Integrating Microorganism-Based Therapy and Emerging Biotechnology in the Treatment of Intracranial Central Nervous System Diseases
Abstract
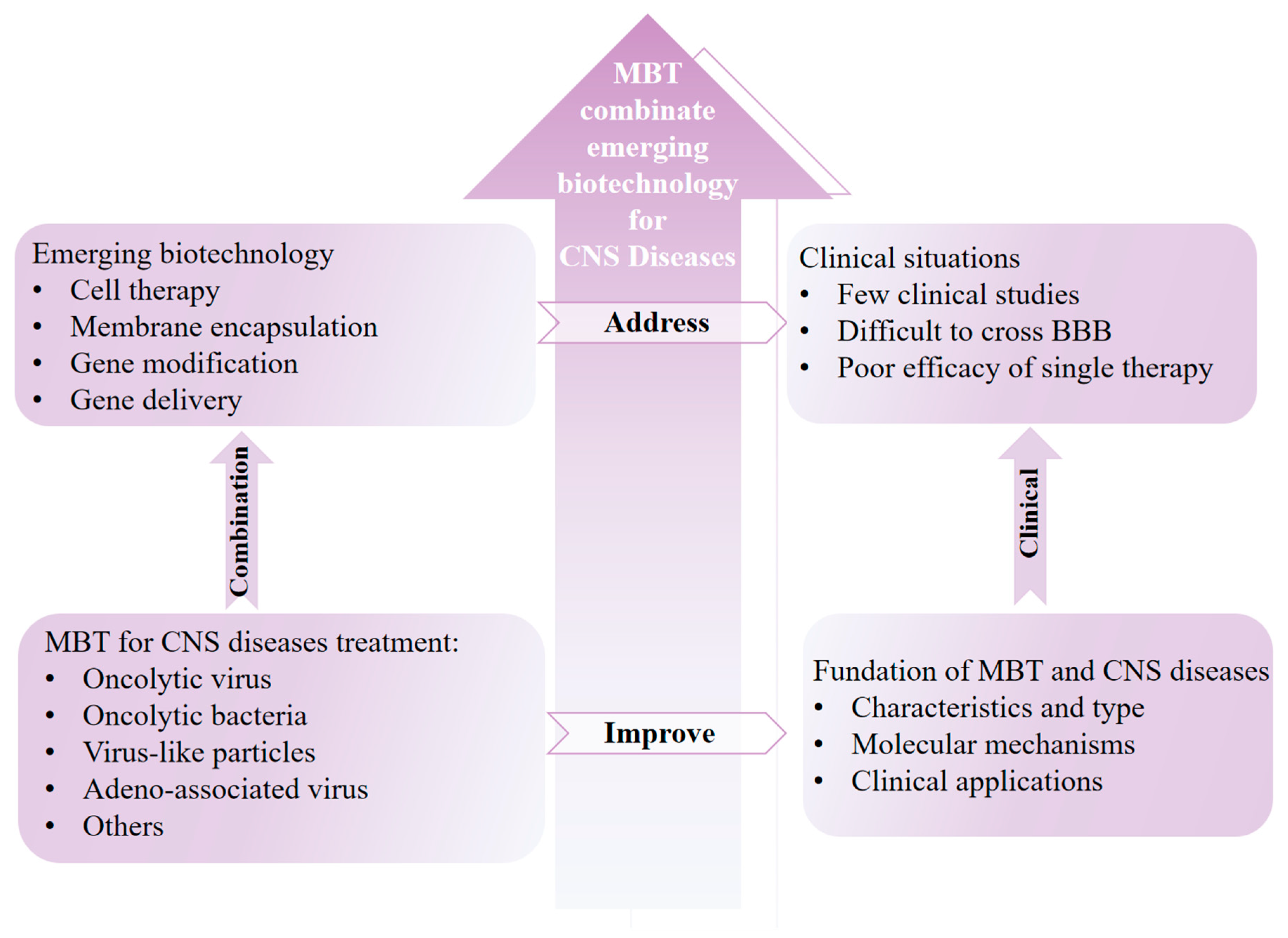
1. Introduction
2. Foundation of Microorganism-Based CNS Disease Therapeutics
2.1. CNS Barriers and Anatomy
2.2. Outline of CNS Diseases
2.2.1. Intracranial Cancer
2.2.2. CNS Inflammation
2.2.3. Neurodegenerative Diseases
2.2.4. Stroke
2.3. Cross-Talk Between CNS Diseases and Microorganisms
3. Emerging Microorganism-Based Therapy for Intracranial Cancer Treatment
3.1. Integrating Virus-Associated Therapy and Emerging Biotechnology Against Intracranial Cancer
3.1.1. Integrating Oncolytic Virus into Cell-Based Technologies Against Intracranial Cancer
3.1.2. Integrating OVs into Membrane Encapsulation Technology Against Intracranial Cancer
3.1.3. Integrating OVs into Gene Modification Technology Against Intracranial Cancer
3.1.4. Integrating OVs into Other Biomedical Technologies Against Intracranial Cancer
3.1.5. Virus-Based Gene Delivery Technologies Against Intracranial Cancer
3.2. Integrating Virus-like Particle-Associated Therapy into Emerging Biotechnology Against Intracranial Cancer
3.3. Integrating Bacteria-Associated Therapy into Emerging Biotechnology Against Intracranial Cancer
4. Emerging Microorganism-Based Therapy for Other CNS Diseases
4.1. Emerging Microorganism-Based Therapy for CNS Inflammation
4.2. Emerging Microorganism-Based Therapy for Neurodegenerative Diseases
4.2.1. Emerging Microorganism-Based Therapy for Parkinson’s Disease
4.2.2. Emerging Microorganism-Based Therapy for Alzheimer’s Disease
4.3. Emerging Microorganism-Based Therapy for Stroke
5. Concluding Remarks
6. Clinical Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Gao, W.; Lichti, C.F.; Gu, X.; Dykstra, T.; Cao, J.; Smirnov, I.; Boskovic, P.; Kleverov, D.; Salvador, A.F.M.; et al. Endogenous self-peptides guard immune privilege of the central nervous system. Nature 2024, 637, 176–183. [Google Scholar] [CrossRef]
- Yalamandala, B.N.; Chen, Y.J.; Lin, Y.H.; Huynh, T.M.H.; Chiang, W.H.; Chou, T.C.; Liu, H.W.; Huang, C.C.; Lu, Y.J.; Chiang, C.S.; et al. A Self-Cascade Penetrating Brain Tumor Immunotherapy Mediated by Near-Infrared II Cell Membrane-Disrupting Nanoflakes via Detained Dendritic Cells. ACS Nano 2024, 18, 18712–18728. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Minami, K.; Yamamoto, T.; Sonoda, H.; Yamaoka, M.; Tachibana, K.; Hirato, T.; Sato, Y. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019, 27, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.J.; Kerklaan, B.M.; Aftimos, P.; Altintas, S.; Jager, A.; Gladdines, W.; Lonnqvist, F.; Soetekouw, P.; Verheul, H.; Awada, A.; et al. Abstract CT216: Phase I dose escalating study of 2B3-101, glutathione PEGylated liposomal doxorubicin, in patients with solid tumors and brain metastases or recurrent malignant glioma. Cancer Res. 2014, 74 (Suppl. 19), CT216. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Li, X.; Qi, L.; Yang, D.; Hao, S.; Zhang, F.; Zhu, X.; Sun, Y.; Chen, C.; Ye, J.; Yang, J.; et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat. Neurosci. 2022, 25, 577–587. [Google Scholar] [CrossRef]
- Huang, Q.; Chan, K.Y.; Wu, J.; Botticello-Romero, N.R.; Zheng, Q.; Lou, S.; Keyes, C.; Svanbergsson, A.; Johnston, J.; Mills, A.; et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science 2024, 384, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Gu, L.; Chen, R.; Zhu, H.; Zhang, X.; Zhang, Y.; Feng, S.; Qiu, S.; Jian, Z.; et al. Gene Targets of CAR-T Cell Therapy for Glioblastoma. Cancers 2023, 15, 2351. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Du, W.; Seah, I.; Bougazzoul, O.; Choi, G.; Meeth, K.; Bosenberg, M.W.; Wakimoto, H.; Fisher, D.; Shah, K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl. Acad. Sci. USA 2017, 114, E6157–E6165. [Google Scholar] [CrossRef]
- István, S.; Dóra, B.; Zsolt, É.; Linda, L.; Zsuzsa, B. Cardiovascular disease and microbiome: Focus on ischemic stroke. Pol. Arch. Intern. Med. 2025, 135, 17088. [Google Scholar]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic Lipopolysaccharide-Free Bacterial Outer Membrane-Functionalized Nanoparticles for Brain-Targeted Drug Delivery. Adv. Sci. 2022, 9, e2105854. [Google Scholar] [CrossRef]
- Smyth, L.C.D.; Xu, D.; Okar, S.V.; Dykstra, T.; Rustenhoven, J.; Papadopoulos, Z.; Bhasiin, K.; Kim, M.W.; Drieu, A.; Mamuladze, T.; et al. Identification of direct connections between the dura and the brain. Nature 2024, 627, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Umpierre, A.D.; Wu, L.J. Tuning neural circuits and behaviors by microglia in the adult brain. Trends Neurosci. 2024, 47, 181–194. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control Release 2020, 321, 372–415. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Motegi, H.; Ishi, Y.; Okamoto, M.; Sawaya, R.; Kobayashi, H.; Terasaka, S.; Houkin, K. Clinical Outcome of Cytoreductive Surgery Prior to Bevacizumab for Patients with Recurrent Glioblastoma: A Single-center Retrospective Analysis. Neurol. Med. Chir. 2021, 61, 245–252. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. Jama 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Lin, N.U.; Overmoyer, B.; Wen, P.Y.; Nayak, L.; Cohen, J.V.; Dietrich, J.; et al. Pembrolizumab in brain metastases of diverse histologies: Phase 2 trial results. Nat. Med. 2023, 29, 1728–1737. [Google Scholar] [CrossRef]
- Alibek, K.; Kakpenova, A.; Baiken, Y. Role of infectious agents in the carcinogenesis of brain and head and neck cancers. Infect. Agents Cancer 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, M.S.; Atwood, W.J. JC virus: An oncogenic virus in animals and humans? Semin. Cancer Biol. 2009, 19, 261–269. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.; Chen, T.; Chang, L.J.; et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol. Cancer 2023, 22, 3. [Google Scholar] [CrossRef]
- Steiner, S.; Kratzel, A.; Barut, G.T.; Lang, R.M.; Aguiar Moreira, E.; Thomann, L.; Kelly, J.N.; Thiel, V. SARS-CoV-2 biology and host interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef]
- Lecca, D.; Jung, Y.J.; Scerba, M.T.; Hwang, I.; Kim, Y.K.; Kim, S.; Modrow, S.; Tweedie, D.; Hsueh, S.C.; Liu, D.; et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimers Dement. 2022, 18, 2327–2340. [Google Scholar] [CrossRef]
- Temple, S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 2023, 111, 1086–1093.e1082. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Hill, L.; Popov, J.; Figueiredo, M.; Caputi, V.; Hartung, E.; Moshkovich, M.; Pai, N. Protocol for a systematic review on the role of the gut microbiome in paediatric neurological disorders. Acta Neuropsychiatr. 2021, 33, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Meng, C.; Deng, P.; Miao, R.; Tang, H.; Li, Y.; Wang, J.; Wu, J.; Wang, W.; Liu, S.; Xia, J.; et al. Gut microbiome and risk of ischaemic stroke: A comprehensive Mendelian randomization study. Eur. J. Prev. Cardiol. 2023, 30, 613–620. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Chervinets, V.M.; Chervinets, Y.V.; Chichanovskaja, L.V.; Ganzja, D.V.; Grigoryants, E.O.; Belyaev, V.S.; Mironov, A.Y. The microbiome of oral cavity patients with periodontitis, adhesive and biofilm forming properties. Klin. Lab. Diagn. 2022, 67, 163–169. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, e2101526. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Khan, N.A.; Shin, S.; Chung, J.W.; Kim, K.J.; Elliott, S.; Wang, Y.; Kim, K.S. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 2003, 35, 35–42. [Google Scholar] [CrossRef]
- Coureuil, M.; Lécuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef]
- Koebnik, R. Structural and functional roles of the surface-exposed loops of the beta-barrel membrane protein OmpA from Escherichia coli. J. Bacteriol. 1999, 181, 3688–3694. [Google Scholar] [CrossRef]
- Chung, J.W.; Hong, S.J.; Kim, K.J.; Goti, D.; Stins, M.F.; Shin, S.; Dawson, V.L.; Dawson, T.M.; Kim, K.S. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003, 278, 16857–16862. [Google Scholar] [CrossRef]
- Cheng, Z.; Zheng, Y.; Yang, W.; Sun, H.; Zhou, F.; Huang, C.; Zhang, S.; Song, Y.; Liang, Q.; Yang, N.; et al. Pathogenic bacteria exploit transferrin receptor transcytosis to penetrate the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2023, 120, e2307899120. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Deng, L.; Neel, D.V.; Erdogan, O.; Basu, H.; Yang, D.; Choi, S.; Walker, A.J.; Carneiro-Nascimento, S.; He, K.; et al. Bacteria hijack a meningeal neuroimmune axis to facilitate brain invasion. Nature 2023, 615, 472–481. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Spellerberg, B.; Adam, R.; Vogel, M.; Kim, K.S.; Schroten, H. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 2007, 9, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.H.; Jäger, V.; Butler, P.J.; van den Heuvel, J.; Schmidt, S.; Ferraris, D.; Gherardi, E.; Heinz, D.W. Structure of the human receptor tyrosine kinase met in complex with the Listeria invasion protein InlB. Cell 2007, 130, 235–246. [Google Scholar] [CrossRef]
- Radin, J.N.; Orihuela, C.J.; Murti, G.; Guglielmo, C.; Murray, P.J.; Tuomanen, E.I. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 2005, 73, 7827–7835. [Google Scholar] [CrossRef]
- Unkmeir, A.; Latsch, K.; Dietrich, G.; Wintermeyer, E.; Schinke, B.; Schwender, S.; Kim, K.S.; Eigenthaler, M.; Frosch, M. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 2002, 46, 933–946. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Ring, A.; Weiser, J.N.; Tuomanen, E.I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Investig. 1998, 102, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Khan, A.N.; Kim, K.J.; Stins, M.; Kim, K.S. Transforming growth factor-beta increases Escherichia coli K1 adherence, invasion, and transcytosis in human brain microvascular endothelial cells. Cell Tissue Res. 2002, 309, 281–286. [Google Scholar]
- Sun, R.; Liu, M.; Lu, J.; Chu, B.; Yang, Y.; Song, B.; Wang, H.; He, Y. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 2022, 13, 5127. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Vietzen, H.; Berger, S.M.; Kühner, L.M.; Furlano, P.L.; Bsteh, G.; Berger, T.; Rommer, P.; Puchhammer-Stöckl, E. Ineffective control of Epstein-Barr-virus-induced autoimmunity increases the risk for multiple sclerosis. Cell 2023, 186, 5705–5718.e5713. [Google Scholar] [CrossRef]
- McGavern, D.B.; Kang, S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011, 11, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Antar, A.A.; Konopka, J.L.; Campbell, J.A.; Henry, R.A.; Perdigoto, A.L.; Carter, B.D.; Pozzi, A.; Abel, T.W.; Dermody, T.S. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 2009, 5, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.V.; Ozden, S.; Cumont, M.C.; Seilhean, D.; Cartier, L.; Rezaie, P.; Mason, S.; Lambert, S.; Huerre, M.; Gessain, A.; et al. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008, 4, e1000205. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct. Target. Ther. 2021, 6, 337. [Google Scholar] [CrossRef]
- Chapagain, M.L.; Verma, S.; Mercier, F.; Yanagihara, R.; Nerurkar, V.R. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology 2007, 364, 55–63. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Ashley, S.L.; Dixon, S.D.; Spindler, K.R. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J. Virol. 2009, 83, 9398–9410. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, M.; Gurjav, U.; Lum, S.; Nerurkar, V.R. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010, 397, 130–138. [Google Scholar] [CrossRef]
- Salinas, S.; Schiavo, G.; Kremer, E.J. A hitchhiker’s guide to the nervous system: The complex journey of viruses and toxins. Nat. Rev. Microbiol. 2010, 8, 645–655. [Google Scholar] [CrossRef]
- Curanovic, D.; Enquist, L. Directional transneuronal spread of α-herpesvirus infection. Future Virol. 2009, 4, 591–603. [Google Scholar] [CrossRef]
- Mori, I.; Nishiyama, Y.; Yokochi, T.; Kimura, Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, A.; Wigdahl, B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008, 4, e1000215. [Google Scholar] [CrossRef] [PubMed]
- Clay, C.C.; Rodrigues, D.S.; Ho, Y.S.; Fallert, B.A.; Janatpour, K.; Reinhart, T.A.; Esser, U. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J. Virol. 2007, 81, 12040–12048. [Google Scholar] [CrossRef] [PubMed]
- Shay, T.F.; Sullivan, E.E.; Ding, X.; Chen, X.; Ravindra Kumar, S.; Goertsen, D.; Brown, D.; Crosby, A.; Vielmetter, J.; Borsos, M.; et al. Primate-conserved carbonic anhydrase IV and murine-restricted LY6C1 enable blood-brain barrier crossing by engineered viral vectors. Sci. Adv. 2023, 9, eadg6618. [Google Scholar] [CrossRef]
- Chacko, A.; Delbaz, A.; Walkden, H.; Basu, S.; Armitage, C.W.; Eindorf, T.; Trim, L.K.; Miller, E.; West, N.P.; St John, J.A.; et al. Chlamydia pneumoniae can infect the central nervous system via the olfactory and trigeminal nerves and contributes to Alzheimer’s disease risk. Sci. Rep. 2022, 12, 2759. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, X.; Bao, W.; Liu, G.; Wei, W.; Ping, Y. An oncolytic virus-T cell chimera for cancer immunotherapy. Nat. Biotechnol. 2024, 42, 1876–1887. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- He, J.; Connors, J.; Meador, A.; Xu, S.; Meador, H.; Jiang, H.; Fueyo, J.; Gomez-Manzano, C.; Friedman, G.K.; Zaky, W.; et al. Immunotherapy-Related Neurotoxicity in the Central Nervous System of Children with Cancer. Neuro Oncol. 2024, 27, 625–643. [Google Scholar] [CrossRef]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Xin, M.; Kahkoska, A.R.; Wang, J.; Gu, Z. Cell-drug conjugates. Nat. Biomed. Eng. 2024, 8, 1347–1365. [Google Scholar] [CrossRef]
- Vincent, R.L.; Gurbatri, C.R.; Li, F.; Vardoshvili, A.; Coker, C.; Im, J.; Ballister, E.R.; Rouanne, M.; Savage, T.; de Los Santos-Alexis, K.; et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science 2023, 382, 211–218. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Gao, Y.; Chen, Y.; Yin, W.; Liu, Z.; Cui, W.; Li, Y.; Sun, J.; Yang, Y.; et al. Framework Nucleic Acid-Based Selective Cell Catcher for Endogenous Stem Cell Recruitment. Adv. Mater. 2024, 36, e2406118. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Lang, F.F.; Alonso, M.M. Hitchhiking to brain tumours: Stem cell delivery of oncolytic viruses. Lancet Oncol. 2021, 22, 1049–1051. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Thaci, B.; Alexiades, N.G.; Han, Y.; Qian, S.; Liu, F.; Balyasnikova, I.V.; Ulasov, I.Y.; Aboody, K.S.; Lesniak, M.S. Neural Stem Cell-based Cell Carriers Enhance Therapeutic Efficacy of an Oncolytic Adenovirus in an Orthotopic Mouse Model of Human Glioblastoma. Mol. Ther. 2011, 19, 1714–1726. [Google Scholar] [CrossRef]
- Thaci, B.; Ahmed, A.U.; Ulasov, I.V.; Tobias, A.L.; Han, Y.; Aboody, K.S.; Lesniak, M.S. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: Targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012, 19, 431–442. [Google Scholar] [CrossRef]
- Tyler, M.A.; Ulasov, I.V.; Sonabend, A.M.; Nandi, S.; Han, Y.; Marler, S.; Roth, J.; Lesniak, M.S. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009, 16, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincón, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Dey, M.; Yu, D.; Kanojia, D.; Li, G.; Sukhanova, M.; Spencer, D.A.; Pituch, K.C.; Zhang, L.; Han, Y.; Ahmed, A.U.; et al. Intranasal Oncolytic Virotherapy with CXCR4-Enhanced Stem Cells Extends Survival in Mouse Model of Glioma. Stem Cell Rep. 2016, 7, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.; Yu, D.; Morshed, R.A.; Li, G.; Pituch, K.C.; Gao, D.X.; Bertolino, N.; Procissi, D.; Lesniak, M.S.; Balyasnikova, I.V. Pharmacologic modulation of nasal epithelium augments neural stem cell targeting of glioblastoma. Theranostics 2019, 9, 2071–2083. [Google Scholar] [CrossRef]
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, J.; Zhang, Q.; Jin, G.; Su, X.; Liu, S.; Liu, F. Enhancement of CD70-specific CAR T treatment by IFN-γ released from oHSV-1-infected glioblastoma. Cancer Immunol. Immunother. 2022, 71, 2433–2448. [Google Scholar] [CrossRef]
- Chen, L.; Qin, H.; Zhao, R.; Zhao, X.; Lin, L.; Chen, Y.; Lin, Y.; Li, Y.; Qin, Y.; Li, Y.; et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021, 13, eabc2816. [Google Scholar] [CrossRef]
- Huang, H.; Sun, M.; Liu, M.; Pan, S.; Liu, P.; Cheng, Z.; Li, J.; Xu, H.; Liu, F.; Pang, Z. Full encapsulation of oncolytic virus using hybrid erythroctye-liposome membranes for augmented anti-refractory tumor effectiveness. Nano Today 2022, 47, 101671. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Feng, X.; Liu, W.; Wang, X.; Li, F.; Liu, X.; Yu, J.; Yu, B.; Yu, X. Cell Membrane-Coated Oncolytic Adenovirus for Targeted Treatment of Glioblastoma. Nano Lett. 2023, 23, 11120–11128. [Google Scholar] [CrossRef]
- Liu, F.; Xin, M.; Feng, H.; Zhang, W.; Liao, Z.; Sheng, T.; Wen, P.; Wu, Q.; Liang, T.; Shi, J.; et al. Cryo-shocked tumor cells deliver CRISPR-Cas9 for lung cancer regression by synthetic lethality. Sci. Adv. 2024, 10, eadk8264. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Park, B.H.; Hwang, T.; Liu, T.C.; Sze, D.Y.; Kim, J.S.; Kwon, H.C.; Oh, S.Y.; Han, S.Y.; Yoon, J.H.; Hong, S.H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, B.; Chen, Y.; Li, Z.; Wang, J.; Zhang, J.; Ma, R.; Cao, S.; Hu, W.; Chiocca, E.A.; et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity. Nat. Cancer 2022, 3, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.A. Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J. Virol. 2005, 79, 8707–8715. [Google Scholar] [CrossRef]
- Puigdelloses, M.; Garcia-Moure, M.; Labiano, S.; Laspidea, V.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; De la Nava, D.; Ausejo, I.; et al. CD137 and PD-L1 targeting with immunovirotherapy induces a potent and durable antitumor immune response in glioblastoma models. J. Immunother. Cancer 2021, 9, e002644. [Google Scholar] [CrossRef] [PubMed]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ichikawa, T.; Wakimoto, H.; Silver, J.S.; Deisboeck, T.S.; Finkelstein, D.; Harsh, G.R.; Louis, D.N.; Bartus, R.T.; Hochberg, F.H.; et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999, 5, 881–887. [Google Scholar] [CrossRef]
- Liu, J.; Piranlioglu, R.; Ye, F.; Shu, K.; Lei, T.; Nakashima, H. Immunosuppressive cells in oncolytic virotherapy for glioma: Challenges and solutions. Front. Cell. Infect. Microbiol. 2023, 13, 1141034. [Google Scholar] [CrossRef]
- Gao, J.; Gunasekar, S.; Xia, Z.J.; Shalin, K.; Jiang, C.; Chen, H.; Lee, D.; Lee, S.; Pisal, N.D.; Luo, J.N.; et al. Gene therapy for CNS disorders: Modalities, delivery and translational challenges. Nat. Rev. Neurosci. 2024, 25, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Huang, C.Y.; Shih, C.M.; Lin, Y.W.; Huang, P.H.; Lin, S.J.; Liu, C.W.; Lin, C.Y.; Lin, F.Y. Tumor Necrosis Factor Superfamily 14 (LIGHT) Restricts Neovascularization by Decreasing Circulating Endothelial Progenitor Cells and Function. Int. J. Mol. Sci. 2023, 24, 6997. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, M.; Vaccaro, A.; van de Walle, T.; Georganaki, M.; Lugano, R.; Vemuri, K.; Kourougkiaouri, D.; Vazaios, K.; Hedlund, M.; Tsaridou, G.; et al. Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma. Cancer Cell 2023, 41, 1134–1151.e1110. [Google Scholar] [CrossRef]
- von Roemeling, C.; Yegorov, O.; Yang, C.; Klippel, K.; Russell, R.; Trivedi, V.; Bhatia, A.; Doonan, B.; Carpenter, S.; Ryu, D.; et al. CXCL9 recombinant adeno-associated virus (AAV) virotherapy sensitizes glioblastoma (GBM) to anti-PD-1 immune checkpoint blockade. Res. Sq. 2023, 15, 5871. [Google Scholar]
- Strecker, M.I.; Wlotzka, K.; Strassheimer, F.; Roller, B.; Ludmirski, G.; König, S.; Röder, J.; Opitz, C.; Alekseeva, T.; Reul, J.; et al. AAV-mediated gene transfer of a checkpoint inhibitor in combination with HER2-targeted CAR-NK cells as experimental therapy for glioblastoma. Oncoimmunology 2022, 11, 2127508. [Google Scholar] [CrossRef]
- Volak, A.; LeRoy, S.G.; Natasan, J.S.; Park, D.J.; Cheah, P.S.; Maus, A.; Fitzpatrick, Z.; Hudry, E.; Pinkham, K.; Gandhi, S.; et al. Virus vector-mediated genetic modification of brain tumor stromal cells after intravenous delivery. J. Neurooncol. 2018, 139, 293–305. [Google Scholar] [CrossRef]
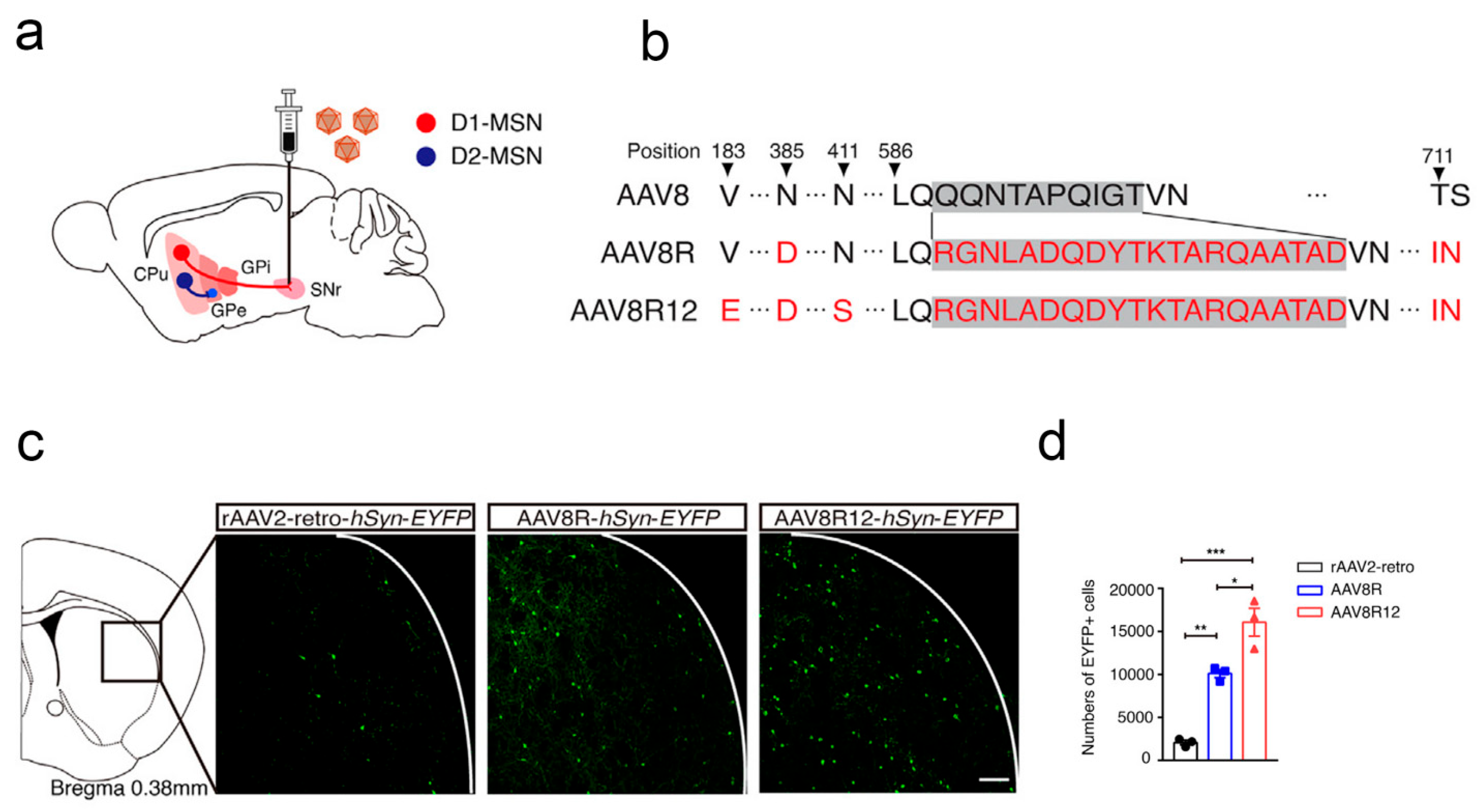
- Yao, Y.; Wang, J.; Liu, Y.; Qu, Y.; Wang, K.; Zhang, Y.; Chang, Y.; Yang, Z.; Wan, J.; Liu, J.; et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat. Biomed. Eng. 2022, 6, 1257–1271. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Guo, C.; Li, J.; Liang, G. Cancer-associated fibroblast-associated gene IGFBP2 promotes glioma progression through induction of M2 macrophage polarization. Am. J. Physiol. Cell Physiol. 2024, 326, C252–C268. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Liu, Y.; Xu, L.; Sun, Z.; Zheng, D.; Liu, X.; Song, C.; Zhang, Y.; Liang, H.; et al. Tumor-associated astrocytes promote tumor progression of Sonic Hedgehog medulloblastoma by secreting lipocalin. Brain Pathol. 2024, 34, e13212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Wu, J.; Zhang, M.Q.; Chai, J.L.; Cao, C. G protein inhibitory α subunit 2 is a molecular oncotarget of human glioma. Int. J. Biol. Sci. 2023, 19, 865–879. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Chen, G.; Huang, M.; Wang, Y.; Liu, Y.Y.; Jiang, Q.; Cao, C.; Liu, F. TIMM44 is a potential therapeutic target of human glioma. Theranostics 2022, 12, 7586–7602. [Google Scholar] [CrossRef] [PubMed]
- Bhere, D.; Arghiani, N.; Lechtich, E.R.; Yao, Y.; Alsaab, S.; Bei, F.; Matin, M.M.; Shah, K. Simultaneous downregulation of miR-21 and upregulation of miR-7 has anti-tumor efficacy. Sci. Rep. 2020, 10, 1779. [Google Scholar] [CrossRef]
- Ye, L.; Park, J.J.; Dong, M.B.; Yang, Q.; Chow, R.D.; Peng, L.; Du, Y.; Guo, J.; Dai, X.; Wang, G.; et al. In vivo CRISPR screening in CD8 T cells with AAV-Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat. Biotechnol. 2019, 37, 1302–1313. [Google Scholar] [CrossRef]
- Chow, R.D.; Guzman, C.D.; Wang, G.; Schmidt, F.; Youngblood, M.W.; Ye, L.; Errami, Y.; Dong, M.B.; Martinez, M.A.; Zhang, S.; et al. AAV-mediated direct in vivo CRISPR screen identifies functional suppressors in glioblastoma. Nat. Neurosci. 2017, 20, 1329–1341. [Google Scholar] [CrossRef]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M.; et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492. [Google Scholar] [CrossRef]
- Zhao, F.H.; Wu, T.; Hu, Y.M.; Wei, L.H.; Li, M.Q.; Huang, W.J.; Chen, W.; Huang, S.J.; Pan, Q.J.; Zhang, X.; et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: End-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect. Dis. 2022, 22, 1756–1768. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Ling, S.; Lu, S.; Wang, X.; Jiang, Z.; Wang, J.; Dai, Y.; Tian, X.; Huang, Q.; et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat. Biomed. Eng. 2024, 9, 185–200. [Google Scholar] [CrossRef]
- Ye, D.; Zimmermann, T.; Demina, V.; Sotnikov, S.; Ried, C.L.; Rahn, H.; Stapf, M.; Untucht, C.; Rohe, M.; Terstappen, G.C.; et al. Trafficking of JC virus-like particles across the blood-brain barrier. Nanoscale Adv. 2021, 3, 2488–2500. [Google Scholar] [CrossRef]
- Madigan, V.; Zhang, F.; Dahlman, J.E. Drug delivery systems for CRISPR-based genome editors. Nat. Rev. Drug Discov. 2023, 22, 875–894. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Gu, W.; Luozhong, S.; Cai, S.; Londhe, K.; Elkasri, N.; Hawkins, R.; Yuan, Z.; Su-Greene, K.; Yin, Y.; Cruz, M.; et al. Extracellular vesicles incorporating retrovirus-like capsids for the enhanced packaging and systemic delivery of mRNA into neurons. Nat. Biomed. Eng. 2024, 8, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wang, C.; Beiss, V.; Cai, H.; Washington, T., 2nd; Murray, A.A.; Gong, X.; Zhao, Z.; Masarapu, H.; Zlotnick, A.; et al. The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomater. Sci. 2020, 8, 5489–5503. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter-Fogle, A.; Shukla, S.; Wang, C.; Beiss, V.; Harris, P.L.R.; Sloan, A.E.; Steinmetz, N.F. Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy. Cancers 2019, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.H.; Huang, C.Y.; Chen, P.Y.; Li, N.S.; Hsu, Y.P.; Wu, J.K.; Fan, H.F.; Wei, K.C.; Yang, H.W. Bioengineered Bacteriophage-Like Nanoparticles as RNAi Therapeutics to Enhance Radiotherapy against Glioblastomas. ACS Nano 2023, 17, 10407–10422. [Google Scholar] [CrossRef]
- Daddacha, W.; Monroe, D.; Carver, K.; Usoro, E.R.; Alptekin, A.; Xu, H.; Osuka, S.; Arbab, A.S.; Sakamuro, D. Viral Particle-Mediated SAMHD1 Depletion Sensitizes Refractory Glioblastoma to DNA-Damaging Therapeutics by Impairing Homologous Recombination. Cancers 2022, 14, 4490. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Liu, Y.; Zhang, X.; Shan, W.; Ye, S.; Zhou, X.; Ge, Y.; Wang, X.; Ren, L. Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine 2020, 15, 1391–1409. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Le, D.H.T.; Lomonossoff, G.P.; Steinmetz, N.F. Bluetongue Virus Particles as Nanoreactors for Enzyme Delivery and Cancer Therapy. Mol. Pharm. 2021, 18, 1150–1156. [Google Scholar] [CrossRef]
- Pang, H.H.; Chen, P.Y.; Wei, K.C.; Huang, C.W.; Shiue, Y.L.; Huang, C.Y.; Yang, H.W. Convection-Enhanced Delivery of a Virus-Like Nanotherapeutic Agent with Dual-Modal Imaging for Besiegement and Eradication of Brain Tumors. Theranostics 2019, 9, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.H.; Huang, C.Y.; Chou, Y.W.; Lin, C.J.; Zhou, Z.L.; Shiue, Y.L.; Wei, K.C.; Yang, H.W. Bioengineering fluorescent virus-like particle/RNAi nanocomplexes act synergistically with temozolomide to eradicate brain tumors. Nanoscale 2019, 11, 8102–8109. [Google Scholar] [CrossRef]
- Chao, C.N.; Yang, Y.H.; Wu, M.S.; Chou, M.C.; Fang, C.Y.; Lin, M.C.; Tai, C.K.; Shen, C.H.; Chen, P.L.; Chang, D.; et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci. Rep. 2018, 8, 2213. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Yu, Y.; Yang, S.; Feng, J.; Zhu, Y.; Huang, W.; Qin, B.; Guan, X.; He, Z.; et al. Micro-to-Nano Oncolytic Microbial System Shifts from Tumor Killing to Tumor Draining Lymph Nodes Remolding for Enhanced Immunotherapy. Adv. Mater. 2024, 36, e2306488. [Google Scholar] [CrossRef]
- Sun, M.; Yang, S.; Huang, H.; Gao, P.; Pan, S.; Cheng, Z.; He, Z.; Wang, Z.; Sun, J.; Liu, F. Boarding Oncolytic Viruses onto Tumor-Homing Bacterium-Vessels for Augmented Cancer Immunotherapy. Nano Lett. 2022, 22, 5055–5064. [Google Scholar] [CrossRef] [PubMed]
- Ban, W.; Sun, M.; Huang, H.; Huang, W.; Pan, S.; Liu, P.; Li, B.; Cheng, Z.; He, Z.; Liu, F.; et al. Engineered bacterial outer membrane vesicles encapsulating oncolytic adenoviruses enhance the efficacy of cancer virotherapy by augmenting tumor cell autophagy. Nat. Commun. 2023, 14, 2933. [Google Scholar] [CrossRef]
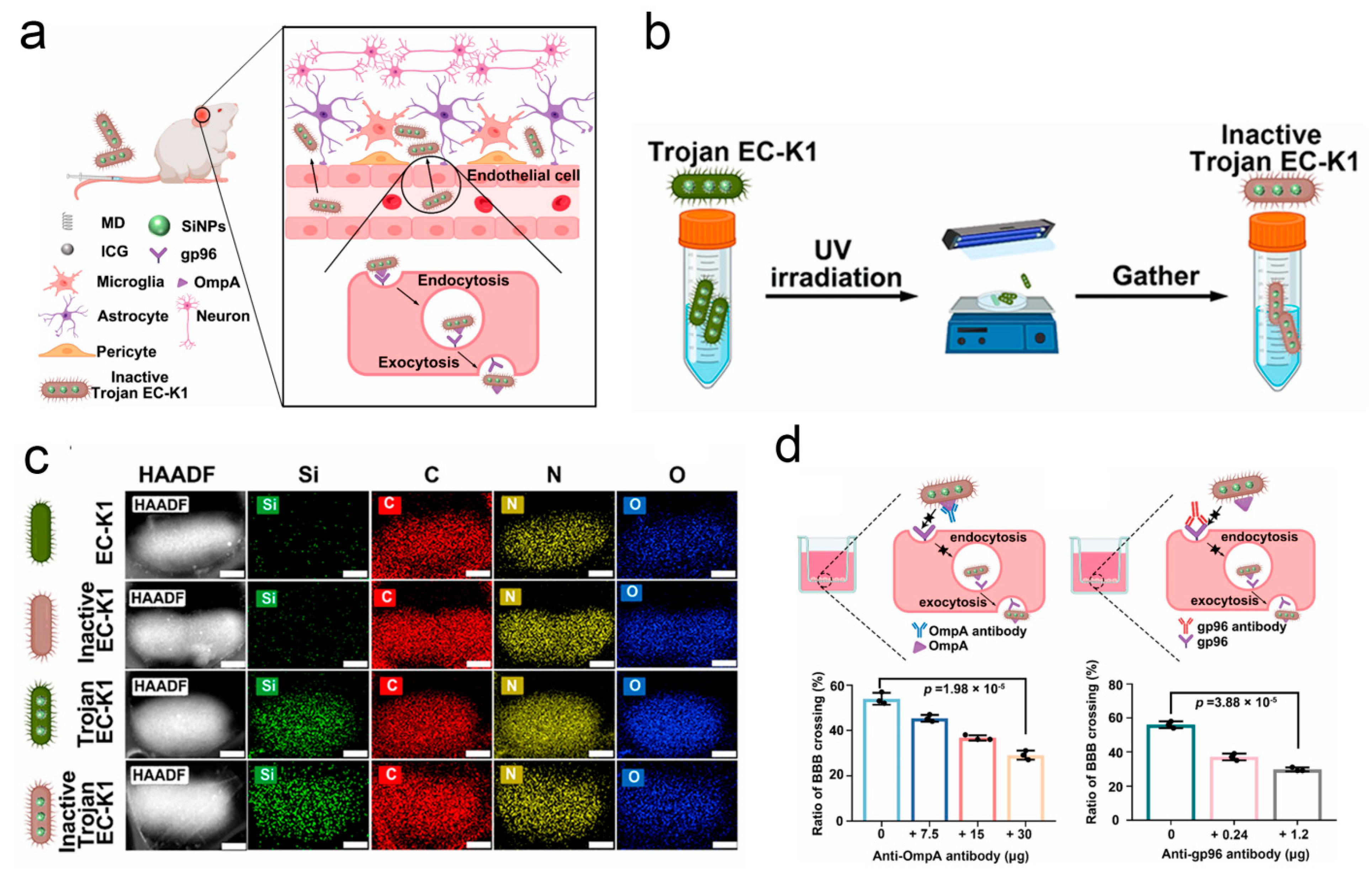
- Lu, J.; Ding, J.; Chu, B.; Ji, C.; Zhang, Q.; Xu, Y.; Song, B.; Wang, H.; He, Y. Inactive Trojan Bacteria as Safe Drug Delivery Vehicles Crossing the Blood-Brain Barrier. Nano Lett. 2023, 23, 4326–4333. [Google Scholar] [CrossRef]
- Johann, L.; Soldati, S.; Müller, K.; Lampe, J.; Marini, F.; Klein, M.; Schramm, E.; Ries, N.; Schelmbauer, C.; Palagi, I.; et al. A20 regulates lymphocyte adhesion in murine neuroinflammation by restricting endothelial ICOSL expression in the CNS. J. Clin. Investig. 2023, 133, e168314. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Bas-Orth, C.; Bruehl, C.; Draguhn, A.; Fairless, R. Glutamate transporter contribution to retinal ganglion cell vulnerability in a rat model of multiple sclerosis. Neurobiol. Dis. 2023, 187, 106306. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Jiang, H.; Huang, H.; Liu, L.; Fang, F.; Li, L.; Feng, X.; Liu, D.; Dalal, R.; et al. Differential effects of SARM1 inhibition in traumatic glaucoma and EAE optic neuropathies. Mol. Ther. Nucleic Acids 2023, 32, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, C.; Hu, L.; Zheng, C.; Li, X.; Zheng, W.; Weng, Y.; Lin, H. Targeted Knockdown of Genes in the Choroid Plexus. J. Vis. Exp. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, H.; Song, H.; Chen, B.; Fu, J.; Sun, M.; Zhou, H.; Bai, W.; Wei, S.; Li, H. Upregulation of C-X-C motif chemokine 12 in the spinal cord alleviated the symptoms of experimental autoimmune encephalomyelitis in Lewis rats. Front. Neurosci. 2023, 17, 1105530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Zeng, Q.; Feng, J.; Fu, H.; Luo, Z.; Xiao, B.; Yang, H.; Wu, M. LRRC4 functions as a neuron-protective role in experimental autoimmune encephalomyelitis. Mol. Med. 2021, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Edo, Á.; Calvo-Barreiro, L.; Eixarch, H.; Bosch, A.; Chillón, M.; Espejo, C. Therapeutic Effect of IL-21 Blockage by Gene Therapy in Experimental Autoimmune Encephalomyelitis. Neurotherapeutics 2022, 19, 1617–1633. [Google Scholar] [CrossRef]
- Alrashdi, B.; Dawod, B.; Schampel, A.; Tacke, S.; Kuerten, S.; Marshall, J.S.; Côté, P.D. Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis. J. Neuroinflammation 2019, 16, 215. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, Z.; Wang, J.; Liu, K.; Liu, J.; Lin, J.; Feng, S.; Zhang, T.; Shan, L.; Liu, T.; et al. Circuit-specific gene therapy reverses core symptoms in a primate Parkinson’s disease model. Cell 2023, 186, 5394–5410.e5318. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Peng, H.; Chou, S.C.; Mehrabani-Tabari, A.A.; Song, J.J.; Yin, X.; Karuppagounder, S.S.; Umanah, G.K.; Rao, A.V.S.; et al. PAAN/MIF nuclease inhibition prevents neurodegeneration in Parkinson’s disease. Cell 2022, 185, 1943–1959.e1921. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: An open label, phase I trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Rico, A.J.; Corcho, A.; Chocarro, J.; Ariznabarreta, G.; Roda, E.; Honrubia, A.; Arnaiz, P.; Lanciego, J.L. Development and characterization of a non-human primate model of disseminated synucleinopathy. Front. Neuroanat. 2024, 18, 1355940. [Google Scholar] [CrossRef]
- Niu, X.Y.; Xie, X.X.; Tuo, H.Z.; Lv, C.P.; Huang, Y.R.; Zhu, J.; Liang, S.Y.; Du, X.Y.; Yang, C.G.; Hou, S.J.; et al. Thrombomodulin reduces α-synuclein generation and ameliorates neuropathology in a mouse model of Parkinson’s disease. Cell Death Discov. 2024, 10, 167. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef]
- Pérez-Sisqués, L.; Sancho-Balsells, A.; Solana-Balaguer, J.; Campoy-Campos, G.; Vives-Isern, M.; Soler-Palazón, F.; Anglada-Huguet, M.; López-Toledano, M.; Mandelkow, E.M.; Alberch, J.; et al. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021, 12, 616. [Google Scholar] [CrossRef]
- Ng, R.C.; Jian, M.; Ma, O.K.; Xiang, A.W.; Bunting, M.; Kwan, J.S.; Wong, C.W.; Yick, L.W.; Chung, S.K.; Lam, K.S.; et al. Liver-specific adiponectin gene therapy suppresses microglial NLRP3-inflammasome activation for treating Alzheimer’s disease. J. Neuroinflammation 2024, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Wang, L.; Zhan, H.; Luo, X.; Zeng, Y.; Wu, W.; Zhang, X.; Wang, F. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging 2020, 12, 20862–20879. [Google Scholar] [CrossRef] [PubMed]
- Boisserand, L.S.B.; Geraldo, L.H.; Bouchart, J.; El Kamouh, M.R.; Lee, S.; Sanganahalli, B.G.; Spajer, M.; Zhang, S.; Lee, S.; Parent, M.; et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J. Exp. Med. 2024, 221, e20221983. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tang, Y.; Huang, T.; Ke, L.; Huang, E. In situ direct reprogramming of astrocytes to neurons via polypyrimidine tract-binding protein 1 knockdown in a mouse model of ischemic stroke. Neural. Regen. Res. 2024, 19, 2240–2248. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhao, Y.; Huang, Y.; Shi, Z.; Wang, H.; Cao, H.; Wang, C.; Wang, Y.; Chen, D.; et al. Arresting the bad seed: HDAC3 regulates proliferation of different microglia after ischemic stroke. Sci. Adv. 2024, 10, eade6900. [Google Scholar] [CrossRef]
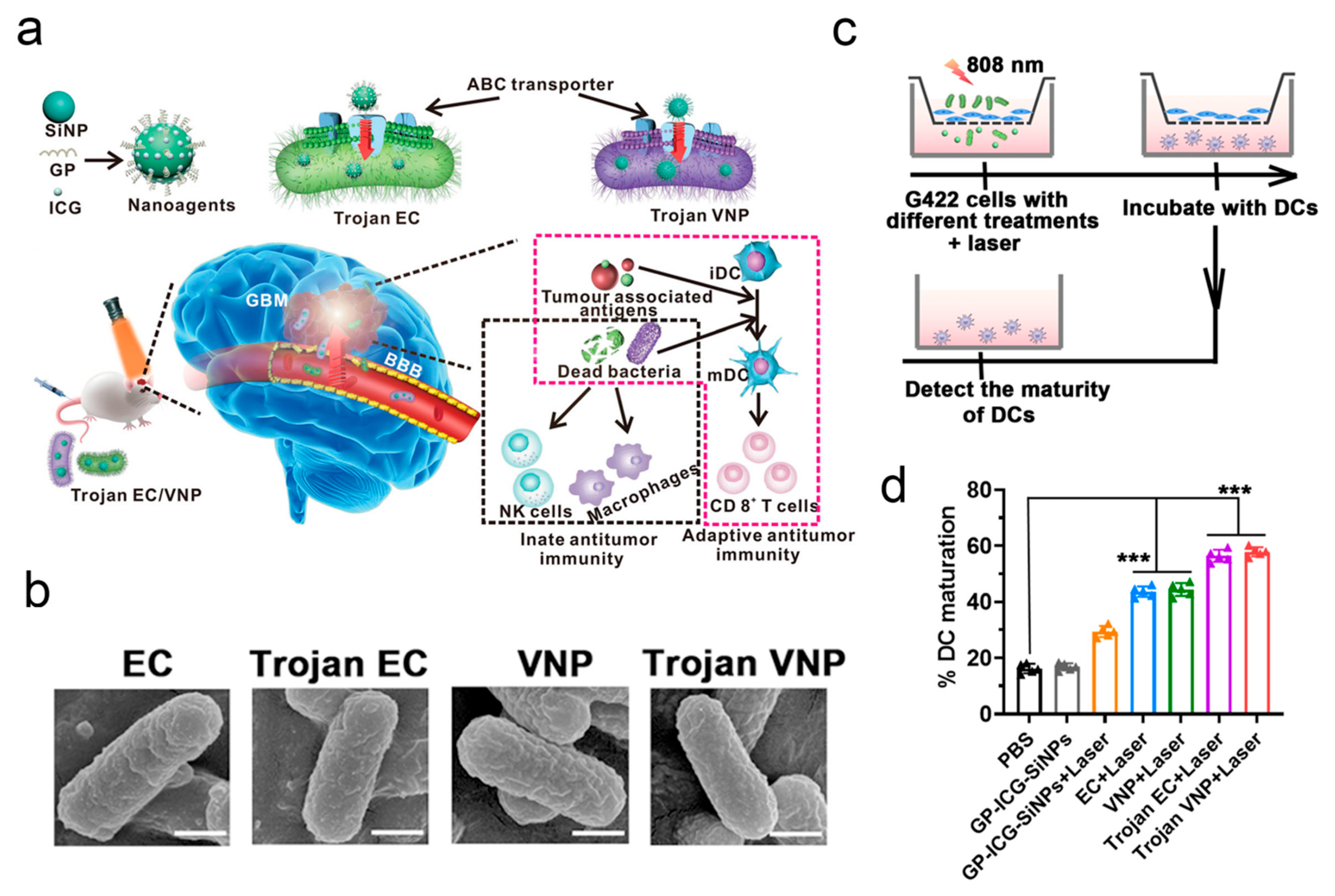
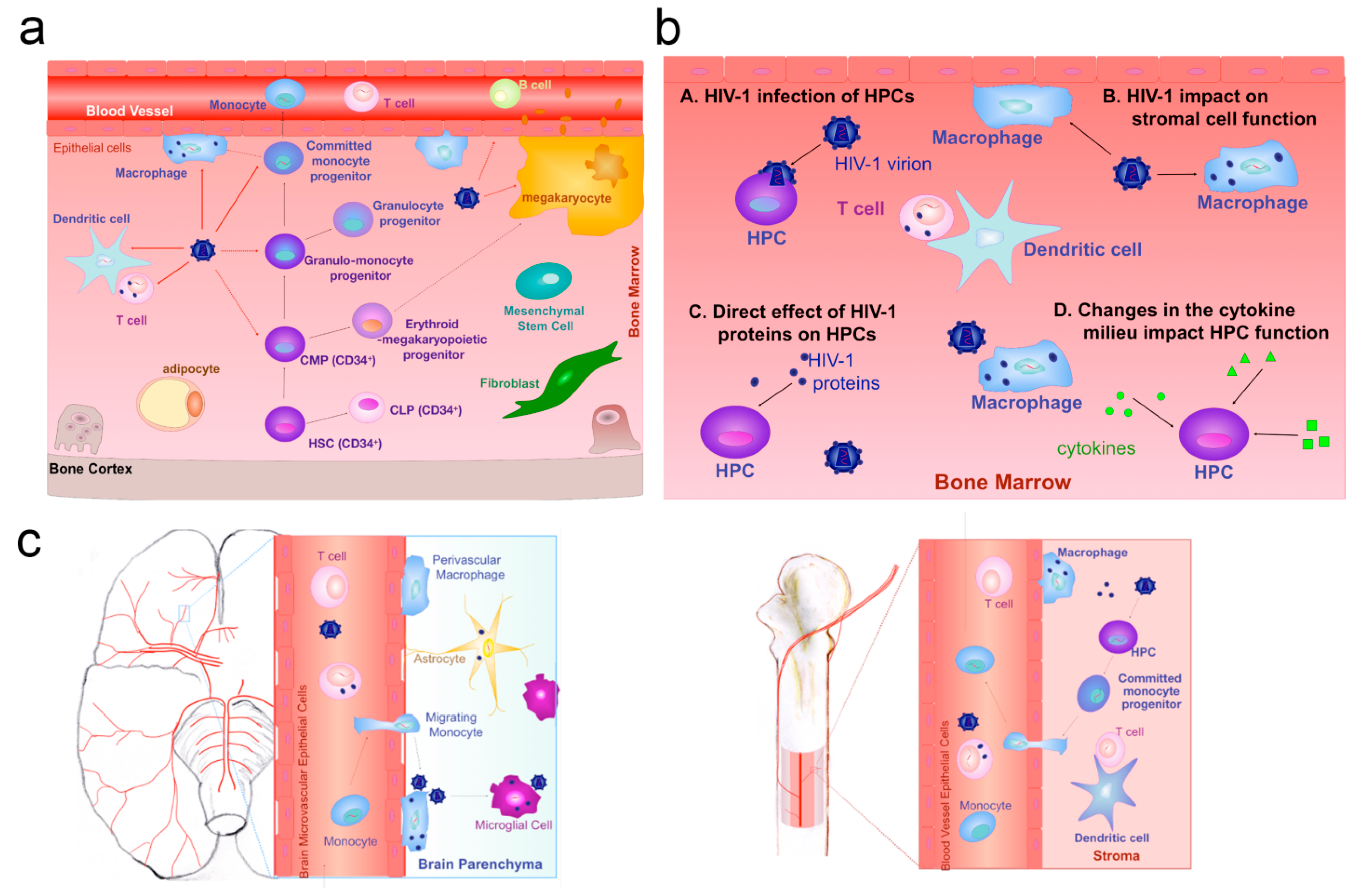
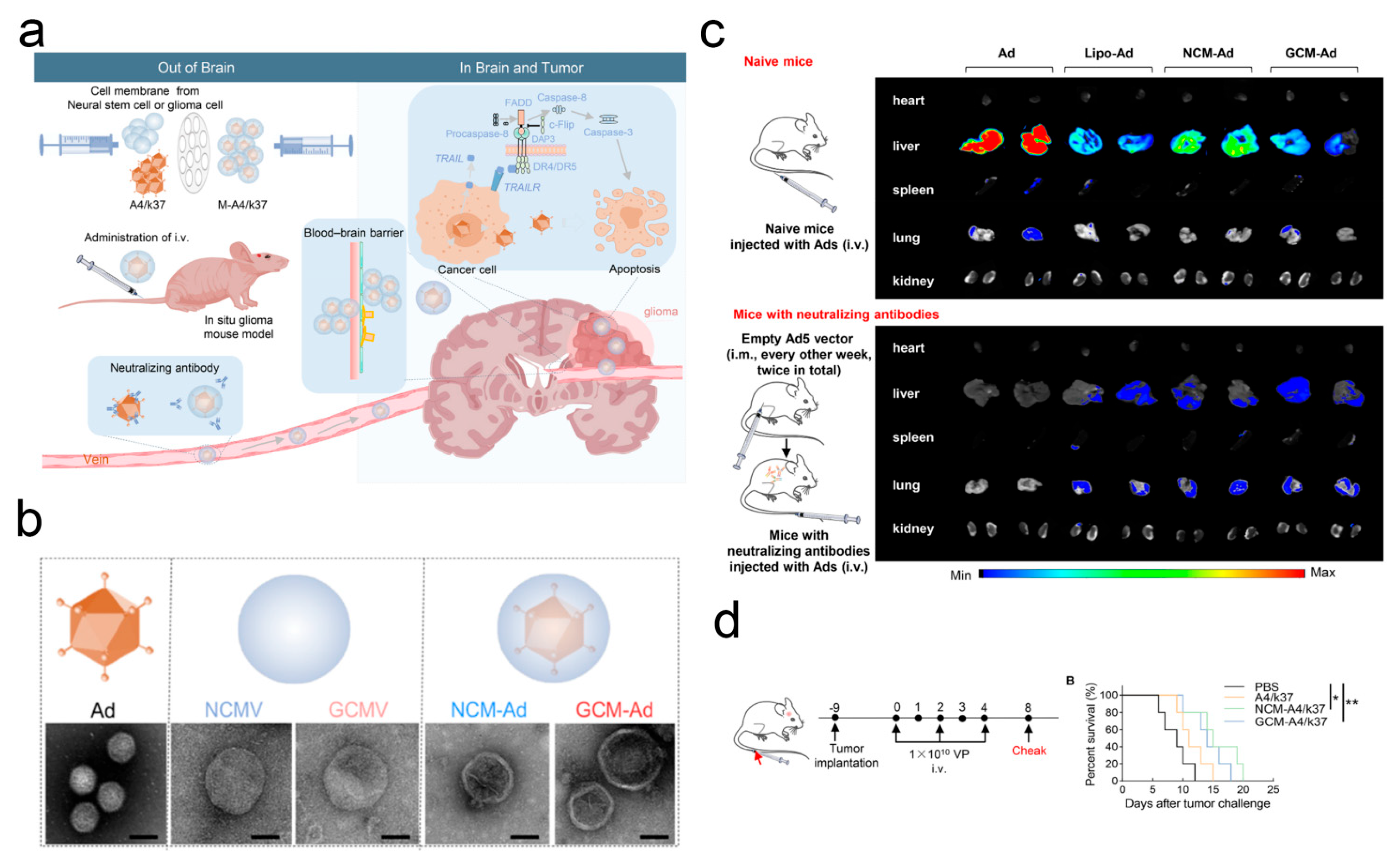
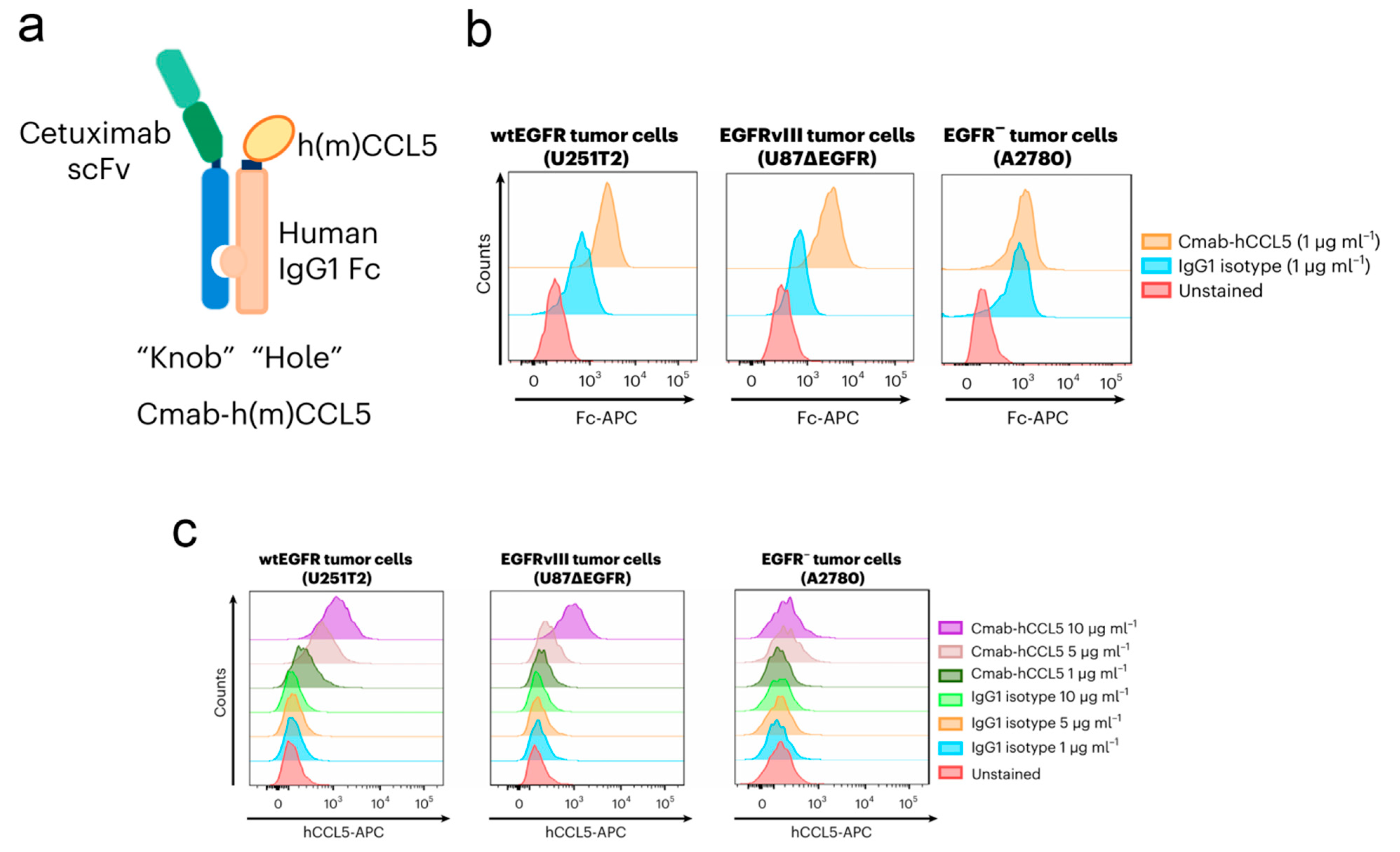
| Microbial Type | Disease Type | Entry Mode | Concrete Pathway | Reference |
|---|---|---|---|---|
| EC-K1 | suppurative meningitis | receptor-ligand binding | OmpA-gp96 | [47] |
| EC-K1 | suppurative meningitis | receptor-ligand binding | CNF1 protein | [48] |
| Streptococcus pneumoniae group B, Streptococcus neonatal meningitis, and Escherichia coli | bacterial meningitis and cerebral palsy | hijacking the iron transporter receptor | transporter receptor | [49] |
| Streptococcus pneumoniae | bacterial meningitis | hijacking the polymeric immunoglobulin receptor | polymeric immunoglobulin receptor | [50] |
| S. agalactiae | bacterial meningitis | receptor-ligand binding | Lmb, FbsA, and IagA | [51] |
| L. monocytogenes | bacterial meningitis | receptor-ligand binding | InlB | [52] |
| S. pneumoniae | bacterial meningitis | receptor-ligand binding | phosphorylcholine and PAF receptor | [53] |
| N. meningitidis | bacterial meningitis | receptor-ligand binding | protein Opc-fibronectin | [54] |
| M. tuberculosis | tuberculous meningitis | hijacking immune cells | scavenger receptors | [55] |
| S. pneumoniae | bacterial meningitis | increasing TNF-α level | phosphorylcholine relative receptor | [56] |
| Reovirus | viral meningitis | receptor-ligand binding | JAM-A | [62] |
| Retrovirus HTLV1 | viral meningitis | receptor-ligand binding | GLUT | [63] |
| SARS-CoV-2 | viral meningitis | upregulation of chemokines | upregulation of the MMP9 protein | [64] |
| Polyomavirus | viral meningitis | receptor-ligand binding | serotonin receptor 2A | [65] |
| Mouse adenovirus type 1 | acute encephalomyelitis | BBB destruction | BBB-destroying E3 protein | [65] |
| West Nile virus | encephalitis | BBB destruction | MMP-9 | [67] |
| Poliovirus | encephalitis | receptor-ligand binding | CD155 | [68] |
| Adenovirus | encephalitis | receptor-ligand binding | CAR | [68] |
| Rabies virus | encephalitis | receptor-ligand binding | NCAM | [68] |
| Herpes virus and pseudorabies virus | encephalitis | receptor-ligand binding | PVRL1, 2 | [69] |
| Trojan horse | encephalitis | Trojan horse | infection of monocytes and macrophages | [69] |
| Chlamydia pneumoniae | Alzheimer’s disease | through the nasal nerves | nasal nerves | [74] |
| Country | Type of OV | Years | Administration Route | Biomedical Technologies | Clinical Stage | Type of Disease | References |
|---|---|---|---|---|---|---|---|
| USA | Ad (DNX-24-RGD) | 2016 | i.t. | Combined with pembrolizumab | II | Malignant brain tumor | NCT02798406 |
| USA | HSV (G207) | 2023 | MRI-guided infusion | Gene modification | II | High-grade glioma | NCT04482933 |
| USA | PVSRIPO | 2017 | i.t. | Gene modification | II | Malignant glioma | NCT02986178 |
| Japan | HSV (G47 delta) | 2014 | i.t. | Gene modification | II | Glioblastoma | UMIN000015995 |
| USA | HSV (M032) | 2022 | Infusion | Gene modification | II | Glioblastoma multiforme | NCT05084430 |
| China | HSV (OH2) | 2021 | Ommaya reservoir injection | Gene modification | II | CNS tumor | NCT05235074 |
| Germany | H-1 parvovirus | 2011 | i.t./i.v. | Gene modification | II | Glioblastoma multiforme | NCT01301430 |
| France | TG6002 | 2017 | i.v. | Gene modification | II | Glioblastoma | NCT03294486 |
| The Netherlands | Ad (DNX-24-RGD) | 2010 | Convection-enhanced delivery | / | II | Recurrent glioblastoma | NCT01582516 |
| Spain | Ad (ICOVIR-5) | 2021 | Infusion | Stem cell therapy | II | Medulloblastoma | NCT04758533 |
| USA | PVSRIPO | 2017 | Convection-enhanced delivery | / | Ib | Malignant glioma | NCT03043391 |
| USA | HSV (G207) | 2019 | MRI-guided infusion | Gene modification | I | Glioblastoma multiforme | NCT03911388 |
| USA | REOLYSIN® | 2006 | i.t. | / | I | Malignant glioma | NCT00528684 |
| China | Ad-TD-nsIL12 | 2023 | i.t. | Gene modification | I | DIPG | NCT05717712 |
| Spain | DNX-24-RGD | 2017 | Cerebellar peduncle infusion | Gene modification | I | Brainstem glioma | NCT03178032 |
| USA | DNX-24-RGD | 2019 | Infusion | Stem cell therapy | I | High-grade glioma | NCT03896568 |
| USA | DNX-24-RGD | 2014 | i.t. | Combined with IFNγ | I | Recurrent glioblastoma | NCT02197169 |
| Spain | DNX-24-RGD | 2013 | i.t. or resected cavity | Combined with temozolomide | I | Recurrent glioblastoma | NCT01956734 |
| USA | CRAd-S-pk7 | 2017 | Resected cavity | Stem cell therapy | I | High-grade glioma | NCT03072134 |
| USA | CRAd-S-pk7 | 2023 | i.c. | / | I | Recurrent high-grade glioma | NCT05139056 |
| Spain | DNX-2440 | 2018 | i.t. | / | I | Recurrent glioblastoma | NCT03714334 |
| USA | Ad5-yCD | 2022 | i.t. | Gene modification | I | Malignant glioma | NCT05686798 |
| USA | HSV (G207) | 2019 | Infusion | Gene modification | I | Glioblastoma multiforme | NCT03911388 |
| USA | rQNestin | 2017 | i.t. | Gene modification | I | Malignant glioma | NCT03152318 |
| USA | HSV (C134) | 2019 | i.t. | / | I | Recurrent glioblastoma | NCT03657576 |
| USA | HSV (M032) | 2013 | Infusion | Gene modification | I | Recurrent glioblastoma | NCT02062827 |
| USA | HSV (MVR-C5252) | 2023 | i.t. | Gene modification | I | Recurrent glioblastoma | NCT05095441 |
| USA | PVSRIPO | 2012 | i.t. | / | I | Glioblastoma | NCT01491893 |
| Microbial Type | Disease Type | Emerging Biotechnology | Specific Empowerment | Reference |
|---|---|---|---|---|
| CRAd-S-pk7 | Intracranial glioma | NSC therapy | Tumor tropism | [17] |
| OVs | Intracranial glioma | NSC therapy | Crossing the BBB, tumor targeting | [88] |
| Δ24-RGD | Intracranial glioma | MSC therapy | Crossing the BBB, tumor targeting | [90] |
| oHSV | Melanoma brain metastases | MSC therapy | Crossing the BBB, tumor targeting | [16] |
| OVs | Glioblastoma | CAR-T therapy | Enhancing the anti-tumor efficacy | [91] |
| A4/k37 | Glioblastoma | Membrane encapsulation | Crossing the BBB | [94] |
| Ads | Glioblastoma | Gene modification | Enhancing the anti-tumor efficacy | [96] |
| OVs | Glioblastoma | Gene modification | Enhancing the anti-tumor efficacy | [97] |
| OVs | Glioblastoma | Gene modification | Enhancing the anti-tumor efficacy | [98] |
| OVs | Glioblastoma | Gene modification | Enhancing OV replication | [99] |
| Delta-24-ACT | Glioblastoma | Gene modification | Enhancing the anti-tumor efficacy | [100] |
| DNX-2401 | DIPG | Combination with radiotherapy | Enhancing the anti-tumor efficacy | [101] |
| OVs | Glioblastoma | Combination with CPA | Inhibition of antiviral response | [102] |
| AAV-LIGHT | Glioblastoma | Gene delivery (LIGHT) | Enhancing the anti-tumor efficacy | [107] |
| HER2-AAV | Glioblastoma | Gene delivery (aPD-1) | Enhancing the anti-tumor efficacy | [109] |
| AAV | Glioblastoma | Gene delivery (IFN-β) | Tumor targeting, enhancing the anti-tumor efficacy | [110] |
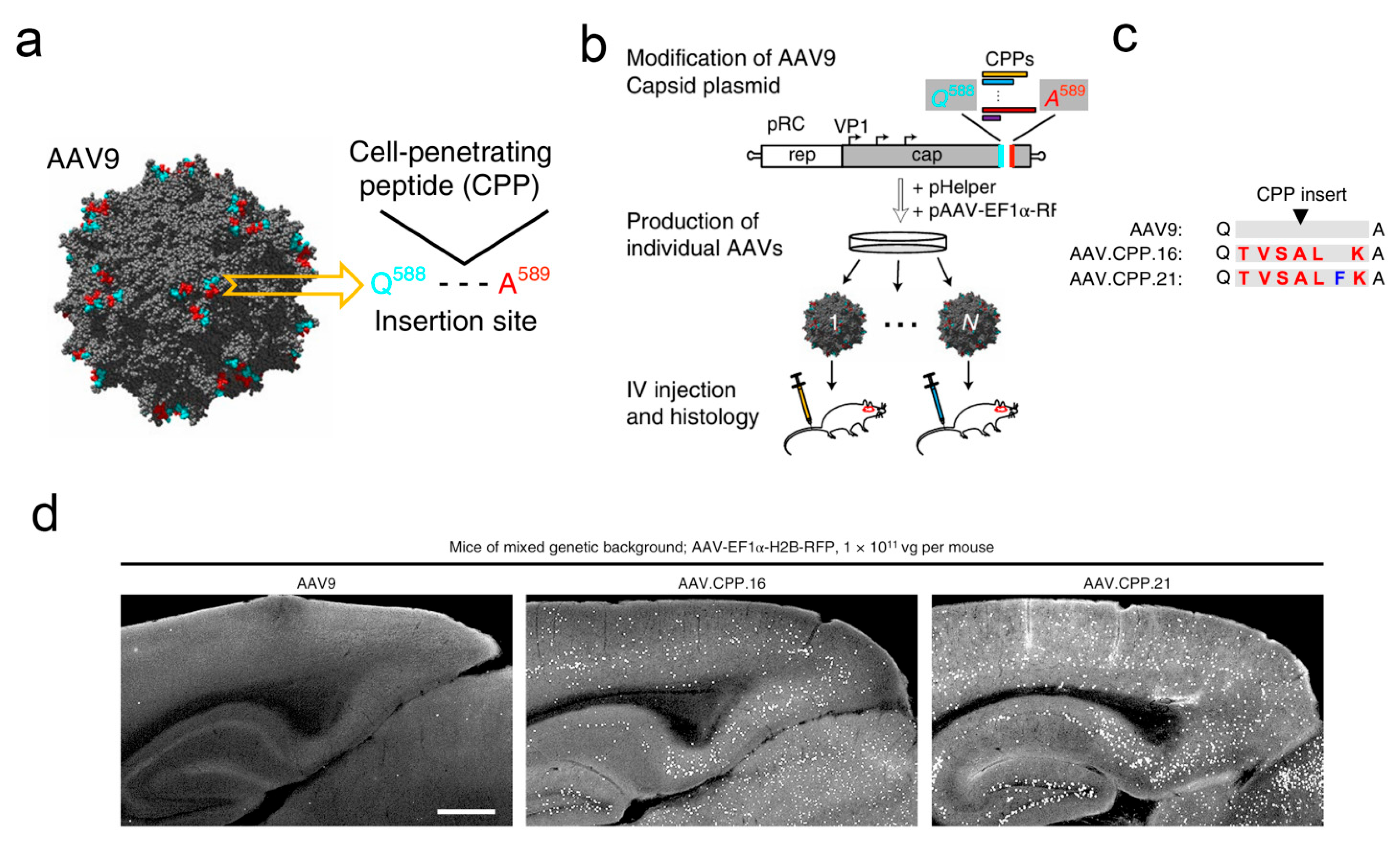
| AAV.CPP.16 | Glioblastoma | Gene delivery (membrane-penetrating peptide) | Crossing the BBB | [13] |
| AAV | Glioblastoma | Gene delivery (IGFBP2) | Exploring novel anti-tumor mechanisms | [112] |
| AAV | Medulloblastoma | Gene delivery (lipocalin-2) | Exploring novel anti-tumor mechanisms | [113] |
| AAV | Glioblastoma | Gene delivery (Gαi2) | Exploring novel anti-tumor mechanisms | [114] |
| AAV | Glioblastoma | Gene delivery (TIMM44) | Exploring novel anti-tumor mechanisms | [115] |
| AAV | Glioblastoma | Gene delivery (miRzip-21) | Exploring novel anti-tumor mechanisms | [116] |
| Hybrid AAV/phage | Glioblastoma | Gene delivery (RGD4C) | Enhancing the anti-tumor efficacy | [119] |
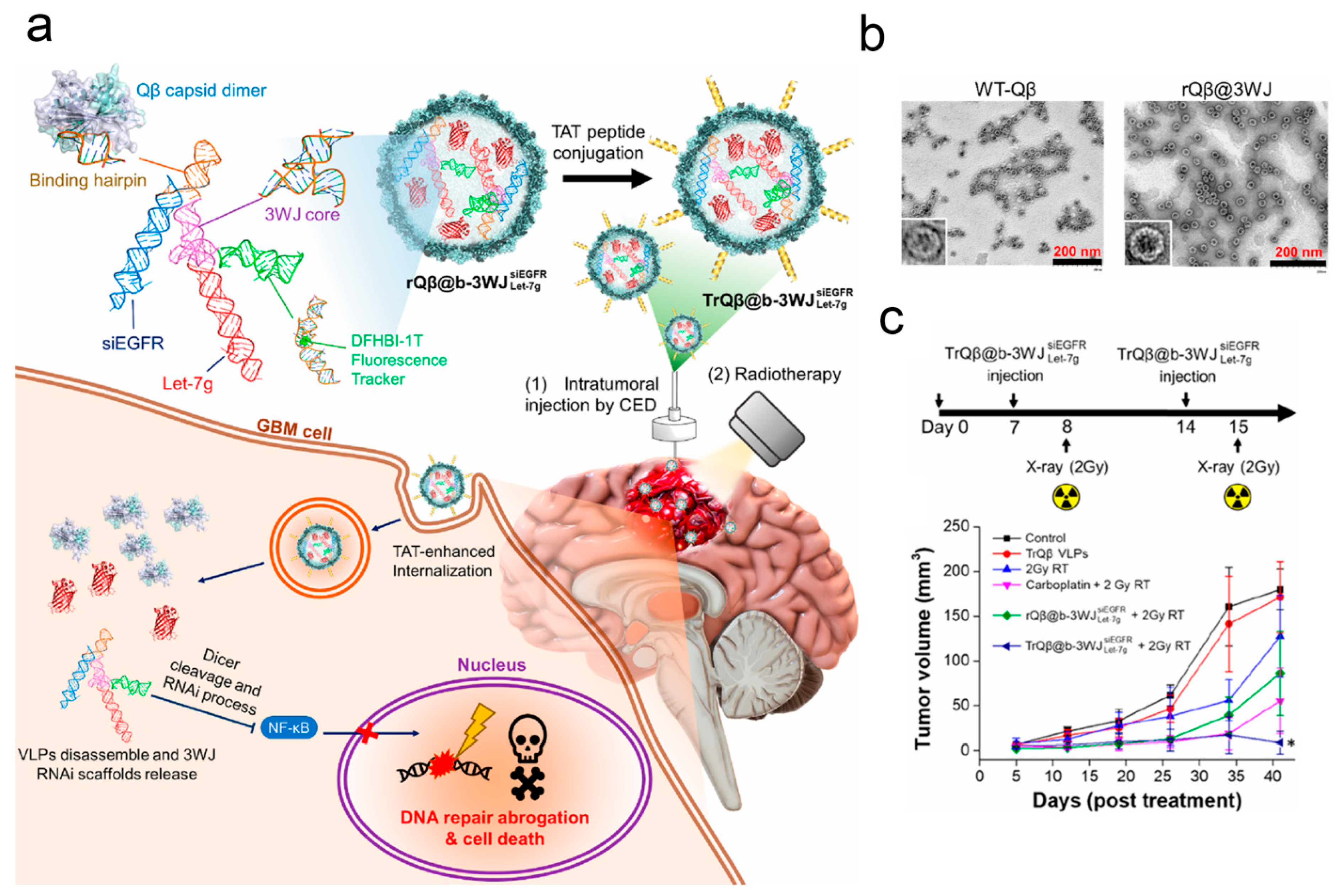
| Bacteriophage Qβ | Glioblastoma | Gene delivery (b-3WJ) | Enhancing the anti-tumor efficacy | [127] |
| VLPs | Glioblastoma | Gene delivery (SAMHD1) | Enhancing the anti-tumor efficacy | [129] |
| VLPs | Glioblastoma | Gene delivery (YAP) | Crossing the BBB, tumor targeting | [130] |
| VLPs | Glioblastoma | Gene delivery (TK-VLPs) | Enhancing the anti-tumor efficacy | [131] |
| VLPs | Glioblastoma | Gene delivery (Ga-DOTA) | Enhancing the anti-tumor efficacy | [131] |
| VLPs | Glioblastoma | Gene delivery (apolipoprotein E peptide) | Crossing the BBB, enhancing the anti-tumor efficacy | [133] |
| VLPs | Glioblastoma | Gene delivery (JCPYV) | Crossing the BBB, enhancing the anti-tumor efficacy | [134] |
| Bacteria | Glioblastoma | Combination with silicon nanoparticles | Crossing the BBB | [58] |
| EC-K1 | Glioblastoma | Combination with photodynamic therapy | Crossing the BBB | [138] |
| EC-K1 OMVs | Glioblastoma | Combination with PLGA nanoparticles | Crossing the BBB | [18] |
| AAV | Multiple sclerosis | Gene delivery (ICOSL) | Enhancing efficacy | [139] |
| AAV | Encephalomyelitis | Gene delivery (GLAST) | Enhancing efficacy | [140] |
| AAV | Encephalomyelitis | Gene delivery (sterile α) | Enhancing efficacy | [141] |
| AAV | Encephalomyelitis | Gene delivery (A2A) | Enhancing efficacy | [142] |
| AAV | Encephalomyelitis | Gene delivery (CXCL12) | Enhancing efficacy | [143] |
| AAV | Encephalomyelitis | Gene delivery (LRRC4) | Enhancing efficacy | [144] |
| AAV | Encephalomyelitis | Gene delivery (IL-21) | Enhancing efficacy | [145] |
| AAV | Parkinson’s disease | Gene delivery (D1-MSN) | Enhancing efficacy | [147] |
| AAV | Parkinson’s disease | Gene delivery (pathologic α-synuclein) | Enhancing efficacy | [148] |
| AAV | Parkinson’s disease | Gene delivery (glutamic acid decarboxylase) | Enhancing efficacy | [149] |
| AAV | Parkinson’s disease | Gene delivery (α-synuclein) | Exploring novel mechanisms | [150] |
| AAV | Parkinson’s disease | Gene delivery (IL-21) | Exploring novel mechanisms | [151] |
| AAV | Parkinson’s disease | Gene delivery (Bl-hTFR1) | Exploring novel mechanisms | [12] |
| AAV | Alzheimer’s disease | Gene delivery (RTP801) | Exploring novel mechanisms | [153] |
| AAV2/8-APN | Alzheimer’s disease | Gene delivery (APN) | Enhancing efficacy | [154] |
| AAV | Alzheimer’s disease | Gene delivery (TREM2) | Enhancing efficacy | [155] |
| AAV-mVEGF-C | Stroke | Gene delivery (VEGF-C) | Enhancing efficacy | [156] |
| AAV | Stroke | Gene delivery (PTB) | Enhancing efficacy | [157] |
| AAV | Stroke | Gene delivery (HDAC3/PU.1) | Enhancing efficacy | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yang, S.; Su, L. Integrating Microorganism-Based Therapy and Emerging Biotechnology in the Treatment of Intracranial Central Nervous System Diseases. Pharmaceutics 2025, 17, 1175. https://doi.org/10.3390/pharmaceutics17091175
Li Z, Yang S, Su L. Integrating Microorganism-Based Therapy and Emerging Biotechnology in the Treatment of Intracranial Central Nervous System Diseases. Pharmaceutics. 2025; 17(9):1175. https://doi.org/10.3390/pharmaceutics17091175
Chicago/Turabian StyleLi, Zifan, Shihua Yang, and Lida Su. 2025. "Integrating Microorganism-Based Therapy and Emerging Biotechnology in the Treatment of Intracranial Central Nervous System Diseases" Pharmaceutics 17, no. 9: 1175. https://doi.org/10.3390/pharmaceutics17091175
APA StyleLi, Z., Yang, S., & Su, L. (2025). Integrating Microorganism-Based Therapy and Emerging Biotechnology in the Treatment of Intracranial Central Nervous System Diseases. Pharmaceutics, 17(9), 1175. https://doi.org/10.3390/pharmaceutics17091175





