Bioactive Chalcone-Loaded Mesoporous Silica KIT-6 Nanocarrier: A Promising Strategy for Inflammation and Pain Management in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the KIT-6 Mesoporous Material
2.2. Encapsulation of 4-Chloro-Chalcone Within KIT-6
2.3. Characterization
2.4. Synthesis of Dibenzoylacetone (1E,4E)-1,5-Bis(4-Chlorophenyl)Penta-1,4-Dien-3-One (4-Cl)
2.5. Assessment of Biological Activities
Animals
2.6. Assessment of Toxicological Effects
2.6.1. Cytotoxicity
2.6.2. Acute Toxicity
2.7. Formalin-Induced Nociceptive Behavior
2.8. Anti-Inflammatory Activity
Induction of Abdominal Edema by Carrageenan
2.9. Statistical Analysis
3. Results
3.1. Structural Data of Chalcone 4-Cl
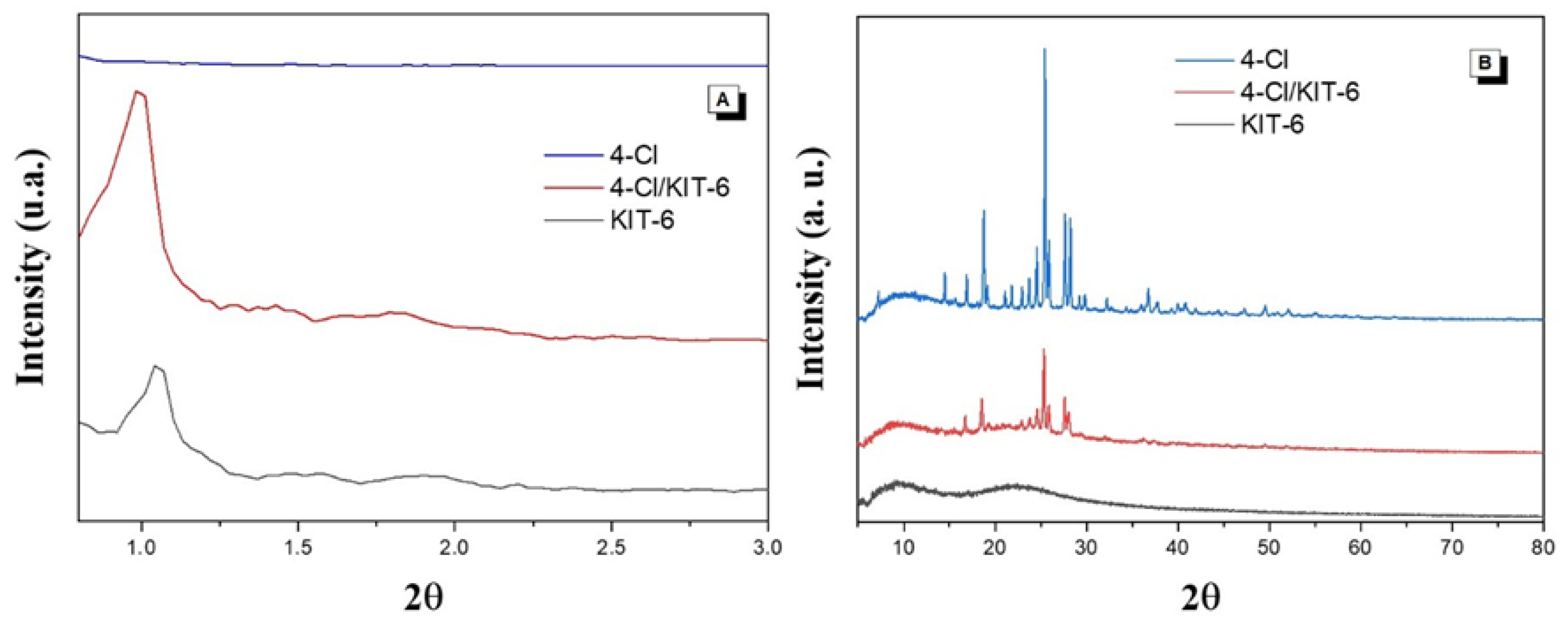
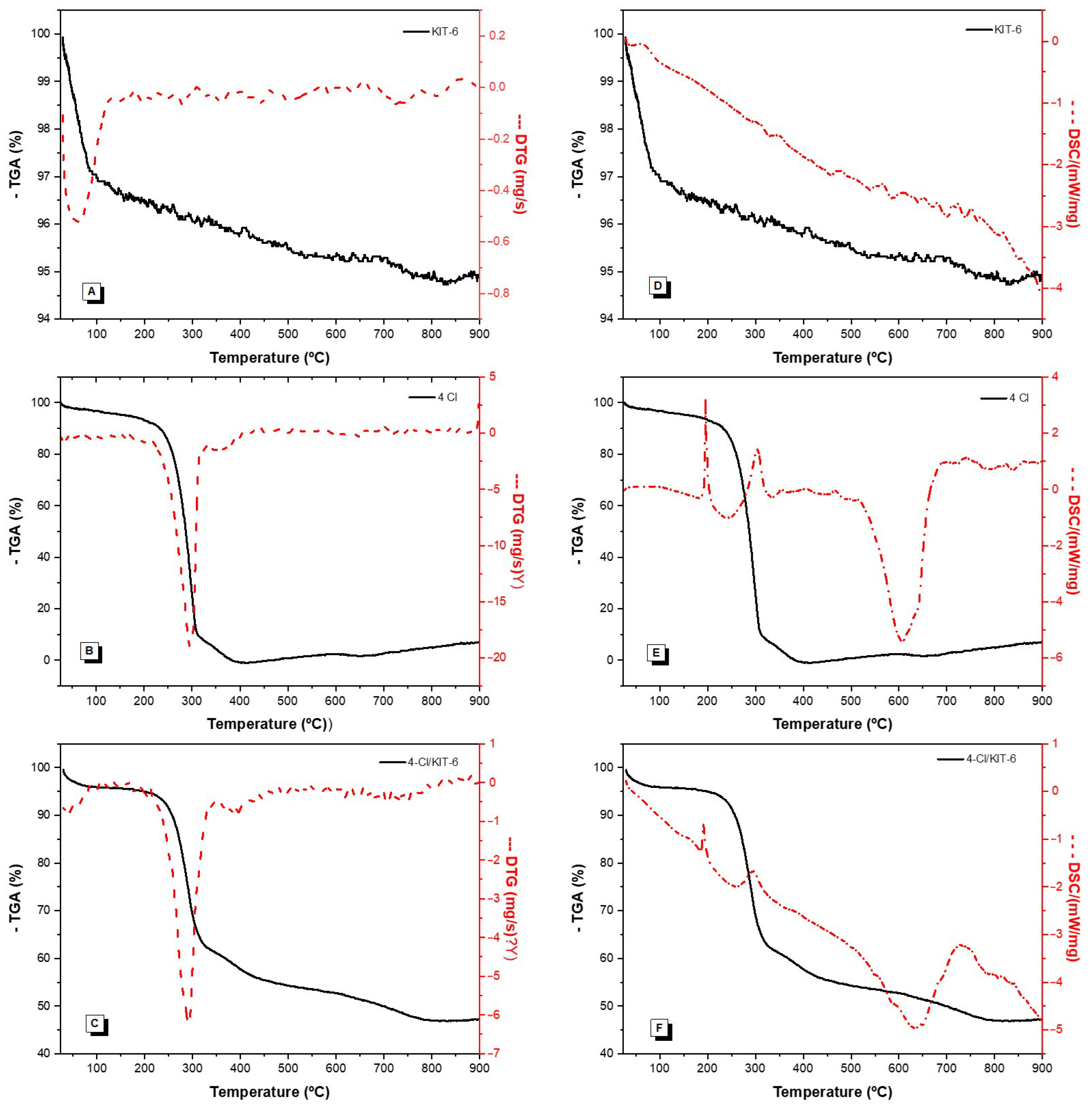
3.2. Characterization
3.3. In Vitro Cytotoxicity Assessment
3.4. Toxicological Assessment
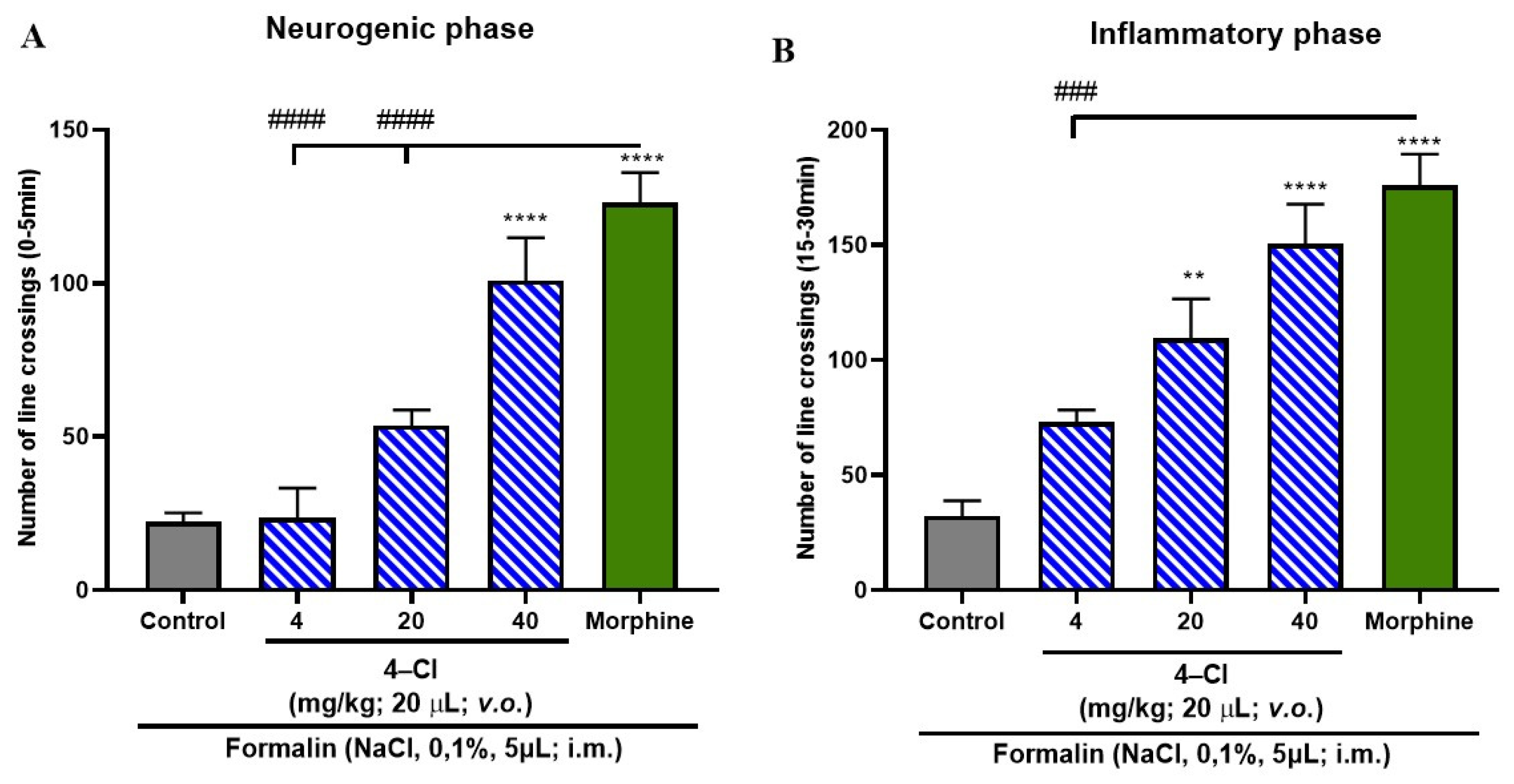
3.5. Formalin-Induced Nociceptive Behavior
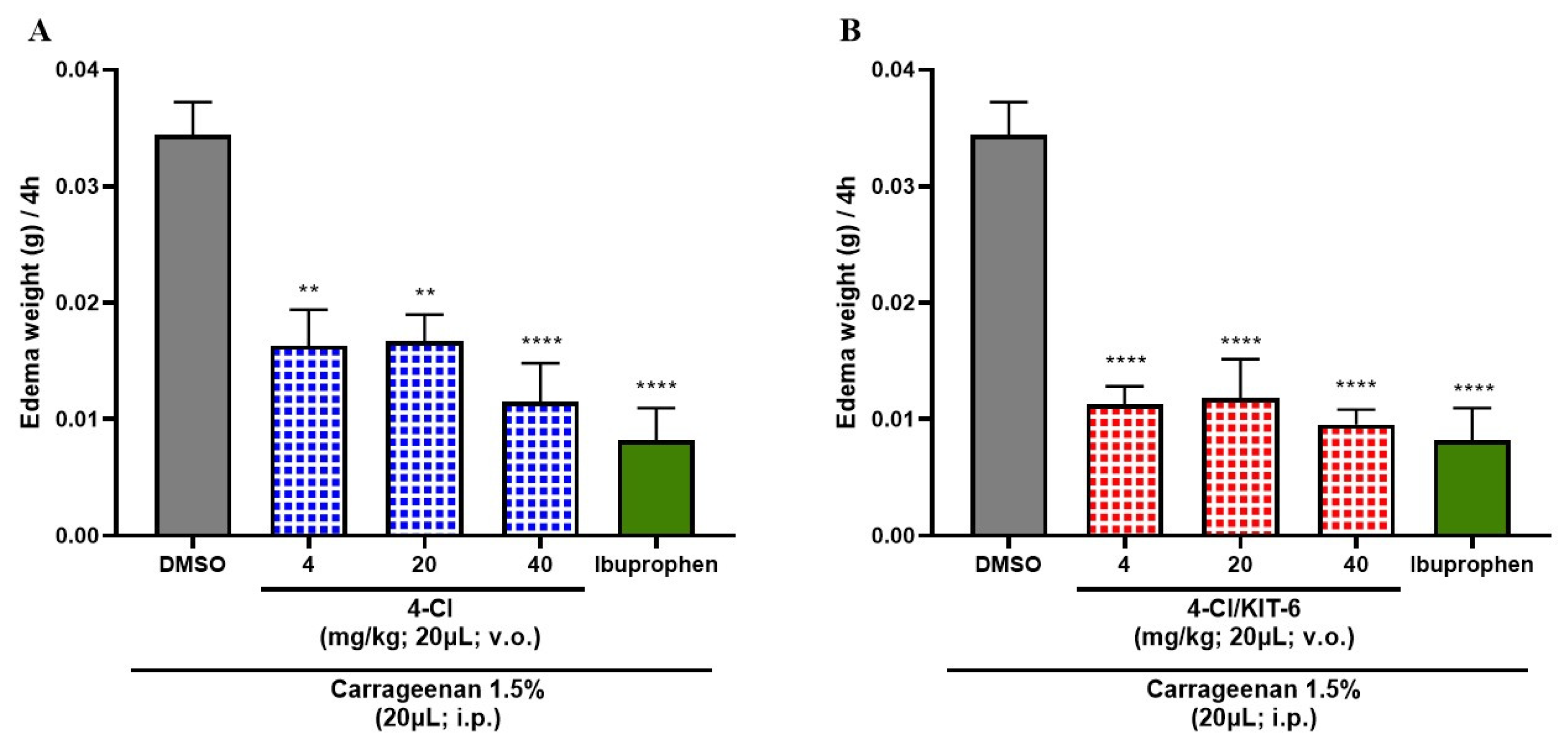
3.6. Anti-Inflammatory Effect of 4-Cl and 4-Cl/KIT-6 System
4. Discussion
4.1. Characterization
4.2. Cytotoxicity
4.3. Acute Toxicity 4-Cl, KIT-6 and 4-Cl/KIT-6
4.4. Formalin-Induced Antinociceptive Effect
4.5. Analysis of the Anti-Inflammatory Effects of 4-Cl and Its Controlled Release via KIT-6
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Noor, F.; Nabil, M.; Saeed, K. Network pharmacology approach for medicinal plants: Review and assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, G.; Xu, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.S.F.; Mendes, A.B.; Tanimaru, A.N.; Gonzalez, F.G.; Carvalho, C.F.F. Non-steroidal anti-inflammatory drugs (NSAIDs) x microbiota: An intestinal symbiosis or dysbiosis? Rev. Med. 2024, 103, e-210041. [Google Scholar] [CrossRef]
- Cohen, M.J.; Jangro, W.C.; Neff, D. Pathophysiology of Pain. In Challenging Neuropathic Pain Syndromes; Elsevier: Philadelphia, PA, USA, 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah, S.A.; Abdul, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ezike, T.C.; Yusuf, S.; Omoboyowa, T.V. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e16875. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Umeh, C.D.; Nwajiobi-Princewill, O.E. Phytochemical characterization, anti-diarrhoeal, analgesic, anti-inflammatory activities and toxicity profile of Ananas comosus (L.) Merr (pineapple) leaf in albino rats. J. Ethnopharmacol. 2024, 319, 117224. [Google Scholar] [CrossRef]
- Ekweogu, C.N.; Ozioko, J.C.; Ugbogu, E.A. Phytochemical profiling, toxicity studies, wound healing, analgesic and anti-inflammatory activities of Musa paradisiaca L. Musaceae (Plantain) stem extract in rats. J. Ethnopharmacol. 2024, 322, 117639. [Google Scholar] [CrossRef]
- Singh, K.; Yadav, A.; Sharma, N.; Saini, V. Nanomedicine and drug delivery: A comprehensive review of applications and challenges. Nano-Struct. Nano-Objects 2024, 40, 101403. [Google Scholar] [CrossRef]
- Zhu, Y.-S.; Wu, J.; Zhi, F. Advances in conjugate drug delivery system: Opportunities and challenges. Int. J. Pharm. 2024, 644, 124867. [Google Scholar] [CrossRef]
- Lang, X.; Yan, X.; Yu, H.; Li, M. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Aziz, M.A.; El-Said, W.A. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Di Stefano, A. Nanotechnology in targeted drug delivery. Int. J. Mol. Sci. 2023, 24, 8194. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.C.; Akhtar, M.; Jain, P. Biotechnology and nanotechnology drug delivery: A review. J. Pharm. Pharmacol. 2021, 9, 127–132. [Google Scholar] [CrossRef]
- Razavi, S.; Thakur, A.; Agrawal, A.; Rai, P. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef]
- Montané, X.; Pujol, M.; Fornaguera, C.; Solans, C. Encapsulation for cancer therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef]
- Zare, M.; Hashemi, A.; Ghasemi, Y.; Ghaee, A. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Ahmed, A.; Jassam, N.; Mahdi, A.A. Drug loading methods and kinetic release models using of mesoporous silica nanoparticles as a drug delivery system: A review. S. Afr. J. Chem. Eng. 2024, 50, 187–198. [Google Scholar] [CrossRef]
- Pablos, J.L.; López-Noriega, A.; Vallet-Regí, M. Regenerative medicine: Hydrogels and mesoporous silica nanoparticles. Mater. Today Bio 2024, 25, 101342. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.M.; Fernandes, F.R.D.; dos Santos, A.P.B.; dos Santos Neto, M.M.; Teixeira, A.M.R.; Marinho, E.S.; de Menezes, J.E.S.A.; Garcia, K.G.V.; Santos, A.G.D.; dos Santos, H.S. NiO and ZrO2 impregnation in KIT-6 by excess solvent and mechanochemistry: A comparison. Mater. Lett. 2023, 345, 134512. [Google Scholar] [CrossRef]
- Ferreira, M.K.A.; Da Silva, A.W.; Moura, A.L.S.; Sales, K.V.B.; Marinho, E.M.; Cardoso, J.N.M.; Marinho, M.M.; Bandeira, P.N.; Magalhães, F.E.; Marinho, E.S.; et al. Chalcones reverse the anxiety and convulsive behavior of adult zebrafish. Epilepsy Behav. 2021, 117, 107881. [Google Scholar] [CrossRef]
- Dan, W.; Dai, J. Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur. J. Med. Chem. 2020, 187, 111980. [Google Scholar] [CrossRef]
- Maluleka, M.M.; Mphahlele, M.J. Crystal structure of (1E,4E)-1,5-bis(4-chlorophenyl) penta-1,4-dien-3-one, C17H12Cl2O. Z. Kristallogr. NCS 2017, 232, 1049–1050. [Google Scholar] [CrossRef]
- McEntire, D.M.; Kirkpatrick, D.R.; Dueck, N.P.; Kerfeld, M.J.; Smith, T.A.; Nelson, T.J.; Reisbig, M.D.; Agrawal, D.K. Pain transduction: A pharmacologic perspective. Expert Rev. Clin. Pharmacol. 2016, 9, 1069–1080. [Google Scholar] [CrossRef]
- Kleitz, F. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 3, 2136–2137. [Google Scholar] [CrossRef]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.P.N.; et al. Electrosprayed mesoporous particles for improved aqueous solubility of a poorly water soluble anticancer agent: In vitro and ex vivo evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef]
- Pinto, L.M.A.; Cavalcanti, B.C.; de Moura, M.A.; da Silva, T.D.; de Lima, J.G.; de Menezes, J.E.S.A.; Teixeira, A.M.R. Preparation and characterization of a synthetic curcumin analog inclusion complex and preliminary evaluation of in vitro antileishmanial activity. Int. J. Pharm. 2020, 589, 119764. [Google Scholar] [CrossRef]
- Ferreira, M.K.A.; da Silva, A.P.; de Moura, M.A.; Cavalcanti, B.C.; de Lima, J.G.; de Menezes, J.E.S.A.; Teixeira, A.M.R. Anxiolytic-like effect of chalcone N-{4’[(2E)-3-(3-nitrophenyl)-1-(phenyl)prop-2-en-1-one]} acetamide on adult zebrafish (Danio rerio): Involvement of the 5-HT system. Biochem. Biophys. Res. Commun. 2020, 526, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 1992; No. 203. [Google Scholar]
- Bezerra, A.J.N.; Sousa, D.F.; Bandeira, P.N.; Lima, B.S.; Marinho, E.M.; de Menezes, J.E.S.A.; dos Santos, H.S. Antinociceptive effect of triterpene acetyl aleuritolic acid isolated from Croton zehntneri in adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2021, 534, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.W.D.S.; Sousa, B.L.; Moura, L.F.W.G.; Rebouças, E.L.; Coutinho, M.R.; Silva, A.W.; Chaves, R.P.; Carneiro, R.F.; Bezerra, E.H.S.; Guedes, M.I.F.; et al. Codium isthmocladum lectin 1 (CiL-1): Interaction with N-glycans explains antinociceptive and anti-inflammatory activities in adult zebrafish (Danio rerio). Int. J. Biol. Macromol. 2022, 208, 1082–1089. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449. [Google Scholar] [CrossRef]
- Pinto, L.G.; Pinho-Ribeiro, F.A.; Verri, W.A. Editorial: Cytokines and Pain. Front. Immunol. 2021, 12, 788578. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, L.; He, H.; Zhang, X.; Zhang, W.; Wang, X.; Zhang, H.; Hu, L. 4-Methylesculetin attenuates inflammatory pain via inhibition of Sp1-TRPV1 and inflammation-related signaling pathways. Int. Immunopharmacol. 2025, 152, 114379. [Google Scholar] [CrossRef]
- Agostinho, J.D.L.; da Silva, L.S.; Bezerra, R.R.; de Sousa, D.F.; Bandeira, P.N.; Lima, B.S.; de Lima, S.G.; Marinho, E.M.; de Menezes, J.E.S.A.; Santos, H.S.; et al. Chemical diversity of the herbal decoction of Plectranthus ornatus and its anti-nociceptive and anti-inflammatory activities in zebrafish models. J. Ethnopharmacol. 2025, 340, 119235. [Google Scholar] [CrossRef]
- Park, J.; Lee, C.; Kim, Y.T. Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation. Pharmaceuticals 2023, 16, 941. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined chemoresistive and in situ FTIR spectroscopy study of nanoporous NiO films for light-activated nitrogen dioxide and acetone gas sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]
- Renuka, V.; Revathi, B.K.; Jonathan, D.R.; Priya, M.K.; Asirvatham, P.S. Synthesis, growth and characterization of a new NLO active chalcone derivative–4-chloro-N-{3-[(2E)-3-(methoxyphenyl)prop-2-enoyl]phenyl}benzamide monohydrate. J. Mol. Struct. 2019, 1176, 838–846. [Google Scholar] [CrossRef]
- Subhan, F.; Khan, M.S.; Kim, I.S. Facile functionalization of 3-D ordered KIT-6 with cuprous oxide for deep desulfurization. Chem. Eng. J. 2017, 330, 372–382. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Hozayen, W.G.; Abdel-Wahhab, M.A.; Ahmed, F.; El-Sherbiny, M. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Hosseini, S.M.; Madadlou, A. Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials 2022, 12, 2016. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Zhang, Y.; Yang, M.; Liang, J.; Wang, X.; Wang, Y.; Jin, M. NAD+-Carrying Mesoporous Silica Nanoparticles Can Prevent Oxidative Stress-Induced Energy Failures of Both Rodent Astrocytes and PC12 Cells. PLoS ONE 2013, 8, e74100. [Google Scholar] [CrossRef]
- Takac, P.; Horvathova, K.; Jurikova, M.; Hudecova, D.; Balkova, J.; Mojzisova, G.; Ondrias, K.; Kollar, P. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Wang, Z.; Zhang, X.; Liu, Y.; Xu, H.; Li, X.; Chen, X.; Guo, L.; Zhang, L. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci. Rep. 2017, 7, 9873. [Google Scholar] [CrossRef]
- Kar, S.; Yang, S. Introducing third-generation periodic table descriptors for nano-qRASTR modeling of zebrafish toxicity of metal oxide nanoparticles. Beilstein J. Nanotechnol. 2024, 15, 1142–1152. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhang, Y.; Liu, J.; Wu, J.; Gao, Y.; Chen, G. Machine learning models for quantitatively prediction of toxicity in macrophages induced by metal oxide nanoparticles. Chemosphere 2025, 370, 143923. [Google Scholar] [CrossRef]
- Bhatane, D.; Patel, A.; Patel, S.; Jani, G.; Tandel, V.; Patel, K. Potential applications of mesoporous silica nanoparticles for the treatment of neurological disorders. J. Drug Deliv. Sci. Technol. 2023, 89, 104970. [Google Scholar] [CrossRef]
- Sharif, F.; Ahmad, Z.; Azam, A.; Masoud, M.S.; Imran, M.; Hussain, A.; Arshad, M.; Ali, I.; Khan, Z.A. Mesoporous silica nanoparticles as a compound delivery system in zebrafish embryos. Int. J. Nanomed. 2012, 7, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. The Controlled Release of Drugs and Bioactive Compounds from Mesoporous Silica Nanoparticles. Curr. Drug Deliv. 2016, 13, 839–856. [Google Scholar] [CrossRef]
- Ahmed, T.; Siddiqui, M.N.; Ullah, M.F.; Khan, M.M.; Ali, A.; Afzal, O.; Shah, S.; Shahid, M. Synthesis, characterization, molecular docking, analgesic, antiplatelet and anticoagulant effects of dibenzylidene ketone derivatives. Chem. Cent. J. 2018, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.M.; Liu, J.W.; Zhao, S.L.; Yang, Y.; Xu, H.Y.; Wang, Q.; Li, J.X.; Wang, Z.Y. Antinociceptive activities of a novel diarylpentanoid analogue, 2-benzoyl-6-(3-bromo-4-hydroxybenzylidene)cyclohexen-1-ol, and its possible mechanisms of action in mice. Sci. Rep. 2021, 11, 24121. [Google Scholar] [CrossRef]
- da Silva, A.; Lepetre-Mouelhi, S.; Couvreur, P. Micro- and nanocarriers for pain alleviation. Adv. Drug Deliv. Rev. 2022, 187, 114359. [Google Scholar] [CrossRef] [PubMed]
- Almeida Junior, S.; dos Santos, P.; Lima, J.P.; Cavalcanti, B.C.; Silva, A.P.; de Moura, M.A.; de Lima, J.G.; Teixeira, A.M.R. Incorporation of indomethacin into a mesoporous silica nanoparticle enhances the anti-inflammatory effect. Eur. J. Pharm. Sci. 2021, 157, 105601. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Yu, M.; Lu, X.; Jin, Y.; Zhang, Z.; Hu, Y.; Wu, Z.; Yu, Q.; Gong, P. An injectable mesoporous silica-based analgesic delivery system prolongs the duration of sciatic nerve block in mice with minimal toxicity. Acta Biomater. 2021, 135, 638–649. [Google Scholar] [CrossRef]
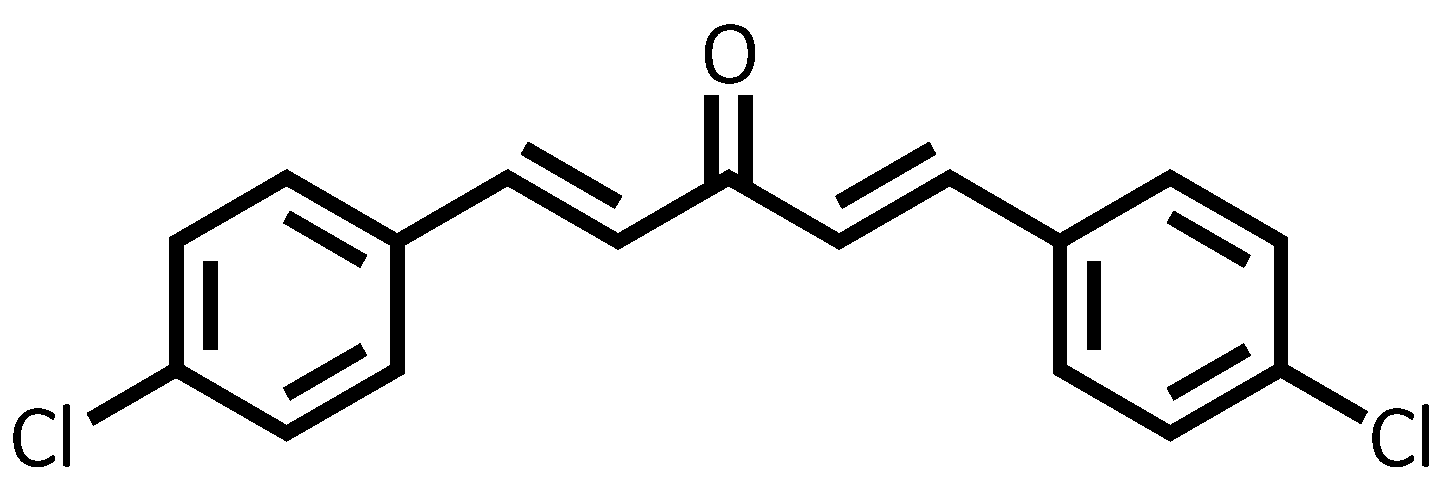







| C | δC | δH |
|---|---|---|
| 1 | 133.2 | |
| 2/6 | 129.3 | 7.40 (d, J = 8.0 Hz) |
| 3/5 | 129.5 | 7.55 (d, J = 8.0 Hz) |
| 4 | 136.5 | |
| Cα | 125.7 | 7.04 (d, J = 15.9 Hz) |
| Cβ | 142.0 | 7.69 (d, J = 15.9 Hz) |
| C=O | 188.3 |
| Samples | Mass Loss (%) | ||||
|---|---|---|---|---|---|
| I | II | I | II | Residue | |
| KIT-6 | 25–132 | - | 4 | - | 96 |
| 4-Cl | 25–331 | 331–408 | 95 | 5 | 0 |
| 4-Cl/KIT-6 | 25–90 | 90–343 | 5 | 34 | 61 |
| Sample | SBET (m2/g) a | VT (cm3/g) b | dP (nm) c |
|---|---|---|---|
| KIT-6 | 404 | 0.403 | 5.38 |
| 4-Cl/KIT-6 | 60 | 0.111 | 7.07 |
| Sample | Zeta Potential (mV) |
|---|---|
| 4-Cl | −20.3 ± 0.98 |
| KIT-6 | −35.3 ± 2.30 |
| 4-Cl/KIT-6 | −44.0 ± 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.K.A.; Mendes, F.R.S.; Marinho, E.S.; Lima de Albuquerque, R.; Guedes, J.M.; Teixeira, I.M.M.; Menezes, R.R.P.P.B.d.; Caldeira, V.P.S.; Santos, A.G.D.; Frederico, M.J.S.; et al. Bioactive Chalcone-Loaded Mesoporous Silica KIT-6 Nanocarrier: A Promising Strategy for Inflammation and Pain Management in Zebrafish. Pharmaceutics 2025, 17, 981. https://doi.org/10.3390/pharmaceutics17080981
Ferreira MKA, Mendes FRS, Marinho ES, Lima de Albuquerque R, Guedes JM, Teixeira IMM, Menezes RRPPBd, Caldeira VPS, Santos AGD, Frederico MJS, et al. Bioactive Chalcone-Loaded Mesoporous Silica KIT-6 Nanocarrier: A Promising Strategy for Inflammation and Pain Management in Zebrafish. Pharmaceutics. 2025; 17(8):981. https://doi.org/10.3390/pharmaceutics17080981
Chicago/Turabian StyleFerreira, Maria Kueirislene Amâncio, Francisco Rogenio Silva Mendes, Emmanuel Silva Marinho, Roberto Lima de Albuquerque, Jesyka Macedo Guedes, Izabell Maria Martins Teixeira, Ramon Róseo Paula Pessoa Bezerra de Menezes, Vinicius Patricio Santos Caldeira, Anne Gabriella Dias Santos, Marisa Jádna Silva Frederico, and et al. 2025. "Bioactive Chalcone-Loaded Mesoporous Silica KIT-6 Nanocarrier: A Promising Strategy for Inflammation and Pain Management in Zebrafish" Pharmaceutics 17, no. 8: 981. https://doi.org/10.3390/pharmaceutics17080981
APA StyleFerreira, M. K. A., Mendes, F. R. S., Marinho, E. S., Lima de Albuquerque, R., Guedes, J. M., Teixeira, I. M. M., Menezes, R. R. P. P. B. d., Caldeira, V. P. S., Santos, A. G. D., Frederico, M. J. S., Barreto, A. C. H., Domingues, I., Rodrigues, T. H. S., Menezes, J. E. S. A. d., & Santos, H. S. d. (2025). Bioactive Chalcone-Loaded Mesoporous Silica KIT-6 Nanocarrier: A Promising Strategy for Inflammation and Pain Management in Zebrafish. Pharmaceutics, 17(8), 981. https://doi.org/10.3390/pharmaceutics17080981








