Whole-Body Physiologically Based Pharmacokinetic–Pharmacodynamic Modeling for Interspecies Translation and Mechanistic Characterization of Plasma and Tissue Disposition of GalNAc-siRNAs
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Software
2.3. General Model Development and Assumptions
2.4. Model Validation
2.5. Species Translation
2.6. PK-PD Relationship
3. Model Evaluation
3.1. Sensitivity Analysis
3.2. Model Performance
4. Results
4.1. Model Validation and Characterization of General Tissue Distribution
4.2. Model Species Translation
4.3. Clinical PK-PD Relationship
4.4. Sensitivity Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Gupta, S.; Qin, J.; Racie, T.; He, G.; Lentini, S.; Malone, R.; Yu, M.; Matsuda, S.; Shulga-Morskaya, S.; et al. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020, 48, 11827–11844. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Guthrie, E.H.; Huang, M.T.; Taxman, D.J. Short hairpin RNA (shRNA): Design, delivery, and assessment of gene knockdown. Methods Mol. Biol. 2010, 629, 141–158. [Google Scholar]
- McDougall, R.; Ramsden, D.; Agarwal, S.; Agarwal, S.; Aluri, K.; Arciprete, M.; Brown, C.R.; Castellanos-Rizaldos, E.; Charisse, K.; Chong, S.; et al. The Nonclinical Disposition and Pharmacokinetic/Pharmacodynamic Properties of N-Acetylgalactosamine-Conjugated Small Interfering RNA Are Highly Predictable and Build Confidence in Translation to Human. Drug Metab. Dispos. 2022, 50, 781–797. [Google Scholar] [CrossRef]
- Bon, C.; Hofer, T.; Bousquet-Mélou, A.; Davies, M.R.; Krippendorff, B.F. Capacity limits of asialoglycoprotein receptor-mediated liver targeting. MAbs 2017, 9, 1360–1369. [Google Scholar] [CrossRef]
- Willoughby, J.L.S.; Chan, A.; Sehgal, A.; Butler, J.S.; Nair, J.K.; Racie, T.; Shulga-Morskaya, S.; Nguyen, T.; Qian, K.; Yucius, K.; et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Ther. 2018, 26, 105–114. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Yu, R.Z.; Graham, M.J.; Post, N.; Riney, S.; Zanardi, T.; Hall, S.; Burkey, J.; Shemesh, C.S.; Prakash, T.P.; Seth, P.P.; et al. Disposition and Pharmacology of a GalNAc3-conjugated ASO Targeting Human Lipoprotein (a) in Mice. Mol. Ther. Nucleic Acids 2016, 5, e317. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Q.; Ji, J.L.; Zheng, S.; Du, L.; Meng, L.; Wu, Y.; Zhao, D.; Wang, X.; Lai, L.; et al. Pharmacokinetic Behaviors of Intravenously Administered siRNA in Glandular Tissues. Theranostics 2016, 6, 1528–1541. [Google Scholar] [CrossRef]
- Salim, E.L.; Kristensen, K.; Sjögren, E. Whole-Body Physiologically Based Pharmacokinetic Modeling of GalNAc-Conjugated siRNAs. Pharmaceutics 2025, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Niederalt, C.; Kuepfer, L.; Solodenko, J.; Eissing, T.; Siegmund, H.U.; Block, M.; Willmann, S.; Lippert, J. A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J. Pharmacokinet. Pharmacodyn. 2018, 45, 235–257. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- Krzyzanski, W.; Harrold, J.M.; Wu, L.S.; Perez-Ruixo, J.J. A cell-level model of pharmacodynamics-mediated drug disposition. J. Pharmacokinet. Pharmacodyn. 2016, 43, 513–527. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Song, D.; Zheng, S.; Carpenter, T.; Heald, D.L. Minimal Physiologically Based Pharmacokinetic-Pharmacodynamic (mPBPK-PD) Model of N-Acetylgalactosamine-Conjugated Small Interfering RNA Disposition and Gene Silencing in Preclinical Species and Humans. J. Pharmacol. Exp. Ther. 2021, 379, 134–146. [Google Scholar] [CrossRef]
- Lumen, A.; Zhang, X.; Dutta, S.; Upreti, V.V. Predicting Clinical Pharmacokinetics/Pharmacodynamics and Impact of Organ Impairment on siRNA-Based Therapeutics Using a Mechanistic Physiologically-Based Pharmacokinetic-Pharmacodynamic Model. Clin. Pharmacol. Ther. 2024, 115, 1054–1064. [Google Scholar] [CrossRef]
- WHO. Harmonization Document No. 9. Characterization and Application of Physiologically Based Pharmacokinetic Models in Risk Assessment 2010, World Health Organization, International Programme on Chemical Safety 2010. Available online: https://www.inchem.org/documents/harmproj/harmproj/harmproj9.pdf (accessed on 1 April 2025).
- Sato, H.; Kato, Y.; Hayasi, E.; Tabata, T.; Suzuki, M.; Takahara, Y.; Sugiyama, Y. A novel hepatic-targeting system for therapeutic cytokines that delivers to the hepatic asialoglycoprotein receptor, but avoids receptor-mediated endocytosis. Pharm. Res. 2002, 19, 1736–1744. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Fridovich, S.E.; Lodish, H.F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J. Biol. Chem. 1982, 257, 4230–4237. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; O’Loughlin, E.; Lee, T.; Houel, S.; O’Carroll, D.; Tarakhovsky, A.; Ahn, N.G.; Yi, R. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012, 26, 693–704. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Davis, M.E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006, 34, 322–333. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Sten, S.; Cardilin, T.; Antonsson, M.; Gennemark, P. Plasma Pharmacokinetics of N-Acetylgalactosamine-Conjugated Small-Interfering Ribonucleic Acids (GalNAc-Conjugated siRNAs). Clin Pharmacokinet. 2023, 62, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.R. Gastrointestinal: Liver. In Systemic lupus Erythematosus; Elsevier: Amsterdam, The Netherlands, 2011; pp. 865–886. [Google Scholar]
- Janas, M.M.; Harbison, C.E.; Perry, V.K.; Carito, B.; Sutherland, J.E.; Vaishnaw, A.K.; Keirstead, N.D.; Warner, G. The Nonclinical Safety Profile of GalNAc-conjugated RNAi Therapeutics in Subacute Studies. Toxicol. Pathol. 2018, 46, 735–745. [Google Scholar] [CrossRef]
- An, G. Pharmacokinetics and Pharmacodynamics of GalNAc-Conjugated siRNAs. J. Clin. Pharmacol. 2023, 64, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Ayyar, V.S.; Song, D. Mechanistic Pharmacokinetics and Pharmacodynamics of GalNAc-siRNA: Translational Model Involving Competitive Receptor-Mediated Disposition and RISC-Dependent Gene Silencing Applied to Givosiran. J. Pharm. Sci. 2024, 113, 176–190. [Google Scholar] [CrossRef]
- Hock, J.; Meister, G. The Argonaute protein family. Genome Biol. 2008, 9, 210. [Google Scholar] [CrossRef]
- Tsubaki, K.; Hammill, M.L.; Varley, A.J.; Kitamura, M.; Okauchi, T.; Desaulniers, J.P. Synthesis and Evaluation of Neutral Phosphate Triester Backbone-Modified siRNAs. ACS Med. Chem. Lett. 2020, 11, 1457–1462. [Google Scholar] [CrossRef]

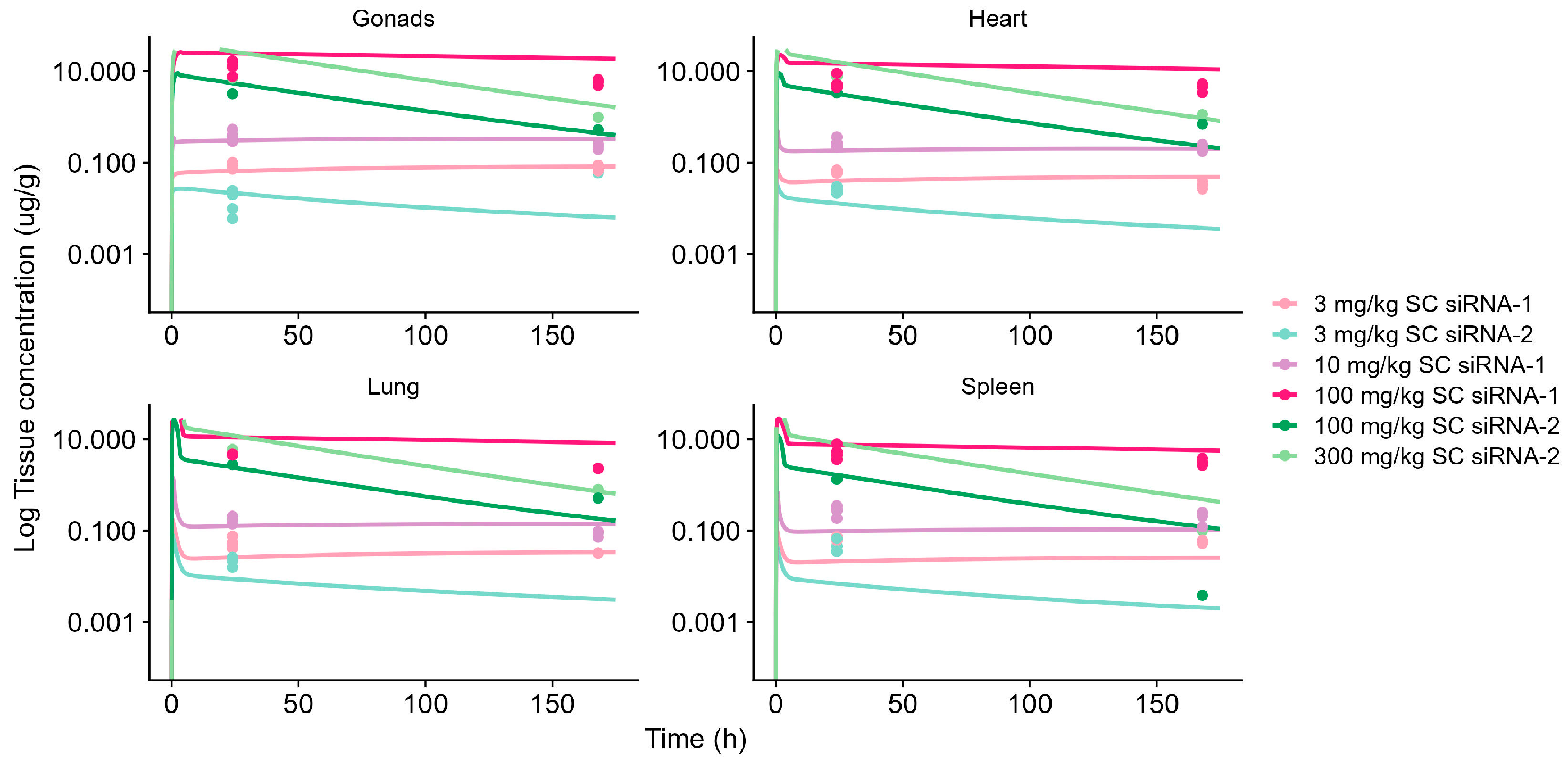
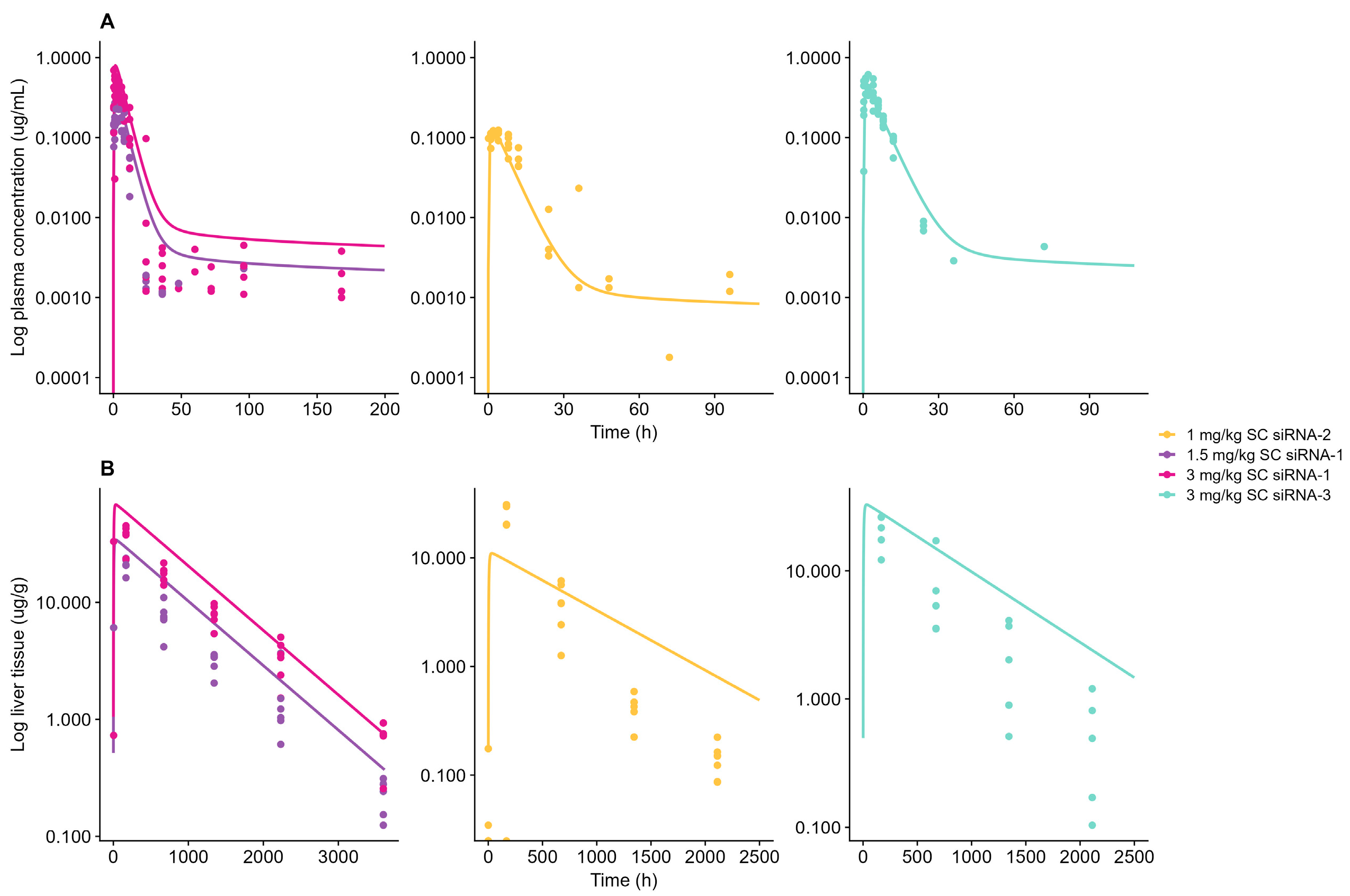
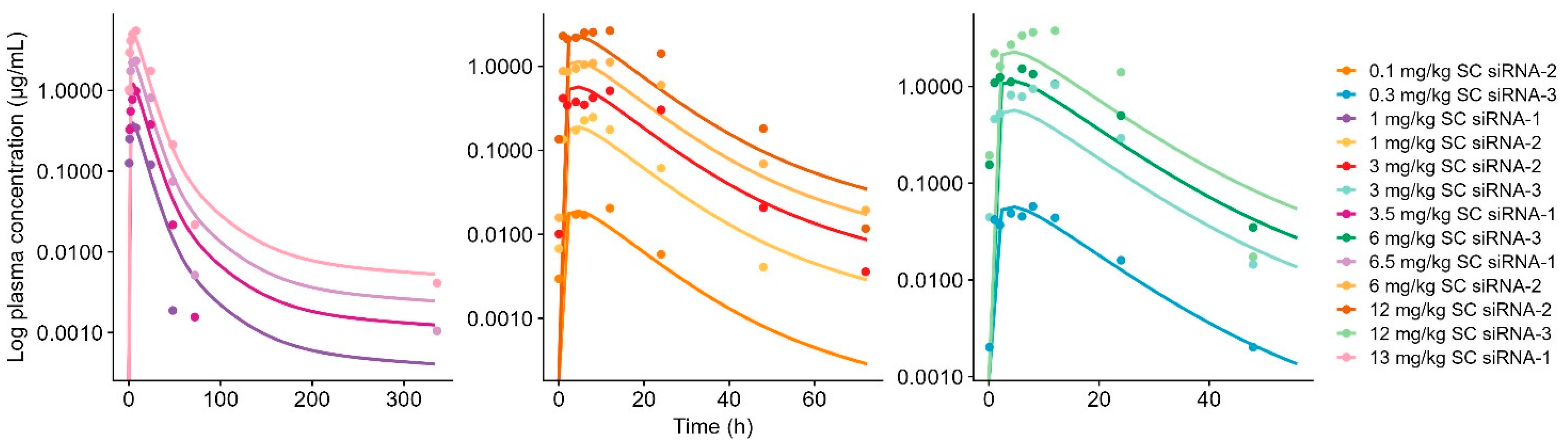

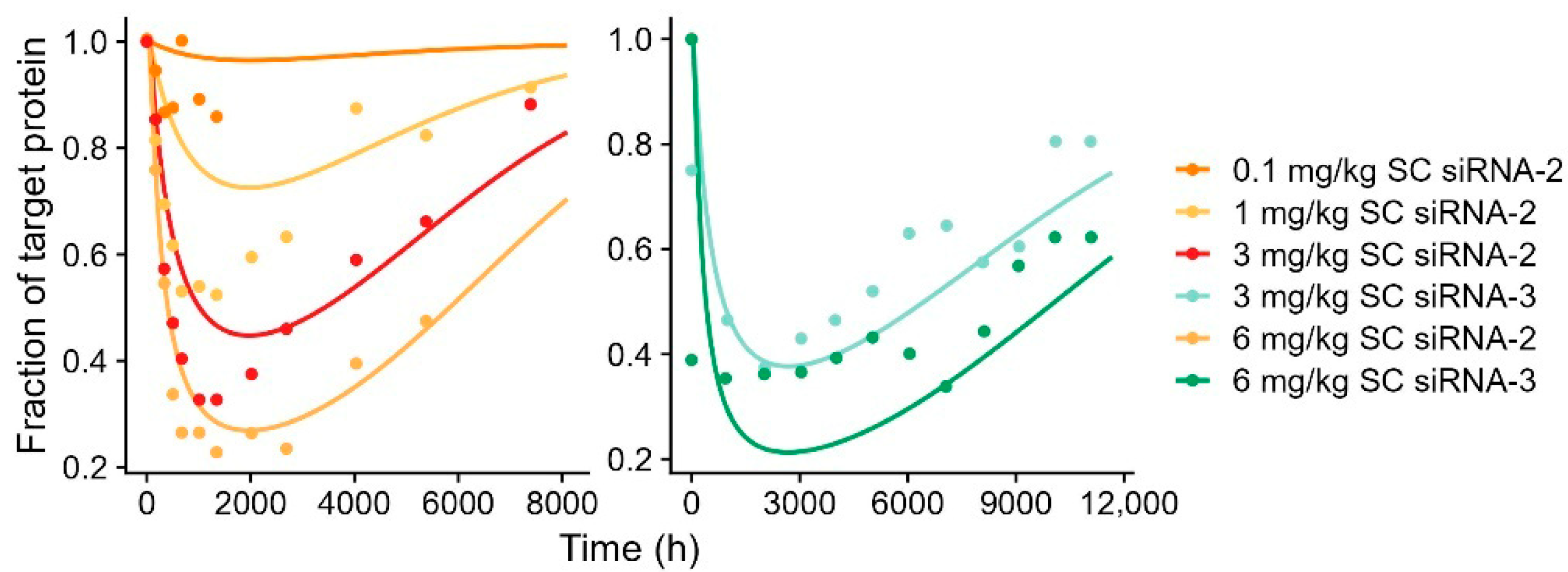

| Compound | Chemical Stabilization Design | Species | Dose Administration b | Measurements c | Modeling Steps | Reference |
|---|---|---|---|---|---|---|
| siRNA-2 | Hairpin Loop | Mouse | 3, 100, 300 mg/kg | Plasma/Liver/ Kidney/Gonads/Lung/Spleen | Validation of legacy model and off-target tissue PK | Internal Data |
| Monkey | 1 mg/kg | Plasma/Liver/ mRNA | Species translation | |||
| Human | 0.1, 1, 3, 6, 12 mg/kg | Plasma/ Target protein | Species translation, clinical PK-PD | |||
| siRNA-3 | Hairpin Loop | Mouse | 3, 100 mg/kg | Plasma/Liver/ Kidney | Validation of legacy model and off-target tissue PK | Internal Data |
| Monkey | 3 mg/kg | Plasma/Liver/ mRNA | Species translation | |||
| Human | 1.5, 3, 6 mg/kg | Plasma/ Target protein | Species translation, clinical PK-PD | |||
| siRNA-1 | Hairpin Loop | Mouse | 3, 10, 100 mg/kg | Plasma/Liver/ Kidney/Gonads/Lung/Spleen/ mRNA | Validation of legacy model and off-target tissue PK | Internal Data |
| Monkey | 3 mg/kg | Plasma/Liver/mRNA | Species translation | |||
| Human | 1, 3.5, 6.5, 13 mg/kg | Plasma | Species translation, clinical PK-PD | |||
| Olpasiran© | ECS a | Monkey | 10 mg/kg | Plasma/ Target protein | Evaluation of key considerations in clinical PK-PD, species translation, and RISC formation | Koren et al. 2022 [13] |
| Human | 3, 9, 30, 75, 225 mg |
| Model Parameter (Unit) | Parameter Description | Mouse | Monkey | Human |
|---|---|---|---|---|
| ka (h−1) | Absorption rate constant | 0.84 (Salim et al., 2025) [11]. | 4.57 (Optimized) | 7.73 (Optimized) |
| Pliver (cm/min) | Endothelial permeability | 0.02 (Salim et al., 2025) [11] | 3.05 × 10−3 (Optimized) | 1.21 × 10−4 (Optimized) |
| fu | Fraction of free GalNAc-siRNA in plasma | 1.0 (Salim et al., 2025) [11]. | ||
| kupatke.liver (min−1) | Liver endosomal uptake rate constant in remaining tissue | 0.29 (Niederalt et al. 2018) [12] | ||
| krecycling.liver (min−1) | Liver endosomal recycling rate constant in remaining tissue | 1.33 × 10−3 (Optimized) | 8.22 × 10−4 (Optimized) | 4.27 × 10−5 (Optimized) |
| kkid.uptake (min−1) | Kidney endosomal uptake rate constant in remaining tissue | 24.98 (Optimized) | ||
| kkid.recycling (min−1) | Kidney endosomal recycling rate constant in remaining tissue | 3.90 × 10−4 (Salim et al., 2025) [11] | ||
| kgonads.uptake (min−1) | Gonad endosomal uptake rate constant in remaining tissue | 1.48 (Optimized) | ||
| klung.uptake (min−1) | Lung endosomal uptake rate constant in remaining tissue | 0.06 (Optimized) | ||
| Kheart.uptake (min−1) | Heart endosomal uptake rate constant in remaining tissue | 0.39 (Optimized) | ||
| kspleen.uptake (min−1) | Spleen endosomal uptake rate constant in remaining tissue | 0.17 (Optimized) | ||
| kRNase (h−1) | Ribonuclease degradation rate constant | 1.21 × 10−4 (Salim et al., 2025) [11]. | ||
| RNasekidney (μmol/l) | Ribonuclease concentration in kidney tissue | 1.17 (Optimized) | ||
| RNaseremaining (μmol/l) | Ribonuclease concentration in remaining tissue | 2.75 × 10−2 (Optimized) | ||
| Rtot (μmol/l) | Total ASGPR density | 5.23 (Salim et al., 2025) [11]. | 2.46 a | 2.46 a |
| kon (l/nmol/h) | Association rate constant between GalNAc-siRNA and ASGPR | 0.53 (Ayyar et al., 2021) [15]. | ||
| koff (h−1) | Dissociation rate constant between GalNAc-siRNA and ASGPR | 1.53 (Sato et al., 2002) [18]. | ||
| kdeg.R (h−1) | Degradation rate constant of ASGPR in cytoplasm | 1.53 (Schwartz et al., 1982) [19]. | ||
| kdeg (h−1) | Degradation rate constant of ASGPR on hepatocyte | 1.52 (Salim et al., 2025) [11]. | ||
| ksyn (h−1) | Synthesis rate constant of ASGPR | 7.94 (ksyn = Rtot × kdeg) | ||
| kint (h−1) | Internalization rate constant of GalNAc-siRNA-ASGPR complex | 5.14 (Salim et al. 2025) [11]. | ||
| kcle (h−1) | Cleavage rate constant of GalNAc-siRNA in liver endosome | 1.32 (Prakash et al., 2014) [20]. | ||
| krec (h−1) | Recycling rate constant of ASGPR | 13.8 (Schwartz et al., 1982) [19]. | ||
| kendosome (h−1) | Liver endosomal degradation rate for siRNA | 5.0 × 10−3 (Optimized) | −0.25 b | −0.25 b |
| fescape | Fraction of antisense strand escaping the liver endosome into cytoplasm | 0.01 (McDougall et al. 2022) [5] | ||
| kdeg.C (h−1) | siRNA degradation rate constant in cytoplasm | 0.1 (Ayyar et al. 2021) [15]. | ||
| RISCtot (umol/l) | Total RISC concentration | 0.0003 (Wang et al., 2012) [21]. | ||
| konRISC (h−1) | Association rate constant of siRNA antisense strand and RISC | 2.73 × 10−4 (Salim et al., 2025) [11] | 1.68 × 10−5 (Optimized) | |
| koff.RISC (h−1) | Dissociation rate constant of siRNA antisense strand and RISC | 1 × 10−7 (Barlett and Davis 2006) [22]. | ||
| Smax | Maximum stimulation of mRNA degradation | 58.84 | ||
| SC50 (nmol/L) | RISC-loaded siRNA at half-maximal stimulation | 3.52 | ||
| kdeg.mRNA (h−1) | Degradation rate constant for mRNA | 0.06 (Ayyar et al. 2021) [15]. | ||
| kdeg.protein (h−1) | Degradation rate target for protein | 0.05 (Ayyar et al. 2021) [15]. | ||
| Gamma (γ) | 1.5 (Ayyar et al. 2021) [15]. | |||
| Parameter (Unit) | Parameter Description | Compound | Species | Value |
|---|---|---|---|---|
| F (%) | Bioavailability | siRNA-1 | Mouse | 47 |
| Monkey | ||||
| Human | ||||
| siRNA-2 | Mouse | 22 | ||
| Monkey | ||||
| Human | ||||
| siRNA-3 | Mouse | |||
| Monkey | ||||
| Human | ||||
| krecycle.tissue (min−1) | Endosomal recycling rate constant in remaining tissue | siRNA-1 | Mouse | 3.23 × 10−5 |
| Monkey | ||||
| Human | ||||
| siRNA-2 | Mouse | 3.49 × 10−4 | ||
| Monkey | ||||
| Human | ||||
| siRNA-3 | Mouse | 3.23 × 10−5 | ||
| Monkey | ||||
| Human | ||||
| kDR (h−1) | Degradation rate constant of RISC complex | siRNA-1 | Mouse | 9.72 × 10−3 |
| Monkey | 8.02 × 10−3 | |||
| Human | - | |||
| siRNA-2 | Mouse | - | ||
| Monkey | 5.28 × 10−3 | |||
| Human | 4.17 × 10−4 | |||
| siRNA-3 | Mouse | - | ||
| Monkey | 1.94 × 10−3 | |||
| Human | 2.02 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim, E.L.; Kristensen, K.; Chopda, G.; Sjögren, E. Whole-Body Physiologically Based Pharmacokinetic–Pharmacodynamic Modeling for Interspecies Translation and Mechanistic Characterization of Plasma and Tissue Disposition of GalNAc-siRNAs. Pharmaceutics 2025, 17, 1154. https://doi.org/10.3390/pharmaceutics17091154
Salim EL, Kristensen K, Chopda G, Sjögren E. Whole-Body Physiologically Based Pharmacokinetic–Pharmacodynamic Modeling for Interspecies Translation and Mechanistic Characterization of Plasma and Tissue Disposition of GalNAc-siRNAs. Pharmaceutics. 2025; 17(9):1154. https://doi.org/10.3390/pharmaceutics17091154
Chicago/Turabian StyleSalim, Emilie Langeskov, Kim Kristensen, Girish Chopda, and Erik Sjögren. 2025. "Whole-Body Physiologically Based Pharmacokinetic–Pharmacodynamic Modeling for Interspecies Translation and Mechanistic Characterization of Plasma and Tissue Disposition of GalNAc-siRNAs" Pharmaceutics 17, no. 9: 1154. https://doi.org/10.3390/pharmaceutics17091154
APA StyleSalim, E. L., Kristensen, K., Chopda, G., & Sjögren, E. (2025). Whole-Body Physiologically Based Pharmacokinetic–Pharmacodynamic Modeling for Interspecies Translation and Mechanistic Characterization of Plasma and Tissue Disposition of GalNAc-siRNAs. Pharmaceutics, 17(9), 1154. https://doi.org/10.3390/pharmaceutics17091154








