Effects of Polysaccharide Coating on Cell-Surface Association and Endocytic Uptake of PLGA Nanomicelles in MCF-7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Natural Synthesis of Halomonas Levan (HL)
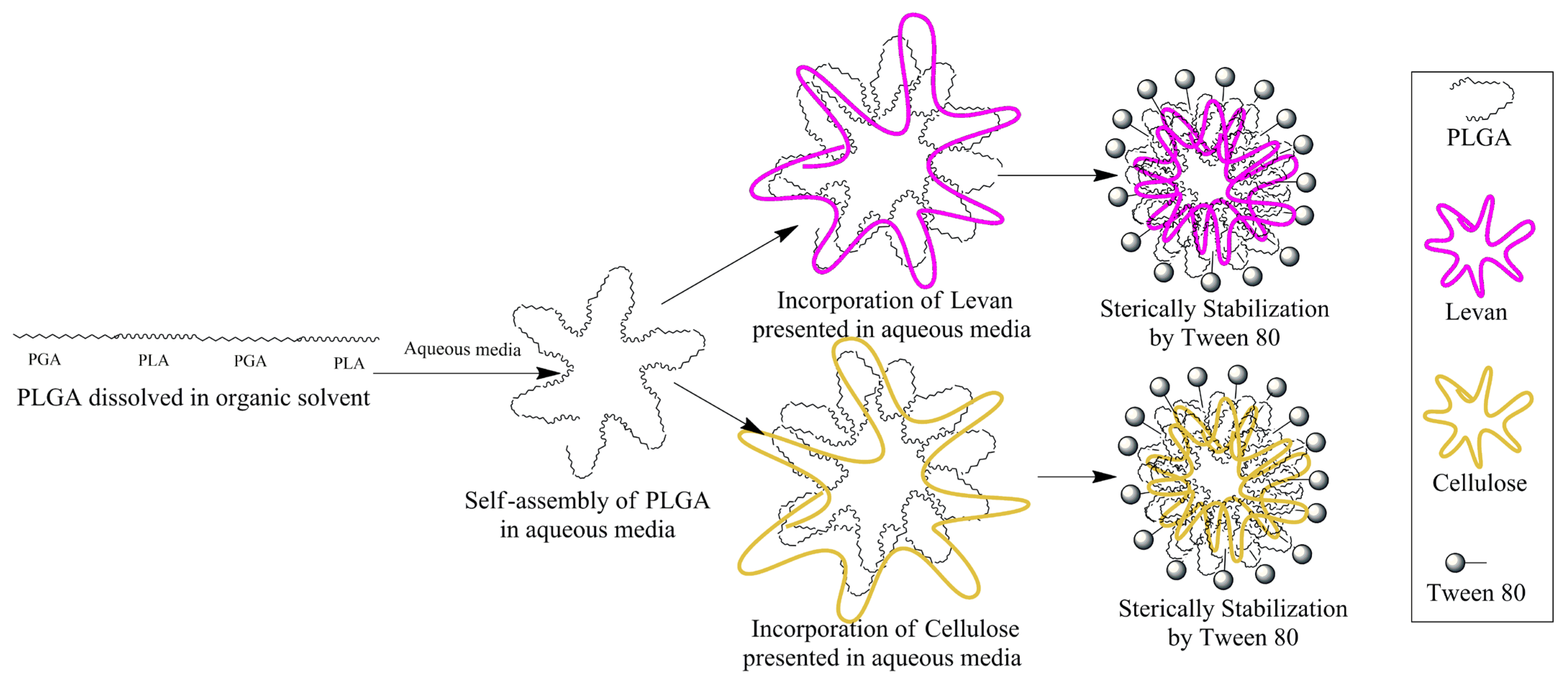
2.2.2. Fabrication of Nanomicelles of PLGA (PM), FITC Labelled PLGA (FPM), PLGA-Levan (FPLM), and PLGA-Cellulose (FPCM)
2.2.3. Characterization of NPs
2.2.4. Cell Culture
2.2.5. Toxicity Assays
2.2.6. Determination of the Cell Internalization Period of NPs
2.2.7. Cell Internalization Mechanism of NPs
2.2.8. TEM Analysis
2.2.9. Statistical Analysis
3. Results
3.1. Particle Size Distribution and Zeta Potential of NPs
3.2. Determination of Incorporation of PLGA with Polysaccharides Using DSC
3.3. Toxicity Assays
3.4. Determination of Max. Cell Internalization Period of NPs
3.5. Cell Internalization Mechanism of NPs
3.6. TEM Images of NDDS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLGA | Poly (D, L-lactide-co-glycolide) |
| NDDS | Nanodrug delivery system |
| CME | Clathrin-mediated endocytosis |
| CavME | Caveolae-mediated endocytosis |
| MPC | Macropinocytosis |
| C | Cellulose |
| HL | Halomonas Levan |
| FPM | FITC-labeled PLGA |
| FPCM | FITC-labeled PLGA decorated with Cellulose |
| FPLM | FITC-labeled PLGA decorated with Halomonas Levan |
| DLS | Dynamic Light Scattering |
| DSC | Differential Scanning Calorimetry |
References
- Eskandari, Z.; Bahadori, F.; Celik, B.; Onyuksel, H. Targeted nanomedicines for cancer therapy, from basics to clinical trials. J. Pharm. Pharm. Sci. 2020, 23, 132–157. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yao, Q.; Zhang, H.; Chu, M.; Bhutia, Y.D.; Chen, R.; Ganapathy, V. Transporter-targeted nano-sized vehicles for enhanced and site-specific drug delivery. Cancers 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, F.-K.; Dang, Q.-F.; Liang, X.-G.; Chen, X.-G. Glucose-conjugated chitosan nanoparticles for targeted drug delivery and their specific interaction with tumor cells. Front. Mater. Sci. 2014, 8, 363–372. [Google Scholar] [CrossRef]
- Xie, F.; Yao, N.; Qin, Y.; Zhang, Q.; Chen, H.; Yuan, M.; Tang, J.; Li, X.; Fan, W.; Zhang, Q. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 2012, 7, 163. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.-Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin-and caveolae-mediated endocytosis in gene transfer mediated by lipo-and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef]
- Rejman, J.; Conese, M.; Hoekstra, D. Gene transfer by means of lipo-and polyplexes: Role of clathrin and caveolae-mediated endocytosis. J. Liposome Res. 2006, 16, 237–247. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials 2009, 30, 2790–2798. [Google Scholar] [CrossRef]
- Feiner-Gracia, N.; Dols-Perez, A.; Royo, M.; Solans, C.; Garcia-Celma, M.; Fornaguera, C. Cell penetrating peptide grafting of PLGA nanoparticles to enhance cell uptake. Eur. Polym. J. 2018, 108, 429–438. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Mao, Z.; Gao, C. Study of the Selective Uptake Progress of Aptamer-M odified PLGA Particles by Liver Cells. Macromol. Biosci. 2013, 13, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Oner, E.T.; Hemberger, J.; Demirci, T.; Erginer, M.; Yıldız, S.Y. Glycan-Based Nanocarriers in Drug Delivery. In Drug Delivery Approaches and Nanosystems; Apple Academic Press: Palm Bay, FL, USA, 2017; Volume 2, pp. 191–228. [Google Scholar]
- Öner, E.T.; Hernández, L.; Combie, J. Review of levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Kazak Sarilmiser, H.; Toksoy Oner, E. Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem. Eng. J. 2014, 92, 28–34. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233. [Google Scholar]
- Carotenuto, P.; Roma, C.; Rachiglio, A.M.; Botti, G.; D’Alessio, A.; Normanno, N. Triple negative breast cancer: From molecular portrait to therapeutic intervention. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 17–34. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S. Targeting CSC in a most aggressive subtype of breast cancer TNBC. Breast Cancer Metastasis Drug Resist. Chall. Prog. 2019, 1152, 311–334. [Google Scholar]
- Erkorkmaz, B.A.; Kırtel, O.; Duru, Ö.A.; Öner, E.T. Development of a cost-effective production process for Halomonas levan. Bioprocess Biosyst. Eng. 2018, 41, 1247–1259. [Google Scholar] [CrossRef]
- Bahadori, F.; Eskandari, Z.; Ebrahimi, N.; Bostan, M.S.; Eroğlu, M.S.; Oner, E.T. Development and optimization of a novel PLGA-Levan based drug delivery system for curcumin, using a quality-by-design approach. Eur. J. Pharm. Sci. 2019, 138, 105037. [Google Scholar] [CrossRef]
- Spink, C.H. Differential scanning calorimetry. Methods Cell Biol. 2008, 84, 115–141. [Google Scholar] [PubMed]
- Bahadori, F.; Topçu, G.; Eroğlu, M.S.; Önyüksel, H. A new lipid-based nano formulation of vinorelbine. AAPS PharmSciTech 2014, 15, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, Z.; Bahadori, F.; Yenigun, V.B.; Demiray, M.; Eroğlu, M.S.; Kocyigit, A.; Oner, E.T. Levan enhanced the NF-κB suppression activity of an oral nano PLGA-curcumin formulation in breast cancer treatment. Int. J. Biol. Macromol. 2021, 189, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.; R Filho, G.; Ribeiro, S.J.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of methylcellulose on the properties and morphology of polysulfone membranes prepared by phase inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, W.; Zhu, Q.; Ke, K.; Zhu, Q.; Jin, W.; Yu, S.; Yang, Z.; Li, L.; Sun, X. The role and therapeutic potential of macropinocytosis in cancer. Front. Pharmacol. 2022, 13, 919819. [Google Scholar] [CrossRef]
- Canton, J. Macropinocytosis: New insights into its underappreciated role in innate immune cell surveillance. Front. Immunol. 2018, 9, 2286. [Google Scholar] [CrossRef]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Asp. Med. 2022, 83, 100993. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Hunter, M.R.; Kapustin, A.N. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180156. [Google Scholar] [CrossRef]
- Li, R.; Peng, Y.; Pu, Y.; Zhao, Y.; Nie, R.; Guo, L.; Wu, Y. Fructose and biotin co-modified liposomes for dual-targeting breast cancer. J. Liposome Res. 2022, 32, 119–128. [Google Scholar] [CrossRef]
- Prichard, K.L.; O’Brien, N.S.; Murcia, S.R.; Baker, J.R.; McCluskey, A. Role of clathrin and dynamin in clathrin mediated endocytosis/synaptic vesicle recycling and implications in neurological diseases. Front. Cell. Neurosci. 2022, 15, 754110. [Google Scholar] [CrossRef]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore-not just a dynamin inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Fishman, P.H. Filipin-dependent inhibition of cholera toxin: Evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998, 141, 905. [Google Scholar] [CrossRef]
- Parton, R.G.; Taraska, J.W.; Lundmark, R. Is endocytosis by caveolae dependent on dynamin? Nat. Rev. Mol. Cell Biol. 2024, 25, 511–512. [Google Scholar] [CrossRef]
- Ganot, P.; Tambutte, E.; Caminiti-Segonds, N.; Toullec, G.; Allemand, D.; Tambutte, S. Ubiquitous macropinocytosis in anthozoans. eLife 2020, 9, e50022. [Google Scholar] [CrossRef]
- Nicolete, R.; dos Santos, D.F.; Faccioli, L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011, 11, 1557–1563. [Google Scholar] [CrossRef]
- Mo, Y.; Lim, L.-Y. Mechanistic study of the uptake of wheat germ agglutinin-conjugated PLGA nanoparticles by A549 cells. J. Pharm. Sci. 2004, 93, 20–28. [Google Scholar] [CrossRef]
- Steinbach, J.M.; Seo, Y.-E.; Saltzman, W.M. Cell penetrating peptide-modified poly (lactic-co-glycolic acid) nanoparticles with enhanced cell internalization. Acta Biomater. 2016, 30, 49–61. [Google Scholar] [CrossRef]
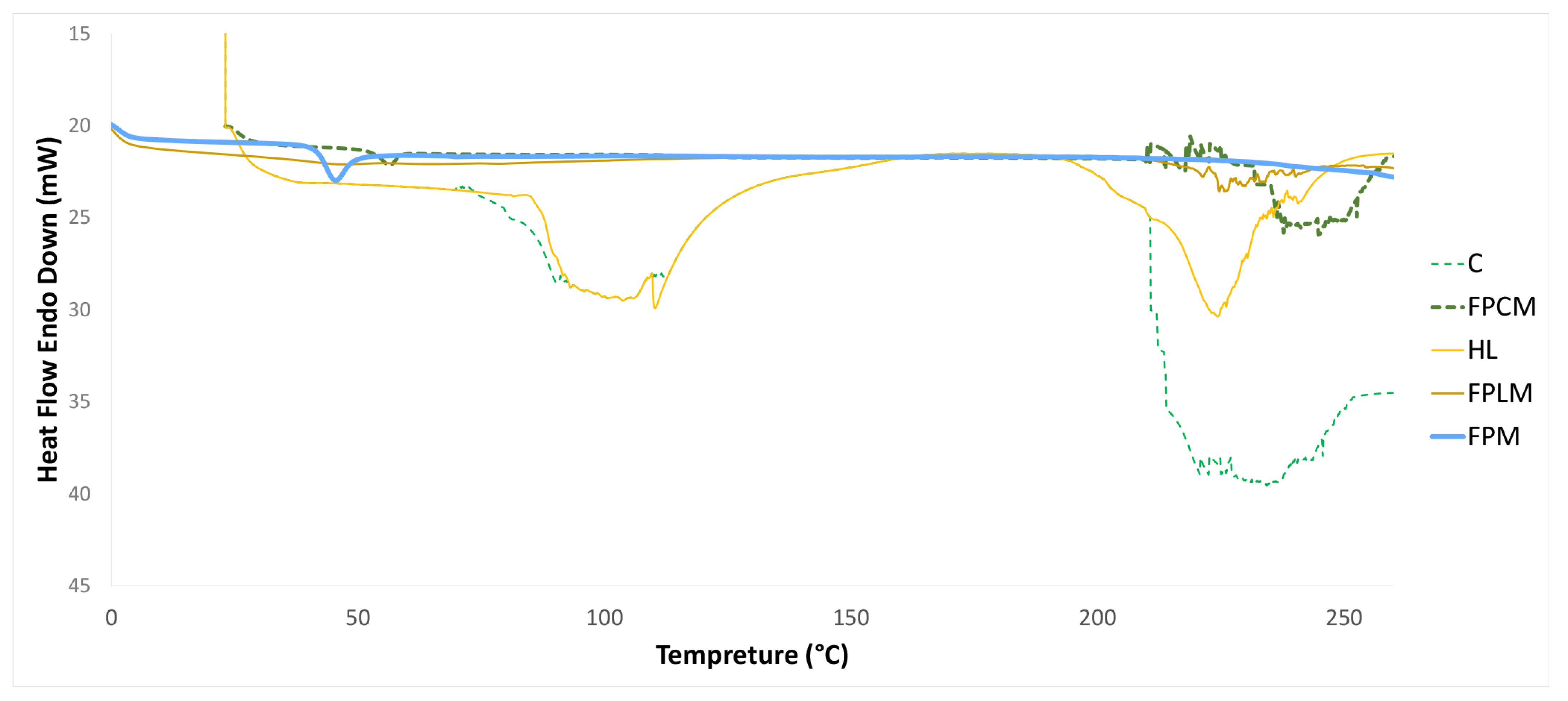

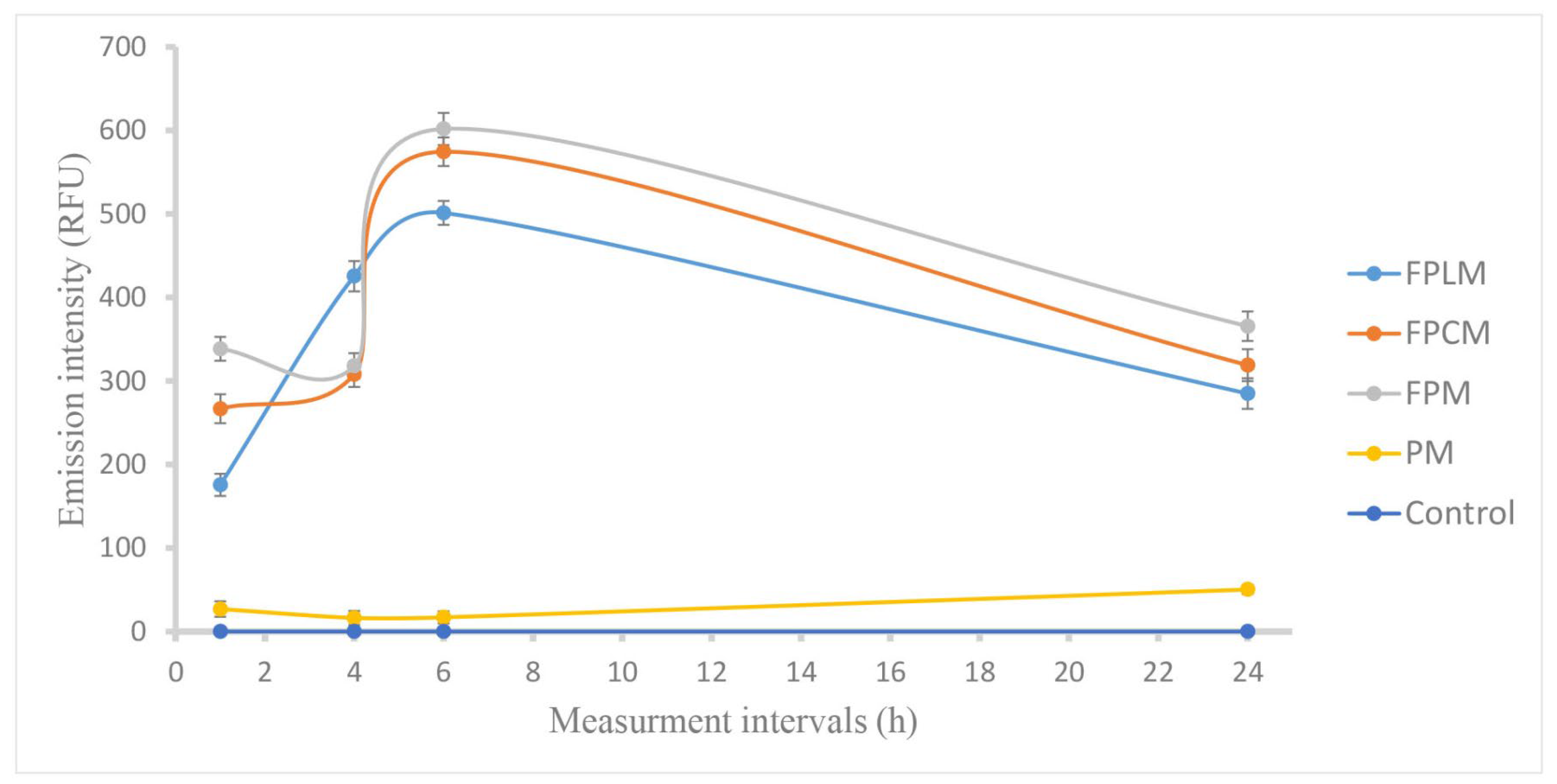



| NP | Particle Size Distribution (Nm) by | ||||
|---|---|---|---|---|---|
| Volume | Number | Intensity | PDI | Zeta Potential (mv) | |
| PM | 142.7 | 115.5 | 151.3 | 0.054 | −3.90 |
| FPM | 270.6 | 137.9 | 285.3 | 0.208 | −9.64 |
| FPLM | 280.6 | 120.8 | 270.4 | 0.182 | −9.28 |
| FPCM | 308.0 | 209.3 | 292.8 | 0.179 | −6.19 |
| Pharmacological Inhibitor | Dynsaore | Genistein | EIPA | Chlorpromazine | Cyclodextrin |
|---|---|---|---|---|---|
| Non-toxic dose (μg/mL) | 28.8 | 72 | 2.5 | 28.8 | 5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Alkan, A.B.; Develi Arslanhan, E.N.; Bahadori, F.; Kasapoglu, M.Z.; Akbas, F.; Susgun, S.; Eskandari, Z.; Oner, E.T. Effects of Polysaccharide Coating on Cell-Surface Association and Endocytic Uptake of PLGA Nanomicelles in MCF-7 Cells. Pharmaceutics 2026, 18, 17. https://doi.org/10.3390/pharmaceutics18010017
Alkan AB, Develi Arslanhan EN, Bahadori F, Kasapoglu MZ, Akbas F, Susgun S, Eskandari Z, Oner ET. Effects of Polysaccharide Coating on Cell-Surface Association and Endocytic Uptake of PLGA Nanomicelles in MCF-7 Cells. Pharmaceutics. 2026; 18(1):17. https://doi.org/10.3390/pharmaceutics18010017
Chicago/Turabian StyleAlkan, Abdulkadir Bahadir, Esma Nur Develi Arslanhan, Fatemeh Bahadori, Muhammed Zahid Kasapoglu, Fahri Akbas, Seda Susgun, Zahra Eskandari, and Ebru Toksoy Oner. 2026. "Effects of Polysaccharide Coating on Cell-Surface Association and Endocytic Uptake of PLGA Nanomicelles in MCF-7 Cells" Pharmaceutics 18, no. 1: 17. https://doi.org/10.3390/pharmaceutics18010017
APA StyleAlkan, A. B., Develi Arslanhan, E. N., Bahadori, F., Kasapoglu, M. Z., Akbas, F., Susgun, S., Eskandari, Z., & Oner, E. T. (2026). Effects of Polysaccharide Coating on Cell-Surface Association and Endocytic Uptake of PLGA Nanomicelles in MCF-7 Cells. Pharmaceutics, 18(1), 17. https://doi.org/10.3390/pharmaceutics18010017






