Formulation, Optimization, and Evaluation of Transferosomes Co-Loaded with Methotrexate and Sorafenib for Anti-Arthritic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of MTX-SRF-TFS
2.4. Optimization of SRF-MTX-TFS
2.5. Particle Size, Zeta Potential, Polydispersability Index, and External Morphology of SRF-MTX-TFS
2.6. SRF and MTX Entrapment Efficiency of SRF-MTX-TFS
2.7. Designing and Assessing of SRF-MTX-TFS Gel
2.8. Skin Permeation and Deposition Studies
2.9. In Vivo Antiarthritic Activity
- ➢
- Negative Control (immunized with CFA and kept untreated).
- ➢
- Normal group (not injected with CFA and treated with normal saline).
- ➢
- Plain MTX-SRF gel (injected with CFA, followed by the treatment) (MTX dose 0.5 mg/kg/day and SRF 10 mg/kg/day).
- ➢
- MTX-SRF-TFS gel (injected with CFA followed by the treatment) (MTX dose 0.5 mg/kg/day and SRF 1.75 mg/kg/day).
2.10. Radiology Studies
2.11. Animal Joint Tissue Histology
2.12. Statistical Analysis
3. Results and Discussion
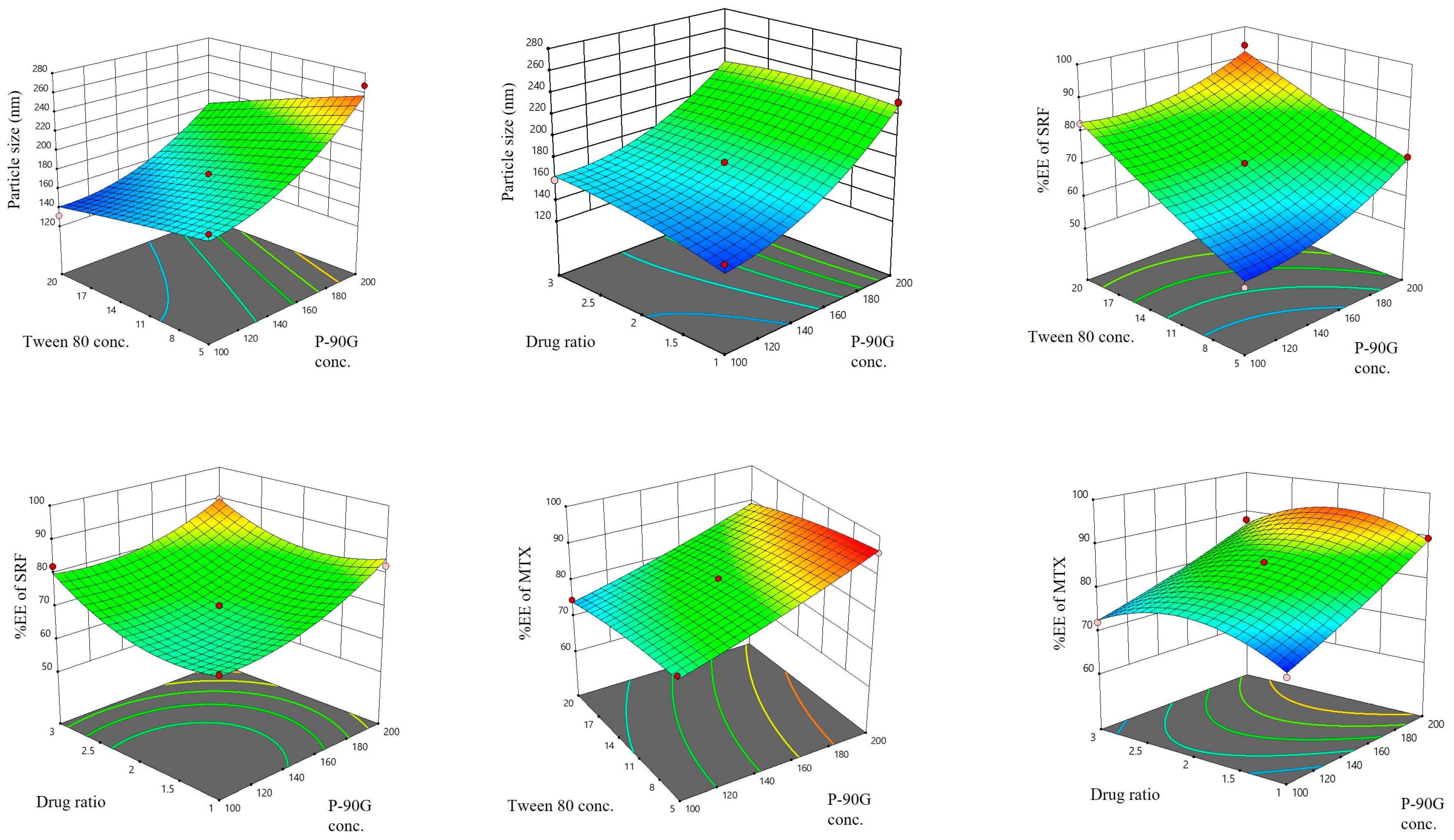
3.1. Box–Behnken Design for SRF-MTX-TFS Optimization
3.1.1. Effect of Independent Factors on the SRF-MTX-TFS Particle Size
3.1.2. Effect of Independent Factors on the %EE of SRF
3.1.3. Effect of Independent Factors on the %EE of MTX
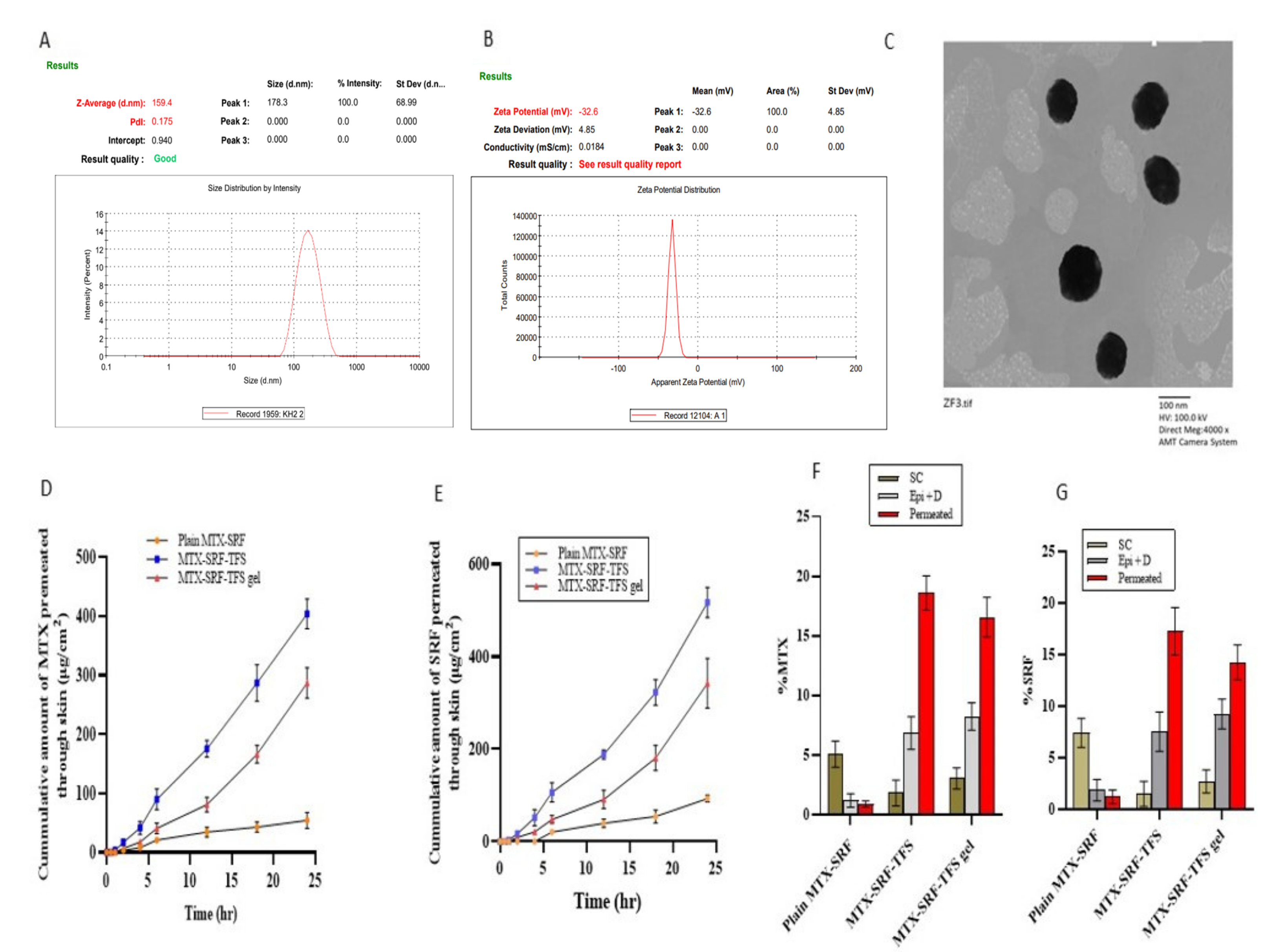
3.2. Zeta Potential and Polydispersability Index of Optimized SRF-MTX-TFS
3.3. External Morphology of Optimized SRF-MTX-TFS
3.4. Ex Vivo Permeation and Retention Assay
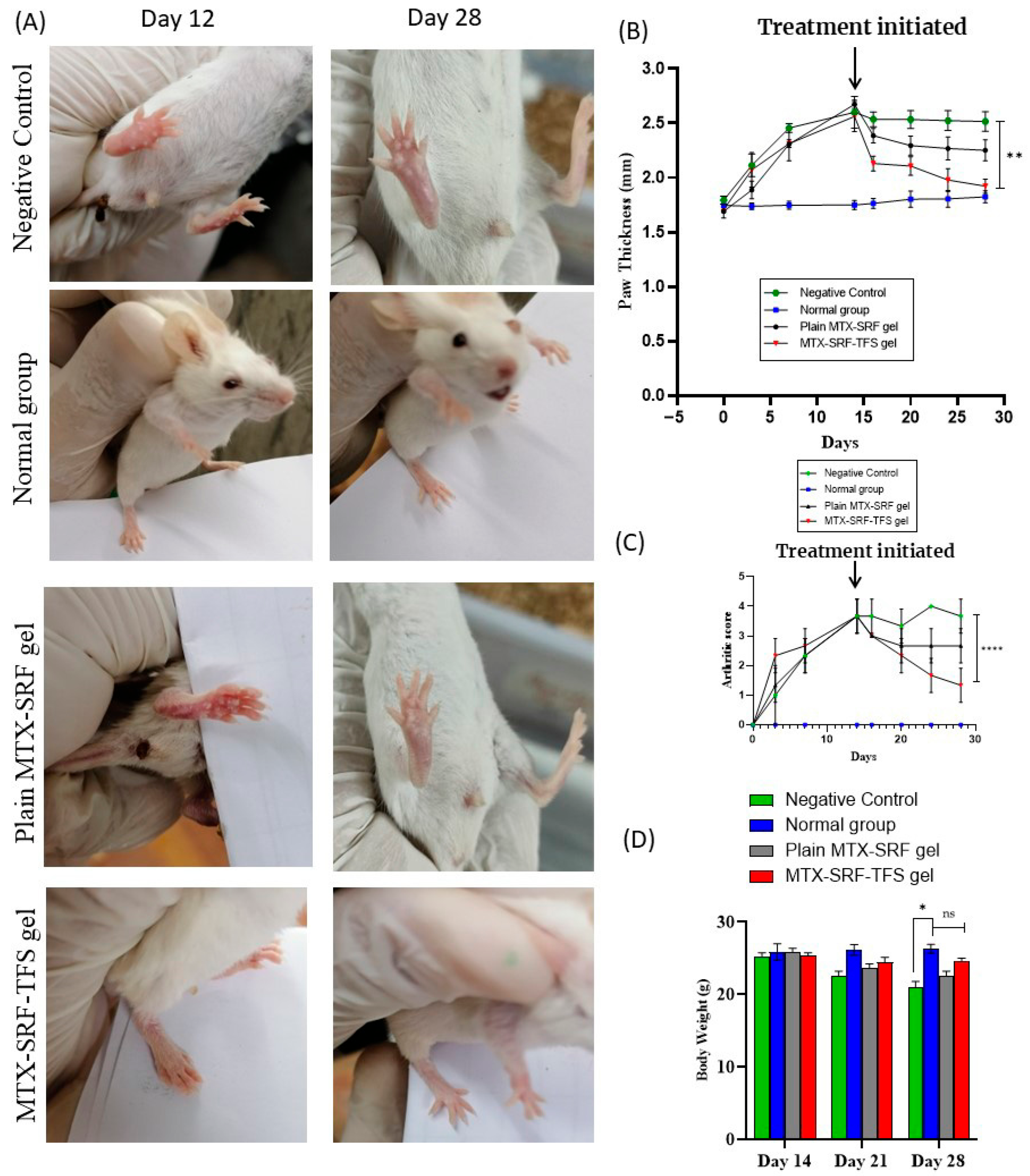
3.5. In Vivo Anti-Arthritic Study: Arthritic Score and Paw Thickness
3.6. Effect on BALB/c Mice Body Weight
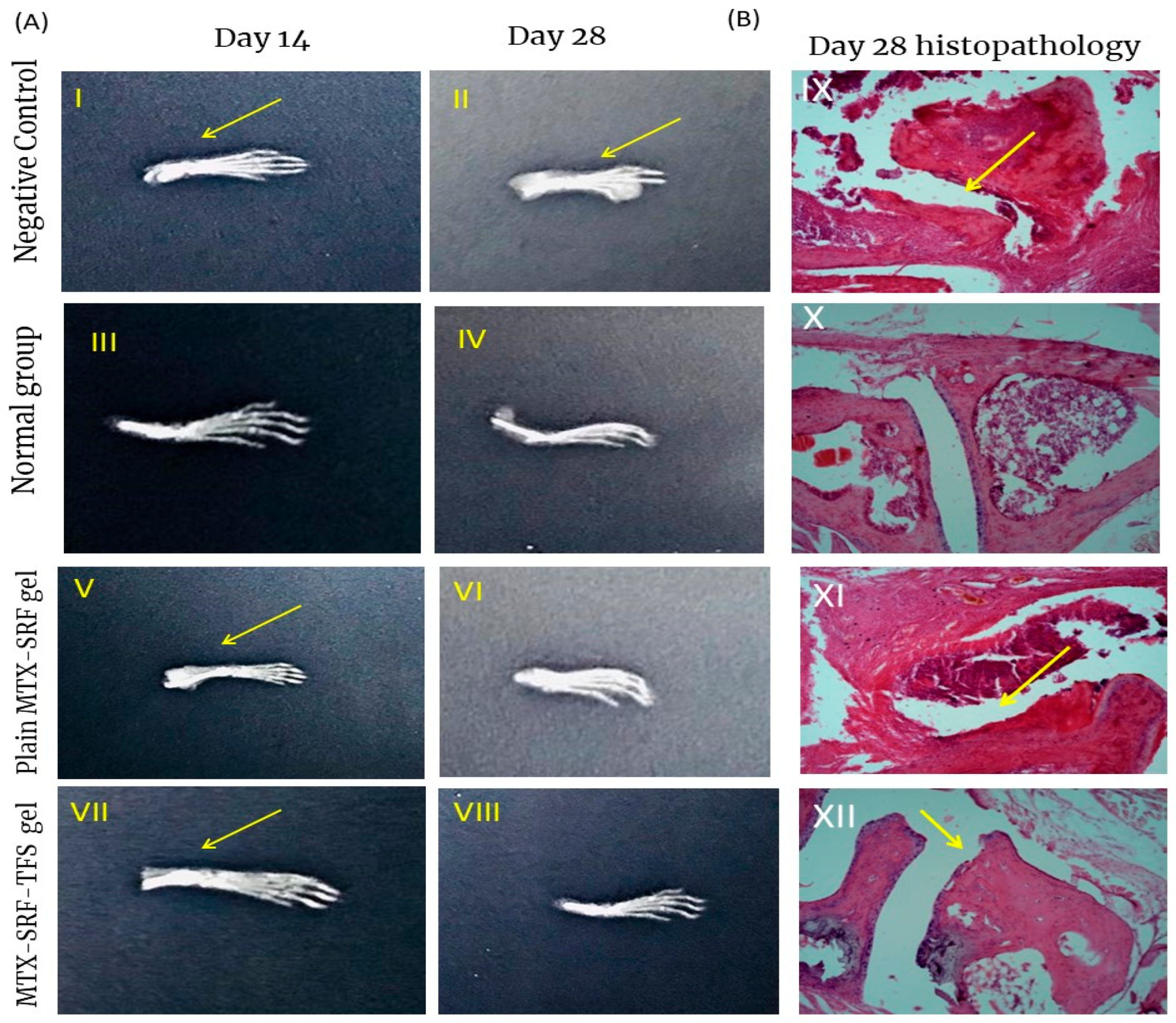
3.7. X-Ray Radiographs
3.8. Bone and Cartilage Erosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar]
- Haroon, N.; Aggarwal, A.; Lawrence, A.; Agarwal, V.; Misra, R. Impact of rheumatoid arthritis on quality of life. Mod. Rheumatol. 2007, 17, 290–295. [Google Scholar] [CrossRef]
- Derksen, V.; Huizinga, T.W.J.; Van Der Woude, D. The Role of Autoantibodies in the Pathophysiology of Rheumatoid Arthritis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 437–446. [Google Scholar]
- Cooles, F.A.H.; Isaacs, J.D. Pathophysiology of rheumatoid arthritis. Curr. Opin. Rheumatol. 2011, 23, 233–240. [Google Scholar] [CrossRef]
- Gibofsky, A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am. J. Manag. Care 2012, 18, S295–S302. [Google Scholar]
- Alam, M.M.; Han, H.S.; Sung, S.; Kang, J.H.; Sa, K.H.; Al Faruque, H.; Hong, J.; Nam, E.J.; San Kim, I.; Park, J.H. Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis. J. Control. Release 2017, 252, 62–72. [Google Scholar]
- Qindeel, M.; Khan, D.; Ahmed, N.; Khan, S.; Asimur, R. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano 2020, 14, 4662–4681. [Google Scholar] [CrossRef] [PubMed]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152. [Google Scholar] [CrossRef] [PubMed]
- Gözel, N.; Çakirer, M.; Karataş, A.; Tuzcu, M.; Özdemir, F.A.; Dağli, A.F.; Şahin, K.; Koca, S.S. Sorafenib reveals anti-arthritic potentials in collagen induced experimental arthritis model. Arch. Rheumatol. 2018, 33, 309. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, L.; Liu, J.; Lian, D.; Li, X. Sorafenib-loaded nanoparticles based on biodegradable dendritic polymers for enhanced therapy of hepatocellular carcinoma. Int. J. Nanomed. 2020, 15, 1469. [Google Scholar] [CrossRef]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes-A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, H.; Khan, G.M. Nanotechnology based solutions for anti-leishmanial impediments: A detailed insight. J. Nanobiotechnol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Akram, M.W.; Jamshaid, H.; Rehman, F.U.; Zaeem, M.; Zeb, A. Transfersomes: A Revolutionary Nanosystem for Efficient Transdermal Drug Delivery. AAPS PharmSciTech 2022, 23, 7. [Google Scholar]
- Khan, A.U.; Jamshaid, H.; ud Din, F.; Zeb, A.; Khan, G.M. Designing, optimization and characterization of Trifluralin transfersomal gel to passively target cutaneous leishmaniasis. J. Pharm. Sci. 2022, 111, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Abd El-Maboud, M.M.; Said, A.S.; Salem, M.N.; Sabry, D.; Hussain, N.; El-Ghafar, O.A.A.; Hussein, R.R. Nano methotrexate versus methotrexate in targeting rheumatoid arthritis. Pharmaceuticals 2022, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.J.; Din, F.U.; Khan, G.M. Sodium stibogluconate loaded nano-deformable liposomes for topical treatment of leishmaniasis: Macrophage as a target cell. Drug Deliv. 2018, 25, 1595–1606. [Google Scholar] [CrossRef]
- Sabbagh, F. pH-Responsive Transdermal Release from Poly (vinyl alcohol)-Coated Liposomes and Transethosomes: Investigating the Role of Coating in Delayed Drug Delivery. ACS Appl. Bio Mater. 2025, 8, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Sana, E.; Zeeshan, M.; Ain, Q.U.; Khan, A.U.; Hussain, I.; Khan, S.; Lepeltier, E.; Ali, H. Topical delivery of curcumin-loaded transfersomes gel ameliorated rheumatoid arthritis by inhibiting NF-κβ pathway. Nanomedicine 2021, 16, 819–837. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar]
- Batool, S.; Zahid, F.; Ud-Din, F.; Naz, S.S.; Dar, M.J.; Khan, M.W.; Zeb, A.; Khan, G.M. Macrophage targeting with the novel carbopol-based miltefosine-loaded transfersomal gel for the treatment of cutaneous leishmaniasis: In vitro and in vivo analyses. Drug Dev. Ind. Pharm. 2021, 47, 440–453. [Google Scholar] [CrossRef]
- Kavian, Z.; Alavizadeh, S.H.; Golmohamadzadeh, S.; Badiee, A.; Khamesipour, A.; Jaafari, M.R. Development of topical liposomes containing miltefosine for the treatment of Leishmania major infection in susceptible BALB/c mice. Acta Trop. 2019, 196, 142–149. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef]
- Dar, M.J.; Khalid, S.; Varikuti, S.; Satoskar, A.R.; Khan, G.M. Nano-elastic liposomes as multidrug carrier of sodium stibogluconate and ketoconazole: A potential new approach for the topical treatment of cutaneous Leishmaniasis. Eur. J. Pharm. Sci. 2020, 145, 105256. [Google Scholar] [CrossRef]
- Alzarea, S.I.; Alasmari, A.F.; Alanazi, A.S.; Alzarea, A.I.; Alharbi, M.; Alshammari, A.; Kazmi, I.; Aljoufi, F.A.; Sayyed, N.; Afzal, M. Butin attenuates arthritis in complete Freund’s adjuvant-treated arthritic rats: Possibly mediated by its antioxidant and anti-inflammatory actions. Front. Pharmacol. 2022, 13, 810052. [Google Scholar]
- Radojčić, M.R.; Thudium, C.S.; Henriksen, K.; Tan, K.; Karlsten, R.; Dudley, A.; Chessell, I.; Karsdal, M.A.; Bay-Jensen, A.-C.; Crema, M.D. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain 2017, 158, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
| Independent Variables | Levels Assessed | ||
|---|---|---|---|
| Low Level (−1) | Medium Level (0) | High Level (+1) | |
| A1: Lipid Conc. (mg) | 100 | 150 | 200 |
| A2: Tween80 Conc. (% w/w) | 5 | 12.5 | 20 |
| A3: Drug Ratio | 1:1 | 1:2 | 2:1 |
| Dependent Factors | Required response | ||
| B1: Particle Size (nm) | Minimize | ||
| B2: Entrapment Efficiency of SRF (%) | Maximize | ||
| B3: Entrapment Efficiency MTX (%) | Maximize | ||
| Formulation | Independent Variables | Dependent Factors | ||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | B1 | B2 | B3 | |
| F1 | 100 | 12.5 | 1:1 | 145.70 ± 2.61 | 68.44 ± 2.17 | 69.54 ± 1.84 |
| F2 | 100 | 5 | 1:2 | 174.76 ± 1.87 | 52.93 ± 2.01 | 79.68 ± 2.06 |
| F3 | 100 | 12.5 | 2:1 | 159.44 ± 3.89 | 82.16 ± 4.88 | 72.12 ± 3.21 |
| F4 | 150 | 20 | 2:1 | 162.20 ± 2.80 | 92.16 ± 4.95 | 81.54 ± 3.23 |
| F5 | 150 | 20 | 1:1 | 139.64 ± 1.56 | 84.27 ± 3.67 | 73.92 ± 2.65 |
| F6 | 200 | 12.5 | 2:1 | 220.02 ± 6.54 | 89.62 ± 3.33 | 87.89 ± 4.44 |
| F7 | 100 | 20 | 1:2 | 131.30 ± 2.87 | 82.39 ± 1.72 | 74.73 ± 1.76 |
| F8 | 150 | 5 | 1:1 | 179.05 ± 3.65 | 62.76 ± 2.13 | 82.67 ± 5.24 |
| F9 | 150 | 5 | 2:1 | 185.44 ± 3.65 | 68.32 ± 3.21 | 78.78 ± 3.38 |
| F10 | 150 | 12.5 | 1:2 | 176.27 ± 4.13 | 70.45 ± 5.92 | 84.95 ± 2.99 |
| F11 | 200 | 5 | 1:2 | 267.44 ± 6.78 | 72.33 ± 2.72 | 95.75 ± 5.64 |
| F12 | 200 | 20 | 1:2 | 204.29 ± 3.22 | 93.84 ± 5.04 | 88.45 ± 3.05 |
| F13 | 200 | 12.5 | 1:1 | 231.55 ± 4.98 | 82.32 ± 3.16 | 89.56 ± 2.82 |
| Dependent Responses | R2 | Adjusted R2 | Significant Factors | S. D | Adequate Precision | Expected Optimized Parameters | Actual Optimized Parameters |
|---|---|---|---|---|---|---|---|
| Particle Size (nm) | 0.9538 | 0.8150 | A1, A2 | 16.55 | 8.303 | 161.77 | 159.40 |
| EE% of SRF | 0.9876 | 0.9503 | A1, A2, A3 | 2.73 | 16.262 | 85.60 | 92.16 |
| EE% of MTX | 0.9928 | 0.9713 | A1, A2 | 1.33 | 22.130 | 84.17 | 81.54 |
| Formulation | Total Drug Amount Permeated in 24 h Q (μg/cm2) | Flux Jmax (μg/cm2/h) | Enhancement Ratio | |||
|---|---|---|---|---|---|---|
| SRF | MTX | SRF | MTX | SRF | MTX | |
| MTX-SRFgel | 92.84 ± 7.98 | 54.29 ± 13.53 | 3.86 ± 0.33 | 2.27 ± 0.57 | 1 | 1 |
| MTX-SRF-TFS | 517.15 ± 32.56 | 403.81 ± 25.5 | 21.46 ± 1.35 | 16.73 ± 0.96 | 5.55 | 7.37 |
| MTX-SRF-TFS gel | 342.12 ± 53.93 | 287.12 ± 26.12 | 14.25 ±2.24 | 11.92 ± 1.15 | 3.69 | 5.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Ahmad, L.; Dar, M.J.; Jamshaid, H.; Noman, M.; Faheem, M. Formulation, Optimization, and Evaluation of Transferosomes Co-Loaded with Methotrexate and Sorafenib for Anti-Arthritic Activity. Pharmaceutics 2025, 17, 1196. https://doi.org/10.3390/pharmaceutics17091196
Adnan M, Ahmad L, Dar MJ, Jamshaid H, Noman M, Faheem M. Formulation, Optimization, and Evaluation of Transferosomes Co-Loaded with Methotrexate and Sorafenib for Anti-Arthritic Activity. Pharmaceutics. 2025; 17(9):1196. https://doi.org/10.3390/pharmaceutics17091196
Chicago/Turabian StyleAdnan, Muhammad, Lateef Ahmad, Muhammad Junaid Dar, Humzah Jamshaid, Muhammad Noman, and Muhammad Faheem. 2025. "Formulation, Optimization, and Evaluation of Transferosomes Co-Loaded with Methotrexate and Sorafenib for Anti-Arthritic Activity" Pharmaceutics 17, no. 9: 1196. https://doi.org/10.3390/pharmaceutics17091196
APA StyleAdnan, M., Ahmad, L., Dar, M. J., Jamshaid, H., Noman, M., & Faheem, M. (2025). Formulation, Optimization, and Evaluation of Transferosomes Co-Loaded with Methotrexate and Sorafenib for Anti-Arthritic Activity. Pharmaceutics, 17(9), 1196. https://doi.org/10.3390/pharmaceutics17091196







