Development Strategies for Herbal Products Reducing the Influence of Natural Variance in Dry Mass on Tableting Properties and Tablet Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Liquid Echinacea Purpurea Extract
2.2.2. Preparation of the Granulate
| Dry mass in tincture (%) | Dry mass in concentrate (%) | Liquid amount (g) | Carrier amount (MCC) (g) | |
|---|---|---|---|---|
| Treatment 1: fixed concentration—fixed carrier standardisation | ||||
| A | 1.2 | 43.0 | 14.5 | 25.0 |
| B | 1.7 | 43.0 | 16.3 | 25.0 |
| C | 2.2 | 43.0 | 16.9 | 25.0 |
| D | 2.8 | 43.0 | 18.9 | 25.0 |
| Treatment 2: fixed concentration—fixed carrier with compensation standardisation | ||||
| E | 1.3 | 43.0 | 14.4 | 21.0 |
| F | 1.5 | 43.0 | 15.4 | 25.0 |
| G | 2.0 | 43.0 | 15.8 | 33.0 |
| H | 2.5 | 43.0 | 16.9 | 42.0 |
| Treatment 3: fixed volume—fixed carrier standardisation | ||||
| I | 1.3 | 32.0 | 15.0 | 25.0 |
| J | 1.5 | 37.5 | 14.2 | 25.0 |
| K | 2.0 | 50.0 | 12.2 | 25.0 |
| L | 2.5 | 62.5 | 10.2 | 25.0 |
| Treatment 4: fixed volume—fixed liquid to carrier ratio standardisation | ||||
| M | 1.3 | 32.0 | 16.4 | 27.3 |
| N | 1.5 | 37.5 | 15.3 | 25.5 |
| O | 2.0 | 50.0 | 14.7 | 24.5 |
| P | 2.5 | 62.5 | 13.5 | 22.5 |
2.2.3. Preparation of Tablets
2.2.4. Characterization of Granulate
2.2.4.1. Particle Size Distribution

2.2.4.2. Moisture Content
2.2.4.3. Bulk and Tapped Density

2.2.5. Tablets Characterization
2.2.5.1. Disintegration Time
2.2.5.2. Compaction Properties
2.3. Examining the Effect of Different Liquid to Carrier Ratios in the Granulation System
| Sample name | Liquid amount (g) | MCC (g) |
|---|---|---|
| 1 | 8.7 | 12.5 |
| 2 | 8.7 | 17.5 |
| 3 | 8.7 | 25 |
| 4 | 12.1 | 12.5 |
| 5 | 12.1 | 17.5 |
| 6 | 12.1 | 25 |
| 7 | 15.4 | 12.5 |
| 8 | 15.4 | 17.5 |
| 9 | 15.4 | 25 |
2.4. Concentration Settings Depending on the Dry Mass
2.4.1. Fixed Concentration Standardization (FCS)
2.4.1.1. Treatment (1) Fixed Carrier
2.4.1.2. Treatment (2) Fixed Carrier with Compensation
2.4.2. Fixed Volume Standardization (FVS)
2.4.2.1. Treatment (3) Fixed carrier
2.4.2.2. Treatment (4) Fixed liquid to carrier ratio
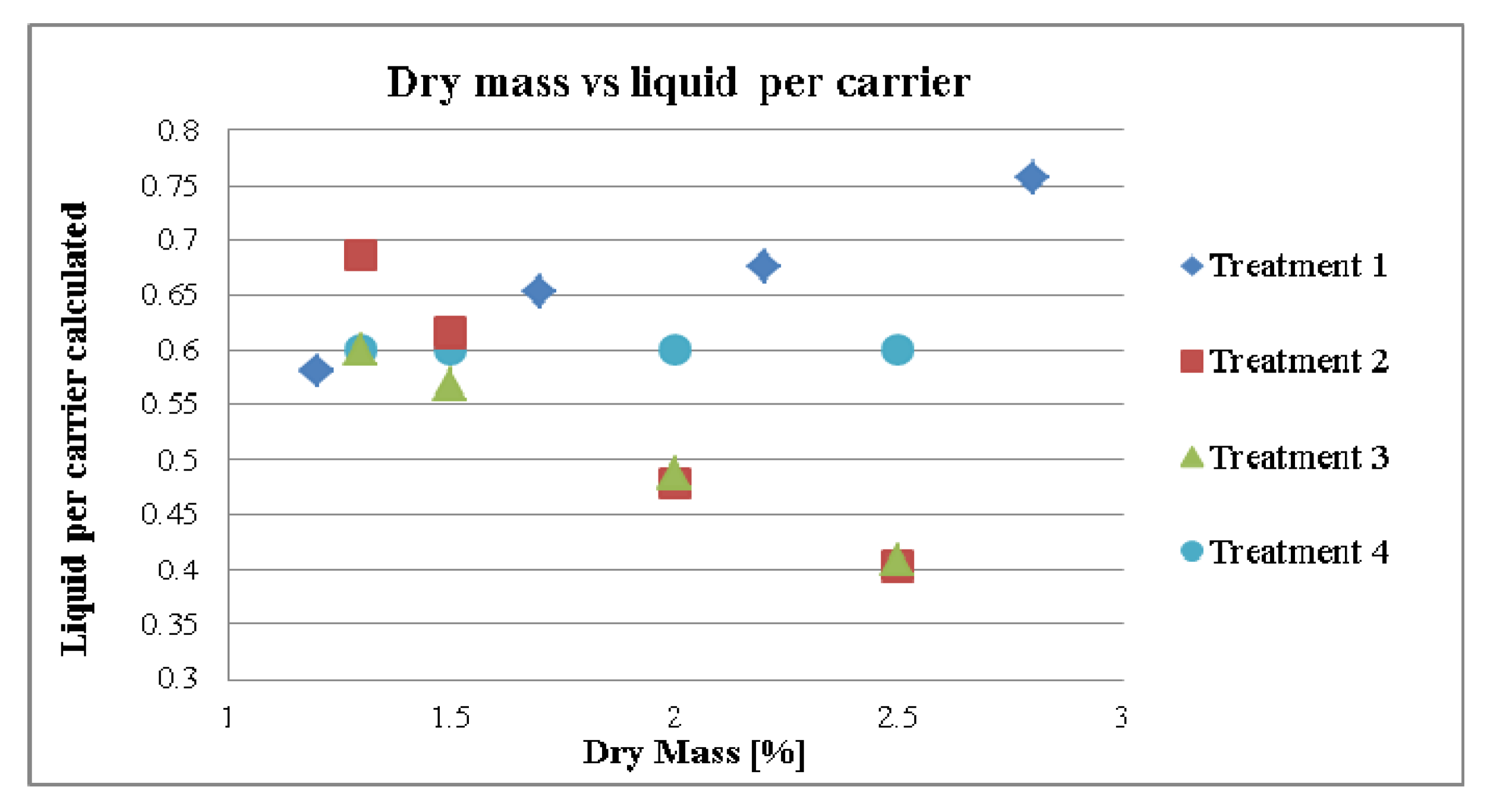
3. Results and Discussion
3.1. Influence of the Liquid per MCC on the Compaction Properties and Disintegration Time

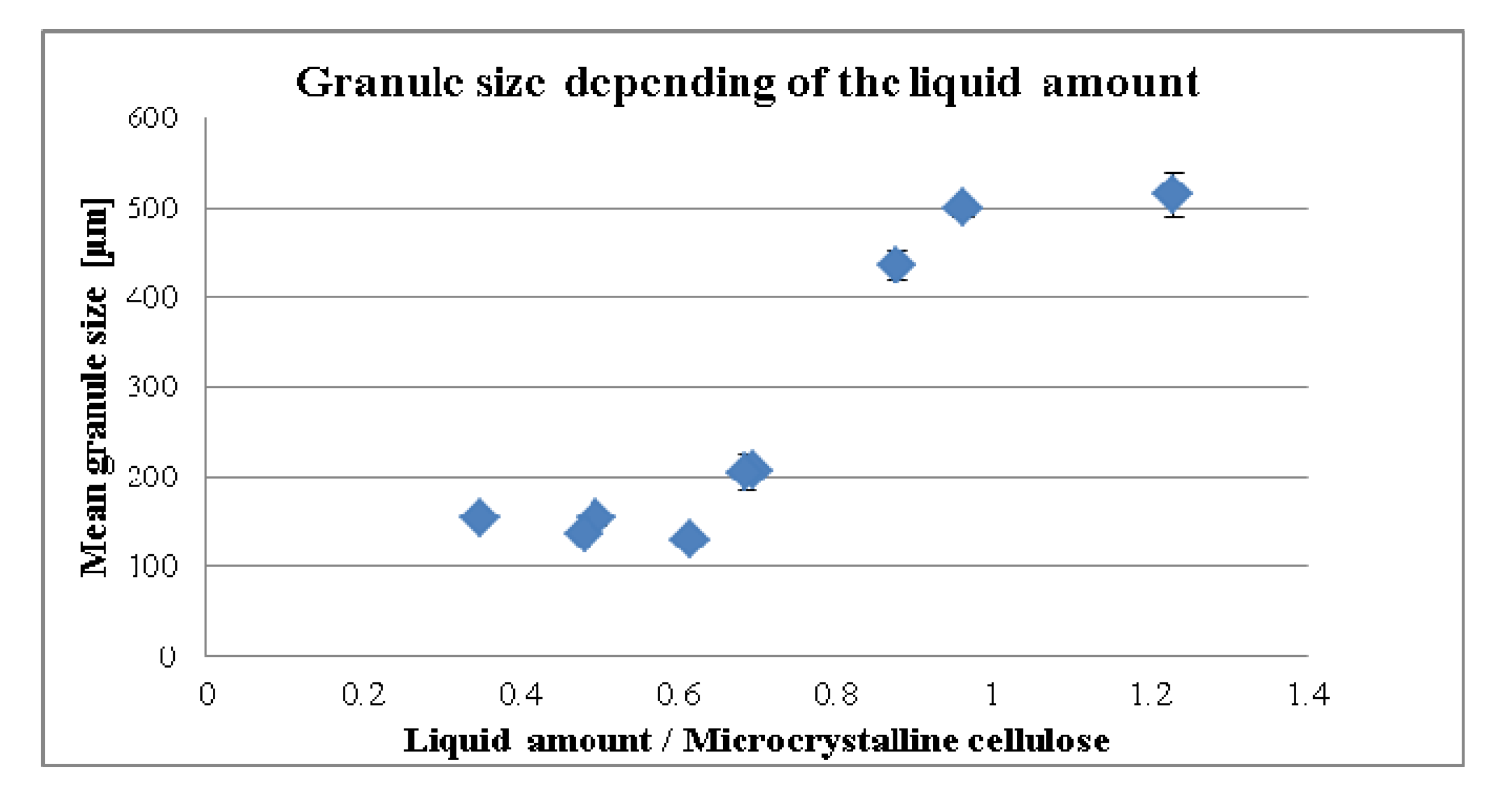
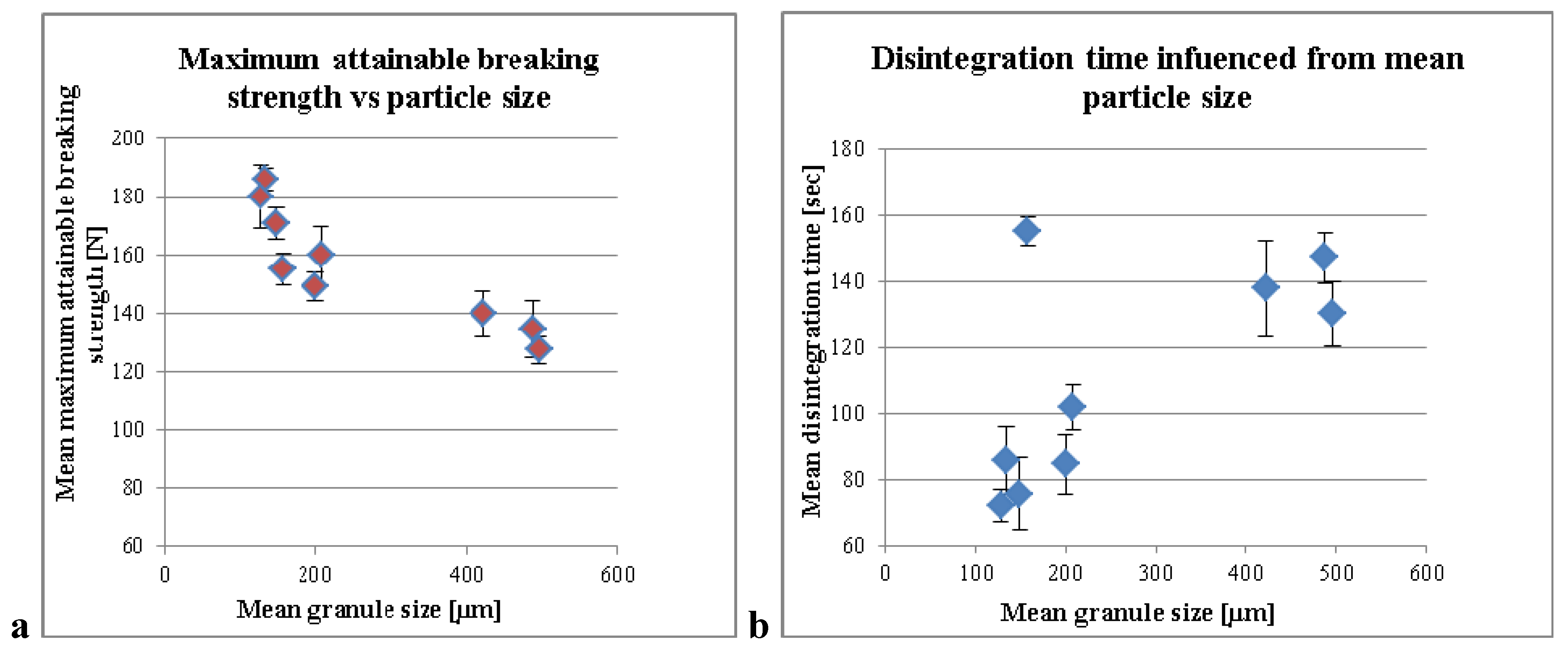
3.2. Standardization Strategies
3.2.1. Effect of Dry Mass of the Tincture on Granule size (MPSD) for Different Treatment Strategies
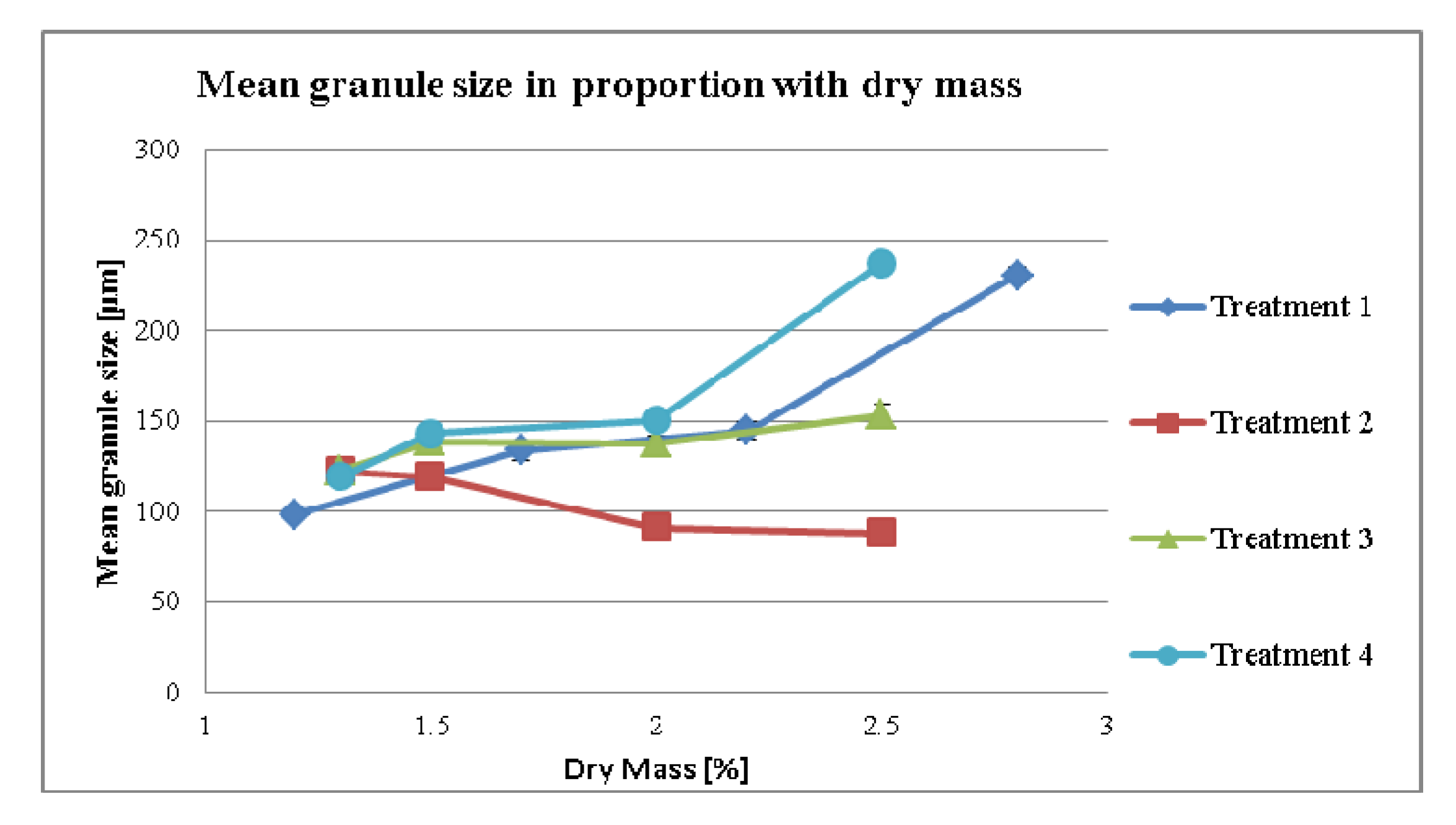
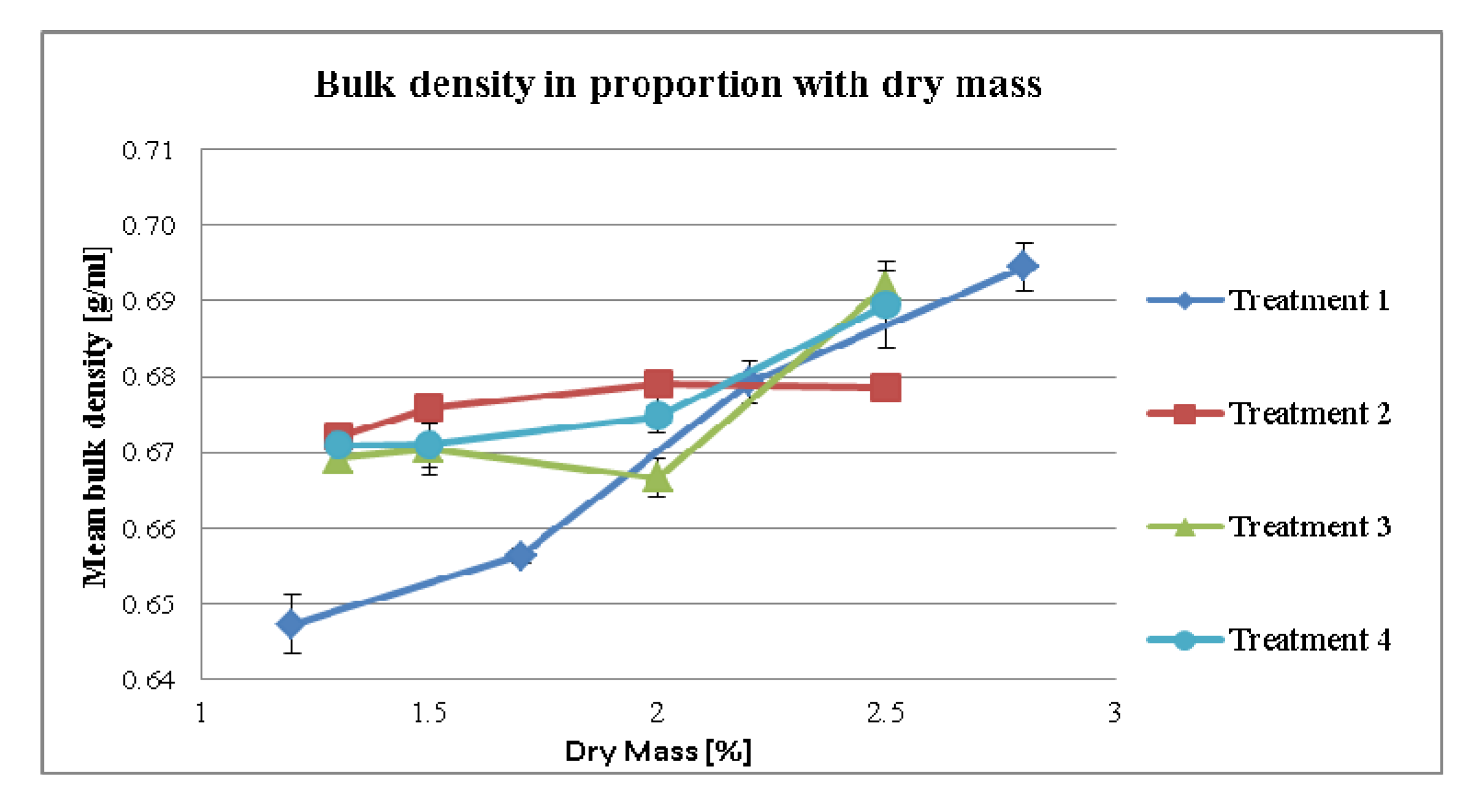
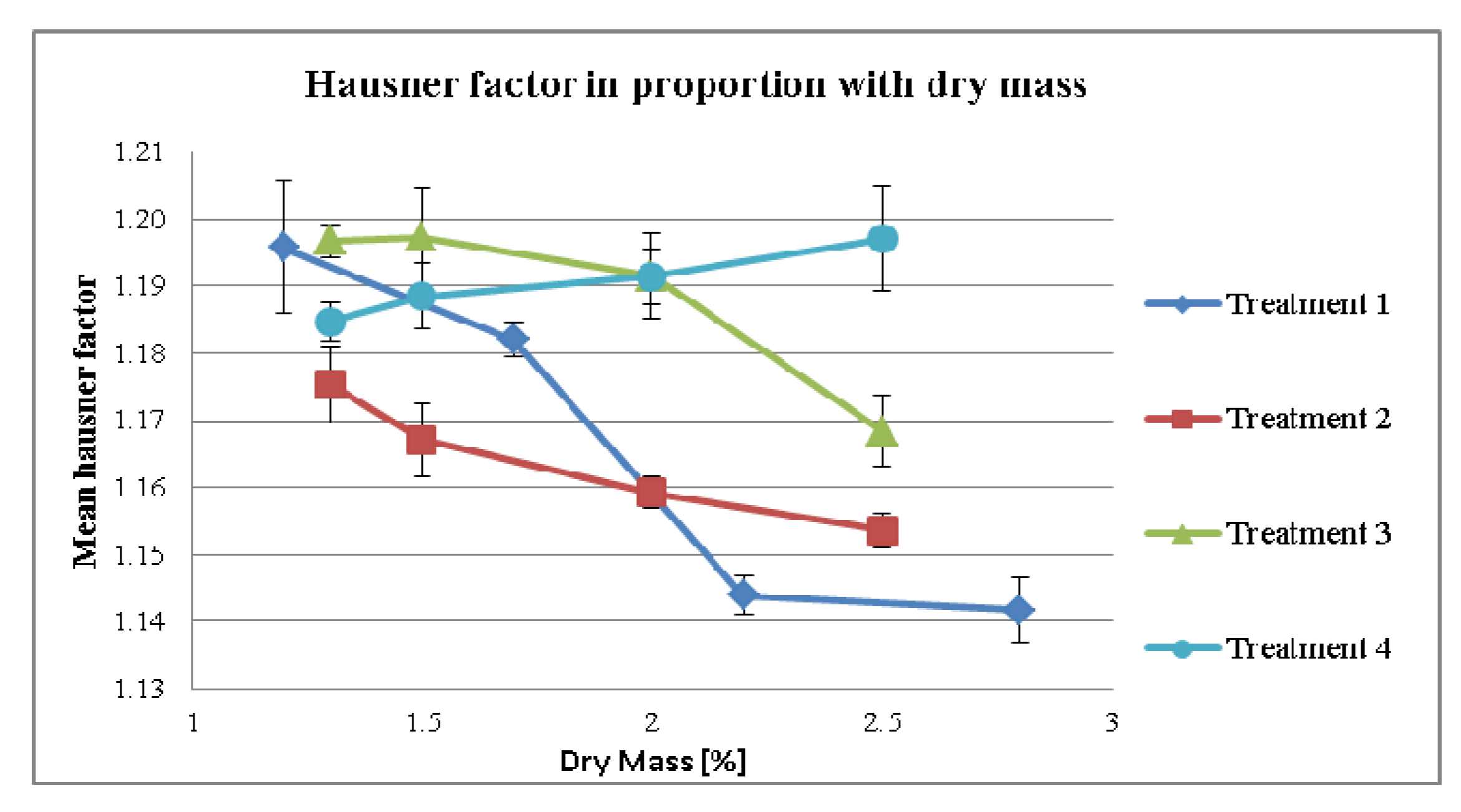
3.2.2. Effect of Dry Mass of the Tincture on Compaction Properties for Different Treatment Strategies

| Dry Mass (%) | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 |
|---|---|---|---|---|
| 1.2 | 3.1 | |||
| 1.7 | 3.4 | |||
| 2.2 | 2.72 | |||
| 2.8 | 3.96 | |||
| 1.3 | 2.92 | 2.96 | 2.77 | |
| 1.5 | 2.96 | 3.04 | 2.48 | |
| 2 | 3.13 | 2.79 | 2.52 | |
| 2.5 | 2.27 | 3.36 | 3.74 |
3.2.3. Effect of Dry Mass of the Tincture on Disintegration Time for Different Treatment Strategies
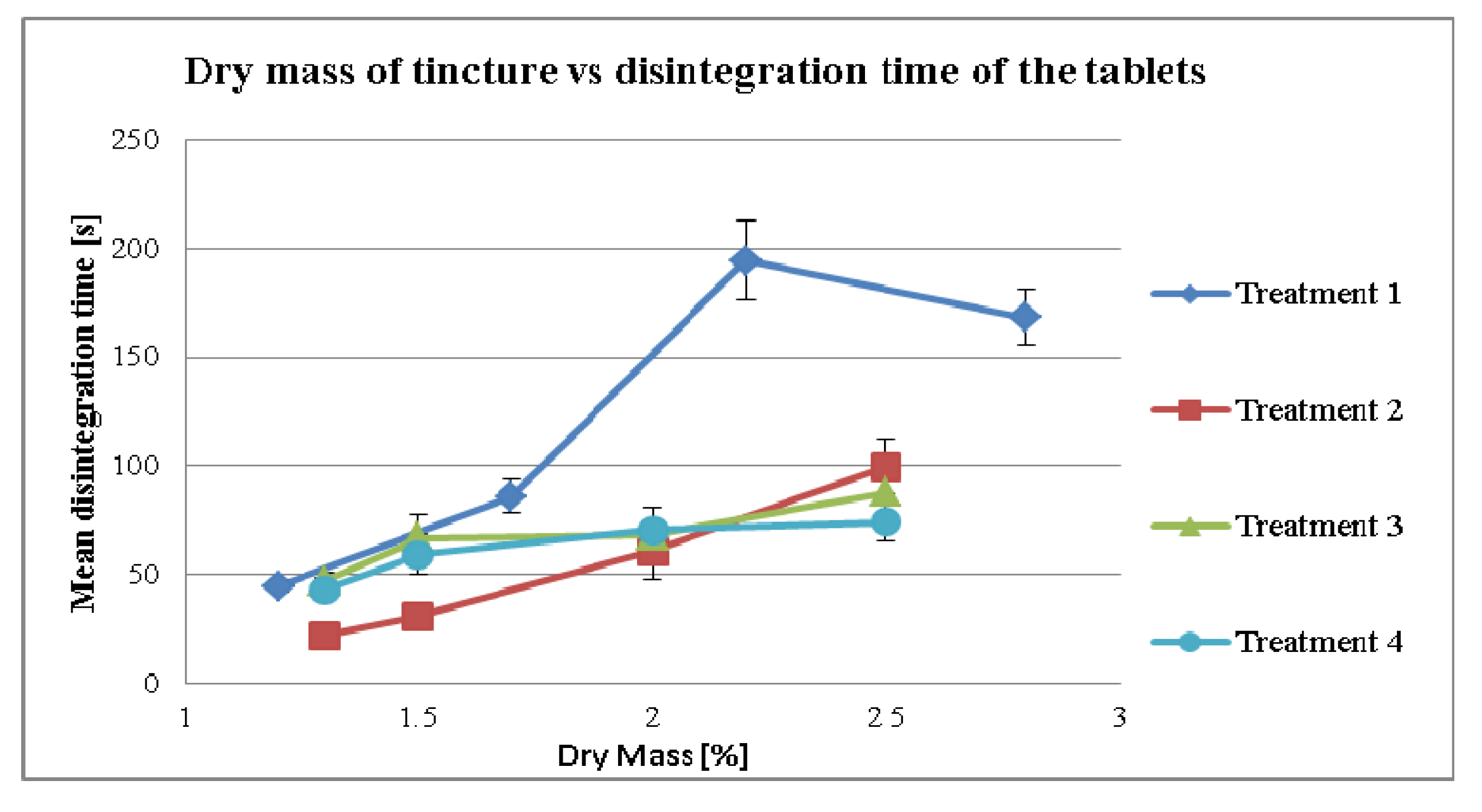
4. Conclusion
Acknowledgements
References
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Raghavendra, H.; Yogesh, H.; Gopalakrishna, B.; Chandrashekhar, V.; Kumar Sathish, B.; Kumar, V. An overview of Herbal Medicine. Int. J. Pharm. Sci. 2009, 1, 1–20. [Google Scholar]
- Okunlola, A.; Babatunde, A.A.; Oluwatoyin, A.O. Evaluation of Pharmaceutical and Microbial Qualities of Some Herbal Medicinal Products in South Western Nigeria. Trop. J. Pharm. Res. 2007, 6, 661–670. [Google Scholar]
- Runha, F.P.; Cordeiro, D.S.; Pereira, C.A.M.; Vilegas, J.; Oliveira, W.P. Production of Dry Extracts of Medicinal Brazilian Plants by Spouted Bed Process: Development of the Process and Evaluation of Thermal Degradation During the Drying Operation. Food Bioprod. Proc. 2001, 79, 160–168. [Google Scholar] [CrossRef]
- Palma, S.; Lujan, C.; Llabot, J.M.; Barboza, G.; Manzo, R.H.; Allemandi, D.A. Design of Peumus boldus tablets by direct compression using a novel dry plant extract. Int. J. Pharm. 2002, 233, 191–198. [Google Scholar] [CrossRef]
- Autralian Government, Guidance on equivalente of herbal extract in complementary medicine. 1.0, pp. 1–16, Department of Health and Ageing. Therapeutic Goods Administration, 2011.
- Brinkeborn, R.M.; Shah, D.V.; Degenring, F.H. Echinaforce and other Echinacea fresh plant preparations in the treatment of the common cold. A randomized, placebo controlled, double-blind clinical trial. Phytomedicine 1999, 6, 1–6. [Google Scholar] [CrossRef]
- Gajdziok, J.; Bernatoniene, J.; Muselik, J.; Masteikova, R.; Dvorackova, K.; Petkeviciute, Z.; Lazauskas, R.; Kalveniene, Z.; Bernatoniene, R. The evaluation of formulations for the preparation of new formula pellets. Pharm. Dev. Technol. 2011, 16, 520–528. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Benali, M.; Gerbaud, V.; Hemati, M. Effect of operating conditions and physico–chemical properties on the wet granulation kinetics in high shear mixer. Powd. Technol. 2009, 190, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Forlano, A.J.; Chavkin, L. The effect of granule size upon disintegration time and capping in compressed tablets. J. Am. Pharm. Assoc. 1960, 49, 67–69. [Google Scholar]
- Bauer, R.; Netsch, M.; Kreuter, M.H. Physiological function of phytopharmaceuticals and its utilization II—Echinacea purpurea. Food Process. Ingred. 2001, 36, 58–63. [Google Scholar]
- Bauer, R.; Wagner, H. Echinacea species as potential immunostimulatory drugs. Econ. Med. Plants Res. 1991, 5, 253–321. [Google Scholar]
- Ohno, I.; Hasegawa, S.; Yada, S.; Kusai, A.; Moribe, K.; Yamamoto, K. Importance of evaluating the consolidation of granules manufactured by high shear mixer. Int. J. Pharm. 2007, 338, 79–86. [Google Scholar] [CrossRef]
- Schiller, M.; Von der Heydt, H.; März, F.; Schmidt, P.C. Enhanced processing properties and stability of film-coated tablets prepared from roller-compacted and ion-exchanged Eschscholtzia californica Cham. dry extracts. STP Pharma. Sci. 2003, 13, 111–118. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qusaj, Y.; Leng, A.; Alshihabi, F.; Krasniqi, B.; Vandamme, T. Development Strategies for Herbal Products Reducing the Influence of Natural Variance in Dry Mass on Tableting Properties and Tablet Characteristics. Pharmaceutics 2012, 4, 501-516. https://doi.org/10.3390/pharmaceutics4040501
Qusaj Y, Leng A, Alshihabi F, Krasniqi B, Vandamme T. Development Strategies for Herbal Products Reducing the Influence of Natural Variance in Dry Mass on Tableting Properties and Tablet Characteristics. Pharmaceutics. 2012; 4(4):501-516. https://doi.org/10.3390/pharmaceutics4040501
Chicago/Turabian StyleQusaj, Ylber, Andreas Leng, Firas Alshihabi, Blerim Krasniqi, and Thierry Vandamme. 2012. "Development Strategies for Herbal Products Reducing the Influence of Natural Variance in Dry Mass on Tableting Properties and Tablet Characteristics" Pharmaceutics 4, no. 4: 501-516. https://doi.org/10.3390/pharmaceutics4040501
APA StyleQusaj, Y., Leng, A., Alshihabi, F., Krasniqi, B., & Vandamme, T. (2012). Development Strategies for Herbal Products Reducing the Influence of Natural Variance in Dry Mass on Tableting Properties and Tablet Characteristics. Pharmaceutics, 4(4), 501-516. https://doi.org/10.3390/pharmaceutics4040501





