Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Skin
2.2. Microemulsion (μE) Preparation
| Component | Composition (% w/w) |
|---|---|
| 0.9% sodium chloride in de-ionized water | 36 |
| PEG-6-caprylic/capric glycerides | 10 |
| Decaglycerol monocaprylate/caprate | 4 |
| Lecithin | 5 |
| Sorbitan monooleate (SMO) | 2-10 |
| Isopropyl myristate (IPM) | To 100% |
2.3. MBG Preparation
2.4. Ternary Phase Diagrams
2.5. Physiochemical Characterization
2.6. Rheological Characterization
2.7. In Vitro Transport of Lidocaine
2.7.1. Transport of Lidocaine in the Skin
2.7.2. Transmembrane Transport of Lidocaine
| CaCl2 ∙ 2H2O NaCl H2O (pH 7.2) | 0.008 g 0.658 g To 100 g |
2.8. Lidocaine Quantification
3. Results and Discussion
3.1. Phase Behaviour of Lecithin-Linker Microemulsions (μEs)
3.1.1. Sorbitan Monooleate (SMO) Phase Scan

| % SMO | 2% | 4% | 6% | 8% | 10% |
|---|---|---|---|---|---|
| Conductivity (μS/cm) of microemulsions (μEs) and excess phases | |||||
| Top | 0.0 ± 0.0 | 0.0 ± 0.0 | 1500 ± 11 | 40.3 ± 1.5 | 30.7 ± 2.1 |
| Bottom | 1660 ± 10 | 1600 ± 10 | 1320 ± 6 | 1220 ± 6 | |
| Hydrodynamic radii (nm) of μEs | |||||
| 1.7 ± 1 | 5.9 ± 3.9 | 2.5 ± 1.6 | N.M. | N.M. | |
| Viscosity (mPa∙s) of μEs | |||||
| 28.3 ± 0.0 | 63.2 ± 0.5 | 126.8 ± 2.7 | N.M | N.M | |
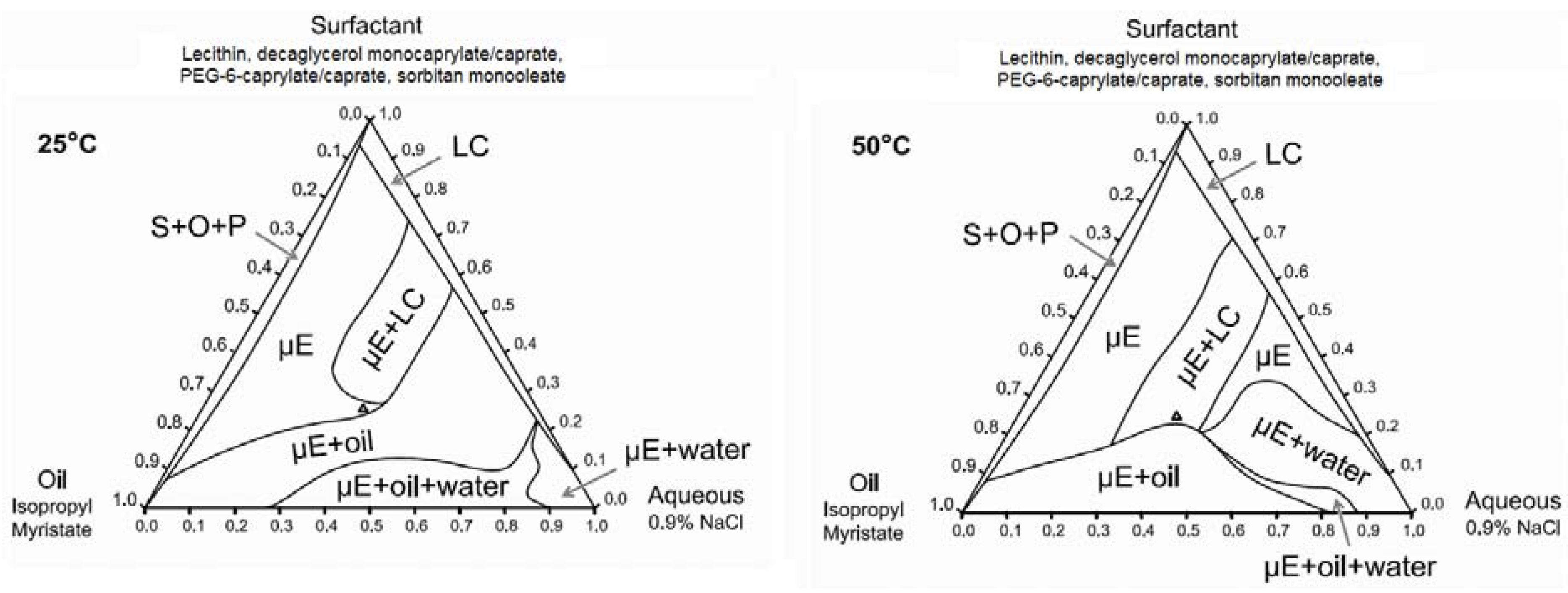
3.1.2. Ternary Phase Diagrams
3.2. Phase Behaviour of Lecithin-Linker MBGs
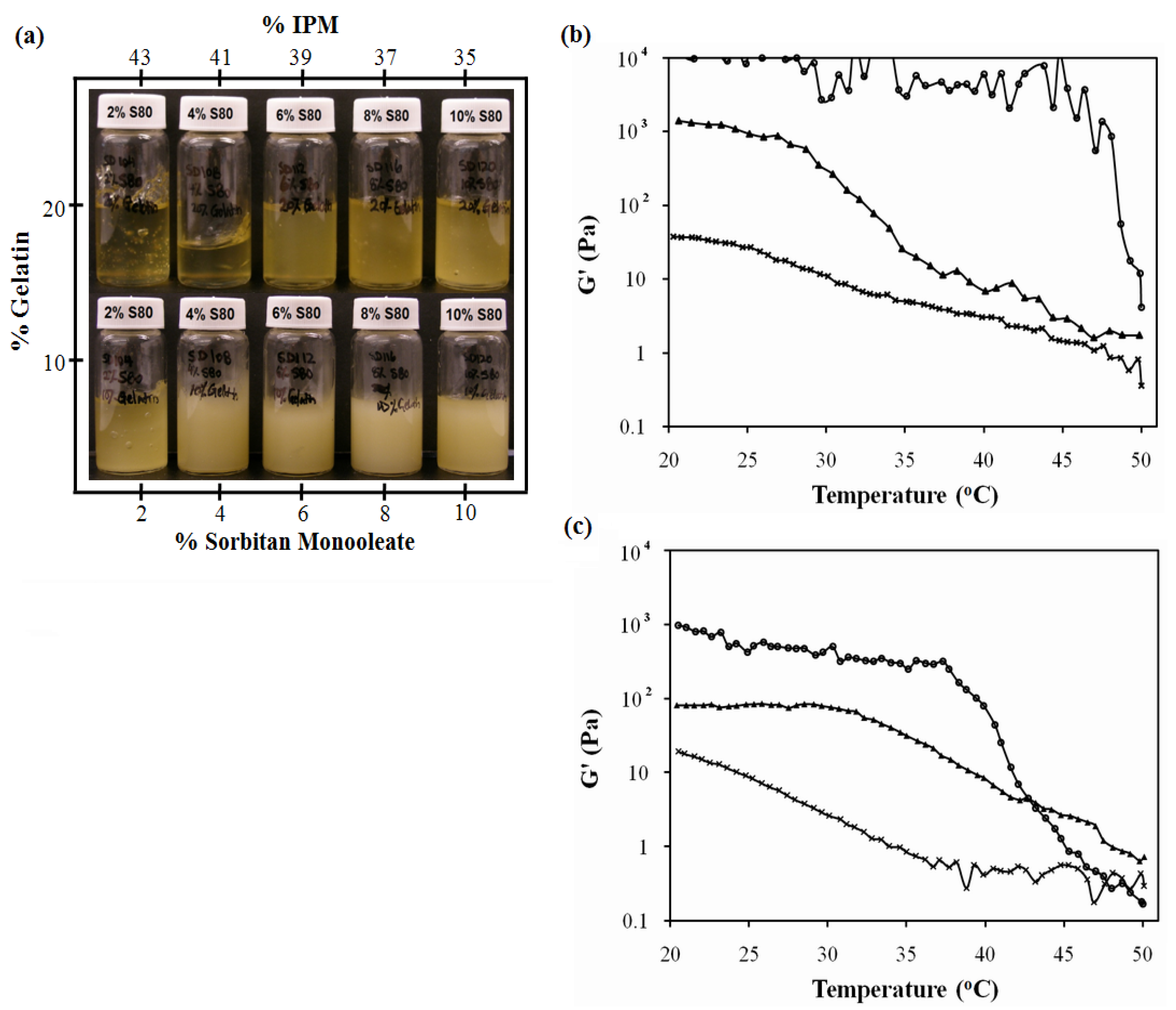
3.2.1. Sorbitan Monooleate (SMO) Phase Scan
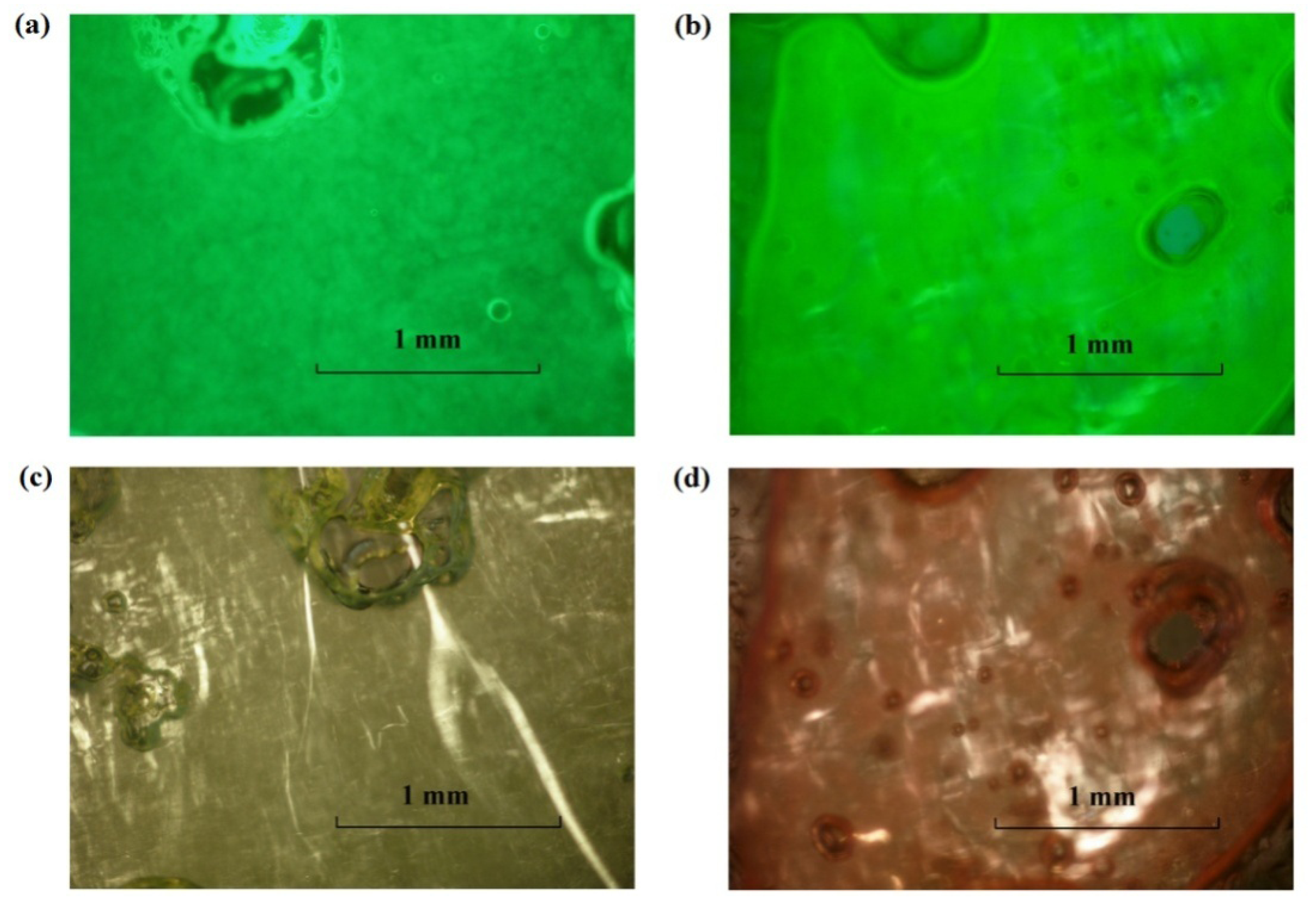
3.2.2. Ternary Phase Diagrams
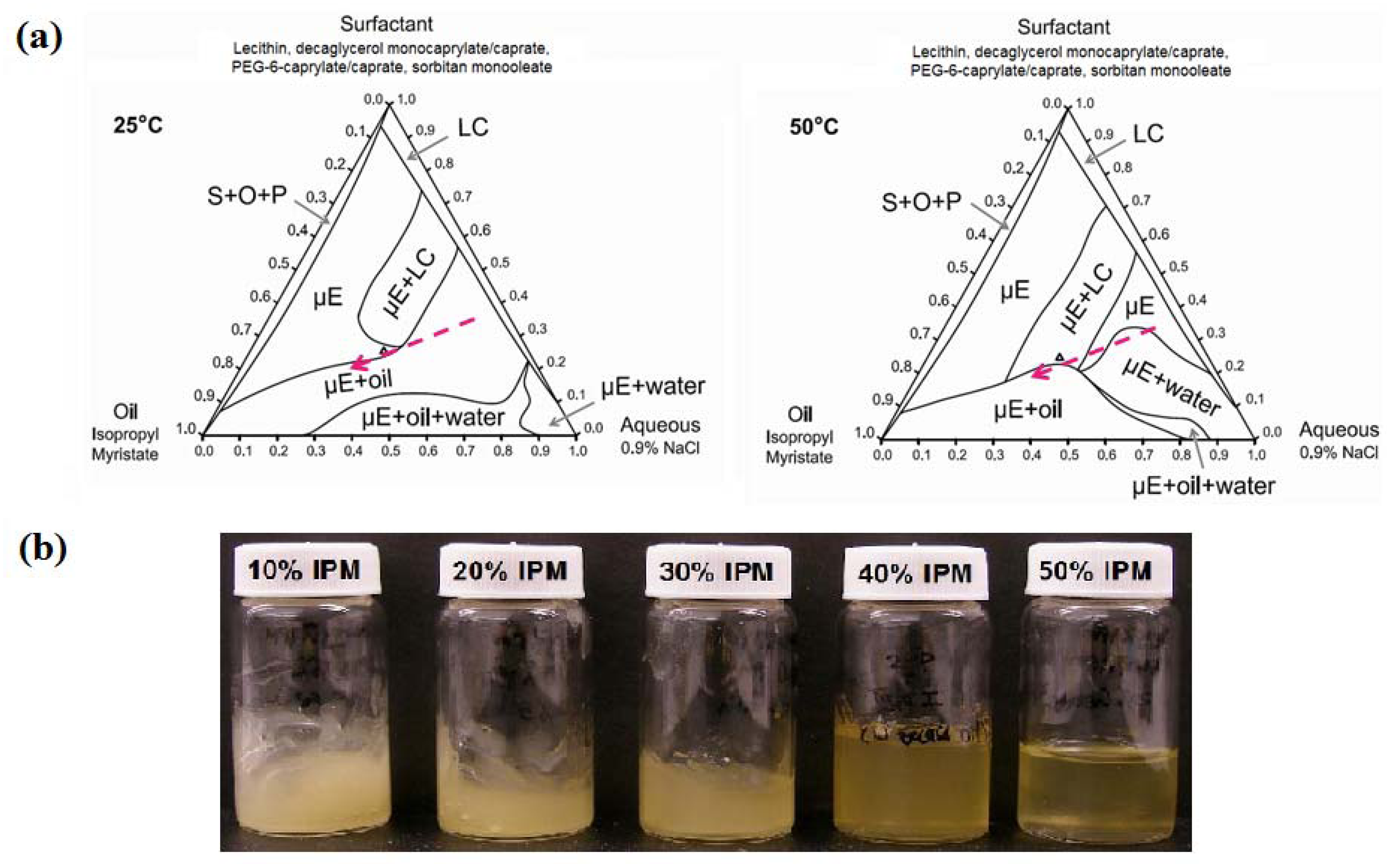
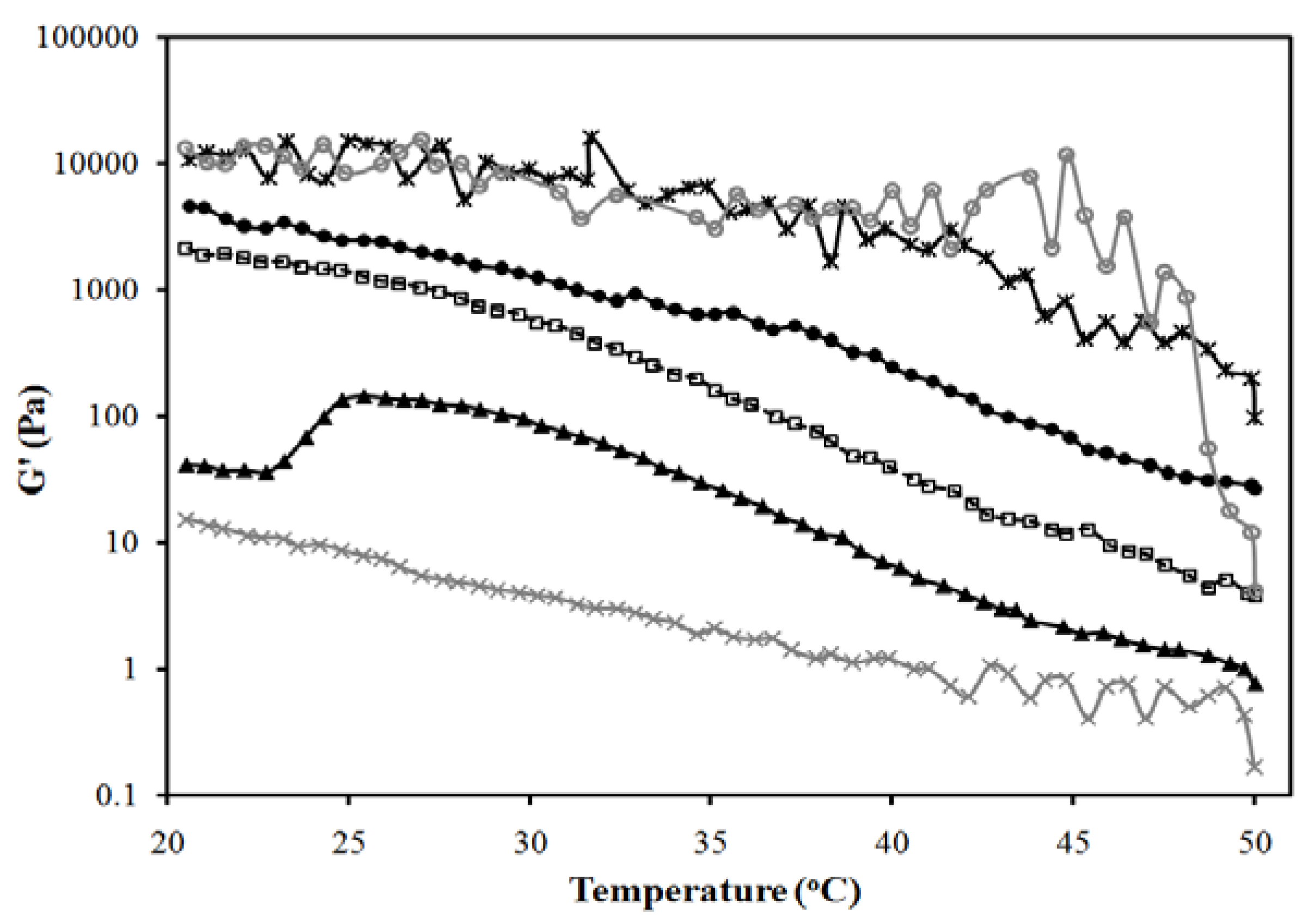

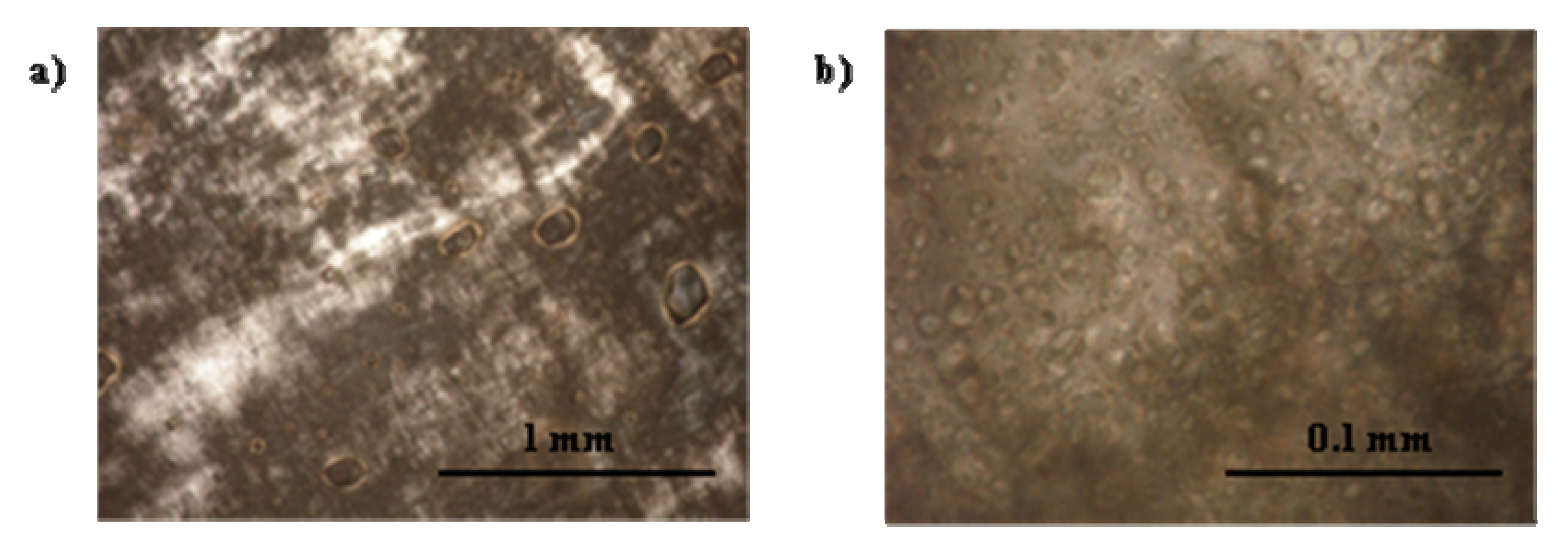
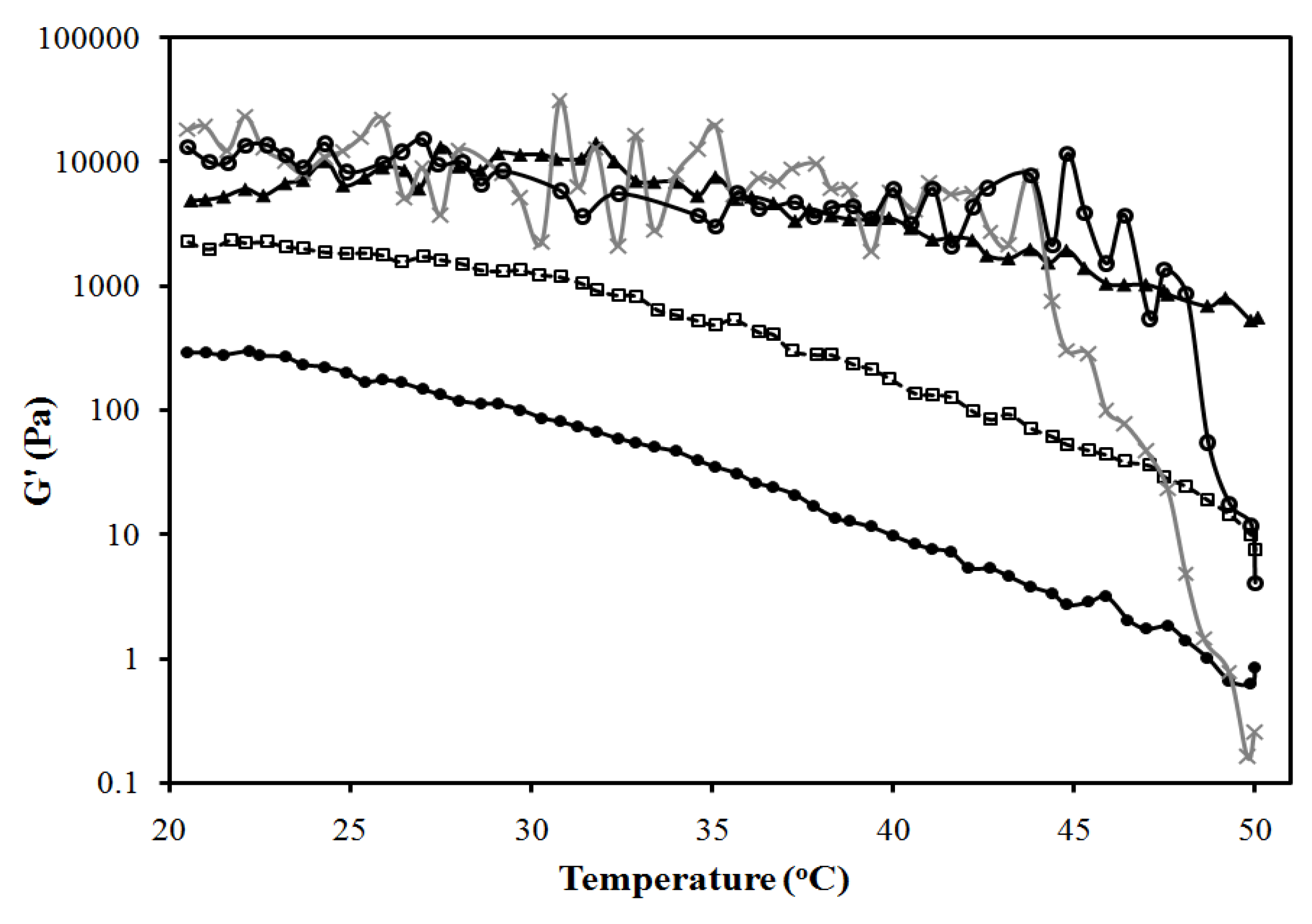
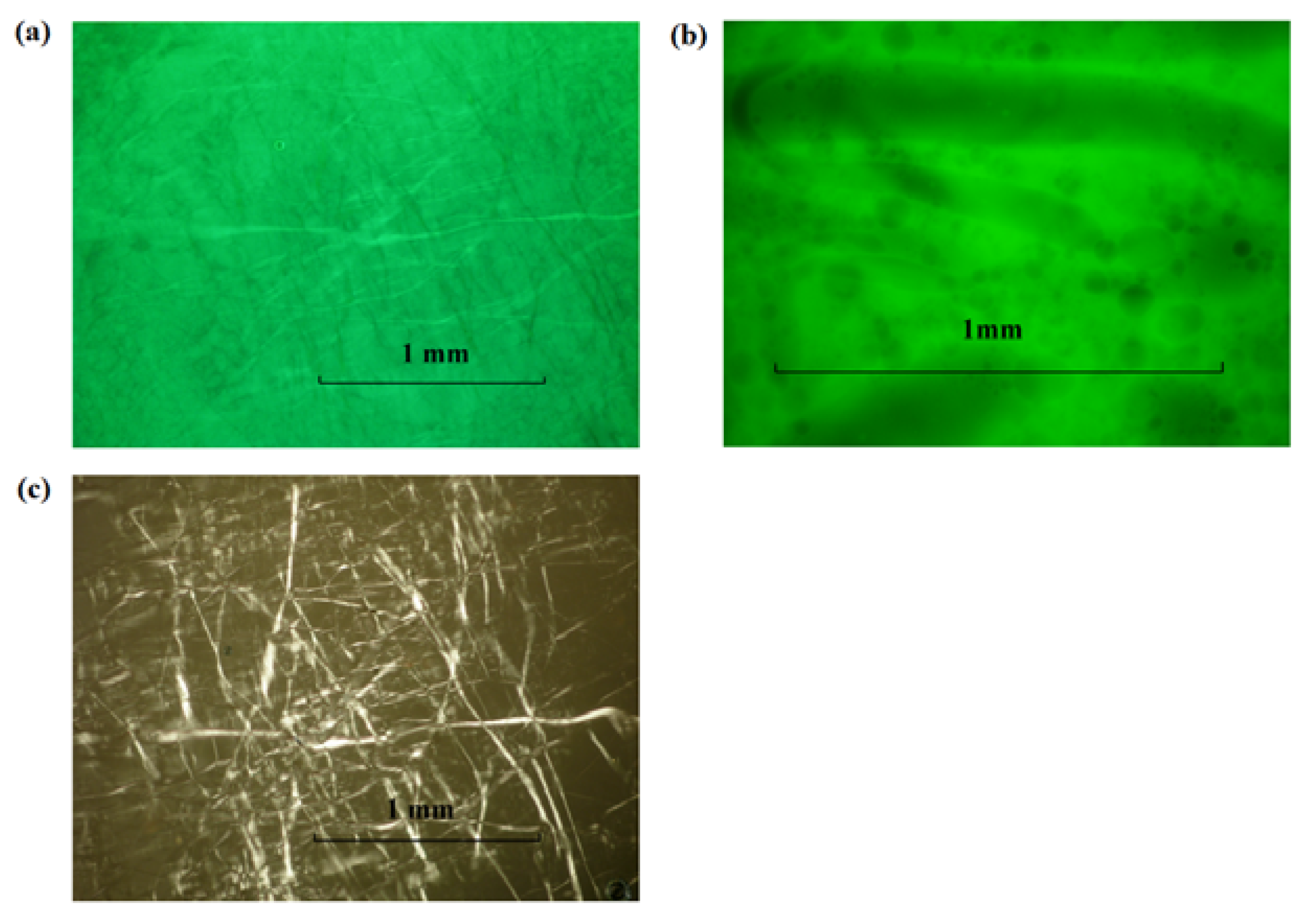
3.2.3. Lecithin-Linker Gels
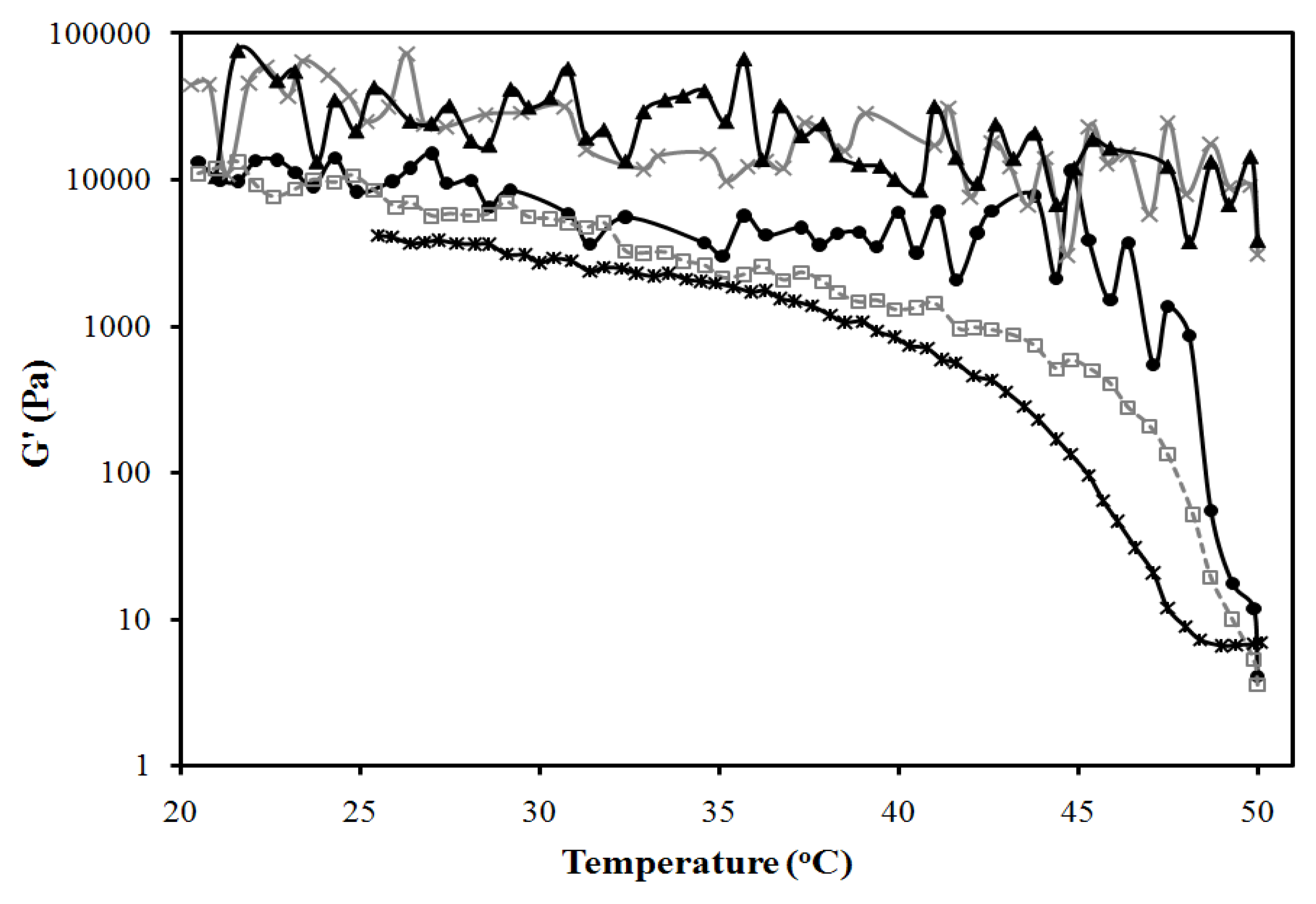
3.3. In Vitro Transport Studies
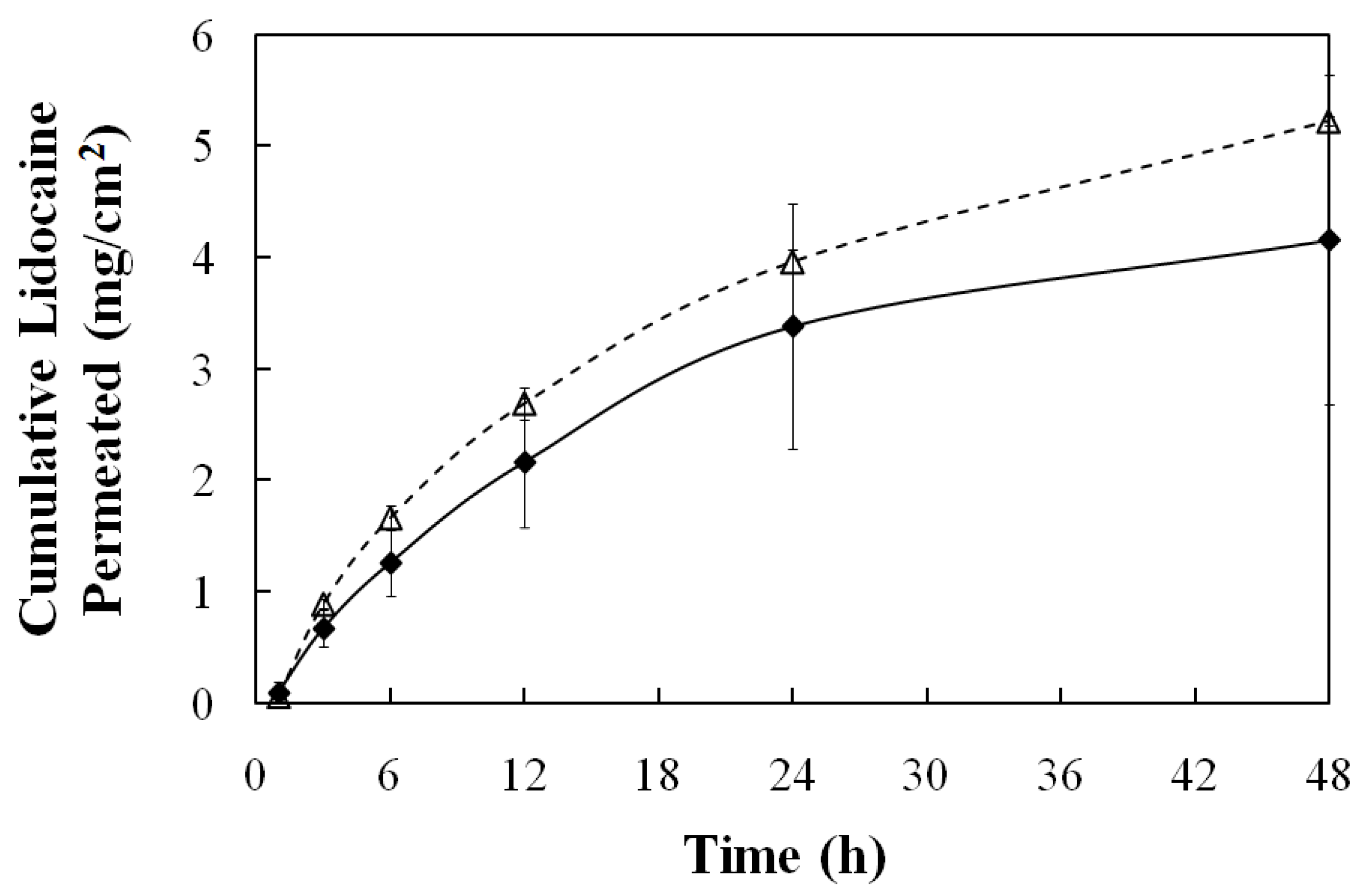
3.3.1. Transport in the Skin

3.3.2. Transmembrane Transport
4. Conclusions
Acknowledgements
References
- Kogan, A.; Garti, N. Microemulsion as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef]
- Walters, K.A. Dermatological and Transdermal Formulations; Marcel Dekker: New York, NY, USA, 2002; p. 4. [Google Scholar]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Delivery Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Kanicky, J.R.; Palla, B.J.; Patanjali, P.K.; Shah, D.O. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit. Rev. Ther. Drug Carrier Syst. 2001, 18, 77–140. [Google Scholar]
- Kreilgaard, M.; Pedersen, E.J.; Jaroszewski, J.W. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J. Control. Release 2000, 69, 421–433. [Google Scholar] [CrossRef]
- Tenjarla, S. Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Ther. Drug. 1999, 16, 461–521. [Google Scholar]
- Kreilgaard, M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Del. Rev. 2002, 54, S77–S98. [Google Scholar] [CrossRef]
- Peltola, S.; Saarinen-Savolainen, P.; Kiesvaara, J.; Suhonen, T.M.; Urtti, A. Microemulsions for topical delivery of estradiol. Int. J. Pharm. 2003, 254, 99–107. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Iglesias-Vilas, G.; Blanco-Méndez, J.; López-Quintela, M.A.; Marty, J.-P.; Guy, R.H. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur. J. Pharm. Biopharm. 1997, 43, 37–42. [Google Scholar] [CrossRef]
- Sintov, A.C.; Shapiro, L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J. Control. Release 2004, 95, 173–183. [Google Scholar] [CrossRef]
- Lee, P.J.; Langer, R.; Shastri, V.P. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm. Res. 2003, 20, 264–269. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef]
- Davies, N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 2000, 27, 558–562. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Dispersion of microemulsion drops in HEMA hydrogel: A potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Benita, S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef]
- Mitra, A.K.; Anand, B.S.; Duvvuri, S. Drug Delivery to the Eye. In Advances in Organ Biology Volume 10: The Biology of the Eye; Elsevier B.V.: Amsterdam, The Netherlands, 2006; pp. 307–351. [Google Scholar]
- Vandamme, Th.F. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34. [Google Scholar] [CrossRef]
- Ligório Fialho, S.; da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef]
- Haße, A.; Keipert, S. Development and characterization of microemulsions for ocular application. Eur. J. Pharm. Biopharm. 1997, 43, 179–183. [Google Scholar] [CrossRef]
- Acosta, E.J.; Nguyen, T.; Witthayapanyanon, A.; Harwell, J.H.; Sabatini, D.A. Linker-based bio-compatible microemulsions. Environ. Sci. Technol. 2005, 39, 1275–1282. [Google Scholar]
- McKarns, S.C.; Hansch, C.; Caldwell, W.S.; Morgan, W.T.; Moore, S.K.; Doolittle, D.J. Correlations between hydrophobicity of short-chain aliphatic alcohols and their ability to alter plasma membrane integrity. Toxicol. Sci. 1997, 36, 62–70. [Google Scholar] [CrossRef]
- Yuan, J.S.; Ansari, M.; Samaan, M.; Acosta, E.J. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int. J. Pharm. 2008, 349, 130–143. [Google Scholar] [CrossRef]
- Yuan, J.S.; Acosta, E.J. Extended release of lidocaine from linker-based lecithin microemulsions. Int. J. Pharm. 2009, 368, 63–71. [Google Scholar] [CrossRef]
- Sabatini, D.A.; Acosta, E.J.; Harwell, J.H. Linker molecules in surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2003, 8, 316–326. [Google Scholar] [CrossRef]
- Acosta, E.J.; Harwell, J.; Sabatini, D. Self-assembly in linker-modified microemulsions. J. Colloid Interface Sci. 2004, 274, 652–664. [Google Scholar] [CrossRef]
- Uchiyama, H.; Acosta, E.J.; Tran, S.; Sabatini, D.; Harwell, J. Supersolubilization in chlorinated hydrocarbon microemulsions: Solubilization enhancement by lipophilic and hydrophilic linkers. Ind. Eng. Chem. Res. 2000, 39, 2704–2708. [Google Scholar] [CrossRef]
- Acosta, E.; Chung, O.; Xuan, X.Y. Lecithin-linker microemulsions in transdermal delivery. J. Drug Deliv. Sci. Technol. 2011, 21, 77–87. [Google Scholar]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drugdelivery. Adv. Drug Deliv. Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef]
- Bapatla, K.M.; Hecht, G. Ophthalmic Ointments and Suspensions. In Pharmaceutical Dosage Forms: Disperse Systems; Marcel Dekker: New York, NY, USA, 2006; pp. 357–397. [Google Scholar]
- Yuan, J.S.; Yip, A.; Nguyen, N.; Chu, J.; Wen, X.-Y.; Acosta, E.J. Effect of surfactant concentration on transdermal lidocaine delivery with linker microemulsions. Int. J. Pharm. 2010, 392, 274–284. [Google Scholar] [CrossRef]
- Luisi, P.L.; Scartazzini, R.; Haering, G.; Schurtenberger, P. Organogels from water-in-oil microemulsions. Colloid Polym. Sci. 1990, 268, 356–374. [Google Scholar] [CrossRef]
- Haering, G.; Luisi, P.L. Hydrocarbon gels from water-in-oil microemulsions. J. Phys. Chem. 1986, 90, 5892–5895. [Google Scholar] [CrossRef]
- Kantaria, S.; Rees, G.D.; Lawrence, M.J. Gelatin-stabilised microemulsion-based organogels: rheology and application in iontophoretic transdermal drug delivery. J. Control. Release 1999, 60, 355–365. [Google Scholar] [CrossRef]
- Murdan, S. Organogels in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 489–505. [Google Scholar] [CrossRef]
- Rees, G.D.; Jenta, T.R.; Nascimento, M.G. Use of water-in-oil microemulsions and gelatin-containing microemulsion-based gels for lipase-catalysed ester synthesis in organic solvents. Indian J. Chem. 1993, 32B, 30–34. [Google Scholar]
- Stamatis, H.; Xenakis, A. Biocatalysis using microemulsion-based polymer gels containing lipase. J. Mol. Catal. B: Enzym. 1999, 6, 399–406. [Google Scholar] [CrossRef]
- Blattner, C.; Zoumpanioti, M.; Kroner, J.; Schmeer, G.; Xenakis, A.; Kunz, W. Biocatalysis using lipase encapsulated in microemulsion-based organogels in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 36, 182–193. [Google Scholar]
- Zhao, X.Y.; Xu, J.; Zheng, L.Q.; Li, X.W. Preparation of temperature-sensitive microemulsion-based gels formed from a triblock copolymer. Colloids Surf. A 2007, 307, 100–107. [Google Scholar] [CrossRef]
- Caldararu, H.; Timmins, G.S.; Gilbert, B.C. The structure of gelatin-water/oil microemulsion sols and gels. An EPR spin-probe and spin-labelling study. Phys. Chem. Chem. Phys. 1999, 1, 5686–5695. [Google Scholar]
- Atkinson, P.J.; Robinson, B.H.; Howe, A.M.; Heenan, R.K. Structure and stability of microemulsion-based organogels. J. Chem. Soc. Faraday Trans. 1991, 87, 3389–3397. [Google Scholar] [CrossRef]
- Petit, C.; Zemb, Th.; Pileni, M.P. Structural study of microemulsion-based gels at the saturation point. Langmuir 1991, 7, 223–231. [Google Scholar] [CrossRef]
- Trickett, K.; Brice, H.; Myakonkaya, O.; Eastoe, J.; Rogers, S.E.; Heenan, R.K.; Grillo, I. Microemulsion-based organogels containing inorganic nanoparticles. Soft Matter. 2010, 6, 1291–1296. [Google Scholar]
- Patravale, V.B.; Date, A.A. Microemulsions: Applications in transdermal and dermal delivery. Crit. Rev. Ther. Drug Carrier Syst. 2007, 24, 547–596. [Google Scholar]
- Zoumpanioti, M.; Stamatis, H.; Xenakis, A. Microemulsion-based organogels as matrices for lipase immobilization. Biotechnol. Adv. 2010, 28, 395–406. [Google Scholar] [CrossRef]
- Nagayama, K.; Yamasaki, N.; Imai, M. Fatty acid esterification catalyzed by Candida rugosa lipase in lecithin microemulsion-based organogels. Biochem. Eng. J. 2002, 12, 231–236. [Google Scholar] [CrossRef]
- Zoumpanioti, M.; Karavas, E.; Skopelitis, C.; Stamatis, H.; Xenakis, A. Lecithin organogels as model carriers of pharmaceuticals. Prog. Colloid Polym. Sci. 2004, 123, 199–202. [Google Scholar]
- Willimann, H.; Walde, P.; Luisi, P.L.; Gazzaniga, A.; Stroppolo, F. Lecithin organogel as matrix for transdermal transport of drugs. J. Pharm. Sci. 1992, 81, 871–874. [Google Scholar] [CrossRef]
- Jenta, T.R.-J.; Batts, G.; Rees, G.D.; Robinson, B.H. Biocatalysis using gelatin microemulsion-based organogels containing immobilized Chromobacterium viscosum lipase. Biotechnol. Bioeng. 1997, 53, 121–131. [Google Scholar] [CrossRef]
- Uemasu, I.; Hinze, W.L. Enantioselective esterification of 2-methylbutyric acid catalyzed via lipase immobilized in microemulsion-based organogels. Chirality 1994, 6, 649–653. [Google Scholar] [CrossRef]
- Kantaria, S.; Rees, G.D.; Lawrence, M.J. Formulation of electrically conducting microemulsion-based organogels. Int. J. Pharm. 2003, 250, 65–83. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Han, F.; Yao, H.; Li, S. Gelatin-stabilised microemulsion-based organogels facilitates percutaneous penetration of cyclosporin A in vitro and dermal pharmacokinetics in vivo. J. Pharm. Sci. 2007, 96, 3000–3009. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Cao, Q.; Zheng, L.-Q.; Zhang, G.-Y. Rheological properties and microstructures of gelatin-containing microemulsion-based organogels. Colloids Surf. A 2006, 281, 67–73. [Google Scholar] [CrossRef]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- Scholfield, C.R. Composition of soybean lecithin. J. Am. Oil Chem. Soc. 1981, 58, 889–892. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Bronaugh, R.L.; Maibach, H.I. In Vitro Percutaneous Absorption: Principles, Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 1991; p. 139. [Google Scholar]
- Aboofazeli, R.; Lawrence, M.J. Investigations into the formation and characterization of phospholipid microemulsions. I. Pseudo-ternary phase diagrams of systems containing water-lecithin-alcohol-isopropyl myristate. Int. J. Pharm. 1993, 93, 161–175. [Google Scholar] [CrossRef]
- Moreno, M.A.; Ballesteros, M.P.; Frutos, P. Lecithin-based oil-in-water microemulsions for parenteral use: Pseudoternary phase diagrams, characterization and toxicity studies. J. Pharm. Sci. 2003, 92, 1428–1437. [Google Scholar] [CrossRef]
- Tetzlaff, J.E. Clinical Pharmacology of Local Anesthetics; Butterworth-Heinemann: Boston, MA, USA, 2000; p. 85. [Google Scholar]
- Behndig, A; Linden, C. Aqueous humor lidocaine concentrations in topical and intracameral anesthesia. J. Cataract. Refract. Surg. 1998, 24, 1598–1601. [Google Scholar]
- Assia, E.I.; Pras, E.; Yehezkel, M.; Rotenstreich, Y.; Jager-Roshu, S. Topicalanesthesia using lidocaine gel for cataract surgery. J. Cataract. Refract. Surg. 1999, 25, 635–639. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981, 26, 84–95. [Google Scholar] [CrossRef]
- Kiran, S.K.; Acosta, E.J. Predicting the morphology and viscosity of microemulsions using the HLD-NAC model. Ind. Eng. Chem. Res. 2010, 49, 3424–3432. [Google Scholar]
- Adeyeye, M.C.; Jain, A.C.; Ghorab, M.K.; Reilly, W.J., Jr. Viscoelastic evaluation of topical creams containing microcrystalline cellulose/sodium carboxymethyl cellulose as stabilizer. AAPS Pharm. Sci. Tech. 2002, 3, E8. [Google Scholar]
- Acosta, E.; Uchiyama, H.; Sabatini, D.A.; Harwell, J.H. The role of hydrophilic linkers. J. Surfactants Deterg. 2002, 5, 151–157. [Google Scholar] [CrossRef]
- Tyrode, E.; Johnson, C.M.; Rutland, M.W.; Claesson, P.M. Structure and hydration of poly(ethylene oxide) surfactants at the air/liquid interface. A vibrational sum frequency spectroscopy study. J. Phys. Chem. C 2007, 111, 11642–11652. [Google Scholar]
- Tyrode, E.; Johnson, C.M.; Kumpulainen, A.; Rutland, M.W.; Claesson, P.M. Hydration state of nonionic surfactant monolayers at the liquid/vapor interface: Structure determination by vibrational sum frequency spectroscopy. J. Am. Chem. Soc. 2005, 127, 16848–16859. [Google Scholar]
- Acosta, E.J.; Bhakta, A.S. The HLD-NAC model for mixtures of ionic and nonionic surfactants. J. Surfactants Deterg. 2009, 12, 7–19. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Robinson, B.H.; Howe, A.M.; Pitt, A.R. Characterisation of water-in-oil microemulsions and organo-gels based on sulphonate surfactants. Colloids Surf. A. 1995, 94, 231–242. [Google Scholar] [CrossRef]
- Pasero, C.L.; Davies, P.; Faucett, J.; Wong, D.L. Pain control. Am. J. Nurs. 1995, 95, 22–24. [Google Scholar]
- Skaaret, I. Extended Release of Lidocaine Hydrochloride from a New Formulation. Available online: http://www.farmfak.uu.se/farm/galfarmweb/DiplomaWork/IngridSkaaret.pdf (accessed in May 2011). Undergraduate Thesis, Department of Pharmacy, Uppsala University, Uppsala, Sweden, 2006..
- Qian, Y.; Wang, F.; Li, R.; Zhang, Q.; Xu, Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev. Ind. Pharm. 2010, 36, 1340–1347. [Google Scholar] [CrossRef]
- Chan, J.; Maghraby, G.M.M.E.; Craig, J.P.; Alany, R.G. Phase transition water-in-oil microemulsions as ocular drug delivery systems: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 328, 65–71. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan-gelatin mucoadhesive systems for ion-exchange based ophthalmic delivery: In vitro and preliminary in vivo studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef]
- Hämäläinen, K.M.; Kananen, K.; Auriola, S.; Kontturi, K.; Urtti, A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest. Ophthalmol. Vis. Sci. 1997, 38, 627–634. [Google Scholar]
- Trier, K. The Sclera. In Advances in Organ Biology Volume 10: The Biology of the Eye; Elsevier B.V.: Amsterdam, The Netherlands, 2006; pp. 353–373. [Google Scholar]
- Cruysberg, L.P.J.; Nuijts, R.M.M.A.; Geroski, D.H.; Koole, L.H.; Hendrikse, F.; Edelhauser, H.F. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6g and the use of a coated coil as a new drug delivery system. J. Ocul. Pharm. Ther. 2002, 18, 559–569. [Google Scholar] [CrossRef]
- Amsden, B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xuan, X.-Y.; Cheng, Y.-L.; Acosta, E. Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery. Pharmaceutics 2012, 4, 104-129. https://doi.org/10.3390/pharmaceutics4010104
Xuan X-Y, Cheng Y-L, Acosta E. Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery. Pharmaceutics. 2012; 4(1):104-129. https://doi.org/10.3390/pharmaceutics4010104
Chicago/Turabian StyleXuan, Xiao-Yue, Yu-Ling Cheng, and Edgar Acosta. 2012. "Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery" Pharmaceutics 4, no. 1: 104-129. https://doi.org/10.3390/pharmaceutics4010104
APA StyleXuan, X.-Y., Cheng, Y.-L., & Acosta, E. (2012). Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery. Pharmaceutics, 4(1), 104-129. https://doi.org/10.3390/pharmaceutics4010104