1. Introduction
Localized scleroderma (LoS) is a rare and complex autoimmune connective tissue disorder characterized by cutaneous fibrosis and chronic inflammation, with an estimated prevalence of fewer than six cases per 100,000 individuals and substantial associated morbidity [
1,
2]. The pathogenesis of LoS involves vasculopathy and dysregulation of both innate and adaptive immune responses, ultimately leading to excessive inflammatory cell infiltration and aberrant extracellular matrix (ECM) deposition [
3]. Clinically, LoS progresses through three distinct phases: the edematous, fibrotic, and atrophic stages [
4]. Current therapeutic strategies primarily rely on immunosuppressive agents such as glucocorticoids and methotrexate; however, their clinical efficacy is limited, and long-term use is associated with significant adverse effects [
5]. Fat grafting has emerged as a promising alternative therapy for LoS, offering multiple benefits including anti-inflammatory activity, attenuation of pathological ECM accumulation, and restoration of soft tissue volume [
6]. Clinical studies have reported an efficacy rate of 87.3% for fat grafting in LoS patients, highlighting its substantial potential to improve clinical outcomes [
7].
The therapeutic effects of fat grafting are mediated by its cellular components, including adipocytes, stromal vascular fraction (SVF), and adipose-derived mesenchymal stem cells (ADSCs). Dedifferentiated and redifferentiated adipocytes contribute to subcutaneous fat regeneration and reversal of skin fibrosis [
8]. The SVF contains a heterogeneous population of cells that secrete paracrine factors with pro-angiogenic and anti-inflammatory properties [
9]. In addition, ADSCs have demonstrated strong anti-fibrotic and immunosuppressive effects in models of skin fibrosis, evidenced by reduced immune cell infiltration and downregulation of fibrogenic markers [
10]. Our previous work identified cell-free fat extract (CEFFE), derived from adipose tissue, as a protein-rich product capable of modulating inflammation and promoting tissue repair [
11,
12,
13]. Studies in multiple animal models and clinical trials suggest that CEFFE mimics the paracrine activity of stem cells [
14,
15,
16,
17,
18]. For example, clinical evaluation of CEFFE in post-inflammatory hyperpigmentation secondary to radiation exposure demonstrated improved skin texture, supporting its anti-inflammatory and anti-fibrotic properties [
19]. However, CEFFE is a complex liquid mixture containing more than 1700 protein components, which poses a challenge for therapeutic standardization. To address this, we employed protein fractionation and bioactivity-guided screening to identify annexin A5 (AnxA5) as a key anti-inflammatory component of CEFFE [
20].
Annexin A5 (AnxA5) is a calcium-dependent phospholipid-binding protein that interacts with negatively charged phospholipids on the cell membrane [
21,
22]. In various inflammatory disease models, AnxA5 has been shown to play a key role in regulating macrophage-mediated immune responses, underscoring its potent anti-inflammatory properties [
23,
24,
25]. Specifically, AnxA5 suppresses M1 macrophage polarization while promoting M2 polarization. It has also been reported to downregulate the expression of α-smooth muscle actin (α-SMA) and transforming growth factor-beta (TGF-β), thereby reducing collagen deposition and mitigating the progression of hepatic fibrosis in models of nonalcoholic steatohepatitis [
23]. Although these findings clarify the anti-inflammatory mechanisms of AnxA5, its potential anti-fibrotic effects and associated molecular pathways remain to be fully elucidated.
In fibrotic skin lesions, the infiltration of immune cells triggers the release of inflammatory mediators such as TGF-β, platelet-derived growth factor (PDGF), and interleukin-13 (IL-13), which act in a paracrine manner on resident skin cells [
26]. Fibroblasts, the key effector cells in fibrotic processes, become activated and differentiate into myofibroblasts, leading to the excessive synthesis and secretion of extracellular matrix (ECM) components, particularly type I collagen (COL I), type III collagen (COL III), and fibronectin (FN) [
27]. The TGF-β/Smad signaling pathway plays a central role in regulating fibrogenesis [
28]. Upon binding of TGF-β ligands to their cognate receptors, intracellular signaling is initiated through the phosphorylation of Smad2 and Smad3, which then translocate into the nucleus to activate the transcription of pro-fibrotic genes [
29]. Given its pivotal role in driving fibroblast activation and ECM production, targeted modulation of the TGF-β/Smad pathway has emerged as a key therapeutic approach in the treatment of fibrotic diseases. In this study, we investigated the anti-fibrotic effects of AnxA5 and its regulatory role in TGF-β/Smad signaling in dermal fibroblasts. Our results demonstrated that AnxA5 suppresses TGF-β-induced phosphorylation and nuclear translocation of Smad2, thereby inhibiting fibroblast activation and extracellular matrix secretion. To model localized scleroderma (LoS), mice were subjected to repeated subcutaneous bleomycin injections [
30]. Using both preventative and therapeutic treatment strategies, we systematically evaluated the efficacy of AnxA5 in modulating fibrotic progression and inflammatory responses in bleomycin-induced LoS murine models.
2. Methods and Materials
2.1. Cell Culture
Primary human dermal fibroblasts (HDFs) were isolated from skin tissue samples obtained from five pediatric patients (aged 6–10 years) undergoing routine circumcision at Shanghai 9th People’s Hospital, with informed consent obtained from all patients and their guardians. The dermal fibroblasts were isolated through enzymatic digestion using neutral protease and collagenase, and subsequently cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator with 5% CO2. Cells at passages 1 to 3 were used for all experiments. Culture medium was refreshed every two days, and cells were used once they reached 80–90% confluency after passaging. For in vitro assays, HDFs were treated with recombinant human AnxA5 (0, 2.5, 5, or 10 μg/mL; SEME Cell Technology Co., Ltd., Shanghai, China) and the simultaneous treatment of 10 ng/mL recombinant human TGF-β (PeproTech, Rocky Hill, NJ, USA). The human immortalized keratinocyte cell line HaCaT (ATCC, Manassas, VA, USA) was cultured under the same conditions as HDFs. HaCaT cells were treated with recombinant human AnxA5 (0, 2.5, 5, or 10 μg/mL) in the presence or absence of a pro-inflammatory cytokine cocktail composed of IL-17A, IL-22, and TNF-α (each at 10 ng/mL; PeproTech, Rocky Hill, NJ, USA). All cell culture experiments were conducted at 37 °C in a humidified 5% CO2 atmosphere, with medium changes every 48 h.
2.2. Cell Counting Kit 8 (CCK-8) Assay
For cell proliferation assays, HDFs or HaCaT cells were seeded in 96-well culture plates at a density of 3 × 103 cells per well and allowed to adhere for 24 h under standard culture conditions. Following treatments, cellular proliferation was assessed at 24, 48, and 72-h time points using the CCK-8 kit (Dojindo Molecular Technologies, Kumamoto, Japan). The kit quantifies the number of live cells through a water-soluble tetrazolium salt, producing an orange formazan. Briefly, culture medium was aspirated and replaced with 100 μL of 1:10 diluted CCK-8 reagent in serum-free medium. Plates were subsequently incubated at 37 °C for 2 h in a humidified 5% CO2 atmosphere. Absorbance was measured at 450 nm using a SpectraMax® i3x microplate reader (Molecular Devices, San Jose, CA, USA). Relative optical density (O.D.) values were calculated using the following formula: Relative O.D. = (O.D. experimental group) − (O.D. blank control).
2.3. Quantitative Real-Time PCR
Total RNA was extracted from cultured cells using the RNA Purification Kit (B0004DP; EZ Bioscience, College Park, MD, USA) according to the manufacturer’s instructions. RNA concentration and purity were determined spectrophotometrically. Subsequently, 1 μg of total RNA was reverse transcribed into complementary DNA (cDNA) using the 4× Reverse Transcription Master Mix (A0010CGQ; EZBioscience
®). Quantitative real-time PCR (qPCR) was performed using SYBR™ Green qPCR Master Mix (A0012-R2; EZBioscience
®) and gene-specific primers (sequences provided in
Supplementary Table S1) on a real-time PCR detection system. All reactions were conducted in technical triplicates. Relative gene expression levels were normalized to GAPDH as an internal control and calculated using the 2
−ΔΔCt method, with results expressed as fold-changes relative to control groups.
2.4. Western Blotting
HDFs were seeded into six-well plates at a density of 1 × 105 cells per well and allowed to adhere overnight. Protein lysates were collected 72 h post-treatment and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by membrane transfer and blocking. Membranes were incubated with primary antibodies overnight at 4 °C and then with secondary antibodies for 1 h at room temperature prior to visualization. The primary antibodies used included: anti-COL I antibody (1:1000; 14695-1-AP; Proteintech, Wuhan, China), anti-α-SMA antibody (1:1000; ab5694; Abcam, Cambridge, UK), anti-p-Smad2 antibody (1:1000; 18338S; CST, Danvers, MA, USA), anti-p-Smad3 antibody (1:1000; 9520S; CST), and anti-Smad2/3 antibody (1:1000; 8685S; CST). The secondary antibody used was a horseradish peroxidase-conjugated rabbit antibody (1:10,000; SA00001-2; Proteintech, Wuhan, China). Protein bands were acquired and quantified using iBright Imager and iBright Analysis Software 5.4.0 (Thermo Fisher Scientific, Waltham, MA, USA), with results (Local Bg. Corr. Vol.) normalized to GAPDH and presented as a ratio relative to the control group.
2.5. Immunofluorescence Staining
Immunofluorescence staining was performed on cell coverslips and skin tissue sections following standard immunofluorescence procedures. Samples were incubated with primary antibodies overnight at 4 °C, followed by incubation with fluorescently labeled secondary antibodies for 1 h at room temperature. Cell nuclei were counterstained with DAPI (P0131; Beyotime, Shanghai, China). The primary antibodies used included: anti-α-SMA antibody (1:200; ab7817; Abcam, Cambridge, UK), anti-PCNA antibody (1:500; ab29; Abcam, Cambridge, UK), anti-CD68 antibody (1:100; ab303565; Abcam, Cambridge, UK), anti-CD86 antibody (1:200; 19589S; CST), anti-COL I antibody (1:200; 14695-1-AP; Proteintech, Wuhan, China), and anti-p-Smad2 antibody (1:200; 18338; CST). The secondary antibodies were Alexa Fluor® 488-conjugated mouse anti-rabbit (1:1000; CST) and Alexa Fluor® 594-conjugated rabbit anti-mouse (1:1000; CST). Images were acquired using a confocal fluorescence microscope (Leica, Wetzlar, Germany) equipped with the Leica LAS X system. Quantitative analysis of fluorescence intensity (/mm2) or positive cell counts was performed using ImageJ software V.1.8.0 (NIH). Three sections were analyzed for each sample.
2.6. Establishment and Treatment in Los Murine Models
All animal procedures were approved by the Animal Care and Experiment Committee of Shanghai Jiao Tong University School of conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Male C57BL/6 mice (6–8 weeks old, 23.48 ± 1.85 g) were obtained from Vital River Laboratories (Beijing, China) and housed under specific pathogen-free conditions. Mice were provided ad libitum access to food and water and maintained under controlled environmental conditions: temperature (22 ± 1 °C), relative humidity (55 ± 5%), and a 12 h light/dark cycle. After a 7-day acclimatization period, mice were anesthetized via intraperitoneal injection of pentobarbital sodium (50 mg/kg). A 1 cm2 area on the dorsal skin was shaved and marked to define the injection site. To establish the localized scleroderma (LoS) model, mice were randomly divided into two groups. The control group (CTRL, n = 3) receieved subcutaneous injections of 100 μL sterile normal saline (NS, 0.9% NaCl) while the model group (BLM, n = 3) received subcutaneous injections of 100 μL bleomycin solution (0.5 mg/mL; Nippon Kayaku Co., Ltd., Tokyo, Japan) into the marked dorsal area every other day. Dorsal skin samples were harvested at week 3 post-injection for validation of model establishment.
To evaluate the therapeutic efficacy of systemic AnxA5 administration, mice were randomly assigned to four experimental groups (
n = 6 per group). Group 1 (CTRL) received subcutaneous injections of 100 μL normal saline (NS) every other day. Group 2 (BLM) received subcutaneous injections of 100 μL bleomycin solution (0.5 mg/mL) and intraperitoneal injections of 200 μL NS every other day. Group 3 (AnxA5-6w) received subcutaneous injections of 100 μL bleomycin solution (0.5 mg/mL) and intraperitoneal injections of 200 μL AnxA5 solution (0.2 mg/mL) every other day for 6 weeks. Group 4 (AnxA5-3w) received subcutaneous bleomycin injections (100 μL, 0.5 mg/mL) every other day for 6 weeks and intraperitoneal AnxA5 injections (200 μL, 0.2 mg/mL) every other day from weeks 4 to 6. The dosage of AnxA5 was based on prior studies in osteoarthritis models [
20] and aligned with protocols used in other bleomycin-induced skin fibrosis models [
31]. At the end of week 6, dorsal skin samples were collected from all groups for further analysis.
For topical AnxA5 application studies, mice were randomly assigned to four groups (n = 6 per group): Group 1 (CTRL) received subcutaneous injections of 100 μL normal saline (NS) every other day. Group 2 (BLM) received subcutaneous injections of 100 μL bleomycin solution (0.5 mg/mL) along with subcutaneous injections of 200 μL NS every other day. Group 3 (AnxA5-6w) received subcutaneous injections of 100 μL bleomycin solution (0.5 mg/mL) and topical subcutaneous injections of 10 μL AnxA5 solution (2 mg/mL) every other day for 6 weeks. Group 4 (AnxA5-3w) received subcutaneous bleomycin injections (100 μL, 0.5 mg/mL) every other day for 6 weeks, and topical subcutaneous injections of 10 μL AnxA5 solution (2 mg/mL) every other day from week 4 to week 6. At the end of the sixth week, dorsal skin samples were harvested from all groups for further evaluation.
2.7. Histological Staining
Following tissue collection, skin samples were fixed in 4% paraformaldehyde (PFA), processed through standard dehydration procedures, and embedded in paraffin. Paraffin blocks were sectioned into 5 μm thick slices using a microtome. Tissue sections were then subjected to hematoxylin and eosin (H&E), Masson’s trichrome, and Sirius red staining according to the manufacturer’s protocols. Sections were sequentially dewaxed, rehydrated, and stained with hematoxylin for 10 min. For H&E staining, sections were counterstained with eosin for 3 min. For Masson’s trichrome staining, sections were blued in Masson bluing solution for 3 min, stained with Ponceau-fuchsin solution for 5 min, differentiated in phosphomolybdic acid solution for 1 min, and stained with aniline blue for 2 min. For Sirius red staining, sections were incubated in Sirius red solution for 30 min. All stained sections were mounted with neutral resin and imaged using an optical microscope (Nikon Instruments, Tokyo, Japan).
2.8. Collagen Content Detection
Skin tissues were hydrolyzed in lysis buffer, and the pH of the resulting lysates was adjusted to 6–8 using sodium hydroxide (NaOH). Hydroxyproline content was quantified using a commercial hydroxyproline assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Briefly, 100 μL of each sample was mixed with assay reagents and incubated at 60 °C for 15 min. Absorbance was measured at 550 nm using a microplate reader. A standard curve was generated using hydroxyproline standards ranging from 0 to 200 μg/mL. Hydroxyproline content was calculated using the following formula:
All samples were measured in triplicate, and results were normalized to tissue wet weight. Hydroxyproline content was used as an indicator of total collagen content, with a conversion factor of 7.46 (1 μg hydroxyproline ≈ 7.46 μg collagen).
2.9. Immunohistochemical Staining
Skin tissue samples were fixed in 4% PFA for 24 h at 4 °C, followed by standard tissue processing through graded ethanol dehydration, xylene clearing, and paraffin embedding. The pre-cooled paraffin blocks were sectioned into slices with a thickness of 5 μm. Sections were dewaxed, rehydrated, and subjected to antigen retrieval by immersion in citrate buffer, followed by microwave heating at high power for 2 min and incubation in a 98 °C water bath for 30 min. Permeabilization was performed by soaking the sections in 0.2% Triton X-100 solution for 5 min. Endogenous peroxidase activity was blocked using hydrogen peroxide for 10 min. Sections were then incubated in 5% goat serum for 30 min to block non-specific binding sites. Primary antibodies anti-fibronectin (FN) (1:600; 15613-AP; Proteintech, Wuhan, China) were applied and incubated overnight at 4 °C. The next day, HRP-conjugated secondary antibodies (rabbit anti-mouse, 1:1000; goat anti-rabbit, 1:1000) were applied for 30 min at room temperature. Color development was achieved using 3,3′-diaminobenzidine (DAB) solution, and the reaction was stopped at the appropriate endpoint. Sections were mounted with neutral resin and visualized under an optical microscope. Quantitative analysis of immunohistochemical staining was performed using ImageJ software.
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
Skin tissue samples were homogenized in ice-cold RIPA buffer supplemented with protease inhibitors using a tissue homogenizer. The homogenates were centrifuged at 12,000× g for 15 min at 4 °C, and the resulting supernatants were collected for further analysis. Total protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Beyotime Biotechnology, Shanghai, China). Cytokine levels were measured using commercial ELISA kits (Elabscience Biotechnology, Houston, TX, USA) according to the manufacturer’s protocols. Briefly, 100 μL of tissue lysates or standards were added to antibody-precoated 96-well plates and incubated at 37 °C for 2 h. Following washes, biotinylated detection antibodies were added and incubated for 1 h at 37 °C, followed by incubation with streptavidin-HRP conjugate for 30 min. Signal development was performed using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate and stopped with 2 N sulfuric acid. Absorbance was measured at 450 nm using a microplate reader. The concentrations of TNF-α, IL-6, IL-17A, and IL-22 were calculated from standard curves and normalized to total protein content, expressed as pg cytokine/mg total protein. All samples were analyzed in duplicate to ensure reproducibility.
2.11. Statistical Analysis
All quantitative data are presented as mean ± standard error of the mean (SEM) from at least three independent experiments. Statistical analyses were conducted using GraphPad Prism version 9.3 (GraphPad Software, San Diego, CA, USA). Comparisons between two groups were performed using unpaired two-tailed Student’s t-tests. For comparisons among multiple groups, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was applied. Statistical significance was defined as p < 0.05, with the following notation used to indicate significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
4. Discussion
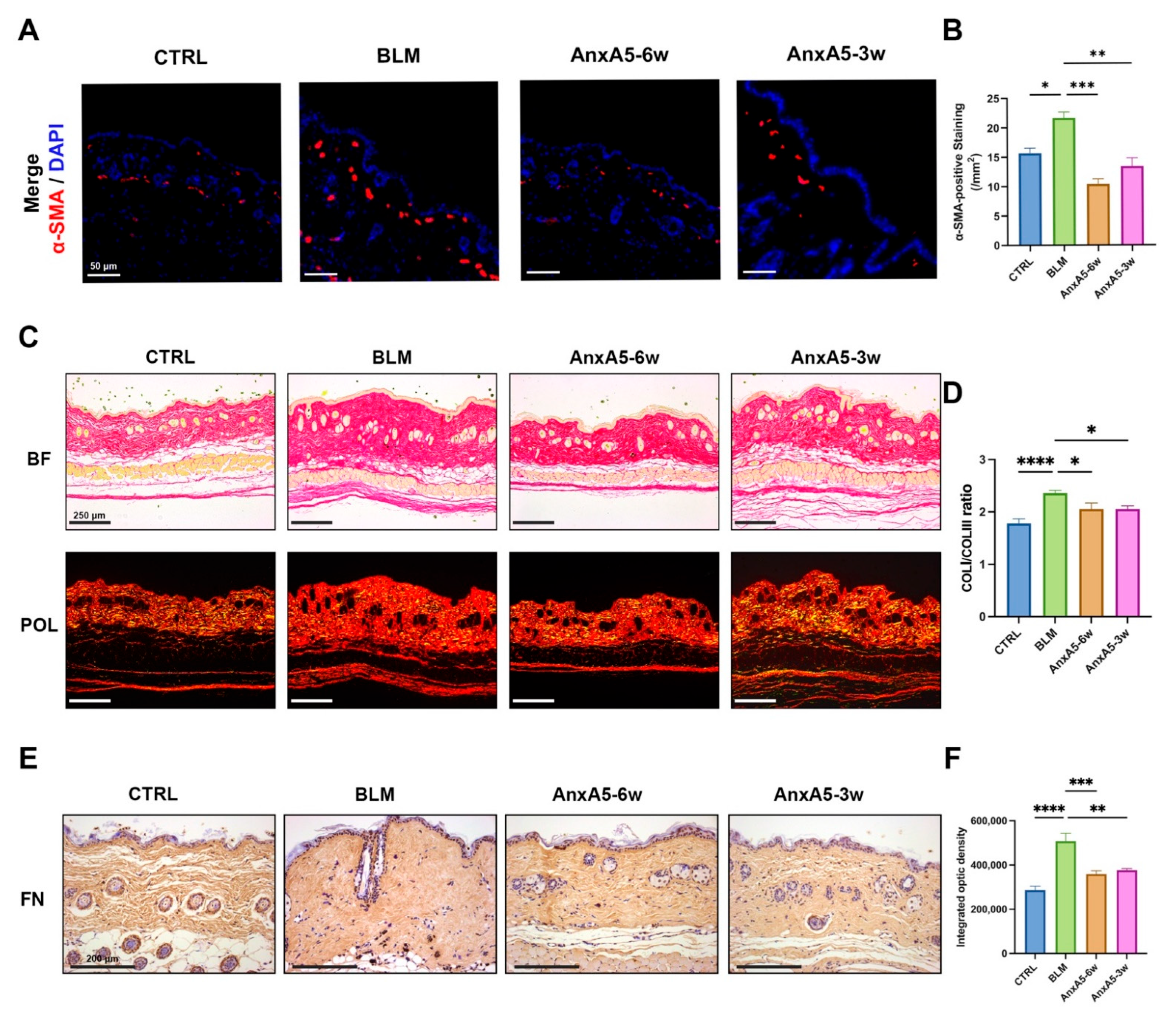
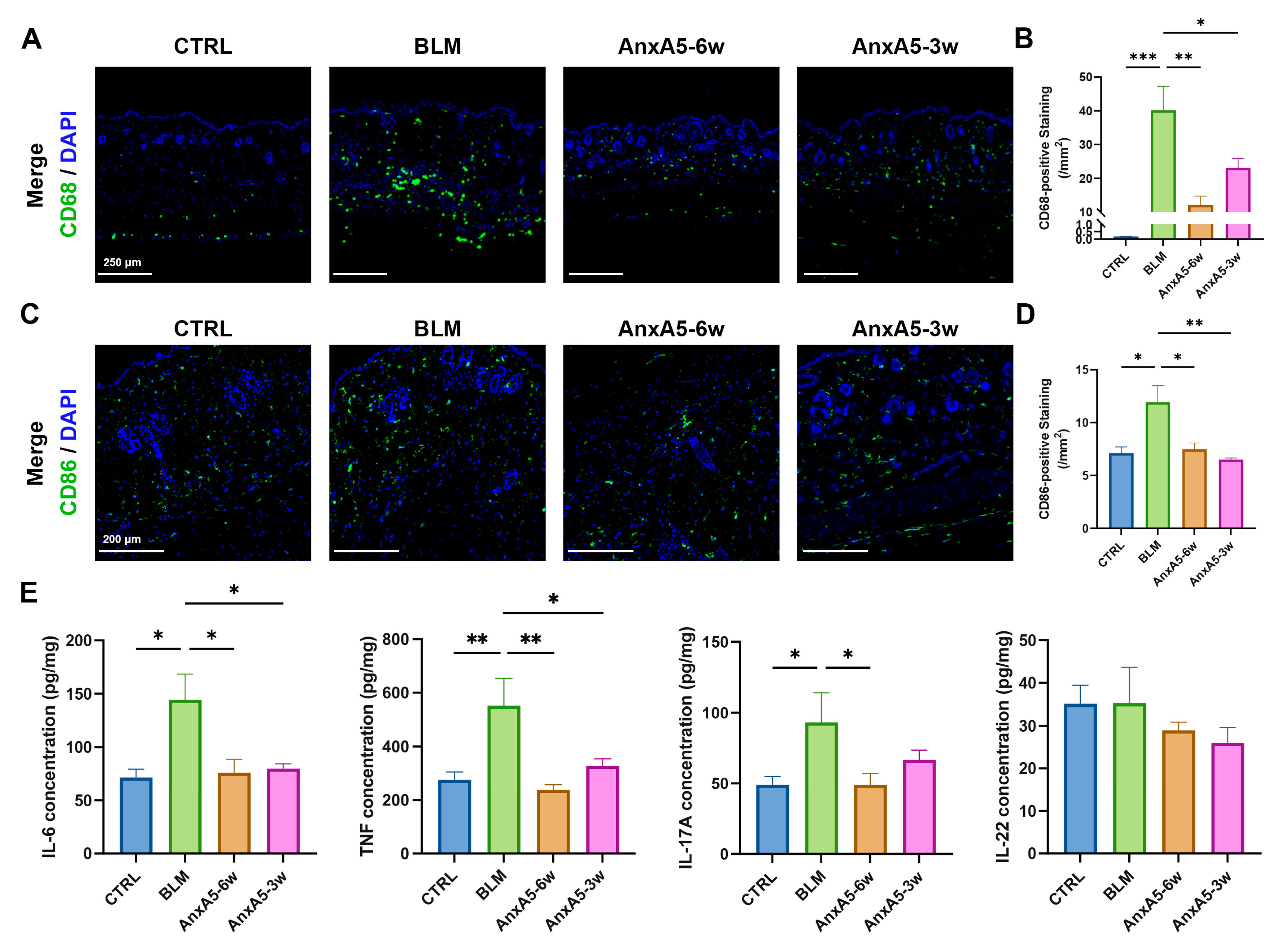
Localized scleroderma (LoS), though rare, has a profound impact on skin tissue structure and function, particularly when affecting the face. Effective treatment strategies for LoS focus on controlling both fibrotic progression and inflammation. In this study, we provide the first evidence that AnxA5 possesses anti-fibrotic activity by regulating extracellular matrix (ECM) synthesis and degradation through inhibition of the TGF-β/Smad2 signaling pathway in dermal fibroblasts. In bleomycin-induced murine models, systemic application of AnxA5 alleviated LoS pathology by modulating both fibroblasts and macrophages activity. Specifically, AnxA5 suppressed the expression of α-SMA, a myofibroblast marker, thereby reducing collagen synthesis and secretion. Concurrently, AnxA5 downregulated the expressions of markers of pro-inflammatory M1 macrophages (CD68 and CD86), leading to decreased production of inflammatory cytokines. These findings suggest that AnxA5, as a single-molecule therapeutic agent, exerts dual anti-fibrotic and immunomodulatory effects. Through both preventative and therapeutic administration, AnxA5 shows promising potential to improve outcomes in LoS and other fibrotic skin diseases.
LoS is characterized by persistent inflammation and progressive fibrosis involving both the dermis and subcutaneous adipose tissue. As the disease progresses, excessive extracellular matrix (ECM) deposition invades and replaces adipose tissue, ultimately compromising the structural and functional integrity of the skin [
36]. Previous studies have reported that AnxA5 is abundantly expressed in adipose tissue but is significantly downregulated in the skin [
37], suggesting a potential deficiency of endogenous AnxA5 in LoS-affected skin lesions. In this study, we demonstrated that exogenous supplementation of recombinant AnxA5 effectively attenuated both fibrosis and inflammation in LoS murine models. This finding is consistent with previous reports that AnxA5 attenuates hepatic fibrosis and ECM deposition in high-fat diet-induced nonalcoholic steatohepatitis by downregulating α-SMA, COL III, and TGF-β expression [
23]. We further validated the anti-fibrotic properties of AnxA5 in dermal fibroblasts (
Figure 1 and
Figure 2). TGF-β signaling includes both canonical (Smad-dependent) and non-canonical (JNK, ERK, and PI3K/AKT) pathways. While these pathways exhibit cross-talk with Smad signaling and ultimately converge on nuclear Smad complexes, the canonical TGF-β/Smad pathway remains the primary mediator of fibrogenesis [
38]. In this study, we specifically detected Smad2/3 activation following AnxA5 treatment. Under TGF-β stimulation, AnxA5 significantly inhibited Smad2 phosphorylation and its nuclear translocation, thereby disrupting the canonical TGF-β/Smad2 signaling pathway (
Figure 2). As a result, AnxA5 suppressed the expression of pro-fibrotic markers including α-SMA, CTGF, and TGF-β, preventing fibroblast activation and myofibroblast differentiation. In addition, AnxA5 reduced the synthesis and secretion of ECM components such as COL I and FN, while promoting ECM degradation by increasing the MMP1/TIMP1 ratio (
Figure 1). While our data demonstrate AnxA5’s selective inhibition of canonical TGF-β/Smad2 signaling (
Figure 2), we cannot exclude potential modulation of non-canonical pathways that may synergistically contribute to its anti-fibrotic effects. Future studies could systematically evaluate AnxA5’s broader signaling regulation while maintaining therapeutic specificity. These findings uncover a novel biological function of AnxA5 as a potent anti-fibrotic regulator in dermal fibroblasts and highlight its therapeutic potential for the treatment of fibrotic skin disorders such as LoS.
LoS is generally recognized as a biphasic disease, consisting of an initial inflammatory stage followed by a fibrotic phase [
39]. The early phase is characterized by aberrant immune activation and heightened inflammatory responses, with prominent infiltration of immune cells and increased secretion of pro-inflammatory cytokines [
40]. Current therapeutic strategies emphasize the importance of early immunomodulatory intervention to impede disease progression and prevent late-stage fibrosis [
41]. In this study, we evaluated the therapeutic efficacy of AnxA5 in bleomycin-induced LoS models using both preventative and regressive treatment approaches. Comparative analysis revealed that preventative treatment (AnxA5-6w group) demonstrated superior immunomodulatory effects, as evidenced by a greater reduction in CD86+ M1 macrophage infiltration and more pronounced suppression of pro-inflammatory cytokine production compared to the regressive treatment group (AnxA5-3w) (
Figure 6). In terms of fibrotic outcomes, the AnxA5-6w group also showed enhanced therapeutic benefit, including greater reductions in epidermal and dermal thickness, decreased collagen content, and attenuated ECM deposition (
Figure 1 and
Figure 2). These results suggest that early intervention with AnxA5 more effectively inhibits dermal fibroblast activation and myofibroblast differentiation, while also modulating M1 macrophage-dominated inflammation. While the current study establishes AnxA5’s capacity to reduce macrophage infiltration and pro-inflammatory cytokine secretion in LoS lesions at the phenotypic level, detailed mechanistic insights into AnxA5-mediated macrophage polarization are systematically characterized in our other studies [
20,
42]. The superior efficacy observed with preventative treatment highlights the critical importance of early therapeutic intervention in LoS and supports the potential of AnxA5 as a preventative strategy to halt disease progression.
In addition to systemic administration, we evaluated the therapeutic potential of topical AnxA5 application in bleomycin-induced LoS models. This localized delivery strategy offers distinct pharmacological advantages, including enhanced drug concentration at the lesion site and reduced risk of systemic adverse effects [
43]. Multiple dermal delivery systems exist for recombinant proteins. Due to their large molecular weight and poor biological membrane permeability, techniques such as fractional laser ablation or microneedle rollers can enhance transdermal delivery by creating micropore arrays [
44,
45]. Liposome-encapsulated recombinant human growth hormone modified with hyaluronic acid and incorporated into carbomer gel significantly increased the secretion of COL I and ameliorated skin photoaging [
46]. Similarly, microneedle-mediated delivery of recombinant human epidermal growth factor accelerated wound healing [
47]. In this study, recombinant human AnxA5 was administered via subcutaneous injection to treat localized scleroderma. Our findings demonstrated that topical AnxA5 administration significantly reduced collagen deposition and suppressed cutaneous inflammation, effectively ameliorating LoS under both preventative and therapeutic administration regimens (
Figure 7). Collectively, our study provides comprehensive evidence supporting the efficacy of AnxA5 in LoS management through both systemic and topical delivery strategies. In the bleomycin-induced LoS model, both approaches exhibited robust anti-inflammatory and anti-fibrotic effects. While recombinant protein therapeutics offer significant potential, challenges remain regarding limited storage stability and potential immunogenicity. Recent advances in protein engineering, including chemical modifications and innovative transdermal delivery systems, have shown promise in mitigating these limitations while maintaining therapeutic efficacy [
48]. Nevertheless, further research is warranted to optimize therapeutic parameters such as administration route, dosage, and delivery formulation to maximize efficacy and support clinical translation. In particular, the development of AnxA5-based topical formulations with improved skin penetration and sustained release properties represents a promising avenue for future investigation.
AnxA5 has been extensively studied for its diverse biological functions, including anti-coagulant, anti-inflammatory, and anti-apoptotic activities [
49], positioning it as a novel therapeutic candidate for inflammation-associated disorders. The clinical translation of recombinant human AnxA5 is supported by completed phase I trials (NCT04217629, NCT04850399) demonstrating the safety of intravenous administration at doses ranging from 0.75 to 20 mg in healthy volunteers. Pharmacokinetic studies have further established that AnxA5 administration at 50–100 μg/kg in patients with normal renal function results in dose-dependent plasma concentration increases, rapid clearance, and no significant impact on coagulation parameters over a 7-day observation period [
50]. These data provide a strong foundation for continued clinical development of AnxA5-based therapies. Given its broad biological activities, the therapeutic potential of AnxA5 may extend beyond LoS to include a wide range of inflammatory and fibrotic conditions. We hypothesize that AnxA5 may be beneficial for treating hypertrophic scars, keloids, and radiation-induced skin injury. Preliminary, unpublished data from a bleomycin-induced pulmonary fibrosis model further support the anti-fibrotic efficacy of AnxA5 across multiple tissue types. Importantly, recombinant protein technology enables scalable production of AnxA5 with consistent quality and well-defined mechanisms of action, making it a feasible candidate for clinical application. Future studies should aim to optimize delivery platforms, establish precise dose-response relationships, and evaluate combination therapies to enhance treatment efficacy. These efforts will be critical in advancing AnxA5 as a viable therapeutic agent for fibrotic and inflammation-related diseases.