Advances in Transdermal Delivery Systems for Treating Androgenetic Alopecia
Abstract
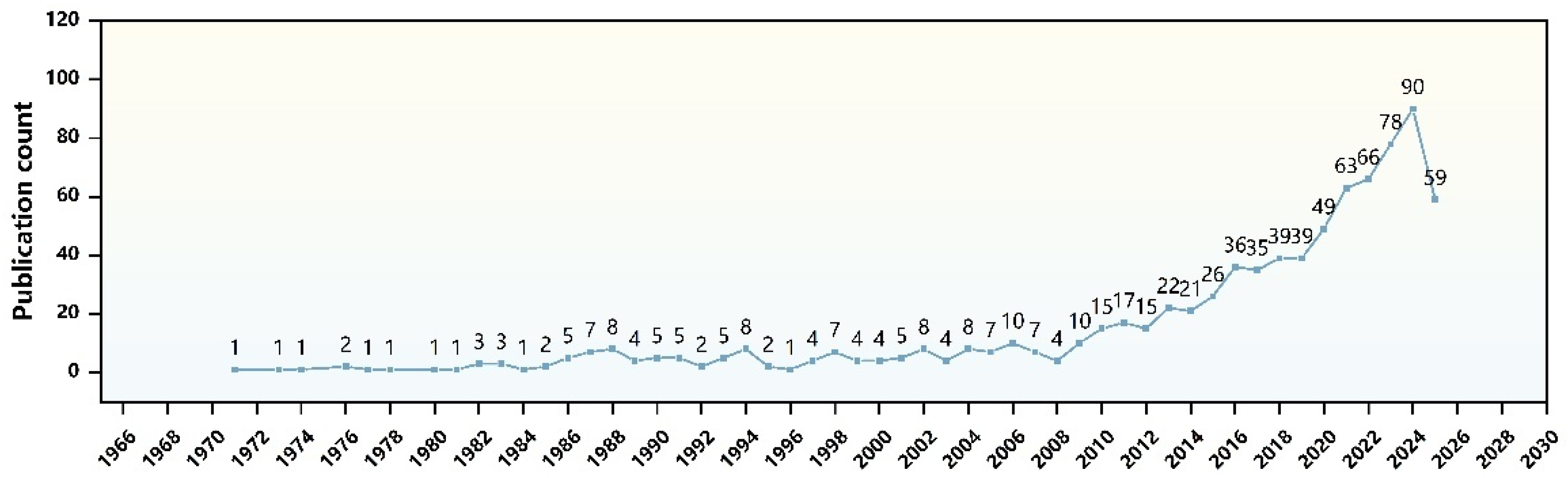
1. Introduction
2. Pathogenesis and Common Treatments in Androgenetic Alopecia
2.1. Pathogenesis
2.1.1. Androgen Metabolism
2.1.2. Immune Inflammation
2.2. Common Treatments
2.2.1. Topical Minoxidil
2.2.2. Oral Finasteride
2.2.3. Hair Transplant
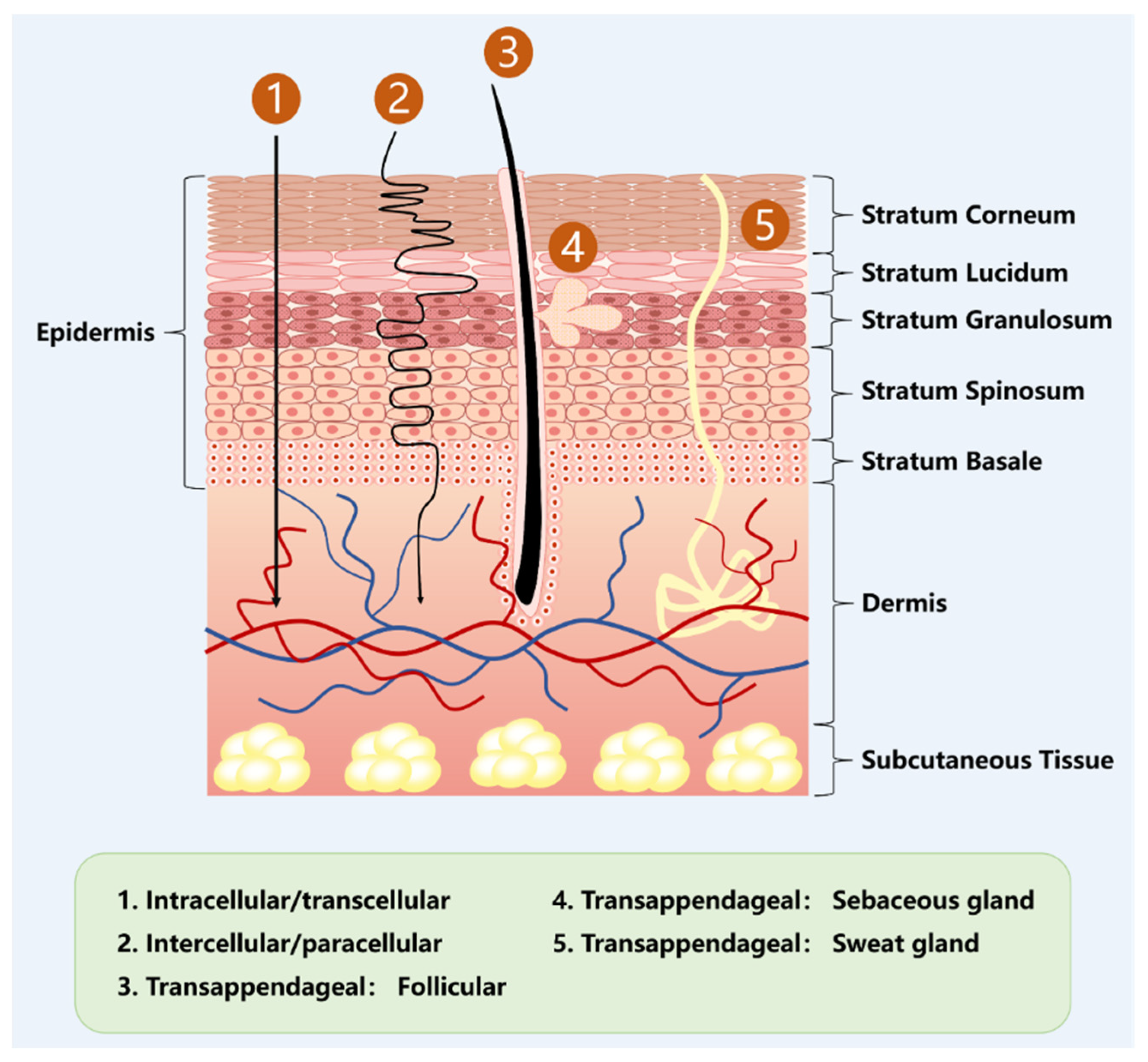
3. Transdermal Drug Delivery (TDDS)
3.1. Microneedles (MNs)
| TDDS | Characteristics | Applications | Reference | |
|---|---|---|---|---|
| Name | Components and Functions | |||
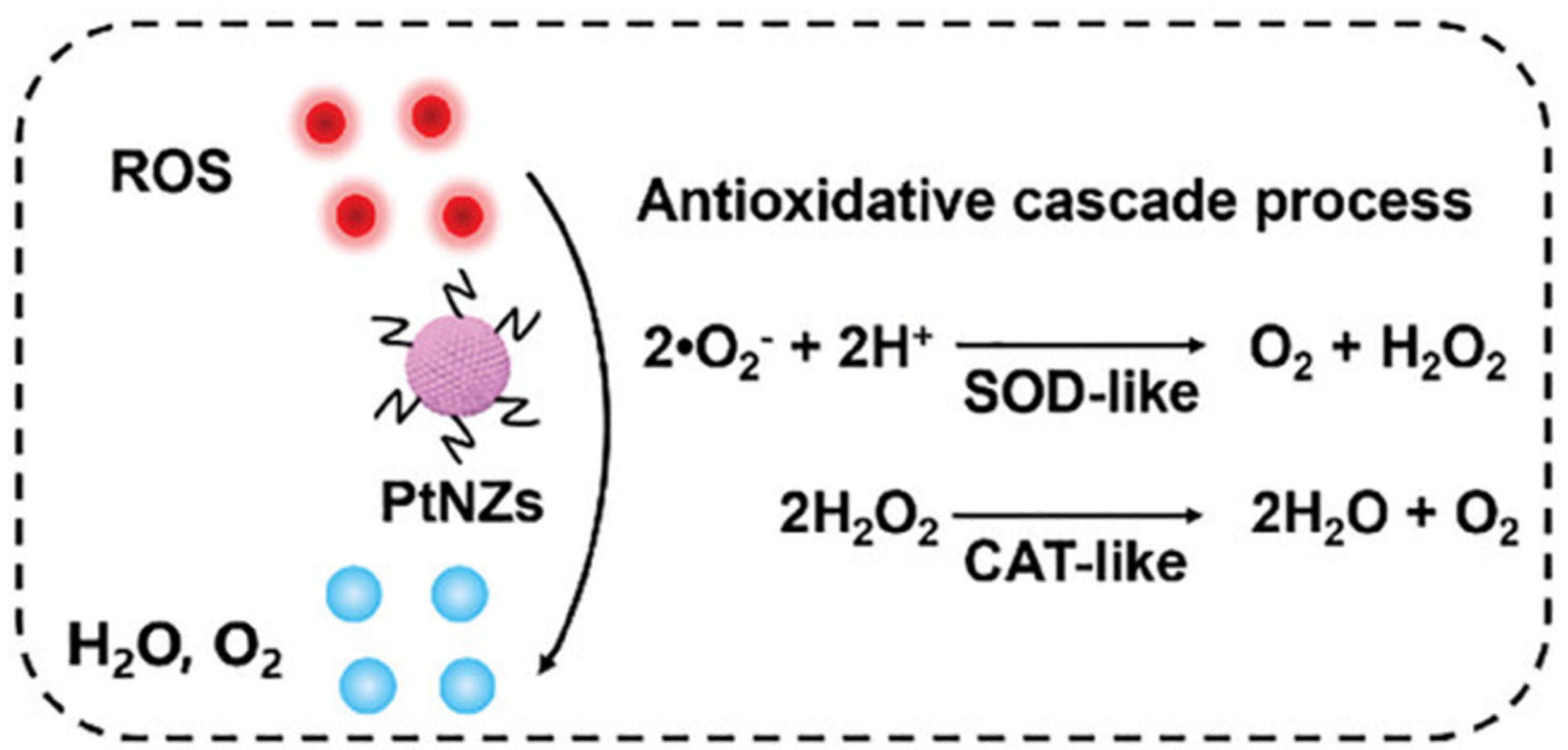
| Microneedles (MNs) | Micron-sized needle-like structures can quickly penetrate the SC and form reversible microchannels to help active drugs reach the dermis. | Pt-MN | PtNZ: Eliminate ROS, improve the pathological microenvironment of AGA, and promote hair follicle stem cell differentiation. Dissolving MNs: Load PtNZ, penetrate the SC, dissolve, and release it. | [47] |
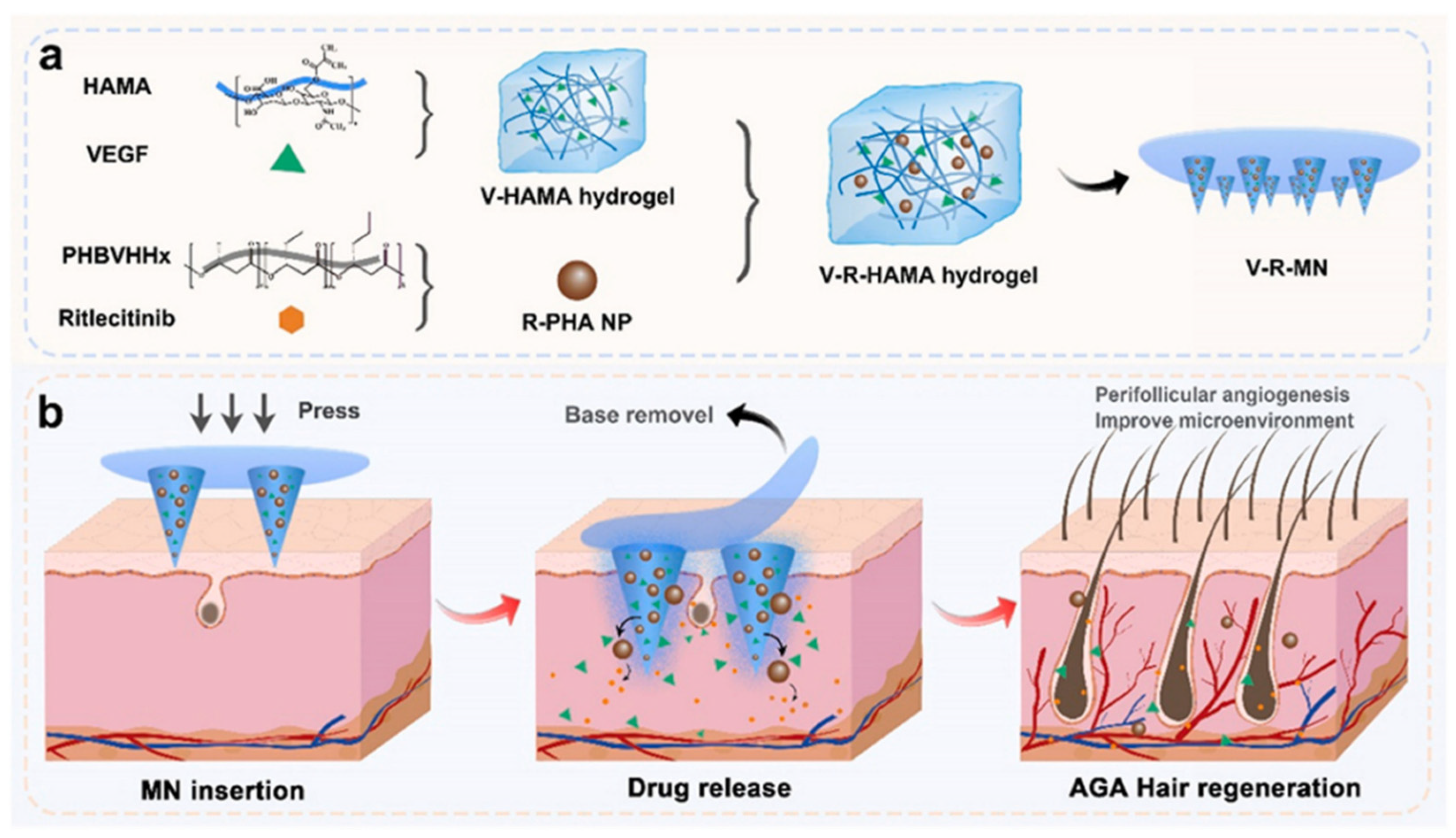
| V-R-MN | Ritlecitinib: Inhibits JAK kinase activity, blocks excessive activation of the immune system that triggers attacks on hair follicles, and promotes hair regrowth. R-PHA NPs: Loaded with Ritlecitinib and combined with HA hydrogel to increase the mechanical properties of MNs. VEGF: Promotes blood vessel formation in hair follicles and provides nutrients for hair regeneration. Activates hair follicle stem cells and promotes hair growth by pushing hair follicles into the growth phase. HA-MN: Load and deliver the above functional ingredients. | [48] | ||
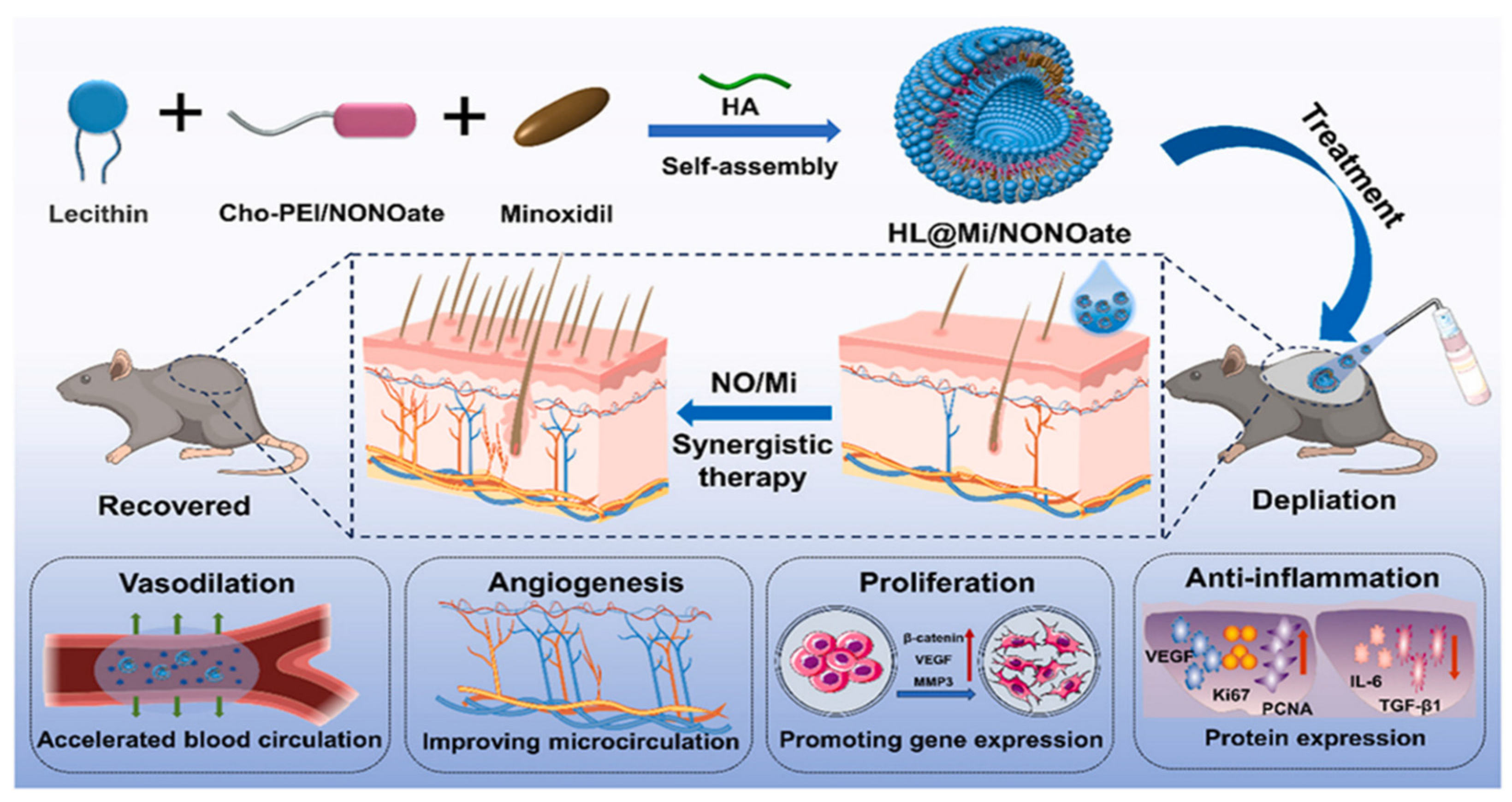
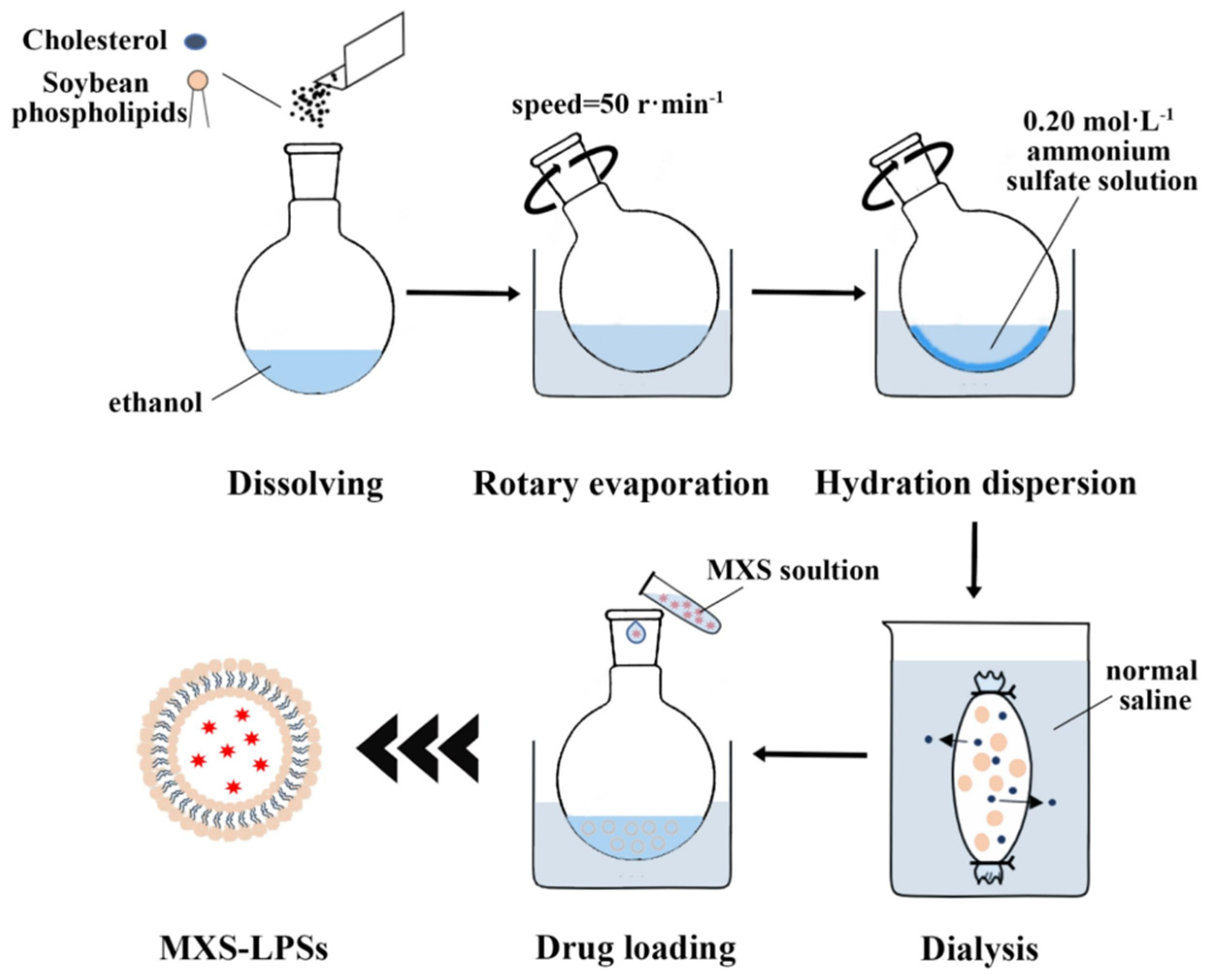
| Liposome | Liposomes are primarily composed of phospholipids and cholesterol. They form closed vesicles with a bilayer membrane structure through self-assembly in aqueous solutions, capable of encapsulating both hydrophilic and hydrophobic molecules simultaneously. They exhibit excellent biocompatibility, stability, deformability, and high drug-loading capacity. | HL@Mi/ NON Oate | Lecithin: Basic components of liposome membranes. Cho-PEI/ONOate: Cho forms a liposome membrane structure. PEI is a cationic polymer that regulates the surface charge of the carrier and adsorbs HA. Nitric oxide donors produce nitric oxide, which promotes vasodilation, enhances skin permeability, and increases the retention of minoxidil. At the same time, it stimulates angiogenesis, improves microcirculation in the hair follicles, and promotes the proliferation of dermal papilla cells. HA: Stabilize the lipid membrane structure. Improve the transdermal penetration efficiency of drugs. Minoxidil: By expanding the blood vessels in the scalp to increase the supply of nutrients and oxygen to the hair follicles, it promotes hair follicle cell proliferation and differentiation. | [49] |
| CAR@Lip | Lipoid S75 and Cho: Membrane materials for liposomes, which encapsulate CAR to form a stable liposome structure. CAR: Regulating the expression of growth factors associated with the hair follicle cycle, such as IGF-1, VEGF, KGF, and TGF-β; participating in the SHH/Gli and Wnt/β-catenin signaling pathways, thereby promoting the transition of hair follicles from the telogen phase to the anagen phase and promoting hair regrowth. However, CAR has poor water solubility and low bioavailability, limiting its application in transdermal delivery. Therefore, it is encapsulated in liposomes. | [50] | ||
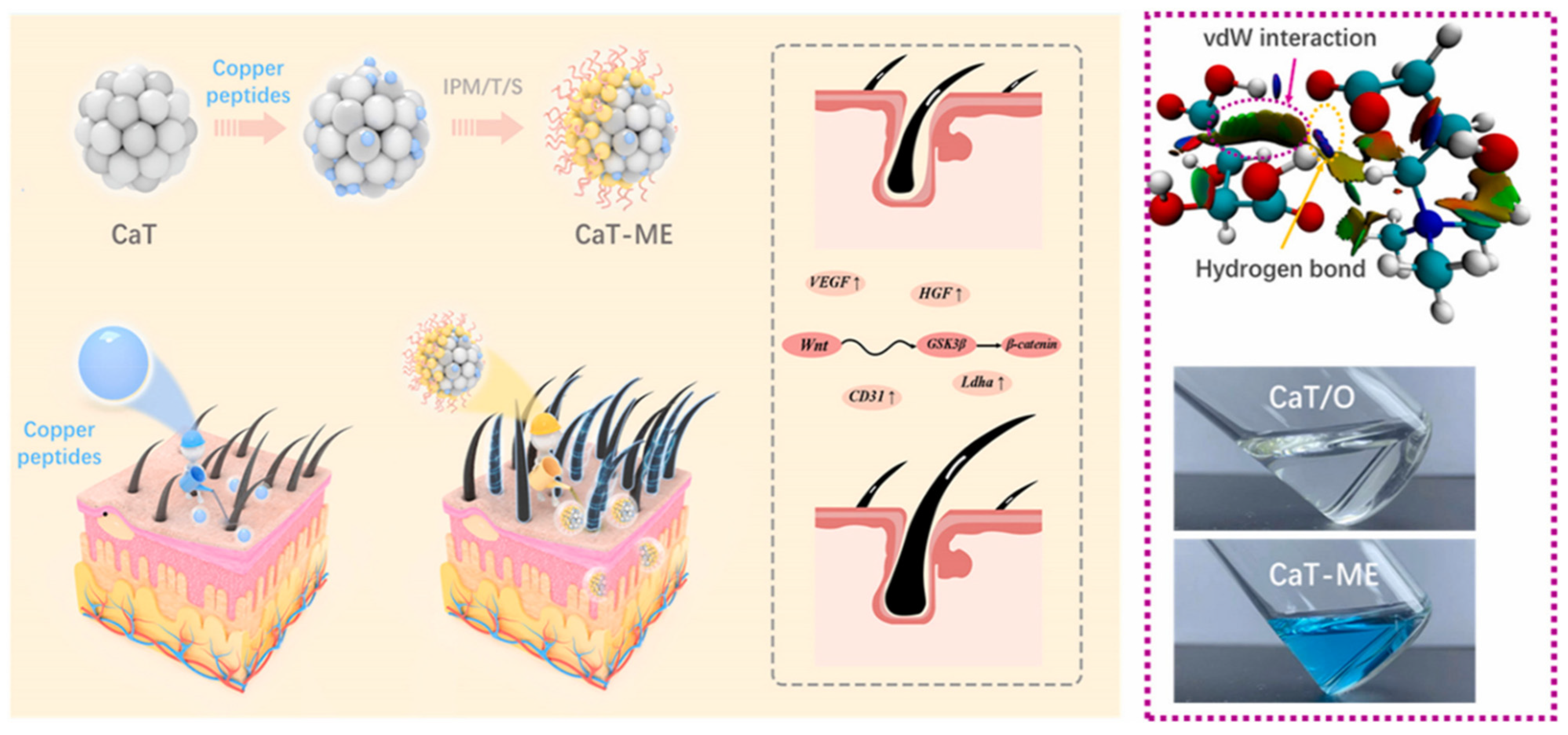
| Ionic liquid (IL) | Ionic liquids are composed of organic cations and organic/inorganic anions. On the one hand, anions and cations can interact with keratin layer lipids through electrostatic forces or hydrogen bonds, disrupting the ordered structure of lipids and enhancing their fluidity. On the other hand, ionic liquids can both increase the solubility of hydrophobic drugs and reduce the charge barrier of drugs by forming neutral ion pairs. These properties enable ionic liquids to combine the high efficiency of traditional permeation enhancers with excellent biocompatibility. | GHK-Cu/ CaT-ME | GHK-Cu: Increases VEGF expression. Promotes new blood vessel formation, providing nutrients for hair growth; promotes hair papilla cell proliferation and inhibits apoptosis; inhibits TGF-β production, preventing hair follicles from prematurely transitioning from the growth phase to the resting phase. CaT: An ionic liquid composed of tartaric acid and L-carnitine, which has hair growth properties. It can also act as a polar phase to form a microemulsion with IPM, improving the local delivery efficiency and bioavailability of copper peptides. IPM: As a nonpolar phase, it forms microemulsions with CaT and surfactants. | [51] |
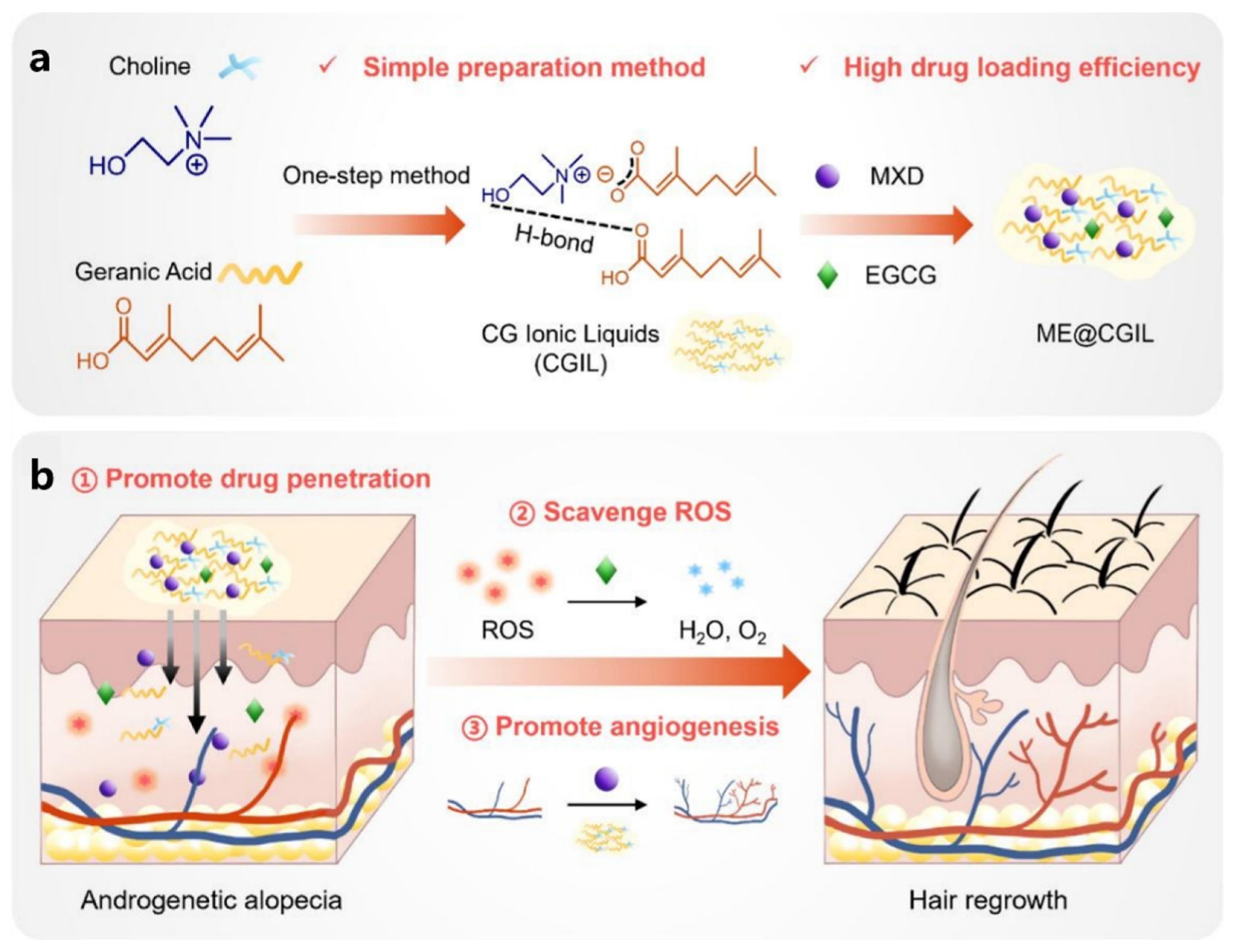
| ME@CGIL | CGIL: Choline and geranic acid are synthesized into IL through a double decomposition reaction in a single step. This process enhances the solubility of MXD and EGCG, improves their transdermal penetration, and prolongs their retention time in the skin. Additionally, CGIL itself possesses certain hair growth-promoting functions, stimulating angiogenesis around hair follicles. EGCG: A natural plant extract with excellent antioxidant activity that effectively reduces ROS levels and inhibits ROS-induced cell apoptosis, regulates oxidative stress in the hair follicle microenvironment. Minoxidil: By expanding the blood vessels in the scalp to increase the supply of nutrients and oxygen to the hair follicles, it promotes hair follicle cell proliferation and differentiation. | [52] | ||
| Nanostructured lipid carrier (NLC) | Nano-structured lipid carriers (NLC) are composed of an aqueous phase, a solid-liquid mixed lipid phase, and surfactants acting in synergy. When applied to the skin surface, the water in the NLC evaporates, and the lipid components spontaneously arrange themselves through intermolecular forces, forming a dense and continuous film. This reduces water loss, improves skin hydration, and promotes drug penetration. The lipid components can also disrupt the lipid arrangement in the stratum corneum, temporarily breaking down its tight structure to form microchannels, thereby enhancing drug diffusion. | DST-NLC | Stearic acid: Forming a lipid matrix, it is one of the main lipid components of NLC. Phosal® 53 MCT: Together with stearic acid, it forms a stable lipid matrix structure. Lutrol® micro68: Surfactants reduce interfacial tension. CSO-LA: Synthesized from chitosan oligomers and lauric acid, it is used to coat DST-NLC. Chitosan oligomers carry a positive charge due to their amino groups, promoting the adsorption and penetration of the carrier in the skin and hair follicle areas. Lauric acid has strong anti-androgenic activity, inhibiting 5α-reductase activity, and synergistically with dutasteride to prevent hair loss and promote hair growth. Dutasteride: A dual type I and type II 5α-reductase inhibitor that reduces serum DHT levels. | [53] |
| SL-NLC | Compritol®888 ATO: As a solid lipid, it forms NLC. It can also dissolve lipophilic drugs, helping to increase the loading capacity of SL. Olive oil: A liquid lipid in NLC that can interfere with the crystal order of solid lipids during preparation, increasing the space available for drug molecules. Transcutol®P: Added to NLCs as a penetration enhancer, it increases drug penetration into the skin. It also reduces the surface tension of oil droplets, promoting the formation of a uniform emulsion and the preparation of nanoparticles with uniform particle size. SL: Has powerful anti-androgenic properties, acting by reducing androgen production and competitively blocking androgen receptors in target tissues. | [54] | ||
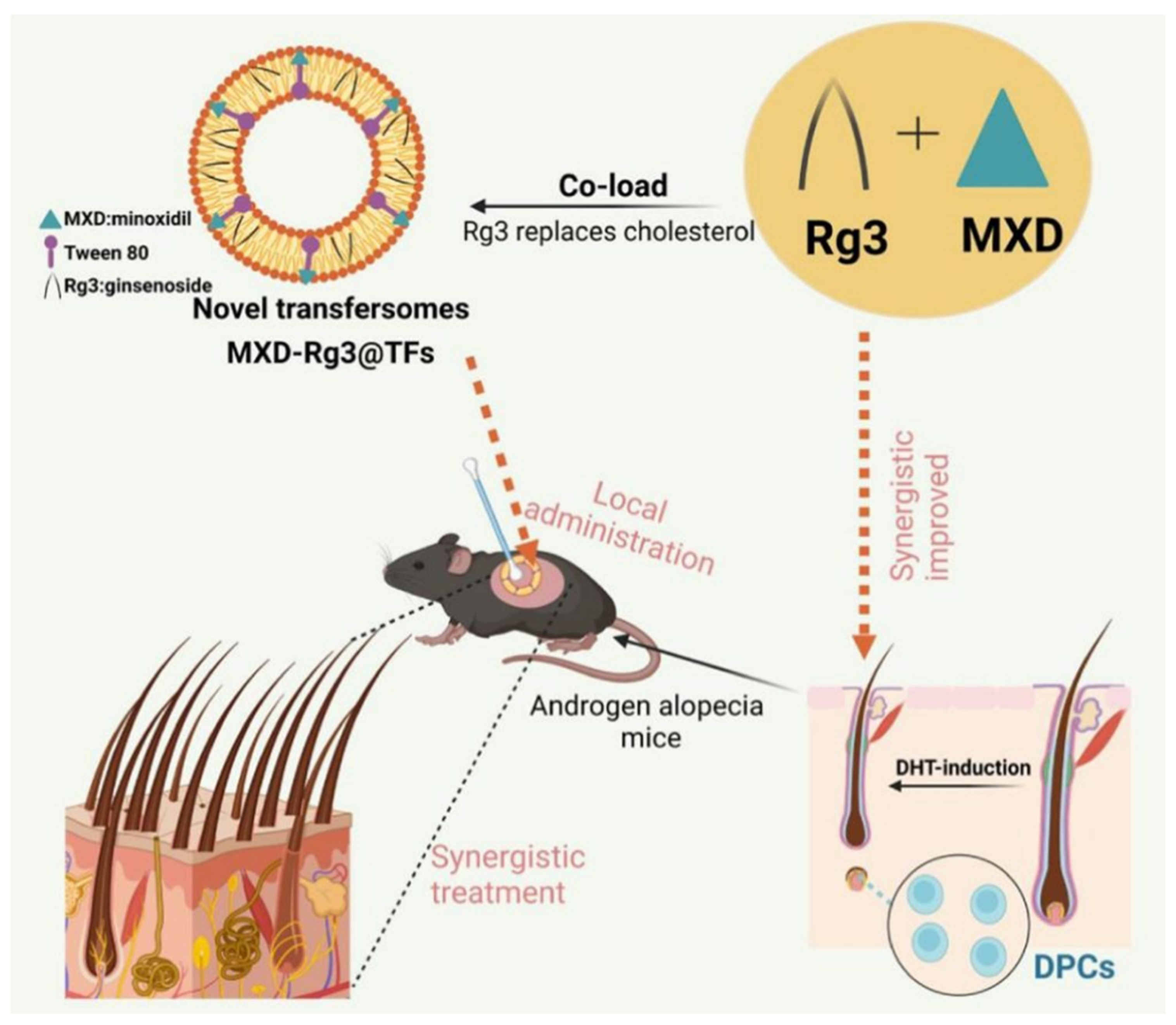
| Transformers (TFs) | Its structure is similar to that of liposomes, but edge active agents are embedded in the lipid bilayer. It can endow TFs with additional flexibility, enabling them to deform under the drive of the osmotic pressure gradient of the skin, overcome the size limitations of traditional nanoparticles, and penetrate pores in the stratum corneum that are much smaller in diameter, thereby achieving targeted drug delivery to the deep layers of the skin. | MXD-Rg3@TFs | Rg3: By targeting the PI3K/AKT and MAPK/NF-κB signaling pathways and inflammatory factors, it potentially regulates inflammation and oxidative stress-related pathways. Its steroid structure is similar to cholesterol, and it can replace cholesterol in the preparation of TF, thereby avoiding the disruption of steroid homeostasis or steroid-sensitive signaling pathways in the body that may be caused by cholesterol accumulation in hair follicles. Minoxidil dilates blood vessels, increases blood flow to hair follicles, stimulates dermal cells to produce growth factors, and activates the β-catenin pathway, thereby inducing and prolonging the growth phase of hair and promoting hair growth. | [55] |
3.2. Liposome
3.3. Ionic Liquid (IL)
3.4. Nanostructured Lipid Carrier (NLC)
3.5. Transfersomes (TFs)
4. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamilton, J.B. Patterned Loss of Hair in Man: Types and Incidence. Ann. N. Y. Acad. Sci. 2010, 53, 708–728. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Androgenetic Alopecia in Women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Caro, G. Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: A prospective, randomized, controlled, assessor blinded, 3-arm, pilot trial. J. Cosmet. Dermatol. 2023, 23, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Design. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Iamsumang, W.; Leerunyakul, K.; Suchonwanit, P. Finasteride and Its Potential for the Treatment of Female Pattern Hair Loss: Evidence to Date. Drug Des. Dev. Ther. 2020, 14, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Restoration, F.H. Facial Plastic Surgery Clinics of North America. Reconstruction 2013, 21, 407–417. [Google Scholar]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Ruby, P.K.; Pathak, S.M.; Aggarwal, D. Critical attributes of transdermal drug delivery system (TDDS)—A generic product development review. Drug Dev. Ind. Pharm. 2014, 40, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Pandey, V.; Maheshwari, R.; Ghode, P.; Tekade, R.K. Chapter 15—Cutaneous and Transdermal Drug Delivery: Techniques and Delivery Systems. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 595–650. [Google Scholar]
- Goren, A.; Kovacevic, M.; McCoy, J.; Shapiro, J. 841 Minoxidil dose response study in female pattern hair loss patients determined to be non-responders to 5% topical Minoxidil. J. Investig. Dermatol. 2017, 137, S144. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-On-A-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Ski. Res. Technol. 2020, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ventrelli, L.; Strambini, L.M.; Barillaro, G. Microneedles for Transdermal Biosensing: Current Picture and Future Direction. Adv. Healthc. Mater. 2015, 4, 2606–2640. [Google Scholar] [CrossRef] [PubMed]
- Czekalla, C.; Schönborn, K.H.; Lademann, J.; Meinke, M.C. Noninvasive Determination of Epidermal and Stratum Corneum Thickness in vivo Using Two-Photon Microscopy and Optical Coherence Tomography: Impact of Body Area, Age, and Gender. Ski. Pharmacol. Physiol. 2019, 32, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Wakabayashi, R.; Kamiya, N.; Goto, M. Solid-in-oil nanodispersions for transdermal drug delivery systems. Biotechnol. J. 2016, 11, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Ngawhirunpat, T.; Subongkot, T.; Rojanarata, T.; Opanasopit, P.; Pamornpathomkul, B. Investigation of the mechanism of enhanced skin penetration by ultradeformable liposomes. Int. J. Nanomed. 2014, 9, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Androgen actions on the human hair follicle: Perspectives. Exp. Dermatol. 2012, 22, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Deng, S.; Yang, X.; Yao, X. Immune and Non-immune Interactions in the Pathogenesis of Androgenetic Alopecia. Clin. Rev. Allergy Immunol. 2025, 68, 22. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Thiboutot, D.; Zouboulis, C.C. Cutaneous Androgen Metabolism: Basic Research and Clinical Perspectives. J. Investig. Dermatol. 2002, 119, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Lattanand, A.; Johnson, W.C. Male Pattern Alopecia A Histopathologic and Histochemical Study. J. Cutan. Pathol. 2006, 2, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sueki, H.; Stoudemayer, T.; Kligman, A.M.; Murphy, G.F. Quantitative and Ultrastructural Analysis of Inflammatory Infiltrates in Male Pattern Alopecia. Acta Derm.-Venereol. 1999, 79, 347–350. [Google Scholar] [PubMed]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Hillmann, K.; Dietz, E.; Canfield, D.; Bartels, N.G. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J. Am. Acad. Dermatol. 2011, 65, 1126–1134.e1122. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Naccarato, T. Minoxidil in the treatment of androgenetic alopecia. Dermatol. Ther. 2018, 31, e12686. [Google Scholar] [CrossRef] [PubMed]
- Asilian, A.; Farmani, A.; Saber, M. Clinical efficacy and safety of low-dose oral minoxidil versus topical solution in the improvement of androgenetic alopecia: A randomized controlled trial. J. Cosmet. Dermatol. 2023, 23, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cauhe, J.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Hermosa-Gelbard, A.; Moreno-Arrones, O.M.; Fernandez-Nieto, D.; Vaño-Galvan, S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J. Am. Acad. Dermatol. 2019, 81, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Arca, E.; Açıkgöz, G.; Taştan, H.B.; Köse, O.; Kurumlu, Z. An Open, Randomized, Comparative Study of Oral Finasteride and 5% Topical Minoxidil in Male Androgenetic Alopecia. Dermatology 2004, 209, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Coskuner, E.R.; Ozkan, B.; Culha, M.G. Sexual Problems of Men with Androgenic Alopecia Treated with 5-Alpha Reductase Inhibitors. Sex. Med. Rev. 2019, 7, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Welk, B.; McArthur, E.; Ordon, M.; Anderson, K.K.; Hayward, J.; Dixon, S. Association of Suicidality and Depression with 5α-Reductase Inhibitors. JAMA Intern. Med. 2017, 177, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ranjan, A. Follicular Unit Extraction (FUE) Hair Transplant: Curves Ahead. J. Maxillofac. Oral Surg. 2019, 18, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, B.; Neema, S.; Ghosh, K.; Singh, S.; Khera, A. Hair transplantation by follicular unit extraction for male androgenetic alopecia: A retrospective observational study from two centers. Med. J. Armed Forces India 2020, 76, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Miao, Y.; Li, X.; Qu, Q.; Liu, Y.; Li, K.; Feng, C.; Hu, Z. The relationship between self-esteem and hair transplantation satisfaction in male androgenetic alopecia patients. J. Cosmet. Dermatol. 2018, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Xu, L.J.; McCarty, J.C.; Xiao, R.; Chen, J.X.; Lee, L.N. A Scoping Review on Complications in Modern Hair Transplantation: More than Just Splitting Hairs. Aesthetic Plast. Surg. 2024, 49, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Cai, R.; Zheng, H.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-based transdermal detection and sensing devices. Lab A Chip 2023, 23, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zeng, Y.; Liu, H.-Q.; Zhang, W.; Wang, C.; Chen, C.; Li, W. Dissolving Microneedle Patch Integrated with Microspheres for Long-Acting Hair Regrowth Therapy. ACS Appl. Mater. Interfaces 2023, 15, 17532–17542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, L.; Zhou, D.; Chen, G.; Fu, Q.; Li, N. Advances in microneedles research based on promoting hair regrowth. J. Control. Release 2023, 353, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Shi, S.; Xu, Y.; Zhang, Y.; Hu, J.; Bian, Q.; Ma, X.; Ye, Y.; Yang, S.; Sheng, X.; et al. Platinum Nanozyme-Loaded Dissolving Microneedles Scavenge ROS and Promote Lineage Progression for Androgenetic Alopecia Treatment. Small Methods 2024, 9, e2401176. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Li, Y.; Zhang, Z.-W.; Dao, J.-W.; Wei, D.-X. Hydrogel forming microneedles loaded with VEGF and Ritlecitinib/polyhydroxyalkanoates nanoparticles for mini-invasive androgenetic alopecia treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Peng, H.; Yang, Y.; Lv, K.; Zhou, S.; Pan, X.; Wang, J.; Hu, Y.; Li, G.; Ma, D. Nitric oxide synergizes minoxidil delivered by transdermal hyaluronic acid liposomes for multimodal androgenetic-alopecia therapy. Bioact. Mater. 2024, 32, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, Z.; Ai, X.; Guo, T.; Feng, N. Cardamonin-loaded liposomal formulation for improving percutaneous penetration and follicular delivery for androgenetic alopecia. Drug Deliv. Transl. Res. 2024, 14, 2444–2460. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Zhao, X.; Zhang, L.; Wang, W.; Bai, D.; Liao, Y.; Wang, Z.; Wang, M.; Zhang, J. Thermodynamically stable ionic liquid microemulsions pioneer pathways for topical delivery and peptide application. Bioact. Mater. 2024, 32, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.; Batur, S.; Fu, C.; Chen, W.; Xie, Q.; Yang, C.; Zhang, Z. Multifunctional ionic liquids-mediated topical delivery of minoxidil and EGCG for treating androgenic alopecia through promoting angiogenesis and scavenging ROS. Chem. Eng. J. 2024, 491, 151888. [Google Scholar] [CrossRef]
- Noor, N.M.; Abdul-Aziz, A.; Sheikh, K.; Somavarapu, S.; Taylor, K.M.G. In Vitro Performance of Dutasteride-Nanostructured Lipid Carriers Coated with Lauric Acid-Chitosan Oligomer for Dermal Delivery. Pharmaceutics 2020, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.J.; De Souza, B.; Flynn, E.; Hagigeorges, D.; Senna, M.M. Spironolactone for treatment of female pattern hair loss. J. Am. Acad. Dermatol. 2020, 83, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kong, X.; Xu, L.; Su, Y.; Xu, S.; Pang, X.; Wang, R.; Ma, Y.; Tian, Q.; Han, L. Synergistic therapeutic effect of ginsenoside Rg3 modified minoxidil transfersomes (MXD-Rg3@TFs) on androgenic alopecia in C57BL/6 mice. Int. J. Pharm. 2024, 654, 123963. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Magar, K.T.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Stoicea, N.; Fiorda-Diaz, J.; Joseph, N.; Shabsigh, M.; Arias-Morales, C.; Gonzalez-Zacarias, A.A.; Mavarez-Martinez, A.; Marjoribanks, S.; Bergese, S.D. Advanced Analgesic Drug Delivery and Nanobiotechnology. Drugs 2017, 77, 1069–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, Y.; Blanco, A.; Tosti, A. Androgenetic Alopecia: An Update of Treatment Options. Drugs 2016, 76, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Buhl, A.E.; Waldon, D.J.; Kawabe, T.T.; Holland, J.M. Minoxidil Stimulates Mouse Vibrissae Follicles in Organ Culture. J. Investig. Dermatol. 1989, 92, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.O.; Tolentino, S.; Gratieri, T.; Cunha-Filho, M.; Lopez, R.; Gelfuso, G. Topical Treatment for Scarring and Non-Scarring Alopecia: An Overview of the Current Evidence. Clin. Cosmet. Investig. Dermatol. 2021, 14, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Xu, C.; Guo, Y.; Wen, L.; Zhou, S.; Fang, L.; Xu, J.; Zheng, H. Liposomes enhance the hair follicle delivery of minoxidil sulfate with improved treatment of androgenic alopecia. Int. J. Pharm. 2025, 677, 125642. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ye, X.; Lu, Y.; Yu, Y.; Mo, W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal. Chim. Acta 2009, 640, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Moshikur, R.M.; Shimul, I.M.; Uddin, S.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Transformation of Hydrophilic Drug into Oil-Miscible Ionic Liquids for Transdermal Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 55332–55341. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.M.; Mitragotri, S. Mechanistic study of transdermal delivery of macromolecules assisted by ionic liquids. J. Control. Release 2019, 311–312, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kiick, K.L.; Sullivan, M.O. VEGF-Encoding, Gene-Activated Collagen-Based Matrices Promote Blood Vessel Formation and Improved Wound Repair. ACS Appl. Mater. Interfaces 2023, 15, 16434–16447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, T.T.; Barber, C.L.; Jordan, M.C.; Murdock, J.; Desai, S.; Ferrara, N.; Nagy, A.; Roos, K.P.; Iruela-Arispe, M.L. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell 2007, 130, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Heloterä, H.; Alitalo, K. The VEGF Family, the Inside Story. Cell 2007, 130, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Kang, D.; Zhou, Y.; Du, L.; Qu, Q.; Wang, J.; Wen, L.; Fu, D.; Hu, Z.; et al. Microenvironmental reprogramming of human dermal papilla cells for hair follicle tissue engineering. Acta Biomater. 2023, 165, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guerrero-Juarez, C.F.; Xiao, F.; Shettigar, N.U.; Ramos, R.; Kuan, C.-H.; Lin, Y.-C.; Lomeli, L.d.J.M.; Park, J.W.; Oh, R.; et al. Hedgehog signaling reprograms hair follicle niche fibroblasts to a hyper-activated state. Dev. Cell 2022, 57, 1758–1775.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, X.; Liang, Y.; Yu, J.; Li, H.; Shokhirev, M.N.; Zheng, Y. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat. Immunol. 2022, 23, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-β1in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sowden, H.M.; Karoo, R.O.S.; Tobin, D.J. Transforming growth factor-β receptor II is preferentially expressed in the companion layer of the human anagen hair follicle. Br. J. Dermatol. 2007, 157, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Lu, B.; Li, D.; Bo, Y.; Wang, W.; Wang, Z.; Liao, Y.; Zhan, J.; Wu, C.; et al. Noninvasive and Efficient Peptide Delivery by a Novel Biocompatible Ionic Liquid. ACS Sustain. Chem. Eng. 2022, 10, 16611–16623. [Google Scholar] [CrossRef]
- Tian, L.-W.; Luo, D.; Chen, D.; Zhou, H.; Zhang, X.-C.; Yang, X.-L.; Wang, Y.-L.; Liu, W. Co-delivery of bioactive peptides by nanoliposomes for promotion of hair growth. J. Drug Deliv. Sci. Technol. 2022, 72, 103381. [Google Scholar] [CrossRef]
- Emer, J.; Levy, L.L.L. Female pattern alopecia: Current perspectives. Int. J. Women’s Health 2013, 5, 541–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jose, S.; Hughbanks, M.L.; Binder, B.Y.K.; Ingavle, G.C.; Leach, J.K. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014, 10, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tang, X.; Yang, M.; Yang, D.; Liu, J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: Preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int. J. Pharm. 2019, 554, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Schwabe, K.; Müller, R.H.; Keck, C.M. Preservation of nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Melcangi, R.C.; Bortolato, M.; Garcia-Segura, L.M.; Zitzmann, M. Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Rev. Endocr. Metab. Disord. 2015, 16, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Yazdabadi, A.; Green, J.; Sinclair, R. Successful treatment of female-pattern hair loss with spironolactone in a 9-year-old girl. Australas. J. Dermatol. 2009, 50, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Shamma, R.; Aburahma, M. Follicular delivery of spironolactone via nanostructured lipid carriers for management of alopecia. Int. J. Nanomed. 2014, 9, 5449–5460. [Google Scholar]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a transdermal drug delivery system: Dermal kinetics and recent developments. WIREs Nanomed. Nanobiotechnol. 2023, 16, e1918. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Andrade, J.F.M.; Matos, B.N.; Rocho, R.V.; Barbalho, G.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment. Pharmaceutics 2024, 16, 1524. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Cutrignelli, A.; Denora, N.; Laquintana, V.; Lopalco, A.; Selva, S.; Ragni, L.; Tongiani, S.; Franco, M. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-β-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev. Ind. Pharm. 2014, 41, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-J.; Park, J.-Y.; Choi, S.; Lee, J.-B.; Jung, H.; Kim, T.-D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.-J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Blakeborough, L.; Harries, M.; Haslam, I.S. Cholesterol homeostasis: Links to hair follicle biology and hair disorders. Exp. Dermatol. 2019, 29, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Smart, E.; Haslam, I.S. Localisation and regulation of cholesterol transporters in the human hair follicle: Mapping changes across the hair cycle. Histochem. Cell Biol. 2021, 155, 529–545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Zhou, L.; Zhao, H.; Li, S. Advances in Transdermal Delivery Systems for Treating Androgenetic Alopecia. Pharmaceutics 2025, 17, 984. https://doi.org/10.3390/pharmaceutics17080984
Xu S, Zhou L, Zhao H, Li S. Advances in Transdermal Delivery Systems for Treating Androgenetic Alopecia. Pharmaceutics. 2025; 17(8):984. https://doi.org/10.3390/pharmaceutics17080984
Chicago/Turabian StyleXu, Shilong, Lian Zhou, Haodong Zhao, and Siwen Li. 2025. "Advances in Transdermal Delivery Systems for Treating Androgenetic Alopecia" Pharmaceutics 17, no. 8: 984. https://doi.org/10.3390/pharmaceutics17080984
APA StyleXu, S., Zhou, L., Zhao, H., & Li, S. (2025). Advances in Transdermal Delivery Systems for Treating Androgenetic Alopecia. Pharmaceutics, 17(8), 984. https://doi.org/10.3390/pharmaceutics17080984






