence.
The calculations regarding the efficacy of lifestyle intervention were made by taking into account weight loss among the patients who reached a certain weight reduction level [22]. For the small molecule treatment strategy, we assumed that it supported regression to NAFL from each corresponding NASH stage. Also, it reduced the risk of fibrosis progression in each stage of NASH and advanced fibrosis (F3). The biological therapy aimed to limit the progression from F3 and F4 into more severe stages of the disease. The curative therapy introduced the possibility of regressing into an early stage of NAFL from the F4 stage of the disease. Table 1 displays the breakdown of the key parameters’ values used in the model.

Table 1.
Summary of model inputs.
Liver-related and other-cause mortality were considered in the model together with the risk of fatal CVE based on the age- and sex-specific rates from the recent life tables [24] and cause-of-death data [25] published by the CDC. Liver-related mortality was adjusted in the model by incorporating a relative risk increase for patients with advanced cirrhosis (F3 or F4), DC, HCC, or after LT [13]. Additionally, to adjust the risk of fatal CVE, hazard ratios (HRs) were differentiated between stages F0 to F2, F3, and F4, based on a recent meta-analysis [26].
In this exploratory cost-effectiveness analysis, we investigate the potential for innovative therapy strategies similar to those presented by Binda et al. [27], showing a significant decrease in NAS score and fibrotic area. To test the impact on ICER, we investigated the efficacy of hypothetical therapies at different threshold values of 50%, 70%, and 90%.
Utilities and costs for the model health states were retrieved from the Younossi et al. study [2], which used the micro-costing method to calculate costs and reported utilities elicited from Short Form-6D (SF-6D) in NAFLD patients. Age-adjusted utilities in the US population were taken from the evidence report published by the ICER US [13]. The costs related to lifestyle intervention and other treatment strategies were collected from the previously published cost-utility analyses [19,20]. Costs were adjusted to 2023 US dollars [28]. For the exploratory analysis, we assumed that the initial prices of biological and curative treatment were the same and equal to $500,000. The breakeven price of each therapy was calculated in the model considering different WTP thresholds of $50,000, $100,000, and $150,000 per QALY gained [29].
For deterministic sensitivity analyses (DSA), one-way sensitivity analyses were run by changing a single variable or assumption at a time. The DSA were conducted for all model parameters associated with uncertainty. Outcomes were computed using low and high model parameter values specified by confidence interval bounds when applicable (Table 1).
For probabilistic sensitivity analysis (PSA), appropriate statistical distributions were assigned to input parameters. Values were drawn randomly from statistical distributions, including beta, gamma, Dirichlet, normal, and lognormal distributions. When it was not possible to obtain all the distribution parameters, a calibration based on the lower or higher bound of the DSA inputs was performed. Values were drawn from the prespecified distributions iteratively 10,000 times to generate distributions for ICERs. The results are presented graphically in (Figure 3, Figure 4 and Figure 5). The cost-effectiveness acceptability curves (CEAC) are available in the appendix (Figure A1), and the cost-effectiveness plane displaying each treatment in the shared plane is shown in (Figure A2).
4. Results
4.1. Cost-Effectiveness Results
The analysis performed showed that the hypothetical curative therapy provided the best results in terms of health outcomes. An extreme example of comparison showed that it was possible to achieve an additional increase of 1.58 QALYs for curative therapy compared with lifestyle intervention. The small molecule treatment yielded gains in QALYs and cost savings compared with lifestyle intervention, making it the dominant treatment. The results of the model’s base case scenarios are presented in Table 2.

Table 2.
Base case results of NAFL/NASH cohort model.
The distribution of causes of death obtained in the model was similar between lifestyle intervention and small molecule therapy. However, in treatment with biological therapy, a specific decrease in liver-related deaths and an increase in deaths caused by fatal CVE were observed. The results of the curative therapy showed a significant reduction in deaths caused by the consequences of NASH/NAFL disease (Table 3).

Table 3.
Distribution of events and causes of deaths observed in the model.
The results shown in Table 4 indicate that all the outcomes behaved linearly depending on the efficacy. The reached values of incremental QALYs and costs highlight that the curative therapy, while holding comparable cost to the biological treatment, is reaching around five times higher incremental QALY gains.

Table 4.
Incremental discounted results for different levels of efficacy.
Table 5 shows the impact of the price of the therapy on the ICER. The price of a curative therapy could be more than twice the cost of a biological therapy.

Table 5.
Economically justifiable price at different levels of WTP and treatment efficacy.
The resultant difference between strategies was highlighted in a comparison of lifetime QALYs depending on the initial state of the disease (Figure 3). The performance of the curative therapy was not substantially impacted by any analyzed initial stage of the disease. However, compared with the other treatments, it had the highest difference once implemented in the late F4 stage. This was most likely caused by the curative therapy initialization stage, which started from the F4 stage.
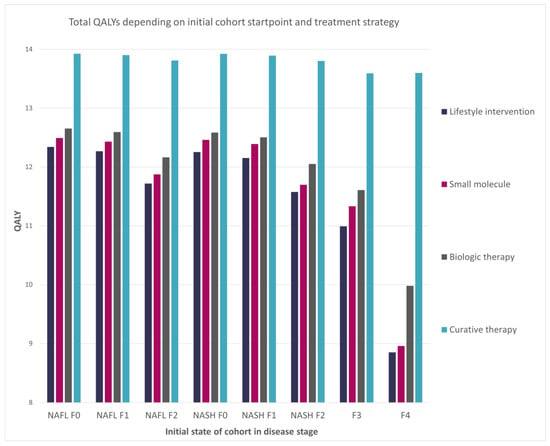
Figure 3.
Impact of the cohort initial disease stage on the total QALYs per patient.
4.2. Analysis of Key Drivers of Cost-Effectiveness (DSA–PSA)
While performing deterministic sensitivity analysis, we analyzed the influence of input parameters on model outcomes such as ICER, QALYs, and costs. Due to the dominance in the base case, we could not generate the tornado plot for the ICER variable for comparison of lifestyle intervention with small molecule therapy (Figure 4A,B). However, we observed for this comparison that utility and costs of particular health states had the most significant impact on the model results. When analyzing the comparison with biological therapy, we observed that one of the most influential factors was its assumed effectiveness (Figure 4C–E). The curative therapy behaved similarly to the small molecule therapy (Figure 4F–H) regarding the influence of different parameters. However, it is worth pointing out that modification of the curative therapy effectiveness and utility of patients with NAFL F0 substantially influenced QALY gained. This outcome was expected because those are the dimensions that curative therapy depends on.
The performed probabilistic sensitivity analysis showed how model outcomes are sensitive to parameter changes within the assumed distributions. As observed when comparing the small molecule with lifestyle intervention, most results were located in the fourth quarter of the CE plane (Figure 5A). The majority of the simulation outputs fell below the willingness-to-pay threshold. In the analysis of the biological therapy, all of the simulation results were within the first quarter of the graph (Figure 5B). However, there was a noticeable split between outputs divided by the willingness-to-pay threshold line. The output from comparing the curative therapy with lifestyle intervention was also located in the first quarter of the plot (Figure 5C) with all the simulation outcomes below the willingness-to-pay threshold.
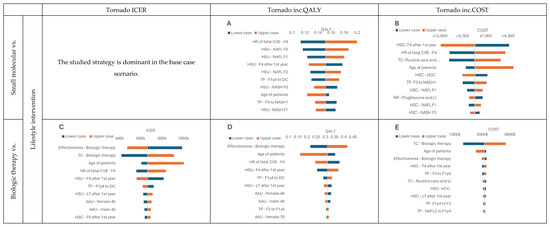
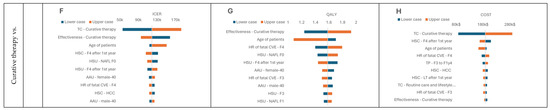
Figure 4.
DSA results. (A) Tornado inc.QALY graph, Small molecular vs. Lifestyle intervention; (B) Tornado inc.COST graph, Small molecular vs. Lifestyle intervention; (C) Tornado ICER graph, Biologic therapy vs. Lifestyle intervention; (D) Tornado inc.QALY graph, Biologic therapy vs. Lifestyle intervention; (E) Tornado inc.COST graph, Biologic therapy vs. Lifestyle intervention; (F) Tornado ICER graph, Curative therapy vs. Lifestyle intervention; (G) Tornado inc.QALY graph, Curative therapy vs. Lifestyle intervention; (H) Tornado inc.COST graph Curative therapy vs. Lifestyle intervention Health state utility—HSU, Transition probabilities—TP, Health state costs—HSC, Treatment costs—TC, NASH resolution—NR, lifestyle intervention —LI, Age-adjusted utility—AAU.
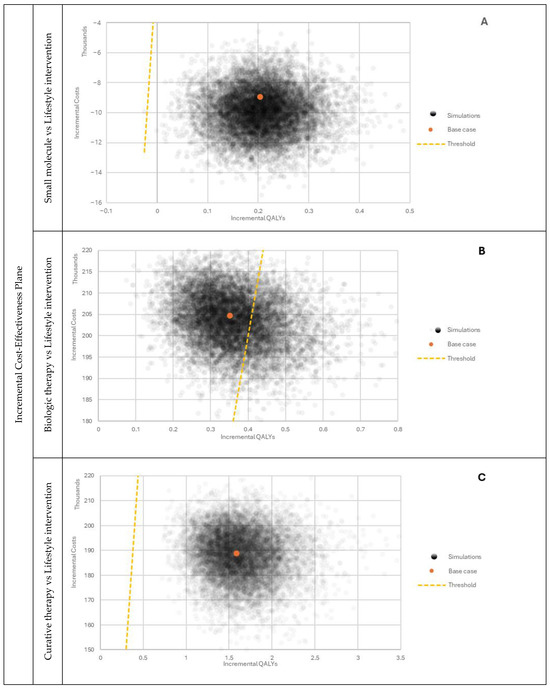
Figure 5.
PSA outcomes comparing selected therapies with lifestyle intervention. (A) Incremental Cost-Effectiveness Plane, Small molecule vs. Lifestyle intervention; (B) Incremental Cost-Effectiveness Plane, Biologic therapy vs Lifestyle intervention; (C) Incremental Cost-Effectiveness Plane, Curative therapy vs. Lifestyle intervention.
5. Discussion
The study revealed that the curative treatment involving cell-based therapy, with the assumed efficacy of 70% of patients cured and assumed price of $500,000, was a cost-effective treatment compared with the other strategies. Although it incurred higher costs, it also generated higher QALY gains, resulting in an ICER below the commonly accepted willingness-to-pay threshold of $150,000 per QALY in the US. This suggested that curative therapy could be viable for patients with NAFL or NASH, especially those at higher risk of disease progression. The study highlighted the potential significance of therapies aimed at curing rather than merely halting disease progression. Nonetheless, each of the analyzed therapies with the assumed efficacy could be cost-effective compared with lifestyle intervention at a relatively high price.
The sensitivity analyses showed that the results were robust to variations in model parameters. Finally, to check the performance of our model, we compared the results of lifestyle intervention from our model with the initial data used in other reviewed models [13,14]. After adopting input values to be the same as those in the compared models (while including a similar model structure), we obtained results for comparison. The incremental QALYs between small molecule and lifestyle interventions were similar between our model and the ICER US model (0.70 vs. 0.61). It is worth noting that small differences in reaching particular QALY values could have occurred because the compared models were not the same but shared similarities in a core structure. In particular our model did not include the cardiovascular events. Nevertheless, we concluded that, despite the small disparity, the results obtained confirm the reliability of our model.
Moreover, our model can be considered conservative due to the further inclusion of health states corresponding to NAFL, which were not considered by ICER US. Once we had introduced the extension of the NAFL and NASH stages, we observed that lifestyle intervention kept subjects in less advanced states for longer periods, which affected the QALY results. When considering the possibility of regression towards NAFL in the early fibrosis stages, our model estimated a much lower difference in QALYs between small molecule and lifestyle interventions (0.20) than the model developed by ICER US (0.61).
However, we acknowledge some limitations of the study. One limitation was that the model did not consider the potential side effects and long-term safety of the treatments, especially for biological and curative therapy. Additionally, the efficacy and costs of some treatments were based on limited evidence, which could have influenced the results. Moreover, the model only considered a US healthcare system perspective, and the results may not be generalizable to other healthcare systems. Lastly, the lifestyle intervention therapy primarily focused on subjects’ weight loss and did not encompass the entire spectrum of parameters that could be addressed in NAFL or NASH. We acknowledge the simplification of subject behavioral change; however, such an approach is considered standard in the cost-effectiveness modeling for NASH.
This finding could have important implications, highlighting the potential benefits of innovative therapies for managing NAFL and NASH and their cost-effectiveness. However, further research is needed to validate the efficacy and safety of such therapies and evaluate their long-term outcomes.
The presented methodology had novelty in extending the model population by including NAFL-diseased subjects. Thus, a broader spectrum of potential patients was included in the evaluation compared with previous studies. In addition, the previous version of the model had a different approach to calculating transitions within the early stages of NASH progression. Despite initial differences, the model structure was able to reach similar outcomes to the model presented by ICER US, and can be considered as its extension through the inclusion of NAFL health states and consideration of new therapies.
As the treatments evaluated in the model are theoretical, certain assumptions have been made concerning the fixed treatment cost within the model. This limitation is inherent in the study, and one of its consequences pertains to the interpretation of PSA results. In practice, some treatment outcomes, such as life extension, may be associated with an increase in treatment cost, a factor not currently observable. This limitation is expected to be addressed when more information on the posology of potential treatments becomes available.
Upon reviewing the literature, we concluded that assuming the direct results of Singh, S. et al. [19] as a distribution source held a certain level of error due to the limited number of observations and cases enlisted in the study. Thus, we considered the data as a foundation for further development. Furthermore, as we reviewed models available in the literature, the methodology presented by ICER US was deemed to have the most appropriate approach for mitigating limited access to data on disease progression. Therefore, we adapted the methodology proposed by ICER US in our calculations.
The other opinion about ICER US methodology was found while reviewing Javanbakht, M. et al. [14]. The authors pointed out that, despite such an approach being a valid option, it considered at least one step progression of the fibrosis stage at a time. The origin of such interpretation might have had a source in the multiplication weight value that was applied in the calculation procedure. The wage to calculate the transition probabilities was taken from Younossi, Z.M. et al. [12], representing the transition probability at least in one step of fibrosis. The wage values for improvement and worsening varied. On one hand, it was possible to claim that there was uncertainty regarding the assumption that the potential distribution of accelerated transition was unknown if we used such a wage. On the other hand, the data distillation procedure to obtain the progression proportion included numerous studies and participants that surpassed, in terms of amount, the analyzed participants in Singh, S. et al. [19]. Thus, it significantly reduced the coincidence of group proportion in analyzing patients, for example, the lack of connection in the Singh, S. et al. [19] NAFL matrix between F1 to F4, although there was a connection between F0 and F4. Due to the highly specific studies, access to the data was limited, highly anticipated, and usually challenging to obtain. Thus, formulating models that operate on a population level necessitated specific generalization layers in calculations.
It has to be noted that, due to the exploratory nature of this analysis, we did not consider potential side effects or safety issues related to new therapies. Their inclusion would require more precise data, which are not available. The inclusion of any side effects in the model would affect tested treatments by reducing incremental gains of QALYs, increasing the total cost of the investigated treatment. Consequently, it might impact the overall cost-effectiveness.
In the current version of our model, we did not include additional cardiovascular events triggered by the accumulation of pharmacological compounds due to their dosage, as shown in ICER US [13] and Javanbakht, M. et al. [14]. It should also be noted that this model version was a simplification of the actual clinical scenario, as it was shown in a publication on post-liver transplantation outcomes by Anstee, Q. M. et al. [30] that patients who underwent LT had a significant risk of NASH/NAFL re-emergence due to the difficulty in evaluating the health status of the donor’s liver. Therefore, such events were not included in the model for this study but were considered in future development steps.
It is important to note that we assumed that published results omitted any misinterpretation, which could have been a potential source of inaccuracy. As shown by Anstee Q.M et al. [31], there is a space for biased judgment while evaluating fibrosis stage classification. Depending on a specialization background, the practitioner could have had a slightly different interpretation of the stage of disease progression. Such a phenomenon was not considered in the current stage of model development. However, the impact of the initial stage of the disease on model results was widely explored.
Furthermore, the current state of the art of our model included theoretical treatments and their outcomes. In contrast, several potential treatments for NAFL and NASH have been under development for a couple of years. Extending the modeling exercise to account for these therapies could have been a field for additional research.
Author Contributions
Conceptualization, M.P., S.A., E.C., I.Z., M.T. and B.B.; Methodology, M.P., J.W.D., S.A., E.C., I.Z., M.T. and B.B.; Software, M.P. and J.W.D.; Validation, M.P., J.W.D., S.A., E.C., I.Z., M.T. and B.B.; Formal analysis, M.P. and J.W.D.; Investigation, M.P. and J.W.D.; Resources, M.P., J.W.D., E.C. and I.Z.; Data curation, M.P. and J.W.D.; Writing—original draft, M.P. and J.W.D.; Writing—review & editing, S.A., E.C., I.Z., M.T. and B.B.; Visualization, M.P. and J.W.D.; Supervision, M.T. and B.B.; Project administration, M.P. and J.W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
M.P., I.Z. and J.W.D. are employed by company Assignity; S.A. and M.T. are employed by company InovIntell; E.C. is employed by company Clever-Access; B.B. is employed by company Prescriptia. Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
List of Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| NAFL | non-alcoholic fatty liver |
| NASH | non-alcoholic steatohepatitis |
| F0 | initial stage of fibrosis progression in NAFL or NASH, considered as a healthy person |
| F1 | first stage of fibrosis progression in NAFL or NASH |
| F2 | second stage of fibrosis progression in NAFL or NASH |
| F3 | third stage of fibrosis progression |
| F4 | fourth stage of fibrosis progression or compensated cirrhosis |
| DC | Decompensated Cirrhosis |
| HCC | Hepatocellular Carcinoma |
| LT | Liver transplant |
| PLT | Post-liver transplant |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| LY | life years |
| ICER | incremental cost-effectiveness ratio |
| CVE | fatal cardiovascular events |
| QALY | quality-adjusted life-years |
| DSA | deterministic sensitivity analyses |
| PSA | probabilistic sensitivity analysis |
| NAS | NAFLD activity score |
| CC | compensated cirrhosis |
| SF-6D | Short Form-6D |
| CEAC | cost-effectiveness acceptability curve |
| HR | hazard ratio |
| ICER US | Institute for Clinical and Economic Review |
| FDA | US Food and Drugs Administration |
Appendix A

Table A1.
Summary of all Input values.
Table A1.
Summary of all Input values.
| Parameter | Base Case | Distribution | Low Value | High Value | Source |
|---|---|---|---|---|---|
| General settings | |||||
| Discount rate for costs | 3.00% | Normal | 1.50% | 5.00% | Weinstein 1996 [23] |
| Discount rate for outcomes | 3.00% | Normal | 1.50% | 5.00% | Weinstein 1996 [23] |
| Percentage of female | 56% | Beta | 50% | 61% | Brunt 2011 [21] |
| Age of patients | 47.70 | Normal | 45.90 | 56.10 | Brunt 2011 [21] |
| Costs | |||||
| Treatment costs | |||||
| Treatment costs—Curative therapy | $500,000 | Lognormal | $350,000 | $650,000 | Assumed |
| Treatment costs—Routine care and pioglitazone | $2311.38 | Lognormal | $1617.96 | $3004.79 | Tapper 2016 [18] |
| Treatment costs—Routine care and lifestyle intervention | $2083.47 | Lognormal | $1458.43 | $2708.51 | Zhang 2015 [20] |
| Treatment costs—Biologic therapy | $500,000 | Lognormal | $350,000 | $650,000 | Assumed |
| Health state costs | |||||
| Health state costs—NAFL F0 | $2882.60 | Gamma | $2017.82 | $3747.38 | Younossi 2016 [3] |
| Health state costs—NAFL F1 | $5765.20 | Gamma | $4035.64 | $7494.76 | |
| Health state costs—NAFL F2 | $8647.80 | Gamma | $6053.46 | $11,242.14 | |
| Health state costs—NASH F0 | $4118.00 | Gamma | $2882.60 | $5353.40 | |
| Health state costs—NASH F1 | $8236.00 | Gamma | $5765.20 | $10,706.80 | |
| Health state costs—NASH F2 | $12,354.00 | Gamma | $8647.80 | $16,060.20 | |
| Health state costs—F3 | $17,904.74 | Gamma | $12,533.32 | $23,276.16 | |
| Health state costs—F4 1st year | $29,688.12 | Gamma | $20,781.68 | $38,594.56 | |
| Health state costs—F4 after 1st year | $29,688.12 | Gamma | $20,781.68 | $38,594.56 | |
| Health state costs—DC | $106,370.53 | Gamma | $74,459.37 | $138,281.69 | |
| Health state costs—HCC | $215,504.24 | Gamma | $150,852.97 | $280,155.51 | |
| Health state costs—LT 1st year after DC | $215,504.24 | Gamma | $150,852.97 | $280,155.51 | |
| Health state costs—LT 1st year after HCC | $215,504.24 | Gamma | $150,852.97 | $280,155.51 | |
| Health state costs—LT after 1st year | $53,043.06 | Gamma | $37,130.14 | $68,955.98 | |
| Transition probabilities & HRs | |||||
| Transition probabilities | |||||
| Transition probabilities—NAFL0 to NAFL1 | 14.66% | Dirichlet | 13.19% | 16.12% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NAFL0 to NAFL2 | 7.33% | Dirichlet | 6.60% | 8.06% | |
| Transition probabilities—NAFL0 to NASH0 | 1.98% | Dirichlet | 1.79% | 2.18% | Singh 2015 [19], Younossi et al., 2019 [12], Tapper 2016 [18] |
| Transition probabilities—NAFL0 to NASH1 | 0.47% | Dirichlet | 0.43% | 0.52% | |
| Transition probabilities—NAFL0 to NASH2 | 0.14% | Dirichlet | 0.12% | 0.15% | |
| Transition probabilities—NAFL0 to F3 | 3.66% | Dirichlet | 3.30% | 4.03% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NAFL0 to F1y4 | 0.92% | Dirichlet | 0.82% | 1.01% | |
| Transition probabilities—NAFL1 to F3 | 6.14% | Dirichlet | 5.53% | 6.76% | |
| Transition probabilities—NAFL1 to F1y4 | 0.00% | Dirichlet | 0.00% | 0.00% | |
| Transition probabilities—NAFL2 to F3 | 13.59% | Dirichlet | 12.23% | 14.95% | |
| Transition probabilities—NAFL2 to F1y4 | 6.79% | Dirichlet | 6.11% | 7.47% | |
| Transition probabilities—NAFL1 to NAFL0 | 22.42% | Dirichlet | 20.18% | 24.67% | |
| Transition probabilities—NAFL1 to NAFL2 | 14.33% | Dirichlet | 12.90% | 15.77% | |
| Transition probabilities—NAFL1 to NASH0 | 0.63% | Dirichlet | 0.57% | 0.69% | Singh 2015 [19], Younossi et al., 2019 [12], Tapper 2016 [18] |
| Transition probabilities—NAFL1 to NASH1 | 1.53% | Dirichlet | 1.38% | 1.68% | |
| Transition probabilities—NAFL1 to NASH2 | 0.34% | Dirichlet | 0.31% | 0.38% | |
| Transition probabilities—NAFL2 to NAFL0 | 9.02% | Dirichlet | 8.12% | 9.92% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NAFL2 to NAFL1 | 13.53% | Dirichlet | 12.18% | 14.88% | |
| Transition probabilities—NAFL2 to NASH0 | 0.15% | Dirichlet | 0.13% | 0.16% | Singh 2015 [19], Younossi et al., 2019 [12], Tapper 2016 [18] |
| Transition probabilities—NAFL2 to NASH1 | 0.49% | Dirichlet | 0.44% | 0.53% | |
| Transition probabilities—NAFL2 to NASH2 | 1.54% | Dirichlet | 1.38% | 1.69% | |
| Transition probabilities—NASH0 to NAFL0 | 19.88% | Dirichlet | 17.89% | 21.87% | |
| Transition probabilities—NASH0 to NAFL1 | 0.55% | Dirichlet | 0.50% | 0.61% | |
| Transition probabilities—NASH0 to NAFL2 | 0.28% | Dirichlet | 0.25% | 0.30% | |
| Transition probabilities—NASH0 to NASH1 | 16.75% | Dirichlet | 15.07% | 18.42% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NASH0 to NASH2 | 4.79% | Dirichlet | 4.31% | 5.26% | |
| Transition probabilities—NASH0 to F3 | 2.39% | Dirichlet | 2.15% | 2.63% | |
| Transition probabilities—NASH0 to F1y4 | 2.39% | Dirichlet | 2.15% | 2.63% | |
| Transition probabilities—NASH1 to F3 | 6.76% | Dirichlet | 6.08% | 7.44% | |
| Transition probabilities—NASH1 to F1y4 | 1.35% | Dirichlet | 1.22% | 1.49% | |
| Transition probabilities—NASH2 to F3 | 10.19% | Dirichlet | 9.17% | 11.21% | |
| Transition probabilities—NASH2 to F1y4 | 10.19% | Dirichlet | 9.17% | 11.21% | |
| Transition probabilities—NASH1 to NAFL0 | 0.84% | Dirichlet | 0.76% | 0.93% | Singh 2015 [19], Younossi et al., 2019 [12], Tapper 2016 [18] |
| Transition probabilities—NASH1 to NAFL1 | 19.88% | Dirichlet | 17.89% | 21.87% | |
| Transition probabilities—NASH1 to NAFL2 | 0.54% | Dirichlet | 0.49% | 0.59% | |
| Transition probabilities—NASH1 to NASH0 | 22.21% | Dirichlet | 19.99% | 24.43% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NASH1 to NASH2 | 12.17% | Dirichlet | 10.95% | 13.38% | |
| Transition probabilities—NASH2 to NAFL0 | 0.34% | Dirichlet | 0.31% | 0.37% | Singh 2015 [19], Younossi et al., 2019, Tapper 2016 [18] |
| Transition probabilities—NASH2 to NAFL1 | 0.51% | Dirichlet | 0.46% | 0.56% | |
| Transition probabilities—NASH2 to NAFL2 | 19.88% | Dirichlet | 17.89% | 21.87% | |
| Transition probabilities—NASH2 to NASH0 | 5.15% | Dirichlet | 4.64% | 5.67% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—NASH2 to NASH1 | 17.18% | Dirichlet | 15.46% | 18.89% | |
| Transition probabilities—F3 to NASH1 | 5.62% | Dirichlet | 5.06% | 6.18% | |
| Transition probabilities—F3 to NASH2 | 5.62% | Dirichlet | 5.06% | 6.18% | |
| Transition probabilities—F3 to F1y4 | 10.26% | Dirichlet | 9.24% | 11.29% | |
| Transition probabilities—F3 to DC | 0.32% | Dirichlet | 0.29% | 0.35% | ICER NASH Draft Report 2023 [13] |
| Transition probabilities—F3 to HCC | 0.24% | Dirichlet | 0.22% | 0.26% | |
| Transition probabilities—NASH_B to NAFL1 | 52.70% | Dirichlet | 47.43% | 57.97% | Zhang 2015 [20] |
| Transition probabilities—NASH_B0 to NASH_B1 | 13.23% | Dirichlet | 11.91% | 14.55% | |
| Transition probabilities—NASH_B0 to NASH_B2 | 3.78% | Dirichlet | 3.40% | 4.16% | |
| Transition probabilities—NASH_B1 to NASH_B2 | 9.61% | Dirichlet | 8.65% | 10.57% | |
| Transition probabilities—NASH_B0 to F3 | 1.89% | Dirichlet | 1.70% | 2.08% | |
| Transition probabilities—NASH_B1 to F3 | 5.34% | Dirichlet | 4.81% | 5.87% | |
| Transition probabilities—NASH_B2 to F3 | 8.05% | Dirichlet | 7.25% | 8.86% | |
| Transition probabilities—F1y4 to F3 | 27.27% | Dirichlet | 24.55% | 30.00% | Singh 2015 [19], Younossi et al., 2019 [12] |
| Transition probabilities—F1y4 to DC | 4.22% | Dirichlet | 3.79% | 4.64% | ICER NASH Draft Report 2023 [13] |
| Transition probabilities—F1y4 to HCC | 2.11% | Dirichlet | 1.90% | 2.32% | |
| Transition probabilities—F_B3 to F1y4 | 4.80% | Dirichlet | 4.32% | 5.28% | Zhang 2015 [20] |
| Transition probabilities—DC to HCC | 3.45% | Dirichlet | 3.11% | 3.80% | ICER NASH Draft Report 2023 [13] |
| Transition probabilities—DC to LT | 37.45% | Dirichlet | 33.71% | 41.20% | |
| Transition probabilities—HCC to LT | 35.20% | Dirichlet | 31.68% | 38.72% | |
| Hazard ratios | |||||
| HR of fatal CVE—NAFL F0 | 1.00 | Normal | 0.65 | 1.16 | Hagström, H. et. al. [26] |
| HR of fatal CVE—NAFL F1 | 1.01 | Normal | 0.70 | 1.46 | |
| HR of fatal CVE—NAFL F2 | 1.60 | Normal | 1.09 | 2.39 | |
| HR of fatal CVE—NASH F0 | 1.00 | Normal | 0.65 | 1.16 | |
| HR of fatal CVE—NASH F1 | 1.01 | Normal | 0.61 | 1.36 | |
| HR of fatal CVE—NASH F2 | 1.85 | Normal | 0.70 | 1.76 | |
| HR of fatal CVE—F3 | 3.04 | Normal | 1.94 | 4.78 | |
| HR of fatal CVE—F4 | 6.53 | Normal | 3.55 | 12.03 | |
| Utilities | |||||
| Health state utility | |||||
| Health state utility—NAFL F0 | 0.76 | Beta | 0.68 | 0.84 | Younossi 2016 [3] |
| Health state utility—NAFL F1 | 0.76 | Beta | 0.68 | 0.84 | |
| Health state utility—NAFL F2 | 0.76 | Beta | 0.68 | 0.84 | |
| Health state utility—NASH F0 | 0.76 | Beta | 0.68 | 0.84 | |
| Health state utility—NASH F1 | 0.76 | Beta | 0.68 | 0.84 | |
| Health state utility—NASH F2 | 0.76 | Beta | 0.68 | 0.84 | |
| Health state utility—F3 | 0.73 | Beta | 0.66 | 0.80 | |
| Health state utility—F4 1st year | 0.66 | Beta | 0.59 | 0.73 | |
| Health state utility—F4 after 1st year | 0.66 | Beta | 0.59 | 0.73 | |
| Health state utility—DC | 0.57 | Beta | 0.51 | 0.63 | |
| Health state utility—HCC | 0.50 | Beta | 0.45 | 0.55 | |
| Health state utility—LT 1st year after DC | 0.73 | Beta | 0.66 | 0.80 | |
| Health state utility—LT 1st year after HCC | 0.73 | Beta | 0.66 | 0.80 | |
| Health state utility—LT after 1st year | 0.73 | Beta | 0.66 | 0.80 | |
| Age-adjusted utilities | |||||
| Age-adjusted utility—male-40 | 0.89 | Beta | 0.80 | 0.98 | Tapper 2016 [18] |
| Age-adjusted utility—male-50 | 0.86 | Beta | 0.78 | 0.95 | |
| Age-adjusted utility—male-60 | 0.84 | Beta | 0.76 | 0.92 | |
| Age-adjusted utility—male-70 | 0.80 | Beta | 0.72 | 0.88 | |
| Age-adjusted utility—male-80 | 0.78 | Beta | 0.70 | 0.86 | |
| Age-adjusted utility—female-40 | 0.86 | Beta | 0.78 | 0.95 | |
| Age-adjusted utility—female-50 | 0.84 | Beta | 0.75 | 0.92 | |
| Age-adjusted utility—female-60 | 0.81 | Beta | 0.73 | 0.89 | |
| Age-adjusted utility—female-70 | 0.77 | Beta | 0.69 | 0.85 | |
| Age-adjusted utility—female-80 | 0.72 | Beta | 0.65 | 0.80 | |
| Efficacy | |||||
| NASH resolution | |||||
| NASH resolution—Lifestyle intervention—Weightloss < 5% | 10.24% | Beta | 8.20% | 12.29% | Tapper 2016 [18] |
| NASH resolution—Lifestyle intervention—Weightloss 5–10% | 42.37% | Beta | 33.90% | 50.85% | |
| NASH resolution—Lifestyle intervention—Weightloss > 10% | 89.66% | Beta | 71.72% | 107.59% | |
| NASH resolution—Pioglitazone and lifestyle intervention | 52.70% | Beta | 42.16% | 63.24% | Zhang 2015 [20] |
| Fibrosis progression | |||||
| Fibrosis progression—Lifestyle intervention—Weightloss < 5% | 100.00% | Beta | 80.00% | 120.00% | Tapper 2016 [18] |
| Fibrosis progression—Lifestyle intervention—Weightloss 5–10% | 98.93% | Beta | 79.15% | 118.72% | |
| Fibrosis progression—Lifestyle intervention—Weightloss > 10% | 40.81% | Beta | 32.65% | 48.98% | |
| Fibrosis progression—Small molecular | 40.81% | Beta | 32.65% | 48.98% | |
| Probability of reversing cirrhosis/avoiding further progression | |||||
| Effectiveness—Biologic therapy | 70.00% | Beta | 56.00% | 84.00% | Assumed |
| Effectiveness—Curative therapy | 70.00% | Beta | 56.00% | 84.00% | Assumed |
| Weight loss in patients with lifestyle intervention | |||||
| Percentage—Weightloss < 5% | 80.19% | Beta | 64.16% | 96.23% | Tapper 2016 [18] |
| Percentage—Weightloss 5–10% | 12.88% | Beta | 10.30% | 15.45% | |
| Percentage—Weightloss > 10% | 6.93% | Beta | 5.54% | 8.31% | |
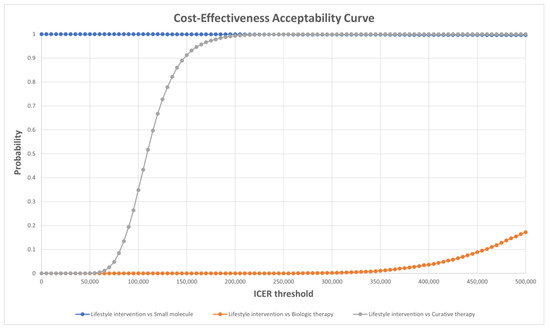
Figure A1.
Cost-effectiveness acceptability curve of each treatment.
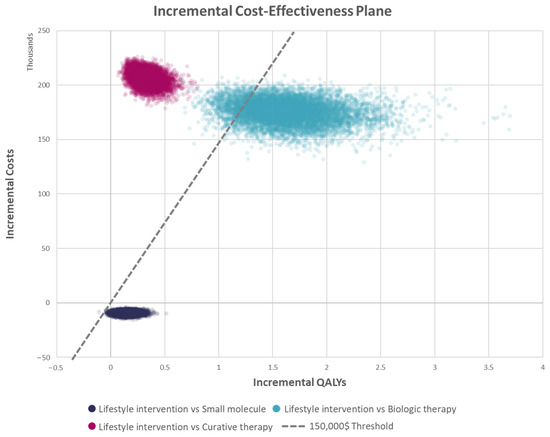
Figure A2.
Incremental cost−effectiveness plane of each treatment.
References
- EASL; EASD; EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2011, 46 (Suppl. S1), 63–69. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.E.; Rajagopal, V.; Smith, C.; Cohick, E.; Whissell, G.; Gamboa, M.; Pai, R.; Sigova, A.; Grossman, I.; Bumcrot, D.; et al. Discovery and Targeting of the Signaling Controls of PNPLA3 to Effectively Reduce Transcription, Expression, and Function in Pre-Clinical NAFLD/NASH Settings. Cells 2020, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03963921, Safety and Tolerability of HepaStem in Patients With Cirrhotic and Pre-cirrhotic NASH Patients (PANASH). Available online: https://clinicaltrials.gov/study/NCT03963921?cond=HepaStem&start=2019-04-01_&aggFilters=phase:2%201&rank=1 (accessed on 6 December 2023).
- Smith, J. First Stem Cell Therapy for Liver Failure Shows Promise in First Human Trials 2019. Available online: https://www.labiotech.eu/trends-news/promethera-stem-cell-therapy/ (accessed on 6 December 2023).
- Li, T.T.; Wang, Z.R.; Yao, W.Q.; Linghu, E.Q.; Wang, F.S.; Shi, L. Stem Cell Therapies for Chronic Liver Diseases: Progress and Challenges. Stem Cells Transl. Med. 2022, 11, 900–911. [Google Scholar] [CrossRef]
- Khan, S.; Khan, R.S.; Newsome, P.N. Cell Therapy for Liver Disease: From Promise to Reality. Semin. Liver Dis. 2020, 40, 411–426. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Tice, J.A.; Fahim, S.M.; Richardson, M.; Herce-Hagiwara, B.; Chu, J.N.; Pearson, S.D.; Rind, D.M.; Suh, K.; Carlson, J.; Dickerson, R. Resmetirom and Obeticholic Acid for Non-Alcoholic Steatohepatitis (NASH); Institute for Clinical and Economic Review: 2023. Available online: https://icer.org/wp-content/uploads/2022/10/NASH-Final-Report_For-Publication_052623 (accessed on 6 December 2023).
- Javanbakht, M.; Fishman, J.; Moloney, E.; Rydqvist, P.; Ansaripour, A. Early Cost-Effectiveness and Price Threshold Analyses of Resmetirom: An Investigational Treatment for Management of Nonalcoholic Steatohepatitis. Pharmacoecon Open 2023, 7, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Aballea, S.; Karwala, P.; Zerda, I.; Toumi, M.; Pochopien, M.; Han, R.; Borissov, B.; Clay, E. EE283 Cost-Effectiveness Analysis of Cell-Based Therapy for Patients with Non-Alcoholic Fatty Liver Disease. Value Health 2022, 25, S389. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef] [PubMed]
- Administration FaD. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Noncirrhotic Nonalcoholic Steatohepatitis with Liver Fibrosis, Developing Drugs for Treatment. In Services USDoHaH; Administration FaD: Rockville, MA, USA, 2018. [Google Scholar]
- Tapper, E.B.; Hunink, M.G.; Afdhal, N.H.; Lai, M.; Sengupta, N. Cost-Effectiveness Analysis: Risk Stratification of Nonalcoholic Fatty Liver Disease (NAFLD) by the Primary Care Physician Using the NAFLD Fibrosis Score. PLoS ONE 2016, 11, e0147237. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654. [Google Scholar] [CrossRef]
- Zhang, E.; Wartelle-Bladou, C.; Lepanto, L.; Lachaine, J.; Cloutier, G.; Tang, A. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur. Radiol. 2015, 25, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Dudekula, A.; Rachakonda, V.; Shaik, B.; Behari, J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS ONE 2014, 9, e111808. [Google Scholar] [CrossRef]
- Weinstein, M.C.; Siegel, J.E.; Gold, M.R.; Kamlet, M.S.; Russell, L.B. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996, 276, 1253–1258. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Center for Health Statistics, United States Life Tables, 2001–2011. In Statistics NCfH; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2011. Available online: https://www.cdc.gov/nchs/products/life_tables.htm (accessed on 6 December 2023).
- Centers for Disease Control and Prevention, National Vital Statistics System. Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in 5-year Age Groups, by Race and Sex: United States, 1999–2015. In Statistics NCfH; Centers for Disease Control and Prevention, National Vital Statistics System: Atlanta, GA, USA, 2015. Available online: https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm (accessed on 6 December 2023).
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Binda, M.M.; Menchi, L.; Baran, T.; Greiling, Y.; Michel, S.; Tchelingerian, J. Clinical-grade human liver mesenchymal stem cells reduce NAS score and fibrosis progression in advanced stage NASH pre-clinical model through immunomodulation. J. Hepatol. 2018, 68, S345–S346. [Google Scholar] [CrossRef]
- Medical Care Price Inflation Calculator; Official Data Foundation/Alioth LLC. Available online: https://www.in2013dollars.com/Medical-care/price-inflation/ (accessed on 6 December 2023).
- Dubois, R.W. Cost-effectiveness thresholds in the USA: Are they coming? Are they already here? J. Comp. Eff. Res. 2016, 5, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Hallsworth, K.; Lynch, N.; Hauvespre, A.; Mansour, E.; Kozma, S.; Bottomley, J.; Milligan, G.; Piercy, J.; Higgins, V. Alignment of Physician-Stated vs Clinically Derived Reference Fibrosis Score in Patients with Non-Alcoholic Steatohepatitis: A Real-World European Survey. Pragmat. Obs. Res. 2023, 14, 13–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).