Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies
Abstract
:1. Introduction
1.1. The origins and applications of radioisotopes
1.2. Radiolabelling in the lab
| Radioisotope | Decay emission | Half-life | Application |
|---|---|---|---|
| 3H | β | 12.3 years | Amino acid labelling, cell proliferation assays |
| 14C | β | 5730 years | Amino acid labelling |
| 35S | β | 87.4 days | Nucleotide, amino acid labelling |
| 51Cr | γ | 27.7 days | Cytotoxicity assays |
| 32P | β | 14.3 days | Nucleotide labelling |
| 33P | β | 25.4 days | Nucleotide labelling |
| 90Y | β, γ | 2.67 days | Therapeutic |
| 125I | γ | 60.1 days | Protein labelling |
1.3. Application of radioisotopes in vaccine studies
1.4. Practicalities of using radioisotopes
2. Materials and Methods
2.1. Materials
2.2. Radiolabelling of liposomes
2.3. Radiolabelling of antigen

| Protein | Size (kDa) | pI | No Tyrosine residues | 125I-labelling Buffer/pH | 125I-labelling efficiency | % adsorption to DDA:TDB liposomes |
|---|---|---|---|---|---|---|
| OVA | 45 | 4.5 | 9 | Tris/7.4 | +++ | 88 %a |
| BSA | 66 | 4.7 | 21 | Tris/7.4 | +++ | N/D |
| Lysozyme | 14.7 | 11 | 3 | Tris/4 | ++ | 29 %b, [22] |
| Ag85B-ESAT-6 | 44 | 4.9 | 13 | Tris/7.4 | +++ | 97 %b, [22] |
2.4. Stability studies using 3H-labelled liposomes or 125I-labelled antigen
2.5. Processing of tissues containing 125I and 3H
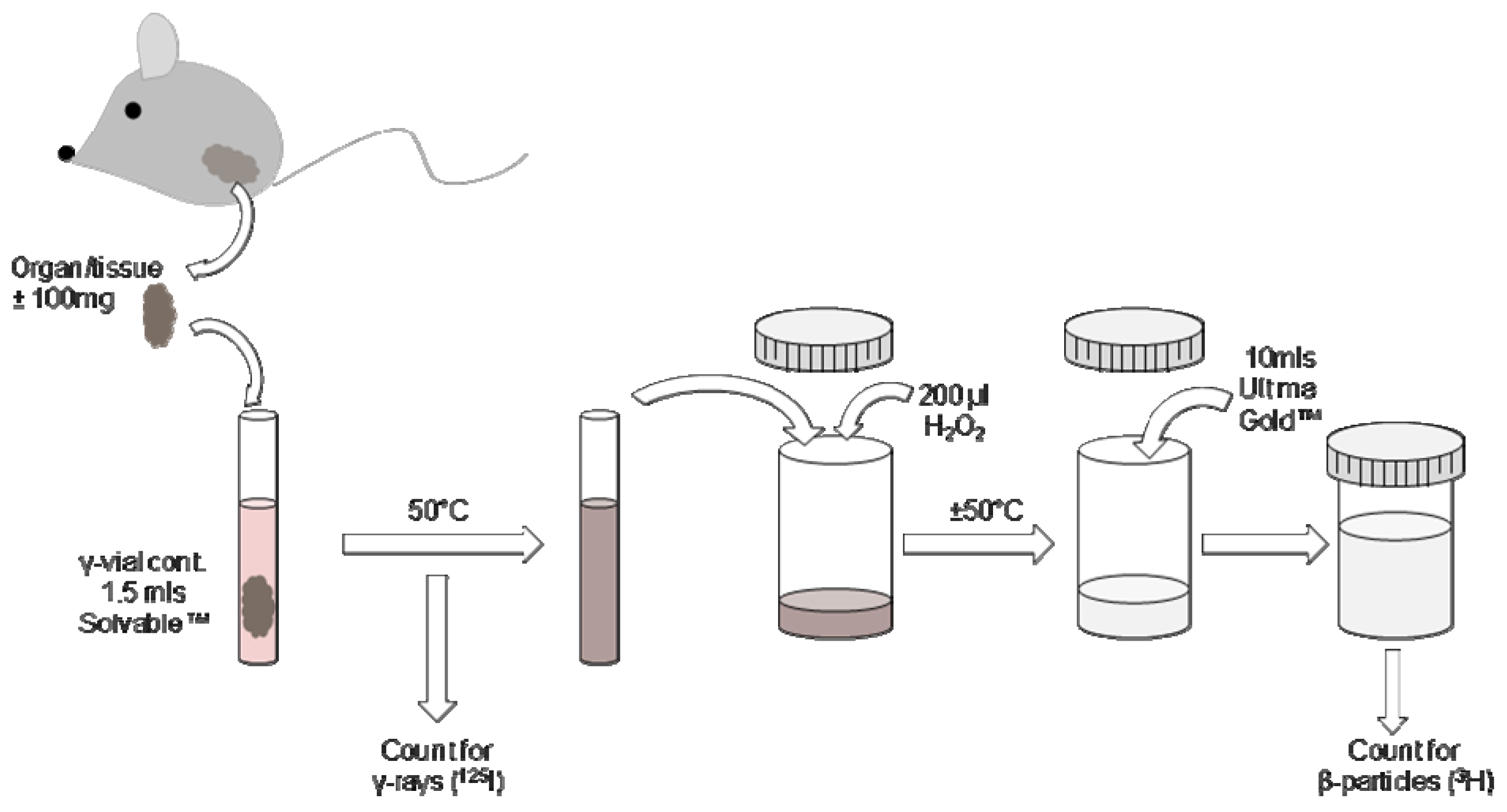
3. Results and Discussion
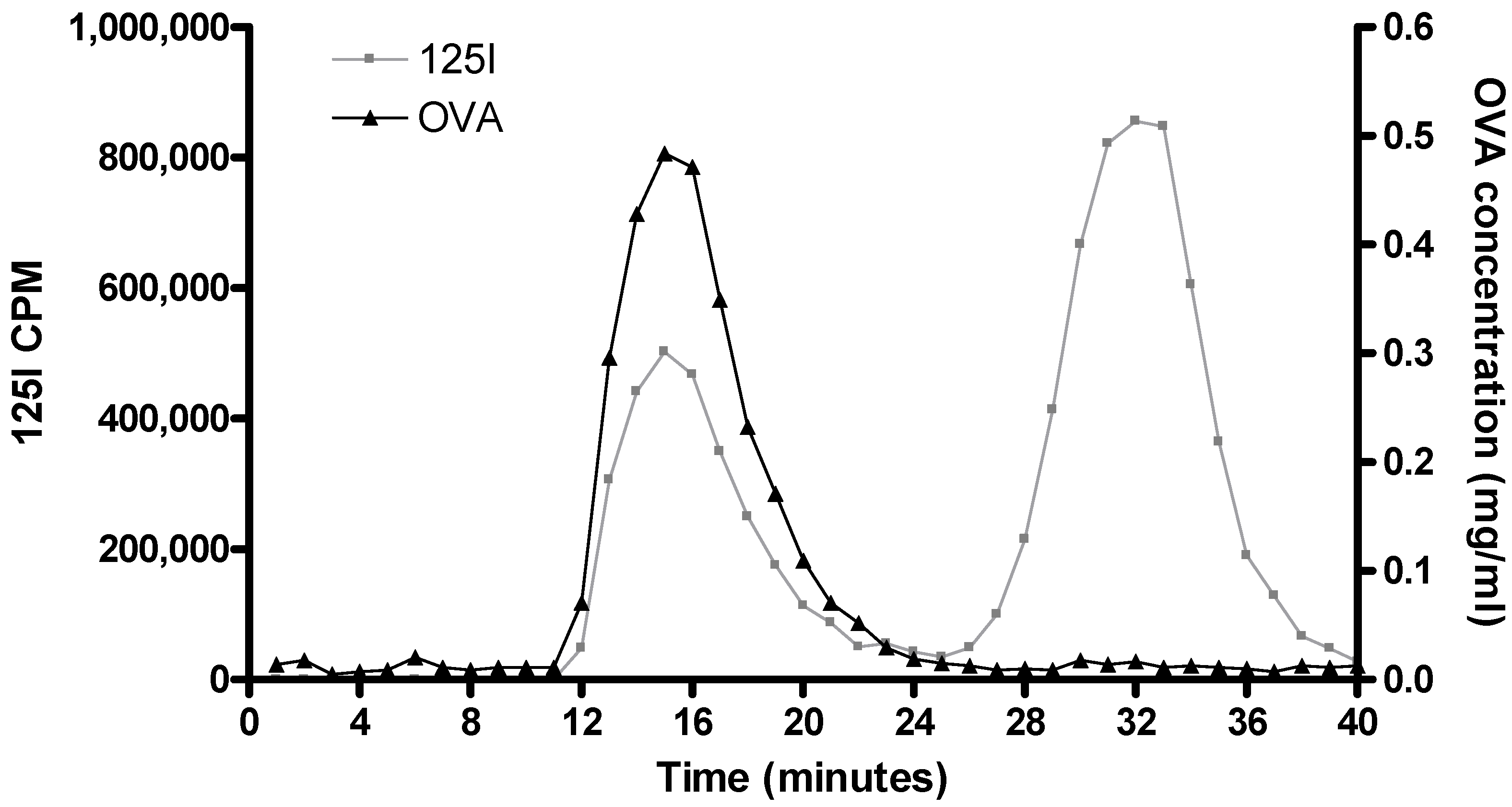
3.1. Radiolabelling of proteins
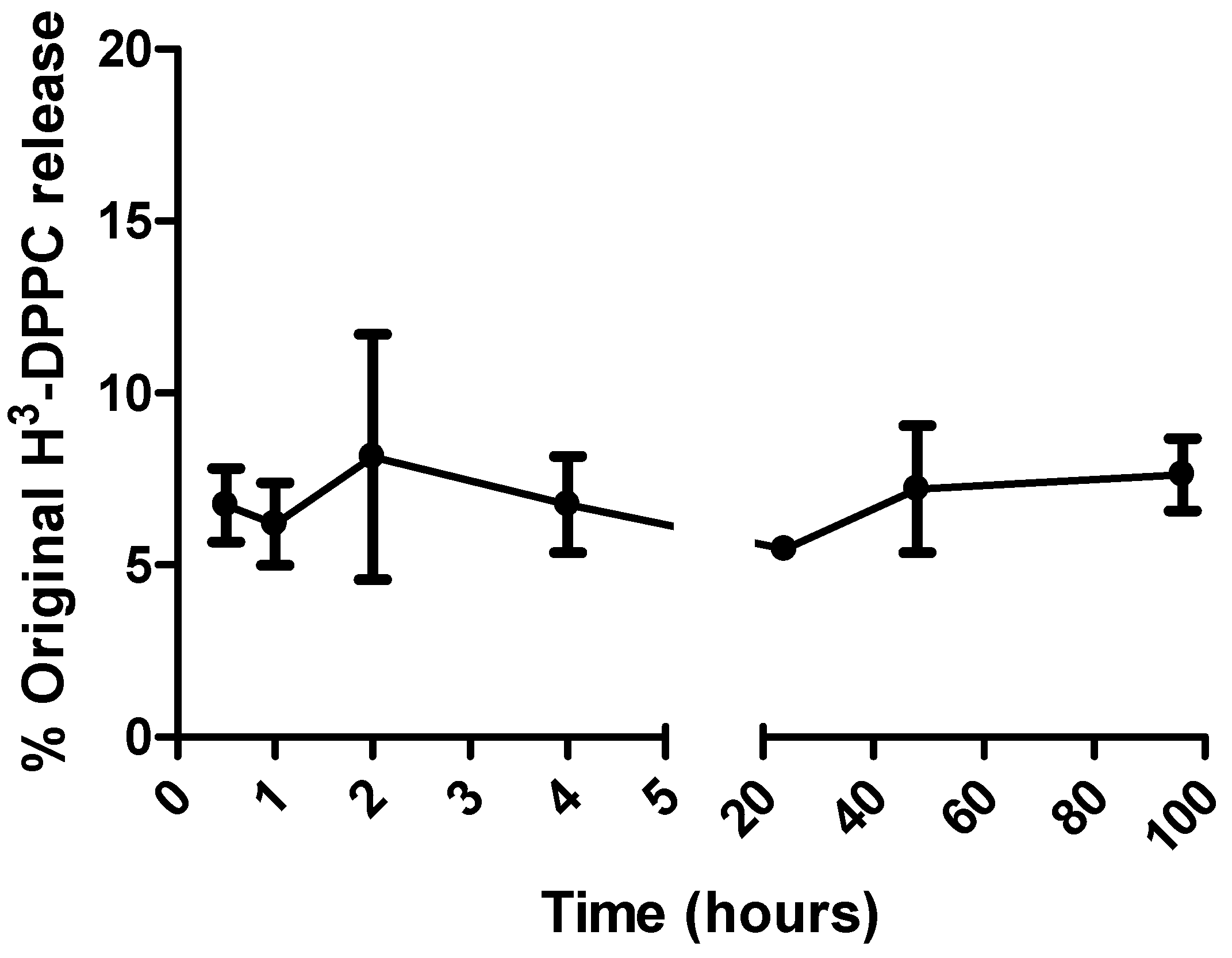
3.2. Radiolabelling of liposomes
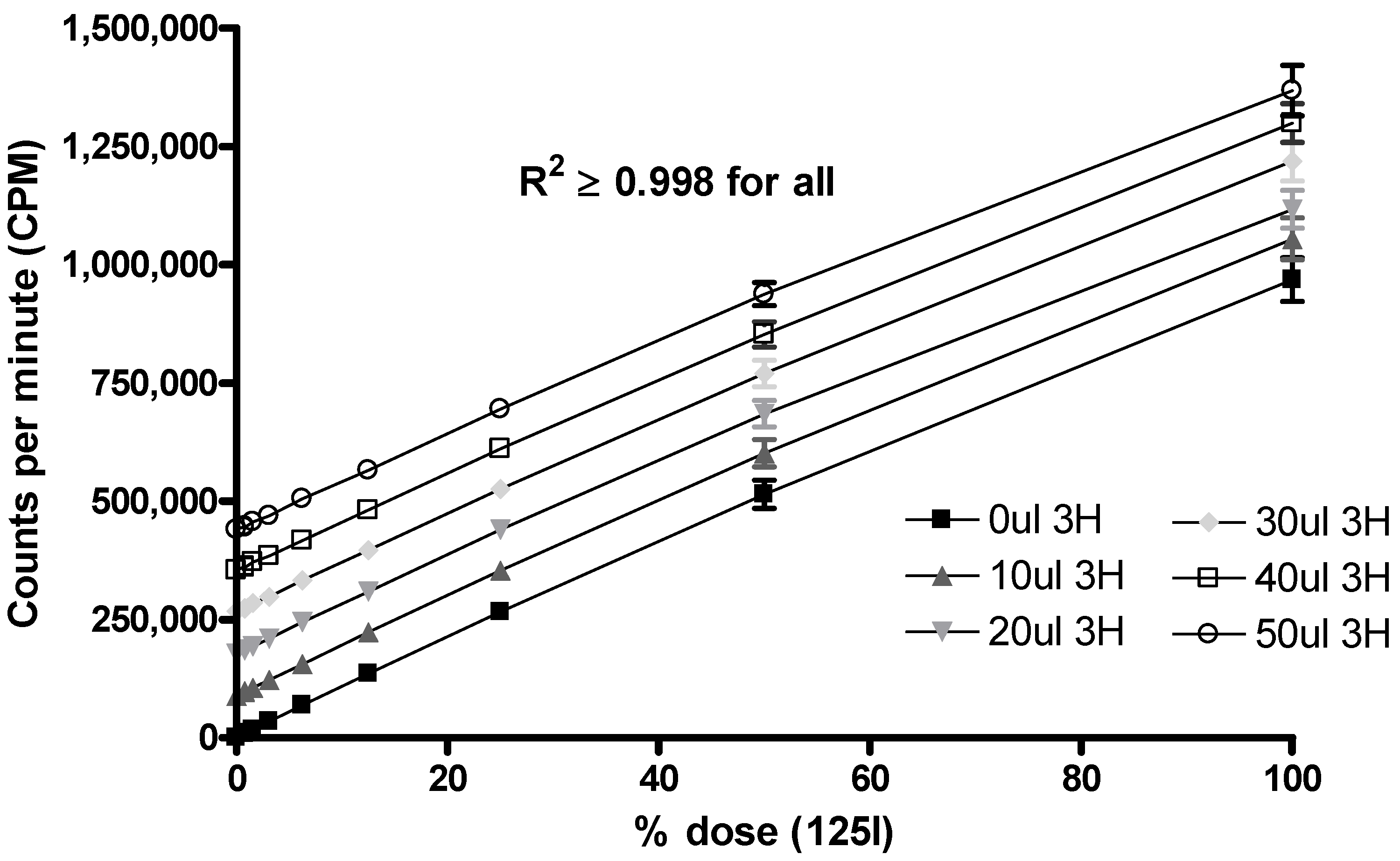
3.3. Measuring dual-radiolabelled preparations in tissues
3.4. Tracking the In Vivo Fate of Radiolabelled Systems
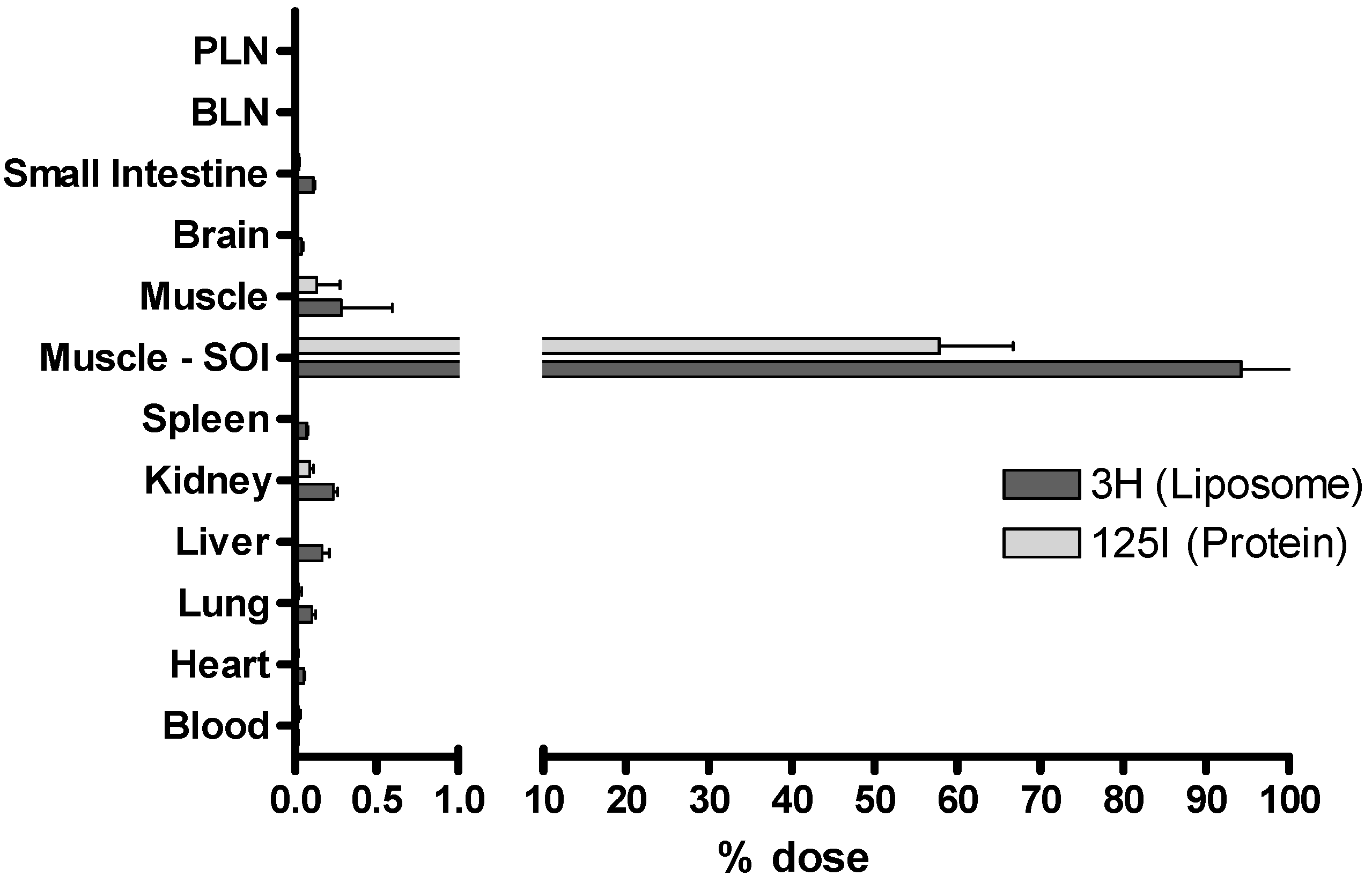
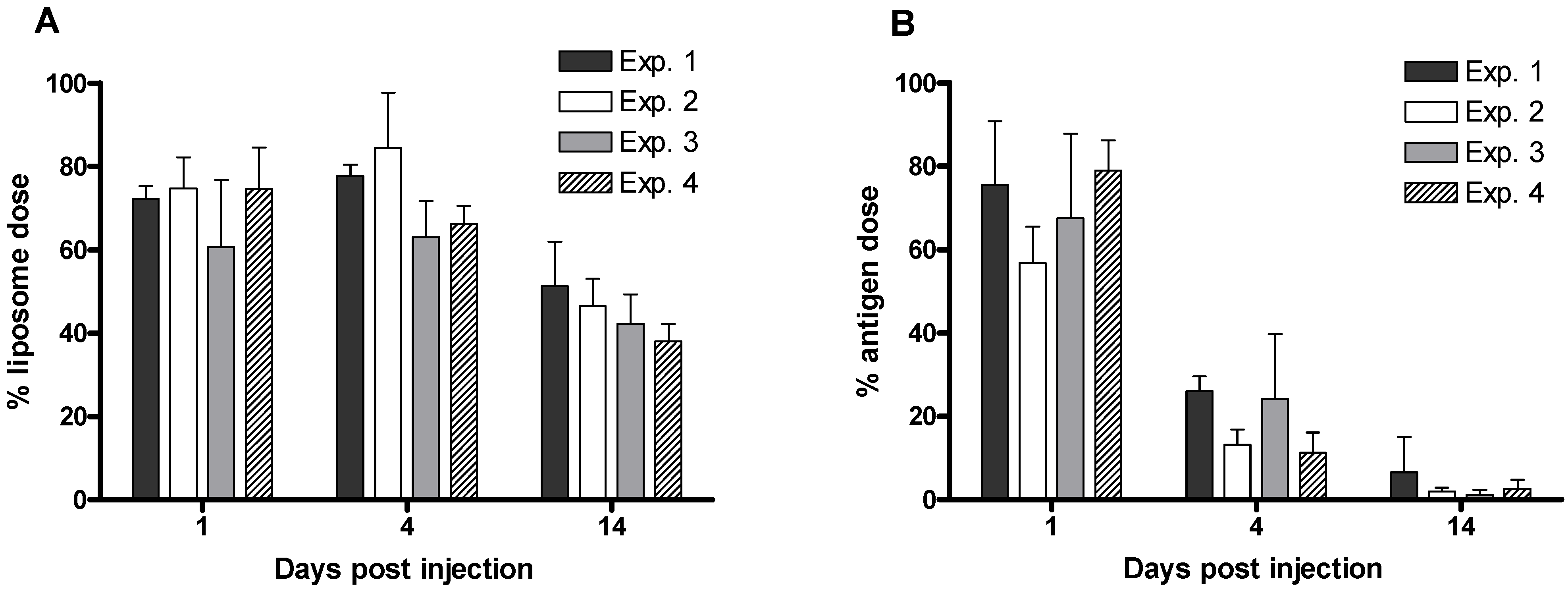
4. Conclusion
References
- Hevesy, G.d.; Paneth, F. The solubility of lead sulphide and lead chromate. Z. Anorg. Chem. 1913, 82, 322. [Google Scholar]
- Hevesy, G.d.; Chievitz, O. Radioactive indicators in the study of phosphorous in the metabolism in rats. Nature 1935, 136, 754–755. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C. Immunological concepts of vaccine adjuvant activity. Curr. Opin. Immunol. 2000, 12, 456–463. [Google Scholar] [CrossRef]
- Guidelines on Adjuvants in Vaccines for Human Use; European Medicine Agency: London, UK, 2005; p. 18.
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterisation of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6'-dibehenate) - A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta. 2005, 1718, 22–31. [Google Scholar] [CrossRef]
- Vangala, A.; Bramwell, V.W.; McNeil, S.; Christensen, D.; Agger, E.M.; Perrie, Y. Comparison of vesicle based antigen delivery systems for delivery of hepatitis B surface antigen. J. Control. Release 2007, 119, 102–110. [Google Scholar] [CrossRef]
- Perrie, Y.; Gregoriadis, G. Liposome-entrapped plasmid DNA: characterisation studies. Biochim. Biophys. Acta. 2000, 1475, 125–132. [Google Scholar] [CrossRef]
- Bhowruth, V.; Minnikin, D.E.; Agger, E.M.; Andersen, P.; Bramwell, V.W.; Perrie, Y.; Besra, G.S. Adjuvant Properties of a Simplified C32 Monomycolyl Glycerol Analogue. Bioorg. Med. Chem. Lett. 2009, 19, 2029–2032. [Google Scholar]
- Holmes, K.L.; Lantz, L.M. Protein labeling with fluorescent probes. Methods Cell Biol. 2001, 63, 185–204. [Google Scholar] [CrossRef]
- Medina, L.A.; Klipper, R.; Phillips, W.T.; Goins, B. Pharmacokinetics and biodistribution of [111In]-avidin and [99mTc]-biotin-liposomes injected in the pleural space for the targeting of mediastinal nodes. Nucl. Med. Biol. 2004, 31, 41–51. [Google Scholar] [CrossRef]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.A.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochimica et Biophysica Acta 1997, 1328, 261–272. [Google Scholar] [CrossRef]
- Oussoren, C.; Velinova, M.; Scherphof, G.; Want, J.J.v.d.; Rooijen, N.v.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. IV. Fate of liposomes in reginal lymph nodes. Biochimica et Biophysica Acta 1998, 1370, 259–272. [Google Scholar] [CrossRef]
- Niven, R.; Pearlman, R.; Wedeking, T.; Mackeigen, J.; Noker, P.; Simpson-Herren, L.; Smith, J.G. Biodistribution of Radiolabeled Lipid-DNA Complexes and DNA in Mice. J. Pharm. Sci. 1998, 87, 1292–1299. [Google Scholar] [CrossRef]
- Soundararajana, A.; Bao, A.; Phillips, W.T.; III, R.P.; Goins, B.A. [186Re]Liposomal doxorubicin (Doxil): in vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl. Med. Biol. 2009, 36, 515–524. [Google Scholar] [CrossRef]
- Kirby, D.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.A.; Perrie, Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J. Drug Target 2008, 16, 543–554. [Google Scholar]
- Vangala, A.; Kirby, D.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Perrie, Y. A Comparative study of cationic liposome and niosome-based systems for protein subunit vaccines: characterisation, environmental scanning electron microscopy and immunisation studies in mice. J. Pharm. Pharmacol. 2006, 58, 787–799. [Google Scholar] [CrossRef]
- Perrie, Y.; Mohammed, A.R.; Vangala, A.; McNeil, S. Environmental scanning electron microscopy offers real-time morphological analysis of liposomes and niosomes. J. Liposome Res. 2007, 17, 27–37. [Google Scholar] [CrossRef]
- Kirby, D.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.A.; Perrie, Y. PLGA microspheres for the delivery of a novel subunit TB vaccine. J Drug Target. 2008, 16:4, 282–293. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Salacinski, P.R.P.; McLean, C.; Sykes, J.E.C.; Clement-Jones, V.V.; Lowry, P.J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen). Anal. Biochem. 1981, 117, 136–146. [Google Scholar] [CrossRef]
- Pause, E.; Bormer, O.; Nustad, K. Radioiodination of proteins with the IODO-GEN method. In Radioimmunoassay and Related Procedures in Medicine; I.A. Agency: Vienna, Austria, 1982; pp. 161–171. [Google Scholar]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and immunogenicity of the vaccine. J. Control. Release 2010, in press. [Google Scholar]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release 2009, 142, 180–186. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Henriksen-Lacey, M.; Bramwell, V.; Perrie, Y. Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies. Pharmaceutics 2010, 2, 91-104. https://doi.org/10.3390/pharmaceutics2020091
Henriksen-Lacey M, Bramwell V, Perrie Y. Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies. Pharmaceutics. 2010; 2(2):91-104. https://doi.org/10.3390/pharmaceutics2020091
Chicago/Turabian StyleHenriksen-Lacey, Malou, Vincent Bramwell, and Yvonne Perrie. 2010. "Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies" Pharmaceutics 2, no. 2: 91-104. https://doi.org/10.3390/pharmaceutics2020091
APA StyleHenriksen-Lacey, M., Bramwell, V., & Perrie, Y. (2010). Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies. Pharmaceutics, 2(2), 91-104. https://doi.org/10.3390/pharmaceutics2020091




