Selenium Nanoparticles: Synthesis, Stability and In Vitro Evaluation in Human Lens Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Dynamic Light Scattering (DLS) and Stability Measurements
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.4. X-Ray Diffraction (XRD)
2.2.5. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
2.2.6. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.2.7. Cell Viability
2.2.8. ATP Assay
2.2.9. BCA Assay
2.2.10. Live/Dead Assay
2.2.11. GPx Assay
2.2.12. GSH Assay
2.2.13. MDA Assay
2.2.14. TrxR Assay
2.2.15. 3-AT Assay
2.2.16. Statistical Analysis
3. Results
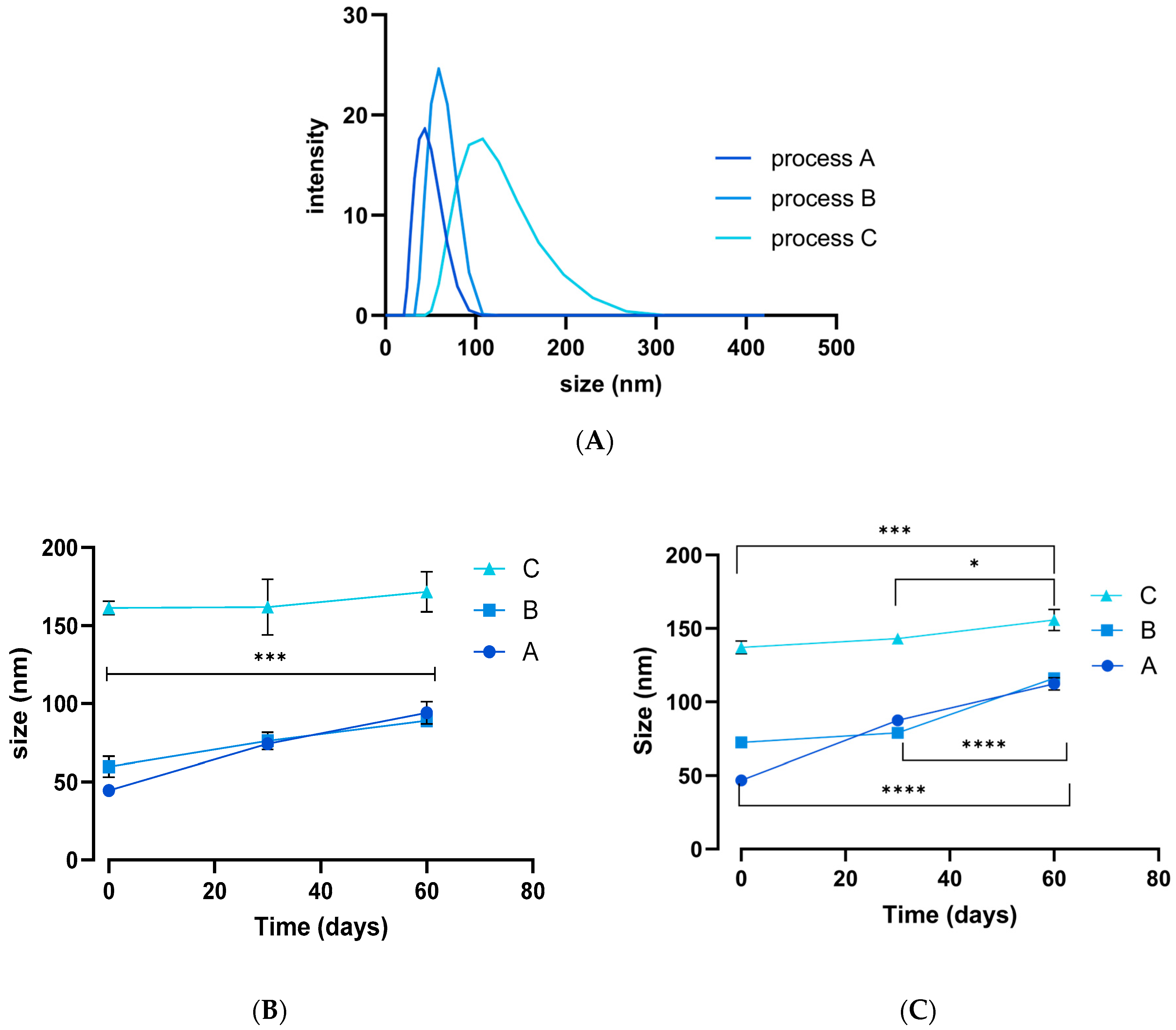
3.1. NP Synthesis
3.2. Antioxidant Activity
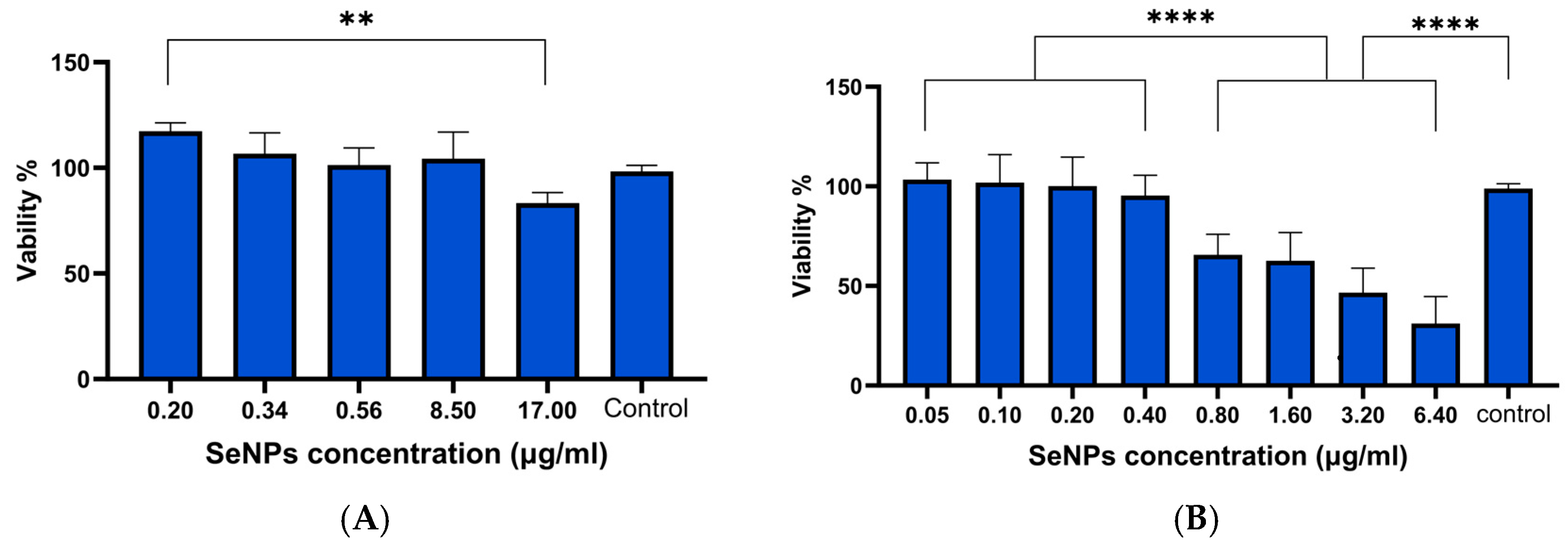
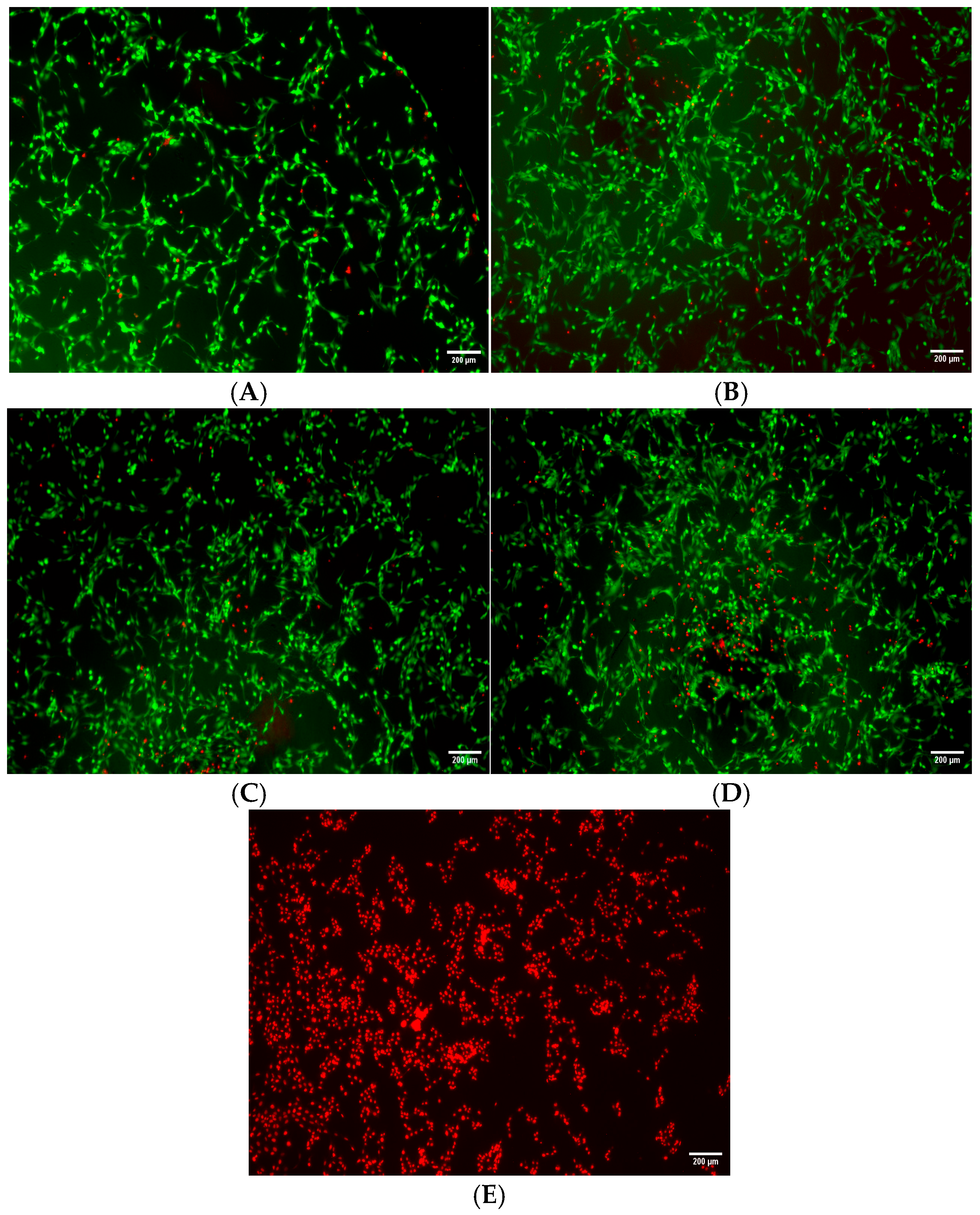
3.3. Cell Viability Results
3.4. GPx Assay Results
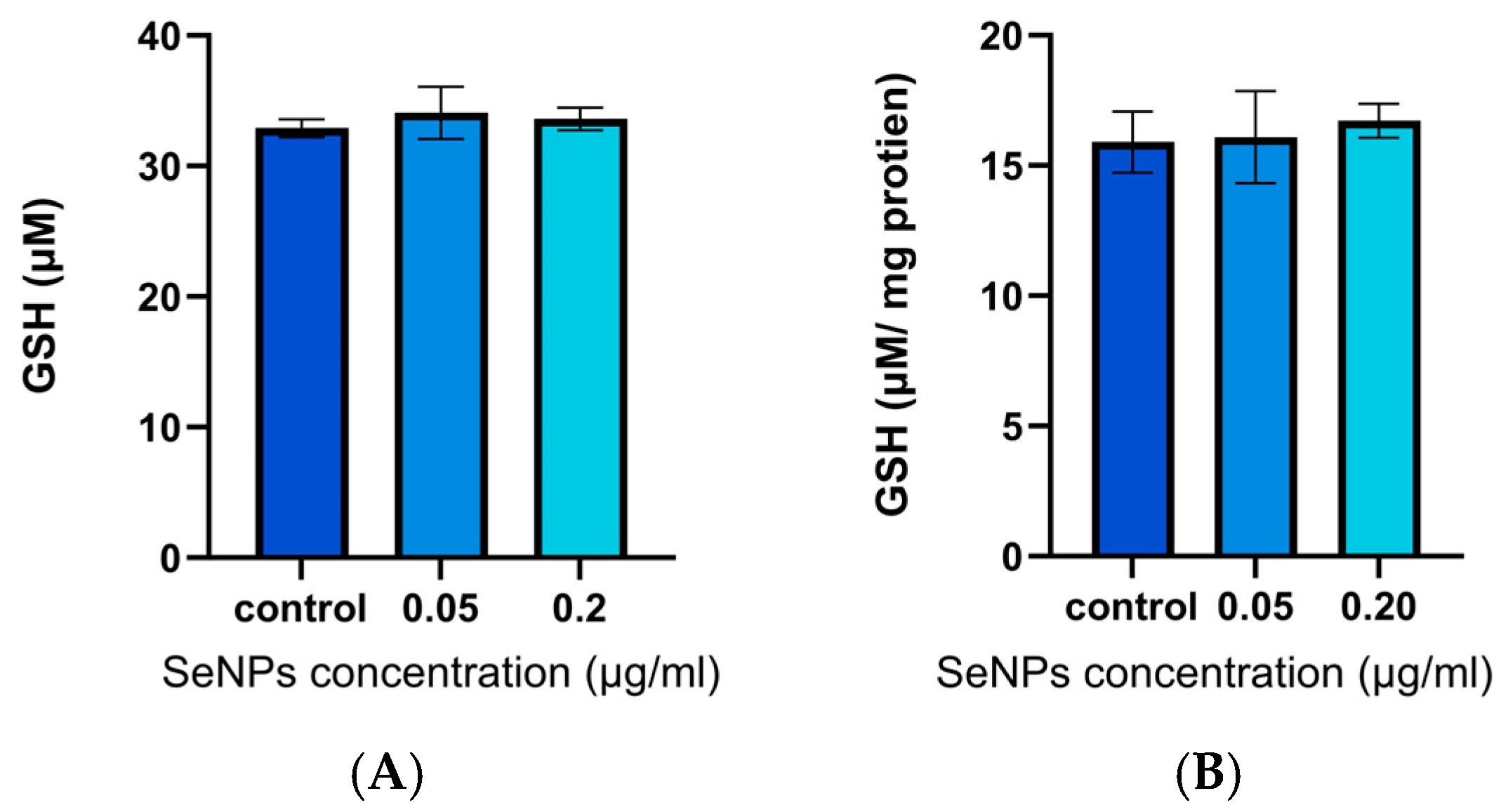
3.5. GSH Assay Results
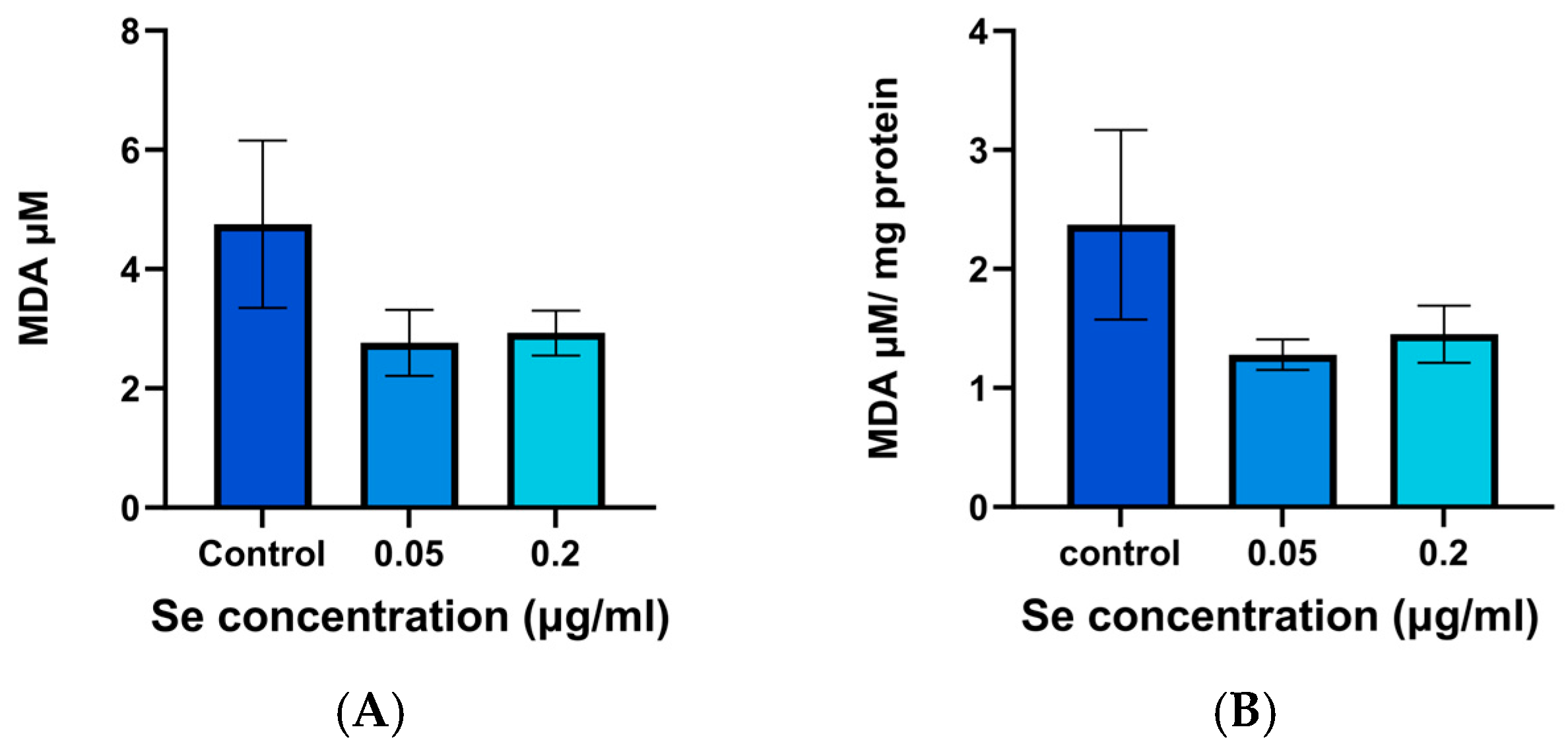
3.6. MDA Assay Results
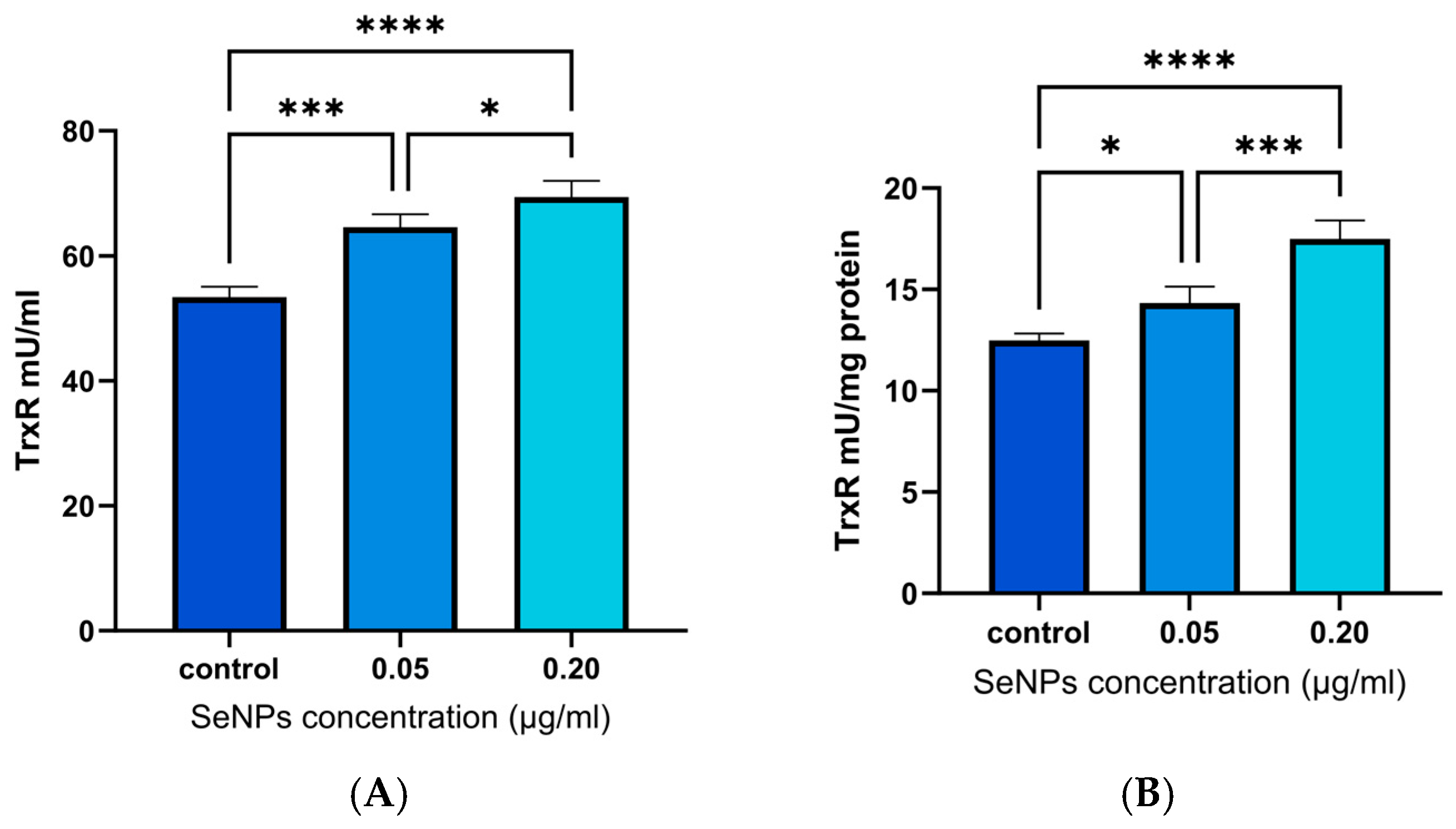
3.7. TrxR Assay Results
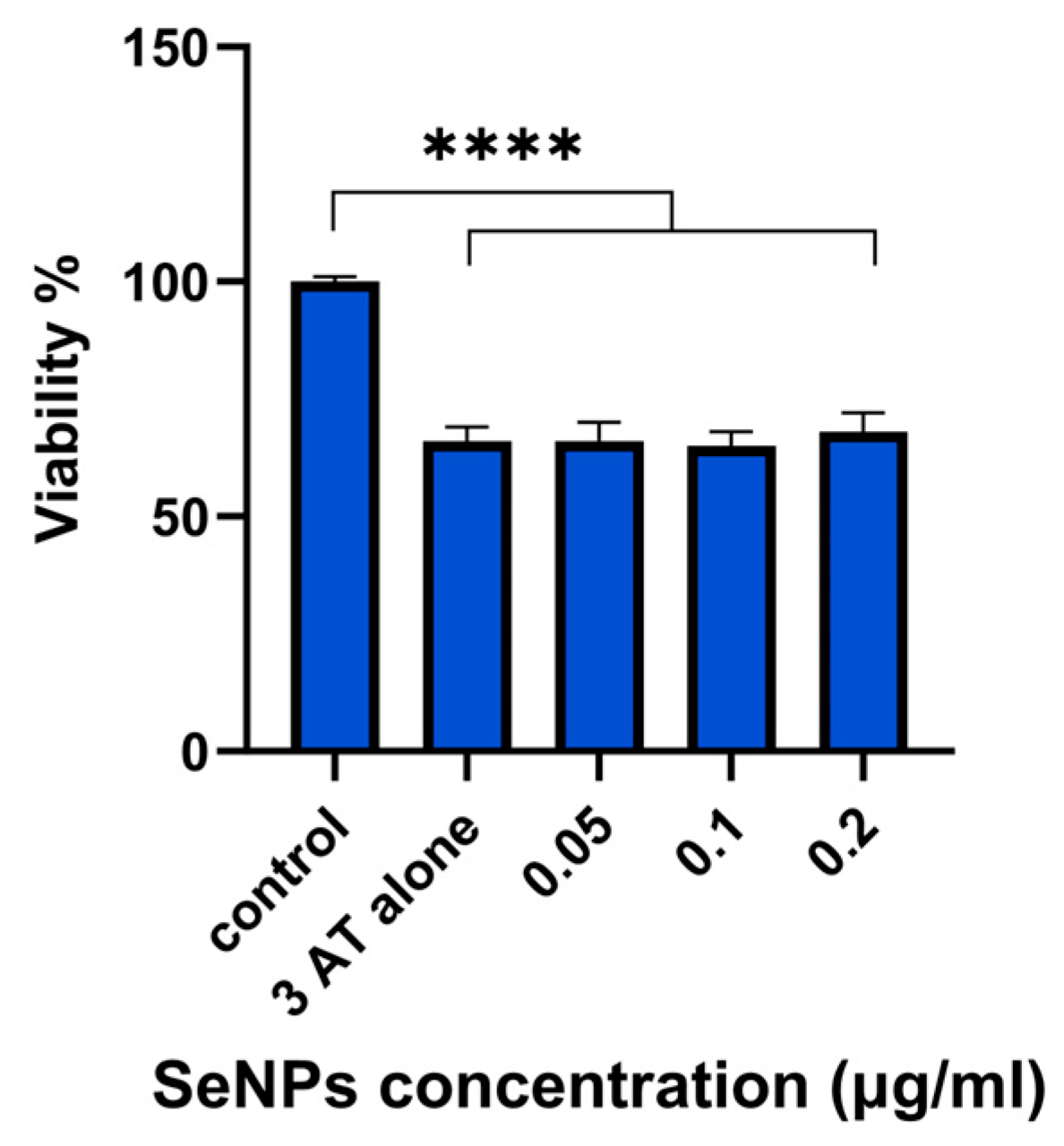
3.8. 3-AT Assay Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARPE | Adult Retinal Pigment Epithelial |
| ATP | Adenosine Triphosphate |
| DLS | Dynamic Light Scattering |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GPx | Glutathione Peroxidase |
| GSH | Reduced Glutathione |
| GSSG | Oxidised Glutathione |
| HLE | Human Lens Epithelial |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| MDA | Malondialdehyde |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| ROS | Reactive Oxygen Species |
| SeNPs | Selenium Nanoparticles |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TPGS | D-α-Tocopheryl Polyethylene Glycol Succinate |
| TrxR | Thioredoxin Reductase |
| XRD | X-ray Diffraction |
| 3-AT | 3-amino-1,2,4-triazole |
References
- Chen, Y.P.; Mehta, G.M.V.S.; Vasiliou, V.P. Antioxidant Defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Al-Bassam, L.; Shearman, G.C.; Brocchini, S.; Alany, R.G.; Williams, G.R. The Potential of Selenium-Based Therapies for Ocular Oxidative Stress. Pharmaceutics 2024, 16, 631. [Google Scholar] [CrossRef]
- Lim, J.C.; Grey, A.C.; Zahraei, A.; Donaldson, P.J. Age-dependent changes in glutathione metabolism pathways in the lens: New insights into therapeutic strategies to prevent cataract formation-A review. Clin. Exp. Ophthalmol. 2020, 48, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.F.; Augusteyn, R.C. Oxidation-Induced Mixed Disulfide and Cataract Formation: A Review. Antioxidants 2025, 14, 425. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Xia, I.F.; Kong, H.K.; Wu, M.M.H.; Lu, Y.; Wong, K.H.; Kwok, K.W.H. Selenium Nanoparticles (SeNPs) Immunomodulation Is More Than Redox Improvement: Serum Proteomics and Transcriptomic Analyses. Antioxidants 2022, 11, 964. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and Selenoproteins: Mechanisms, Health Functions, and Emerging Applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Alvarez-Barrios, A.; Alvarez, L.; Garcia, M.; Artime, E.; Pereiro, R.; Gonzalez-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Agmon, E.; Stockwell, B.R. Lipid homeostasis and regulated cell death. Curr. Opin. Chem. Biol. 2017, 39, 83–89. [Google Scholar] [CrossRef]
- Lopes Junior, E.; Leite, H.P.; Konstantyner, T. Selenium and selenoproteins: From endothelial cytoprotection to clinical outcomes. Transl. Res. 2019, 208, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-X.; Jin, S.-S.; Wu, K.-S.; Yu, G.-C.; Tu, L.-L.; Liu, L. Effect of nano-selenium loaded with Lycium barbarum polysaccharide on the proliferation of lens epithelial cells after UVB damage in vitro. Int. J. Ophthalmol. 2022, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Short, S.P.; Williams, C.S. Selenoproteins in Tumorigenesis and Cancer Progression. In Advances in Cancer Research; Tew, K.D., Galli, F., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 136, Chapter 2; pp. 49–83. [Google Scholar]
- Dannenmann, B.; Lehle, S.; Hildebrand, D.G.; Kübler, A.; Grondona, P.; Schmid, V.; Holzer, K.; Fröschl, M.; Essmann, F.; Rothfuss, O.; et al. High Glutathione and Glutathione Peroxidase-2 Levels Mediate Cell-Type-Specific DNA Damage Protection in Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2015, 4, 886–898. [Google Scholar] [CrossRef]
- Kisić, B.; Mirić, D.; Žorić, L.; Ilić, A.; Dragojević, I. Reduced glutathione level and GSH-dependent enzyme activities in corticonuclear blocks of lenses in patients with senile cataract. Srp. Arh. Celok. Lek. 2012, 140, 563–570. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Chen, X.S.; Zhu, L.Z.; Xue, A.N.; Li, W.X.; Wang, S.Q.; Piao, J.H.; Li, J.; Sun, C.P.; Wu, K.; et al. Biochemical and morphological changes in the lenses of selenium and/or vitamin E deficient rats. Biomed. Environ. Sci. 1994, 7, 109–115. [Google Scholar]
- Flohé, L. Selenium, Selenoproteins and Vision. In Nutrition and the Eye: Basic and Clinical Research; Karger: Basel, Switzerland, 2005; pp. 89–102. [Google Scholar]
- Xu, B.; Liu, Z.; Zhao, J.; Yu, Z. Selenium intake help prevent age-related cataract formation: Evidence from NHANES 2001–2008. Front. Nutr. 2023, 10, 1042893. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Fernando, M.R.; Lou, M.F. Induction of Thioltransferase and Thioredoxin/Thioredoxin Reductase Systems in Cultured Porcine Lenses under Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3783–3789. [Google Scholar] [CrossRef]
- Venardos, K.; Harrison, G.; Headrick, J.; Perkins, A. Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia–reperfusion. J. Trace Elem. Med. Biol. 2004, 18, 81–88. [Google Scholar] [CrossRef]
- Gromer, S.; Johansson, L.; Bauer, H.; Arscott, L.D.; Rauch, S.; Ballou, D.P.; Williams, C.H.; Schirmer, R.H.; Arnér, E.S.J. Active sites of thioredoxin reductases: Why selenoproteins? Proc. Natl. Acad. Sci. USA 2003, 100, 12618–12623. [Google Scholar] [CrossRef]
- Bhuyan, K.C.; Reddy, P.G.; Bhuyan, D.K. [40]—Thioredoxin Genes in Lens: Regulation by Oxidative Stress. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 347, pp. 421–435. [Google Scholar]
- Wei, M.; Xing, K.-Y.; Fan, Y.-C.; Libondi, T.; Lou, M.F. Loss of Thiol Repair Systems in Human Cataractous Lenses. Investig. Ophthalmol. Vis. Sci. 2015, 56, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Y.; Ge, J.; Wang, X.; Liu, L.; Bu, Z.; Liu, P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol. Vis. 2010, 16, 1467–1474. [Google Scholar]
- Ateş, N.A.; Yildirim, Ö.; Tamer, L.; Ünlü, A.; Ercan, B.; Muşlu, N.; Kanik, A.; Hatungil, R.; Atik, U. Plasma catalase activity and malondialdehyde level in patients with cataract. Eye 2004, 18, 785–788. [Google Scholar] [CrossRef]
- Cekić, S.; Zlatanović, G.; Cvetković, T.; Petrović, B. Oxidative stress in cataractogenesis. Bosn. J. Basic Med. Sci. 2010, 10, 265–269. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Wu, C.-R.; Liu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rastegar Moghaddam, S.H.; Hosseini, M.; Sabzi, F.; Hojjati Fard, F.; Marefati, N.; Beheshti, F.; Darroudi, M.; Ebrahimzadeh Bideskan, A.; Anaeigoudari, A. Cardiovascular protective effect of nano selenium in hypothyroid rats: Protection against oxidative stress and cardiac fibrosis. Clin. Exp. Hypertens. 2022, 44, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef]
- Higuchi, A.; Takahashi, K.; Hirashima, M.; Kawakita, T.; Tsubota, K. Selenoprotein P controls oxidative stress in cornea. PLoS ONE 2010, 5, e9911. [Google Scholar] [CrossRef]
- Higuchi, A.; Inoue, H.; Kawakita, T.; Ogishima, T.; Tsubota, K. Selenium Compound Protects Corneal Epithelium against Oxidative Stress. PLoS ONE 2012, 7, e45612. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Inoue, H.; Kaneko, Y.; Oonishi, E.; Tsubota, K. Selenium-binding lactoferrin is taken into corneal epithelial cells by a receptor and prevents corneal damage in dry eye model animals. Sci. Rep. 2016, 6, 36903. [Google Scholar] [CrossRef]
- Ou, L.; Wu, Z.; Hu, X.; Huang, J.; Yi, Z.; Gong, Z.; Li, H.; Peng, K.; Shu, C.; Koole, L.H. A tissue-adhesive F127 hydrogel delivers antioxidative copper-selenide nanoparticles for the treatment of dry eye disease. Acta Biomater. 2024, 175, 353–368. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, Y.; Xu, L.; Wang, Y.; Wang, M.; Zhou, W.; Zhu, H.; Zhao, M.; Wang, S.; Zhang, H.; et al. Selenium nanoparticles attenuate retinal pathological angiogenesis by disrupting cell cycle distribution. Nanomedicine 2025, 20, 803–816. [Google Scholar] [CrossRef]
- Omerović, N.; Vranić, E. Application of nanoparticles in ocular drug delivery systems. Health Technol. 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Abbasi, M.; Aghamollaei, H.; Vaez, A.; Amani, A.M.; Kamyab, H.; Chelliapan, S.; Jamalpour, S.; Zambrano-Dávila, R. Bringing ophthalmology into the scientific world: Novel nanoparticle-based strategies for ocular drug delivery. Ocul. Surf. 2025, 37, 140–172. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Roy, N.; T., N.; Paira, P.; Chakrabarty, R. Selenium-based nanomaterials: Green and conventional synthesis methods, applications, and advances in dye degradation. RSC Adv. 2025, 15, 3008–3025. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C Nanomater. Interfaces 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, S.; Feng, S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, H.; Zeng, X.; Wang, Z.; Zhang, X.; Wu, Y.; Gao, Y.; Zhang, J.; Liu, K.; Liu, R.; et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance. Biomaterials 2013, 35, 2391–2400. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.; Aljaeid, B.M. Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Lucca, L.G.; Govoni, P.; Padula, C.; Favero, E.D.; Cantu, L.; Santi, P.; Nicoli, S. Ex Vivo Conjunctival Retention and Transconjunctival Transport of Poorly Soluble Drugs Using Polymeric Micelles. Pharmaceutics 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef]
- Cholkar, K.; Hariharan, S.; Gunda, S.; Mitra, A.K. Optimization of Dexamethasone Mixed Nanomicellar Formulation. AAPS PharmSciTech 2014, 15, 1454–1467. [Google Scholar] [CrossRef]
- Cholkar, K.; Gunda, S.; Earla, R.; Pal, D.; Mitra, A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech 2014, 16, 610–622. [Google Scholar] [CrossRef]
- Duan, Y.; Cai, X.; Du, H.; Zhai, G. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf. B Biointerfaces 2015, 128, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Alkholief, M.; Albasit, H.; Alhowyan, A.; Alshehri, S.; Raish, M.; Abul Kalam, M.; Alshamsan, A. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of Acyclovir. Saudi Pharm. J. 2019, 27, 293–302. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenger activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.-L.; Kim, H.Y.; Guillen Quispe, Y.N.; Wang, Z.-Q.; Hwang, S.H.; Shin, K.-O.; Lim, S.S. Efficient Separation of Phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by Polarity-Stepwise Elution Counter-Current Chromatography and Their Antioxidant, Antiglycation, and Aldose Reductase Inhibition Potentials. Molecules 2021, 26, 224. [Google Scholar] [CrossRef] [PubMed]
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.G.; Almahri, A.; Ahmed, M.K.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Gomez-Llorente, C.; Aguilera, C.M.; Gil, A.; Rupérez, A.I. Impact of 3-Amino-1,2,4-Triazole (3-AT)-Derived Increase in Hydrogen Peroxide Levels on Inflammation and Metabolism in Human Differentiated Adipocytes. PLoS ONE 2016, 11, e0152550. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surf. B Biointerfaces 2019, 175, 625–635. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-nanoparticles-loaded chitosan/chitooligosaccharide microparticles and their antioxidant potential: A chemical and in vivo investigation. Pharmaceutics 2020, 12, 43. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol. Rep. 2021, 30, e00615. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Glutathione Peroxidase Assay Kit. Cayman Chemical. Available online: https://www.caymanchem.com/product/703102/glutathione-peroxidase-assay-kit?srsltid=AfmBOop3mWHKaOZul6MDbwcCtjVSC9ii73UWCnBfNXys0_rFdoIlY1gL (accessed on 1 May 2023).
- Ganea, E.; Harding, J.J. Glutathione-Related Enzymes and the Eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Rupenthal, I.D.; Räsch, S.S.; Lim, J.C.; Morton, J.D.; Bunt, C.R. Drug delivery to the lens for the management of cataracts. Adv. Drug Deliv. Rev. 2018, 126, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Raj Rai, S.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-α-tocopheryl polyethylene glycol succinate: A review of multifarious applications in nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Bakshi, M.S. How Surfactants Control Crystal Growth of Nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133. [Google Scholar] [CrossRef]
- Chen, H.-I.; Chang, H.-Y. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Zhong, Z.; Lan, Y.; Chen, J.; Ping, L.; Li, X.; Wang, Q.; Zhuang, X.; Qiu, Z.; Yuan, T.; Guo, Q.; et al. Optimizing Paclitaxel Oral Absorption and Bioavailability: TPGS Co-Coating via Supercritical Anti-Solvent Fluidized Bed Technology. Pharmaceuticals 2024, 17, 412. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; K.S., S.D.; Agarwal, H.; Shanmugam, V.K. Efficacy of Biogenic Selenium Nanoparticles from an Extract of Ginger towards Evaluation on Anti-Microbial and Anti-Oxidant Activities. Colloid. Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium nanoparticles: Synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 2019, 6, 0850d0858. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Liang, X.; Liu, G.; Wen, C.; Liang, L.; Liu, X.; Li, Y.; Xu, X. Synthesis and characterization of selenium nanoparticles stabilized by Grifola frondosa polysaccharides and gallic acid conjugates. Int. J. Biol. Macromol. 2024, 278, 134787. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Galić, E.; Ilić, K.; Hartl, S.; Tetyczka, C.; Kasemets, K.; Kurvet, I.; Milić, M.; Barbir, R.; Pem, B.; Erceg, I.; et al. Impact of surface functionalization on the toxicity and antimicrobial effects of selenium nanoparticles considering different routes of entry. Food Chem. Toxicol. 2020, 144, 111621. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.; Newport, D.; McGourty, K. Cell specific variation in viability in suspension in in vitro Poiseuille flow conditions. Sci. Rep. 2021, 11, 13997. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. The Influence of Synthesis Conditions on the Antioxidant Activity of Selenium Nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 25. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M. Hepatocellular-Targeted mRNA Delivery Using Functionalized Selenium Nanoparticles In Vitro. Pharmaceutics 2021, 13, 298. [Google Scholar] [CrossRef]
- Liu, X.; Huang, N.; Li, H.; Jin, Q.; Ji, J. Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells. Langmuir 2013, 29, 9138–9148. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, L.; Feng, L.; Wang, S.; Su, H.; Liu, H.; Liu, H.; Wu, Y. Mitochondrion-targeted selenium nanoparticles enhance reactive oxygen species-mediated cell death. Nanoscale 2020, 12, 1389–1396. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, N.-H.; Patel, K.D.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells. Nanomaterials 2021, 11, 557. [Google Scholar] [CrossRef]
- Ansari, J.A.; Malik, J.A.; Ahmed, S.; Manzoor, M.; Ahemad, N.; Anwar, S. Recent advances in the therapeutic applications of selenium nanoparticles. Mol. Biol. Rep. 2024, 51, 688. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, X.; Huang, Q. Protective effects of Cordyceps sinensis exopolysaccharide-selenium nanoparticles on H2O2-induced oxidative stress in HepG2 cells. Int. J. Biol. Macromol. 2022, 213, 339–351. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, Y. Selenium Supplementation Can Slow the Development of Naphthalene Cataract. Curr. Eye Res. 2012, 37, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ringuet, M.T.; Hunne, B.; Lenz, M.; Bravo, D.M.; Furness, J.B. Analysis of Bioavailability and Induction of Glutathione Peroxidase by Dietary Nanoelemental, Organic and Inorganic Selenium. Nutrients 2021, 13, 1073. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Liu, L.; Tao, W.; Wang, G.; Sun, W.; Pei, X.; Xiao, Z.; Jin, Y.; Wang, M. Prooxidation and Cytotoxicity of Selenium Nanoparticles at Nonlethal Level in Sprague-Dawley Rats and Buffalo Rat Liver Cells. Oxidative Med. Cell. Longev. 2020, 2020, 7680276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Boostani, A.; Sadeghi, A.A.; Mousavi, S.N.; Chamani, M.; Kashan, N. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest. Sci. 2015, 178, 330–336. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, L.; Yang, Z.Y.; Zhang, Z.Y.; Jiang, Y.; Gao, F.; Zhou, G.H. Effects of Different Selenium Sources on Growth Performance, Antioxidant Capacity and Meat Quality of Local Chinese Subei Chickens. Biol. Trace Elem. Res. 2018, 181, 340–346. [Google Scholar] [CrossRef]
- Wang, H.; Wei, W.; Zhang, S.Y.; Shen, Y.X.; Yue, L.; Wang, N.P.; Xu, S.Y. Melatonin-selenium nanoparticles inhibit oxidative stress and protect against hepatic injury induced by Bacillus Calmette-Guérin/lipopolysaccharide in mice. J. Pineal Res. 2005, 39, 156–163. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Plasma selenium in specific and non-specific forms. BioFactors 2001, 14, 107–114. [Google Scholar] [CrossRef]
- Combs, G.F.; Watts, J.C.; Jackson, M.I.; Johnson, L.K.; Zeng, H.; Scheett, A.J.; Uthus, E.O.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; et al. Determinants of selenium status in healthy adults. Nutr. J. 2011, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Arteel, G.E.; Sies, H. The biochemistry of selenium and the glutathione system. Environ. Toxicol. Pharmacol. 2001, 10, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Pilawa, S.; Flohé, L. Trends in selenium biochemistry. Nat. Product. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Khalilov, R.A.; Dzhafarova, A.M.; Rabadanova, Z.G.; Dzhafarov, M.B. Effects of Dihydroquercetin on the Intensity of Oxydative Stress in Rat Liver Mitochondria at Hypothermia. J. Evol. Biochem. Physiol. 2024, 60, 1039–1049. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Khelfi, A. Biomarkers of Oxidative Damage. In Biomarkers of Oxidative Stress: Basics and Measurement of Oxidative Stress; Andreescu, S., Henkel, R., Khelfi, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 69–127. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Sies, H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox Signal 2013, 19, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Kondaparthi, P.; Deore, M.; Naqvi, S.; Flora, S.J.S. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ. Sci. Pollut. Res. 2021, 28, 53034–53044. [Google Scholar] [CrossRef]
- Hendrickx, W.; Decock, J.; Mulholland, F.; Fairweather-Tait, S.; Bao, Y.-P. Selenium Biomarkers in Prostate Cancer Cell Lines and Influence of Selenium on Invasive Potential of PC3 Cells. Front. Oncol. 2013, 3, 239. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Alili, L.; Bilgic, E.; Sies, H.; Brenneisen, P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic. Biol. Med. 2006, 40, 1513–1523. [Google Scholar] [CrossRef]
- Varadaraj, K.; Gao, J.; Mathias, R.T.; Kumari, S.S. GPX1 knockout, not catalase knockout, causes accelerated abnormal optical aberrations and cataract in the aging lens. Mol. Vis. 2022, 28, 11–20. [Google Scholar] [PubMed]
- Xing, K.-Y.; Lou, M.F. Effect of Age on the Thioltransferase (Glutaredoxin) and Thioredoxin Systems in the Human Lens. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6598–6604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Buonfiglio, F.; Zeng, Y.; Pfeiffer, N.; Gericke, A. Oxidative Stress in Cataract Formation: Is There a Treatment Approach on the Horizon? Antioxidants 2024, 13, 1249. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bassam, L.; Naiyer, M.M.; Morris, C.J.; Brocchini, S.; Williams, G.R. Selenium Nanoparticles: Synthesis, Stability and In Vitro Evaluation in Human Lens Epithelial Cells. Pharmaceutics 2025, 17, 1157. https://doi.org/10.3390/pharmaceutics17091157
Al-Bassam L, Naiyer MM, Morris CJ, Brocchini S, Williams GR. Selenium Nanoparticles: Synthesis, Stability and In Vitro Evaluation in Human Lens Epithelial Cells. Pharmaceutics. 2025; 17(9):1157. https://doi.org/10.3390/pharmaceutics17091157
Chicago/Turabian StyleAl-Bassam, Lulwah, Mohammed M. Naiyer, Christopher J. Morris, Steve Brocchini, and Gareth R. Williams. 2025. "Selenium Nanoparticles: Synthesis, Stability and In Vitro Evaluation in Human Lens Epithelial Cells" Pharmaceutics 17, no. 9: 1157. https://doi.org/10.3390/pharmaceutics17091157
APA StyleAl-Bassam, L., Naiyer, M. M., Morris, C. J., Brocchini, S., & Williams, G. R. (2025). Selenium Nanoparticles: Synthesis, Stability and In Vitro Evaluation in Human Lens Epithelial Cells. Pharmaceutics, 17(9), 1157. https://doi.org/10.3390/pharmaceutics17091157






