State of the Art of Cyclic Lipopeptide–Membrane Interactions: Pore Formation and Bilayer Permeability
Abstract
1. Introduction
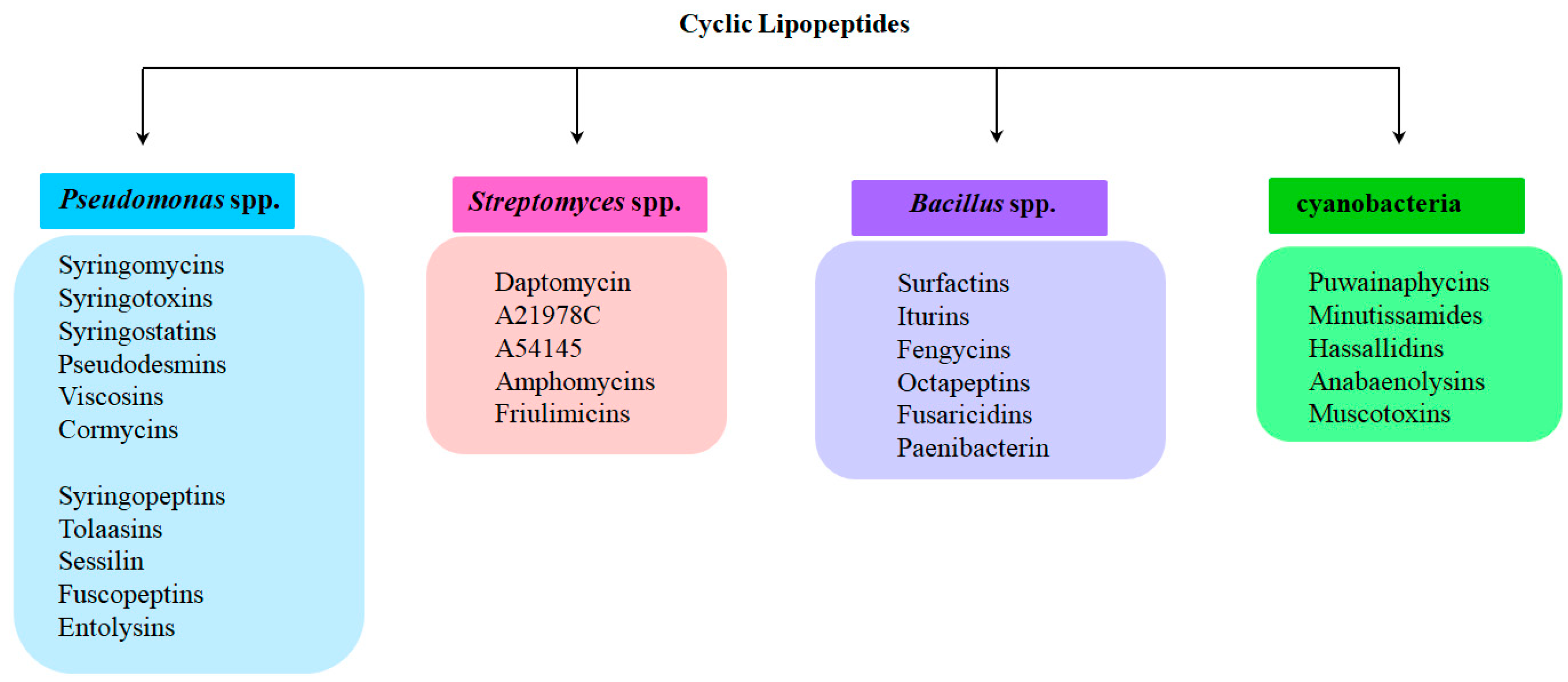
2. Cyclic Lipopeptides Produced by Pseudomonas spp.
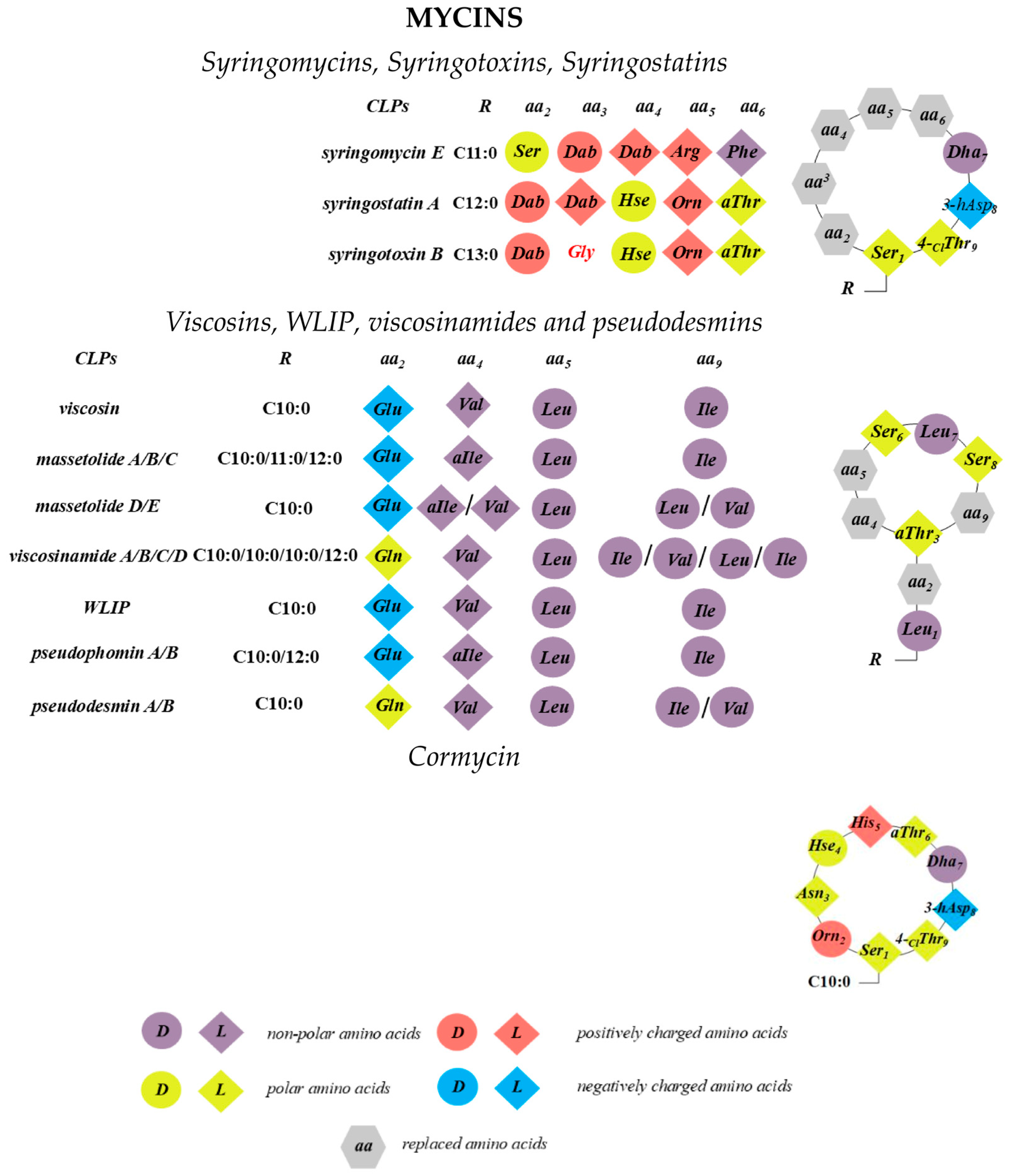
2.1. Syringomycins, Syringotoxins, Syringostatins
2.2. Viscosins, WLIP, Viscosinamides, and Pseudodesmins
2.3. Cormycins
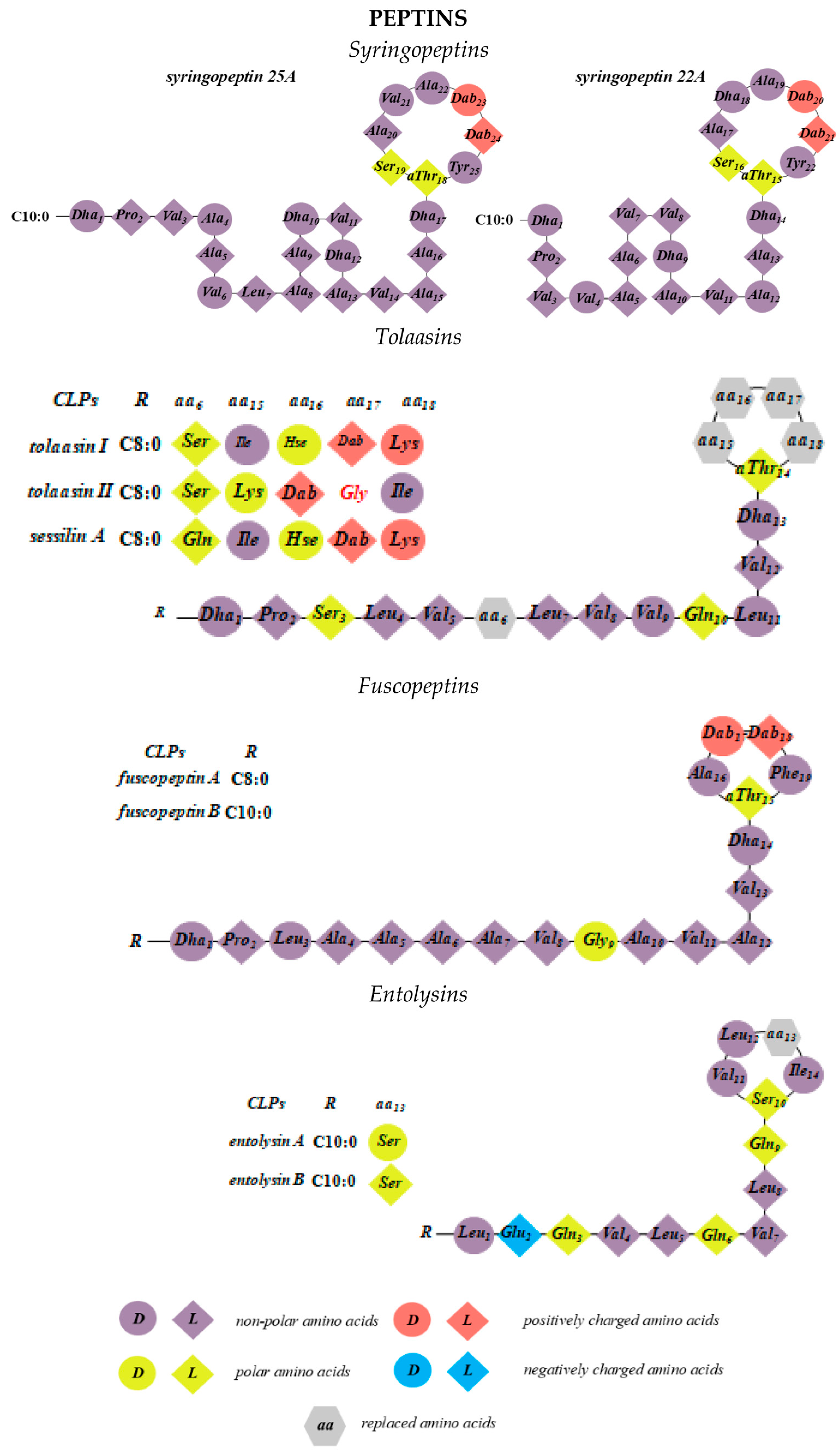
2.4. Syringopeptins
2.5. Tolaasins
2.6. Fuscopeptins
2.7. Entolysins
3. Cyclic Lipopeptides from Streptomyces spp.
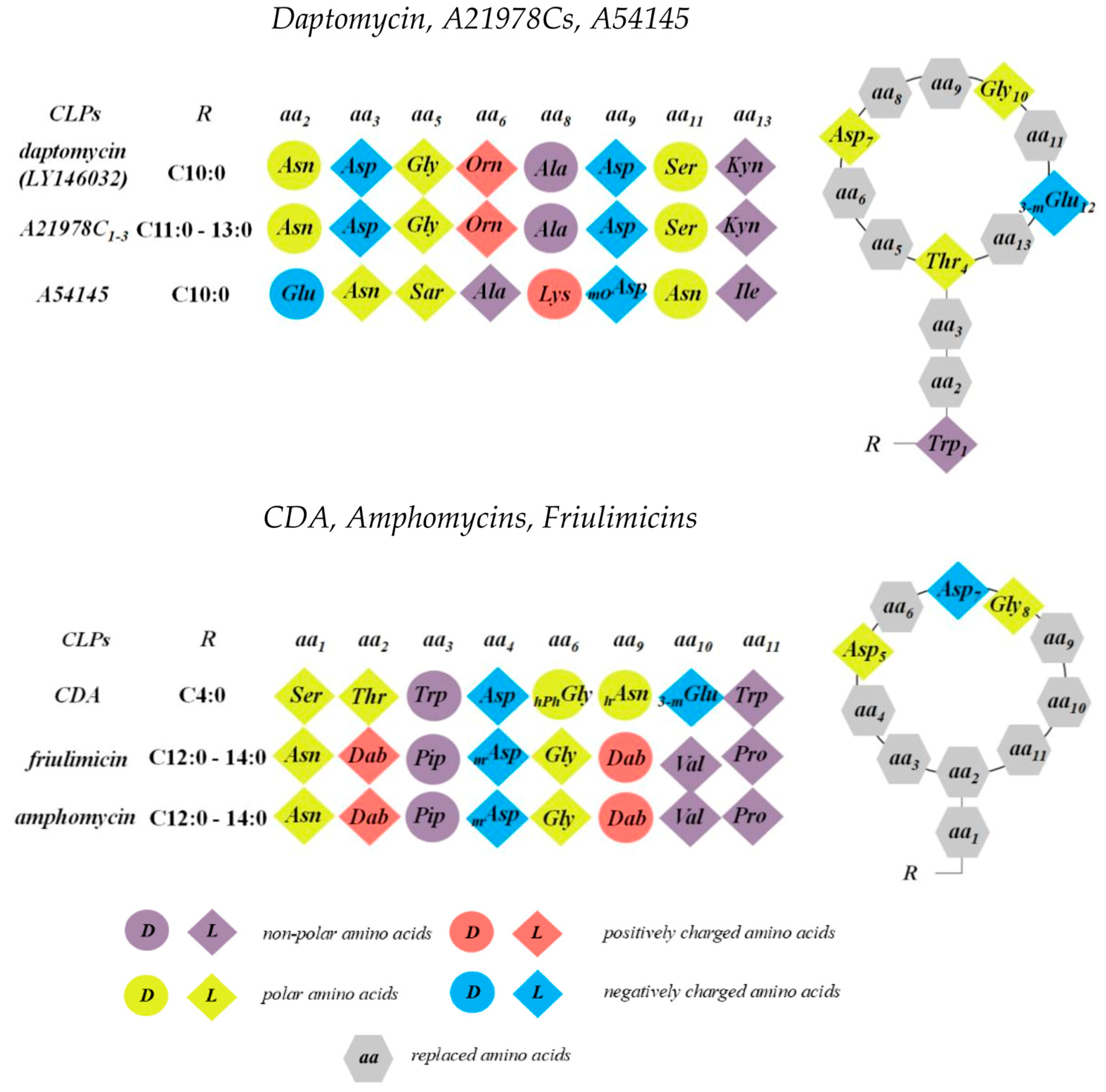
3.1. Daptomycin and the A21978C Complex
3.2. A54145
3.3. Calcium-Dependent Antibiotics
3.4. Amphomycin and Friulimicin
4. Cyclic Lipopeptides from Bacillus spp.
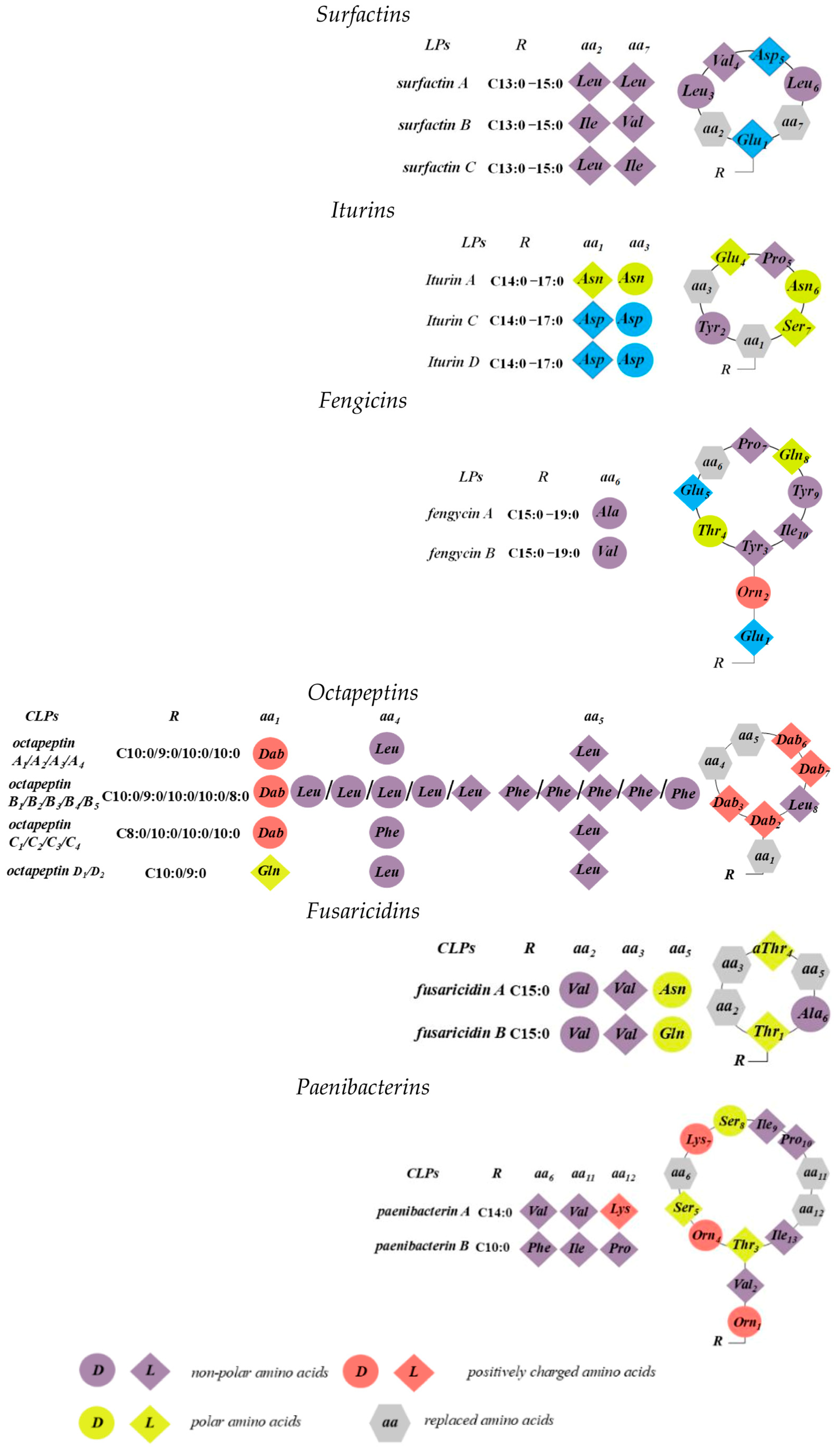
4.1. Surfactins, Iturins, and Fengicins
4.2. Octapeptins
4.3. Fusaricidins
4.4. Paenibacterin
5. Cyclic Lipopeptides from Cyanobacteria
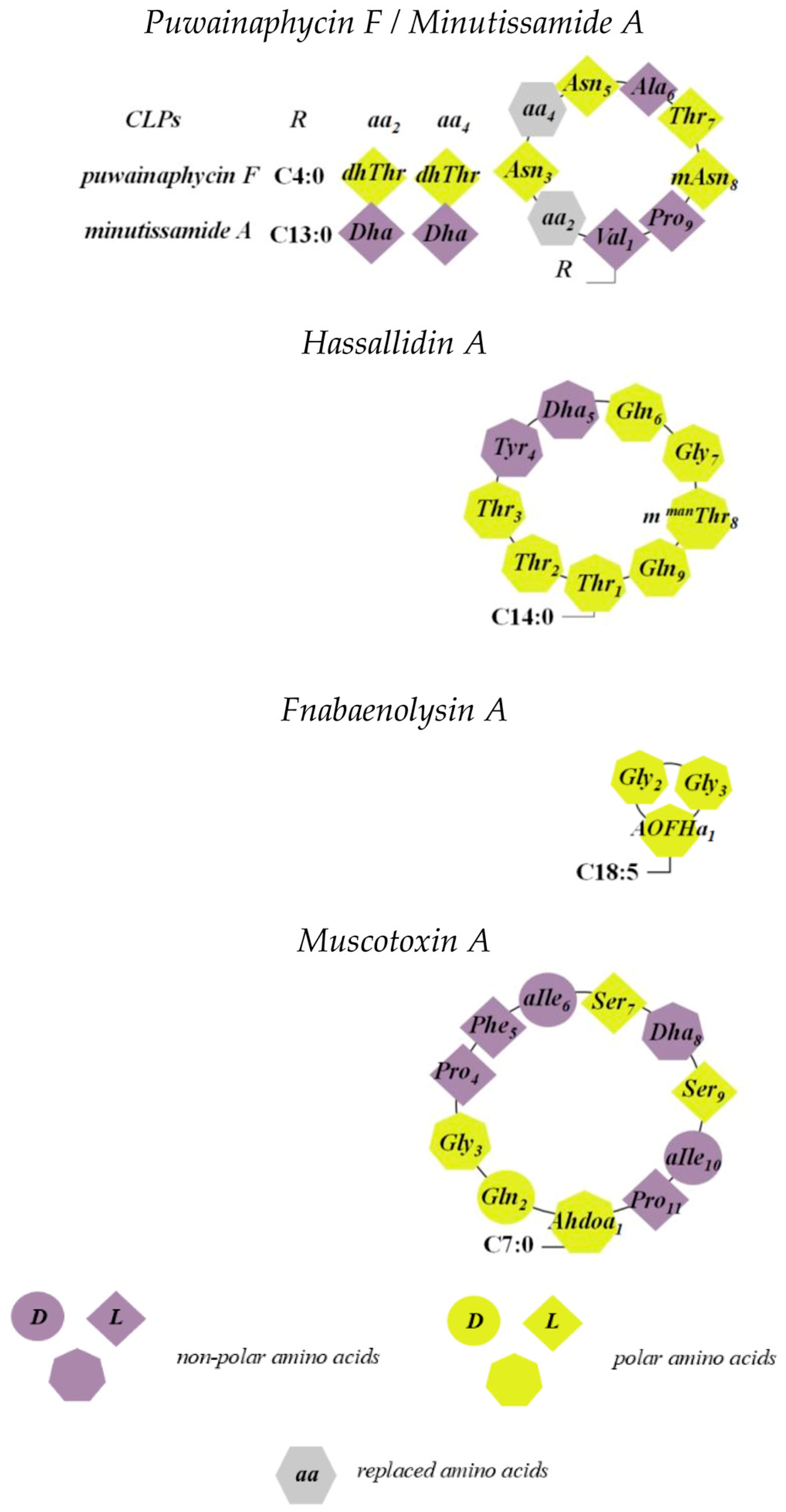
5.1. Puwainaphycins and Minutissamides
5.2. Hassallidins
5.3. Anabaenolysins
5.4. Muscotoxins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nybroe, O.; Sørensen, J. Production of cyclic lipopeptides by fluorescent pseudomonads. Pseudomonas 2004, 3, 147–172. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; de Bruijn, I.; de Kock, M.J. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: Diversity, activity, biosynthesis, and regulation. Mol. Plant Microbe Interact. 2006, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Marahiel, M.A.; Stachelhaus, T.; Mootz, H.D. Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis. Chem. Rev. 1997, 97, 2651–2674. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Fischbach, M.A.; Chu, F.; Losick, R.; Kolter, R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2009, 106, 280–285. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Losick, R.; Kolter, R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol. Microbiol. 2009, 74, 609–618. [Google Scholar] [CrossRef]
- Groupe, V.; Pugh, L.H.; Weiss, D.; Kochi, M. Observations on antiviral activity of viscosin. Proc. Soc. Exp. Biol. Med. 1951, 78, 354–358. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Ozel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing., and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Evans, M.E.; Feola, D.J.; Rapp, R.P. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 1999, 33, 960–967. [Google Scholar] [CrossRef]
- Hermsen, E.D.; Sullivan, C.J.; Rotschafer, J.C. Polymyxins: Pharmacology, pharmacokinetics., pharmacodynamics, and clinical applications. Infect. Dis. Clin. N. Am. 2003, 17, 545–562. [Google Scholar] [CrossRef]
- Gales, A.C.; Reis, A.O.; Jones, R.N. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 2001, 39, 183–190. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Global assessment of the antimicrobial activity of polymyxin B against 54,731 clinical isolates of Gram-negative bacilli: Report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin. Microbiol. Infect. 2006, 12, 315–321. [Google Scholar] [CrossRef]
- Storm, D.R.; Rosenthal, K.S.; Swanson, P.E. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 1977, 46, 723–763. [Google Scholar] [CrossRef]
- Dugourd, D.; Yang, H.; Elliott, M.; Siu, R.; Clement, J.J.; Straus, S.K.; Hancock, R.E.; Rubinchik, E. Antimicrobial properties of MX-2401, an expanded-spectrum lipopeptide active in the presence of lung surfactant. Antimicrob. Agents Chemother. 2011, 55, 3720–3728. [Google Scholar] [CrossRef][Green Version]
- Reder-Christ, K.; Falkenstein-Paul, H.; Klocek, G.; Al-Kaddah, S.; Bakowsky, U.; Bendas, G. Model membrane approaches to determine the role of calcium for the antimicrobial activity of friulimicin. Int. J. Antimicrob. Agents 2011, 37, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, T.H. Anti-infective natural products from cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar] [CrossRef]
- Shishido, T.K.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.; Fiore, M.F.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef]
- Tan, L.T.; Salleh, N.F. Marine cyanobacteria: A rich source of structurally unique anti-infectives for drug development. Molecules 2024, 29, 5307. [Google Scholar] [CrossRef]
- Jafari, P.S.; Konur, O.; Nowruzi, B. Cyanobacterial natural products as sources for antiviral drug discovery against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 7629–7644. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Tester, M.A.; Gross, D.C. Role of biosurfactant and ion channel-forming activities of syringomycin in transmembrane ion flux: A model for the mechanism of action in the plant-pathogen interaction. Mol. Plant Microbe Interact. 1995, 8, 610–620. [Google Scholar] [CrossRef]
- Feigin, A.M.; Takemoto, J.Y.; Wangspa, R.; Teeter, J.H.; Brand, J.G. Properties of voltage-gated ion channels formed by syringomycin E in planar lipid bilayers. J. Membr. Biol. 1996, 149, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, F.A.; Kaulin, Y.A.; Tikhomirova, A.V.; Wangspa, R.; Takemoto, D.; Malev, V.V.; Shchagina, L.V. Activity of toxins produced by Pseudomonas syringae pv. syringae in model and cellular membranes. Tsitologiya 2002, 44, 296–304. (In Russian) [Google Scholar]
- Menestrina, G.; Coraiola, M.; Fogliano, V.; Fiore, A.; Grgurina, I.; Carpaneto, A.; Gambale, F.; Serra, M. Antimicrobial lipodepsipeptides from Pseudomonas spp: A comparison of their activity on model membranes. In Pseudomonas Syringae and Related Pathogens: Biology and Genetic; Springer: Dordrecht, The Netherlands, 2003; pp. 185–198. [Google Scholar] [CrossRef]
- Dalla Serra, M.; Fagiuoli, G.; Nordera, P.; Bernhart, I.; Della Volpe, C.; Di Giorgio, D.; Ballio, A.; Menestrina, G. The interaction of lipodepsipeptide toxins from Pseudomonas syringae pv. syringae with biological and model membranes: A comparison of syringotoxin, syringomycin, and two syringopeptins. Mol. Plant Microbe Interact. 1999, 12, 391–400. [Google Scholar] [CrossRef]
- Feigin, A.M.; Schagina, L.V.; Takemoto, J.Y.; Teeter, J.H.; Brand, J.G. The effect of sterols on the sensitivity of membranes to the channel-forming antifungal antibiotic, syringomycin E. Biochim. Biophys. Acta 1997, 1324, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, W.; Pavlovkin, J.; Pokorny, J. Effect of syringotoxin on the permeability of bilayer lipid membranes. Biologia 1984, 39, 693–699. [Google Scholar]
- Szabó, Z.; Gróf, P.; Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Mátyus, E.; Blaskó, K. Syringotoxin pore formation and inactivation in human red blood cell and model bilayer lipid membranes. Biochim. Biophys. Acta 2002, 1567, 143–149. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Malev, V.V.; Shchagina, L.V. Cooperativeness of functioning of ion channels formed by phytotoxins, syringomycin E and syringostatin A. Biol. Membr. 2006, 23, 412–419. (In Russian) [Google Scholar]
- Geudens, N.; Nasir, M.N.; Crowet, J.M.; Raaijmakers, J.M.; Fehér, K.; Coenye, T.; Martins, J.C.; Lins, L.; Sinnaeve, D.; Deleu, M. Membrane interactions of natural cyclic lipodepsipeptides of the viscosin group. Biochim. Biophys. Acta Biomembr. 2017, 1859, 331–339. [Google Scholar] [CrossRef]
- Steigenberger, J.; Verleysen, Y.; Geudens, N.; Madder, A.; Martins, J.C.; Heerklotz, H. Complex electrostatic effects on the selectivity of membrane-permeabilizing cyclic lipopeptides. Biophys. J. 2023, 122, 950–963. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Lazzaroni, S.; Coraiola, M.; Dalla Serra, M.; Cafarchia, C.; Evidente, A.; Lacobellis, N.S. Biological characterization of white line-inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Mol. Plant Microbe Interact. 2006, 19, 1113–1120. [Google Scholar] [CrossRef]
- Scaloni, A.; Dalla Serra, M.; Amodeo, P.; Mannina, L.; Vitale, R.M.; Segre, A.L.; Cruciani, O.; Lodovichetti, F.; Greco, M.L.; Fiore, A.; et al. Structure, conformation and biological activity of a novel lipodepsipeptide from Pseudomonas corrugata: Cormycin A. Biochem. J. 2004, 384 Pt 1, 25–36. [Google Scholar] [CrossRef]
- Agner, G.; Kaulin, Y.A.; Gurnev, P.A.; Szabo, Z.; Schagina, L.V.; Takemoto, J.Y.; Blasko, K. Membrane-permeabilizing activities of cyclic lipodepsipeptides, syringopeptin 22A and syringomycin E from Pseudomonas syringae pv. syringae in human red blood cells and in bilayer lipid membranes. Bioelectrochemistry 2000, 52, 161–167. [Google Scholar] [CrossRef]
- Bensaci, M.F.; Gurnev, P.A.; Bezrukov, S.M.; Takemoto, J.Y. Fungicidal activities and mechanisms of action of Pseudomonas syringae pv. syringae lipodepsipeptide syringopeptins 22A and 25A. Front. Microbiol. 2011, 2, 216. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Gross, D.C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: Comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol. Plant Microbe Interact. 1997, 10, 347–3354. [Google Scholar] [CrossRef]
- Carpaneto, A.; Dalla Serra, M.; Menestrina, G.; Fogliano, V.; Gambale, F. The phytotoxic lipodepsipeptide syringopeptin 25A from Pseudomonas syringae pv syringae forms ion channels in sugar beet vacuoles. J. Membr. Biol. 2002, 188, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Brodey, C.L.; Rainey, P.B.; Tester, M.; Johnstone, K. Bacterial blotch disease of the cultivated mushroom ls caused by an lon channel forming lipodepsipeptide toxin. Mol. Plant-Microbe Interact. 1991, 4, 407–411. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, Y.K. Two types of ion channel formation of tolaasin, a Pseudomonas peptide toxin. FEMS Microbiol. Lett. 2003, 221, 221–226. [Google Scholar] [CrossRef]
- Jo, G.; Hwang, D.; Lee, S.; Woo, Y.; Hyun, J.; Yong, Y.; Kang, K.; Kim, D.W.; Lim, Y. In silico study of the ion channel formed by tolaasin I produced by Pseudomonas tolaasii. J. Microbiol. Biotechnol. 2011, 21, 1097–1100. [Google Scholar] [CrossRef]
- Coraiola, M.; Paletti, R.; Fiore, A.; Fogliano, V.; Dalla Serra, M. Fuscopeptins, antimicrobial lipodepsipeptides from Pseudomonas fuscovaginae, are channel forming peptides active on biological and model membranes. J. Pept. Sci. 2008, 14, 496–502. [Google Scholar] [CrossRef]
- Reder-Christ, K.; Schmidt, Y.; Dörr, M.; Sahl, H.G.; Josten, M.; Raaijmakers, J.M.; Gross, H.; Bendas, G. Model membrane studies for characterization of different antibiotic activities of lipopeptides from Pseudomonas. Biochim. Biophys. Acta 2012, 1818, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Seydlová, G.; Sokol, A.; Lišková, P.; Konopásek, I. Fišer, R Daptomycin pore formation and stoichiometry depend on membrane potential of target membrane. Antimicrob. Agents Chemother. 2018, 63, e01589-18. [Google Scholar] [CrossRef] [PubMed]
- Machhua, P.; Unnithan, V.G.; Liu, Y.; Jiang, Y.; Zhang, L.; Guo, Z. Daptomycin forms a stable complex with phosphatidylglycerol for selective uptake to bacterial membrane. Elife Biochem. Chem. Biol. 2024, 13, RP93267. [Google Scholar] [CrossRef]
- Lakey, J.H.; Lea, E.J.; Rudd, B.A.; Wright, H.M.; Hopwood, D.A. A new channel-forming antibiotic from Streptomyces coelicolor A3(2) which requires calcium for its activity. J. Gen. Microbiol. 1983, 129, 3565–3573. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Jumarie, C.; Cooper, D.G.; Laprade, R. Ionic channels induced by surfactin in planar lipid bilayer membranes. Biochim. Biophys. Acta 1991, 1064, 13–23. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Malev, V.V.; Ilin, M.G.; Schagina, L.V. Surfactin activity depends on the membrane dipole potential. Langmuir 2010, 26, 15092–15097. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Ptak, M.; Peypoux, F.; Michel, G. Effect of the O-methylation of tyrosine on the pore-forming properties of iturins. Biochim. Biophys. Acta 1987, 898, 1–5. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Heitz, F.; Ptak, M.; Peypoux, F.; Guinand, M. Bacterial lipopeptides induce ion-conducting pores in planar bilayers. Biochem. Biophys. Res. Commun. 1985, 129, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, A.A.; Efimova, S.S.; Malev, V.V.; Ostroumova, O.S. Fengycin induces ion channels in lipid bilayers mimicking target fungal cell membranes. Sci. Rep. 2019, 9, 16034. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B. Antibiotics 2022, 11, 1445. [Google Scholar] [CrossRef]
- Mikkola, R.; Andersson, M.A.; Grigoriev, P.; Heinonen, M.; Salkinoja-Salonen, M.S. The toxic mode of action of cyclic lipodepsipeptide fusaricidins, produced by Paenibacillus polymyxa, toward mammalian cells. J. Appl. Microbiol. 2017, 123, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, R.; Andersson, M.; Kharechkina, E.; Kruglova, S.; Kruglov, A. Fusaricidin-type compounds create pores in mitochondrial and plasma membranes of mammalian cells. Biomolecules 2019, 9, 433. [Google Scholar] [CrossRef]
- Hájek, J.; Bieringer, S.; Voráčová, K.; Macho, M.; Saurav, K.; Delawská, K.; Divoká, P.; Fišer, R.; Mikušová, G.; Cheel, J.; et al. Semi-synthetic puwainaphycin/minutissamide cyclic lipopeptides with improved antifungal activity and limited cytotoxicity. RSC Adv. 2021, 11, 30873–30886. [Google Scholar] [CrossRef]
- Sinden, S.L.; DeVay, J.E.; Backman, P.A. Properties of syringomycin, a wide spectrum antibiotic and phytotoxin produced by Pseudomonas syringae, and its role in the bacterial canker disease of peach trees. Physiol. Plant Pathol. 1971, 1, 199–213. [Google Scholar] [CrossRef]
- Backman, P.A.; DeVay, J.E. Studies on the mode of action and biogenesis of the phytotoxin syringomycin. Physiol. Plant Pathol. 1971, 1, 215–233. [Google Scholar] [CrossRef]
- Devay, J.E.; Lukezic, F.L.; Sinden, S.L.; English, H.; Coplin, D.L. A biocide produced by pathogenic isolates of Pseudomonas vringae and its possible role in the bacterial canker disease of peach trees. Phytopatholopy 1968, 58, 95–101. [Google Scholar]
- Ballio, A.; Barra, D.; Bossa, F.; Collina, A.; Grgurina, I.; Marino, G.; Moneti, G.; Paci, M.; Pucci, P.; Segre, A.; et al. Syringopeptins, new phytotoxic lipodepsipeptides of Pseudomonas syringae pv. syringae. FEBS Lett. 1991, 291, 109–112. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lavermicocca, P.; Grgurina, I.; Simmaco, M.; Ballio, A. Phytotoxic properties of Pseudomonas syringae pv. syringae toxins. Physiol. Mol. Plant Pathol. 1992, 40, 107–116. [Google Scholar] [CrossRef]
- Takemoto, J.Y.; Brand, J.G.; Kaulin, Y.A.; Malev, V.V.; Schagina, L.V.; Blasko, K. The syringomycins: Lipodepsipeptide pore formers from plant bacterium Pseudomonas syringae. In Pore-Forming Peptides and Protein Toxins; Menestrina, G., Dalla Serra, M., Lazarovici, P., Eds.; Taylor and Francis: London, UK, 2003; pp. 260–271. [Google Scholar]
- Bender, C.L.; Alarcón-Chaidez, F.; Gross, D.C. Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [CrossRef] [PubMed]
- Rokni-Zadeh, H.; Li, W.; Yilma, E.; Sanchez-Rodriguez, A.; De Mot, R. Distinct lipopeptide production systems for WLIP (white line-inducing principle) in Pseudomonas fluorescens and Pseudomonas putida. Environ. Microbiol. Rep. 2013, 5, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rokni-Zadeh, H.; Li, W.; Sanchez-Rodriguez, A.; Sinnaeve, D.; Rozenski, J.; Martins, J.C.; De Mot, R. Genetic and functional characterization of cyclic lipopeptide white-line-inducing principle (WLIP) production by rice rhizosphere isolate Pseudomonas putida RW10S2. Appl. Environ. Microbiol. 2012, 78, 4826–4834. [Google Scholar] [CrossRef]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem. Int. Ed. Engl. 2013, 52, 7098–7124. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Di Giorgio, D.; Ferranti, P.; Paci, M.; Pucci, P.; Scaloni, A.; Segre, A.; Strobel, G.A. Novel bioactive lipodepsipeptides from Pseudomonas syringae: The pseudomycins. FEBS Lett. 1994, 355, 96–100. [Google Scholar] [CrossRef]
- Segre, A.; Bachmann, R.C.; Ballio, A.; Bossa, F.; Grgurina, I.; Iacobellis, N.S.; Marino, G.; Pucci, P.; Simmaco, M.; Takemoto, J.Y. The structure of syringomycins A1, E and G. FEBS Lett. 1989, 255, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Scaloni, A.; Backmann, R.C.; Takemoto, J.Y.; Barra, D.; Simmaco, M.; Ballio, A. Stereochemical structure of syringomycin, a phytotoxic metabolites of Pseudomonas syringae pv. syringae. Nat. Prod. Lett. 1994, 4, 159–164. [Google Scholar] [CrossRef]
- Fukuchi, N.A.; Isogai, J.; Nakayama, S.; Takayama, S.; Yamashita, K.; Suyama, J.Y.; Takemoto, J.Y.; Suzuki, A. Structures and stereochemistry of three phytotoxins, syringomycin, syringotoxin and syringostatin, produced by Pseudomonas syringae pv. syringae. J. Chem. Soc. Perkin Trans. 1992, 1, 1149–1157. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Collina, A.; Gallo, M.; Iacobellis, N.S.; Paci, M.; Pucci, P.; Scaloni, A.; Segre, A.; Simmaco, M. Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett. 1990, 269, 377–380. [Google Scholar] [CrossRef]
- Michelsen, C.F.; Watrous, J.; Glaring, M.A.; Kersten, R.; Koyama, N.; Dorrestein, P.C.; Stougaard, P. Nonribosomal peptides, key biocontrol components for Pseudomonas fluorescens In5, isolated from a Greenlandic suppressive soil. mBio 2015, 6, e00079. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.W.; Skinnider, M.A.; Wyatt, M.A.; Li, X.; Ranieri, M.R.; Yang, L.; Zechel, D.L.; Ma, B.; Magarvey, N.A. An automated Genomes-to-Natural Products platform (GNP) for the discovery of modular natural products. Nat. Commun. 2015, 6, 8421. [Google Scholar] [CrossRef] [PubMed]
- Mátyus, E.; Blaskó, K.; Fidy, J.; Tieleman, D.P. Structure and dynamics of the antifungal molecules Syringotoxin-B and Syringopeptin-25A from molecular dynamics simulation. Eur. Biophys. J. 2008, 37, 495–502. [Google Scholar] [CrossRef]
- Janek, T.; Łukaszewicz, M.; Rezanka, T.; Krasowska, A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour. Technol. 2010, 101, 6118–6123. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Melnik, A.V.; Koyama, N.; Lu, X.; Schorn, M.; Fang, J.; Aguinaldo, K.; Lincecum, T.L., Jr.; Ghequire, M.G.; Carrion, V.J.; et al. Indexing the Pseudomonas specialized metabolome enabled the discovery of poaeamide B and the bananamides. Nat. Microbiol. 2016, 2, 16197. [Google Scholar] [CrossRef] [PubMed]
- Cautain, B.; de Pedro, N.; Schulz, C.; Pascual, J.; da S. Sousa, T.; Martin, J.; Pérez-Victoria, I.; Asensio, F.; González, I.; Bills, G.F.; et al. Identification of the lipodepsipeptide MDN-0066, a novel inhibitor of VHL/HIF pathway produced by a new pseudomonas species. PLoS ONE 2015, 10, e0125221. [Google Scholar] [CrossRef]
- Geudens, N.; Martins, J.C. Cyclic lipodepsipeptides from Pseudomonas spp.—Biological Swiss-Army Knives. Front. Microbiol. 2018, 9, 1867. [Google Scholar] [CrossRef]
- Tran, H.; Ficke, A.; Asiimwe, T.; Höfte, M.; Raaijmakers, J.M. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef]
- Ma, Z.; Hua, G.K.H.; Ongena, M.; Höfte, M. Role of phenazines and cyclic lipopeptides produced by Pseudomonas sp. CMR12a in induced systemic resistance on rice and bean. Environ. Microbiol. Rep. 2016, 8, 896–904. [Google Scholar] [CrossRef]
- Bull, C.T.; Wadsworth, M.L.; Sorensen, K.N.; Takemoto, J.Y.; Austin, R.K.; Smilanick, J.L. Syringomycin E produced by biological control agents controls green mold on lemons. Biol. Control 1998, 12, 89–95. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Nischwitz, C.; Grilley, M.M.; Jones, J.; Brown, J.D.; Takemoto, J.Y. Production and application of syringomycin E as an organic fungicide seed protectant against pythium damping-off. J. Phytopathol. 2016, 164, 801–810. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Sante Iacobellis, N.; Simmaco, M.; Graniti, A. Biological properties and spectrum of activity of Pseudomonas syringaepv syringaetoxins. Physiol. Mol. Plant Pathol. 1997, 50, 129–140. [Google Scholar] [CrossRef]
- Sorensen, K.N.; Kim, K.H.; Takemoto, J.Y. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob. Agents Chemother. 1996, 40, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Batoko, H.; de Kerchove d’Exaerde, A.; Kinet, J.M.; Bouharmont, J.; Gage, R.A.; Maraite, H.; Boutry, M. Modulation of plant plasma membrane H+-ATPase by phytotoxic lipodepsipeptides produced by the plant pathogen Pseudomonas fuscovaginae. Biochim. Biophys. Acta 1998, 1372, 216–226. [Google Scholar] [CrossRef]
- Bassarello, C.; Lazzaroni, S.; Bifulco, G.; Lo Cantore, P.; Iacobellis, N.S.; Riccio, R.; Gomez-Paloma, L.; Evidente, A. Tolaasins A–E, five new lipodepsipeptides produced by Pseudomonas tolaasii. J. Nat. Prod. 2004, 67, 811–816. [Google Scholar] [CrossRef]
- Pedras, M.S.; Ismail, N.; Quail, J.W.; Boyetchko, S.M. Structure, chemistry, and biological activity of pseudophomins A and B, new cyclic lipodepsipeptides isolated from the biocontrol bacterium Pseudomonas fluorescens. Phytochemistry 2003, 62, 1105–1114. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Sørensen, D.; Tobiasen, C.; Andersen, J.B.; Christophersen, C.; Givskov, M.; Sørensen, J. Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl. Environ. Microbiol. 2002, 68, 3416–3423. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Sørensen, J. Production of cyclic lipopeptides by Pseudomonas fluorescens strains in bulk soil and in the sugar beet rhizosphere. Appl. Environ. Microbiol. 2003, 69, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Grgurina, I.; Barca, A.; Cervigni, S.; Gallo, M.; Scaloni, A.; Pucci, P. Relevance of chlorine-substituent for the antifungal activity of syringomycin and syringotoxin, metabolites of the phytopathogenic bacterium Pseudomonas syringae pv. syringae. Experientia 1994, 50, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.L.; Scholz-Schroeder, B.K. New insights into the biosynthesis, mode of action, and regulation of syringomycin, syringopeptin, and coronatine. In Virulence and Gene Regulation; Springer: Boston, MA, USA, 2004; pp. 125–158. [Google Scholar] [CrossRef]
- Zhang, L.; Takemoto, J.Y. Mechanism of action of Pseudomonas syringae phytotoxin, syringomycin. Interaction with the plasma membrane of wild-type and respiratory-deficient strains of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1986, 861, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Paynter, V.A.; Alconero, R. A specific fluorescent antibody for detection of syringomycin in infected peach tree tissues. Phytopathology 1979, 69, 493–496. [Google Scholar] [CrossRef]
- Reidl, H.H.; Takemoto, J.Y. Mechanism of action of bacterial phytotoxin, syringomycin. Simultaneous measurement of early responses in yeast and maize. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 898, 59–69. [Google Scholar] [CrossRef]
- Bidwai, A.P.; Zhang, L.; Bachmann, R.C.; Takemoto, J.Y. Mechanism of Action of Pseudomonas syringae Phytotoxin, Syringomycin: Stimulation of Red Beet Plasma Membrane ATPase Activity. Plant Physiol. 1987, 83, 39–43. [Google Scholar] [CrossRef]
- Che, F.S.; Kasamo, K.; Fukuchi, N.; Isogai, A.; Suzuki, A. Bacterial phytotoxins, syringomycin, syringostatin and syringotoxin, exert their effect on the plasma membrane H+-ATPase partly by a detergent-like action and partly by inhibition of the enzyme. Physiol. Plant. 1992, 86, 518–524. [Google Scholar] [CrossRef]
- Surico, G.; DeVay, J.E. Effect of syringomycin and syringotoxin produced by Pseudomonas syringae pv. syringae on structure and function of mitochondria isolated from holcus spot resistant and susceptible maize lines. Physiol. Plant Pathol. 1982, 21, 39–48. [Google Scholar] [CrossRef]
- Takemoto, J.Y.; Giannini, J.L.; Vassey, T.; Briskin, D.P. Syringomycin Effects on Plasma Membrane Ca+2 Transport. In Phytotoxins and Plant Pathogenesis; Springer: Berlin/Heidelberg, Germany, 1989; Volume 27. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Malev, V.V.; Kaulin, Y.A.; Gurnev, P.A.; Takemoto, J.Y.; Schagina, L.V. Voltage-dependent synchronization of gating of syringomycin E ion channels. FEBS Lett. 2005, 579, 5675–5679. [Google Scholar] [CrossRef][Green Version]
- Schagina, L.V.; Kaulin, Y.A.; Feigin, A.M.; Takemoto, J.Y.; Brand, J.G.; Malev, V.V. Properties of ionic channels formed by the antibiotic syringomycin E in lipid bilayers: Dependence on the electrolyte concentration in the bathing solution. Membr. Cell Biol. 1998, 12, 537–555. [Google Scholar][Green Version]
- Kaulin, Y.A.; Schagina, L.V.; Bezrukov, S.M.; Malev, V.V.; Feigin, A.M.; Takemoto, J.Y.; Teeter, J.H.; Brand, J.G. Cluster organization of ion channels formed by the antibiotic syringomycin E in bilayer lipid membranes. Biophys. J. 1998, 74, 2918–2925. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Gurnev, P.A.; Schagina, L.V.; Bezrukov, S.M. Asymmetry of syringomycin E channel studied by polymer partitioning. FEBS Lett. 2007, 581, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Malev, V.V.; Kaulin, Y.A.; Bezrukov, S.M.; Gurnev, P.A.; Takemoto, J.Y.; Shchagina, L.V. Kinetics of opening and closure of syringomycin E channels formed in lipid bilayers. Membr. Cell Biol. 2001, 14, 813–829. [Google Scholar] [PubMed]
- Gurnev, P.A.; Bessonov, A.N.; Takemoto, J.Y.; Shchagina, L.V.; Malev, V.V. Conductance of ion channels induced by syringomycin E in lipid bilayers with asymmetrically distributed surface charge. Biol. Membr. 2004, 21, 325–332. (In Russian) [Google Scholar]
- Malev, V.V.; Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Nestorovich, E.M.; Bezrukov, S.M. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophys. J. 2002, 82, 1985–1994. [Google Scholar] [CrossRef]
- Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Malev, V.V. Effective gating charge of ion channels induced by toxin syringomycin E in lipid bilayers. Bioelectrochemistry 2003, 60, 21–27. [Google Scholar] [CrossRef]
- Ziegler, W.; Pavlovkin, J.; Remis, D.; Pokorny, J. The anion/ cation selectivity of the syringotoxin channel. Biologia 1986, 41, 1091–1096. [Google Scholar]
- Mortishire-Smith, R.J.; Nutkins, J.C.; Packman, L.C.; Brodey, C.L.; Rainey, P.B.; Johnstone, K.; Williams, D.H. Determination of the structure of an extracellular peptide produced by the mushroom saprotroph pseudomonas reactans. Tetrahedron 1991, 47, 3645–3654. [Google Scholar] [CrossRef]
- Gerard, J.; Lloyd, R.; Barsby, T.; Haden, P.; Kelly, M.T.; Andersen, R.J. Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J. Nat. Prod. 1997, 60, 223–229. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Christophersen, C.; Anthoni, U.; Sørensen, J. Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J. Appl. Microbiol. 1999, 87, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Geudens, N.; De Vleeschouwer, M.; Fehér, K.; Rokni-Zadeh, H.; Ghequire, M.G.; Madder, A.; De Mot, R.; Martins, J.C.; Sinnaeve, D. Impact of a stereocentre inversion in cyclic lipodepsipeptides from the viscosin group: A comparative study of the viscosinamide and pseudodesmin conformation and self-assembly. ChemBioChem 2014, 15, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Sinnaeve, D.; Hendrickx, P.M.; Van Hemel, J.; Peys, E.; Kieffer, B.; Martins, J.C. The solution structure and self-association properties of the cyclic lipodepsipeptide pseudodesmin A support its pore-forming potential. Chemistry 2009, 15, 12653–12662. [Google Scholar] [CrossRef] [PubMed]
- Sinnaeve, D.; Michaux, C.; Vandenkerckhove, J.; Peys, E.; Borremans, F.A.M.; Sas, B.; Wouters, J.; Martins, J.C. Structure and X-ray conformation of pseudodesmins A and B, two new cyclic lipodepsipeptides from Pseudomonas bacteria. Tetrahedron 2009, 65, 4173–4181. [Google Scholar] [CrossRef]
- Quail, J.W.; Ismail, N.; Pedras, M.S.; Boyetchko, S.M. Pseudophomins A and B, a class of cyclic lipodepsipeptides isolated from a Pseudomonas species. Cryst. Struct. Commun. 2002, 58, o268–o271. [Google Scholar] [CrossRef] [PubMed]
- Geudens, N.; Sinnaeve, D.; Martins, J.C. Cyclic lipodepsipeptides: Time for a concerted action to unlock their application potential? Future Med. Chem. 2018, 10, 479–481. [Google Scholar] [CrossRef]
- Thrane, C.; Olsson, S.; Harder Nielsen, T.; Sørensen, J. Vital fluorescent stains for detection of stress in Pythium ultimum and Rhizoctonia solani challenged with viscosinamide from Pseudomonas fluorescens DR54. FEMS Microbiol. Ecol. 1999, 30, 11–23. [Google Scholar] [CrossRef]
- Khattari, Z.; Al-Abdullah, T.; Maghrabi, M.; Khasim, S.; Fasfous, A.R.I. Interaction study of lipopeptide biosurfactant viscosin with DPPC and cholesterol by Langmuir monolayer technique. Soft Mater. 2015, 13, 254–262. [Google Scholar] [CrossRef]
- Coraiola, M.; Lo Cantore, P.; Lazzaroni, S.; Evidente, A.; Iacobellis, N.S.; Dalla Serra, M. WLIP and tolaasin I, lipodepsipeptides from Pseudomonas reactans and Pseudomonas tolaasii, permeabilise model membranes. Biochim. Biophys. Acta 2006, 1758, 1713–1722. [Google Scholar] [CrossRef]
- Sinnaeve, D.; Delsuc, M.-A.; Martins, J.C.; Kieffer, B. Insight into peptide self-assembly from anisotropic rotational diffusion derived from 13C NMR relaxation. Chem. Sci. 2012, 3, 1284. [Google Scholar] [CrossRef]
- Chun, W.; Leary, J.V. Novel toxin produced by Pseudomonas corrugata, the causal agent of tomato pith necrosis; Determination of its Role in Virulence and the Genetics of Production. Phytotoxins Plant Pathog. 1989, 27, 93–116. [Google Scholar] [CrossRef]
- Vassilev, V.; Lavermicocca, P.; Di Giorgio, C.; Iacobellis, N. Production of syringomycins and syringopeptins by Pseudomonas syringae pv. atrofaciens. Plant Pathol. 1996, 45, 316–322. [Google Scholar] [CrossRef]
- Grgurina, I.; Bensaci, M.; Pocsfalvi, G.; Mannina, L.; Cruciani, O.; Fiore, A.; Fogliano, V.; Sorensen, K.N.; Takemoto, J.Y. Novel cyclic lipodepsipeptide from Pseudomonas syringae pv. lachrymans strain 508 and syringopeptin antimicrobial activities. Antimicrob. Agents Chemother. 2005, 49, 5037–5045. [Google Scholar] [CrossRef]
- Isogai, A.; Iguchi, H.; Nakayama, J.; Kusai, A.; Takemoto, J.Y.; Suzuki, A. Structural analysis of new syringopeptins by tandem mass spectroscopy. Biosci. Biotech. Biochem. 1995, 59, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Grgurina, I.; Mariotti, F.; Fogliano, V.; Gallo, M.; Scaloni, A.; Iacobellis, N.S.; Lo Cantore, P.; Mannina, L.; van Axel Castelli, V.; Greco, M.L.; et al. A new syringopeptin produced by bean strains of Pseudomonas syringae pv. syringae. Biochim. Biophys. Acta 2002, 1597, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Adetuyi, F.C.; Isogai, A.; Di Giorgio, D.; Ballio, A.; Takemoto, J.Y. Saprophytic Pseudomonas syringae strain M1 of wheat produces cyclic lipodepsipeptides. FEMS Microbiol. Lett. 1995, 131, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Scaloni, A.; Camoni, L.; Di Giorgio, D.; Scortichini, M.; Cozzolini, R.; Ballio, A. A new syringopeptin produced by a Pseudomonas syringae pv. syringae strain isolated from diseased twigs of laurel. Physiol. Mol. Plant Pathol. 1997, 51, 259–264. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.; Raden, D.; Racker, E.; Katchalski-Katzir, E. Phosphorylation of synthetic random polypeptides by protein kinase P and other protein-serine (threonine) kinases and stimulation or inhibition of kinase activities by microbial toxins. Proc. Natl. Acad. Sci. USA 1988, 85, 1408–1411. [Google Scholar] [CrossRef]
- Fukuchi, N.; Isogai, A.; Nakayama, J.; Suzuki, A. Structure of syringotoxin B, a phytotoxin produced by citrus isolates of Pseudomonas syringae pv. syringae. Agric. Biol. Chem. 1990, 54, 3377–3379. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.K.C.; Aswathanarayan, J.B.; Vittal, R.R. Endophytic peptides—A source of therapeutic agents. Curr.Protein Pept. Sci. 2017, 18, 284–290. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Di Giorgio, D.; Di Nola, A.; Manetti, C.; Paci, M.; Scaloni, A.; Segre, A.L. Solution conformation of the Pseudomonas syringae pv. syringae phytotoxic lipodepsipeptide syringopeptin 25-A. Two-dimensional NMR, distance geometry and molecular dynamics. Eur. J. Biochem. 1995, 234, 747–758. [Google Scholar] [CrossRef]
- Di Giorgio, D.; Lavermicocca, P.; Marchiafava, C.; Camoni, L.; Surico, G.; Ballio, A. Effect of syringomycin-E and syringopeptins on isolated plant mitochondria. Physiol. Mol. Plant Pathol. 1996, 48, 325–334. [Google Scholar] [CrossRef]
- Mott, K.A.; Takemoto, J.Y. Syringomycin, a bacterial phytotoxin, closes stomata. Plant Physiol. 1989, 90, 1435–1439. [Google Scholar] [CrossRef]
- Camoni, L.; Di Giorgio, D.; Marra, M.; Aducci, P.; Ballio, A. Pseudomonas syringae pv. syringae phytotoxins reversibly inhibit the plasma membrane H(+)-ATPase and disrupt unilamellar liposomes. Biochem. Biophys. Res. Commun. 1995, 214, 118–124. [Google Scholar] [CrossRef]
- Szabó, Z.; Budai, M.; Blaskó, K.; Gróf, P. Molecular dynamics of the cyclic lipodepsipeptides’ action on model membranes: Effects of syringopeptin22A, syringomycin E, and syringotoxin studied by EPR technique. Biochim. Biophys. Acta 2004, 1660, 118–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nutkins, J.C.; Mortishire-Smith, R.J.; Packman, L.C.; Brodey, C.L.; Rainey, P.B.; Johnstone, K.; Williams, D.H. Structure determination of tolaasin, an extracellular lipodepsipeptide produced by the mushroom pathogen Pseudomonas tolaasii Paine. J. Am. Chem. Soc. 1991, 113, 2621–2627. [Google Scholar] [CrossRef]
- Osdaghi, E.; Martins, S.J.; Ramos-Sepulveda, L.; Vieira, F.R.; Pecchia, J.A.; Beyer, D.M.; Bell, T.H.; Yang, Y.; Hockett, K.L.; Bull, C.T. 100 Years since tolaas: Bacterial blotch of mushrooms in the 21st century. Plant. Dis. 2019, 103, 2714–2732. [Google Scholar] [CrossRef]
- Scherlach, K.; Lackner, G.; Graupner, K.; Pidot, S.; Bretschneider, T.; Hertweck, C. Biosynthesis and mass spectrometric imaging of tolaasin, the virulence factor of brown blotch mushroom disease. ChemBioChem 2013, 14, 2439–2443. [Google Scholar] [CrossRef]
- D’aes, J.; Kieu, N.P.; Léclère, V.; Tokarski, C.; Olorunleke, F.E.; De Maeyer, K.; Jacques, P.; Höfte, M.; Ongena, M. To settle or to move? The interplay between two classes of cyclic lipopeptides in the biocontrol strain Pseudomonas CMR12a. Environ. Microbiol. 2014, 16, 2282–2300. [Google Scholar] [CrossRef]
- Wong, W.C.; Preece, T.F. Identification ofPseudomonas tolaasi:the White Line in Agar and Mushroom Tissue Block Rapid Pitting Tests. J. Appl. Bacteriol. 1979, 47, 401–407. [Google Scholar] [CrossRef]
- Rainey, P.B.; Brodey, C.L.; Johnstone, K. Biological properties and spectrum of activity of tolaasin, a lipodepsipeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol. Mol. Plant Pathol. 1991, 39, 57–70. [Google Scholar] [CrossRef]
- Oni, F.E.; Olorunleke, O.F.; Höfte, M. Phenazines and cyclic lipopeptides produced by Pseudomonas sp. CMR12a are involved in the biological control of Pythium myriotylum on cocoyam (Xanthosoma sagittifolium). Biol. Control 2018, 129, 109–114. [Google Scholar] [CrossRef]
- Cole, A.L.J.; Skellerup, M.V. Ultrastructure of the interaction of Agaricus bisporus and Pseudomonas tolaasii. Trans. Br. Mycol. Soc. 1986, 87, 314–316. [Google Scholar] [CrossRef]
- Ferrarini, E.; De Roo, V.; Geudens, N.; Martins, J.C.; Höfte, M. Altering in vivo membrane sterol composition affects the activity of the cyclic lipopeptides tolaasin and sessilin against Pythium. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184008. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Johnstone, K. Evidence for the involvement of the surface active properties of the extracellular toxin tolaasin in the manifestation of brown blotch disease symptoms by Pseudomonas tolaasii on Agaricus bisporus. Physiol. Mol. Plant Pathol. 1993, 42, 373–384. [Google Scholar] [CrossRef]
- Jourdan, F.; Lazzaroni, S.; Méndez, B.L.; Lo Cantore, P.; de Julio, M.; Amodeo, P.; Iacobellis, N.S.; Evidente, A.; Motta, A. A left-handed alpha-helix containing both L- and D-amino acids: The solution structure of the antimicrobial lipodepsipeptide tolaasin. Proteins 2003, 52, 534–543. [Google Scholar] [CrossRef]
- Steigenberger, J.; Mergen, C.; De Roo, V.; Geudens, N.; Martins, J.C.; Heerklotz, H. The effect of membrane thickness on the membrane permeabilizing activity of the cyclic lipopeptide tolaasin II. Front. Mol. Biosci. 2022, 9, 1064742. [Google Scholar] [CrossRef] [PubMed]
- Ballio, A.; Bossa, F.; Camoni, L.; Di Giorgio, D.; Flamand, M.C.; Maraite, H.; Nitti, G.; Pucci, P.; Scaloni, A. Structure of fuscopeptins, phytotoxic metabolites of Pseudomonas fuscovaginae. FEBS Lett. 1996, 381, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A. Bioactive lipodepsipeptides produced by bacteria and fungi. Int. J. Mol. Sci. 2022, 23, 12342. [Google Scholar] [CrossRef]
- Baré, S.; Coiro, V.M.; Scaloni, A.; Di Nola, A.; Paci, M.; Segre, A.L.; Ballio, A. Conformations in solution of the fuscopeptins. Phytotoxic metabolites of Pseudomonas fuscovaginae. Eur. J. Biochem. 1999, 266, 484–492. [Google Scholar] [CrossRef][Green Version]
- Vallet-Gely, I.; Novikov, A.; Augusto, L.; Liehl, P.; Bolbach, G.; Péchy-Tarr, M.; Cosson, P.; Keel, C.; Caroff, M.; Lemaitre, B. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Appl. Environ. Microbiol. 2010, 76, 910–921. [Google Scholar] [CrossRef]
- Bode, H.B.; Reimer, D.; Fuchs, S.W.; Kirchner, F.; Dauth, C.; Kegler, C.; Lorenzen, W.; Brachmann, A.O.; Grün, P. Determination of the absolute configuration of peptide natural products by using stable isotope labeling and mass spectrometry. Chemistry 2012, 18, 2342–2348. [Google Scholar] [CrossRef]
- Oni, F.E.; Geudens, N.; Omoboye, O.O.; Bertier, L.; Hua, H.G.K.; Adiobo, A.; Sinnaeve, D.; Martins, J.C.; Höfte, M. Fluorescent Pseudomonas and cyclic lipopeptide diversity in the rhizosphere of cocoyam (Xanthosoma sagittifolium). Environ. Microbiol. 2019, 21, 1019–1034. [Google Scholar] [CrossRef]
- Muangkaew, P.; De Roo, V.; Zhou, L.; Girard, L.; Cesa-Luna, C.; Höfte, M.; De Mot, R.; Madder, A.; Geudens, N.; Martins, J.C. Stereomeric lipopeptides from a single non-ribosomal peptide synthetase as an additional source of structural and functional diversification in Pseudomonas Lipopeptide Biosynthesis. Int. J. Mol. Sci. 2023, 24, 14302. [Google Scholar] [CrossRef] [PubMed]
- Boeck, L.D.; Papiska, H.R.; Wetzel, R.W.; Mynderse, J.S.; Fukuda, D.S.; Mertz, F.P.; Berry, D.M. A54145, a new lipopeptide antibiotic complex: Discovery, taxonomy, fermentation and HPLC. J. Antibiot. 1990, 43, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Strieker, M.; Marahiel, M.A. The structural diversity of acidic lipopeptide antibiotics. ChemBioChem 2009, 10, 607–616. [Google Scholar] [CrossRef]
- Wood, T.M.; Martin, N.I. The calcium-dependent lipopeptide antibiotics: Structure, mechanism, & medicinal chemistry. MedChemComm 2019, 10, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Abbott, B.J.; Molloy, R.M.; Fukuda, D.S.; Hunt, A.H.; Daupert, V.M.; Counter, F.T.; Ott, J.L.; Carrell, C.B.; Howard, L.C.; et al. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: The synthesis and evaluation of daptomycin (LY146032). J. Antibiot. 1988, 41, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H.; Miao, V.; Wrigley, S.K. Natural products to drugs: Daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 2005, 22, 717–741. [Google Scholar] [CrossRef]
- Wale, L.J.; Shelton, A.P.; Greenwood, D. Scanning electronmicroscopy of Staphylococcus aureus and Enterococcus faecalis exposed to daptomycin. J. Med. Microbiol. 1989, 30, 45–49. [Google Scholar] [CrossRef]
- Allen, N.E.; Alborn, W.E., Jr.; Hobbs, J.N., Jr. Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob. Agents Chemother. 1991, 35, 2639–2642. [Google Scholar] [CrossRef][Green Version]
- Alborn, W.E., Jr.; Allen, N.E.; Preston, D.A. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob. Agents Chemother. 1991, 35, 2282–2287. [Google Scholar] [CrossRef]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef]
- Allen, N.E.; Hobbs, J.N.; Alborn, W.E., Jr. Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob. Agents Chemother. 1987, 31, 1093–1099. [Google Scholar] [CrossRef]
- Zhang, J.; Scoten, K.; Straus, S.K. Daptomycin leakage is selective. ACS Infect. Dis. 2016, 2, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberger, M.A.; Pokorny, A.; Almeida, P.F. Daptomycin-Phosphatidylglycerol Domains in Lipid Membranes. Langmuir 2017, 33, 13669–13679. [Google Scholar] [CrossRef]
- Lakey, J.H.; Lea, E.J. The role of acyl chain character and other determinants on the bilayer activity of A21978C an acidic lipopeptide antibiotic. Biochim. Biophys. Acta 1986, 859, 219–226. [Google Scholar] [CrossRef]
- Ball, L.J.; Goult, C.M.; Donarski, J.A.; Micklefield, J.; Ramesh, V. NMR structure determination and calcium binding effects of lipopeptide antibiotic daptomycin. Org. Biomol. Chem. 2004, 2, 1872–1878. [Google Scholar] [CrossRef]
- Jung, D.; Rozek, A.; Okon, M.; Hancock, R.E. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 2004, 11, 949–957. [Google Scholar] [CrossRef]
- Straus, S.K.; Hancock, R.E. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: Comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 2006, 1758, 1215–1223. [Google Scholar] [CrossRef]
- Ho, S.W.; Jung, D.; Calhoun, J.R.; Lear, J.D.; Okon, M.; Scott, W.R.; Hancock, R.E.; Straus, S.K. Effect of divalent cations on the structure of the antibiotic daptomycin. Eur. Biophys. J. 2008, 37, 421–433. [Google Scholar] [CrossRef]
- Taylor, R.; Butt, K.; Scott, B.; Zhang, T.; Muraih, J.K.; Mintzer, E.; Taylor, S.; Palmer, M. Two successive calcium-dependent transitions mediate membrane binding and oligomerization of daptomycin and the related antibiotic A54145. Biochim. Biophys. Acta 2016, 1858, 1999–2005. [Google Scholar] [CrossRef]
- Lee, M.T.; Hung, W.C.; Hsieh, M.H.; Chen, H.; Chang, Y.Y.; Huang, H.W. Molecular State of the Membrane-Active Antibiotic Daptomycin. Biophys. J. 2017, 113, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Muraih, J.K.; Pearson, A.; Silverman, J.; Palmer, M. Oligomerization of daptomycin on membranes. Biochim. Biophys. Acta 2011, 1808, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Daptomycin: Mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Muraih, J.K.; Harris, J.; Taylor, S.D.; Palmer, M. Characterization of daptomycin oligomerization with perylene excimer fluorescence: Stoichiometric binding of phosphatidylglycerol triggers oligomer formation. Biochim. Biophys. Acta 2012, 1818, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Juhaniewicz-Dębińska, J.; Dziubak, D.; Sęk, S. Physicochemical characterization of daptomycin interaction with negatively charged lipid membranes. Langmuir 2020, 36, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Balleza, D.; Mescola, A.; Alessandrini, A. Model lipid systems and their use to evaluate the phase state of biomembranes, their mechanical properties and the effect of non-conventional antibiotics: The case of daptomycin. Eur. Biophys. J. 2020, 49, 401–408. [Google Scholar] [CrossRef]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef]
- Epand, R.M.; Rotem, S.; Mor, A.; Berno, B.; Epand, R.F. Bacterial membranes as predictors of antimicrobial potency. J. Am. Chem. Soc. 2008, 130, 14346–14352. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Ishibe, N.; Ueha, M.; Nakata, S.; Miyajima, K.; Epand, R.M. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry 1998, 37, 11856–11863. [Google Scholar] [CrossRef]
- Taylor, R.; Beriashvili, D.; Taylor, S.; Palmer, M. Daptomycin Pore Formation Is Restricted by Lipid Acyl Chain Composition. ACS Infect. Dis. 2017, 3, 797–801. [Google Scholar] [CrossRef]
- Beriashvili, D.; Taylor, R.; Kralt, B.; Abu Mazen, N.; Taylor, S.D.; Palmer, M. Mechanistic studies on the effect of membrane lipid acyl chain composition on daptomycin pore formation. Chem. Phys. Lipids 2018, 216, 73–79. [Google Scholar] [CrossRef]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef]
- Zhang, T.; Taylor, S.D.; Palmer, M.; Duhamel, J. Membrane binding and oligomerization of the lipopeptide A54145 studied by pyrene fluorescence. Biophys. J. 2016, 111, 1267–1277. [Google Scholar] [CrossRef][Green Version]
- Kara, J.A.; Carter, G.P.; Howden, B.P.; Turner, A.M.; Paulin, O.K.A.; Swarbrick, J.D.; Baker, M.A.; Li, J.; Velkov, T. Structure-activity relationships of daptomycin lipopeptides. J. Med. Chem. 2020, 63, 13266–13290. [Google Scholar] [CrossRef]
- Goodyear, J.; Diamandas, M.; Moreira, R.; Taylor, S.D. The calcium-dependent antibiotics: Structure-activity relationships and determination of their lipid target. ACS Infect. Dis. 2025, 11, 226–237. [Google Scholar] [CrossRef]
- Tyurin, A.P.; Alferova, V.A.; Paramonov, A.S.; Shuvalov, M.V.; Kudryakova, G.K.; Rogozhin, E.A.; Zherebker, A.Y.; Brylev, V.A.; Chistov, A.A.; Baranova, A.A.; et al. Gausemycins A,B: Cyclic lipoglycopeptides from Streptomyces sp. Angew. Chem. Int. Ed. Engl. 2021, 60, 18694–18703. [Google Scholar] [CrossRef]
- Poshvina, D.V.; Dilbaryan, D.S.; Kasyanov, S.P.; Sadykova, V.S.; Lapchinskaya, O.A.; Rogozhin, E.A.; Vasilchenko, A.S. Staphylococcus aureus is able to generate resistance to novel lipoglycopeptide antibiotic gausemycin A. Front. Microbiol. 2022, 13, 963979. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, T.V.; Paramonov, A.S.; Kudzhaev, A.M.; Efimova, S.S.; Khorev, A.S.; Kudryakova, G.K.; Ivanov, I.A.; Chistov, A.A.; Baranova, A.A.; Krasilnikov, M.S.; et al. Gausemycin antibiotic family acts via Ca2+-dependent membrane targeting. J. Nat. Prod. 2024, 87, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Kalmankar, N.V.; Guida, M.; Bennett, J.L.; Poce, G.; Bolla, J.R.; Robinson, C.V. Lipopeptide antibiotics disrupt interactions of undecaprenyl phosphate with UptA. Proc. Natl. Acad. Sci. USA 2024, 121, e2408315121. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.M.; Zeronian, M.R.; Buijs, N.; Bertheussen, K.; Abedian, H.K.; Johnson, A.V.; Pearce, N.M.; Lutz, M.; Kemmink, J.; Seirsma, T.; et al. Mechanistic insights into the C55-P targeting lipopeptide antibiotics revealed by structure-activity studies and high-resolution crystal structures. Chem. Sci. 2022, 13, 2985–2991. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A gold mine of antibiotic candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Velkov, T.; Gallardo-Godoy, A.; Swarbrick, J.D.; Blaskovich, M.A.T.; Elliott, A.G.; Han, M.; Thompson, P.E.; Roberts, K.D.; Huang, J.X.; Becker, B.; et al. Structure, function, and biosynthetic origin of octapeptin antibiotics active against extensively drug-resistant Gram-negative bacteria. Cell Chem. Biol. 2018, 25, 380–391.e5. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Lohans, C.T.; Brandelli, J.R.; Mulvey, G.; Armstrong, G.D.; Vederas, J.C. Synthesis and structure-activity relationship studies of N-terminal analogues of the antimicrobial peptide tridecaptin A(1). J. Med. Chem. 2014, 57, 1127–1131. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.S.R.; Yang, H.; Jin, M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef]
- Qian, C.D.; Wu, X.C.; Teng, Y.; Zhao, W.P.; Li, O.; Fang, S.G.; Huang, Z.H.; Gao, H.C. Battacin (Octapeptin B5), a new cyclic lipopeptide antibiotic from Paenibacillus tianmuensis active against multidrug-resistant Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1458–1465. [Google Scholar] [CrossRef]
- Huang, E.; Guo, Y.; Yousef, A.E. Biosynthesis of the new broad-spectrum lipopeptide antibiotic paenibacterin in Paenibacillus thiaminolyticus OSY-SE. Res. Microbiol. 2014, 165, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maget-Dana, R.; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef]
- Schneider, J.; Taraz, K.; Budzikiewicz, H.; Deleu, M.; Thonart, P.; Jacques, P. The structure of two fengycins from Bacillus subtilis S499. Z. Naturforsch. C J. Biosci. 1999, 54, 859–865. [Google Scholar] [CrossRef]
- Tsukagoshi, N.; Tamura, G.; Arima, K. A novel protoplast-bursting factor (surfactin) obtained from bacillus subtilis IAM 1213. II. The interaction of surfactin with bacterial membranes and lipids. Biochim. Biophys. Acta 1970, 196, 211–214. [Google Scholar] [CrossRef]
- Besson, F.; Peypoux, F.; Michel, G. Interactions between bacterial membranes and peptidolipids: Lysis of micrococcus luteus protoplasts by derivatives of peptidolipidic antibiotics from Bacillus subtilis. Biochim. Biophys. Acta 1979, 552, 558–562. [Google Scholar] [CrossRef]
- Kracht, M.; Rokos, H.; Ozel, M.; Kowall, M.; Pauli, G.; Vater, J. Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J. Antibiot. 1999, 52, 613–619. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Pauli, G.; Ozel, M.; Vater, J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl. Environ. Microbiol. 1997, 63, 44–49. [Google Scholar] [CrossRef]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin—A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides. 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Balleza, D.; Alessandrini, A.; Beltrán García, M.J. Role of lipid composition, physicochemical interactions, and membrane mechanics in the molecular actions of microbial cyclic lipopeptides. J. Membr. Biol. 2019, 252, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, S.M.; Rishi, N.; Singh, R. Microbial lipopeptides: Properties, mechanics and engineering for novel lipopeptides. Microbiol. Res. 2023, 271, 127363. [Google Scholar] [CrossRef] [PubMed]
- Grifé-Ruiz, M.; Hierrezuelo-León, J.; de Vicente, A.; Pérez-García, A.; Romero, D. Diversification of lipopeptide analogues drives versatility in biological activities. J. Agric. Food Chem. 2025, 73, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.; Parker, W.L.; Brown, W.E. A nomenclature proposal for the octapeptin antibiotics. J. Antibiot. 1976, 29, 1241–1242. [Google Scholar] [CrossRef]
- Konishi, M.; Sugawara, K.; Tomita, K.; Matsumoto, K.; Miyaki, T.; Fujisawa, K.; Tsukiura, H.; Kawaguchi, H. Bu-2470, a new peptide antibiotic complex. I. Production, isolation and properties of Bu-2470 A, B1 and B2. J. Antibiot. 1983, 36, 625–633. [Google Scholar] [CrossRef]
- Parker, W.L.; Rathnum, M.L. EM49, a new peptide antibiotic IV. The structure of EM49. J. Antibiot. 1975, 28, 379–389. [Google Scholar] [CrossRef][Green Version]
- Sugawara, K.; Yonemoto, T.; Konishi, M.; Matsumoto, K.; Miyaki, T.; Kawaguchi, H. Bu-2470, a new peptide antibiotic complex. II. Structure determination of Bu-2470 A, B1, B2a and B2b. J. Antibiot. 1983, 36, 634–638. [Google Scholar] [CrossRef]
- Han, M.L.; Shen, H.H.; Hansford, K.A.; Schneider, E.K.; Sivanesan, S.; Roberts, K.D.; Thompson, P.E.; Le Brun, A.P.; Zhu, Y.; Sani, M.A.; et al. Investigating the interaction of octapeptin A3 with model bacterial membranes. ACS Infect. Dis. 2017, 3, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.E.; Paddy, M.R.; Dahlquist, F.W.; Storm, D.R. Characterization of octapeptin-membrane interactions using spin-labeled octapeptin. Biochemistry 1980, 19, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, W.; Galla, H.J.; Sackmann, E. Polymyxin binding to charged lipid membranes. An example of cooperative lipid-protein interaction. Biochim. Biophys. Acta 1978, 510, 124–139. [Google Scholar] [CrossRef]
- Santos, D.E.S.; Pol-Fachin, L.; Lins, R.D.; Soares, T.A. Polymyxin Binding to the Bacterial Outer Membrane Reveals Cation Displacement and Increasing Membrane Curvature in Susceptible but Not in Resistant Lipopolysaccharide Chemotypes. J. Chem. Inf. Model. 2017, 57, 2181–2193. [Google Scholar] [CrossRef]
- Bionda, N.; Stawikowski, M.; Stawikowska, R.; Cudic, M.; López-Vallejo, F.; Treitl, D.; Medina-Franco, J.; Cudic, P. Effects of cyclic lipodepsipeptide structural modulation on stability, antibacterial activity, and human cell toxicity. ChemMedChem 2012, 7, 871–882. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, E.Y.; Lee, J.M.; Kim, Y.S.; Bang, E.; Kim, B.S. Site-directed modification of the adenylation domain of the fusaricidin nonribosomal peptide synthetase for enhanced production of fusaricidin analogs. Biotechnol. Lett. 2012, 34, 1327–1334. [Google Scholar] [CrossRef]
- Vater, J.; Niu, B.; Dietel, K.; Borriss, R. Characterization of Novel Fusaricidins Produced by Paenibacillus polymyxa-M1 Using MALDI-TOF Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2015, 26, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, K.; Ohba, K.; Arai, T.; Fukushima, K. New peptide antibiotics LI-F03, F04, F05, F07, and F08, produced by Bacillus polymyxa. I. Isolation and characterization. J. Antibiot. 1987, 40, 1506–1514. [Google Scholar] [CrossRef]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef]
- Kajimura, Y.; Kaneda, M. Fusaricidins B, C and D, New Depsipeptide Antibuotics Produced by Bacillus Polymyxa KT-8: Isolation, Structure Elucidation and Biological Activity. J. Antibiot. 1997, 50, 220–228. [Google Scholar] [CrossRef]
- Yu, C.; Yang, X.; Liang, X.; Song, Y.; Zhu, L.; Xing, S.; Yang, Y.; Gu, Q.; Borriss, R.; Dong, S.; et al. Fusaricidin produced by the rhizobacterium Paenibacillus polymyxa NX20 is involved in the biocontrol of postharvest plant-pathogenic oomycete Phytophthora capsica. Postharvest Biol. Technol. 2023, 205, 112545. [Google Scholar] [CrossRef]
- Yu, W.B.; Yin, C.Y.; Zhou, Y.; Ye, B.C. Prediction of the mechanism of action of fusaricidin on Bacillus subtilis. PLoS ONE 2012, 7, e50003. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, E.; Yuan, C.; Zhang, L. Yousef AE. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012, 78, 3156–3165. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Jokela, J.; Oftedal, L.; Herfindal, L.; Permi, P.; Wahlsten, M.; Døskeland, S.O.; Sivonen, K. Anabaenolysins, novel cytolytic lipopeptides from benthic Anabaena cyanobacteria. PLoS ONE 2012, 7, e41222. [Google Scholar] [CrossRef]
- Horgen, F.D.; Yoshida, W.Y.; Scheuer, P.J. Malevamides A-C, new depsipeptides from the marine cyanobacterium Symploca laete-viridis. J. Nat. Prod. 2000, 63, 461–467. [Google Scholar] [CrossRef]
- Horgen, F.D.; Kazmierski, E.B.; Westenburg, H.E.; Yoshida, W.Y.; Scheuer, P.J. Malevamide D: Isolation and structure determination of an isodolastatin H analogue from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 2002, 65, 487–491. [Google Scholar] [CrossRef]
- von Elert, E.; Oberer, L.; Merkel, P.; Huhn, T.; Blom, J.F. Cyanopeptolin 954, a chlorine-containing chymotrypsin inhibitor of Microcystis aeruginosa NIVA Cya 43. J. Nat. Prod. 2005, 68, 1324–1327. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Döhren, H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat. Prod. 2005, 68, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Schmieder, P.; Seibold, M.; Preussel, K.; von Döhren, H. Hassallidin B—Second antifungal member of the Hassallidin family. Bioorg Med. Chem. Lett. 2006, 16, 4220–4222. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Krunic, A.; Shen, Q.; Swanson, S.M.; Orjala, J. Minutissamides A-D, antiproliferative cyclic decapeptides from the cultured cyanobacterium Anabaena minutissima. J. Nat. Prod. 2011, 74, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.H.; Wray, V.; Nimtz, M.; Fossen, T.; Preisitsch, M.; Schröder, G.; Wende, K.; Heiden, S.E.; Mundt, S. Balticidins A-D, antifungal hassallidin-like lipopeptides from the Baltic Sea cyanobacterium Anabaena cylindrica Bio33. J. Nat. Prod. 2014, 77, 1287–1296. [Google Scholar] [CrossRef]
- Mehner, C.; Müller, D.; Krick, A.; Kehraus, S.; Löser, R.; Gütschow, M.; Maier, A.; Fiebig, H.H.; Brun, R.; König, G.M. A Novel β-Amino Acid in Cytotoxic Peptides from the Cyanobacterium tychonema sp. Eur. J. Org. Chem. 2008, 2008, 1732–1739. [Google Scholar] [CrossRef]
- Vestola, J.; Shishido, T.K.; Jokela, J.; Fewer, D.P.; Aitio, O.; Permi, P.; Wahlsten, M.; Wang, H.; Rouhiainen, L.; Sivonen, K. Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway. Proc. Natl. Acad. Sci. USA 2014, 111, E1909–E1917. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Seibold, M.; Thewes, S.; Laue, M.; Han, C.O.; Hube, B.; von Döhren, H. Comparison of susceptibility and transcription profile of the new antifungal hassallidin A with caspofungin. Biochem. Biophys. Res. Commun. 2006, 349, 740–749. [Google Scholar] [CrossRef]
- Oftedal, L.; Myhren, L.; Jokela, J.; Gausdal, G.; Sivonen, K.; Døskeland, S.O.; Herfindal, L. The lipopeptide toxins anabaenolysin A and B target biological membranes in a cholesterol-dependent manner. Biochim. Biophys. Acta 2012, 1818, 3000–3009. [Google Scholar] [CrossRef]
- Shishido, T.K.; Jokela, J.; Kolehmainen, C.T.; Fewer, D.P.; Wahlsten, M.; Wang, H.; Rouhiainen, L.; Rizzi, E.; De Bellis, G.; Permi, P.; et al. Antifungal activity improved by coproduction of cyclodextrins and anabaenolysins in Cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 13669–13674. [Google Scholar] [CrossRef]
- Gregson, J.M.; Chen, J.-L.; Patterson, G.M.L.; Moore, R.E. Structures of puwainaphycins A–E. Tetrahedron 1992, 48, 3727–3734. [Google Scholar] [CrossRef]
- Hrouzek, P.; Kuzma, M.; Černý, J.; Novák, P.; Fišer, R.; Simek, P.; Lukešová, A.; Kopecký, J. The cyanobacterial cyclic lipopeptides puwainaphycins F/G are inducing necrosis via cell membrane permeabilization and subsequent unusual actin relocalization. Chem. Res. Toxicol. 2012, 25, 1203–1211. [Google Scholar] [CrossRef]
- Kang, H.S.; Sturdy, M.; Krunic, A.; Kim, H.; Shen, Q.; Swanson, S.M.; Orjala, J. Minutissamides E-L, antiproliferative cyclic lipodecapeptides from the cultured freshwater cyanobacterium cf. Anabaena sp. Bioorg Med. Chem. 2012, 20, 6134–6143. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Heinilä, L.; Aesoy, R.; Sivonen, K.; Galica, T.; Hrouzek, P.; Herfindal, L. Chemical diversity and cellular effects of antifungal cyclic lipopeptides from cyanobacteria. Physiol. Plant. 2021, 173, 639–650. [Google Scholar] [CrossRef]
- Mareš, J.; Hájek, J.; Urajová, P.; Kust, A.; Jokela, J.; Saurav, K.; Galica, T.; Čapková, K.; Mattila, A.; Haapaniemi, E.; et al. Alternative biosynthetic starter units enhance the structural diversity of cyanobacterial lipopeptides. Appl. Environ. Microbiol. 2019, 85, e02675-18. [Google Scholar] [CrossRef]
- Saurav, K.; Caso, A.; Urajová, P.; Hrouzek, P.; Esposito, G.; Delawská, K.; Macho, M.; Hájek, J.; Cheel, J.; Saha, S.; et al. Fatty acid substitutions modulate the cytotoxicity of puwainaphycins/minutissamides isolated from the Baltic sea cyanobacterium Nodularia harveyana UHCC-0300. ACS Omega 2022, 7, 11818–11828. [Google Scholar] [CrossRef]
- Tomek, P.; Hrouzek, P.; Kuzma, M.; Sýkora, J.; Fiser, R.; Cerný, J.; Novák, P.; Bártová, S.; Simek, P.; Hof, M.; et al. Cytotoxic lipopeptide muscotoxin A, isolated from soil cyanobacterium Desmonostoc muscorum, permeabilizes phospholipid membranes by reducing their fluidity. Chem. Res. Toxicol. 2015, 28, 216–224. [Google Scholar] [CrossRef]
- Pancrace, C.; Jokela, J.; Sassoon, N.; Ganneau, C.; Desnos-Ollivier, M.; Wahlsten, M.; Humisto, A.; Calteau, A.; Bay, S.; Fewer, D.P.; et al. Rearranged biosynthetic gene cluster and synthesis of hassallidin E in planktothrix serta PCC 8927. ACS Chem. Biol. 2017, 12, 1796–1804. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Morphological changes of Candida albicans induced by micafungin (FK463), a water-soluble echinocandin-like lipopeptide. J. Electron. Microsc. 2002, 51, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Cheel, J.; Hájek, J.; Kuzma, M.; Saurav, K.; Smýkalová, I.; Ondráčková, E.; Urajová, P.; Vu, D.L.; Faure, K.; Kopecký, J.; et al. Application of HPCCC combined with polymeric resins and HPLC for the separation of cyclic lipopeptides muscotoxins A–C and their antimicrobial activity. Molecules 2018, 23, 2653. [Google Scholar] [CrossRef] [PubMed]

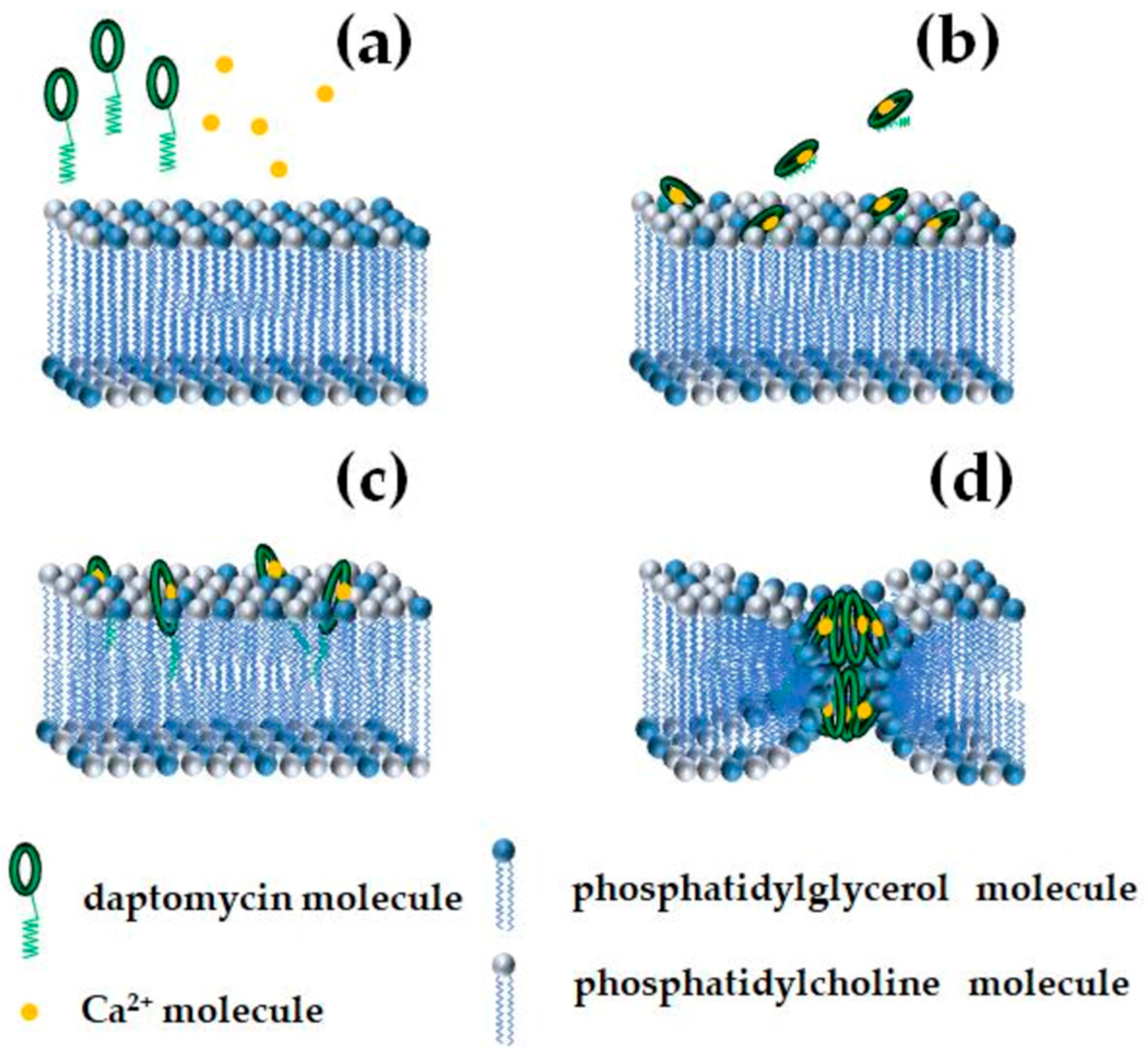

| CLPs | Lipid Composition | C, µM | The Amplitude of Ion-Permeable Pores | m | Reference |
|---|---|---|---|---|---|
| Pseudomonas spp | |||||
| syringomycins | DOPE/DOPS/DOPC/cholesterol (3/1.5/0.5/5) | 0.4–1.6 | 1 pA at 80 mV | ND | [24] |
| DOPS | 0.8–4.1 | 0.7 pA at 100 mV | 5–7 | [25] | |
| DOPC | 0.8–2.5 | −0.8 pA at −100 mV; 1.2 pA at 100 mV | ND | [26] | |
| DPhPC | 10 | −1.5 pA at −180 mV | ND | [27] | |
| red blood cells | ND | ND | 5–7 | [27] | |
| DOPC/sterols (cholesterol, ergosterol, or stigmasterol) (1/1) | ND | ND | 5–7 | [28] | |
| DOPE/DOPS/sterols (cholesterol, ergosterol, or stigmasterol) (1/1/2) | 2.5 | −2.6 pA at −100 mV −12 pA at −200 mV | ND | [29] | |
| syringotoxins | DOPC | 7.9–14.1 | −1.4 pA at −100 mV; 1.6 pA at 100 mV | ND | [26] |
| phospholipids isolated from soybeans | 0.7 | 2 pA at 10 mV | ND | [30] | |
| red blood cells | ND | ND | 4–6 | [27] | |
| ND | ND | 2–6 | [31] | ||
| syringostatins | DOPC | 1.4–2.1 | −1.4 pA at −100 mV; 1.9 pA at 100 mV | ND | [26] |
| DOPE/DOPS (1/1) | 0.6–0.9 | 0.9 pA at 150 mV | ND | [32] | |
| pseudodesmins | DOPE/DOPG/CL (7/2/1) | 3.7 | ND | ND | [33] |
| DOPE/DOPG (3/7) | 0.17 | ND | ND | [34] | |
| viscosins | DOPE/DOPG/CL (7/2/1) | 5.0 | ND | ND | [33] |
| DOPE/DOPG (3/7) | 0.1 | ND | ND | [34] | |
| viscosinamides | DOPE/DOPG/CL (7/2/1) | 3.6 | ND | ND | [33] |
| WLIP | DOPE/DOPG/CL (7/2/1) | 4.7 | ND | ND | [33] |
| red blood cells | ND | ND | 6–10 | [35] | |
| cormycin A | DPhPC/cholesterol (7/3) | <0.05 | −0.6 pA at −150 mV | 6–8 | [36] |
| syringopeptin 22A | DOPE/DOPS (1/1) | 0.1 | 0.2 pA at 100 mV 0.3 pA at −100 mV | 2–4 | [37] |
| DOPC | 0.1 | ND | ND | [38] | |
| syringopeptin 22B | DOPE | 2.3 | 1–1.5 pA at 80 mV | ND | [39] |
| syringopeptin 25A | DOPC/DOPE/DOPS (2/2/1) | 0.004 | −5.5 pA at −140 mV 4.1 pA at 140 mV | 4–5 | [28] |
| DOPC/sterols (cholesterol, ergosterol, or stigmasterol) (1/1) | ND | ND | 4–6 | [28] | |
| asolectin | 0.0125 | 2.2 pA at 140 mV −6.3 pA at −140 mV | ND | [40] | |
| DOPC | 0.1 | ND | ND | [38] | |
| tolaasin I | POPE | 0.3 | 5 pA at 20 mV | ND | [41] |
| PS/PE (1/1) | 0.0159 | 7–12 pA 40 mV | ND | [42] | |
| ND | ND | ND | 6–8 | [43] | |
| red blood cells | ND | ND | 5–7 | [35] | |
| fuscopeptins A | POPC | 0.040 | ND | ND | [44] |
| fuscopeptins B | POPC | 0.003–0.01 | −0.4 pA at −140 mV 1.3 pA at 140 mV | ND | [44] |
| entolysin B | POPC/DOPG (9/1), POPC/DOPG/ergosterol (6/1/3; 4/1/5) | 0.5 | ND | ND | [45] |
| Streptomyces spp. | |||||
| daptomycin | DPhPG | 6.2 | 5 pA at 100 mV | ND | [46] |
| DMPC/DMPG (9/1) | ND | ND | 8 | [47] | |
| calcium-dependent antibiotics | egg lecithin/cholesterol (2/1) | 3 v % | 5 pA at 50 mV | ND | [48] |
| Bacillus spp. | |||||
| surfactin | GMO | 1.4 | 0.5–7 pA at 50 mV | 2 | [49] |
| DPhPC | 0.2–0.4 | 2–240 pA at 25 mV | ND | [50] | |
| iturin | egg lecithin | 0.0001 | 0.7–5 pA at 100 mV | ND | [51] |
| GMO | ND | 0.6–3 pA at 100 mV | 2 | [52] | |
| fengycin | POPC/POPE/POPG/ergosterol (2/2/5/1) | 0.0001–0.0005 | 1 pA at 150 mV | 2–3 | [53] |
| polymyxin B | DOPC/DOPG (1/1) | 5 | 1–5 pA at 50 mV | 2–3 | [54] |
| DOPC/DOPG/Kdo2-Lipid A (1/1/0.02) | 1 | 1–5 pA at 50 mV | 5–7 | ||
| fusaricidins A + B | azolectin | 0.6 | 30 pA at 60 mV | ND | [55] |
| fusaricidins A + B | mitochondrial inner membrane | 11.8 | ND | ND | [56] |
| Cyanobacteria | |||||
| puwainaphycins F | DOPC/DOPE (2/1) | 5 | 500 pA at 50 mV | ND | [57] |
| minutissamid A | DOPC/DOPE (2/1) | 10 | 10 pA at 50 mV | ND | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. State of the Art of Cyclic Lipopeptide–Membrane Interactions: Pore Formation and Bilayer Permeability. Pharmaceutics 2025, 17, 1142. https://doi.org/10.3390/pharmaceutics17091142
Zakharova AA, Efimova SS, Ostroumova OS. State of the Art of Cyclic Lipopeptide–Membrane Interactions: Pore Formation and Bilayer Permeability. Pharmaceutics. 2025; 17(9):1142. https://doi.org/10.3390/pharmaceutics17091142
Chicago/Turabian StyleZakharova, Anastasiia A., Svetlana S. Efimova, and Olga S. Ostroumova. 2025. "State of the Art of Cyclic Lipopeptide–Membrane Interactions: Pore Formation and Bilayer Permeability" Pharmaceutics 17, no. 9: 1142. https://doi.org/10.3390/pharmaceutics17091142
APA StyleZakharova, A. A., Efimova, S. S., & Ostroumova, O. S. (2025). State of the Art of Cyclic Lipopeptide–Membrane Interactions: Pore Formation and Bilayer Permeability. Pharmaceutics, 17(9), 1142. https://doi.org/10.3390/pharmaceutics17091142






