Heparin-Based Growth Factor Delivery Platforms: A Review
Abstract
1. Introduction
2. Heparin Composition and Modification Principles
2.1. Electrostatic Adsorption
2.2. Physical Blending
2.3. Surface Modification
2.4. Network Crosslinking Involving Heparin
2.5. Multiple Mechanisms Involving Heparin
3. Heparin Delivery Platforms
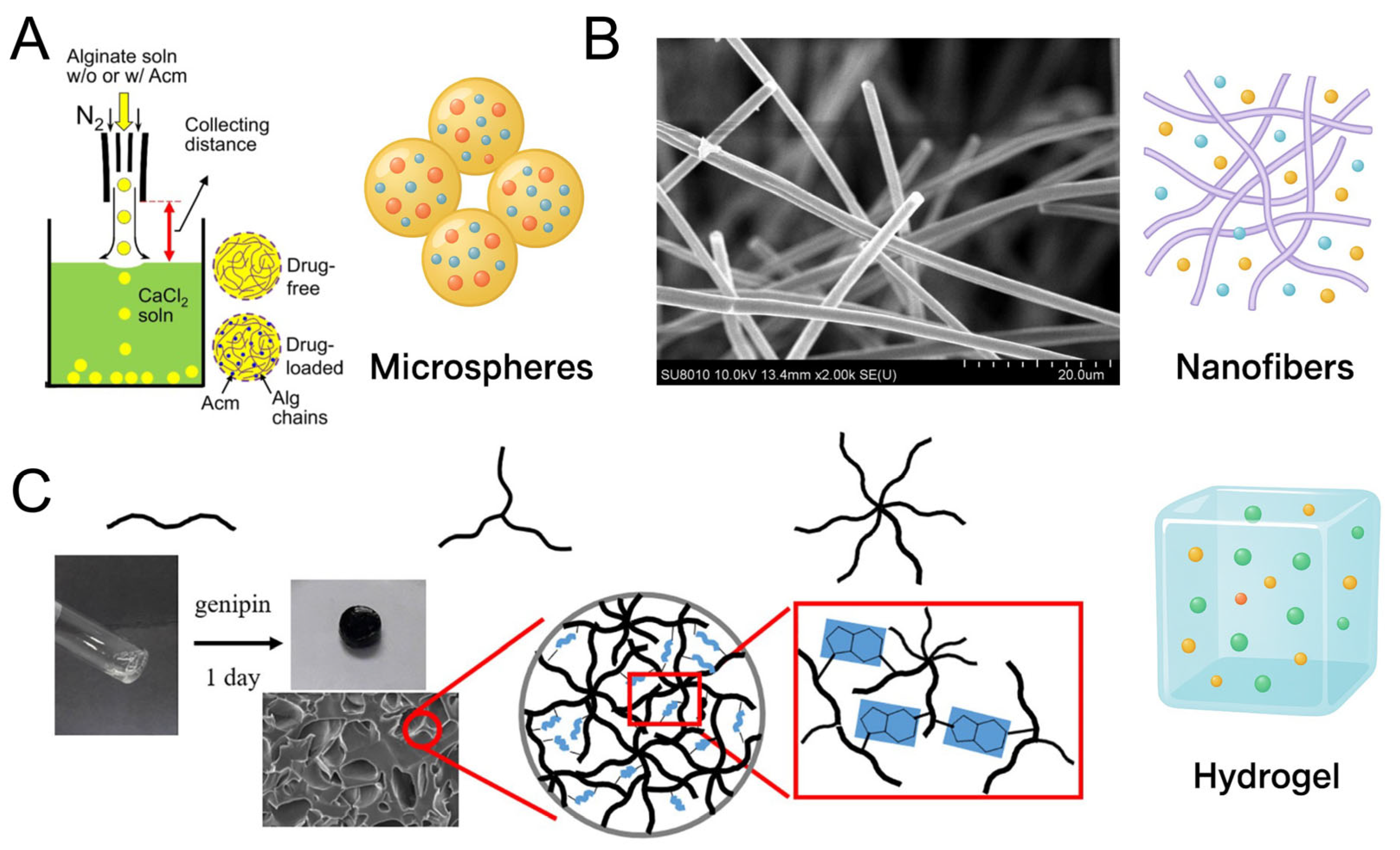
3.1. Heparin-Loaded Microspheres and Fabrication Strategies
3.1.1. Emulsion–Solvent Evaporation/Extraction
3.1.2. Spray Drying
3.1.3. Microfluidics
3.1.4. Coacervation
3.1.5. Electrospraying
3.1.6. Summary
3.2. Heparin-Loaded Nanofibers and Fabrication Strategies
3.2.1. Coaxial Electrospun Core–Shell Nanofibers
3.2.2. Microfluidic and Emulsion Electrospinning
- Microfluidic-Assisted Electrospinning
- Emulsion Electrospinning
3.2.3. Triaxial, Janus, and Multilayer Concentric Nanofibers
- Triaxial Electrospinning
- Janus (Side-by-Side) Electrospinning
- Multilayer Concentric Electrospinning
3.2.4. Another Nanofibers
3.2.5. Summary
3.3. Heparin-Based Hydrogel Platforms
4. Stimuli-Responsive Designs and Therapeutic Applications of Heparin-Based GF Delivery Platforms
4.1. Functional Designs: Stimuli-Responsive Delivery Systems
4.1.1. pH-Responsive Heparin-Based Systems
4.1.2. Thermo-Responsive Systems
4.1.3. Enzyme-Responsive Systems
4.1.4. ROS/Redox-Responsive Systems
4.1.5. Ultrasound-Responsive Systems
4.2. Applications of Heparin-Based GF Delivery Platforms
4.2.1. Soft Tissue Regeneration
4.2.2. Bone and Cartilage Repair
4.2.3. Neural Tissue Engineering
4.2.4. Cardiovascular Regeneration
4.2.5. Wound Healing and Chronic Ulcers
4.2.6. Anti-Fibrotic and Anti-Inflammatory Therapies
4.2.7. Cancer Therapy and TME Modulation
5. Future Perspectives and Research Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Growth Factors and Biological Molecules | |
| VEGF | Vascular Endothelial Growth Factor |
| FGF | Fibroblast Growth Factor |
| bFGF | Basic Fibroblast Growth Factor |
| BMPs | Bone Morphogenetic Proteins |
| TGF-β | Transforming Growth Factor-β |
| PDGF | Platelet-Derived Growth Factor |
| HGF | Hepatocyte Growth Factor |
| NGF | Nerve Growth Factor |
| BDNF | Brain-Derived Neurotrophic Factor |
| HB-EGF | Heparin-Binding Epidermal Growth Factor |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GABA | Gamma Amino Butyric Acid |
| GSH | Glutathione |
| ALP | Alkaline Phosphatase |
| GFs | Growth Factors |
| Materials and Carrier Systems | |
| DBM | Demineralized Bone Matrix |
| PCL | Polycaprolactone |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLLA | Poly(L-lactic acid) |
| PU | Polyurethane |
| H-PHNF | Heparin–Polyurethane Hybrid Nanofibers |
| FN | Fibronectin |
| DMP-BMP-2 | Heparin Microparticle Delivery of Bone Morphogenetic Protein-2 |
| PNIPAM | Poly(N-isopropylacrylamide) |
| HepMA | Heparin Methacrylate |
| PVA | Poly(vinyl alcohol) |
| PEG | Polyethylene Glycol |
| PTFE | Polytetrafluoroethylene |
| Mechanisms and Analytical Technologies | |
| ROS | Reactive Oxygen Species |
| ECM | Extracellular Matrix |
| TME | Tumor Microenvironment |
| TKI | Tyrosine Kinase Inhibitor |
| CED | Convection-Enhanced Delivery |
| MMP | Matrix Metalloproteinase |
| HPMBs | Microbubbles |
| LMWH | Low-Molecular-Weight Heparins |
| Others | |
| IdoA | L-iduronic Acid |
| GlcA | D-glucuronic Acid |
| GlcN | D-glucosamine |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Ngaha, T.Y.S.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Abah, M.O.; Fossa, L.T.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Rusanov, A.S.; Pirogova, Y.N.; et al. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers 2023, 15, 4648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Li, J. Current progress in growth factors and extracellular vesicles in tendon healing. Int. Wound J. 2023, 20, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Bouju, A.; Nusse, R.; Wu, P.V. A primer on the pleiotropic endocrine fibroblast growth factor FGF19/ FGF15. Differentiation 2024, 140, 100816. [Google Scholar] [CrossRef]
- Ao, R.G.L.; Liang, W.; Wang, Z.M.; Li, Q.Y.; Pan, X.Y.; Zhen, Y.H.; An, Y. Delivery Strategies of Growth Factors in Cartilage Tissue Engineering. Tissue Eng. Part B-Rev. 2024, 31, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.J.; Ding, G.; Hao, Z.K.; Li, Y.C.; Deng, A.J.; Zhang, C.M. Elucidating the mechanism of corneal epithelial cell repair: Unraveling the impact of growth factors. Front. Med. 2024, 11, 1384500. [Google Scholar] [CrossRef]
- Ge, Z.M.; Zhang, Q.; Lin, W.; Jiang, X.F.; Zhang, Y.Y. The role of angiogenic growth factors in the immune microenvironment of glioma. Front. Oncol. 2023, 13, 1254694. [Google Scholar] [CrossRef]
- Wu, S.; Nie, Q.; Tan, S.; Liao, G.Y.; Lv, Y.Y.; Lv, C.H.; Chen, G.; Liu, S.C. The immunity modulation of transforming growth factor-β in malaria and other pathological process. Int. Immunopharmacol. 2023, 122, 110658. [Google Scholar] [CrossRef]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7653–7664. [Google Scholar]
- Zhan, L.W.; Zhou, Y.G.; Liu, R.T.; Sun, R.L.; Li, Y.F.; Tian, Y.Z.; Fan, B. Advances in growth factor-containing 3D printed scaffolds in orthopedics. Biomed. Eng. Online 2025, 24, 14. [Google Scholar] [CrossRef]
- Morales, T.I.; Stearns-Yoder, K.A.; Hoffberg, A.S.; Khan, T.K.; Wortzel, H.; Brenner, L.A. Interactions of Glutamate and Gamma Amino Butyric Acid with the insulin-like growth factor system in traumatic brain injury (TBI) and/or cardiovascular accidents (CVA or stroke): A systematic review. Heliyon 2022, 8, e09037. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.; Schwarz, Q.; Wiszniak, S. Endothelial-derived angiocrine factors as instructors of embryonic development. Front. Cell Dev. Biol. 2023, 11, 1172114. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.T.; Schwinghamer, K.M.; Siahaan, T.J. Delivery of Neuroregenerative Proteins to the Brain for Treatments of Neurodegenerative Brain Diseases. Life 2024, 14, 1456. [Google Scholar] [CrossRef]
- Tavares, M.R.; Wasinski, F.; Metzger, M.; Donato, J., Jr. Impact of Growth Hormone on Microglial and Astrocytic Function. J. Integr. Neurosci. 2024, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Y.; Li, J.Y.; Liu, Y.W.; Zhu, X.J.; Shao, W.D. Feasibility of Growth Factor Agent Therapy in Repairing Motor Injury. Front. Pharmacol. 2022, 13, 842775. [Google Scholar] [CrossRef]
- Mullin, J.A.; Rahmani, E.; Kiick, K.L.; Sullivan, M.O. Growth factors and growth factor gene therapies for treating chronic wounds. Bioeng. Transl. Med. 2024, 9, e10642. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2024, 36, e2210707. [Google Scholar] [CrossRef]
- Deepak, P.; Kumar, P.; Arya, D.K.; Pandey, P.; Kumar, S.; Parida, B.P.; Narayan, G.; Singh, S.; Rajinikanth, P.S. c(RGDfK) anchored surface manipulated liposome for tumor-targeted tyrosine kinase inhibitor (TKI) delivery to potentiate liver anticancer activity. Int. J. Pharm. 2023, 642, 123160. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Le, T.M.D.; Hong, J.; Jiao, A.; Yoon, A.R.; Yun, C.O. Smart Accumulating Dual-Targeting Lipid Envelopes Equipping Oncolytic Adenovirus for Enhancing Cancer Gene Therapeutic Efficacy. ACS Nano 2024, 18, 27869–27890. [Google Scholar] [CrossRef]
- Rochon, P.A.; Gurwitz, J.H. Drug therapy. Lancet 1995, 346, 32–36. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110994. [Google Scholar] [CrossRef]
- Jalani, G.; Rizwan, M.; Akram, M.A.; Mujahid, M. Editorial: Cell and therapeutic delivery using injectable hydrogels for tissue engineering applications. Front. Bioeng. Biotechnol. 2023, 11, 1170933. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Su, M.; Qin, X.; Ruan, Q.; Lang, W.; Wu, M.; Chen, Y.; Lv, Q. Progress in the application of sustained-release drug microspheres in tissue engineering. Mater. Today Bio 2022, 16, 100394. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Gupta, S.; Puttaiahgowda, Y.M.; Deiglmayr, L. Recent advances in the design and immobilization of heparin for biomedical application: A review. Int. J. Biol. Macromol. 2024, 264, 130743. [Google Scholar] [CrossRef]
- He, C.; Ji, H.F.; Qian, Y.H.; Wang, Q.; Liu, X.L.; Zhao, W.F.; Zhao, C.S. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Safi, S.Z.; Fazil, S.; Saeed, L.; Shah, H.M.R.; Arshad, M.; Alobaid, H.M.; Rehman, F.; Sharif, F.; Selvaraj, C.; Orakzai, A.H.; et al. Chitosan- and heparin-based advanced hydrogels: Their chemistry, structure and biomedical applications. Chem. Pap. 2024, 78, 9287–9309. [Google Scholar] [CrossRef]
- Chu, H.; Gao, J.; Chen, C.-W.; Huard, J.; Wang, Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 13444–13449. [Google Scholar] [CrossRef]
- Yeh, C.-W.; Wang, Y. Coacervate-Filled Lipid Vesicles for Protein Delivery. Macromol. Biosci. 2023, 23, 2200538. [Google Scholar] [CrossRef]
- Choi, J.S.; Yoo, H.S. Chitosan/Pluronic Hydrogel Containing bFGF/Heparin for Encapsulation of Human Dermal Fibroblasts. J. Biomater. Sci. Polym. Ed. 2013, 24, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, Y.; Kobayashi, J.; Ohashi, K.; Tatsumi, K.; Kim, K.; Akiyama, Y.; Yamato, M.; Okano, T. A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Regen. Ther. 2016, 3, 97–106. [Google Scholar] [CrossRef]
- Voorneveld, J.; Oosthuysen, A.; Franz, T.; Zilla, P.; Bezuidenhout, D. Dual electrospinning with sacrificial fibers for engineered porosity and enhancement of tissue ingrowth. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.F.; Yang, X.L. Progress in Heparin-Functionalized Biomaterials. Chin. J. Org. Chem. 2010, 30, 359–367. [Google Scholar]
- Joung, Y.K.; Bae, J.W.; Park, K.D. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin. Drug Deliv. 2008, 5, 1173–1184. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E. Incorporation of heparin into biomaterials. Acta Biomater. 2014, 10, 1581–1587. [Google Scholar] [CrossRef]
- Liang, Y.K.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef]
- Schmitz, M.G.J.; Ibrahim, D.M.; Bartels, P.A.A.; Twisk, S.A.E.; Smits, A.I.P.M.; Bouten, C.V.C.; Dankers, P.Y.W. Heparin-guided binding of vascular endothelial growth factor to supramolecular biomaterial surfaces. J. Polym. Sci. 2023, 61, 2524–2538. [Google Scholar] [CrossRef]
- Zhao, W.; McCallum, S.; Xiao, Z.; Zhang, F.; Linhardt, R. Binding affinities of vascular endothelial growth factor (VEGF) for heparin-derived oligosaccharides. Biosci. Rep. 2011, 32, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lundin, L.; Larsson, H.; Kreuger, J.; Kanda, S.; Lindahl, U.; Salmivirta, M.; Claesson-Welsh, L. Selectively Desulfated Heparin Inhibits Fibroblast Growth Factor-induced Mitogenicity and Angiogenesis. J. Biol. Chem. 2000, 275, 24653–24660. [Google Scholar] [CrossRef] [PubMed]
- Ashikari-Hada, S.; Habuchi, H.; Kariya, Y.; Itoh, N.; Reddi, A.H.; Kimata, K. Characterization of Growth Factor-binding Structures in Heparin/Heparan Sulfate Using an Octasaccharide Library. J. Biol. Chem. 2004, 279, 12346–12354. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, H.; Liu, J.; Zhai, G. Advanced nanocarriers based on heparin and its derivatives for cancer management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef]
- García-Briones, G.S.; Laga, R.; Cernochová, Z.; Arjona-Ruiz, C.; Janousková, O.; Slouf, M.; Pop-Georgievski, O.; Kubies, D. Polyelectrolyte nanoparticles based on poly N-(2-hydroxypropyl) methacrylamide-block-poly(N-(3-aminopropyl)methacrylamide copolymers for delivery of heparin-binding proteins. Eur. Polym. J. 2023, 191, 111976. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Z.; Liu, W.; Piao, M.; Li, J.; Zhang, H. Controlled Release of Growth Factor from Heparin Embedded Poly(aldehyde guluronate) Hydrogels and Its Effect on Vascularization. Gels 2023, 9, 589. [Google Scholar] [CrossRef]
- Safikhani, M.M.; Asefnejad, A.; Aghdam, R.M.; Rahmati, S. Fabrication, and characterization of crosslinked sodium alginate/hyaluronic acid/gelatin 3Dprinted heparin-loaded scaffold. J. Polym. Res. 2024, 31, 121. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zeng, Y.; Zhao, P.; Liao, J.; Lian, C.; Hu, N.; Luo, X.; et al. Stem Cell-Recruiting Injectable Microgels for Repairing Osteoarthritis. Adv. Funct. Mater. 2021, 31, 2105084. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.Y.; Wu, Q.J.; An, Y.; Wang, K.N.; Xuan, T.X.; Zhang, J.W.; Song, W.X.; He, H.C.; Song, L.W.; et al. Mussel-Inspired Surface Immobilization of Heparin on Magnetic Nanoparticles for Enhanced Wound Repair via Sustained Release of a Growth Factor and M2 Macrophage Polarization. Acs Appl. Mater. Interfaces 2021, 13, 2230–2244. [Google Scholar] [CrossRef]
- Kang, S.; Chi, Y.J.; Cho, K.; Lee, H.J.; Koh, W.G. Dual surface modification of poly(L-lactide) scaffold achieved by thermal incorporation of aligned nanofiber and click immobilization of VEGF to enhance endothelialization and blood compatibility. Appl. Surf. Sci. 2022, 589, 152969. [Google Scholar] [CrossRef]
- Song, H.X.; Wu, T.; Yang, X.T.; Li, Y.Z.; Ye, Y.; Li, B.; Liu, T.; Liu, S.H.; Li, J.H. Surface Modification with NGF-Loaded Chitosan/Heparin Nanoparticles for Improving Biocompatibility of Cardiovascular Stent. Stem Cells Int. 2021, 2021, 941143. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, T.Q.; Weng, W.; Zhu, X.H. Research Progress on Extracellular Matrix-Based Composite Materials in Antibacterial Field. Biomater. Res. 2025, 29, 0128. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Guo, J.H.; Wang, F.F. Heparosan crosslinked hydrogels for injectable dermal fillers: Fabrication, physicochemical properties and biocompatibility. New J. Chem. 2024, 48, 18111–18119. [Google Scholar] [CrossRef]
- Torresan, V.; Gandin, A.; Contessotto, P.; Zanconato, F.; Brusatin, G. Injectable hyaluronic acid-based hydrogel niches to create localized and time-controlled therapy delivery. Mater. Today Bio 2025, 31, 101510. [Google Scholar] [CrossRef]
- Charron, P.N.; Garcia, L.M.; Tahir, I.; Floreani, R.A. Bio-inspired green light crosslinked alginate-heparin hydrogels support HUVEC tube formation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104932. [Google Scholar] [CrossRef]
- Fan, B.; Liu, L.; Zheng, Y.; Xing, Y.; Shen, W.G.; Li, Q.; Wang, R.Y.; Liang, G.X. Novel pH-responsive and mucoadhesive chitosan-based nanoparticles for oral delivery of low molecular weight heparin with enhanced bioavailability and anticoagulant effect. J. Drug Deliv. Sci. Technol. 2022, 78, 103955. [Google Scholar] [CrossRef]
- Wang, D.F.; Wang, X.F.; Zhang, Z.; Wang, L.X.; Li, X.M.; Xu, Y.Y.; Ren, C.H.; Li, Q.; Turng, L.S. Programmed Release of Multimodal, Cross-Linked Vascular Endothelial Growth Factor and Heparin Layers on Electrospun Polycaprolactone Vascular Grafts. ACS Appl. Mater. Inter. 2019, 11, 32533–32542. [Google Scholar] [CrossRef]
- Cho, Y.; Baek, J.; Lee, E.; Im, S.G. Heparin-mediated electrostatic immobilization of bFGF via functional polymer films for enhanced self-renewal of human neural stem cells. J. Mater. Chem. B 2021, 9, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Yuan, W.W.; Liao, G.X.; Yu, Q.Q.; Wang, L.G. Construction of bFGF/heparin and Fe3O4 nanoparticles functionalized scaffolds aiming at vascular repair and magnetic resonance imaging monitoring. Int. J. Biol. Macromol. 2025, 286, 138416. [Google Scholar] [CrossRef]
- Han, U.; Hong, J. Structure of a Multilayer Nanofilm To Increase the Encapsulation Efficiency of Basic Fibroblast Growth Factor. Mol. Pharm. 2018, 15, 1277–1283. [Google Scholar] [CrossRef]
- Heydari, S.; Esmaeili, A. Synthesize of polyurethane/chitosan/Vicia ervilia protein/gelatin/heparin-coated Astragalus gossypinus scaffold for cardiovascular tissue engineering. Process Biochem. 2022, 122, 205–213. [Google Scholar] [CrossRef]
- Hogan, K.J.; Perez, M.R.; Mikos, A.G. Extracellular matrix component-derived nanoparticles for drug delivery and tissue engineering. J. Control. Release 2023, 360, 888–912. [Google Scholar] [CrossRef] [PubMed]
- Aryanti, P.T.P.; Nugroho, F.A.; Kusmala, Y.Y. Heparin and heparin-like modifications in hemodialysis membranes: Current innovations and future directions. Biotechnol. Adv. 2025, 80, 108527. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Liao, H.T.; Lin, W.H.; Darshan, T.G.; Mini, P.A.; Chen, J.P. Braided suture-reinforced fibrous yarn bundles as a scaffold for tendon tissue engineering in extensor digitorum tendon repair. Chem. Eng. J. 2023, 454, 140366. [Google Scholar] [CrossRef]
- Hama, R.; Aytemiz, D.; Moseti, K.O.; Kameda, T.; Nakazawa, Y. Silk Fibroin Conjugated with Heparin Promotes Epithelialization and Wound Healing. Polymers 2022, 14, 3582. [Google Scholar] [CrossRef]
- Li, H.F.; Shang, Y.L.; Feng, Q.; Liu, Y.; Chen, J.L.; Dong, H. A novel bioartificial pancreas fabricated via islets microencapsulation in anti-adhesive core-shell microgels and macroencapsulation in a hydrogel scaffold prevascularized in vivo. Bioact. Mater. 2023, 27, 362–376. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.H.; Hong, Q.X.; Meng, L.J.; Ji, Y.; Wang, L.T.; Zhang, Q.Y.; Lin, J.F.; Pan, C.J. Synthesizing a multifunctional polymer to construct the catalytically NO-generating coating for improving corrosion resistance and biocompatibility of the magnesium alloy stent materials. Prog. Org. Coat. 2024, 186, 108058. [Google Scholar] [CrossRef]
- Sandoval-Castellanos, A.M.; Claeyssens, F.; Haycock, J.W. Bioactive 3D Scaffolds for the Delivery of NGF and BDNF to Improve Nerve Regeneration. Front. Mater. 2021, 8, 734683. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Diaz-Rodriguez, P.; Ardao, I.; Moreira, D.; Montini-Ballarin, F.; Abraham, G.A.; Concheiro, A.; Alvarez-Lorenzo, C. Evaluation of human umbilical vein endothelial cells growth onto heparin-modified electrospun vascular grafts. Int. J. Biol. Macromol. 2021, 179, 567–575. [Google Scholar] [CrossRef]
- Sui, S.; Sun, H.; Ni, G.; Liu, Y.; Zheng, H.; Sun, T.; Kong, L.; Ma, Z.; Yuan, F. Ar-H2O-NH3 plasma grafting and polymerization of dopamine onto polytetrafluoroethylene to promote heparin immobilization. Plasma Process. Polym. 2023, 20, 2200228. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A.; Sabzevari, A. A review of advanced hydrogels for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2024, 12, 1340893. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Carreca, A.P.; Iannolo, G.; Pitarresi, G.; Amico, G.; Giammona, G.; Conaldi, P.G.; Chinnici, C.M. Modulating the release of bioactive molecules of human mesenchymal stromal cell secretome: Heparinization of hyaluronic acid-based hydrogels. Int. J. Pharm. 2024, 653, 123904. [Google Scholar] [CrossRef]
- Yim, W.; Jin, Z.C.; Chang, Y.C.; Brambila, C.; Creyer, M.N.; Ling, C.X.; He, T.Y.; Li, Y.; Retout, M.; Penny, W.F.; et al. Polyphenol-stabilized coacervates for enzyme-triggered drug delivery. Nat. Commun. 2024, 15, 7295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Kuang, D.J.; Ding, K.L.; Huang, X.Y.; Fan, H.S.; Yang, L.; Wang, Y.B.; Zhang, X.D. A functionalized biological heart valve by double bond crosslinking with enhanced biocompatibility and antithrombogenicity. J. Mater. Chem. B 2022, 10, 10001–10017. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Mousavi, A.; Zoetebier, B.; Dijkstra, P.J. Enzymatically crosslinked hyaluronic acid microgels as a vehicle for sustained delivery of cationic proteins. Eur. Polym. J. 2019, 115, 234–243. [Google Scholar] [CrossRef]
- Ding, X.; Gao, J.; Wang, Z.; Awada, H.; Wang, Y. A shear-thinning hydrogel that extends in vivo bioactivity of FGF2. Biomaterials 2016, 111, 80–89. [Google Scholar] [CrossRef]
- Seth, P.; Friedrichs, J.; Limasale, Y.D.P.; Fertala, N.; Freudenberg, U.; Zhang, Y.; Lampel, A.; Werner, C. Interpenetrating Polymer Network Hydrogels with Tunable Viscoelasticity and Proteolytic Cleavability to Direct Stem Cells In Vitro. Adv. Healthc. Mater. 2025, 14, 2402656. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers. Molecules 2019, 24, 4630. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Hettiaratchi, M.; Temenoff, J.; Guldberg, R.; McDevitt, T. Heparin Microparticle Delivery of Bone Morphogenetic Protein-2 (BMP-2) for Bone Regeneration. Tissue Eng. Part A 2014, 20, S39. [Google Scholar]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Thomas, A.M.; Gomez, A.J.; Palma, J.L.; Yap, W.T.; Shea, L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials 2014, 35, 8687–8693. [Google Scholar] [CrossRef]
- Seal, B.L.; Panitch, A. Physical matrices stabilized by enzymatically sensitive covalent crosslinks. Acta Biomater. 2006, 2, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Hu, J.-J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr. Polym. 2021, 269, 118262. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.-H.; Hu, M.-H.; Wang, J.-F.; Hu, J.-J. Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers 2025, 17, 800. [Google Scholar] [CrossRef]
- Hsu, F.-M.; Hu, M.-H.; Jiang, Y.-S.; Lin, B.-Y.; Hu, J.-J.; Jan, J.-S. Antibacterial polypeptide/heparin composite hydrogels carrying growth factor for wound healing. Mater. Sci. Eng. C 2020, 112, 110923. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Wang, D.; Zhang, J.; Yi, K.; Su, Y.; Luo, J.; Deng, X.; Deng, F. Fabrication of polymeric microspheres for biomedical applications. Mater. Horiz. 2024, 11, 2820–2855. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Lin, C.H.; Srioudom, J.R.; Sun, W.; Xing, M.; Yan, S.; Yu, L.; Yang, J. The use of hydrogel microspheres as cell and drug delivery carriers for bone, cartilage, and soft tissue regeneration. Biomater. Transl. 2024, 5, 236–256. [Google Scholar] [CrossRef]
- Gwon, K.; Hong, H.J.; Gonzalez-Suarez, A.M.; Slama, M.Q.; Choi, D.; Hong, J.; Baskaran, H.; Stybayeva, G.; Peterson, Q.P.; Revzin, A. Bioactive hydrogel microcapsules for guiding stem cell fate decisions by release and reloading of growth factors. Bioact. Mater. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Miller, T.; Temenoff, J.S.; Guldberg, R.E.; McDevitt, T.C. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials 2014, 35, 7228–7238. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef]
- Rajabi, M.; Shabani, I.; Ahmadi Tafti, S.H.; Shabani, A. Sustained release of heparin from PLLA micropartricles for tissue engineering applications. Polym. Test. 2024, 140, 108628. [Google Scholar] [CrossRef]
- Yang, Z.; Birkenhauer, P.; Julmy, F.; Chickering, D.; Ranieri, J.P.; Merkle, H.P.; Lüscher, T.F.; Gander, B. Sustained release of heparin from polymeric particles for inhibition of human vascular smooth muscle cell proliferation. J. Control. Release 1999, 60, 269–277. [Google Scholar] [CrossRef]
- Anaya, B.J.; D’Angelo, D.; Bettini, R.; Molina, G.; Sanz-Perez, A.; Dea-Ayuela, M.A.; Galiana, C.; Rodríguez, C.; Tirado, D.F.; Lalatsa, A.; et al. Heparin-azithromycin microparticles show anti-inflammatory effects and inhibit SARS-CoV-2 and bacterial pathogens associated to lung infections. Carbohydr. Polym. 2025, 348, 122930. [Google Scholar] [CrossRef]
- Wu, D.; Yu, Y.; Zhao, C.; Shou, X.; Piao, Y.; Zhao, X.; Zhao, Y.; Wang, S. NK-Cell-Encapsulated Porous Microspheres via Microfluidic Electrospray for Tumor Immunotherapy. ACS Appl. Mater. Interfaces 2019, 11, 33716–33724. [Google Scholar] [CrossRef] [PubMed]
- Headen, D.M.; García, J.R.; García, A.J. Parallel droplet microfluidics for high throughput cell encapsulation and synthetic microgel generation. Microsyst. Nanoeng. 2018, 4, 17076. [Google Scholar] [CrossRef]
- Siltanen, C.; Yaghoobi, M.; Haque, A.; You, J.; Lowen, J.; Soleimani, M.; Revzin, A. Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta Biomater. 2016, 34, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.S. Complex Coacervate-Based Materials for Biomedicine: Recent Advancements and Future Prospects. Ind. Eng. Chem. Res. 2024, 63, 5414–5487. [Google Scholar] [CrossRef]
- Gao, X.; Hwang, M.P.; Wright, N.; Lu, A.; Ruzbarsky, J.J.; Huard, M.; Cheng, H.; Mullen, M.; Ravuri, S.; Wang, B.; et al. The use of heparin/polycation coacervate sustain release system to compare the bone regenerative potentials of 5 BMPs using a critical sized calvarial bone defect model. Biomaterials 2022, 288, 121708. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Ge, P.; Mao, Y.; Wang, L. Anti-acute thrombogenic surface using coaxial electrospraying coating for vascular graft application. Mater. Lett. 2017, 205, 15–19. [Google Scholar] [CrossRef]
- Mao, Y.; Li, C.; Ge, P.; Wang, F.; Wang, L. Efficiently and Conveniently Heparin/ PEG-PCL Core-Shell Microcarriers Fabrication and Optimization via Coaxial-Electrospraying. Mol. Cell. Biomech. 2018, 15, 143–154. [Google Scholar] [CrossRef]
- da Silva, R.Y.P.; de Menezes, D.L.B.; Oliveira, V.D.S.; Converti, A.; de Lima, Á.A. Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 5441. [Google Scholar] [CrossRef]
- Dass, C.R.; Choong, P.F.M. Selective gene delivery for cancer therapy using cationic liposomes: In vivo proof of applicability. J. Control. Release 2006, 113, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Choudhary, C.; Ajazuddin; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef]
- Arunkumar, P.; Dougherty, J.A.; Weist, J.; Kumar, N.; Angelos, M.G.; Powell, H.M.; Khan, M. Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials 2019, 9, 1037. [Google Scholar] [CrossRef]
- Hoffart, V.; Ubrich, N.; Lamprecht, A.; Bachelier, K.; Vigneron, C.; Lecompte, T.; Hoffman, M.; Maincent, P. Microencapsulation of low molecular weight heparin into polymeric particles designed with biodegradable and nonbiodegradable polycationic polymers. Drug Deliv. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, H.K.; Yoon, J.J.; Park, T.G. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm. Res. 2006, 23, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Motlekar, N. Optimization of experimental parameters for the production of LMWH-loaded polymeric microspheres. Drug Des. Devel. Ther. 2009, 2, 39–47. [Google Scholar]
- Tsung, M.; Burgess, D.J. Preparation and stabilization of heparin/gelatin complex coacervate microcapsules. J. Pharm. Sci. 1997, 86, 603–607. [Google Scholar] [CrossRef]
- Yildiz, A.; John, E.; Özsoy, Y.; Araman, A.; Birchall, J.C.; Broadley, K.J.; Gumbleton, M. Inhaled extended-release microparticles of heparin elicit improved pulmonary pharmacodynamics against antigen-mediated airway hyper-reactivity and inflammation. J. Control. Release 2012, 162, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Mori, Y.; Hattori, H.; Fujita, M.; Nakamura, S. Novel experimental and clinical therapeutic uses of low-molecular-weight heparin/protamine microparticles. Pharmaceutics 2012, 4, 42–57. [Google Scholar] [CrossRef]
- Tzannis, S.T.; Prestrelski, S.J. Activity-stability considerations of trypsinogen during spray drying: Effects of sucrose. J. Pharm. Sci. 1999, 88, 351–359. [Google Scholar] [CrossRef]
- Young, C.; Rozario, K.; Serra, C.; Poole-Warren, L.; Martens, P. Poly(vinyl alcohol)-heparin biosynthetic microspheres produced by microfluidics and ultraviolet photopolymerisation. Biomicrofluidics 2013, 7, 44109. [Google Scholar] [CrossRef]
- Ma, C.; Jing, Y.; Sun, H.; Liu, X. Hierarchical Nanofibrous Microspheres with Controlled Growth Factor Delivery for Bone Regeneration. Adv. Heal. Mater. 2015, 4, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, X.; Zhu, Y.; Su, W.; Lv, Q.; Li, D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater. Des. 2022, 215, 110478. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Gan, L.; Zhang, L.; Yang, L.; Wu, P. Application of Biomedical Microspheres in Wound Healing. Int. J. Mol. Sci. 2023, 24, 7319. [Google Scholar] [CrossRef]
- Mouftah, S.; Abdel-Mottaleb, M.M.A.; Lamprecht, A. Buccal delivery of low molecular weight heparin by cationic polymethacrylate nanoparticles. Int. J Pharm. 2016, 515, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, M.; Sun, H.; Zhang, C.; Shen, M.; Ma, J.; Zhang, G.; Shi, X. Electrosprayed core–shell microspheres co-deliver fibronectin and resveratrol for combined treatment of acute lung injury. J. Colloid Interface Sci. 2025, 686, 498–508. [Google Scholar] [CrossRef]
- Shakoor, S.; Kibble, E.; El-Jawhari, J.J. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering 2022, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.; Williams, K.; Phelps, J.; La Boucan, F.; Lewandowski, G.; O’Grady, K.; Yu, B.; Willerth, S.M. Microspheres for 3D bioprinting: A review of fabrication methods and applications. Front. Bioeng. Biotechnol. 2025, 13, 1551199. [Google Scholar] [CrossRef]
- Bonany, M.; del-Mazo-Barbara, L.; Espanol, M.; Ginebra, M.-P. Microsphere incorporation as a strategy to tune the biological performance of bioinks. J. Tissue Eng. 2022, 13, 20417314221119895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, L.; Ma, A.; Bai, X.; Zeng, Y.; Liu, D.; Liu, B.; Zhang, W.; Tang, S. Recent advances in coaxial electrospun nanofibers for wound healing. Mater. Today Bio 2024, 29, 101309. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Khorsandi, D.; Zarepour, A.; Yilmaz, H.; Agarwal, T.; Hooshmand, S.; Mohammadinejad, R.; Ozdemir, F.; Sahin, O.; Adiguzel, S.; et al. Biomedical applications of engineered heparin-based materials. Bioact. Mater. 2024, 31, 87–118. [Google Scholar] [CrossRef]
- Ikegami, Y.; Shafiq, M.; Aishima, S.; Ijima, H. Heparin/growth factors-immobilized aligned electrospun nanofibers promote nerve regeneration in polycaprolactone/gelatin-based nerve guidance conduits. Adv. Fiber Mater. 2023, 5, 554–573. [Google Scholar] [CrossRef]
- Qiu, S.; Du, J.; Zhu, T.; Zhang, H.; Chen, S.; Wang, C.; Chen, D.; Lu, S. Electrospun compliant heparinized elastic vascular graft for improving the patency after implantation. Int. J. Biol. Macromol. 2023, 253, 126598. [Google Scholar] [CrossRef] [PubMed]
- Fahad, M.A.A.; Lee, H.Y.; Park, S.; Choi, M.; Shanto, P.C.; Park, M.; Bae, S.H.; Lee, B.T. Small-diameter vascular graft composing of core-shell structured micro-nanofibers loaded with heparin and VEGF for endothelialization and prevention of neointimal hyperplasia. Biomaterials 2024, 306, 122507. [Google Scholar] [CrossRef]
- Joshi, A.; Xu, Z.; Ikegami, Y.; Yoshida, K.; Sakai, Y.; Joshi, A.; Kaur, T.; Nakao, Y.; Yamashita, Y.-i.; Baba, H. Exploiting synergistic effect of externally loaded bFGF and endogenous growth factors for accelerated wound healing using heparin functionalized PCL/gelatin co-spun nanofibrous patches. Chem. Eng. J. 2021, 404, 126518. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Vasconcelos, F.; Lima, A.C.; Bonani, W.; Silva, C.S.; Reis, R.L.; Motta, A.; Migliaresi, C.; Martins, A.; Neves, N.M. Microfluidic-assisted electrospinning, an alternative to coaxial, as a controlled dual drug release system to treat inflammatory arthritic diseases. Biomater. Adv. 2022, 134, 112585. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Liu, R.; Li, Y.; Zeng, J.; Zhou, M.; Zhao, Y. Bioinspired Vascular Stents with Microfluidic Electrospun Multilayer Coatings for Preventing In-Stent Restenosis. Adv. Healthc. Mater. 2022, 11, e2200965. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, C.; Zhao, Q.; Li, X.; Xu, F.; Yao, X.; Wang, M. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Du, J.; Zhu, T.; Chen, D.; Liu, F.; Dong, Y. Nanofibers with homogeneous heparin distribution and protracted release profile for vascular tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1187914. [Google Scholar] [CrossRef]
- Jiang, S.; Duan, G.; Zussman, E.; Greiner, A.; Agarwal, S. Highly flexible and tough concentric triaxial polystyrene fibers. ACS Appl. Mater. Interfaces 2014, 6, 5918–5923. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.-G. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef]
- Chang, G.; Ullah, W.; Li, A.; Das, S.K.; Lin, L.; Wang, X. Self-constructed side-by-side nanofiber photocatalyst via oppositely charged electrospinning and its photocatalytic degradation of rhodamine B. New J. Chem. 2019, 43, 15405–15412. [Google Scholar] [CrossRef]
- Han, F.; Jia, X.; Dai, D.; Yang, X.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 2013, 34, 7302–7313. [Google Scholar] [CrossRef]
- Wang, J.-F.; Hu, J.-J. Enhanced drug release control in coaxial electrospun fibers via heat pressing: Reducing burst release and achieving dual-phase delivery. Int. J. Pharm. 2025, 674, 125501. [Google Scholar] [CrossRef]
- Chen, L.; Wu, P.; Zhu, Y.; Luo, H.; Tan, Q.; Chen, Y.; Luo, D.; Chen, Z. Electrospinning strategies targeting fibroblast for wound healing of diabetic foot ulcers. APL Bioeng. 2025, 9, 011501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.T.; Wang, Z.; Shiu, B.C.; Lin, J.H.; Lou, C.W. Coaxial microfluidic spinning design produced high strength alginate membranes for antibacterial activity and drug release. Int. J. Biol. Macromol. 2023, 243, 124956. [Google Scholar] [CrossRef]
- Gregory, H.N.; Johnson, L.D.V.; Phillips, J.B. An emulsion electrospun nanofibrous scaffold loaded with glial cell line-derived neurotrophic factor for nerve regeneration. Front. Cell Dev. Biol. 2025, 13, 1567654. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ni, C.; Chase, D.B.; Rabolt, J.F. Preparation of multilayer biodegradable nanofibers by triaxial electrospinning. ACS Macro Lett. 2013, 2, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ye, H.; Deng, D.; Li, J.; Wu, Y. Electrospun metformin-loaded polycaprolactone/chitosan nanofibrous membranes as promoting guided bone regeneration membranes: Preparation and characterization of fibers, drug release, and osteogenic activity in vitro. J. Biomater. Appl. 2020, 34, 1282–1293. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Fang, B.; Ying, Y.; Yu, D.G.; He, H. Three EHDA processes from a detachable spinneret for fabricating drug fast dissolution composites. Macromol. Mater. Eng. 2024, 309, 2300361. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Zhang, Z.; Yu, D.-G.; Bligh, S.W.A. Integrated Janus nanofibers enabled by a co-shell solvent for enhancing icariin delivery efficiency. Int. J. Pharm. 2024, 658, 124180. [Google Scholar] [CrossRef]
- Zhang, A.; van Genderen, A.M.; Liu, B.; Qian, J.; Iamsamang, J.; Wang, Z.; Castilho, M.; Akhavan, B. Surface bio-engineering of melt electrowritten tubular scaffolds via plasma immersion ion implantation (PIII). Mater. Today Bio 2025, 33, 101923. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Leong, W.S.; Wu, S.C.; Ng, K.; Tan, L.P. Electrospun 3D multi-scale fibrous scaffold for enhanced human dermal fibroblast infiltration. Int. J. Bioprint. 2016, 2, 81–92. [Google Scholar] [CrossRef]
- Karimizade, A.; Hasanzadeh, E.; Abasi, M.; Enderami, S.E.; Mirzaei, E.; Annabi, N.; Mellati, A. Collagen short nanofiber-embedded chondroitin sulfate–hyaluronic acid nanocomposite: A cartilage-mimicking in situ-forming hydrogel with fine-tuned properties. Int. J. Biol. Macromol. 2024, 266, 131051. [Google Scholar] [CrossRef]
- Li, L.; Yao, Z.; Salimian, K.J.; Kong, J.; Zaheer, A.; Parian, A.; Gearhart, S.L.; Mao, H.Q.; Selaru, F.M. Extracellular Vesicles Delivered by a Nanofiber-Hydrogel Composite Enhance Healing In Vivo in a Model of Crohn’s Disease Perianal Fistula. Adv. Healthc. Mater. 2025, 14, 2402292. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Abune, L.; Wang, Y. Affinity Hydrogels for Protein Delivery. Trends Pharmacol. Sci. 2021, 42, 300–312. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Zheng, X.; Zheng, Z.; Wu, C.; Hao, Y.; Zhou, B. Progress in the Application of Multifunctional Composite Hydrogels in Promoting Tissue Repair. ACS Omega 2024, 9, 47964–47975. [Google Scholar] [CrossRef] [PubMed]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef]
- Roberts, J.J.; Naudiyal, P.; Jugé, L.; Bilston, L.E.; Granville, A.M.; Martens, P.J. Tailoring Stimuli Responsiveness using Dynamic Covalent Cross-Linking of Poly(vinyl alcohol)-Heparin Hydrogels for Controlled Cell and Growth Factor Delivery. ACS Biomater. Sci. Eng. 2015, 1, 1267–1277. [Google Scholar] [CrossRef]
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 2013, 34, 2252–2264. [Google Scholar] [CrossRef]
- Truong-Thi, N.-H.; Ngoc Hoi, N.; Nguyen, D.; Tang, T.-N.; Nguyen, T.; Nguyen, D.H. pH-Responsive Delivery of Platinum-based Drugs through the Surface Modification of Heparin on Mesoporous Silica Nanoparticles. Eur. Polym. J. 2023, 185, 111818. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef]
- Lu, Y.T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of Stable Cross-Linked Multilayers Based on Thermo-Responsive PNIPAM-Grafted-Chitosan/Heparin to Tailor Their Physiochemical Properties and Biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-I.; Lee, S.-Y.; Tae, G. The effect of heparin on the gellation of Pluronic F-127 hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2006, 284, 480–484. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef]
- Yang, F.; Guo, G.; Wang, Y. Inflammation-triggered dual release of nitroxide radical and growth factor from heparin mimicking hydrogel-tissue composite as cardiovascular implants for anti-coagulation, endothelialization, anti-inflammation, and anti-calcification. Biomaterials 2022, 289, 121761. [Google Scholar] [CrossRef]
- Dogru, S.; Alba, G.M.; Pierce, K.C.; Wang, T.; Kia, D.S.; Albro, M.B. Cell mediated reactions create TGF-β delivery limitations in engineered cartilage. Acta Biomater. 2024, 190, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Nguyễn Thị, H.; Ngoc, S.; Minh, T.; Van, Q.; Bui, V.; Nguyen, N. A heparin-based nanogel system for redox and pH dual-responsive delivery of cisplatin. Biomed. Mater. 2024, 19, 025012. [Google Scholar] [CrossRef]
- Wang, H.; Moon, C.; Shin, M.C.; Wang, Y.; He, H.; Yang, V.C.; Huang, Y. Heparin-Regulated Prodrug-Type Macromolecular Theranostic Systems for Cancer Therapy. Nanotheranostics 2017, 1, 114–130. [Google Scholar] [CrossRef]
- Lin, L.; Xie, S.; Zhao, Y.; Liang, Z.; Wu, Q.; Fang, M.; Teng, X.; Shi, B.; Yang, Y.; Chen, B. Ultrasound-induced destruction of heparin-loaded microbubbles attenuates L-arginine-induced acute pancreatitis. Eur. J. Pharm. Sci. 2023, 180, 106318. [Google Scholar] [CrossRef]
- Erfanian, S.; Mostafaei, F.; Ajalloueian, F.; Baharvand, H.; Rajabi, S.; Ashtiani, M.K. Controlled delivery of PRP from decellularized extracellular matrix enhances skeletal muscle regeneration. Sci. Rep. 2025, 15, 12719. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; Lin, Z.; Nangle, M.R.; Li, Y.; Cai, P.; Liu, D.; Ye, L.; Xiao, Z.; He, C.; et al. Single injection of a novel nerve growth factor coacervate improves structural and functional regeneration after sciatic nerve injury in adult rats. Exp. Neurol. 2017, 288, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Shen, Z.; Han, L.; Wang, J.; Sang, S. A heparin-functionalized bioink with sustained delivery of vascular endothelial growth factor for 3D bioprinting of prevascularized dermal constructs. Int. J. Biol. Macromol. 2024, 262, 130075. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Wang, Y. Controlled delivery of heparin-binding EGF-like growth factor yields fast and comprehensive wound healing. J. Control. Release 2013, 166, 124–129. [Google Scholar] [CrossRef]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef]
- Gansevoort, M.; Oostendorp, C.; Bouwman, L.; Tiemessen, D.; Geutjes, P.; Feitz, W.; Kuppevelt, T.; Daamen, W. Collagen-Heparin-FGF2-VEGF Scaffolds Induce a Regenerative Gene Expression Profile in a Fetal Sheep Wound Model. Tissue Eng. Regen. Med. 2024, 21, 1173–1187. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, Y.; Sun, W.; Chen, B.; Zhang, J.; Zhao, W.; Xiao, Z.; Dai, J. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials 2008, 29, 1189–1197. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.; Wang, W.; Zhang, D.; Ma, Y.; Zhang, Y.; Wang, X. The auxiliary role of heparin in bone regeneration and its application in bone substitute materials. Front. Bioeng. Biotechnol. 2022, 10, 837172. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Hunter, D.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Heparin-binding-affinity-based delivery systems releasing nerve growth factor enhance sciatic nerve regeneration. J. Biomater. Sci. Polym. Ed. 2010, 21, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.F.; Morrison, P.F.; Chen, M.Y.; Harvey-White, J.; Pernaute, R.S.; Phillips, H.; Oldfield, E.; Bankiewicz, K.S. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp. Neurol. 2001, 168, 155–161. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Ma, K.; Chen, M.; Xu, H.; Niu, X.; Gu, H.; Wang, R.; Chen, X.; Sun, H. Collagen/heparin scaffold combined with vascular endothelial growth factor promotes the repair of neurological function in rats with traumatic brain injury. Biomater. Sci. 2021, 9, 745–764. [Google Scholar] [CrossRef]
- Chu, H.; Chen, C.-W.; Huard, J.; Wang, Y. The effect of a heparin-based coacervate of fibroblast growth factor-2 on scarring in the infarcted myocardium. Biomaterials 2013, 34, 1747–1756. [Google Scholar] [CrossRef]
- Wu, J.; Ye, J.; Zhu, J.; Xiao, Z.; He, C.; Shi, H.; Wang, Y.; Lin, C.; Zhang, H.; Zhao, Y.; et al. Heparin-Based Coacervate of FGF2 Improves Dermal Regeneration by Asserting a Synergistic Role with Cell Proliferation and Endogenous Facilitated VEGF for Cutaneous Wound Healing. Biomacromolecules 2016, 17, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kotani, T.; Suzuki, K. Antifibrotic therapy by sustained release of low molecular weight heparin from poly(lactic-co-glycolic acid) microparticles on bleomycin-induced pulmonary fibrosis in mice. Sci. Rep. 2020, 10, 19019. [Google Scholar] [CrossRef]
- Bastard, C.; Günther, D.; Gerardo-Nava, J.; Dewerchin, M.; Sprycha, P.; Licht, C.; Lüken, A.; Wessling, M.; De Laporte, L. How Does Temporal and Sequential Delivery of Multiple Growth Factors Affect Vascularization Inside 3D Hydrogels? Adv. Ther. 2024, 7, 2300091. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, Y.; Huang, J.P.; Wang, J.; Wang, Z.K.; Ding, P.H. Essential elements for spatiotemporal delivery of growth factors within bio-scaffolds: A comprehensive strategy for enhanced tissue regeneration. J. Control. Release 2024, 368, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Mantry, S.; Das, P.K.; Sankaraiah, J.; Panda, S.; Silakabattini, K.; Reddy Devireddy, A.K.; Barik, C.S.; Khalid, M. Advancements on heparin-based hydrogel/scaffolds in biomedical and tissue engineering applications: Delivery carrier and pre-clinical implications. Int. J. Pharm. 2025, 679, 125733. [Google Scholar] [CrossRef] [PubMed]
- Bannigan, P.; Bao, Z.; Hickman, R.J.; Aldeghi, M.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine learning models to accelerate the design of polymeric long-acting injectables. Nat. Commun. 2023, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Kamihira, M. Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals 2024, 17, 1362. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Mostafavi, E. Biomaterials-mediated CRISPR/Cas9 delivery: Recent challenges and opportunities in gene therapy. Front. Chem. 2023, 11, 1259435. [Google Scholar] [CrossRef]
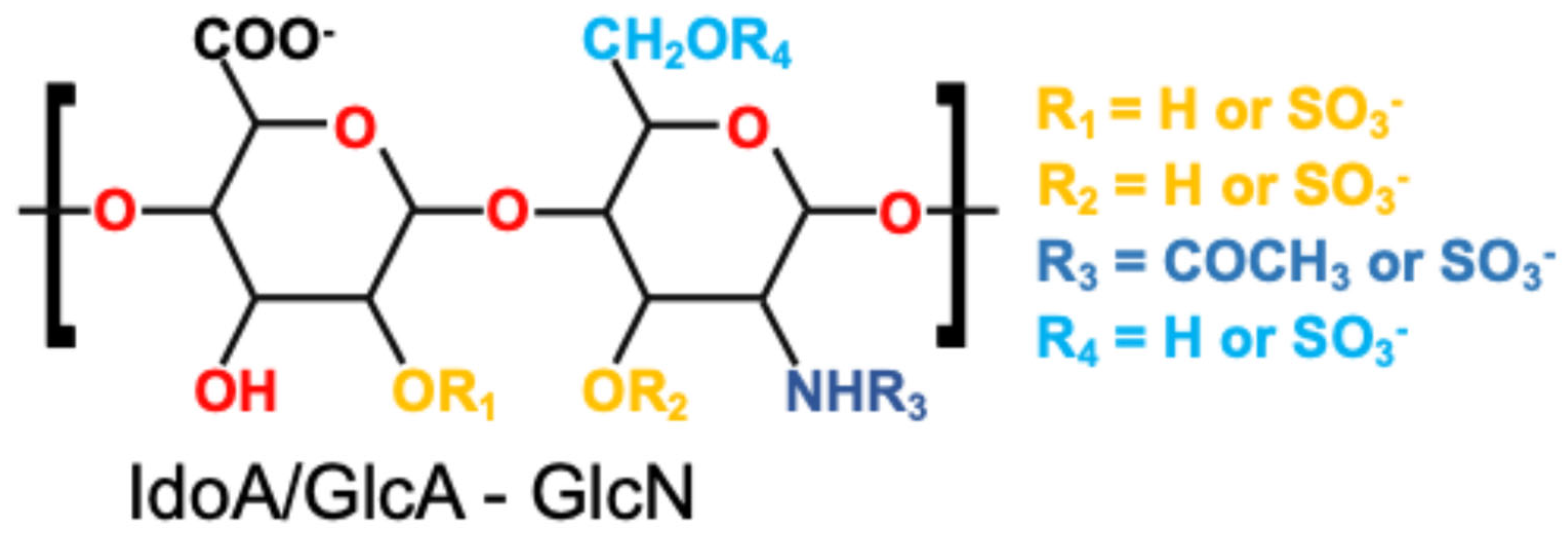
| Functional Group | Chemical Location | Modifiability | Corresponding Design Strategy |
|---|---|---|---|
| –OSO3− | GlcN, IdoA | Involved in electrostatic and ionic interactions | Electrostatic adsorption, ionic crosslinking |
| –COOH | GlcA, IdoA | Can be activated for amide formation | Covalent grafting, chemical crosslinking |
| –OH | GlcN, GlcA, IdoA | Can undergo oxidation, esterification | Covalent grafting, network crosslinking |
| –NH–COCH3 | GlcN | Permits enzymatic or chemical conjugation | Covalent grafting, enzymatic crosslinking |
| Strategy | Schematic | Advantages | Challenges | References |
|---|---|---|---|---|
| Electrostatic Adsorption |  | Simple; preserves bioactivity; reversible and suitable for short-term or in situ delivery | Weak interactions; serum- and ion-sensitive; risk of burst release | [33,34,47] |
| Physical Blending |  | Easy to implement; maintains biomolecule integrity; no harsh reactions | Risk of leaching under physiological conditions; uncontrolled release | [48,49,50] |
| Covalent Conjugation | 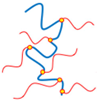 | High stability; long-term retention; site-specific functionalization | Requires reaction control; risk of reduced bioactivity; complex chemistry | [51,52,53] |
| Network Crosslinking | 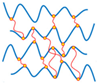 | Forms stable 3D networks; enhances mechanical strength; controls release kinetics | Requires reactants; balance mildness and strength | [54,55,56,57,58] |
| Fabrication | Schematic | Advantages | Challenges | References |
|---|---|---|---|---|
| Emulsion–Solvent Evaporation/ Extraction | 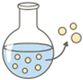 | Excellent size tunability Widely used | Use of organic solvents Possible protein denaturation | [89,94,95] |
| Spray Drying | 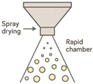 | Scalable Rapid production Suitable for temperature-stable drugs | Not fit for heat-sensitive proteins Limited encapsulation efficiency | [96,97] |
| Microfluidics | 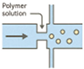 | Precise size control High monodispersity Advanced release design | Equipment complexity Low throughput | [98,99,100] |
| Coacervation | 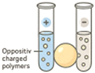 | Mild processing Suitable for proteins Electrostatic compatibility with heparin | Stability of coacervates Batch-to-batch variability | [75,101,102] |
| Electrospraying | 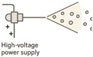 | Suitable for sensitive molecules Can form core–shell structures | Solvent use Optimization complexity | [103,104,105] |
| Fabrication | Schematic | Advantages | Challenges | References |
|---|---|---|---|---|
| Uniaxial Electrospinning |  | Simple setup, widely used, good fiber uniformity | Limited drug loading complexity, fast release profiles | [128,129] |
| Coaxial Electrospinning |  | Precise spatial control, reduced burst release, co-encapsulation possible | Requires precise control of flow and viscosity, low scalability | [130,131,132] |
| Microfluidic-Assisted Electrospinning | 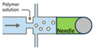 | High precision, allows modular design, compatible with in-line crosslinking | Device complexity, clogging, requires stable flow control | [133,134] |
| Emulsion Electrospinning |  | Simple setup, protects sensitive agents, enables multi-phase release | Polydispersity, irregular structure, lower mechanical strength | [135,136] |
| Triaxial Electrospinning |  | Fine-tuned gradient release, high encapsulation efficiency, structural stability | Complex fabrication, interfacial instability, low throughput | [137,138] |
| Janus Electrospinning |  | Combines incompatible drugs, biphasic release, targeted surface modification | Jet instability, matching viscosity is critical | [139] |
| Multilayer Concentric Electrospinning |  | Highly tailored release, Strong protection for proteins, Improved control | High technical demand, Interfacial stability, Poor scalability | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-F.; Jan, J.-S.; Hu, J.-J. Heparin-Based Growth Factor Delivery Platforms: A Review. Pharmaceutics 2025, 17, 1145. https://doi.org/10.3390/pharmaceutics17091145
Wang J-F, Jan J-S, Hu J-J. Heparin-Based Growth Factor Delivery Platforms: A Review. Pharmaceutics. 2025; 17(9):1145. https://doi.org/10.3390/pharmaceutics17091145
Chicago/Turabian StyleWang, Ji-Feng, Jeng-Shiung Jan, and Jin-Jia Hu. 2025. "Heparin-Based Growth Factor Delivery Platforms: A Review" Pharmaceutics 17, no. 9: 1145. https://doi.org/10.3390/pharmaceutics17091145
APA StyleWang, J.-F., Jan, J.-S., & Hu, J.-J. (2025). Heparin-Based Growth Factor Delivery Platforms: A Review. Pharmaceutics, 17(9), 1145. https://doi.org/10.3390/pharmaceutics17091145








