Abstract
On a worldwide scale, neurodegenerative diseases, including multiple sclerosis, Parkinson’s, and Alzheimer’s, face considerable healthcare challenges demanding the development of novel approaches to early detection and efficient treatment. With its ability to provide real-time patient monitoring, customized medical care, and advanced predictive analytics, artificial intelligence (AI) is fundamentally transforming the way healthcare is provided. Through the integration of wearable physiological sensors, motion sensors, and neurological assessment tools, the NeuroPredict platform harnesses AI and smart sensor technologies to enhance the management of specific neurodegenerative diseases. Machine learning algorithms process these data flows to find patterns that point out disease evolution. This paper covers the design and architecture of the NeuroPredict platform, stressing the ethical and regulatory requirements that guide its development. Initial development of AI algorithms for disease monitoring, technical achievements, and constant enhancements driven by early user feedback are addressed in the discussion section. To ascertain the platform’s trustworthiness and data security, it also points towards risk analysis and mitigation approaches. The NeuroPredict platform’s capability for achieving AI-driven smart healthcare solutions is highlighted, even though it is currently in the development stage. Subsequent research is expected to focus on boosting data integration, expanding AI models, and providing regulatory compliance for clinical application. The current results are based on incremental laboratory tests using simulated user roles, with no clinical patient data involved so far. This study reports an experimental technology evaluation of modular components of the NeuroPredict platform, integrating multimodal sensors and machine learning pipelines in a laboratory-based setting, with future co-design and clinical validation foreseen for a later project phase.
1. Introduction
Multiple sclerosis (MS), Parkinson’s disease (PD), and Alzheimer’s disease (AD) are examples of neurodegenerative diseases (NDDs) that pose an increasing threat to global health. These disorders, which are characterized by a progressive and frequently irreversible decline in neuronal function, cause significant impairments in daily activities, mobility, and cognition. As the population ages, their prevalence is increasing, which necessitates improved diagnostic and treatment approaches in healthcare systems as well as long-term support networks [1,2].
Few effective disease-modifying therapies are available, despite progress in our understanding of the pathophysiology of NDDs. To improve patient outcomes, customize interventions, and slow disease progression, early diagnosis and ongoing monitoring are essential. However, subtle preclinical changes are often missed by traditional diagnostic methods, which are often episodic and rely on complex imaging techniques. Such delays reduce the opportunity for prompt therapeutic intervention in conditions like AD and PD, where neuropathological changes occur years before clinical symptoms appear.
In recent years, artificial intelligence (AI) and wearable technologies have opened new possibilities for the continuous and personalized monitoring of neurodegenerative diseases. By shifting from episodic clinical evaluations to real-time, context-aware analysis, these tools aim to capture subtle changes in motor and cognitive function that would otherwise go undetected. However, many current solutions remain limited in terms of integration, ethical oversight, and regulatory compliance. These gaps are what the NeuroPredict platform seeks to address through its unified and modular design.
The aim of this study is to present and experimentally evaluate, in a laboratory setting, the modular architecture of the NeuroPredict platform, with a specific focus on its fall prevention module. The objectives are: (i) to describe the technological components and their integration; (ii) to report the results of laboratory-based evaluations using simulated user scenarios; and (iii) to discuss the platform’s preparedness for future co-design, clinical validation, and regulatory compliance.
Identified shortcomings and research gap: Previous research in AI-enabled neurodegenerative disease monitoring has shown several shortcomings: (i) fragmented focus on individual domains, such as motor or cognitive assessment, without a unified approach; (ii) insufficient integration of ethical, legal, and regulatory safeguards from the design phase; (iii) lack of participatory or user-centered design methodologies; and (iv) limited readiness for scalable, clinically deployable architectures. These gaps highlight the need for a cohesive, modular platform that can integrate multimodal data acquisition, advanced machine learning pipelines, and compliance-by-design principles for trustworthy neuro-monitoring.
Research questions: To address these gaps, this study investigates the following research questions:
- RQ1: How can a modular, scalable architecture integrate multimodal sensors to support neurodegenerative disease monitoring in a laboratory-based setting?
- RQ2: What is the technical performance of fall detection algorithms implemented on the NeuroPredict platform using simulated user data?
- RQ3: How well does the platform’s architecture align with anticipated ethical, legal, and regulatory requirements, including the General Data Protection Regulation (GDPR), the Medical Device Regulation (MDR), and the emerging European AI Act?
This paradigm is reflected in the modular architecture of the NeuroPredict platform, which facilitates multimodal monitoring in real-world settings, including data pertaining to speech, motor, cognitive, and emotional processes [3,4,5]. The platform is also well-positioned to support geriatric care, where intelligent monitoring tools are becoming increasingly significant for managing long-term, chronic neurodegenerative conditions, by enabling continuous and personalized tracking [6].
AI systems in healthcare must provide explainable outputs and be compatible with clinical workflows in addition to having predictive performance. Adoption in delicate areas like neurodegenerative care requires that clinicians comprehend and have faith in automated recommendations, which is made possible by transparent and interpretable AI models. To improve transparency and clinical relevance, ongoing research focuses on hybrid architectures that combine deep learning with rule-based logic or attention mechanisms [7]. Moreover, ensuring fairness in AI-based neuro-monitoring requires robust validation across diverse populations to prevent algorithmic bias and health disparities [8].
The development of AI-powered healthcare platforms must align with established legal and ethical frameworks to ensure safety, trust, and clinical applicability. This includes compliance with the GDPR, the MDR, and the emerging European AI Act. These requirements are particularly important in neurodegenerative care, where sensitive data, clinical oversight, and algorithmic transparency are critical.
The NeuroPredict platform is an AI-driven digital health system aimed at facilitating timely identification and ongoing monitoring of neurodegenerative diseases, namely PD, AD, and MS. It seeks to overcome existing constraints in managing diseases by supporting extensive, longitudinal gathering and analysis of data by using embedded sensing technology. The NeuroPredict platform is designed with a firm dedication to ethical principles and regulatory compliance, guaranteeing data protection, transparency, and conformity to advancing healthcare standards across its lifecycle. The multifaceted nature and variety of neurodegenerative disorders represent a considerable challenge for precise and accurate monitoring. The NeuroPredict platform addresses this need by integrating multimodal data collection with advanced AI algorithms, enabling more reliable and individualized tracking of disease progression.
Due to project constraints, the NeuroPredict platform’s initial design phase relied on internal expertise, literature-based requirements, and iterative lab testing rather than direct co-design with target user groups. However, the system has been intentionally developed with a scalable, flexible architecture that will facilitate full participatory co-design in future clinical validation phases.
While a variety of AI-enabled digital health platforms have emerged for neurodegenerative conditions, many of them address only partial domains or lack robust compliance-by-design and modular architectures. The NeuroPredict platform aims to fill this gap by integrating multimodal sensing, scalable machine learning pipelines, and advanced privacy and regulatory safeguards in a unified system.
This work should be interpreted as an experimental technology assessment focused on validating a modular architecture that integrates multimodal data acquisition, initial machine learning pipelines, and secure data visualization tools. The broader platform development will continue through subsequent participatory design and clinical validation phases, including post-project time.
2. Related Work
2.1. Advances in Neurodegenerative Disease Research
Recent studies have increasingly focused on the role of advanced neuroimaging and AI-powered analysis in improving diagnostic precision and enabling individualized disease tracking in neurodegenerative disorders. For example, Zhang (2024) emphasizes the need for deeper insights into disease mechanisms to support the development of tailored therapeutic strategies [9,10]. In this context, technological innovations, particularly in high-resolution imaging and machine learning, are being explored to support early detection and more responsive care.
In parallel, there is a growing need for tools that enable continuous, multidimensional assessments in everyday settings. Traditional episodic clinical evaluations often fail to capture early or subtle signs of disease progression, especially in nonmotor domains such as cognition, mood, and behavior [11]. Recent research has highlighted the significant impact of nonmotor symptoms on quality of life in neurodegenerative disorders, reinforcing the importance of integrated monitoring strategies that combine both clinical and psychosocial factors [11,12]. These limitations have increasingly been addressed through digital technologies, particularly wearable and ambient sensors paired with AI models capable of longitudinal data analysis. By capturing patterns in gait variability, tremor dynamics, and cognitive decline, such systems offer promising tools for early detection and personalized intervention in MS, PD, and AD [12,13]. However, despite ongoing innovation, many existing digital health solutions remain limited in terms of cross-domain integration, ethical oversight, and regulatory compliance—issues that newer platforms like NeuroPredict aim to overcome [3,14,15,16,17].
High-resolution imaging and AI-assisted interpretation can identify early structural and functional brain changes, enabling prompt clinical decisions in AD and PD, as reviewed by Akram et al. (2024) [18]. Similarly, Jayaraj (2023) emphasizes how integrating AI with digital platforms allows for automated, scalable neurological condition evaluation, bridging the gap between conventional clinical assessments and functional monitoring in the real world [19].
AI-enhanced digital platforms that integrate wearable technology, smartphone apps, and cloud-based analytics have emerged as a result of this paradigm shift, providing patients and clinicians with remote, real-time support. Examples include the continuous, ecologically valid evaluation of mobility, cognition, and behavior using smartwatches, gait sensors, electroencephalography (EEG) devices, and serious games. A thorough review of sensor-based systems used in gait and postural assessment for MS and PD is given by Das et al. (2022), who also highlight the potential of wearable technology to assist at-home functional evaluation [20]. The increasing use of AI for remote assessment and management of Parkinson’s disease symptoms is also described by Bounsall et al. (2023), who highlight the shift to decentralized care models [21].
Few platforms provide a unified, modular approach that can integrate various physiological and behavioral signals across multiple conditions, even though several systems focus on domains, such as motor assessment or cognitive monitoring. This disparity spurs the creation of cutting-edge solutions like the NeuroPredict platform, which seeks to integrate ethical compliance, intelligent analytics, and real-time sensing within a single scalable framework.
2.2. AI and Wearable Technologies for Diagnosis and Monitoring
Wearable technology and artificial intelligence (AI) have greatly improved the early diagnosis and continuous monitoring of NDDs. Ganjizadeh et al. (2024) highlight the use of AI-driven models to improve diagnostic accuracy by analyzing clinical and neuroimaging data [12].
Among the AI methods applied in this context, convolutional neural networks (CNNs) have shown strong potential for identifying subtle neuroanatomical changes in neuroimaging data, supporting earlier and more accurate diagnoses [18,22]. Beyond neuroimaging, AI systems increasingly generate individualized clinical insights by combining multimodal data sources, including genomic profiles, physiological signals, and electronic health records [23]. When integrated with Internet of Things (IoT) frameworks, such platforms enable real-time, context-aware monitoring and more responsive care planning. In resource-constrained environments, noninvasive approaches—such as voice analysis and facial emotion recognition—have also gained attention for early cognitive impairment detection [24]. Predictive modeling techniques, while still evolving, are becoming increasingly capable of dynamically adapting to individual disease trajectories [25,26].
These technological advancements have broadened the scope of AI in NDD monitoring, moving beyond neuroimaging toward multimodal, real-time evaluation techniques. For instance, recent studies have explored the integration of natural language processing (NLP) models to assess cognitive decline through speech and written language patterns [27,28,29]. Simultaneously, wearable sensors such as smartwatches, EEG headbands, and gait-tracking devices now allow for the continuous recording of motor and physiological activity in nonclinical environments [30,31]. In combination with deep learning algorithms, these devices offer novel ways to detect neurological abnormalities in their early stages and support more scalable, personalized care models.
Mobile applications are valuable tools for supporting patients with central nervous system disorders, facilitating disease monitoring and management due to their accessibility, ease of use, and advanced capabilities [32].
Generative AI techniques such as Generative Adversarial Networks (GANs) and large language models further expand this potential, enabling synthetic EEG augmentation, enhanced imaging, and personalized patient trajectories [33].
The convergence of artificial intelligence and neuroscience is advancing the diagnosis of neurological disorders, including neurodegenerative conditions, using biomarkers such as brain age. Brain age is estimated by AI models that analyze anatomical brain maps to compare the person’s biological age with the patient’s chronological age; significant discrepancies can indicate an increased risk of Alzheimer’s disease [34,35].
AI-driven wearable technologies can enhance diagnostic and monitoring capabilities across various aspects of neurodegenerative disease care, as shown by Faragó et al. [36], Zhao et al. [30], and Yammouri et al. [37]. Based on movement data gathered by embedded sensors, Faragó’s system correctly identified motor characteristics unique to Parkinson’s disease, including bradykinesia, tremor, and irregularities in gait. This allowed for early and customized symptom monitoring outside of clinical settings. Moreover, Zhao’s framework for tracking gait demonstrated that cognitive load can dramatically change locomotor dynamics, especially influencing phase intervals and gait asymmetry. These insights highlight how crucial it is to record both motor and cognitive-motor patterns, particularly in diseases like MS and PD, where these impairments may manifest gradually and at an early age. Beyond movement analysis, wearable biosensors have also been suggested to identify biochemical markers in sweat and saliva, allowing for metabolic and biochemical profiling [37].
Together, these innovations highlight the growing potential of multiparametric, AI-enhanced wearables to deliver real-time, personalized insights into both motor and physiological aspects of neurodegenerative disease progression.
2.3. AI-Powered Digital Tools and Interfaces
In the treatment of NDDs, digital platforms enhanced by AI are increasingly used to maximize real-time monitoring and encourage patient involvement. These systems frequently integrate cloud-based analytics with wearable and ambient sensing devices to support data-driven clinical decisions, personalized care, and early detection.
Platforms like RO-SmartAgeing and PERFORM, which showcase various but complementary technological approaches, serve as examples of this potential. While PERFORM uses embedded microsensors and remote analysis frameworks to enable real-time motor symptom tracking in PD and amyotrophic lateral sclerosis (ALS), RO-SmartAgeing integrates sensor-based cognitive monitoring for aging populations [3,14]. Machine learning-enabled wearable systems that detect motor impairments in real-world settings by gathering multimodal data like posture, tremor frequency, and gait characteristics are examples of further developments [15]. These tools aid in prompt and well-informed therapeutic interventions by identifying functional changes that might not be apparent during routine clinical evaluations, particularly in MS and PD [16]. For example, in MS, AI systems that combine EEG data, physiological metrics, and gait variability have been used to predict relapses and customize treatment plans. Long short-term memory (LSTM)-based models have been used in PD to improve real-time analysis of abnormalities in gait and tremor [17].
Current advancements also include conversational and mobile AI applications for remote cognitive evaluation. To increase accessibility and user engagement, one such system uses chatbot interfaces integrated into mobile apps to help older adults in rural areas with cognitive screening and personalized training [38]. The CarePredict Tempo [39], a wrist-worn AI device, detects behavioral deviations that may indicate cognitive decline or increased fall risk, while radar-based noncontact solutions like Vayyar Home [40] continuously detect fall events by analyzing spatial movement patterns.
To provide users and caregivers with adaptive, patient-specific feedback and encourage more individualized care strategies, multimodal platforms like Dem@Care [41] integrate physiological and environmental data. In the meantime, Smart Aging [42] uses serious games to incorporate cognitive evaluation into 3D virtual environments, comparing gameplay metrics like accuracy, time, and distance with standardized cognitive tests like the MMSE and MoCA. In nonclinical contexts, this gamified method facilitates ongoing, easily accessible, and entertaining cognitive function monitoring.
While these systems represent a growing interest in intelligent, user-centered neurodegenerative care, their degree of integration, technical scope, and clinical applicability vary greatly For instance, PERFORM focuses on motor symptom detection without integrating cognitive elements; Dem@Care offers extensive sensor data fusion but lacks predictive analytics; and Smart Aging, although useful for gamified cognitive screening, does not allow for continuous physiological monitoring. These limitations underscore the need for more comprehensive and ethically sound platforms, such as NeuroPredict, which support multimodal, real-time, and personalized monitoring in line with clinical and regulatory requirements.
The digital solutions illustrate the growing role of AI-powered interfaces in transforming neurodegenerative disease care. From diagnosis to daily support, these technologies contribute to more proactive, efficient, and personalized healthcare delivery. By combining real-time physiological sensing, cognitive evaluation, and AI-powered predictive modeling into a single, modular system, the NeuroPredict platform aims to get around these restrictions. Its design also complies with ethical and legal standards, making it a more complete and scalable option for managing MS, PD, and AD in realistic environments.
2.4. Ethical, Legal, and Regulatory Considerations in AI for NDDs
The responsible development of AI-based healthcare systems—particularly those intended for use in sensitive contexts such as neurodegenerative disease monitoring—requires strict adherence to legal and ethical principles throughout the entire system lifecycle. Foundational values such as fairness, transparency, prevention of harm, and respect for human autonomy have been established at the European level by the High-Level Expert Group on AI (AI HLEG) as core requirements for trustworthy AI [43]. Complementing these principles, UNESCO’s Recommendations on the Ethics of Artificial Intelligence emphasize the need for human dignity and safety in the design and deployment of AI technologies [44].
Binding legal frameworks serve to reinforce these ethical foundations. Strict guidelines for handling sensitive health and personal data are established by the GDPR, which requires strong data security, openness, and explicit consent [45]. The National Authority for the Supervision of Personal Data Processing (ANSPDCP), the appropriate public body in charge of guaranteeing the preservation of people’s fundamental rights to privacy and personal data, made the GDPR mandatory in Romania in 2018 [46].
The legal framework for medical devices, including software-based instruments used for therapeutic or diagnostic purposes, is updated by the MDR, Regulation (EU) 2017/745. It highlights safety, clinical evaluation, and traceability while introducing the Unique Device Identification (UDI) system [47]. Additionally, the European AI Act establishes a risk-based categorization of AI systems into four levels: unacceptable risk (prohibited), high-risk (e.g., healthcare applications), limited or minimal-risk (e.g., video games, spam filters) [48].
There are significant issues with algorithmic transparency, data security, and clinical accountability when using AI systems for neurodegenerative disease monitoring. To address these, adherence to legal and ethical frameworks throughout the entire system lifecycle is necessary. Romania’s National AI Strategy was developed by the Interinstitutional Commission and the Romanian Committee for Artificial Intelligence, which were established by Order No. 20484 (April 2023) [49,50]. These efforts support the governance framework that guides the development of platforms such as NeuroPredict.
Adherence to ethical principles, medical regulations, and data protection laws is necessary for the responsible use of AI in the treatment of neurodegenerative diseases. Informed consent, data minimization, and patient privacy are all guaranteed by GDPR, which is particularly fundamental in situations involving long-term monitoring [51]. Concerns like algorithmic bias, lack of explainability, and opaque decision-making highlight the necessity for strong clinical validation and supervision as AI systems are used in neurology and mental health [52].
To guarantee clinical validity, Fereshtehnejad and Lökk [53] emphasize the necessity of precise regulatory standards. In PD and AD, tools like gait monitoring and predictive algorithms must adhere to MDR and respect safety and equity principles. The need for close supervision is further demonstrated by emerging diagnostics such as brain-aging biomarker analysis [54].
The regulatory challenges at the nexus of the AI Act and MDR are demonstrated by the NeuroPredict platform. Given its integration of wearable biosensors and AI-driven analytics for health condition monitoring, the NeuroPredict platform would likely fall under Class IIa or IIb of the MDR, as it performs functions with diagnostic and monitoring intent. This classification implies the need for formal clinical validation and conformity assessment by a notified body. Simultaneously, under the European AI Act, it would be categorized as a high-risk AI system due to its healthcare application, thus subject to requirements for transparency, human oversight, lifecycle risk management, and the implementation of an AI-specific quality management system.
There are still questions about how third-party AI Act evaluations relate to MDR processes, especially when it comes to opaque models like deep neural networks.
Furthermore, multidisciplinary teams face integration challenges because the AI Act mandates dynamic logging and version control, whereas MDR prioritizes static documentation. To meet these demands, a hybrid regulatory approach that anticipates future harmonization at the EU level and combines clinical rigor with algorithmic governance is required.
2.5. Shortcomings of Existing Approaches and NeuroPredict Platform’s Anticipated Added Value
Several frequent constraints of a great number of related digital health solutions are shown by a thorough examination of current digital health systems aimed at managing neurodegenerative diseases. First, many solutions have limited functionality dimensions, concentrating on distinct cognitive tests, physiological data collection, or gait monitoring with no incorporation of all these elements into a single, time-sensitive architecture. Either interpretation of circumstances or predictability is hampered by this disjointed approach. Also, the overwhelming majority of analyzed solutions make use of static classification algorithms or threshold-based detection logic, which are not properly adaptable to the diversity of individual patients. The systems’ capacity to detect early changes in functional status is diminished by the absence of customized initial conditions and the insufficient use of time-series modeling. Translation into clinical practice and regulatory compliance is hindered by the frequently inadequate handling of algorithmic transparency and lifecycle monitoring.
With a multilayered architecture that integrates motion data, physiological signals, and cognitive performance measurements, the NeuroPredict platform has been designed to make up for these shortcomings. Featuring an emphasis on temporal feature extraction and multimodal correlation, machine learning techniques that can identify anomalies in real-time and model progression are used. The NeuroPredict platform encompasses ethical and regulatory compliance—GDPR, MDR, and AI Act considerations—at the system design level, which sets it apart from other similar digital health solutions. Such features as risk-aware AI governance approaches, consent management infrastructure, and data minimization—all of which are not frequently employed in similar platforms. The NeuroPredict platform proposes a distinctive structure for ongoing, responsive, and ethically responsible monitoring of neurodegenerative diseases in nonclinical settings by overcoming both functional and regulatory shortcomings noted in the available research.
In contrast, only about 3% of the extensive research on inertial sensors for Parkinson’s disease demonstrates clinical utility, with most efforts still in the technical validation stage [55]. The NeuroPredict platform provides a clinically relevant monitoring architecture designed for real-world implementation. While platforms such as Kinesia [56] and HopkinsPD rely solely on smartphones to track activity (achieving ~71% accuracy in detecting medication response), they lack multimodal integration, versioned AI pipelines, and embedded regulatory compliance. The JTrack platform, with its GDPR alignment, marks progress in digital biomarker collection, yet remains limited to passive smartphone data and lacks customizable configurations [57]. Moreover, despite comprehensive EU regulations on AI and healthcare, few platforms integrate regulatory frameworks directly into system design—one of NeuroPredict’s core advantages [58].
While earlier platforms have addressed isolated aspects of neurodegenerative disease management, NeuroPredict uniquely combines multimodal sensing, a regulation-by-design architecture, and explainable machine learning within an integrated, modular framework, an approach rarely implemented in existing solutions.
3. Materials and Methods
The NeuroPredict platform is developed by a team comprising the authors of this paper as members in an ongoing research project. It integrates real-time data collection from wearables (e.g., Withings [59], Oura [60], and Sensoria [61]) and ambient sensors with cognitive interaction modules and cloud-based machine learning pipelines, versus previous studies that concentrated on clinical events or distinct inputs. Timely pattern identification and anomaly detection are made possible by its modular design, which allows continuous data streams comprising physiological, behavioral, and environmental dimensions. The approaches and technologies addressed in this section are based on the platform’s present implementation in a lab setting, where hardware and AI components are iteratively tested and calibrated using experimental datasets and simulated user interactions.
The presented study describes experimental evaluations of the technological components of the NeuroPredict platform in a laboratory environment. These evaluations include modular testing of data acquisition devices, signal preprocessing pipelines, and data visualization dashboards, in the absence of clinical end-user participation at this stage.
3.1. Design and Architecture of NeuroPredict Platform
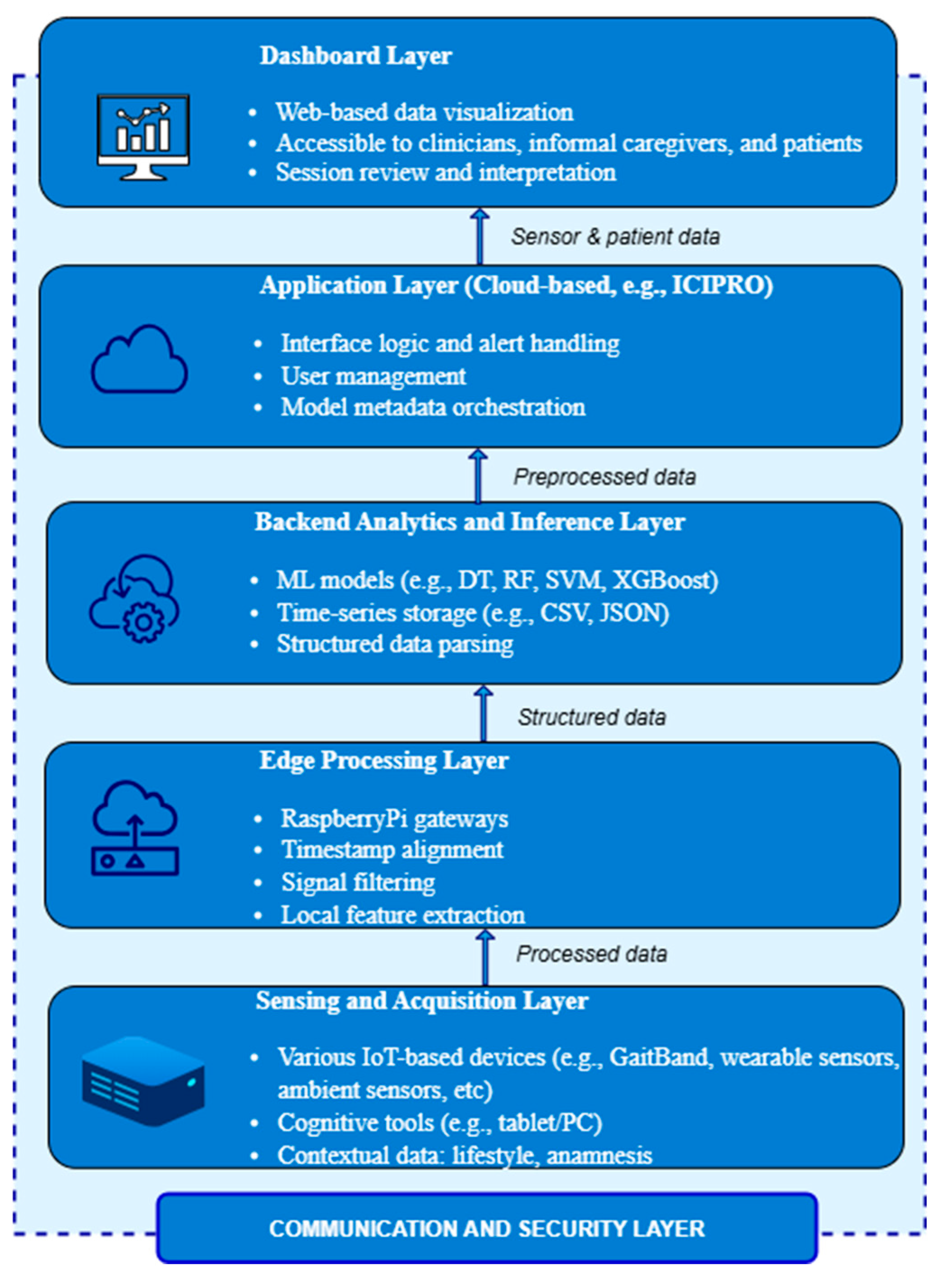
In order to facilitate ongoing, multimodal monitoring and predictive analytics for the detection and management of neurodegenerative diseases, the NeuroPredict platform is based on a modular, vertically tiered architecture (Figure 1). Secure data transmission protocols, centralized machine learning inference, edge-level signal preprocessing, regulatory-compliant governance mechanisms, and wearable and ambient sensor devices are all integrated into its architecture. Simulated end users are being used to provide an array of data situations for iterative testing and model training because the platform achieves an advanced laboratory-level phase of development.
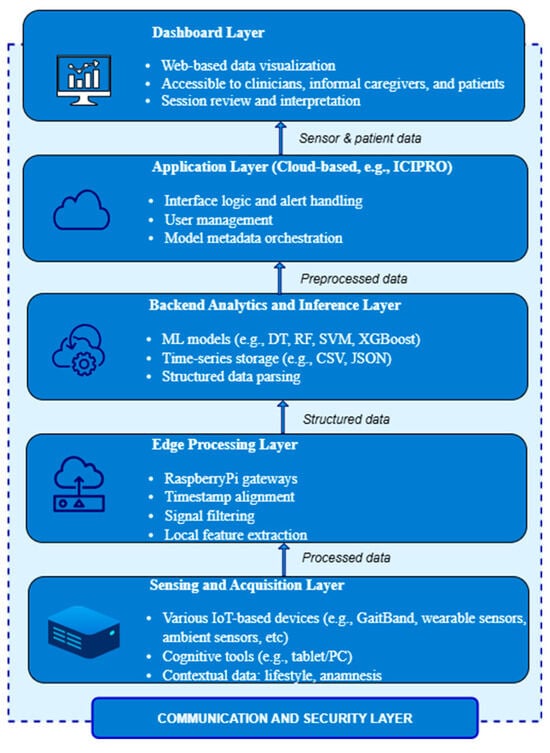
Figure 1.
Modular architecture of the NeuroPredict platform and secure data flow across core functional layers.
The layered architecture was chosen to enable independent scalability, traceable data flow, and flexible updates across hardware and software components. It ensures clear separation of concerns—from sensor data acquisition to cloud-based inference and user interaction—while supporting modular validation and aligning with MDR and AI Act requirements for traceability, lifecycle documentation, and certification readiness.
The Sensing and Acquisition Layer, particularly, is constructed of an interconnected set that includes multiple devices, and is the platform’s core. Such devices are commercial smartwatches for tracking vital signs like skin temperature, heart rate variability, and sleep patterns; wearable sensors like the GaitBand device with triaxial accelerometers for gathering parameters like movements and acceleration magnitude; and environmental IoT-based devices for issues like ambient gas detection. The platform provides software-based cognitive screening tools that may be used on desktop or tablet interfaces. These applications evaluate cognitive abilities, including attention, decision-making, and response speed, under controlled conditions by simulating common activities.
With sample rates set in accordance with device specifications and the needs associated with every monitored parameter, all IoT-based devices are operated on calibrated timers. Time-based assessment and multimodal fusion are made achievable by temporally aligning data streams across categories.
Raspberry-Pi-class microcontrollers, which operate as local gateways for collecting and preprocessing data that is gathered, are used to implement the Edge Processing Layer. The choice of a Raspberry-Pi-class microcontroller for local edge processing was driven by several technical, scientific, and practical considerations. Raspberry Pi platforms combine adequate computational resources (quad-core ARM Cortex-A processors, clock speeds over 1.2 GHz, and RAM up to 8 GB in current versions) with a highly cost-effective footprint, making them ideal for prototyping medical-grade IoT architectures. Their support for standard Linux-based operating systems allows seamless integration of established data acquisition frameworks, advanced Python or C++ libraries for biomedical signal processing, and edge machine learning inference engines (e.g., TensorFlow Lite, ONNX). These features enable the deployment of real-time data preprocessing algorithms—such as timestamp synchronization, noise filtering, and feature extraction—directly on the device, thereby reducing latency, lowering network bandwidth demands, and enhancing system resilience in case of connectivity interruptions.
Raspberry-Pi-class devices provide a wide range of built-in interfaces, including Wi-Fi, Bluetooth, USB, SPI, I2C, and GPIO, supporting flexible integration of heterogeneous sensor modules such as accelerometers, heart rate monitors, or temperature sensors. Their GPIO capabilities and driver ecosystem are especially relevant for medical device prototyping, enabling easy adaptation to a variety of sensor configurations without major hardware redesigns. In addition, these microcontrollers facilitate version-controlled, transparent, and replicable software updates, which are critical for future MDR and AI Act compliance. From a privacy-by-design perspective, local edge computing using Raspberry-Pi-class platforms helps to preprocess and pseudonymize data before transfer to the cloud, addressing key GDPR requirements. Collectively, these technical, operational, and regulatory-aligned characteristics have proven relevant in the development of other solutions by the NeuroPredict platform team, confirming their suitability for the current experimental validation phase.
Initial preprocessing is performed on edge-level devices, which additionally segment activity windows, align timestamps, and normalize across channels. Filtering and segmentation are part of this preprocessing. Depending on the configuration, certain motion characteristics, including acceleration magnitude, are calculated locally or during backend processing. Data are sent from edge nodes to the backend via USB or secured Wi-Fi, depending on the setup. Device type, session ID, sensor approach, firmware version, and other metadata headers are included in every transfer that is sent across encrypted HTTPS channels. Each transmission’s metadata, including the sensor ID and session timestamp, is recorded to assist with data origin traceability and synchronized consistency [5].
The structured data streams are received, indexed, and processed by the Analytics and Inference Layer on the backend. The platform relies on a time-series storage approach based on comma-separated values (CSVs), with metadata and session-level folder patterns kept in corresponding JavaScript Object Notation (JSON) files. This layout makes it easier to integrate with dashboard displays and analytical services and allows subsequent components to parse data consistently. Fast querying and longitudinal comparisons between time points and users are rendered possible by the session-level index in which each dataset is registered. The backend analytics layer incorporates machine learning algorithms to facilitate trend analysis and real-time classification. These methods, which are written in Python and trained on labeled datasets created through simulated user situations, include decision trees (DTs), random forests (RFs), support vector machines (SVMs), and extreme gradient boosting (XGBoost) classifiers. Preprocessed characteristics taken from cognitive interaction modules and multimodal sensor streams are processed by these models. Version control is incorporated into the ML pipeline’s design to ensure consistency for training data, model parameters, and inference outputs.
The Application Layer is associated with the management of the process logic that connects the analytics infrastructure and the user-facing interface, handling alert process flow, and orchestrating user interactions. This layer, which is located in a cloud environment (e.g., ICIPRO), manages user authentication, session control, access rights, and the context-sensitive distribution of alerts and AI-driven insights. It acts as the platform’s functional core, converting machine learning results into structured messages and then making them ready for visualization and processing. This layer controls the spread of metadata, thus guaranteeing that every single output stream has model versions, inference confidence ratings, and data authenticity properly attached. Future deployment scenarios, including asynchronous notifications, user-specific dashboards, and interaction with clinical decision support systems or electronic health records, are supported by the Application Layer’s modular, scalable design.
The NeuroPredict platform’s visualization front end, the Dashboard Layer, is designed for a variety of user types, including patients, physicians, and informal caregivers. It is implemented as a secure web-based interface that transforms Application Layer processing outcomes into comprehensible visual overviews, including trend indicators, classification labels, time-aligned graphs, and feedback on cognitive performance. A review of the sensor inputs, preprocessing pipeline, and AI inference environment is made possible by the appropriate metadata associated with each session. The dashboard facilitates interpretability and accessibility, which are critical for making decisions in healthcare settings subject to the AI Act and MDR. The design provides an adaptable framework for multimodal interaction and feedback, and although it is presently implemented in a controlled laboratory setting, it is anticipated to be scalable for use in clinical and home-based settings.
The Communication and Security Layer performs throughout every single component of the NeuroPredict architecture, allowing for dependable, secure data transfer and preserving system integrity, even though it is not a stand-alone functional layer. All data flows are protected using HTTPS encryption, and communication across layers—for example, from acquisition devices to edge processors and from edge to backend services—is handled via secured channels (such as Wi-Fi and USB). To facilitate traceability and source validation, metadata related to every transmission—such as session ID, device type, and firmware version—is recorded.
This layer is intended to grow with deployment situations that need sporadic or distant connectivity and protects data exchange secrecy and integrity. Despite being visible to users, it is essential for guaranteeing reliable and compatible communication amongst the system’s dispersed components.
The architectural layering of the NeuroPredict platform is purposefully designed to address the unique challenges of neurodegenerative disease management. The Sensing and Acquisition Layer enables continuous and multidimensional data collection, capturing subtle motor and cognitive changes critical for early detection. The Edge Processing Layer supports near-real-time anomaly detection and trend recognition, reducing latency for timely interventions. The Communication and Transport Layer ensures secure and traceable data transmission, preserving the integrity of longitudinal patterns. The Cloud Integration and Data Lake support the aggregation and retrospective analysis of long-term trajectories, enabling clinicians to access high-resolution patient histories for personalized care planning. Finally, the Dashboard Layer delivers interpretable insights and alerts to healthcare professionals and caregivers, facilitating proactive adjustments to therapy or support strategies. This integrated architecture directly supports the continuous, adaptive, and explainable monitoring required for managing complex neurodegenerative disease trajectories.
All the records from the database of the NeuroPredict platform provide retrospective analysis and repeatability, which is consistent with the latest documentation standards under MDR. In accordance with medical device standards, this architecture ensures lifecycle documentation and traceable validation of experimental outcomes.
The regulatory and governance approach, which enforces data protection and ethical design standards, runs across the whole system. GDPR-compliant features, including data minimization, explicit consent, and pseudonymizing personal identifiers, are all included in the platform. To map data flows, evaluate related risks, and direct mitigation actions, a conceptual Data Protection Impact Assessment (DPIA) has been carried out. Role-based restrictions that are in line with internal testing protocols limit access to datasets and system operations.
To prepare for future compliance requirements under the EU AI Act and the MDR, the platform features inference session recording and model version tracking. Future clinical dashboard prototypes are expected to provide functional assistance for comprehensibility, such as including significance depiction. The architecture of the platform can be easily adapted and made for scalable deployment in semi-clinical and remote monitoring settings, despite the fact that it has only been proven in lab-based simulations so far.
While initial requirements were gathered through internal expert workshops and reviews of established clinical and regulatory frameworks, direct involvement of patient and caregiver groups was not feasible at this stage due to funding restrictions. This limitation is addressed in the project roadmap, with structured co-design and participatory evaluation activities planned for the next validation phases.
Although the project did not include clinical or patient-facing partners, the NeuroPredict platform was iteratively evaluated during development through internal testing by team members and technical staff within the institute. Structured questionnaires and scenario-based simulations were used to assess usability, data flow, and interface responsiveness. While not a full co-design process, this internal validation provided early feedback and guided functional refinement. The platform’s modular design supports future co-design sessions with clinicians and patients, planned for the upcoming semi-clinical phase to ensure alignment with real-world user needs.
Other Planned Functional Modules: In addition to the fall detection module described in detail in this paper, the NeuroPredict platform is designed to accommodate a range of complementary services that address multiple dimensions of neurodegenerative disease management. Planned modules include cognitive function screening (based on tablet-based memory and attention tasks), cardiovascular pattern analysis (leveraging heart rate variability, oxygen saturation, and blood pressure measurements), sleep quality monitoring (using nocturnal activity sensors to detect sleep disruptions and apnea risks), and environmental stressor detection (capturing ambient temperature, humidity, and air quality factors). These additional modules are built upon the same multimodal sensing, edge-cloud data pipelines, and explainable AI frameworks described for fall detection, supporting a unified, scalable, and regulation-ready architecture. Their detailed validation and implementation strategies will be reported in future studies as their development progresses.
3.2. Multimodal Sensing Strategy in NeuroPredict Platform
The NeuroPredict platform’s Sensing and Acquisition layer integrates a carefully selected set of physiological and environmental parameters that are significant to the detection and monitoring of MS, PD, or AD, to facilitate high-accuracy, multimodal data gathering. In addition to signal type variation, the approach behind device selection places a strong emphasis on technical trustworthiness, user versatility, and long-term deployment reliability.
Rather than prioritizing generic sensor connectivity, the platform emphasizes clinically relevant measurements and device-level complementarity over conservative connectivity of sensors. Every sensing device assists in accomplishing one or more reasoning targets, which might include contextual environmental assessment, and movement pattern detection. Several symptom categories are covered by the combination of wearable, near-body, and ambient technologies without overburdening the platform with obsolete or low-value inputs. Although some of the devices—like the GaitBand and the Blackbox units—have been designed and built by the NeuroPredict development team to satisfy performance, synchronization, and preprocessing requirements, others are commercially available and Bluetooth-enabled for smooth edge integration.
An outline of the main devices currently included in the platform is laid out in Table 1, together with information on their physiological targets, relevance for disease monitoring, and integration roles within the platform:

Table 1.
IoT-based devices and their functional contributions in the NeuroPredict platform.
Depending on the specific type of signal, ongoing or periodic gathering is possible by enabling the integration alongside time synchronization of all devices via a single data acquisition protocol. Aligning time-stamped segments across sensors enables multimodal fusion, which acts as a framework for reliable downstream analytics and model inference. For instance, the three target conditions—gait variability and movement abnormalities in MS and PD, sleep irregularities and autonomic fluctuations in AD, cardiovascular dysregulation as a common comorbidity, and cognitive decline as a cross-condition indicator—have been linked to distinct manifestations for each sensing method. The assessment of patient status under customized baselines is further facilitated by the incorporation of environmental sensing and body composition assessment. As further detailed, this setup multimodal dataset supports both pattern recognition and temporal inference tasks, acting as the computational foundation for downstream AI pipelines.
In addition to offering high granularity for AI-based modeling, this cautious layout of sensor infrastructure facilitates the platform’s modular extension in later phases of development. The following part delves into detail about the GaitBand device, which is one of the most intricate and technically significant of these components.
3.3. GaitBand: Motion Monitoring Device for Fall Detection
Fall detection in NDDs is essential as individuals with conditions like PD or AD are at an increased risk of falling due to compromised motor skills, balance issues, and cognitive deterioration. Detecting falls in real-time allows for rapid intervention, ensuring timely medical assistance, which is important for preventing further complications like fractures or head injuries. Furthermore, fall detection provides valuable data to monitor disease progression, optimize care plans, and improve patient safety.
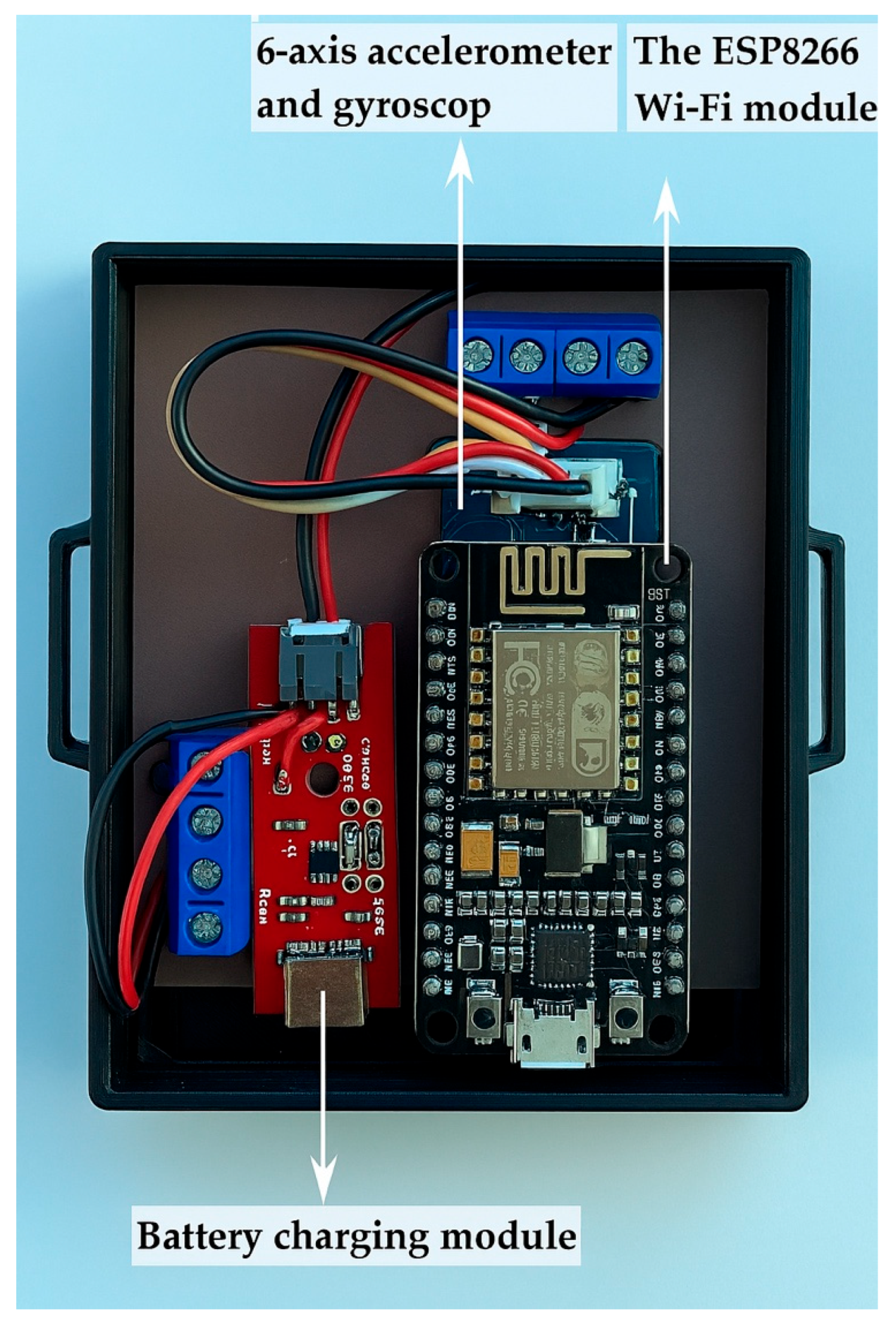
GaitBand is a wearable device used to collect movement data, particularly for applications like fall detection. The main components are the following:
- Battery Charging Module: It manages the power supply of the device by charging the accumulator and ensuring it remains operational for extended periods without needing frequent recharging.
- The ESP8266 Wi-Fi Module: It is responsible for wireless communication. It connects the GaitBand to a Wi-Fi network, allowing the collected movement data to be transmitted to ICIPRO Cloud for further analysis.
- Six-Axis Accelerometer and Gyroscope: It measures movement along six axes (three for acceleration and three for rotational movement). Raw accelerometry data can be extracted to provide information regarding activity intensity, posture, postural transitions, and walking patterns.
Figure 2 illustrates the components of GaitBand. The interconnection of these components is presented in Figure 3.
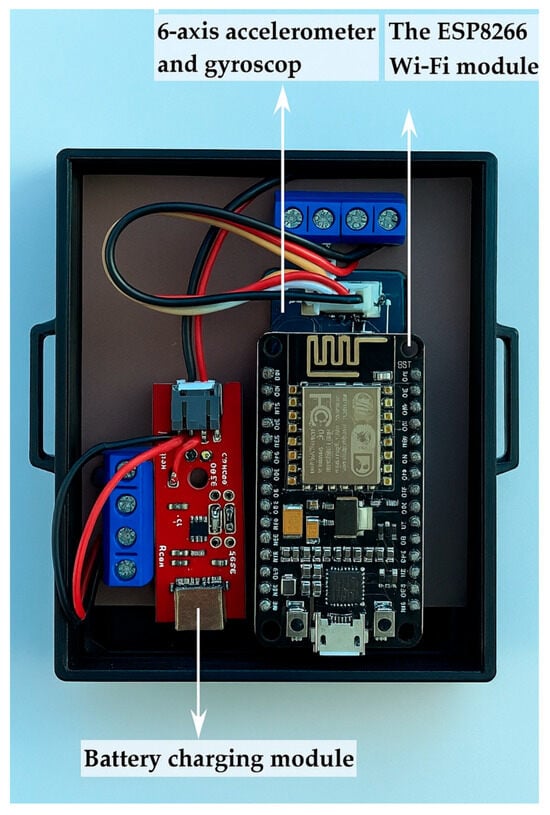
Figure 2.
Components of GaitBand.
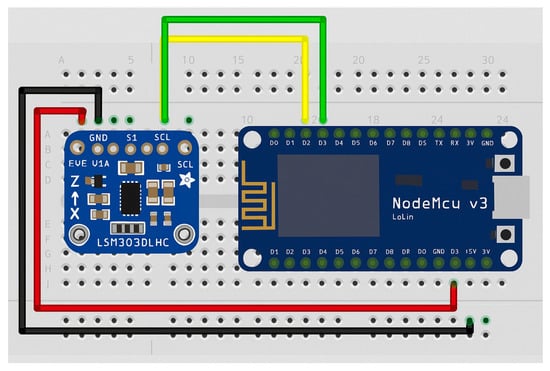
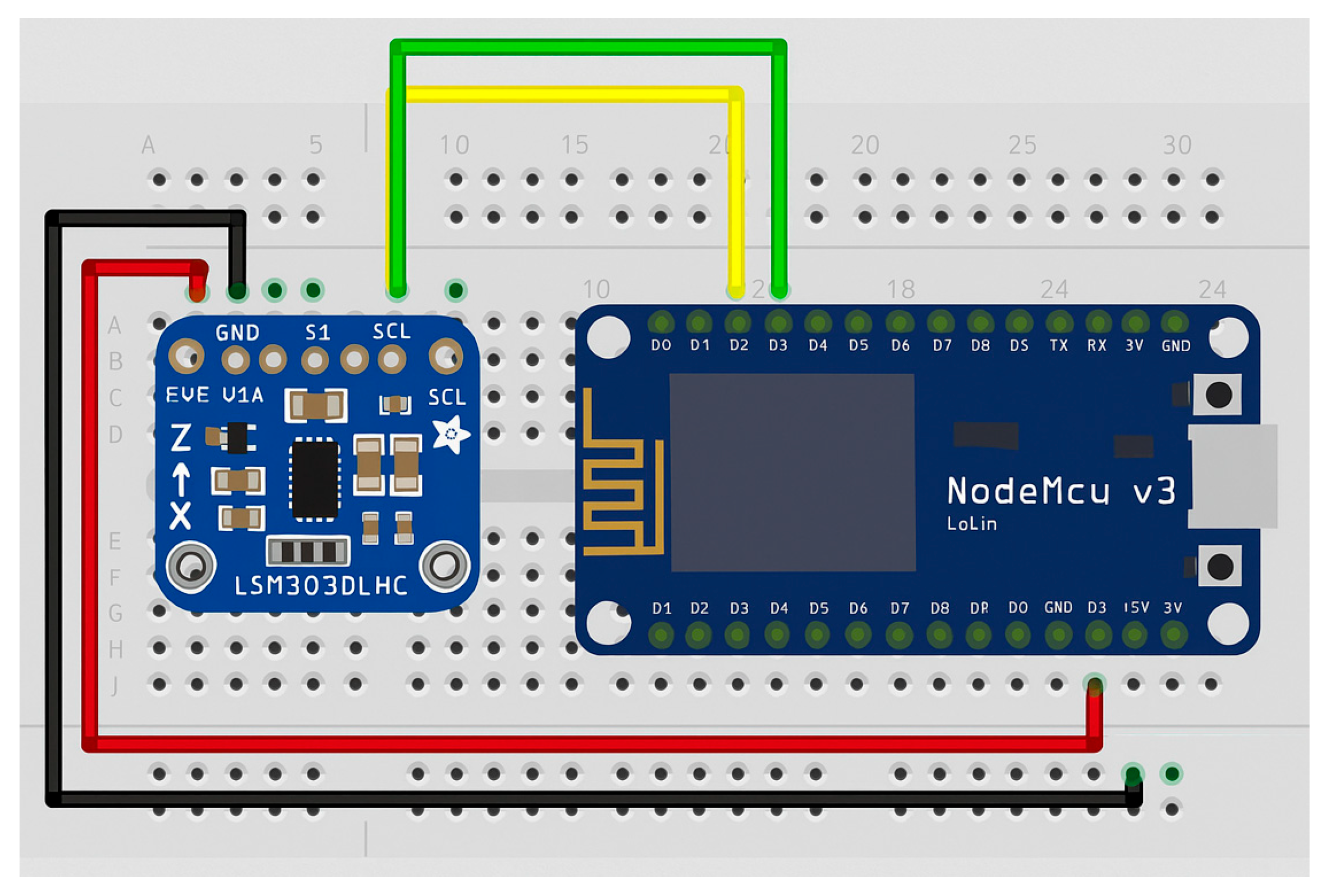
Figure 3.
Interconnection of the components [6].
A controller–peripheral architecture (with the NodeMCU as the controller and the accelerometer sensor as the peripheral) was used to collect data from the sensor. The NodeMCU module handles the connection and transmission of data from the sensor to a database hosted on a Raspberry Pi 4.
On the microcontroller side, pin D1 of the NodeMCU is connected to the serial data line (SDA) of the accelerometer, which is used for data transmission in inter-integrated circuit (I2C) communication. Similarly, pin D2 is connected to the serial clock line (SCL) of the LSM303D accelerometer, providing the clock signal required for data synchronization in I2C communication. The LSM303D sensor was selected for integration into the GaitBand device because of its high sensitivity in detecting small variations of acceleration and orientation, its low intrinsic noise level, and its suitability for continuous wearable fall detection. This sensor balances reliable data acquisition with low power consumption, which is essential for long-term monitoring scenarios. Its compatibility with standard I2C interfaces simplifies integration into the platform’s edge-processing layer, supporting efficient timestamp synchronization and modular system updates.
The 3.3 V output of the NodeMCU supplies power to the accelerometer via its Voltage Input (Vin) pin. Additionally, the ground (GND) pin of the NodeMCU is connected to the GND pin of the accelerometer to ensure a common voltage reference between the two devices.
The connection between the wireless module (acting as the controller) and the accelerometer sensor (acting as the peripheral) is summarized in Table 2.

Table 2.
Node MCU connection module with the LSM303D accelerometer.
The wireless communication module connects upon startup to the Raspberry Pi gateway device and sends HTTP_POST messages, which include the data collected from the sensor to the database server. Once connected, the device starts transmitting the relevant data to the Raspberry Pi, which then forwards it to the ICIPRO Cloud database.
The following advantages of GaitBand can be mentioned:
- Relatively low design costs;
- Continuous data acquisition allows for highly accurate patient monitoring;
- The use of an accumulator enables continuous and proper functioning of the device, with the possibility of fully charging it during the patient’s rest period.
- Although fall detection is a relatively straightforward application and is available in many smartwatches and smartphones, these devices are multifunctional, making them complex, bulky, and expensive. In contrast, GaitBand is specifically designed solely for fall detection, allowing hardware and software to be optimized for this particular purpose. Therefore, our proposal addresses specific needs and offers an efficient, simplified, and cost-effective alternative to existing solutions on the market.
Leveraging Accelerometer Data for Fall Detection Algorithms
Communication between the LSM303D accelerometer sensor and the Node MCU Wi-Fi module takes place periodically, with the collected information being processed and stored in array variables of integer or floating-point types. The values collected from the sensor are then sent to the gateway device via the HTTP_POST method. The data is stored in a MySQL database using PHP.
The data is obtained at one-second intervals and includes accelerations for the X, Y, and Z axes. The activities must involve both daily actions (walking, jumping, running, climbing/descending stairs, lying down, sitting on a chair/bed/armchair) as well as falls that are tested on a mattress.
To collect data, the following activities were performed: the subject lies down on the mattress, gets up and walks, walks at a faster pace, sits down on and gets up from a chair, goes up and down stairs, jumps, falls forward, falls to the right, falls to the left, falls on their knees, and falls on their back. The GaitBand is worn around the waist.
A total of 11,916 accelerations were recorded, with the data labeled as either fall or non-fall activities, and the resultant for each set of three accelerations was calculated separately.
3.4. Machine Learning Algorithms for Fall Detection
This section presents the implementation and evaluation of machine learning algorithms for fall detection using real-time acceleration data collected via the GaitBand device. It describes the rationale for model selection, the use of labeled data for training and testing, and the integration of the final classifiers into the NeuroPredict platform. The study aimed to identify the most effective algorithm for accurate fall detection under simulated user scenarios by comparing multiple classification techniques.
3.4.1. Selected Machine Learning Models
In this study, four machine learning algorithms were implemented for fall detection: DT, RF, SVM, and XGBoost. These algorithms were selected based on their proven effectiveness in classification tasks involving structured data and their widespread application in activity recognition and health monitoring domains [16]. Multiple algorithms were implemented to conduct a comparative study, allowing for a more comprehensive evaluation of different modeling strategies and helping identify which algorithm performs best in the specific context of fall detection using the available data.
A DT algorithm [62] is both a nonparametric and supervised learning method for classification and regression. It continuously divides the data based on feature value selection with the intention of forming subsets that are as homogenous as possible at all nodes. DTs are highly preferred due to their simplicity and interpretability, as well as being capable of working with numerical and categorical data. However, DT models are highly susceptible to overfitting, particularly when they are not restricted in depth.
RF [63] is an ensemble learning method that builds multiple Decision Trees and aggregates their predictions to improve accuracy and robustness. Each tree in the forest is trained on a bootstrapped sample of the dataset, and at each node, a random subset of features is considered for splitting. The final prediction is determined by majority voting in classification tasks. RF reduces the risk of overfitting by leveraging multiple trees, making it highly effective for complex classification problems.
SVM [64] is a widely used supervised learning algorithm designed for both classification and regression tasks. The primary goal of SVM is to find an optimal decision boundary, known as a hyperplane, that best separates different classes in a dataset. This boundary is chosen to maximize the margin, which is the distance between the hyperplane and the nearest data points from each class, known as support vectors. A larger margin generally leads to better generalization and improved classification performance on unseen data.
When data is linearly separable, SVM constructs a straight hyperplane that effectively divides the feature space into distinct regions. However, in cases where the data is not separable in its original space, SVM employs kernel functions to transform the data into a higher-dimensional space where a linear separation becomes possible. Commonly used kernels include:
- Linear Kernel: Applied when the data is linearly separable, as it maintains the original feature space without transformation;
- Polynomial Kernel: Projects input features into a higher-degree polynomial space, enabling the model to capture complex, nonlinear relationships;
- Radial Basis Function (RBF) Kernel: Commonly employed for its ability to map data into an infinite-dimensional space, making it highly effective for modeling intricate and nonlinear patterns;
- Sigmoid Kernel: Derived from neural network activation functions, it is used in scenarios where data exhibits specific probabilistic distributions.
To enhance flexibility, SVM also incorporates a regularization parameter that controls the trade-off between achieving a perfect separation of training data and maintaining generalization to unseen examples. If the parameter is too high, the model may overfit; if too low, it may underfit.
SVM is particularly effective in high-dimensional spaces and is known for its robustness against outliers and noise. However, it can be computationally expensive, especially when applied to large datasets.
XGBoost [65] is an advanced gradient boosting algorithm designed for speed and efficiency. It builds an ensemble of weak decision trees sequentially, where each new tree corrects the errors of the previous ones by minimizing a regularized objective function. XGBoost is particularly effective due to its handling of missing data, built-in regularization to prevent overfitting, and efficient parallel computation, making it one of the most competitive algorithms for structured data problems.
3.4.2. Steps Involved in Developing AI Algorithms for Fall Detection
Data Acquisition with GaitBand: The process begins with collecting movement data using the GaitBand, which captures real-time acceleration data essential for detecting patterns indicative of falls. The raw data consists of multiaxis acceleration signals that capture various types of movements and potential fall events. For the purposes of model training and evaluation, both typical daily activities and simulated fall scenarios were performed and recorded under controlled conditions (e.g., jumping, walking, sitting, standing, and intentional falls in four directions—forward, left side, right side, and backward), resulting in a dataset that is both realistic and suitable for analysis. The data was collected from five adult subjects, all members of the development team, who voluntarily participated in the data recording process. Given that no sensitive personal data was recorded and the participants were fully informed and consenting team members, no formal ethical approval was required for this preliminary study.
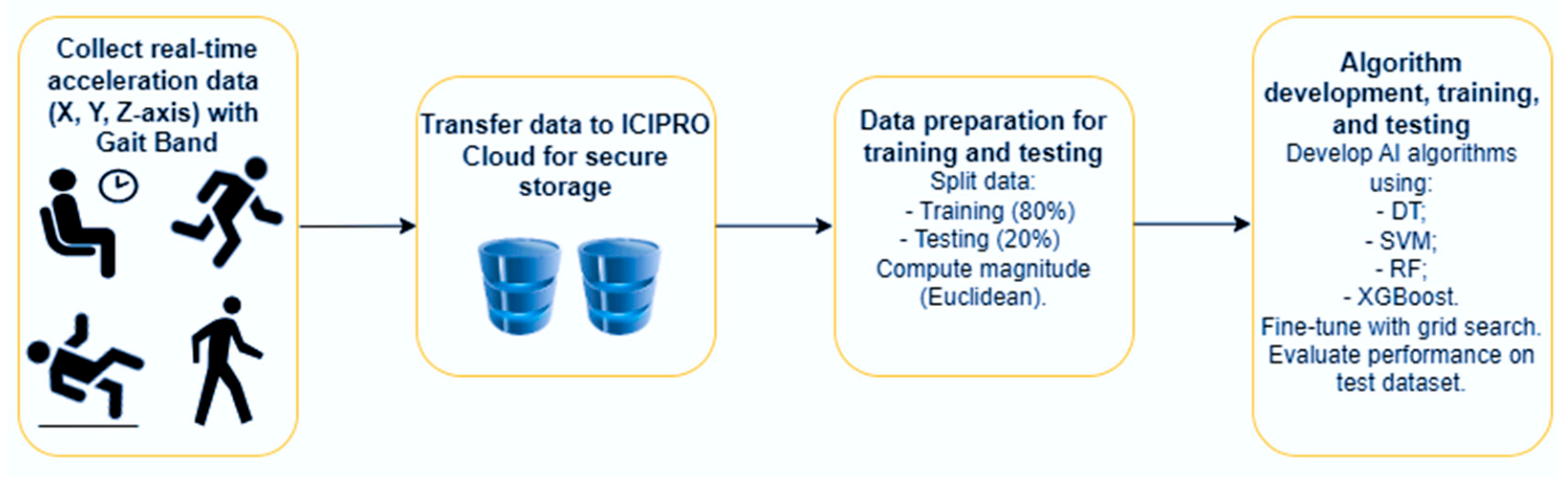
Data Storage in ICIPRO Cloud: Once the data is collected, it is transferred to ICIPRO Cloud for secure storage and management.
Data Preparation for Training and Testing: The next step involves preparing the data for the machine learning algorithms by splitting it into training and testing sets. The raw sensor data, which includes time-stamped X, Y, and Z-axis acceleration values, is transformed. The magnitude of the acceleration is computed using the Euclidean norm, simplifying the data into a single value that reflects overall movement intensity. The dataset is then divided into training (80%) and testing (20%) sets, and a random seed is applied to ensure the split is consistent and reproducible in future runs. The method for calculating total acceleration is presented in Equation (1), where Ax, Ay, and Az represent the acceleration along each axis, and M is the magnitude.
Algorithm Development and Training: Various AI algorithms are then developed and trained using the training dataset. These models are fine-tuned with grid search to optimize hyperparameters for better performance in recognizing fall patterns. To improve the robustness of fall detection and reduce the risk of overfitting, classification decisions were aggregated over sliding windows of 30 s with a step size of 20 s. This window size was selected because fall events generally happen within a 30 s period, allowing the model to adequately represent the temporal progression of a fall.
Model Evaluation using Accuracy: Finally, the trained models are evaluated on the test dataset. Accuracy is the primary metric used to assess how well the model performs in detecting falls, providing insight into the model’s ability to generalize to new, unseen data.
Figure 4 shows the diagram for gathering acceleration data in real time with a GaitBand and analyzing it with machine learning algorithms.
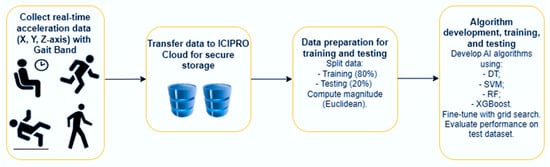
Figure 4.
The workflow for collecting real-time acceleration data using GaitBand and processing it for machine learning algorithms.
To further validate the models during the training phase and to reduce the risk of overfitting, 5-fold cross-validation was employed, applied exclusively to the training set. In this approach, the training data is partitioned into 5 equally sized folds; the model is trained iteratively on 4 folds and validated on the remaining fold. The process is repeated 5 times, with each fold serving once as the validation set.
3.5. Ethical Principles, GDPR Compliance, and Data Security
The NeuroPredict platform was developed in accordance with internationally recognized legal and ethical frameworks, thereby ensuring its compliance and suitability for deployment in sensitive healthcare contexts. Adherence to fundamental principles of data ethics provides a solid and responsible foundation for the development and application of artificial intelligence technologies [44,45,66,67].
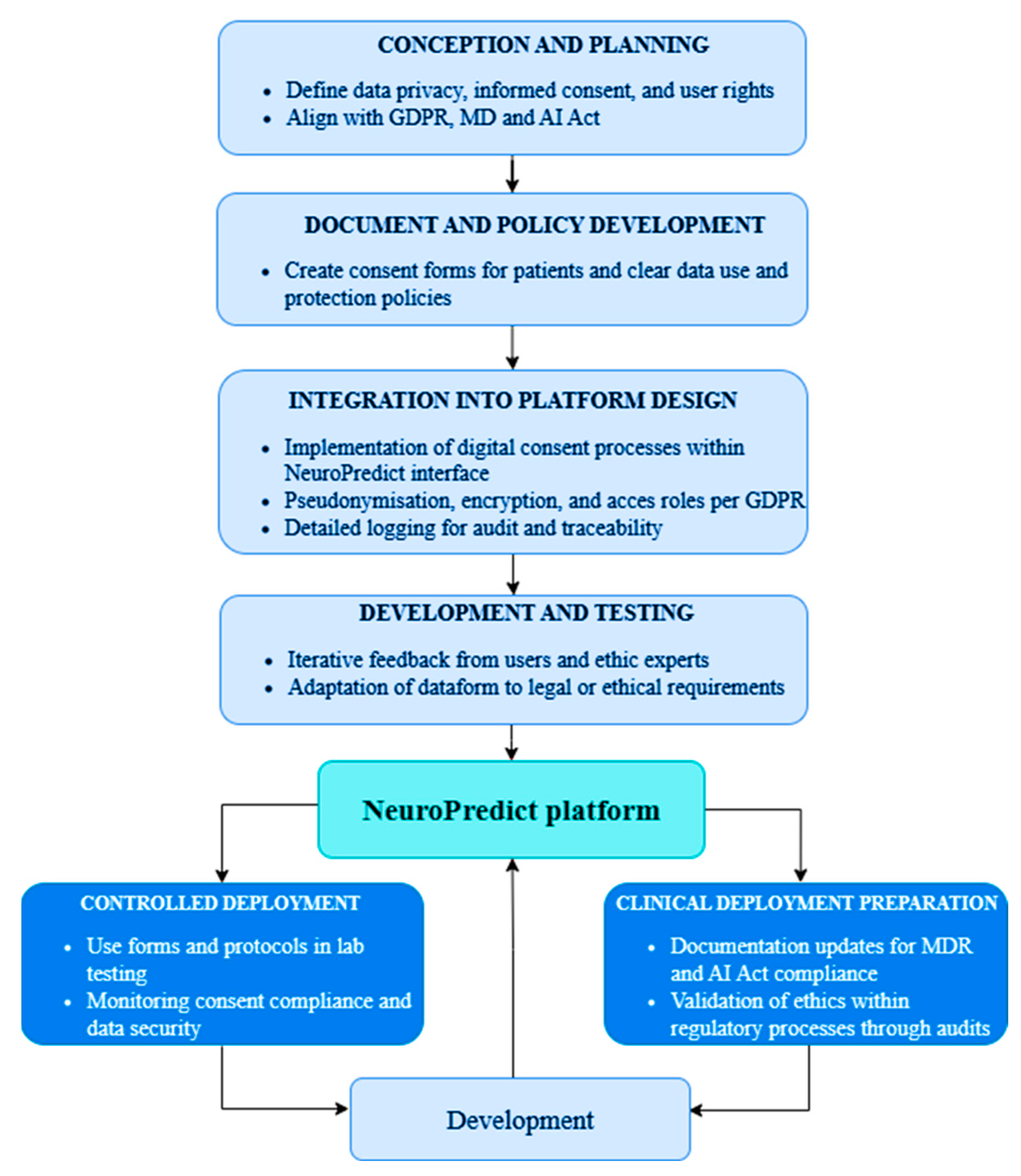
From the very beginning of design to the anticipated clinical deployment, the NeuroPredict platform’s ethical-regulatory development is directed by a systematic, multiphase pipeline that incorporates ethical protections and legal compliance. To ensure that regulatory requirements are integrated into the technical lifecycle rather than applied retroactively, each phase addresses distinct aspects of GDPR, MDR, and AI Act alignment. This step-by-step integration process is depicted in Figure 5.
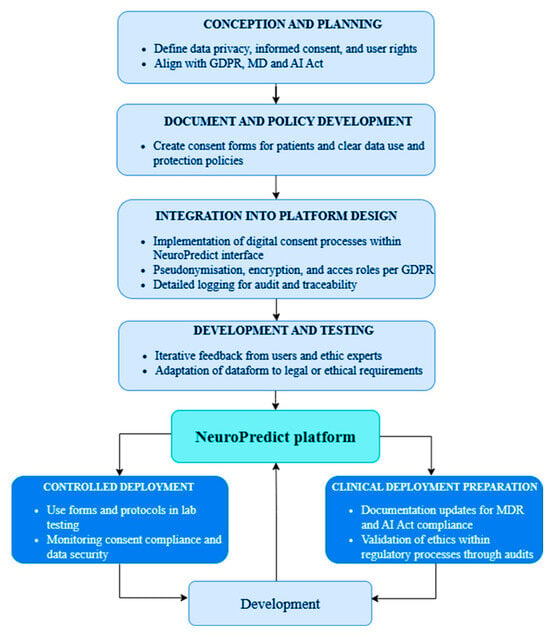
Figure 5.
Ethical-regulatory development pipeline of the NeuroPredict platform, showing the seven phases from conceptualization to clinical deployment.
This process includes:
- Conception and Planning: The design phase incorporated fundamental ethical principles, such as informed consent, user autonomy, and privacy. The platform’s original architecture and data flows were influenced by anticipated GDPR and MDR compliance.
- Document and Policy Development: Data use policies that clearly connect user rights to system functionalities were developed, along with consent forms specifically designed for patients and clinicians.
- Integration into Platform Design: The interface now includes digital informed consent mechanisms. To facilitate auditability and safe data handling, session-level logging, role-based access, encryption, and pseudonymization were enabled.
- Development and Testing: User session simulations confirmed that ethical standards (such as assigning consent to data records, limiting access, and encrypting transmission) were appropriately implemented. Iterative updates were informed by ethics reviewers’ and developers’ feedback.
- Controlled Deployment: Internal audits verified that privacy measures like metadata tracking, secure upload protocols, and AI inference logging were implemented correctly. Internal training procedures and regulatory documentation were started. To guarantee the traceability and explainability of algorithm outputs, specific checkpoints for MDR and AI Act readiness were established.
- Clinical Deployment Preparation: Participants’ actual informed consent will be gathered, and ethical precautions will be continuously observed. Stakeholder feedback will be used to continuously improve platform updates.
Ethical and legal compliance is guaranteed to be an ingrained design principle rather than a post-hoc fix thanks to this lifecycle-based governance. Through trace logs and a conceptual DPIA conducted in compliance with GDPR Article 35, the platform operationalizes all the fundamental GDPR principles (Table 3), including explicit consent, the right of withdrawal, pseudonymization, data minimization, and auditability [45,66].

Table 3.
GDPR principles implemented in the NeuroPredict platform.
The NeuroPredict platform directly integrates privacy-by-design principles into its data architecture to guarantee adherence to GDPR and MDR standards. Using a session-level tokenization system, all personal identifiers are pseudonymized at the time of acquisition to avoid direct identification of the subject during processing or analysis. While data at rest is safeguarded using AES-256 encryption standards [68], end-to-end encryption using HTTPS (Transport Layer Security (TLS) 1.3) [69] secures data transmission between edge devices and the backend infrastructure.
A role-based authentication system is used to enforce access control, limiting identifiable information to authorized development personnel only within controlled test environments. To protect data privacy and confidentiality throughout the processing pipeline, these safeguards have been incorporated into the dashboard interface where inference results are displayed, as well as the data acquisition workflow.
4. Results
The NeuroPredict platform, currently undergoing experimental evaluation in a laboratory setting, has progressed through several development stages, focusing on high-fidelity multimodal data acquisition, modular integration, and AI functionality. Although it has not yet been deployed in clinical environments, the platform has reached a high level of technical maturity, allowing its architecture, signal processing workflows, and inference mechanisms to be evaluated under controlled conditions. Main implementation achievements and technical insights gained throughout the current development phases are included in the findings shown in this section.
All results are derived from internal testing in a lab environment with simulated user interactions. The purpose was to assess the functional readiness of the platform’s core modules—data acquisition, edge/cloud transmission, ML-based inference, and dashboard visualization.
4.1. Development Insights and Technical Achievements
To accomplish functional integration and operational validation of its modular components in a controlled test environment, the NeuroPredict platform was built. This consists of structured processing of multimodal input streams, real-time data transmission to the edge and cloud layers, and synchronized collection from various sensors. Controlled simulations with pre-established user scenarios to evaluate end-to-end workflows, including data visualization and AI-driven inference, were used. This has guaranteed that the current version fulfills the technical requirements and is prepared for implementation in semi-clinical settings.
Real-time acquisition and processing have been successfully shown in laboratory testing scenarios in a variety of representative configurations, such as single-sensor and multisensor sessions. To enable repeatable assessment of system responsiveness, structured activity scripts have been used to create simulated user data that replicates clinically relevant behaviors, such as irregular sleep patterns, gait instability, and periodic changes in cardiovascular patterns. To maintain consistency and validate processing flows retrospectively, all sessions are logged with synchronized timestamps and preprocessing versions.
The entire edge-to-cloud pipeline—which includes on-device filtering, secure transmission, structured storage, and AI-based classification—was used to route sensor data captured during these sessions. Internal monitoring tools confirmed that data integrity was preserved at each transition point. To ensure compliance with the platform’s anomaly detection and trend analysis modules, the completeness of the session was confirmed by automated logging of sensor availability, timestamp continuity, and feature vector density per window.
Model inference was carried out on preprocessed data streams using the backends’ development-stage classifiers. The results included the detection of predefined event signatures, the assessment of temporal patterns, and the export of inference labeling to the secure dashboard interface. Although these conclusions have not yet been validated on patient data, they provide technical evidence of inference readiness, integration stability, and compliance with regulatory norms for traceability and consistency.
Further testing has focused on confirming the platform’s efficiency and reliability in handling synchronized data streams from several sensor inputs. Using a range of wearable and ambient device combinations, simulation sessions have demonstrated the platform’s ability to maintain temporal synchronization across various forms. Sensor-specific features can be included subsequently, thanks to time-stamped segments. Preplanned user actions were used in these sessions to alter walking patterns, heart rates, and ambient variables. This made it possible to internally validate the model’s initial behavior and the stability of data collection.
Development-phase users assessed the outcomes of data using the trustworthy dashboard, demonstrating aligned sensor inputs, and matching AI inference labels, to assess system-level consistency. The outputs may be traced back to their original acquisition sessions thanks to metadata recording for device type, session ID, and preprocessing settings. These characteristics indicate the functional integrity of the data flow pipeline and its availability for additional testing using semi-clinical protocols.
4.1.1. Implementation of ML Algorithms for Fall Detection
Multiple machine learning classifiers are implemented and evaluated for fall detection using Python 3.11, each with its own configuration and hyperparameters. To further optimize the performance of these models, a grid search approach is applied, systematically exploring combinations of hyperparameters to identify the optimal settings for each classifier. This process ensures that the models are fine-tuned beyond their default configurations, allowing for a more rigorous and comprehensive evaluation of their performance.
- The first model utilized is a DT classifier. The splitting criterion is set to Gini (criterion = “gini”), used to evaluate the quality of splits at each node. The splitter is configured to select the best possible split (splitter = “best”). There is no predefined maximum depth (max_depth = None), allowing the tree to grow until all leaves are pure or the node contains fewer than the minimum samples required to split (min_samples_split = 2), and the minimum number of samples for a leaf node is 1 (min_samples_leaf = 1). After training the model on the dataset, predictions are made, and the performance is evaluated using the accuracy metric to assess the model’s effectiveness.
- The second classifier is an RF classifier, an ensemble technique that constructs multiple decision trees and aggregates their predictions. Each model is trained on a bootstrapped sample of the data (bootstrap = True), with a total of 100 trees being generated (n_estimators = 100). Gini is again used as the splitting criterion (criterion = “gini”), and no limit is imposed on the depth of each tree (max_depth = None). The model samples a subset of features at each split, with the number of features determined by the square root of the total features (max_features = “auto”, which defaults to sqrt (n_features) for classification).
- SVM is implemented next, utilizing the radial basis function (RBF) kernel to capture nonlinear relationships in the feature space. The regularization parameter C is set to 1, which controls the trade-off between maximizing the margin and minimizing classification errors. The gamma parameter, controlling the influence of each individual data point, is set to “scale”, meaning it is automatically computed based on the number of features and the variance of the input data.
- Lastly, the XGBoost Classifier is implemented, a gradient boosting algorithm known for its computational efficiency and performance. The model uses logistic regression as the objective function for binary classification (objective = “binary:logistic”). The number of boosting rounds is set to 100 (n_estimators = 100), and the learning rate, which controls the contribution of each tree, is set to 0.3 (learning_rate = 0.3). The maximum depth of each decision tree in the boosting process is limited to 6 (max_depth = 6). XGBoost also automatically handles missing values and uses multiclass log loss (mlogloss) as the evaluation metric during training.
4.1.2. Comparison of ML Models for Fall Detection
Using a sliding window approach with a window size of 30 s and a step size of 20 s, we extracted a total of 595 windows from the 11,916 acceleration samples collected at 1 Hz. The evaluation of the implemented machine learning models for fall detection is conducted using accuracy (2) as the primary performance metric.
where:
TP (True Positives)—the number of correctly classified positive instances;
TN (True Negatives)—the number of correctly classified negative instances;
FP (False Positives)—the number of negative instances incorrectly classified as positive;
FN (False Negatives)—the number of positive instances incorrectly classified as negative.
Table 4 summarizes the accuracy results obtained for the DT, RF, SVM, and XGBoost classifiers. Among these, the RF model demonstrates the highest performance, indicating it is the most suitable algorithm for our fall detection system.

Table 4.
Accuracy comparison of ML models for fall detection.
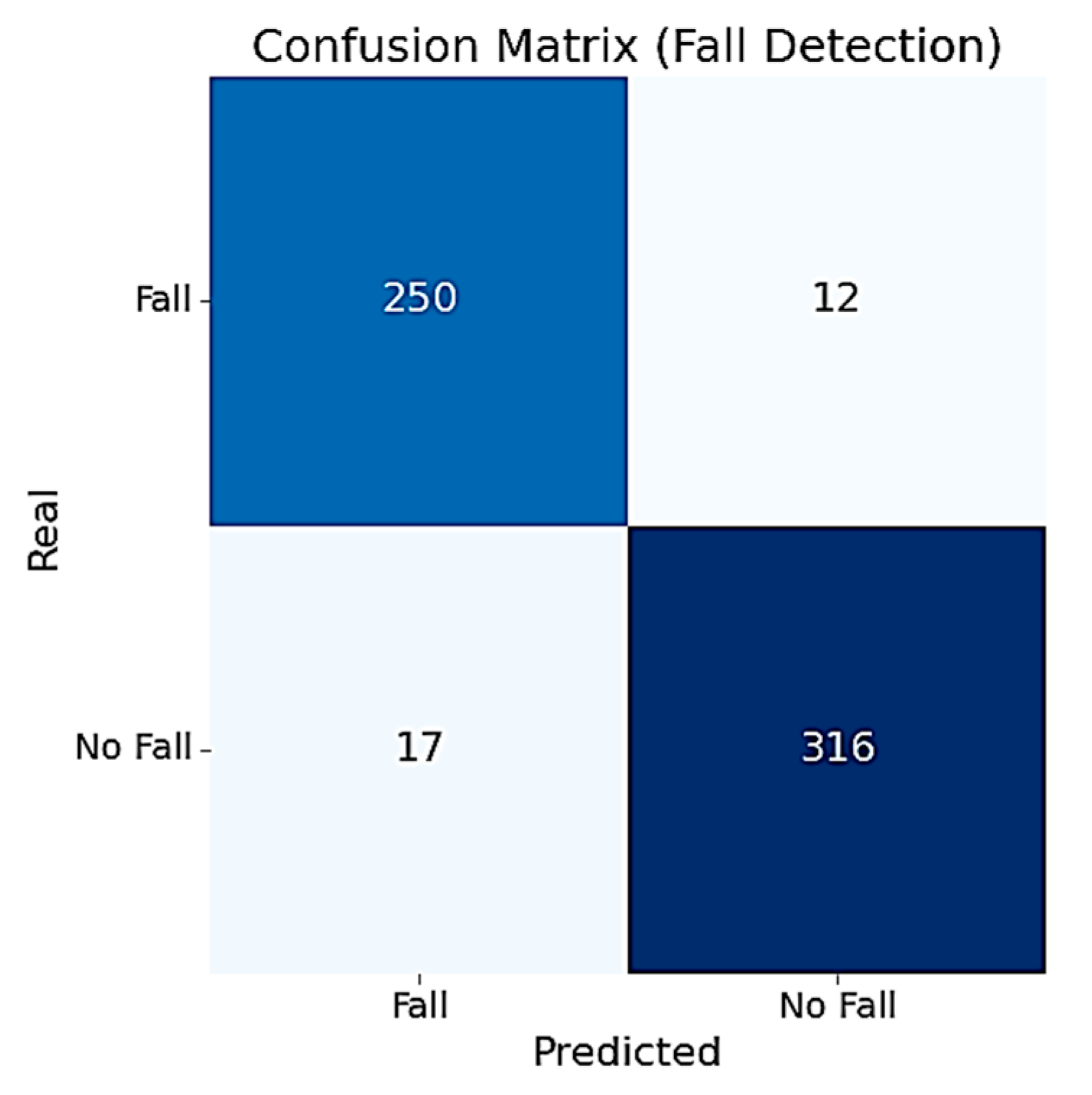
To assess the RF model’s performance, we computed the confusion matrix over the entire set of 595 windows. As shown in Figure 6, the model correctly identified 250 out of 262 fall windows and 316 out of 333 non-fall windows. The confusion matrix reveals that the model maintains a strong balance between sensitivity and specificity.
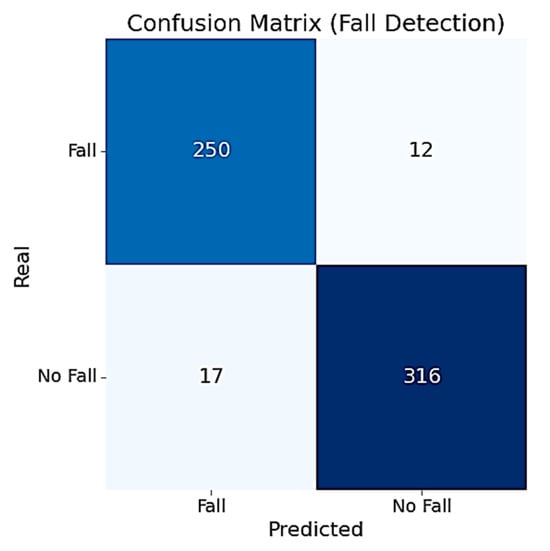
Figure 6.
Confusion matrix of the fall detection RF model.
4.1.3. Algorithm Implementation and Platform Integration
During the implementation phase of the fall detection system, several technical and operational challenges were encountered. One of the primary difficulties was minimizing false positives—situations where regular movements, such as sitting down quickly or jumping, were mistakenly identified as falls. To address this, the training dataset was carefully curated, including a wide range of daily activities alongside both real and simulated fall events, which allowed the algorithm to better distinguish between them.
Once new data is securely transmitted to the ICIPRO Cloud, the system computes the magnitude of acceleration using the Euclidean formula and immediately applies the pretrained model to classify the event as either a fall or a non-fall. If a fall is detected, the platform automatically triggers email alerts to notify caregivers or healthcare providers, enabling a rapid response and ensuring continuous passive monitoring without requiring user input.
4.2. Risk Analysis and Mitigation
During the development of the NeuroPredict platform, risk-related tests were carried out to identify shortcomings in the gathering, processing, and transmission of data under controlled laboratory conditions. These assessments were conducted by simulating potential points of malfunction in the platform workflow and observing how it behaves in specific load and atypical scenarios.
To reduce the risks associated with sensor data integrity, session-level metadata, including sensor type, timestamp intervals, and preprocessing version, were included in all data-gathering modules. This configuration allowed for the finding of missing data segments or discrepancies caused by transitory disconnections or deviations between gathering channels. Internal logging throughout preprocessing demonstrated that most signal anomalies may be detected using vector consistency checks and temporal gap evaluation.
Two edge-layer mitigating approaches hired for guaranteeing data consistency in the context of intermittent connectivity problems were local buffering and backup transmission approaches. Abnormal input structures were identified via preprocessing pipelines, enabling exclusion or reprocessing prior to the inference stage.
By applying the classifier to simulated movements that have been observed to be highly susceptible to false positives, for instance unexpected transitions or high-impact non-fall activities, the fall detection functionality’s performance sensitivity was assessed. Inaccurate classifications were identified and analyzed to facilitate future threshold and activity segmentation algorithm improvements. The development dashboard, which allowed each classification outcome to be linked to the original session and model version, was used to track outputs.
To mitigate any potential security risks, data transmitted from edge devices was encrypted via HTTPS and stored on the ICIPRO private cloud infrastructure. During all testing, system access was restricted to authorized users using role-based permissions, and audit logs were recorded for each data upload and processing event. These findings show that the implemented mitigation approaches operate as intended in the laboratory environment. Although more evaluation of performance in real-world operating environments is required, the current configuration provides a validated basis for proceeding with semi-clinical testing.
4.3. Ethical-Regulatory Validation During Laboratory Testing
The NeuroPredict platform effectively demonstrated the incorporation of ethical and regulatory safeguards in all simulated use scenarios during laboratory testing. Each stage of the ethical-regulatory pipeline, which was established during system development, was operationalized in practice and incorporated into the platform’s everyday operations, according to the validation process. To ensure that data streams contained only anonymized session-level identifiers, the platform implemented encryption and pseudonymization mechanisms across all test sessions. Through internal audit logs and network monitoring tools, secure data transmission via HTTPS (TLS 1.3) [69] and encrypted data storage based on AES-256 standards [68] were verified, ensuring that end-to-end data protection measures were operating as planned.
Only authorized development experts could see sensitive metadata thanks to role-based access controls that were properly tested and implemented. This verified that the platform could maintain data confidentiality in the controlled testing environment while limiting access in accordance with GDPR regulations. Digitally signed forms for fictitious patients and clinicians were used to validate the consent management workflow concurrently. Each session was programmatically associated with these forms, and pertinent consent metadata, such as timestamps, document versions, and related policy references, were captured in the system logs. This confirmed that the platform can support compliance with ethical review processes and enforce traceable and verifiable consent documentation, as mandated by GDPR Article 7.
The effective application of these safeguards in a controlled setting shows that adherence to ethical and legal requirements is ingrained in the NeuroPredict system and is not an afterthought. In addition to meeting future certification requirements under the GDPR, MDR, and the upcoming AI Act, these results offer a solid basis for transitioning to semi-clinical trials.
5. Discussion
This paper does not aim to present the NeuroPredict platform as a fully validated prototype, but rather as an evolving platform currently under laboratory-based testing. The fall prevention module is highlighted as a demonstrative use case, with the remaining functionalities being under active development. It is important to note that the current results represent an experimental evaluation of modular technological components within the NeuroPredict platform, rather than a complete clinical platform deployment. As a next step, participatory co-design and clinical validation will be carried out to ensure usability and acceptance in real-world settings.
This discussion reflects on the technical maturity of the NeuroPredict platform and the implications of its modular architecture, rather than evaluating real-world clinical performance or patient acceptability. Although user interaction is not part of this stage, internal validation using structured questionnaires and simulated sessions helped refine core functionalities.
The platform is designed to incorporate a clear and transparent informed consent process, ensuring that, upon deployment, individuals will understand the nature and scope of data collection and AI-driven decisions. The NeuroPredict platform is distinct from existing digital health systems addressing MS, PD, or AD through its integrated sensing environment and unified inference pipeline. By combining custom-built devices (e.g., GaitBand, Blackbox modules) with commercial wearables, it enables a versatile and high-resolution data acquisition approach. Emphasis on data synchronization, edge preprocessing, and layered compliance strategies supports the system’s adaptability to various deployment scenarios, including semi-clinical and remote monitoring contexts.
As a comprehensive digital health system for managing and monitoring neurodegenerative diseases, the outcomes presented in this paper illustrate the NeuroPredict platform’s technological potential and early functional consistency. Specifically, repeated end-to-end session operation, consistent sensor synchronization, and reliable data transfer across the preprocessing, inference, and data collection components demonstrate this consistency. The platform’s modular architecture allows for real-time collection, preprocessing, and inference throughout a range of ambient and physiological signals while preserving traceability and flexibility in compliance with new regulations pertaining to AI and medical device standards. The base data collection patterns, edge-to-cloud communication protocols, and AI-based classification pipelines have been determined to be functional and in accordance with the platform’s stated clinical use cases by its layered architecture and controlled laboratory testing.
With testing performed solely in controlled laboratory settings, making use of simulated user scenarios, this platform is presently in the preclinical testing stage. The functionalities are still not confirmed on actual patient populations, even though these experiments have shown consistent performance throughout the platform’s layered architecture, including real-time data gathering, edge processing, and machine learning inference. Data alignment, label reliability, traceability visualization, and results consistency monitoring via the secure dashboard interface were all part of the testing procedure.
AI’s role in the NeuroPredict platform is directly aligned with current trends in healthcare, where digital tools are increasingly being used for more precise, real-time monitoring and predictive analytics. In the NeuroPredict platform, AI modules are configured to extract clinically relevant features such as gait variability, cardiovascular fluctuations, and sleep irregularities from multimodal sensor streams, supporting early trend detection and event classification. These continuous assessments enable healthcare providers to detect early signs of NDD progression, improving the likelihood of timely interventions. The AI components of the NeuroPredict platform are built on a structured data acquisition pipeline, synchronized multimodal inputs, and versioned preprocessing workflows, ensuring consistency and traceability across inference stages. Unlike fixed-device ecosystems, the platform supports adaptive, device-agnostic modeling that evolves with incoming data and deployment contexts.
Methodologically, the use of supervised learning models on multimodal sensor data, together with synchronized timestamping, metadata control, and preprocessing validation, provides a solid foundation for reliable AI-assisted inference. Internal test results show that the platform can detect falls with high classification accuracy across many methodologies. The platform’s modular ML architecture, versioned processing pipelines, and secure dashboard interaction platforms may provide visible model traceability and systematic auditability, both of which are required for future regulatory certification.
Supervised learning algorithms (DT, RF, SVM, XGBoost) applied to multimodal sensor data have shown high accuracy in fall detection tasks, operating within version-controlled and traceable pipelines. These models are embedded within traceable, version-controlled pipelines aligned with regulatory expectations for auditability and reproducibility. Clinical validation is still pending, and the AI models remain in the preclinical stage. This stepwise validation approach—focusing first on technical integrity and regulatory alignment before clinical claims—is consistent with best practices in digital health innovation. It positions NeuroPredict for phased clinical deployment while ensuring ethical and performance safeguards are in place.
Although not yet implemented with clinical populations, initial fairness guidelines have been outlined in the algorithm development documents, with bias analysis modules planned for integration during the semi-clinical testing phase. Ensuring generalizability across diverse patient profiles, particularly given the heterogeneity of neurodegenerative disease progression, remains a major challenge. To enhance therapeutic relevance, future research should incorporate real-world datasets, address demographic variability, and implement continuous fairness and bias evaluation. Ongoing model auditing, integration of fairness indicators, and sensitivity analyses are planned for both training and inference. The modular design also allows for iterative retraining when new clinical data becomes available, which is an important step toward equitable and trustworthy AI support in neurodegenerative treatment.
Key ethical and legal frameworks, including the GDPR, MDR, and the upcoming EU AI Act, have been taken into consideration when developing the NeuroPredict platform. Critical compliance elements, including traceability, access control, encryption, pseudonymization, and explicit informed consent, are all incorporated into the architecture and have been checked in laboratory scenarios. Technical capability for secure and accessible data management proved over assessment by the effective implementation of role-based access, session-level metadata monitoring, and functional digital consent procedures.
Such processes represent the cornerstone of an organized compliance pipeline, guaranteeing that ethical principles are included throughout every detail from data collection to AI inference. Traceability, transparency, and lifecycle accountability are all supported by the platform’s framework and constitute a prerequisite for future certification under European AI regulations. Given that the AI-based functionalities act as an assisting rather than an autonomous method, healthcare decisions are still meant to be participatory approaches between patients and practitioners.
Session-level consent mapping and audit logging are functionally satisfactory and have been confirmed to operate over several lab sessions in the current version. Throughout the semi-clinical validation phase, other components that are currently under development—like automatic regulatory notifications and real-time compliance monitoring—will be incorporated. Remodeling role-based dashboards and making user consent reminders clearer are just two examples of how insights from audit log assessments and consent flow simulations are currently impacting platform design improvements.
Setting up regulatory applications, coordinating internal processes with MDR and AI Act compliance protocols, and being ready for the integration of dynamic consent and compliance milestones in practical deployments are all examples of ongoing accomplishments. In line with the recent European Commission regulations for reliable AI, projected improvements also include comprehensive checks for data authenticity and algorithmic transparency.
It is particularly important to underscore that all the findings presented in this research are a result of preclinical, laboratory-controlled testing, deploying simulated user situations to distinguish between validated outcomes and intended improvements. The platform has not yet collected or processed clinical data from actual patient populations. As a result, while some features, like versioned preprocessing, multimodal data acquisition, and fall detection inference, have been successfully tested and deployed, additional characteristics, like responsive training, real-time compliance warnings, and dynamic deployment dashboards, are still being developed or evaluated. To match objectives with the progressive testing and validation plan and to preserve clarity on the system’s present technological maturity, this difference is required.
Despite its demonstrated technical maturity, the NeuroPredict platform has some operational limitations that require more development before clinical implementation. Generalizability to heterogeneous patient populations and real-world clinical variability remains unconfirmed, as the platform has not yet been tested across diverse clinical infrastructures or multisite deployments. Although the platform includes digital informed consent workflows and secure data handling protocols, its effectiveness in dynamic, real-world environments—particularly across varying levels of digital literacy—has not yet been validated. The ability to maintain usability and compliance across user types remains a challenge.
One relevant limitation concerns the absence of end-user involvement during the initial design phase. This was a consequence of the project funding model, which restricted external partnerships. Nevertheless, the NeuroPredict platform was architected to remain adaptable, enabling easy integration of user feedback and participatory co-design approaches in future semi-clinical and clinical trials. The platform was iteratively evaluated during development through internal testing by team members and technical staff within the institute. Structured questionnaires and scenario-based simulations were used to assess usability, data flow, and interface responsiveness. While not a full co-design process, this internal validation provided early feedback and guided functional refinement. The platform’s modular design supports future co-design sessions with clinicians and patients, planned for the upcoming semi-clinical validation phase to ensure alignment with real-world user needs.
The NeuroPredict platform currently lacks real-time feedback and closed-loop decision-making capabilities. This reduces its ability to provide quick, adaptive interventions based on live data. Similarly, user contact is essentially one-way, with no mechanisms for patients or physicians to actively provide feedback or adjust monitoring settings during deployment.
While the fall detection algorithms have achieved high lab accuracy, clinical calibration using gold-standard datasets remains pending. The same applies to broader anomaly detection and trend analysis functions, which require further refinement to ensure clinical interpretability.
To address these limitations, the platform development plan includes co-design sessions with medical professionals and patient representatives, the incorporation of real-time user input modules, and the deployment of fairness monitoring pipelines. Semi-clinical trial preparations will also handle unpredictability in patient behavior and data settings, thereby enabling model retraining and adaptive deployment techniques.
Interoperability with current healthcare information solutions, including electronic health records platforms, is an additional unresolved issue. This is important for data integrity and for a simple integration into real-world healthcare workflows.
Hardware limitations, such as battery life, device durability in daily use, and usability by elderly patients, remain to be systematically tested. In terms of hardware resilience and simplicity of use, initial pilot tests are planned to systematically evaluate battery longevity, device durability, and ergonomic usability for elderly patients and those with motor deficits. These tests will include live usage simulation and extended monitoring durations to measure device performance across living conditions. Feedback from these tests will inform hardware improvements to optimize energy efficiency and user comfort, enabling long-term use and consistent data acquisition in semi-clinical and remote settings. Hardware resilience and accessibility continue to be a core priority in the development roadmap of the platform.
From a regulatory perspective, formal certification procedures are still ongoing even though the architecture complies with GDPR, MDR, and the EU AI Act. Although audit and documentation procedures have been designed with future compliance in mind, they must be continuously adjusted as legal frameworks change. The NeuroPredict platform is getting ready to be used in actual clinical settings as development moves forward, with an emphasis on putting in place dynamic consent procedures, ongoing ethical supervision, and iterative improvement based on feedback from stakeholders.
The future work will focus on advancing the platform beyond laboratory settings and into structured semi-clinical trials. This will involve testing with actual patients from the three target groups—PD, AD, and MD—under real-world use scenarios.
Efforts will also be made to:
- Extend AI modules with federated learning capabilities to allow personalized, decentralized model training on different user profiles;
- Integrate clinician feedback loops and develop interactive visualization modules to support explainability and medical decision support;
- Expand the cognitive monitoring module with more detailed task metrics and neuropsychological correlates;
- Assess long-term device performance, usability, and user acceptance among elderly users and caregivers, with ethnographic and usability testing protocols;
- Operationalizing ethical safeguards and regulatory alignment in real-world settings. This includes validating informed consent flows, data access controls, and explainable AI interfaces through participatory testing with patients, caregivers, and clinicians. A complete DPIA and MDR conformity assessment are planned, alongside alignment with the AI Act through lifecycle monitoring, algorithm traceability, and the implementation of an AI-specific quality management system;
- Begin the technical preparation for regulatory submissions under MDR and future EU AI Act compliance frameworks, including software lifecycle documentation, performance validation protocols, and risk assessments;
- Guarantee an effortless integration into healthcare workflows, future developments will also address compatibility with clinical data infrastructures and electronic health records.
Future research should therefore focus on clinical pilot studies in diverse care settings, participatory co-design processes with end users, and empirical assessments of ethical and regulatory features in practice. Iterative assessment of ethical and regulatory aspects is expected to occur in semi-clinical testing for feasibility and collaborative learning experiments as part of a staged plan to achieve these targets. The subsequent development phases will be conducted in partnership with clinical shareholders and ethics specialists, and technical members from the NeuroPredict development team, with the aim of enhancing the platform’s scientific basis and applied availability as an AI-powered smart healthcare solution for managing neurodegenerative diseases.
6. Conclusions
This paper presents the design, development, and early validation of the NeuroPredict platform, an artificial-intelligence-driven digital health platform designed to facilitate continuous monitoring and management of neurodegenerative diseases (PD, AD, and MS). The platform supports a modular, tiered architecture that integrates heterogeneous IoT-based sensors, edge-level signal preprocessing, secure cloud infrastructures, and machine learning modules for real-time classification and trend analysis. Its design is aligned with compliance-by-design principles under evolving model regulations such as the AI Act and MDR.
Early evaluations in controlled laboratory environments with simulated users demonstrate the platform’s functional reliability, end-to-end data traceability, and promising fall detection performance with various machine learning models. Clinical verification with real patient data remains a necessity for the future. Near-body, wearable, and ambient sensing with a formalized AI inference pipeline supports robust multimodal data integration and anomaly detection capabilities. The platform has been guided by a user-centered and ethics-driven strategy, embedding privacy-by-design safeguards and GDPR-aligned data protection mechanisms, to support future clinical transparency and trust. While not yet validated with patients, its technical maturity and modular scalability offer a robust foundation for upcoming semi-clinical evaluation.
Technical maturity and horizontal scalability of the platform provide a strong basis for future semi-clinical studies and real-world implementation, even though existing validations are limited to simulated data under controlled environments. Future plans call for detailed regulatory documentation for easy certification and continuous compliance, incremental user input integration, and development of AI-powered personalization tools. By integrating methodological rigor, architectural robustness, and ethical foresight, the NeuroPredict platform advances the current state of AI-enabled smart healthcare systems for neurodegenerative disease management. This work represents an important step toward incorporating trustworthy AI solutions into next-generation digital health infrastructures.
The development of the NeuroPredict platform demonstrates how advanced digital health tools can be aligned with ethical and regulatory requirements from the outset. As AI adoption in healthcare accelerates, the need for internationally harmonized standards on certification, auditing, and ethical oversight becomes increasingly urgent. The NeuroPredict platform provides a specific case study of how combining GDPR compliance, algorithmic transparency, and user-centric ethical protections supports responsible innovation and helps to close the gap between technical advancement and regulatory maturity.
These findings should be interpreted as an experimental technology demonstration of the platform’s modular capabilities, paving the way for subsequent participatory validation and real-world deployment.
Author Contributions
Conceptualization, M.I. and A.-M.V.; methodology, L.B., A.-M.V. and M.I.; software, A.-M.V.; validation, L.B., A.-M.V. and M.I.; investigation, M.I., M.G.-M. and C.-G.G.; data curation, A.-M.V.; writing—original draft preparation, M.I., L.B., A.-M.V., M.G.-M. and C.-G.G.; writing—review and editing, L.B., A.-M.V. and M.I.; project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the contribution of the Romanian Ministry of Research, Innovation, and Digitization for funding the development of the NeuroPredict platform inside the project “Advanced Artificial Intelligence Techniques in Science and Applications” (Contract No. 13N/2023 (PN 23 38 05 01)) for the period 2023–2026.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4G | fourth generation (for mobile communication) |
| AD | Alzheimer’s disease |
| AI | artificial intelligence |
| AI Act | Artificial Intelligence Act |
| AI HLEG | European Commission’s High-Level Expert Group on Artificial Intelligence |
| ALS | amyotrophic lateral sclerosis |
| ANCPDCP | National Authority for the Supervision of Personal Data Processing |
| CO2 | carbon dioxide |
| CNN | convolutional neural networks |
| CSV | comma-separated values |
| DT | decision tree |
| DPIA | Data Protection Impact Assessment |
| ECG | electrocardiogram |
| EEG | electroencephalography |
| FN | false negatives |
| FP | false positives |
| GDPR | General Data Protection Regulation |
| GND | ground |
| HRV | heart rate variability |
| IoT | Internet of Things |
| I2C | inter-integrated circuit |
| JSON | JavaScript Object Notation |
| LSTM | long short–term memory |
| MDR | Medical Device Regulation |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Overall Cognitive Assessment |
| MS | multiple sclerosis |
| NDD | Neurodegenerative diseases |
| PD | Parkinson’s disease |
| RBF | radial basis function |
| RF | random forest |
| SpO2 | oxygen saturation |
| SCL | serial clock line |
| SDA | serial data line |
| SVM | support vector machine |
| TLS | transport layer security |
| TN | true negatives |
| TP | true positives |
| UDI | unique device identification system |
| Vin | voltage input |
| Wi-Fi | wireless fidelity |
| XGBoost | extreme gradient boosting |
References
- Zhang, W. Current Therapy and Challenges for Neurodegenerative Diseases. Trans. Mater. Biotechnol. Life Sci. 2024, 7, 132–136. [Google Scholar] [CrossRef]
- Wang, H. Research on an AI Model for Early Alzheimer’s Detection. Appl. Comput. Eng. 2025, 116, 168–173. [Google Scholar] [CrossRef]
- Sandulescu, V.; Ianculescu, M.; Valeanu, L.; Alexandru, A. Integrating IoMT and AI for Proactive Healthcare: Predictive Models and Emotion Detection in Neurodegenerative Diseases. Algorithms 2024, 17, 376. [Google Scholar] [CrossRef]
- Tîrziu, E.; Vasilevschi, A.-M.; Alexandru, A.; Tudora, E. Enhanced Fall Detection Using YOLOv7-W6-Pose for Real-Time Elderly Monitoring. Future Internet 2024, 16, 472. [Google Scholar] [CrossRef]
- Ianculescu, M.; Nicolau, D.; Petrache, C.; Vasilevschi, A. Advancing Neurodegenerative Disease Management: The NeuroPredict Platform Integrating IoT, AI, and Cloud Technologies. In Proceedings of the 2024 15th International Conference on Communications (COMM), Bucharest, Romania, 3 October 2024; IEEE: New York, NJ, USA, 2024; pp. 1–6. [Google Scholar]
- Băjenaru, O.L.; Băjenaru, L.; Ianculescu, M.; Constantin, V.-Ș.; Gușatu, A.-M.; Nuță, C.R. Geriatric Healthcare Supported by Decision-Making Tools Integrated into Digital Health Solutions. Electronics 2024, 13, 3440. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Alizadehsani, R.; CIFCI, M.A.; Kausar, S.; Rehman, R.; Mahanta, P.; Bora, P.K.; Almasri, A.; Alkhawaldeh, R.S.; Hussain, S.; et al. A Review of Explainable Artificial Intelligence in Healthcare. Comput. Electr. Eng. 2024, 118, 109370. [Google Scholar] [CrossRef]
- Chin, M.H.; Afsar-Manesh, N.; Bierman, A.S.; Chang, C.; Colón-Rodríguez, C.J.; Dullabh, P.; Duran, D.G.; Fair, M.; Hernandez-Boussard, T.; Hightower, M.; et al. Guiding Principles to Address the Impact of Algorithm Bias on Racial and Ethnic Disparities in Health and Health Care. JAMA Netw. Open 2023, 6, e2345050. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Yang, A.; Meng, F.; Zhang, J. The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need. Aging Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, Y.; Lu, Y.; Zhang, Y.; Huang, Q.; Yang, Y.; Zhang, K.; Li, M. Impact of Virtual Reality-Based Therapies on Cognition and Mental Health of Stroke Patients: Systematic Review and Meta-Analysis. J. Med. Internet. Res. 2021, 23, e31007. [Google Scholar] [CrossRef] [PubMed]
- Băjenaru, O.L.; Nuță, C.R.; Băjenaru, L.; Balog, A.; Constantinescu, A.; Andronic, O.; Popescu, B.O. Health-Related Quality of Life in Romanian Patients with Dystonia: An Exploratory Study. J. Clin. Med. 2024, 13, 3403. [Google Scholar] [CrossRef] [PubMed]
- Ganjizadeh, A.; Wei, Y.; Erickson, B. From Pixels to Prognosis: AI-Driven Insights into Neurodegenerative Diseases. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Boskovic, P.; Gao, W.; Kipnis, J. Will Cellular Immunotherapies End Neurodegenerative Diseases? Trends Immunol. 2024, 45, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Baga, D.; Fotiadis, D.I.; Konitsiotis, S.; Tsouli, S.; Diakou, M.; Arrendondo, M.T.; Estrada, J.J.; Pansera, M.; Akay, M. PERFORM: A Platform for Monitoring and Management of Chronic Neurodegenerative Diseases: The Parkinson and Amyotrophic Lateral Sclerosis Case. In Proceedings of the 2009 4th International IEEE/EMBS Conference on Neural Engineering, Antalya, Turkey, 29 April–2 May 2009; IEEE: New York, NJ, USA, 2009; pp. 1–5. [Google Scholar]
- Mohammed, I.A.; Venkataraman, S. An Innovative Study for the Development of a Wearable AI Device to Monitor Parkinson’s Disease Using Generative AI and LLM Techniques. Int. J. Creat. Res. Thoughts (IJCRT) 2024, 11, 2320–2882. [Google Scholar]
- Chudzik, A.; Śledzianowski, A.; Przybyszewski, A.W. Machine Learning and Digital Biomarkers Can Detect Early Stages of Neurodegenerative Diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Qi, L.; Dong, J.; Yu, H. Dual Channel LSTM Based Multi-Feature Extraction in Gait for Diagnosis of Neurodegenerative Diseases. Knowl. Based Syst. 2018, 145, 91–97. [Google Scholar] [CrossRef]
- Akram, A.S.; Grezenko, H.; Singh, P.; Ahmed, M.; Hassan, B.D.; Hagenahalli Anand, V.; Elashry, A.A.; Nazir, F.; Khan, R. Advancing the Frontier: Neuroimaging Techniques in the Early Detection and Management of Neurodegenerative Diseases. Cureus 2024, 16, e61335. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, A. The Intersections of Artificial Intelligence, Brain Imaging Tools and Diagnostics for Neurodegenerative Diseases. J. Stud. Res. 2023, 12. [Google Scholar] [CrossRef]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices Toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights From Human Gait. Front. Neurosci. 2022, 16, 859298. [Google Scholar] [CrossRef] [PubMed]
- Bounsall, K.; Milne-Ives, M.; Hall, A.; Carroll, C.; Meinert, E. Artificial Intelligence Applications for Assessment, Monitoring, and Management of Parkinson Disease Symptoms: Protocol for a Systematic Review. JMIR Res. Protoc. 2023, 12, e46581. [Google Scholar] [CrossRef] [PubMed]
- Alhassun, W.H.; Alothman, A.S.; Alfawaz, S.A. The Role of AI in Early Detection of Alzheimer’s and Parkinson’s Diseases: A Literature Survey. Asian J. Res. Comput. Sci. 2025, 18, 186–196. [Google Scholar] [CrossRef]
- Chen, C. AI-Aided Diagnosis For Neurodegenerative Diseases: Prospects And Challenges. Trans. Mater. Biotechnol. Life Sci. 2024, 3, 66–72. [Google Scholar] [CrossRef]
- Goudarzi, N.; Taheri, Z.; Nezhad Salari, A.M.; Kazemzadeh, K.; Tafakhori, A. Recognition and Classification of Facial Expression Using Artificial Intelligence as a Key of Early Detection in Neurological Disorders. Rev. Neurosci. 2025, 36, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Khastehband, S.; Ghasempour Dabbaghi, K.; Khosravirad, Z.; Jamalnia, S.; GhorbaniNia, R.; Mahmoudikohani, F.; Zakeri, H. The Use of Artificial Intelligence in the Management of Neurodegenerative Disorders; Focus on Alzheimer’s Disease. Galen Med. J. 2023, 12, e3061. [Google Scholar] [CrossRef] [PubMed]
- Pasumarthy, S.M.; Katam, D.; Neella, K.; Mylavarapu, S.G. Artificial Intelligence in the Diagnosis of Parkinson’s Disease. J. Pharma Insights Res. 2024, 2, 1–6. [Google Scholar] [CrossRef]
- Patil, A.D.; Biousse, V.; Newman, N.J. Artificial Intelligence in Ophthalmology: An Insight into Neurodegenerative Disease. Curr. Opin. Ophthalmol. 2022, 33, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, R.; Chen, D.; Shi, L.; Xiao, C. Label-Based Topic Modeling to Enhance Medical Triage for Medical Triage Robots. Stud. Inform. Control 2023, 32, 37–48. [Google Scholar] [CrossRef]
- Biró, A.; Cuesta-Vargas, A.I.; Szilágyi, L. Precognition of Mental Health and Neurogenerative Disorders Using AI-Parsed Text and Sentiment Analysis. Acta Univ. Sapientiae. Inform. 2023, 15, 359–403. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Qi, D.; Xie, J.; Cao, J.; Liao, W.H. Wearable Gait Monitoring for Diagnosis of Neurodegenerative Diseases. Measurement 2022, 202, 111839. [Google Scholar] [CrossRef]
- Paraschiv, E.-A.; Băjenaru, L.; Petrache, C.; Bica, O.; Nicolau, D.-N. AI-Driven Neuro-Monitoring: Advancing Schizophrenia Detection and Management Through Deep Learning and EEG Analysis. Future Internet 2024, 16, 424. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Segkouli, S.; Zygouris, S.; Michailidou, C.; Avgerinakis, K.; Fappa, E.; Vassiliades, S.; Bougea, A.; Papagiannakis, N.; Katakis, I.; et al. Mobile App Interventions for Parkinson’s Disease, Multiple Sclerosis and Stroke: A Systematic Literature Review. Sensors 2023, 23, 3396. [Google Scholar] [CrossRef] [PubMed]
- Băjenaru, L.; Tomescu, M.; Grigorovici-Togănel, I. Leveraging Generative Artificial Intelligence for Advanced Healthcare Solutions. Rom. J. Inf. Technol. Autom. Control (Rev. Română De Informatică și Autom.) 2024, 34, 149–164. [Google Scholar] [CrossRef]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E.; Hewage, C. Convergence of Artificial Intelligence and Neuroscience towards the Diagnosis of Neurological Disorders—A Scoping Review. Sensors 2023, 23, 3062. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Imms, P.; Cheng, M.; Amgalan, A.; Chowdhury, N.F.; Massett, R.J.; Chaudhari, N.N.; Chen, X.; Thompson, P.M.; Bogdan, P.; et al. Anatomically Interpretable Deep Learning of Brain Age Captures Domain-Specific Cognitive Impairment. Proc. Natl. Acad. Sci. USA 2023, 120, e2214634120. [Google Scholar] [CrossRef] [PubMed]
- Farago, P.; Popescu, A.-S.; Perju-Dumbrava, L.; Ilesan, R.R. Wearables as Part of Decision Support System in Parkinson’s Disease Prediagnosis: A Case Study. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; IEEE: New York, NJ, USA, 2022; pp. 1–4. [Google Scholar]
- Yammouri, G.; Ait Lahcen, A. AI-Reinforced Wearable Sensors and Intelligent Point-of-Care Tests. J. Pers. Med. 2024, 14, 1088. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.H.; Kim, W.; Ko, H.J.; Park, S.Y.; Kim, J.S.; Kim, G.H.; Kim, B.; Lee, J.H.; Kim, J.W. Development and Effectiveness of an AI Chatbot-Based Mobile Cognitive Screening and Customized Training Application for Preventing Dementia: Older Adults Living in Rural Areas of South Korea. Arch. Des. Res. 2024, 37, 77–90. [Google Scholar] [CrossRef]
- CarePredict Tempo Wearable for Senior Care and Activity Monitoring. Available online: https://www.carepredict.com/ (accessed on 4 June 2025).
- Vayyar Care. Vayyar Home—Fall Detection for Seniors. Available online: https://vayyar.com/care/ (accessed on 4 June 2025).
- Karakostas, A.; Briassouli, A.; Avgerinakis, K.; Kompatsiaris, I.; Tsolaki, M. The Dem@Care Experiments and Datasets: A Technical Report. arXiv 2016, arXiv:1701.01142. [Google Scholar] [CrossRef]
- Bottiroli, S.; Bernini, S.; Cavallini, E.; Sinforiani, E.; Zucchella, C.; Pazzi, S.; Cristiani, P.; Vecchi, T.; Tost, D.; Sandrini, G.; et al. The Smart Aging Platform for Assessing Early Phases of Cognitive Impairment in Patients with Neurodegenerative Diseases. Front. Psychol. 2021, 12, 635410. [Google Scholar] [CrossRef] [PubMed]
- High-Level Expert Group on Artificial Intelligence. Shaping Europe’s Digital Future. Available online: https://digital-strategy.ec.europa.eu/en/policies/expert-group-ai (accessed on 20 September 2024).
- UNESCO. Recommendation on the Ethics of Artificial Intelligence. Available online: https://www.unesco.org/en/articles/recommendation-ethics-artificial-intelligence (accessed on 26 September 2024).
- Regulation—2016/679—EN—Gdpr—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 17 September 2024).
- The National Supervisory Authority for Personal Data Processing. Available online: https://www.dataprotection.ro/index.jsp?page=home&lang=en (accessed on 20 September 2024).
- Regulation—2017/745—EN—Medical Device Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745&lang1=EN&from=RO&lang3=choose&lang2=choose&_csrf=b47c5607-b98c-44da-98d8-35ddd9439253 (accessed on 18 September 2024).
- EU Artificial Intelligence Act|Up-to-Date Developments and Analyses of the EU AI Act. Available online: https://artificialintelligenceact.eu/ (accessed on 18 September 2024).
- ORDIN 20484 13/04/2023—Portal Legislativ. Available online: https://legislatie.just.ro/public/DetaliiDocument/267590 (accessed on 20 September 2024).
- Ministry of Research, Innovation and Digitization. Artificial Intelligence. Available online: https://www.mcid.gov.ro/strategia-inteligenta-artificiala/ (accessed on 20 September 2024).[Green Version]
- Jwa, A.S.; Poldrack, R.A. Addressing Privacy Risk in Neuroscience Data: From Data Protection to Harm Prevention. J. Law Biosci. 2022, 9, lsac025. [Google Scholar] [CrossRef]
- Krittanawong, C. Artificial Intelligence in Clinical Practice: How AI Technologies Impact Medical Research and Clinics; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Fereshtehnejad, S.-M.; Lökk, J. Challenges of Teleneurology in the Care of Complex Neurodegenerative Disorders: The Case of Parkinson’s Disease with Possible Solutions. Healthcare 2023, 11, 3187. [Google Scholar] [CrossRef] [PubMed]
- NAMMDR—National Agency for Medicines and Medical Devices of Romania. Available online: https://www.anm.ro/en/ (accessed on 20 September 2024).
- Sapienza, S.; Tsurkalenko, O.; Giraitis, M.; Mejia, A.C.; Zelimkhanov, G.; Schwaninger, I.; Klucken, J. Assessing the Clinical Utility of Inertial Sensors for Home Monitoring in Parkinson’s Disease: A Comprehensive Review. NPJ Park. Dis. 2024, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on Wearable Sensors for the Management of Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Far, M.S.; Stolz, M.; Fischer, J.M.; Eickhoff, S.B.; Dukart, J. JTrack: A Digital Biomarker Platform for Remote Monitoring of Daily-Life Behaviour in Health and Disease. Front. Public Health 2021, 9, 763621. [Google Scholar] [CrossRef]
- Schmidt, J.; Schutte, N.M.; Buttigieg, S.; Novillo-Ortiz, D.; Sutherland, E.; Anderson, M.; de Witte, B.; Peolsson, M.; Unim, B.; Pavlova, M.; et al. Mapping the Regulatory Landscape for Artificial Intelligence in Health within the European Union. NPJ Digit. Med. 2024, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Smart Scales, Watches and Health Monitoring Devices|Withings. Available online: https://www.withings.com/eu/en/?srsltid=AfmBOoqukJrG5xzJkv0jG8wIYDpPlQAkeA6G8a2Rkp0A57bMY3-cKzQ1 (accessed on 3 June 2025).
- Oura Ring. Smart Ring for Fitness, Stress, Sleep & Health. Available online: https://ouraring.com/?srsltid=AfmBOorQCmOy51pmxDgTd3uSyzjI8Cd2CsxdWjcP1yssVvu59awL4dBV (accessed on 3 June 2025).
- Sensoria Home Page. Available online: https://www.sensoriafitness.com/ (accessed on 3 June 2025).
- Mienye, I.D.; Jere, N. A Survey of Decision Trees: Concepts, Algorithms, and Applications. IEEE Access 2024, 12, 86716–86727. [Google Scholar] [CrossRef]
- Salman, H.A.; Kalakech, A.; Steiti, A. Random Forest Algorithm Overview. Babylon. J. Mach. Learn. 2024, 2024, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Guido, R.; Ferrisi, S.; Lofaro, D.; Conforti, D. An Overview on the Advancements of Support Vector Machine Models in Healthcare Applications: A Review. Information 2024, 15, 235. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Guidelines on Data Protection Impact Assessment (DPIA) (Wp248rev.01). Available online: https://ec.europa.eu/newsroom/article29/items/611236 (accessed on 3 June 2025).
- Gheorghe-Moisii, M.; Gheorghe, C.-G.; Soviany, S. Ethical Considerations on the Use of AI Technology in EHealth Applications for Neurodegenerative Diseases. Rom. J. Inf. Technol. Autom. Control 2024, 34, 97–108. [Google Scholar] [CrossRef]
- Rescorla, E. The Transport Layer Security (TLS) Protocol Version 1.3. RFC 8446. August 2018. Available online: https://www.rfc-editor.org/info/rfc8446 (accessed on 30 May 2025).
- National Institute of Standards and Technology (NIST). Advanced Encryption Standard (AES). FIPS 197. 26 November 2001. Available online: https://nvlpubs.nist.gov/nistpubs/FIPS/NIST.FIPS.197-upd1.pdf (accessed on 30 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).