Design and Biological Evaluation of Delivery Systems Containing Bisphosphonates
Abstract
:1. Introduction
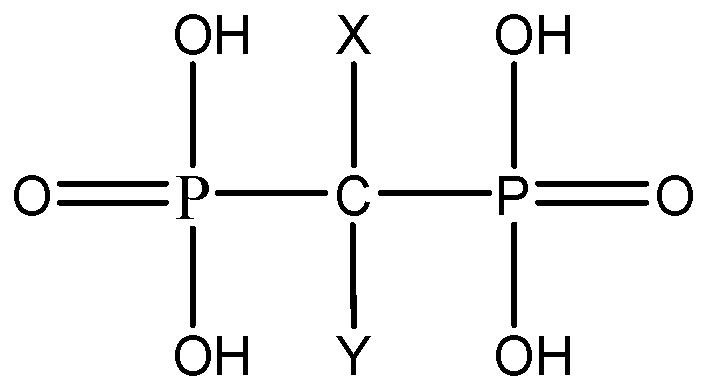
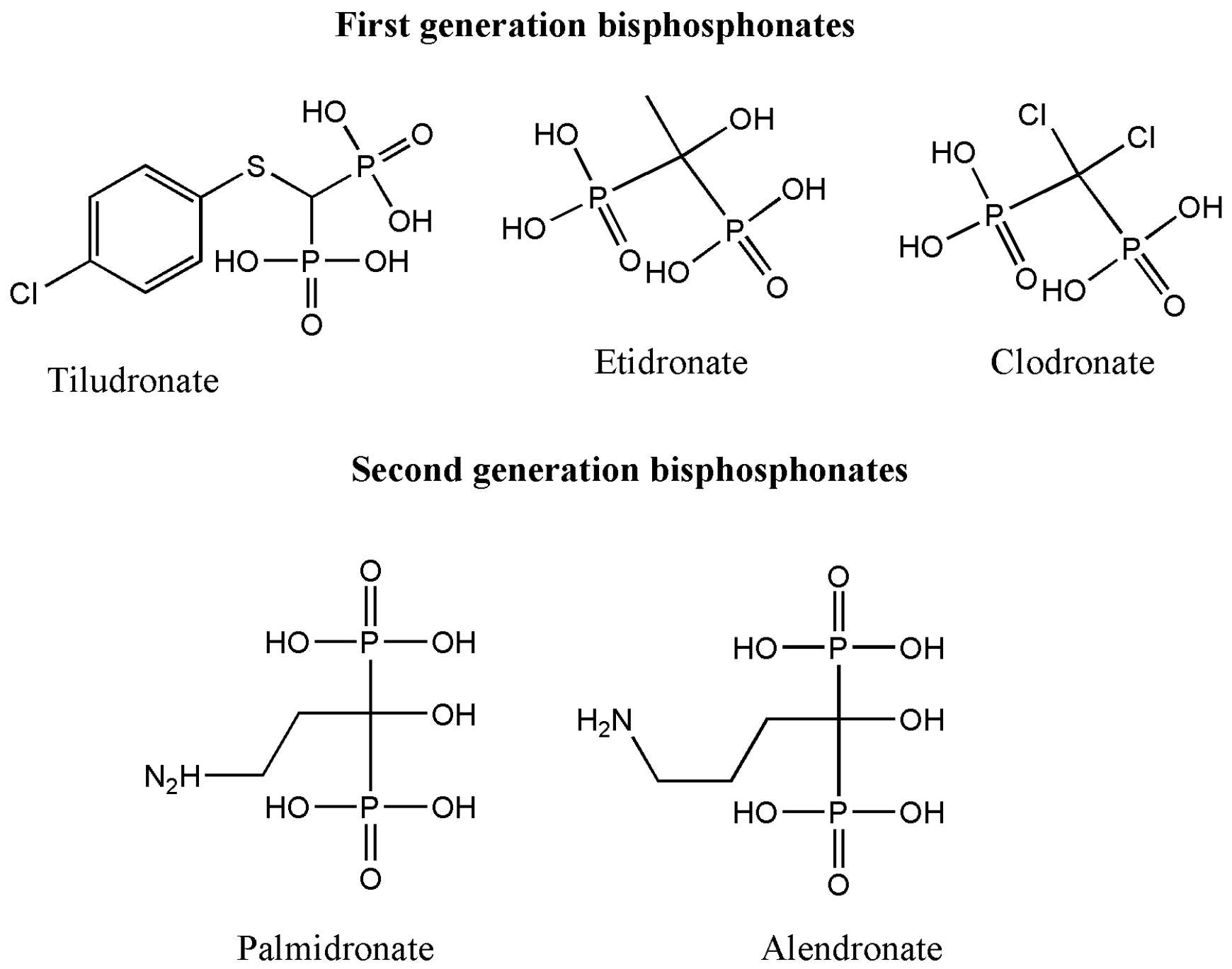
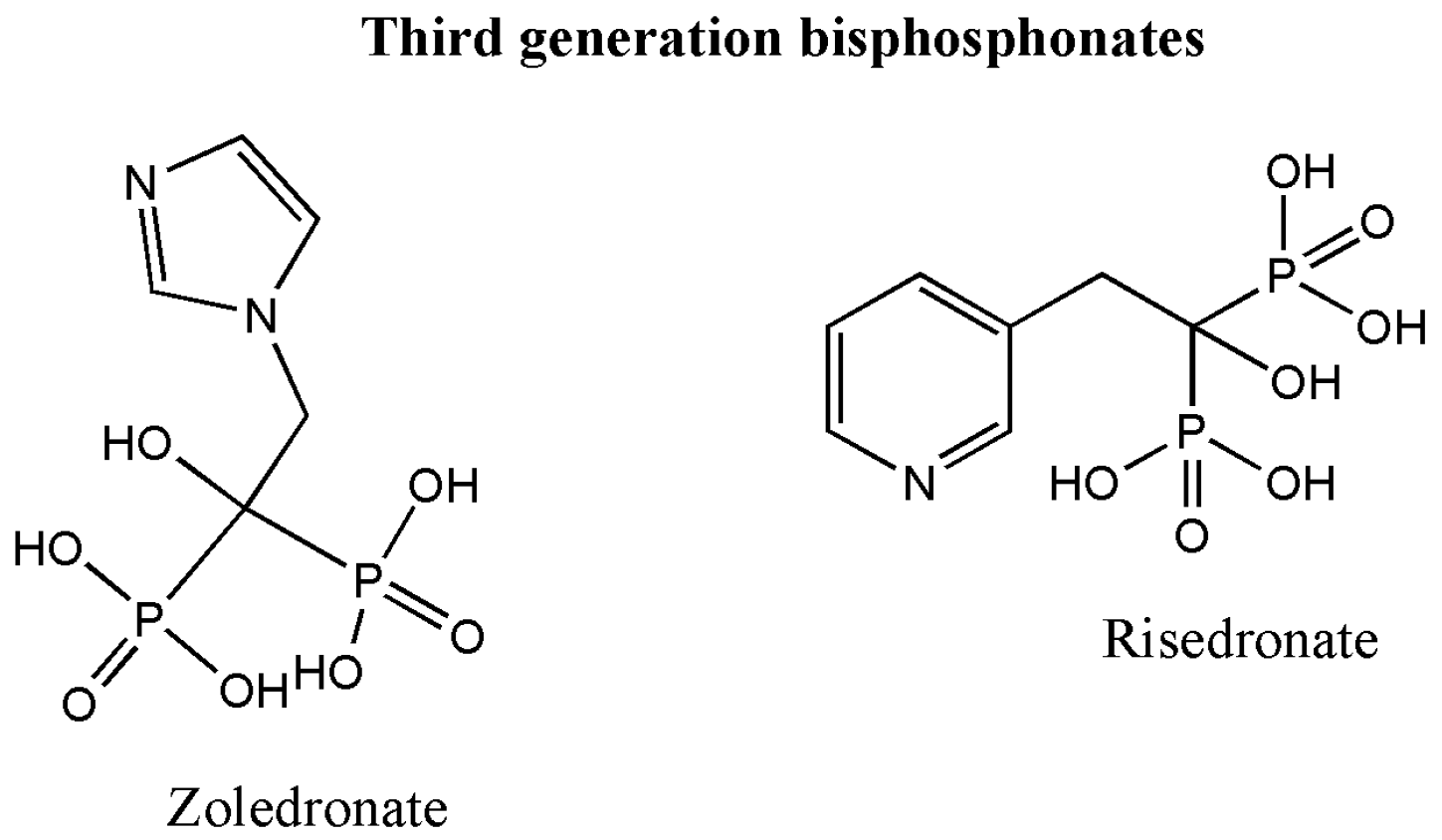
2. Classification of Bisphosphonates
3. Mechanism of Action of Bisphosphonate
4. Application of Bisphosphonates
4.1. Bisphosphonates in Osteoporosis
4.2. Bisphosphonates in Orthopedic Medicine
4.3. Bisphosphonates in Paget’s Disease
4.4. Bisphosphonates in Oncology
4.5. Administration Routes of Bisphosphonates
5. Types of Systems Used for Delivery of Bisphosphonates
5.1. Polymer Drug Conjugates (Copolymers)
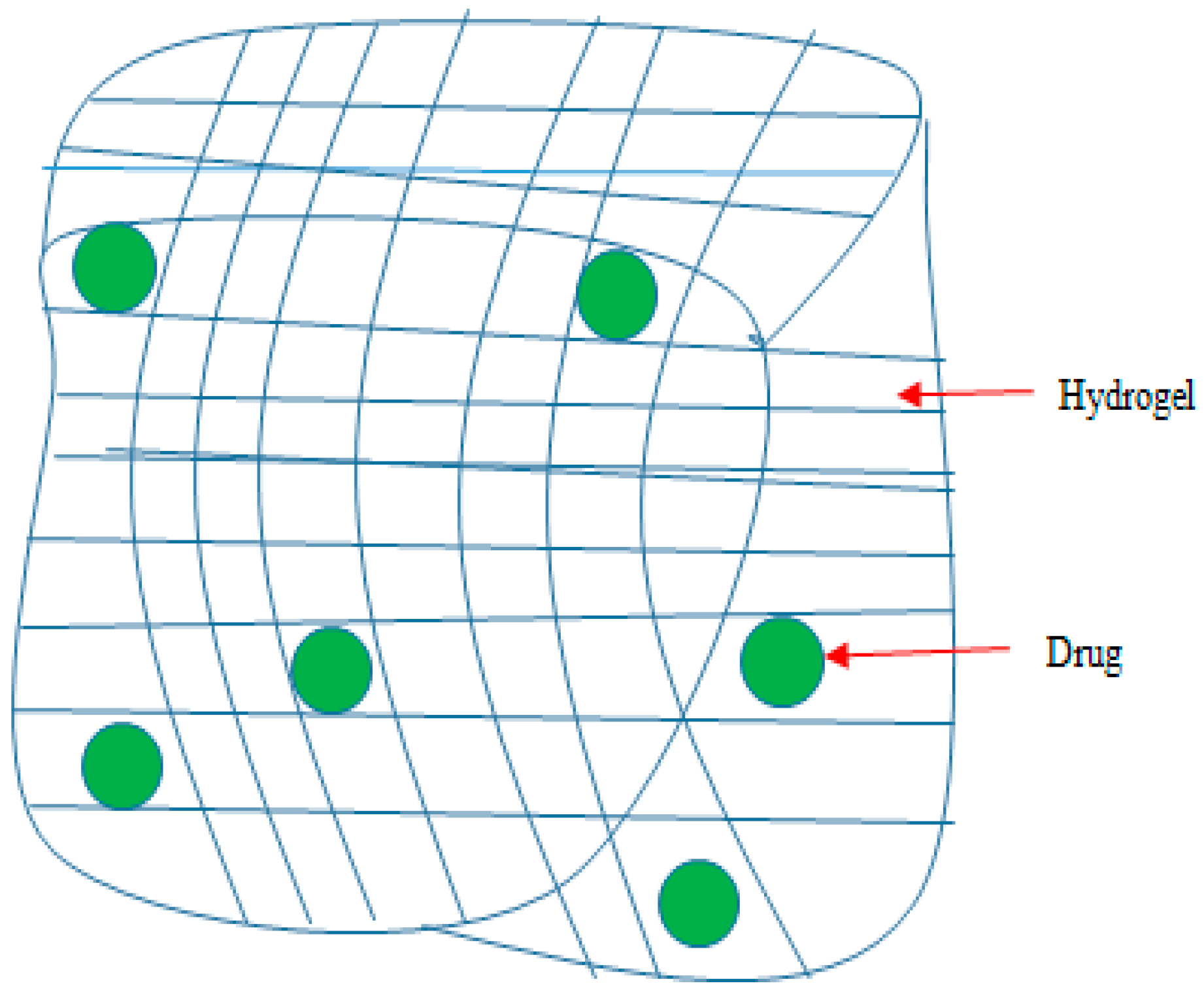
5.2. Hydrogels
5.3. Bioceramics
5.4. Hybrid Compounds
6. Carbon-Based Materials
6.1. Carbon Nanotubes
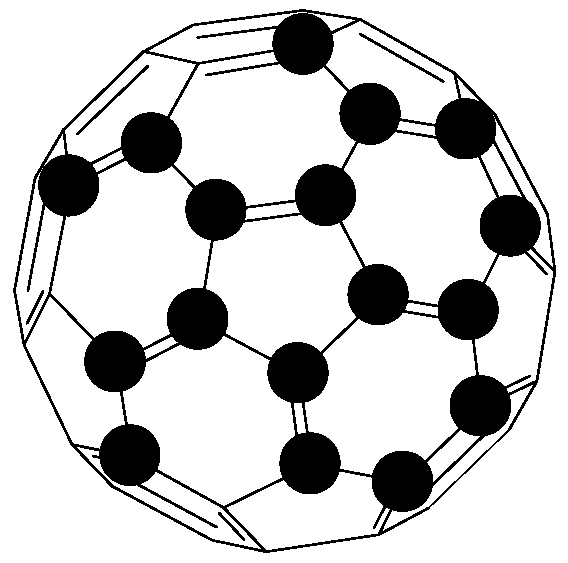
6.2. Fullerenes
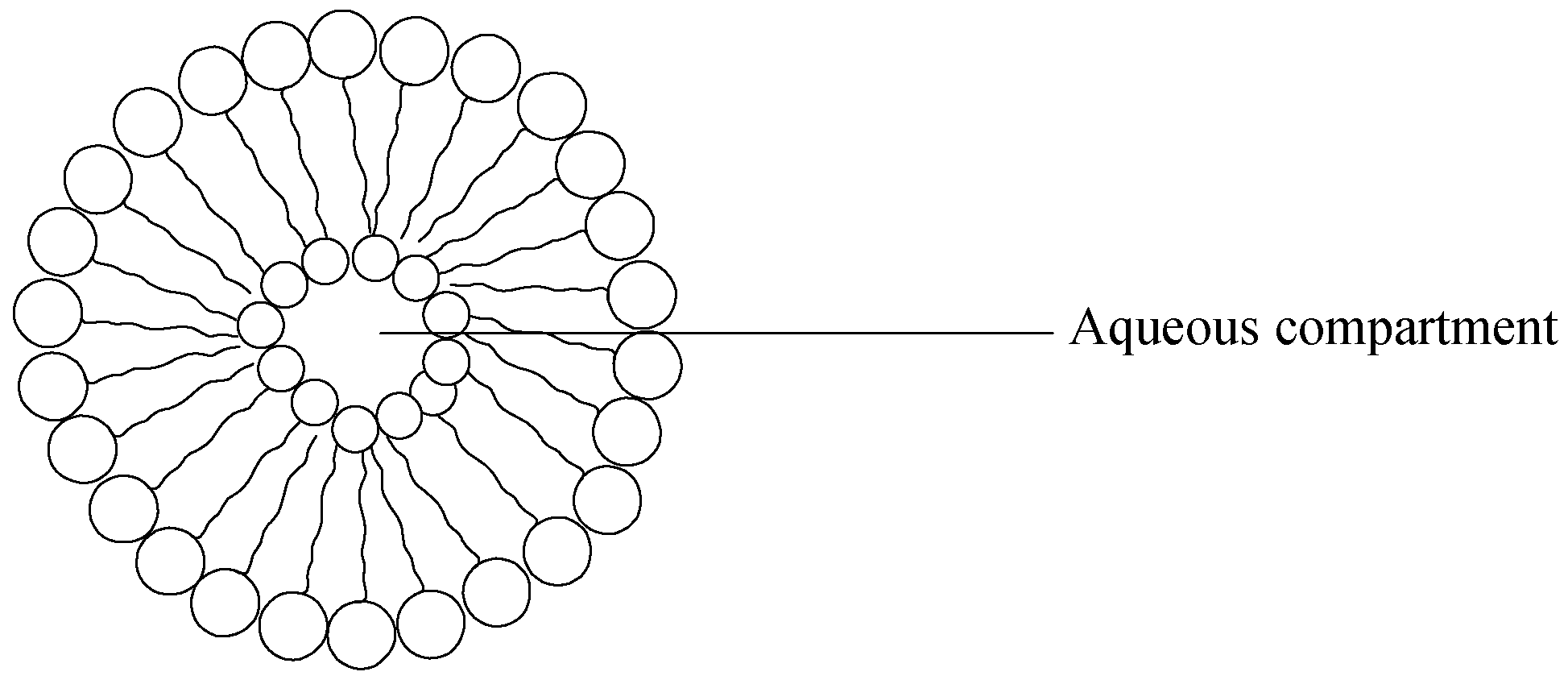
7. Liposomes
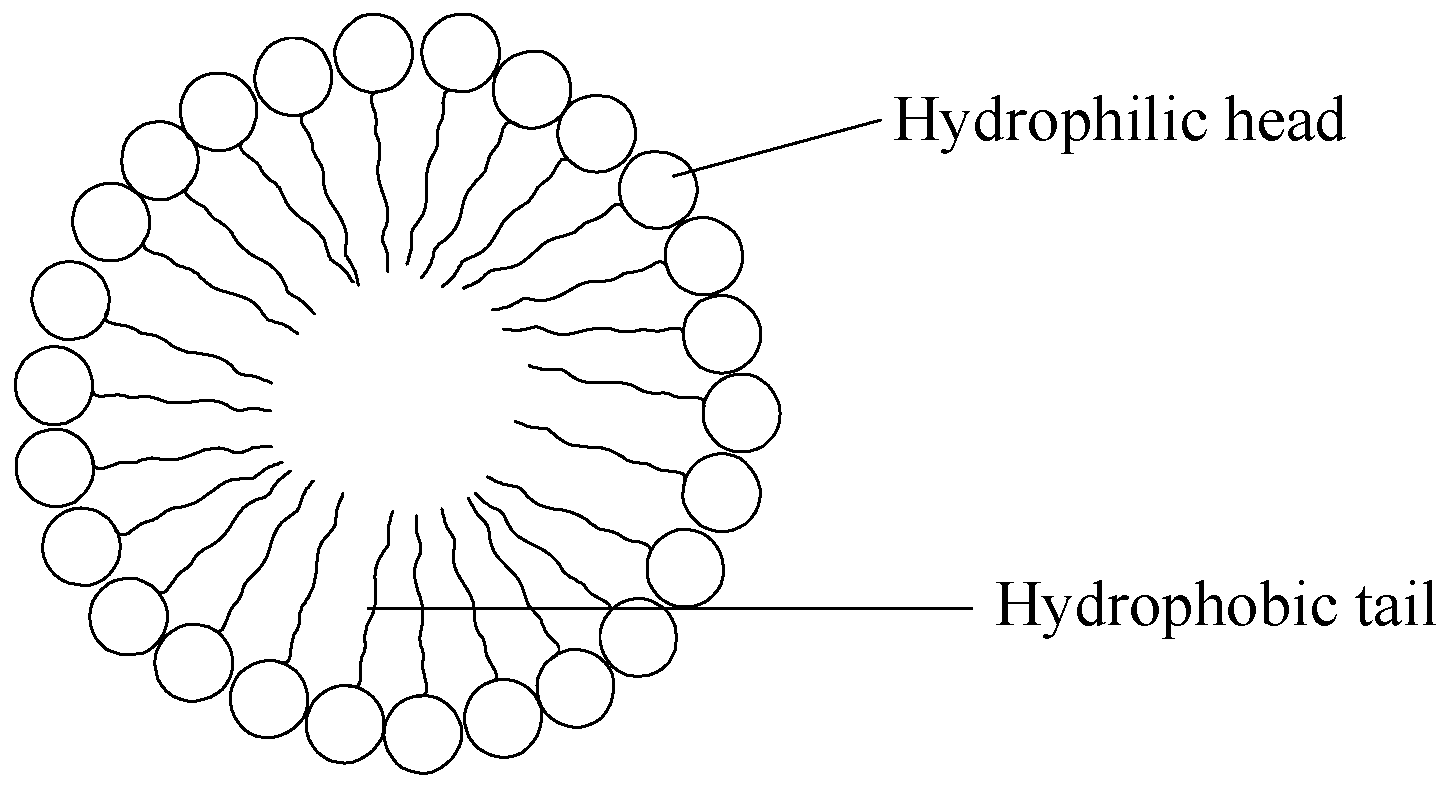
8. Micelles
9. Problems Associated with Delivery Systems Used to Deliver Bisphosphonates
10. Conclusion
Acknowledgments
Conflicts of Interest
References
- Dang, L.; Liu, J.; Li, F.; Wang, L.; Li, D.; Guo, B.; He, X.; Jiang, F.; Liang, C.; Liu, B.; Badshah, S.A. Targeted delivery systems for molecular therapy in skeletal disorders. Int. J. Mol. Sci. 2016, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Busselberg, D. Osteoporosis: An under-recognized public health problem. J. Local Glob. Health Sci. 2016. [Google Scholar] [CrossRef]
- Lin, J.T.; Lane, J.M. Osteoporosis: A review. Clin. Orthop. Relat. Res. 2004, 425, 126–134. [Google Scholar] [CrossRef]
- Bolster, M.B. Osteoporosis. Merc Manual. Available online: https://www.merckmanuals.com/professional/musculoskeletal-and-connective-tissue-disorders/osteoporosis/osteoporosis (accessed on 15 July 2016).
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Osteoporosis: Assessing the Risk of Fragility Fracture; NICE Clinical Guideline: London, UK, 2012.
- Horowitz, M.C. Cytokines and estrogen in bone: Anti-osteoporotic effects. Science 1993, 260, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, T.; Lewis, J.; Collin-Osdoby, P.; Osdoby, P. Estrogen’s bone protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J. Clin. Investig. 1999, 103, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Correa, P.H.S. Bone quality and osteoporosis therapy. Arq. Bras. Endocrinol. Metab. 2010, 54, 186–199. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2012, 11, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.J.; Shepherd, J.; Gaw, A.; Murphy, M.J.; Shephered, J. Clinical Biochemistry: An Illustrated Colour Text; Elsevier: Edinburgh, UK, 2008. [Google Scholar]
- Shuster, S. Osteoporosis, a unitary hypothesis of collagen loss in skin and bone. Med. Hypotheses 2005, 65, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.H. Brittle bones-fragile molecules: Disorders of collagen gene structure and expression. Trends Genet. 1990, 6, 293–300. [Google Scholar] [CrossRef]
- Pesce, V.; Speciale, D.; Sammarco, G.; Patella, S.; Spinarelli, A.; Patella, V. Surgical approach to bone healing in osteoporosis. Clin. Cases Miner. Bone Metab. 2009, 2, 131–135. [Google Scholar]
- Roux, A.; Decroocq, L.; El Batti, S.; Bonnevialle, N.; Moineau, G.; Trojani, C.; Boileau, P.; de Peretti, F. Epidemiology of proximal humerus fractures managed in a trauma center. Orthop. Traumatol. Surg. Res. 2012, 98, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., III. Epidemiology of fractures. In Osteoporosis: Etiology; Diagnosis and Management; Riggs, B.L., Melton, L.J., Eds.; Raven Press: New York, NY, USA, 1988; pp. 133–155. [Google Scholar]
- Vicente-Rodríguez, G.; Ezquerra, J.; Mesana, M.; Fernández-Alvira, J.; ReyLópez, J.; Casajus, J.; Moreno, L. Independent and combined effect of nutrition and exercise on bone mass development. J. Bone Miner. Metab. 2008, 26, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.D.; Einhorn, T.A. Bisphosphonates in orthopaedic surgery. J. Bone Jt. Surg. Am. 2005, 87, 1609–1618. [Google Scholar] [CrossRef]
- Patel, S. Current and potential future drug treatments for osteoporosis. Ann. Rheum Dis. 1996, 55, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Coleman, R.E. Bisphosphonates as treatment of bone metastases. Curr. Pharm. Des. 2010, 16, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, R. Ibandronate provides efficacy and safety in the treatment of metastatic bone disease. Eur. J. Cancer Suppl. 2006, 4, 13–18. [Google Scholar] [CrossRef]
- Barret-Lee, P.; Casbard, A.; Abraham, J.; Hood, K.; Coleman, R.; Simmonds, P.; Timmins, H.; Wheatley, D.; Grieve, R.; Griffiths, G.; et al. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: A randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014, 15, 114–122. [Google Scholar] [CrossRef]
- Seton, M.; Krane, S.M. Use of zoledronic acid in the treatment of Paget’s disease. Ther. Clin. Risk Manag. 2007, 3, 913–918. [Google Scholar] [PubMed]
- Pazianas, M.; Abrahamsen, B.; Ferrari, S.; Russell, R.G.G. Eliminating the need for fasting with oral administration of bisphosphonates. Ther. Clin. Risk Manag. 2013, 9, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; Stone, M.; Rawlinson, F. Bisphosphonates and the treatment of bone disease in the elderly. Drugs Aging 1996, 8, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Gómez, V.; Xiao, S.Y. Alendronate-induced esophagitis in an elderly woman. Int. J. Clin. Exp. Pathol. 2009, 2, 200–203. [Google Scholar] [PubMed]
- Niemi, R.; Vepsalainen, J.; Taipale, H.; Jarvinen, T. Bisphonatesprodrugs: Synthesis and an in vitro evaluation of novel acyloxylated esters of clodronic acid. J. Med. Chem. 1999, 42, 5053–5085. [Google Scholar] [CrossRef] [PubMed]
- Niemi, R.; Vepsalainen, J.; Taipale, H.; Jarvinen, T. Bisphosphonates prodrugs: Synthesis and in vitro evaluation of alkyl and acyloxy esters of etidronic acid as bioreversible prodrugs of etidronate. Eur. J. Pharm. Sci. 2000, 11, 173–180. [Google Scholar] [CrossRef]
- Boulenc, X.; Breul, T.; Gautier, J.C.; Saudemon, P.; Joyeux, H.; Roques, C.; Berger, Y.; Fabre, G. Sodium lauryl sulphate increases tiludronateparacellular transport using epithelial Caco-2 monolayers. Int. J. Pharm. 1995, 123, 71–83. [Google Scholar] [CrossRef]
- Lindmark, T.; Kimura, Y.; Artursson, P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J. Pharmacol. Exp. Ther. 1998, 284, 362–369. [Google Scholar] [PubMed]
- Raiman, J.; Törmälehto, S.; Yritys, K.; Junginger, H.E.; Mönkkönen, J. Effect of various absorption enhancer on transport of clodronate through Caco-2 cells. Int. J. Pharm. 2003, 261, 129–136. [Google Scholar] [CrossRef]
- Low, S.A.; Kopeček, J. Targeting polymer therapeutics to bone. Adv. Drug Deliv. Rev. 2012, 64, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Wysowski, D.K.; Chang, J.T. Alendronate and risedronate: Reports of severe bone, joint, and muscle pain. Arch. Intern. Med. 2005, 165, 346–347. [Google Scholar] [PubMed]
- Penning-Van Beest, F.J.; Goettsch, W.G.; Erkens, J.A.; Herings, R.M. Determinants of persistence with bisphosphonates: A study in women with postmenopausal osteoporosis. Clin. Ther. 2006, 28, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, H.; Ohno, S.; Hattori, H.; Ishigatsubo, Y. Persistence with bisphosphonate therapy including treatment courses with multiple sequential bisphosphonates in the real world. Osteoporos. Int. 2007, 18, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 2009, 4, e6562. [Google Scholar] [CrossRef] [PubMed]
- Perugini, P.; Genta, I.; Conti, B.; Modena, T.; Pavanetto, F. Long-term release of clodronate from biodegradable microspheres. AAPS PharmSciTech 2001, 2, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Tahri, A.; Belbaraka, R. Bisphosphonates and innovative drugs in the prevention of skeletal complications secondary to metastatic prostate cancer. Clin. Cancer Investig. J. 2013, 2, 294–297. [Google Scholar] [CrossRef]
- Rogers, T.L.; Holen, I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 2011, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Happerfield, L.C.; Bobrow, L.G.; Millis, R.R. Angiogenesis and inflammation in invasive carcinoma of the breast. J. Clin. Pathol. 1997, 50, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996, 97, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Stresing, V.; Daubiné, F.; Benzaid, I.; Mönkkönen, H.; Clézardin, P. Bisphosphonates in cancer therapy. Cancer Lett. 2007, 257, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.C.; Coleman, R.E. Bisphosphonates in breast cancer: Teaching old dog new tricks. Curr. Opin. Oncol. 2009, 21, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Gyorgi, E.; Oner, E.T. Lasers in Materials Science; Castillejo, M., Ossi, P.M., Zhigilei, L., Eds.; Springer: Cham, Switzerland, 2011. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: From bench to bedside. Ann. N. Y. Acad. Sci. 2006, 1068, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyldiphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar] [PubMed]
- Cakarer, S.; Selvi, F.; Keskin, C. Bisphosphonates and Bone. Available online: http://www.intechopen.com/books/orthopedic-surgery/bisphosphonates-and-bone (accessed on 15 July 2016).
- Russell, R.G.G. Determinants of structure-function relationships among bisphosphonates. Bone 2007, 40, S21–S25. [Google Scholar] [CrossRef]
- Naidu, A.; Dechow, P.C.; Spears, R.; Wright, J.M.; Kessler, H.P.; Opperman, L.A. The effects of bisphosphonates on osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. 2008, 106, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Diel, I.J.; Fogelman, I.; Al-Nawas, B.; Hoffmeister, B.; Migliorati, C.; Gligorov, J.; Väänänen, K.; Pylkkänen, L.; Pecherstorfer, M.; Aapro, M.S. Pathophysiology risk factors and management of bisphosphonate-associated osteonecrosis of the jaw: Is there a diverse relationship of amino and non-amino bisphosphonates. Crit. Rev. Oncol. Hematol. 2007, 64, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Schindeler, A.; Peacock, L.; Mikulec, K.; Baldock, P.A.; Ruys, A.J.; Little, D.G. In vivo local co-delivery of recombinant human bone morphogenetic protein-7 and pamidronate via poly-d,l-lactic acid. Eur. Cells Mater. 2010, 20, 431–442. [Google Scholar] [CrossRef]
- Green, J.R. Zoledronic acid: Pharmacologic profile of a potent bisphosphonate. J. Organomet. Chem. 2005, 690, 2439–2448. [Google Scholar] [CrossRef]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent posttranslational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Coxon, F.P.; Helfrich, M.H.; Van’t Hof, R.; Sebti, S.; Ralston, S.H.; Hamilton, A.; Rogers, M.J. Protein geranylgeranylation is required for osteoclast formation, function, and survival: Inhibition by bisphosphonates and GGTI-298. J. Bone Miner. Res. 2000, 15, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Mönkkönen, J.; Blackburn, G.; Russell, R.; Rogers, M. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(β,γ-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Bisphosphonates: Mechanisms of action. Endocr. Rev. 1998, 19, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Von Knoch, F.; Jaquiery, C.; Kowalsky, M.; Schaeren, S.; Alabre, C.; Martin, I.; Rubash, H.E.; Shanbhag, A.S. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 2005, 26, 6941–6949. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 18, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, G.G.; Getz, B.; Pederson, L.; Sanders, E.S.; Subramaniam, M.; Ingle, J.N.; Spelsberg, T.C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60, 6001–6007. [Google Scholar] [PubMed]
- Delmas, P.D.; Meunier, P.J. The management of Paget’s disease of bone. N. Engl. J. Med. 1997, 336, 558–566. [Google Scholar] [PubMed]
- Kanis, J.A. Rationale for the use of bisphosphonates in breast cancer. Acta Oncol. 1996, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Bisphosphonates: Preclinical aspects and use in osteoporosis. Ann. Med. 1997, 29, 55–62. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General; US Department of Health & Human Services, Office of the Surgeon General: Rockville, MD, USA, 2004.
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Olszynki, W.; Davison, K. Alendronate for the treatment of osteoporosis in men. Expert Opin. Phamacother. 2008, 9, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.; Ettinger, M.; Weiss, S.; Miller, P.; Kendler, D.; Graham, J.; Adami, S.; Weber, K.; Lorenc, R.; Pietschmann, P. Alendronate for the treatment of osteoporosis in men. N. Engl. J. Med. 2000, 343, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Career Corner Stone Centre. Orthopedic Surgery. Available online: http://www.careercornerstone.org/physsurg/physsurgareas.htm (accessed on 18 October 2016).
- Aggarwal, S.; Gahlot, N.; Saini, U.C.; Dhillon, M.S. Bisphosphonate in orthopedics: Evidence based review of indications and adverse effects. J. Postgrad. Med. Educ. Res. 2016, 50, 75–85. [Google Scholar] [CrossRef]
- Lozano-Calderon, S.A.; Colman, M.W.; Raskin, K.A.; Hornicek, F.J.; Gebhardt, M. Bisphosphonates in Orthopedic Surgery: Pearls and Pitfalls. Orthop. Clin. N. Am. 2014, 45, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Arthritis Research UK. Paget’s disease of bone. Available online: https://www.arthritisresearchuk.org (accessed on 13 June 2016).
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, A. Management of bone metastases. Can. Fam. Physician 2008, 54, 524–527. [Google Scholar] [PubMed]
- Coleman, R.E. Bisphosphonates: Clinical experience. Oncologist 2004, 9, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A. Pathophysiology of bone metastases: How this knowledge may lead to therapeutic intervention. J. Support. Oncol. 2004, 2, 205–220. [Google Scholar] [PubMed]
- Zhu, M.; Liang, R.; Pan, L.H.; Huang, B.; Qian, W.; Zhong, J.H.; Zheng, W.W.; Li, C.L. Zoledronate for metastatic bone disease and pain: A meta-analysis of randomized clinical trials. Pain Med. 2013, 14, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Stockler, M.R.; Pavlakis, N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2012, 2, CD003474. [Google Scholar]
- Lopez-Olivo, M.A.; Shah, N.A.; Pratt, G.; Risser, J.M.; Symanski, E.; Suarez-Almazor, M.E. Bisphosphonates in the treatment of patients with lung cancer and metastatic bone disease: A systematic review and meta-analysis. Support. Care Cancer 2012, 20, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Redzepovic, J.; Wheatley, K.; Clark, O.A.C.; Miladinovic, B.; Glasmacher, A.; Kumar, A.; Djulbegovic, B. Bisphosphonates in multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2012, 5, CD003188. [Google Scholar]
- Tanvetyanon, T.; Stiff, P.J. Management of the adverse effects associated with intravenous bisphosphonates. Ann. Oncol. 2006, 17, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Intranasal Drug Delivery. Available online: http://intranasal.net/overview/default.htm (accessed on 15 November 2016).
- Cruz, L.; Fattal, E.; Tasso, L.; Freitas, G.C.; Carregaro, A.B.; Guterres, S.S.; Pohlmann, A.R.; Tsapis, N. Formulation and in vivo evaluation of sodium alendronate spray-dried microparticles intended for lung delivery. J. Control. Release 2011, 152, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.C.; Engle, K.; Fix, J.A. Intranasal delivery of the bisphosphonate alendronate in the rat and dog. Pharm. Res. 1993, 10, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [PubMed]
- Ueno, M.; Maeno, T.; Nishimura, S.; Ogata, F.; Masubuchi, H.; Hara, K.; Yamaguchi, K.; Aoki, F.; Suga, T.; Nagai, R.; et al. Alendronate inhalation ameliorates elastase induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat. Commun. 2016, 6, 6332. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Nakatani, M.; Sano, J.I.; Abe, M.; Kusamori, K.; Kurihara, M.; Shiota, R.; Takashima, M.; Fujita, T.; Sakane, T.; et al. Absorption and safety of alendronate, a nitrogen-containing bisphosphonate, after intrapulmonary administration in rats. Int. J. Pharm. 2010, 400, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Takashima, M.; Sano, J.I.; Nishiyama, K.; Kitamura, N.; Sakane, T.; Hibi, T.; Yamamoto, A. Development of polyethylene glycol-conjugated alendronate, a novel nitrogen-containing bisphosphonate derivative: Evaluation of absorption, safety, and effects after intrapulmonary administration in rats. J. Pharm. Sci. 2011, 100, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Bhatnager, A.; Sultana, S.; Ahmad, F.J.; Mittal, G.; Talegaonkar, S.; Sultana, S.; Singh, T.; Neeraj, K.; Rashid, A. Process for Preparation of Nanosize Bisphosphonate Particles. Indian Patent 270,668, 8 January 2016. [Google Scholar]
- Sahoo, C.K.; Nayak, P.K.; Sarangi, D.K.; Sahoo, T.K. Intra vaginal drug delivery system: An overview. Am. J. Adv. Drug Deliv. 2013, 1, 43–55. [Google Scholar]
- Harrison, D.C.; Liu, J.H.; Pauletti, G.M.; Ritschel, W.A. Vaginal Delivery of Bisphosphonates. U.S. Patent 6,572,874 B1, 3 June 2003. [Google Scholar]
- Clendening, C.E.; Pauletti, G.M. Improved Formulations for Transmucosal Vaginal Delivery of Bisphosphonates. U.S. Patent 6,905,701, 4 November 2004. [Google Scholar]
- Ilem-Ozdemir, D.; Asikoglu, M.; Guneri, T.; Koseoglu, K.; Ozkilic, H. Evaluation bone uptake of alendronate sodium via vaginal route by gamma scintigraphy, vaginal uptake of alendronate sodium. J. Drug Deliv. Sci. Technol. 2014, 24, 218–221. [Google Scholar] [CrossRef]
- Pauletti, G.M.; Loveland, O.; Clendening, C.E.; Cleves, O. Formulations for Transmucosal Vaginal Delivery of Bisphosphonates. U.S. Patent 20,040,005,345 A1, 8 January 2004. [Google Scholar]
- İlem-Özdemir, D.; Köseoğlu, K.; Aşıkoğlu, M.; Özkılıç, H.; Güneri, T. Comparative bone uptake study of alendronate sodium from vaginal suppositories prepared with polyethylene glycol and massa estarinum bases. Marmara Pharm. J. 2013, 17, 165–169. [Google Scholar] [CrossRef]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release 2012, 164, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.Y.; Wu, C.T.; Chen, J.Z.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases andbone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Giger, E.V.; Castagner, B.; Leroux, J.C. Biomedical applications of bisphosphonates. J. Control. Release 2013, 167, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S. Controlled release scaffolds for bone tissue engineering. J. Pharm. Sci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Roussière, H.; Fayon, F.; Alonso, B.; Rouillon, T.; Schnitzler, V.; Verron, E.; Guicheux, J.; Petit, M.; Massiot, D.; Janvier, P.; et al. Reaction of zoledronate with β-tricalcium phosphate for the design of potential drug device combined systems. Chem. Mater. 2008, 20, 182–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Dusad, A.; Ren, K. Drug delivery strategies for treating osteoporosis. Orthop. Muscular. Syst. 2014, 3, 1–4. [Google Scholar]
- Ezra, A.; Golomb, G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv. Drug Deliv. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Seshima, H.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Inoue, T.; Oda, Y. Control of bisphosphonate release using hydroxyapatite granules. J. Biomed. Mater. Res. B 2006, 78, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Shi, X.; Varshney, R.; Wang, D. Transplantable delivery systems for in situ controlled release of bisphosphonate in orthopedic therapy. Expert Opin. Drug Deliv. 2011, 8, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Josse, S.; Faucheux, C.; Soueidan, A.; Grimandi, G.; Massiot, D.; Alonso, B.; Janvier, P.; Laib, S.; Pilet, P.; Gauthier, O.D.G.; et al. Novel biomaterials for bisphosphonate delivery. Biomaterials 2005, 26, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Karrholm, J.; Borssen, B.; Lowenhielm, G.; Snorrason, F. Does early micromotion of femoral stem prostheses matter? 4–7-Year stereoradiographic follow-up of 84 cemented prostheses. J. Bone Jt. Surg. Br. 1994, 76, 912–917. [Google Scholar]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Auriola, S.; Mönkkönen, J.; Määttä, J. Liposome encapsulated zoledronate favours M1-like behaviour in murine macrophages cultured with soluble factors from breast cancer cells. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011, 49, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Gutman, D.; Golomb, G. Liposomal alendronate for the treatment of restenosis. J. Control. Release. 2012, 161, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zeisberger, S.M.; Odermatt, B.; Marty, C.; Zehnder-Fjallman, A.H.M.; Ballmer-Hofer, K.; Schwendener, R.A. Clodronate-liposome-mediated depletion of tumour associated macrophages: A new and highly effective antiangiogenic therapy approach. Br. J. Cancer 2006, 95, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Marra, M.; Porru, M.; Zappavigna, S.; Abbruzzese, A.; la Rotonda, M.I.; Leonetti, C.; Caraglia, M.; de Rosa, G. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int. J. Pharm. 2011, 403, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Shmeeda, H.; Amitay, Y.; Gorin, J.; Tzemach, D.; Mak, L.; Ogorka, J.; Kumar, S.; Zhang, J.A.; Gabizon, A. Delivery of zoledronic acid encapsulated in folate-targeted liposome results in potent in vitro cytotoxic activity on tumor cells. J. Control. Release 2010, 146, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Neuse, E.W. Synthetic polymers as drug delivery vehicles in medicine. Metal Based Drugs 2008. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Mukaya, H.E. Polymer-therapeutics: Design, application and pharmacokinetics. In Nanoarchitectonics for Smart Delivery and Drug Deliver; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: London, UK, 2016. [Google Scholar]
- Kolmas, J.; Sobczak, M.; Olędzka, E.; Nałęcz-Jawecki, G.; Dębek, C. Synthesis, characterization and in vitro evaluation of new composite bisphosphonate delivery systems. Int. J. Mol. Sci. 2014, 15, 16831–16847. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Park, K. Biomimetic polymers for in vivo drug delivery. In Bioinspired and Biomimetic Polymer Systems for Drug and Gene Delivery; Gu, Z., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Seyfoori, M.R.; Koshkaki, K.; Majidzadeh, A. Nanohybrid stimuli-responsive microgels: A new approach in cancer therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: London, UK, 2016. [Google Scholar]
- Aderibigbe, B.; Sadiku, E.; Jayaramudu, J.; Sinha Ray, S. Controlled dual release study of curcumin and a 4-aminoquinoline analog from gum acacia containing hydrogels. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Paolino, D.; Licciardi, M.; Celia, C.; Giammona, G.; Fresta, M.; Cavallaro, G. Bisphosphonate–polyaspartamide conjugates as bone targeted drug delivery systems. J. Mater. Chem. B 2015, 3, 250–259. [Google Scholar] [CrossRef]
- Cenni, E.; Avnet, S.; Granchi, D.; Fotia, C.; Salerno, M.; Micieli, D.; Sarpietro, M.G.; Pignatello, R.; Castelli, F.; Baldini, N. The effect of poly(d,l-lactide-co-glycolide)-alendronate conjugate nanoparticles on human osteoclast precursors. J. Biomater. Sci. Polym. Ed. 2012, 23, 1285–1300. [Google Scholar] [PubMed]
- Pan, H.; Sima, M.; Kopečková, P.; Wu, K.; Gao, S.; Liu, J.; Wang, D.; Miller, S.C.; Kopecek, J. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl) methacrylamide copolymer–alendronate conjugates. Mol. Pharm. 2008, 25, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Satchi-Fainaro, R.; Miller, K.; Shabat, D.; Erez, R.; Ramot At Tel-Aviv University Ltd. Conjugates of a Polymer, a Bisphosphonate and an Anti-Angiogenesis Agent and Uses thereof in the Treatment and Monitoring of Bone Related Diseases. U.S. Patent 9,095,618, 26 November 2015. [Google Scholar]
- Hrubý, M.; Etrych, T.; Kučka, J.; Forsterová, M.; Ulbrich, K. Hydroxybisphosphonate-containing polymeric drug-delivery systems designed for targeting into bone tissue. J. Appl. Polym. Sci. 2006, 101, 3192–3201. [Google Scholar] [CrossRef]
- Wang, D.; Miller, S.; Kopeckova, P.; Kopecek, J.; University of Utah Research Foundation. Water-Soluble Polymeric Bone-Targeting Drug Delivery System. U.S. Patent 11150,865, 29 December 2005. [Google Scholar]
- Wang, D.; Li, W.; Pechar, M.; Kopečková, P.; Brömme, D.; Kopeček, J.; Cathepsin, K. Inhibitor–polymer conjugates: Potential drugs for the treatment of osteoporosis and rheumatoid arthritis. Int. J. Pharm. 2004, 277, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Merrill, E.W. PVA Hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 441–457. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.; Cui, S.W.; Wang, Q. Polysaccharide gums: Structures, functional properties, and applications. Available online: http://uqu.edu.sa/files2/tiny_mce/plugins/filemanager/files/4300270/1/2/1574_C006.pdf (accessed on 20 June 2013).
- Aderibigbe, B.A.; Varaprasad, K.; Sadiku, E.R.; Ray, S.S.; Mbianda, X.Y.; Fotsing, M.C.; Owonubi, S.J.; Agwuncha, S.C. Kinetic release studies of nitrogen-containing bisphosphonate from gum acacia crosslinked hydrogels. Int. J. Biolmacromol. 2015, 73, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hulsart-Billström, G.; Yuen, P.K.; Marsell, R.; Hilborn, J.; Larsson, S.; Ossipov, D. Bisphosphonate-linked hyaluronic acid hydrogel sequesters and enzymatically releases active bone morphogenetic protein-2 for induction of osteogenic differentiation. Biomacromolecules 2013, 14, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Mahapatra, D.R. Photonic hydrogel beads for controlled release of risedronate. SPIE BiOS 2014. [Google Scholar] [CrossRef]
- Kettenberger, U.; Luginbuehl, V.; Procter, P.; Pioletti, D.P. In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Posadowska, U.; Parizek, M.; Filova, E.; Wlodarczyk-Biegun, M.; Kamperman, M.; Bacakova, L.; Pamula, E. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. 2015, 485, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kootala, S.; Zhang, Y.; Ghalib, S.; Tolmachev, V.; Hilborn, J.; Ossipov, D.A. Control of growth factor binding and release in bisphosphonate functionalized hydrogels guides rapid differentiation of precursor cells in vitro. Biomater. Sci. 2016, 4, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Schüssele, A. Drug Delivery to the Bone-Implant Interface: Functional Hydroxyapatite Surfaces and Particles. Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2006. [Google Scholar]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O.C. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef]
- Joschek, S.; Nies, B.; Krotz, R.; Gopferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Linderbäck, P. Improved Titanium and Steel Implants: Studies on Bisphosphonate, Strontium and Surface Treatments. Master’s Thesis, Linköping University, Linköping, Sweden, 2011. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Buchanan, J.M. 16 Year Review of Hydroxyapatite Ceramic Coated Hip Implants—A Clinical and Histological Evaluation. In Key Engineering Materials; Trans Tech Publications: Heidelberg, Germany, 2005. [Google Scholar]
- Puleo, D.A. Bone-implant interface. In Encyclopedia of Biomaterials and Biomedical Engineering; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Triffitt, J. Osteogenic stem cells and orthopedic engineering: Summary and update. J. Biomed. Mater. Res. 2002, 63, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Rose, F.R.; Oreffo, R.O.C. Bone tissue engineering: Hope vs. hype. Biochem. Biophys. Res. Commun. 2002, 292, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regi, M. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J. Am. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Mbianda, X.Y. Bisphosphonates: Polymer-linked. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor and Francis: London, UK, 2015. [Google Scholar]
- Denissen, H.; van Beek, E.; Löwik, C.; Papapoulos, S.; van den Hooff, A. Ceramic hydroxyapatite implants for the release of bisphosphonate. Bone Miner. 1994, 25, 123–134. [Google Scholar] [CrossRef]
- Sörensen, T.C.; Arnoldi, J.; Procter, P.; Robioneck, B.; Steckel, H. Bone substitute materials delivering zoledronic acid: Physicochemical characterization, drug load, and release properties. J. Biomater. Appl. 2013, 27, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Muller-Schiffmann, A.; Sticht, H.; Korth, C. Hybrid compounds: From simple combinations to nanomachines. Biodrugs 2012, 26, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Lane, N.E. Target delivery of mesenchymal stem cells to bone. Bone 2015, 70, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Bekker, K.S.; Chukanov, N.V.; Grigor’ev, I.A. Synthesis of a bisphosphonate derivative of folic acid. Chem. Nat. Compd. 2013, 49, 495. [Google Scholar] [CrossRef]
- Yang, X.N.; Zeng, J.C.; Song, Y.C.; Zhang, H.; Pei, F.X. Targeted Antiosteosarcoma methotrexate-bisphosphonate conjugate induces apoptosis of osteosarcoma cells in vitro. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2116–2123. [Google Scholar] [PubMed]
- El-Mabhouh, A.A.; Nation, P.N.; Abele, J.T.; Riauka, T.; Postema, E.; McEwan, A.J.B.; Mercer, J.R. A conjugate of gemcitabine with bisphosphonate (Gem/BP) shows potential as a targeted bone-specific therapeutic agent in an animal model of human breast cancer bone metastases. Oncol. Res. 2011, 19, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Nakatake, H.; Ekimoto, H.; Aso, M.; Ogawa, A.; Yamaguchi, A.; Suemune, H. Dialkyl bisphosphonate platinum(II) complex as a potential drug for metastatic bone tumor. Chem. Pham. Bull. 2011, 59, 710–713. [Google Scholar] [CrossRef]
- Liu, Z.; Robinsonb, J.T.; Tabakmanb, S.M.; Yanga, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Singh, P. Recent advances of multifunctional nanomedicine. In Nanomedicine for Drug Delivery and Therapeutics; Mishra, A.K., Ed.; Wiley Scrivener: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Singh, B.G.P.; Baburao, C.; Pispati, V.; Pathipati, H.; Muthy, N.; Prassana, S.R.V.; Rathode, B.G. Carbon nanotubes. A novel drug delivery system. Int. J. Res. Pharm. Chem. 2012, 2, 523–532. [Google Scholar]
- Usui, Y.; Haniu, H.; Tsuruoka, S.; Saito, N. Carbon nanotubes innovate on medical technology. Med. Chem. 2012, 2. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555–577. [Google Scholar] [CrossRef] [PubMed]
- Kateb, B.; Yamamoto, V.; Alizadeh, D.; Zhang, L.; Manohara, H.M.; Bronikowski, M.J.; Badie, B. Multiwalled carbon nanotube (MWCNT) synthesis, preparation, labeling, and functionalization. Methods Mol. Biol. 2010, 651, 307–317. [Google Scholar] [PubMed]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.L.; Mbianda, X.Y.; Szucs, Z.; Zeervaart, J. Synthesis and characterization of bisphosphonate conjugated carbon nanomaterials as potential treatment of secondary bone cancer. Eur. Cells Mater. 2010, 19, 21. [Google Scholar]
- Gonzalez, K.A.; Wilson, L.J.; Wub, W.; Nancollas, G.H. Synthesis and in vitro characterization of a tissue-selective fullerene: Vectoring C60(OH)16AMBPto mineralized bone. Bioorg. Med. Chem. 2002, 10, 1991–1997. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Foley, S.; Crowley, C.; Smaihi, M.; Bonfils, C.; Erlanger, B.F.; Seta, P.; Larroque, C. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002, 294, 116–119. [Google Scholar] [CrossRef]
- Fenske, D.B. Structural and motional properties of vesicles as revealed by nuclear magnetic resonance. Chem. Phys. Lipids 1993, 64, 143–162. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Kesrevani, R.K.; Sharma, A.K. Nanoarchitectured biomaterials: present status and future prospects in drug delivery. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Elsevier B.V.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Amarnath, S.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Huang, L. Antibody-directed liposomes as drug-delivery vehicles. Adv. Drug Deliv. Rev. 1989, 3, 343–389. [Google Scholar] [CrossRef]
- Lasic, D.D. Liposomes: From Physics to Applications; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Romberg, B.; Metselaar, J.M.; de Vringer, T.; Motonage, K.; Bosch, J.J.K.; Oussoren, C.; Storm, G.; Hennink, W.E. Enzymatic degradation of liposomes-grafted poly(hydroxyethyl l-glutamine. Bioconj. Chem. 2006, 17, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Takeda, Y.; Honda, Y.; Sakurai, K.; Suzuki, O. Synthesis of calcium phosphate-binding liposome for drug delivery. Bioorg. Med. Chem. Lett. 2009, 19, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
- Hengst, V.; Oussoren, C.; Kissel, T.; Storm, G. Bone targeting potential of bisphosphonate-targeted liposomes: Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharm. 2007, 331, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Golomb, G. Nanoparticulate delivery system for the therapy of restenosis. In Proceedings of the 15th International Symposium on Microencapsulation, Parma, Italy, 18–21 September 2005.
- Van Rooijen, N.; Van Nieuwnegen, R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome encapsulated dichloromethylene-diphosphonate. An enzyme histochemical study. Cell Tissue Res. 1984, 238, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Barenholz, Y.; Shmeeda, H. Liposomes Co-Encapsulating a Bisphosphonate and an Amphipathic Agent. U.S. Patent 20,140,328,899, 17 January 2013. [Google Scholar]
- Biswas, S.; Vase, O.S.; Movassaghian, S.; Torchilin, V.P. Polymeric micelles for the delivery of poorly soluble drugs. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Douroumis, D., Fahr, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nishiyama, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Cisplatin loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm. Res. 2001, 18, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Okazaki, S.; Cabral, H.; Miyamoto, M.; Kato, Y.; Sugiyama, Y.; Nishio, K.; Matsumura, Y.; Kataoka, K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003, 63, 8977–8983. [Google Scholar] [PubMed]
- Zhang, L.; Eisenberg, A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, M.H.; Fournier, E.; Jones, M.C.; Ranger, M.; Leroux, J.C. Block copolymer micelles-engineering versatile carriers for drugs and biomacromolecules. Bull. Tech. Gattefossé 2003, 96, 87–102. [Google Scholar]
- Gaucher, G.; Dufresne, M.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J. Block copolymer micelles: Preparation, characterization and application in drug delivery. J Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, Y.; Fan, W. Exploring polymeric micelles for improved delivery of anticancer agents: Recent developments in preclinical studies. Pharmaceutics 2013, 5, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludağ, H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J. Biomed. Mater. Res. A 2012, 100, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.L.; Zhao, Y.P.; Li, H.Q.; Na, R.; Li, F.; Mei, Q.B.; Zhao, M.G.; Zhou, S.Y. Doxorubicin-poly(ethylene glycol)-alendronate self-assembled micelles for targeted therapy of bone metastatic cancer. Sci. Rep. 2015, 5, 14614. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Clementi, C.; Polyak, D.; Eldar-Boock, A.; Benayoun, L.; Barshack, I.; Shaked, Y.; Pasut, G.; Satchi-Fainaro, R. Poly(ethylene glycol)–paclitaxel–alendronate self-assembled micelles for the targeted treatment of breast cancer bone metastases. Biomaterials 2013, 34, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Hochdorffer, K.; Abu, A.K.; Schafer-Obodozie, C.; Kratz, F. Development of novel bisphosphonate prodrugs of doxorubicin for targeting bone metastases that are cleaved pH dependently or by cathepsin B: Synthesis, cleavage properties, and binding properties to hydroxyapatite as well as bone matrix. J. Med. Chem. 2012, 55, 7502–7515. [Google Scholar] [CrossRef] [PubMed]
- Klenner, T.; Wingen, F.; Kepper, B.K.; Krempien, B.; Schmahl, D. Anticancer-agent-linked phosphonates with antiosteolytic and antineoplastic properties: A promising perspective in the treatment of bone-related malignancies? J. Cancer Res. Clin. Oncol. 1990, 116, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Canal, F.; Lucas, R.; Vicent, M.J. Polymer-drug conjugates for novel molecular targets. Nanomedicine 2010, 5, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Baabur-Cohen, H.; Omer, L.; Satchi-Fainaro, R. Recent progress in polymer therapeutics as nanomedicines. In Handbook of Harnessing Biomaterials in Nanomedicine: Preparation, Toxicity and Applications; Peer, D., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ferreira Ddos, S.; Boratto, F.A.; Cardoso, V.N.; Serakides, R.; Fernandes, S.O.; Ferreira, L.A.; Oliveira, M.C. Alendronate-coated long-circulating liposomes containing 99mtechnetium-ceftizoxime used to identify osteomyelitis. Int. J. Nanomed. 2015, 10, 2441–2450. [Google Scholar]
- Hench, L.L. Bioceramics. J. Am. Ceram Soc. 1998, 81, 1705–1727. [Google Scholar] [CrossRef]
- Cascone, M.G.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G.; Lazzeri, L. Bioartificial polymeric materials based on polysaccharides. J. Biomater. Sci. 2001, 12, 267–281. [Google Scholar] [CrossRef]

| Drug/Formulation | Carrier | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Neridronic | Polyamidoamine | - | Cancer | - | [120] |
| Bisphosphonates + curcumin | Polyamidoamine | - | Cancer | - | [120] |
| Bisphosphonates | Poly-hydroxy-aspartamide | - | Bone diseases | in vivo | [121] |
| Alendronate | poly(d,l-lactide-co-glycolide) (PLGA) | - | Bone diseases | in vitro | [122] |
| Alendronate | N-(2-hydroxypropyl) methacrylamide copolymer | Intravenous | Bone diseases | in vivo | [123] |
| Bisphosphonate | polyethylenglycol (PEG) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | polyglutamic acid (PGA) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | polylactic acid (PLA) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | polylactic-co-glycolic (PLGA) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | poly(lactide-co-glycolide) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | poly(d,l-lactide-co-glycolide) (PLA/PLGA) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | poly(hydroxyalkylmethaacrylamide) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | polyglycerol, a polyamidoamine (PAMAM) | Intravenous | Bone diseases | in vivo | [124] |
| Bisphosphonate | polyethylenimine (PEI) | Intravenous | Bone diseases | in vivo | [124] |
| Alendronate | poly[N-(2-hydroxypropyl) methacrylamide] | - | Bone diseases | in vitro | [125] |
| Drug/Formulation | Carrier | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Bisphosphonates | Acrylamide + gum acacia | - | Bone Treatment | - | [133] |
| Bisphosphonates | Hyaluronic acid hydrogel | - | Bone regeneration | in vitro | [134] |
| Risedronate sodium | Sodium alginate | - | Bone Treatment | - | [135] |
| Drug/Formulation | Bioceramic | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Alendronate | Mesoporous silica-based materials | - | Bone Treatment | - | [153] |
| Bisphosphonates | Hydroxyapatite (HA) | - | Bone graft substitute | in vitro | [154] |
| Zoledronic acid | HA | - | Bone graft substitute | in vitro | [156] |
| Zoledronic acid | Calcium phosphate (80% tricalcium phosphate, 20% HA) | - | Bone graft substitute | in vitro | [156] |
| Drug/Formulation | Administration | Intended Application | Status | References |
|---|---|---|---|---|
| LLP2A-Ale | Intravenous | Bone diseases | in vivo | [158] |
| Bisphosphonates + folic acid | - | Bone regeneration | in vitro | [159] |
| Bisphosphonate + Methotrexate | - | Osteosarcoma | in vitro | [160] |
| Bisphosphonate + gemcitabine | Intravenous | Bone metatases | in vivo | [161] |
| Bisphosphonate + platinum complexes | - | Bone Treatment | in vitro | [162] |
| Drug/Formulation | Delivery System | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Bisphosphonates | Carbon nanotubes | - | Osteosarcoma | - | [171] |
| Bisphosphonate-fullerenes C60(OH)16AMBP | Fullerene | - | Bone mineralization | in vitro | [172] |
| Drug/Formulation | Delivery System | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Bisphosphonates | Liposome | - | Anticancer | in vitro | [185] |
| Bisphosphonates | Liposome | Intravenous | Treatment of stenotic coronary disease | in vivo | [187] |
| Clodronate | Liposome | Intravenous | Treatment of the spleen | in vivo | [188] |
| Bisphosphonate + PLAD | Liposome | - | Anticancer | in vivo | [189] |
| Drug/Formulation | Delivery System | Administration | Intended Application | Status | References |
|---|---|---|---|---|---|
| Bisphosphonate (thiolBP) + distearoylphospho-ethanolamine-polyethylene glycol | Micelle | - | Bone tissue engineering | in vitro | [197] |
| Doxorubicin-poly (ethylene glycol)-alendronate | Micelle | - | Bone cancer | in vitro | [198] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.; Aderibigbe, I.; Popoola, P. Design and Biological Evaluation of Delivery Systems Containing Bisphosphonates. Pharmaceutics 2017, 9, 2. https://doi.org/10.3390/pharmaceutics9010002
Aderibigbe B, Aderibigbe I, Popoola P. Design and Biological Evaluation of Delivery Systems Containing Bisphosphonates. Pharmaceutics. 2017; 9(1):2. https://doi.org/10.3390/pharmaceutics9010002
Chicago/Turabian StyleAderibigbe, Blessing, Isiaka Aderibigbe, and Patricia Popoola. 2017. "Design and Biological Evaluation of Delivery Systems Containing Bisphosphonates" Pharmaceutics 9, no. 1: 2. https://doi.org/10.3390/pharmaceutics9010002
APA StyleAderibigbe, B., Aderibigbe, I., & Popoola, P. (2017). Design and Biological Evaluation of Delivery Systems Containing Bisphosphonates. Pharmaceutics, 9(1), 2. https://doi.org/10.3390/pharmaceutics9010002






