A New Approach to the Diagnosis and Treatment of Cardiovascular Diseases
Abstract
1. Introduction
2. Pathophysiology of Atherosclerosis
3. Perspectives and Possibilities of Using Nanomedicine in the Context of Cardiovascular Diseases
4. Nanotechnology in the Diagnosis of Cardiovascular Diseases
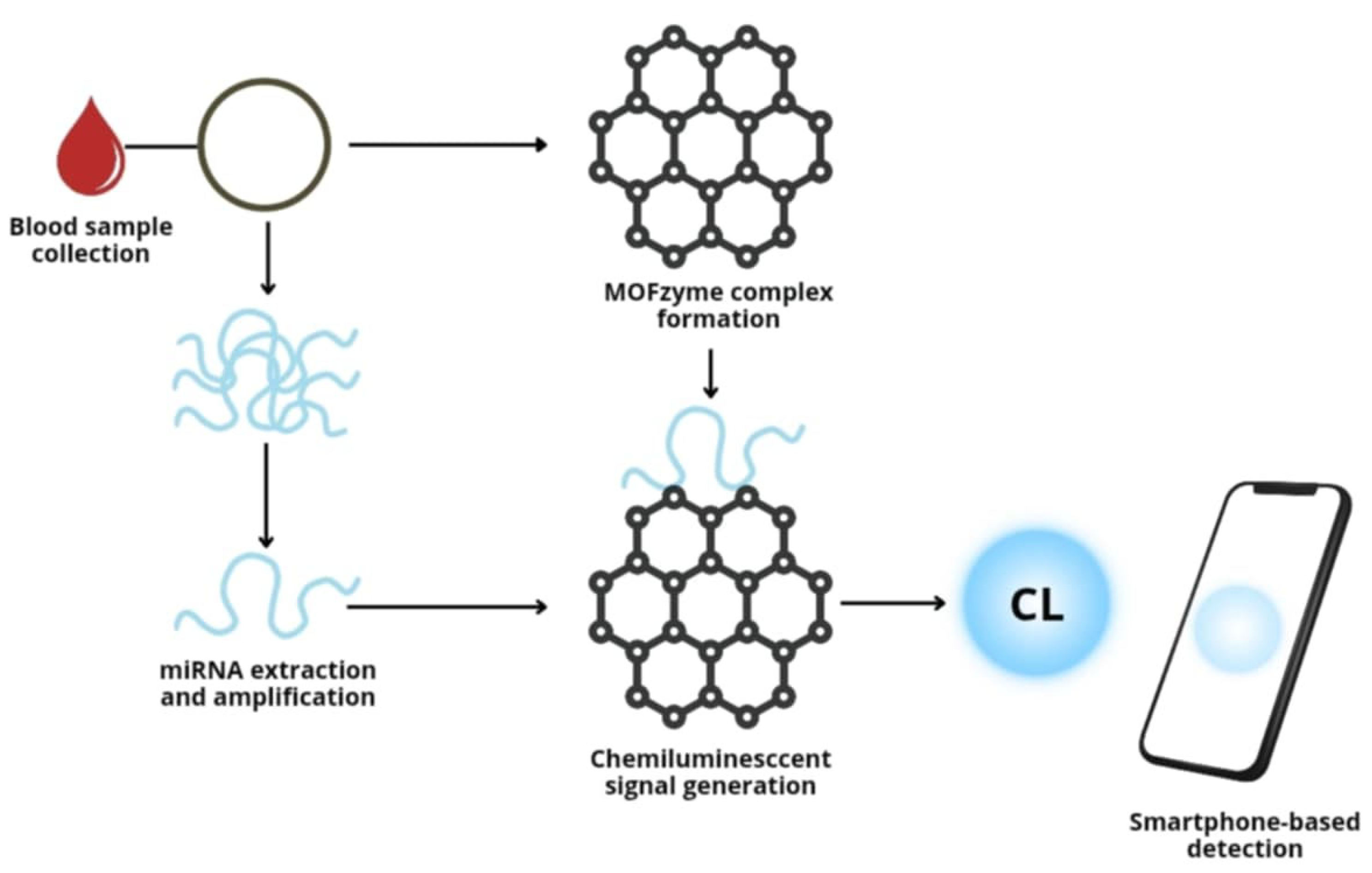
4.1. Detection of Biomarkers of Cardiovascular Diseases
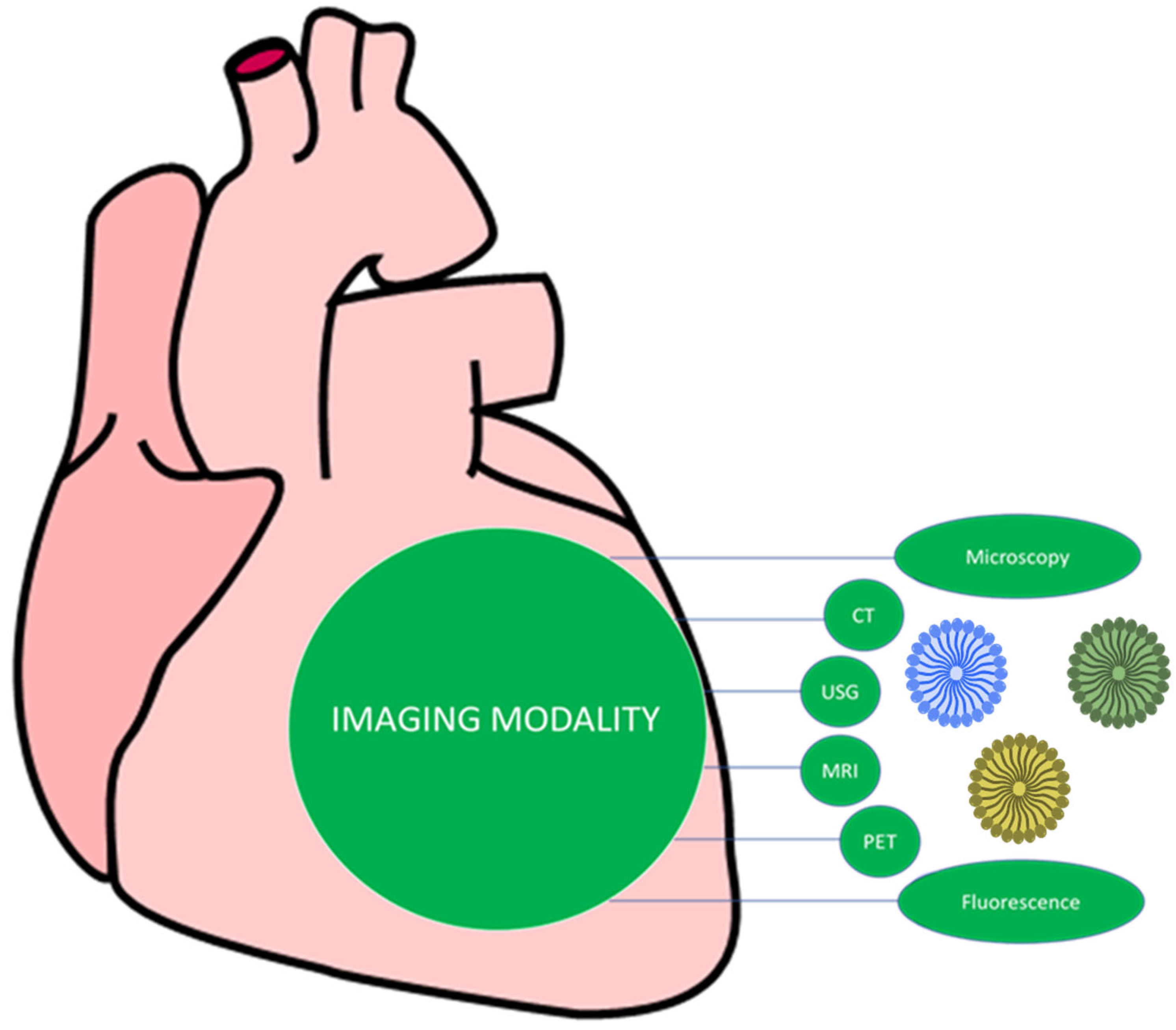
4.2. Imaging Modality for Nanotechnology Used in CVD
4.2.1. Nanoparticles in Magnetic Resonance Imaging
4.2.2. Nanoparticles in Radionuclide-Based Molecular Imaging
4.2.3. Nanoparticles in Optical Imaging
4.2.4. Nanoparticles in Multimodal Imaging
5. Nanoparticles in the Modern Treatment of Circulatory System Diseases
5.1. Nanotechnology in Inhibiting Inflammation in the Atherosclerotic Plaque Microenvironment
5.2. Nanocarriers Transport
5.3. The Interaction Between Nanoparticles and Immune System
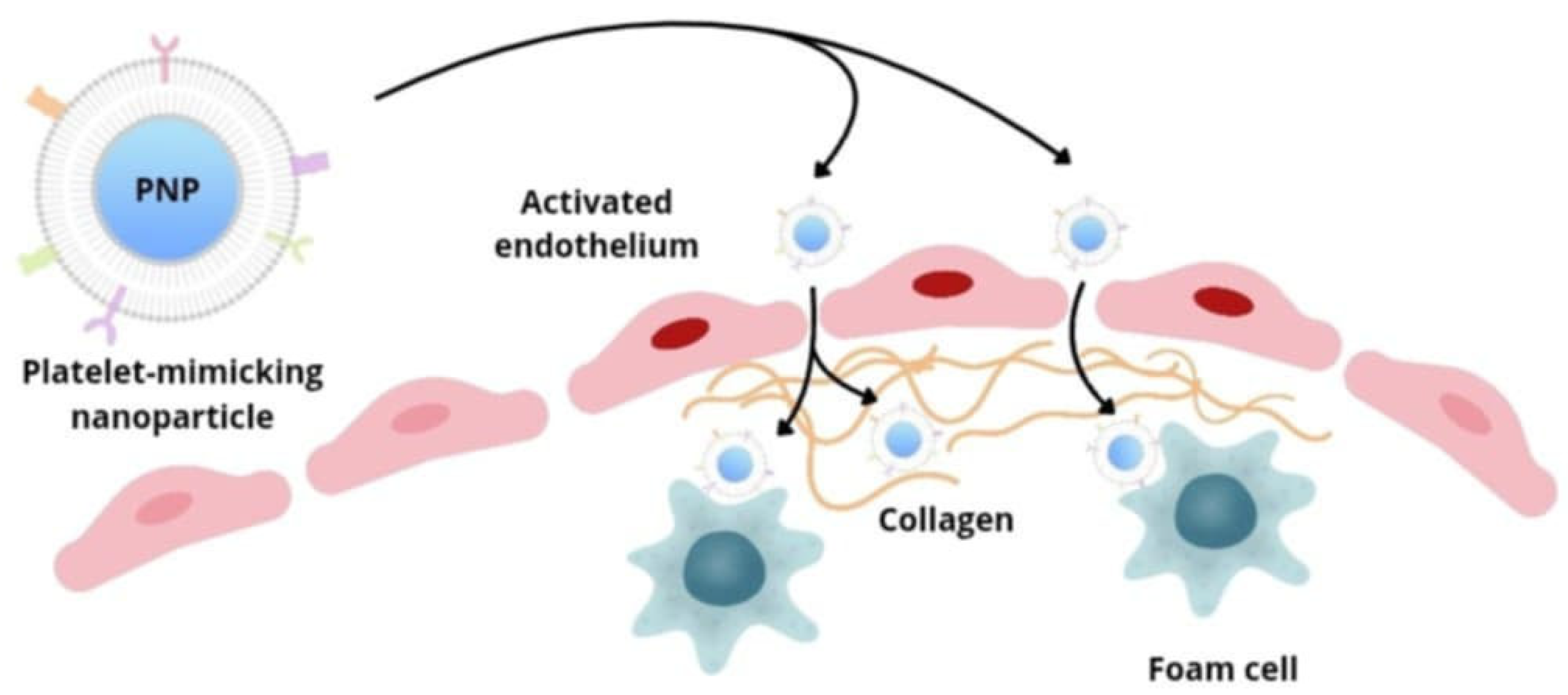
5.4. Nanotechnology in Vascular Treatment
5.5. Nucleic Acid Transporting Nanoparticles
5.5.1. siRNA-Based Therapies
5.5.2. miRNA-Based Therapies
6. Nanocarriers with Therapeutic Properties
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ec.europa.eu. Available online: https://ec.europa.eu/eurostat/statistics-explained/ (accessed on 23 May 2025).
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innovation 2022, 3, 100214. [Google Scholar] [CrossRef]
- Gomez Cardoso, A.; Rahin Ahmed, S.; Keshavarz-Motamed, Z.; Srinivasan, S.; Reza Rajabzadeh, A. Recent advancements of nanomodified electrodes—Towards point-of-care detection of cardiac biomarkers. Bioelectrochemistry 2023, 152, 108440. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, D.; Barman, S.; Ranjan, R.; Stone, H. A Systematic Review of Major Cardiovascular Risk Factors: A Growing Global Health Concern. Cureus 2022, 14, e30119. [Google Scholar] [CrossRef]
- Manners, N.; Priya, V.; Mehata, A.K.; Rawat, M.; Mohan, S.; Makeen, H.A.; Albratty, M.; Albarrati, A.; Meraya, A.M.; Muthu, M.S. Theranostic Nanomedicines for the Treatment of Cardiovascular and Related Diseases: Current Strategies and Future Perspectives. Pharmaceuticals 2022, 15, 441. [Google Scholar] [CrossRef]
- Ou, L.C.; Zhong, S.; Ou, J.S.; Tian, J.W. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol. Sin. 2021, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef]
- Stone, N.J.; Smith, S.C., Jr.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Tu, L.; Zou, Z.; Yang, Y.; Wang, S.; Xing, B.; Feng, J.; Jin, Y.; Cheng, M. Targeted drug delivery systems for atherosclerosis. J. Nanobiotechnol. 2025, 23, 306. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef]
- Colombo, I.; Koster, K.L.; Holer, L.; Haefliger, S.; Rabaglio, M.; Bastian, S.; Schwitter, M.; Eckhardt, K.; Hayoz, S.; Mc Laughlin, A.M.; et al. TLD-1, a novel liposomal doxorubicin, in patients with advanced solid tumors: Dose escalation and expansion part of a multicenter open-label phase I trial (SAKK 65/16). Eur. J. Cancer 2024, 201, 113588. [Google Scholar] [CrossRef]
- Ali, F.; Arshad, K.; Szpunar, S.; Daher, E. Elevated Troponins and Diagnosis of Non-ST-Elevation Myocardial Infarction in the Emergency Department. Cureus 2024, 16, e59910. [Google Scholar] [CrossRef]
- Prajapati, S.; Padhan, B.; Amulyasai, B.; Sarkar, A. Nanotechnology-Based Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Liyanage, T.; Sangha, A.; Sardar, R. Achieving biosensing at attomolar concentrations of cardiac troponin T in human biofluids by developing a label-free nanoplasmonic analytical assay. Analyst 2017, 142, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Kang, M.; Huang, X.; Liu, Y.; Zhou, N.; Zhang, Z. Nanodiamonds and hydrogen-substituted graphdiyne heteronanostructure for the sensitive impedimetric aptasensing of myocardial infarction and cardiac troponin I. Anal. Chim. Acta 2021, 1141, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Sun, Y.; Shi, L.; Li, T. Hemin-Bridged MOF Interface with Double Amplification of G-Quadruplex Payload and DNAzyme Catalysis: Ultrasensitive Lasting Chemiluminescence MicroRNA Imaging. ACS Appl. Mater. Interfaces 2020, 12, 7879–7887. [Google Scholar] [CrossRef]
- Zhu, C.; Sadat, U.; Patterson, A.J.; Teng, Z.; Gillard, J.H.; Graves, M.J. 3D high-resolution contrast enhanced MRI of carotid atheroma--a technical update. Magn. Reson. Imaging 2014, 32, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Luehmann, H.P.; Pressly, E.D.; Detering, L.; Wang, C.; Pierce, R.; Woodard, P.K.; Gropler, R.J.; Hawker, C.J.; Liu, Y. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 629–634. [Google Scholar] [CrossRef]
- Woodard, P.K.; Liu, Y.; Pressly, E.D.; Luehmann, H.P.; Detering, L.; Sultan, D.E.; Laforest, R.; McGrath, A.J.; Gropler, R.J.; Hawker, C.J. Design and Modular Construction of a Polymeric Nanoparticle for Targeted Atherosclerosis Positron Emission Tomography Imaging: A Story of 25% (64)Cu-CANF-Comb. Pharm. Res. 2016, 33, 2400–2410. [Google Scholar] [CrossRef]
- Douma, K.; Megens, R.T.; van Zandvoort, M.A. Optical molecular imaging of atherosclerosis using nanoparticles: Shedding new light on the darkness. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibáñez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated with Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef]
- Beldman, T.J.; Senders, M.L.; Alaarg, A.; Pérez-Medina, C.; Tang, J.; Zhao, Y.; Fay, F.; Deichmöller, J.; Born, B.; Desclos, E.; et al. Hyaluronan Nanoparticles Selectively Target Plaque-Associated Macrophages and Improve Plaque Stability in Atherosclerosis. ACS Nano 2017, 11, 5785–5799. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef]
- Mai, W.; Liao, Y. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar] [CrossRef]
- Song, P.; Yang, C.; Thomsen, J.S.; Dagnæs-Hansen, F.; Jakobsen, M.; Brüel, A.; Deleuran, B.; Kjems, J. Lipidoid-siRNA Nanoparticle-Mediated IL-1β Gene Silencing for Systemic Arthritis Therapy in a Mouse Model. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, C.; You, T.; Zhang, X.; Su, H.; Cao, B.; Anwaier, S.; Xiang, H.; Dai, C.; Long, X.; et al. Injection of ROS-Responsive Hydrogel Loaded with IL-1β-targeted nanobody for ameliorating myocardial infarction. Bioact. Mater. 2024, 46, 273–284. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Banach, M.; Sirtori, C.R.; Corsini, A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2023, 118, 3288–3304. [Google Scholar] [CrossRef]
- Hossaini Nasr, S.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, S.; Kang, D.W.; Jo, H.; Park, J.H. Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy. ACS Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Kurdi, A.; Martinet, W.; De Meyer, G.R.Y. mTOR Inhibition and Cardiovascular Diseases: Dyslipidemia and Atherosclerosis. Transplantation 2018, 102, S44–S46. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Gao, P.; Chen, W.; Zhou, Q. Construction of dual nanomedicines for the imaging and alleviation of atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chekman, I.S.; Simonov, P.V. Structure and function of biological membranes: The impact of nanoparticles. Int. J. Phys. Pathophys. 2012, 3, 187–208. [Google Scholar] [CrossRef]
- Boada, C.; Zinger, A.; Tsao, C.; Zhao, P.; Martinez, J.O.; Hartman, K.; Naoi, T.; Sukhoveshin, R.; Sushnitha, M.; Molinaro, R.; et al. Rapamycin-Loaded Biomimetic Nanoparticles Reverse Vascular Inflammation. Circ. Res. 2020, 126, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Nanoimmunotherapy targeting CD40-TRAF6 signalling to reduce atherosclerosis. Nat. Rev. Cardiol. 2018, 15, 317. [Google Scholar] [CrossRef]
- Seijkens, T.T.P.; van Tiel, C.M.; Kusters, P.J.H.; Atzler, D.; Soehnlein, O.; Zarzycka, B.; Aarts, S.A.B.M.; Lameijer, M.; Gijbels, M.J.; Beckers, L.; et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 527–542. [Google Scholar] [CrossRef]
- Lameijer, M.; Binderup, T.; van Leent, M.M.T.; Senders, M.L.; Fay, F.; Malkus, J.; Sanchez-Gaytan, B.L.; Teunissen, A.J.P.; Karakatsanis, N.; Robson, P.; et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2018, 2, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Yang, X.; Wei, J.; He, Y.; Jing, T.; Li, Y.; Xiao, Y.; Wang, B.; Wang, W.; Zhang, J.; Lin, R. SIRT1 inhibition promotes atherosclerosis through impaired autophagy. Oncotarget 2017, 8, 51447–51461. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhou, Y.; Chen, L.; Wang, X.; Long, C.Y.; Pi, Y.; Gao, C.Y.; Li, J.C.; Zhang, L.L. SIRT1 improves VSMC functions in atherosclerosis. Prog. Biophys. Mol. Biol. 2016, 121, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017, 7, 3541. [Google Scholar] [CrossRef]
- Sun, X.; Kong, B.; Wang, W.; Chandran, P.; Selomulya, C.; Zhang, H.; Zhu, K.; Liu, Y.; Yang, W.; Guo, C.; et al. Mesoporous silica nanoparticles for glutathione-triggered long-range and stable release of hydrogen sulfide. J. Mater. Chem. B 2015, 3, 4451–4457. [Google Scholar] [CrossRef]
- Xia, M.; Wu, Q.; Chen, P.; Qian, C. Regulatory T Cell-Related Gene Biomarkers in the Deterioration of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 661709. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sadoshima, J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc. Med. 2011, 21, 27–32. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Delivering nitric oxide with nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2015, 205, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.S.; Sun, W.; Misener, S.; Wang, J.J.; Zhang, Z.J.; Kibbe, M.R.; Dravid, V.P.; Venkatraman, S.; Thaxton, C.S. Nitric Oxide-Delivering High-Density Lipoprotein-like Nanoparticles as a Biomimetic Nanotherapy for Vascular Diseases. ACS Appl. Mater. Interfaces 2018, 10, 6904–6916. [Google Scholar] [CrossRef]
- Dongó, E.; Hornyák, I.; Benko, Z.; Kiss, L. The cardioprotective potential of hydrogen sulfide in myocardial ischemia/reperfusion injury (review). Acta Physiol. Hung. 2011, 98, 369–381. [Google Scholar] [CrossRef]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Sarzani, R.; Spannella, F.; Di Pentima, C.; Giulietti, F.; Landolfo, M.; Allevi, M. Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension. Int. J. Mol. Sci. 2023, 25, 328. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, S.; Pan, Y.; Zhi, W.; Gu, C.; Guo, T.; Zhai, J.; Li, C.; Chen, Y.Q.; Wang, R. Development, opportunities, and challenges of siRNA nucleic acid drugs. Mol. Ther. Nucleic Acids 2024, 36, 102437. [Google Scholar] [CrossRef]
- Sumara, G.; Belwal, M.; Ricci, R. “Jnking” atherosclerosis. Cell. Mol. Life Sci. CMLS 2005, 62, 2487–2494. [Google Scholar] [CrossRef]
- Pan, H.; Palekar, R.U.; Hou, K.K.; Bacon, J.; Yan, H.; Springer, L.E.; Akk, A.; Yang, L.; Miller, M.J.; Pham, C.T.; et al. Anti-JNK2 peptide-siRNA nanostructures improve plaque endothelium and reduce thrombotic risk in atherosclerotic mice. Int. J. Nanomed. 2018, 13, 5187–5205. [Google Scholar] [CrossRef]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef]
- Sager, H.B.; Dutta, P.; Dahlman, J.E.; Hulsmans, M.; Courties, G.; Sun, Y.; Heidt, T.; Vinegoni, C.; Borodovsky, A.; Fitzgerald, K.; et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 2016, 8, 342ra80. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Doran, A.C.; Ozcan, L.; Cai, B.; Zheng, Z.; Fredman, G.; Rymond, C.C.; Dorweiler, B.; Sluimer, J.C.; Hsieh, J.; Kuriakose, G.; et al. CAMKIIγ suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J. Clin. Investig. 2017, 127, 4075–4089. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kim, J.H.; Oh, G.T.; Lee, B.H.; Kwon, I.C.; Kim, I.S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J. Control. Release Off. J. Control. Release Soc. 2011, 155, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yurdagul, A.; Jr Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Yin, K.; Liao, D.F.; Tang, C.K. ATP-binding membrane cassette transporter A1 (ABCA1): A possible link between inflammation and reverse cholesterol transport. Mol. Med. 2010, 16, 438–449. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.W.; Zeng, P.H.; Zhou, Y.J.; Yin, W.J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 2022, 21, 1121–1139. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Charo, I.F.; Peters, W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation 2003, 10, 259–264. [Google Scholar] [CrossRef]
- Patel, N.; Chin, D.D.; Magee, G.A.; Chung, E.J. Therapeutic Response of miR-145 Micelles on Patient-Derived Vascular Smooth Muscle Cells. Front. Digit. Health 2022, 4, 836579. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Met. Integr. Biometal Sci. 2017, 9, 21–37. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Jia, N.; Qiao, H.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Synthesis and evaluation of a novel water-soluble high Se-enriched Astragalus polysaccharide nanoparticles. Int. J. Biol. Macromol. 2012, 24, 1418–1423. [Google Scholar] [CrossRef]
- Mali, S.B.; Dahivelkar, S.D.; Mahale, S.A. Nanotechnology in photodynamic therapy. Oral Oncol. Rep. 2024, 10, 100307, Erratum in Oral Oncol. Rep. 2025, 10, 100307. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L. The Current Status of Chlorin e6-Based Nanoscale Delivery Systems for Cancer Therapy. Oncologie 2021, 23, 515–531. [Google Scholar] [CrossRef]
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017, 8, e2864. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, Y.; Cai, M.; Zhao, Y.; Cao, W.; Liu, Z.; Cui, G.; Tang, B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 2018, 9, 231. [Google Scholar] [CrossRef]
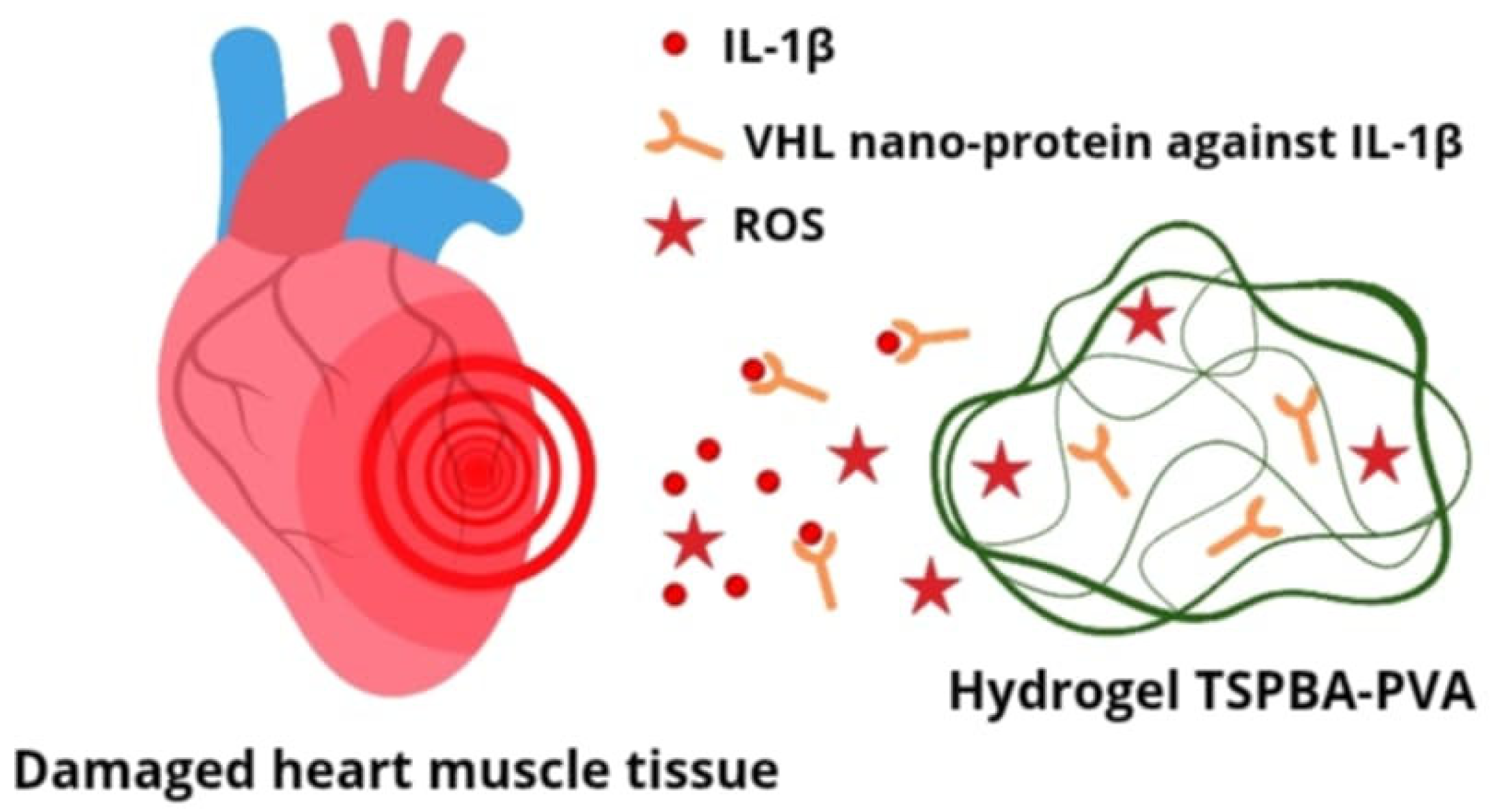
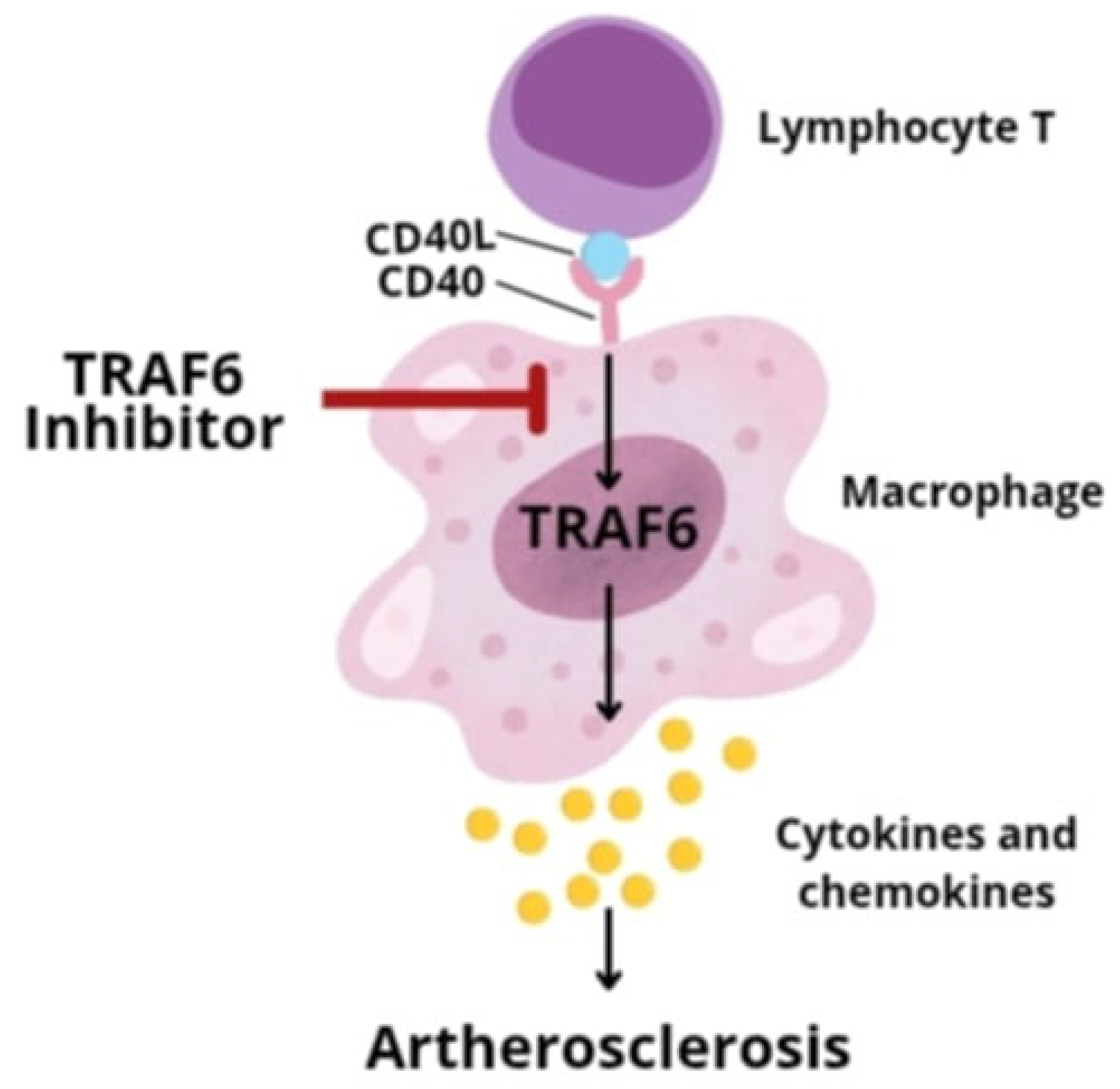
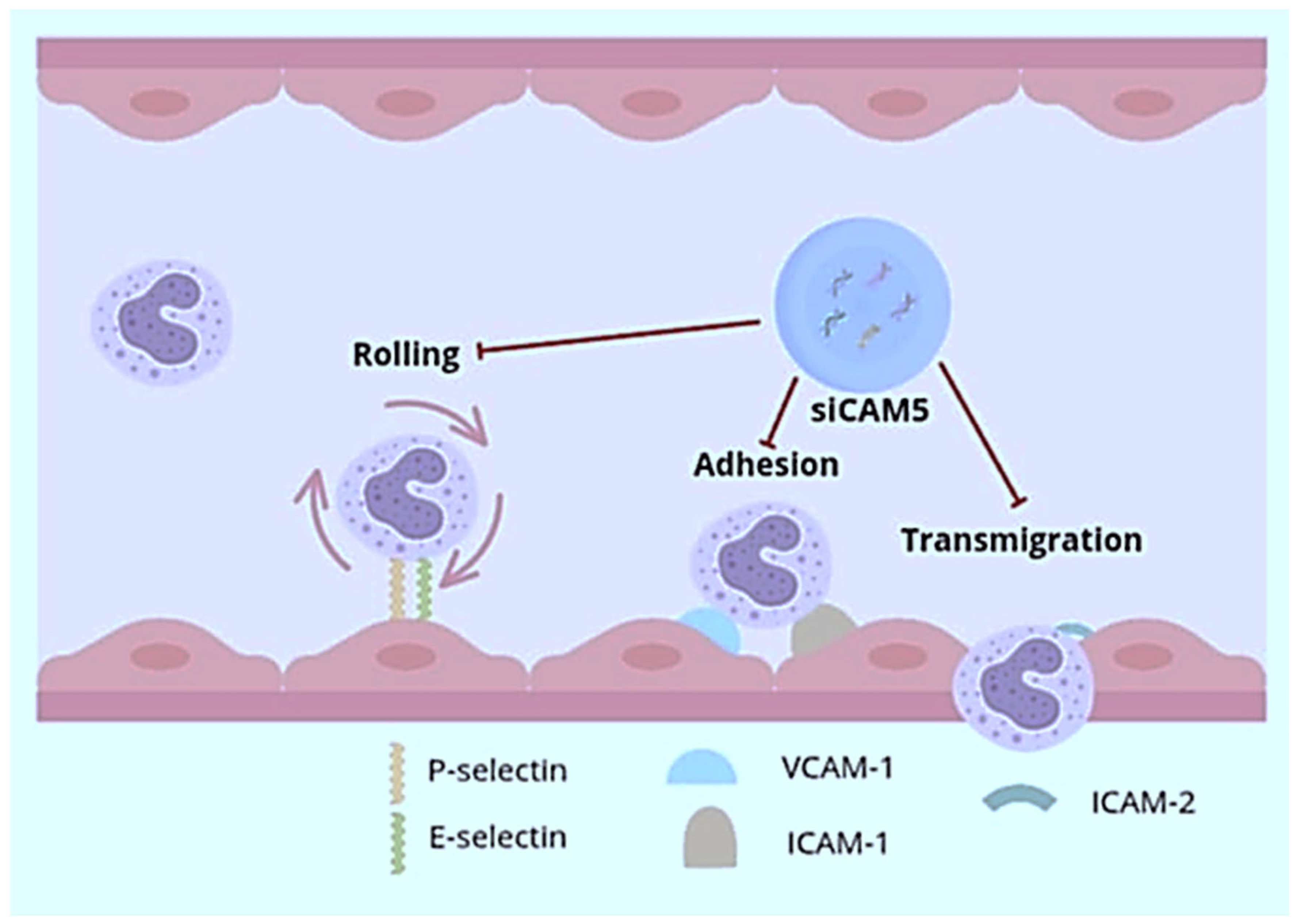
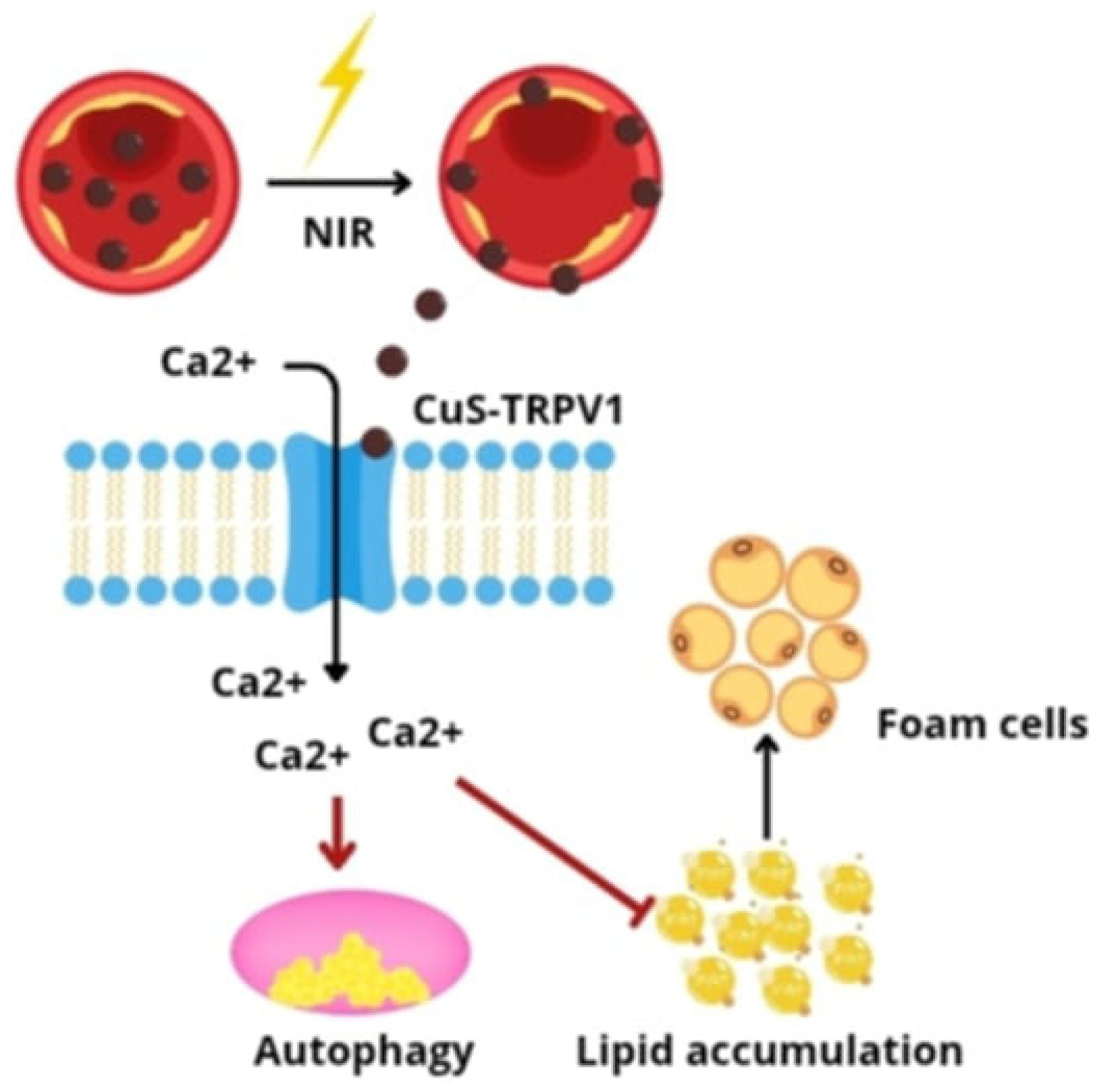
| Advantages | Disadvantages |
|---|---|
| Increased solubility of highly lipophilic drugs | Lack of proper knowledge about the effect of nanoparticles on biochemical pathway and processes in human body |
| Tunable physical and chemical properties | Unpredictable genotoxicity due to insufficient toxicological assessment studies |
| Targeted drug delivery | Carcinogenesis |
| Drug release in a sustained and controllable manner | Elimination and metabolism vary with different types of materials used in nanoparticles synthesis |
| Good biocompatibility and bioavailability | More expensive |
| Imaging Method | Advantages | Disadvantages |
|---|---|---|
| MRI (magnetic resonance imaging) | high spatial resolution | low sensitivity |
| NIFR (nuclear–infrared fluorescence) | high sensitivity low background signal low cost | insufficient tissue penetration depth |
| PET (positron emission tomography) | very high sensitivity | low spatial resolution exposure to ionizing radiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Kotlińska, A.; Koszarska, K.; Aebisher, D. A New Approach to the Diagnosis and Treatment of Cardiovascular Diseases. Pharmaceutics 2025, 17, 1141. https://doi.org/10.3390/pharmaceutics17091141
Bartusik-Aebisher D, Kotlińska A, Koszarska K, Aebisher D. A New Approach to the Diagnosis and Treatment of Cardiovascular Diseases. Pharmaceutics. 2025; 17(9):1141. https://doi.org/10.3390/pharmaceutics17091141
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Aleksandra Kotlińska, Katarzyna Koszarska, and David Aebisher. 2025. "A New Approach to the Diagnosis and Treatment of Cardiovascular Diseases" Pharmaceutics 17, no. 9: 1141. https://doi.org/10.3390/pharmaceutics17091141
APA StyleBartusik-Aebisher, D., Kotlińska, A., Koszarska, K., & Aebisher, D. (2025). A New Approach to the Diagnosis and Treatment of Cardiovascular Diseases. Pharmaceutics, 17(9), 1141. https://doi.org/10.3390/pharmaceutics17091141








