Enhancing Parenteral Nutrition via Supplementation with Antioxidant Lutein in Human Serum Albumin-Based Nanosuspension
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AlbLuteN
2.3. Formulation Characterization
2.3.1. Physicochemical Characterization of AlbLuteN
2.3.2. Lutein Crystallinity
2.3.3. Quantitative Analysis
2.3.4. Lutein Encapsulation in Nanoparticles
2.3.5. Nanoparticle Behavior upon Dilution
2.4. Parenteral Nutrition Stability Study
2.5. Statistical Analysis and Software
3. Results
3.1. AlbLuteN Characterization
3.2. Parenteral Nutrition Stability Study
4. Discussion
- Meet IV drug quality standards to ensure safe clinical application;
- Minimize excipient content to reduce the risk of potential side effects and interactions with PN;
- Ensure a reproducible and scalable formulation process to facilitate large-scale production;
- Efficiently load lutein—the higher the lutein-to-matrix ratio, the lower the total formulation amount required to deliver a given dose, reducing the risk of potential interactions with PN;
- Remain stable post-lyophilization, ideally without requiring additional cryoprotectants that could interfere with PN components.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PN | Parenteral nutrition |
| AlbLuteN | Albumin–lutein nanosuspension |
| PFAT5 | Percentage of fat residing in globules larger than 5 µm |
| Z-average | Intensity-weighted mean hydrodynamic diameter |
| IV | Intravenous |
| IFALD | Intestinal failure-associated liver disease |
| nabTM | Nanoparticle albumin-bound |
| API | Active pharmaceutical ingredient |
| FDA | Food and Drug Administration |
| HPLC | High-performance liquid chromatography |
| PDI | Polydispersity index |
| DSC | Differential scanning calorimetry |
| DAD | Diode-array detection |
| DL% | Drug loading |
| EE% | Entrapment efficiency |
| NL% | Nanoparticle lutein |
| FTIR | Fourier transform infrared |
| LPe | Lipoflex Peri |
| OPe | Omegaflex Peri |
| OSp | Omegaflex Special |
| OPl | Omegaflex Plus |
| T0 | Immediately after sample preparation |
| T24 | After 24 h of storage |
| BCS | Biopharmaceutical classification system |
| GRAS | Generally recognized as safe |
References
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Farhan, M.; McCallion, N.; Bennett, J.; Cram, A.; O’Brien, F. Stability and Compatibility of Parenteral Nutrition Solutions; a Review of Influencing Factors. Eur. J. Pharm. Biopharm. 2023, 187, 87–95. [Google Scholar] [CrossRef]
- Monczka, J.; Ayers, P.; Berger, M.M.; Wischmeyer, P.E. Safety and Quality of Parenteral Nutrition: Areas for Improvement and Future Perspectives. Am. J. Health. Syst. Pharm. 2024, 81, S121–S136. [Google Scholar] [CrossRef]
- Lal, S.; Paine, P.; Tack, J.; Aziz, Q.; Barazzoni, R.; Cuerda, C.; Jeppesen, P.; Joly, F.; Lamprecht, G.; Mundi, M.; et al. Avoiding the Use of Long-Term Parenteral Support in Patients without Intestinal Failure: A Position Paper from the European Society of Clinical Nutrition & Metabolism, the European Society of Neurogastroenterology and Motility and the Rome Foundation for Disorders of Gut–Brain Interaction. Clin. Nutr. 2024, 43, 2279–2282. [Google Scholar] [CrossRef]
- Jing, Z.; Hongyan, X.; Jingjing, M.; Mujuan, P.; Shiyu, M.; Ying, S.; Yan, H. Adverse Events Associated with Parenteral Nutrition Support Therapy: A Pharmacovigilance Study. J. Parenter. Enter. Nutr. 2025, 49, 122–131. [Google Scholar] [CrossRef]
- Kumpf, V.J.; Gray, B.; Monczka, J.; Zeraschi, S.; Klek, S. Parenteral Nutrition at Home/Long-Term Parenteral Nutrition. Am. J. Health. Syst. Pharm. 2024, 81, S112–S120. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rosseel, Z.; De Waele, E.; Vinken, M. Parenteral Nutrition-Associated Liver Injury: Clinical Relevance and Mechanistic Insights. Toxicol. Sci. 2024, 199, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, W. Health Benefits and Future Research of Phytochemicals: A Literature Review. J. Nutr. 2025, 155, 87–101. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Ohia, S.E.; Moriyama, H.; Chakraborty, S.; Wang, C.-K.; Song, Y.S.; Goel, A.; Bagchi, M.; Bagchi, D. Current Insights on the Photoprotective Mechanism of the Macular Carotenoids, Lutein and Zeaxanthin: Safety, Efficacy and Bio-Delivery. J. Am. Nutr. Assoc. 2024, 43, 505–518. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From Carotenoid Intake to Carotenoid Blood and Tissue Concentrations—Implications for Dietary Intake Recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Żółnowska, I.; Gostyńska-Stawna, A.; Stawny, M. Molecular Mechanisms Underlying Hepatoprotective Activity of Lutein in the Context of Intestinal Failure-Associated Liver Disease. Pharmacol. Res. 2024, 209, 107421. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Gao, Y.; Wei, N.; Zhao, X.; Wang, C.; Li, Y.; Xiu, X.; Cui, J. Direct Comparison of Two Albumin-Based Paclitaxel-Loaded Nanoparticle Formulations: Is the Crosslinked Version More Advantageous? Int. J. Pharm. 2014, 468, 15–25. [Google Scholar] [CrossRef]
- Adick, A.; Hoheisel, W.; Schneid, S.; Mulac, D.; Azhdari, S.; Langer, K. Challenges of Nanoparticle Albumin Bound (NabTM) Technology: Comparative Study of Abraxane® with a Newly Developed Albumin-Stabilized Itraconazole Nanosuspension. Eur. J. Pharm. Biopharm. 2023, 193, 129–143. [Google Scholar] [CrossRef]
- Adick, A.; Hoheisel, W.; Schneid, S.; Hester, S.; Langer, K. Development of a Screening Platform for the Formulation of Poorly Water-Soluble Drugs as Albumin-Stabilized Nanosuspensions Using NabTM Technology. Int. J. Pharm. 2024, 662, 124491. [Google Scholar] [CrossRef]
- Anhorn, M.G.; Mahler, H.-C.; Langer, K. Freeze Drying of Human Serum Albumin (HSA) Nanoparticles with Different Excipients. Int. J. Pharm. 2008, 363, 162–169. [Google Scholar] [CrossRef]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Tejada, M.L.S.D. Application of a UV–Vis Detection-HPLC Method for a Rapid Determination of Lycopene and β-Carotene in Vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Yasuda, K.; Maeda, H.; Kinoshita, R.; Minayoshi, Y.; Mizuta, Y.; Nakamura, Y.; Imoto, S.; Nishi, K.; Yamasaki, K.; Sakuragi, M.; et al. Encapsulation of an Antioxidant in Redox-Sensitive Self-Assembled Albumin Nanoparticles for the Treatment of Hepatitis. ACS Nano 2023, 17, 16668–16681. [Google Scholar] [CrossRef]
- Dettlaff, K.; Anglart, G.; Gruszczyńska, A.; Jelińska, A. Compatibility Studies of Selected Multichamber Bag Parenteral Nutrition with Fluconazole. Nutrition 2024, 123, 112417. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability Studies of Parenteral Nutrition with a High Dose of Vitamin C. J. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef]
- Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Stawny, M. Improving the Safety of Clinical Management of COVID-19 Patients Receiving Aminoglycosides and Parenteral Nutrition: Y-Site Compatibility Studies. J. Pharm. Sci. 2023, 112, 2597–2603. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph—Optical Spectroscopy Software. Version 1.2.16.1. 2022. Available online: https://www.effemm2.de/spectragryph/ (accessed on 18 June 2025).
- Song, X.; Luo, Y.; Zhao, W.; Liu, S.; Wang, Y.; Zhang, H. Preparation and Characterization of Lutein Co-Amorphous Formulation with Enhanced Solubility and Dissolution. Foods 2024, 13, 2029. [Google Scholar] [CrossRef]
- Galiyeva, A.; Daribay, A.; Zhumagaliyeva, T.; Zhaparova, L.; Sadyrbekov, D.; Tazhbayev, Y. Human Serum Albumin Nanoparticles: Synthesis, Optimization and Immobilization with Antituberculosis Drugs. Polymers 2023, 15, 2774. [Google Scholar] [CrossRef]
- Anselmo, C.D.S.; Mendes, T.D.C.; Honorio, T.D.S.; Do Carmo, F.A.; Cabral, L.M.; De Sousa, V.P. Development and Validation of a Dissolution Test for Lutein Tablets and Evaluation of Intestinal Permeability. Food Chem. 2016, 210, 63–69. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Pandey, M.; Madheswaran, T.; Kesharwani, P.; Tekade, R.K. Drug–Excipient Interaction and Incompatibilities. In Dosage Form Design Parameters; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–402. ISBN 978-0-12-814421-3. [Google Scholar]
- Peshkovsky, A.S.; Bystryak, S. Continuous-Flow Production of a Pharmaceutical Nanoemulsion by High-Amplitude Ultrasound: Process Scale-Up. Chem. Eng. Process. Process Intensif. 2014, 82, 132–136. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Zhang, J.; Li, Z.; Kang, W.; He, H.; Wu, Z.; Dong, Z. Nanoemulsification of Soybean Oil Using Ultrasonic Microreactor: Process Optimization, Scale-up and Numbering-up in Series. Ultrason. Sonochem. 2023, 97, 106451. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Hoseini, B.; Jaafari, M.R.; Golabpour, A.; Momtazi-Borojeni, A.A.; Karimi, M.; Eslami, S. Application of Ensemble Machine Learning Approach to Assess the Factors Affecting Size and Polydispersity Index of Liposomal Nanoparticles. Sci. Rep. 2023, 13, 18012. [Google Scholar] [CrossRef]
- European Medicines Agency, Abraxane, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane (accessed on 18 June 2025).
- Roethlisberger, D.; Mahler, H.-C.; Altenburger, U.; Pappenberger, A. If Euhydric and Isotonic Do Not Work, What Are Acceptable pH and Osmolality for Parenteral Drug Dosage Forms? J. Pharm. Sci. 2017, 106, 446–456. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, X.; Pang, X.; Pang, Z.; Zhao, J.; Zhang, Z.; Jiang, T.; Xu, W.; Zhang, Q.; Jiang, X. Lapatinib-Loaded Human Serum Albumin Nanoparticles for the Prevention and Treatment of Triple-Negative Breast Cancer Metastasis to the Brain. Oncotarget 2016, 7, 34038–34051. [Google Scholar] [CrossRef]
- Qu, N.; Sun, Y.; Li, Y.; Hao, F.; Qiu, P.; Teng, L.; Xie, J.; Gao, Y. Docetaxel-Loaded Human Serum Albumin (HSA) Nanoparticles: Synthesis, Characterization, and Evaluation. Biomed. Eng. OnLine 2019, 18, 11. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Yao, Y.; Qiu, Q.; Wu, X.-W.; Cai, Z.; Xu, S.; Liang, X. Lutein Supplementation Improves Visual Performance in Chinese Drivers: 1-Year Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2013, 29, 958–964. [Google Scholar] [CrossRef]
- Feng, L.; Nie, K.; Jiang, H.; Fan, W. Effects of Lutein Supplementation in Age-Related Macular Degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar] [CrossRef]
- Yoshida, T.; Takagi, Y.; Igarashi-Yokoi, T.; Ohno-Matsui, K. Efficacy of Lutein Supplements on Macular Pigment Optical Density in Highly Myopic Individuals: A Randomized Controlled Trial. Medicine 2023, 102, e33280. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-evaluation of Lutein Preparations Other than Lutein with High Concentrations of Total Saponified Carotenoids at Levels of at Least 80%. EFSA J. 2011, 9, 2144. [CrossRef]
- Toragall, V.; Srirangam, P.; Jayapala, N.; Baskaran, V. Lutein Encapsulated Oleic—Linoleic Acid Nanoemulsion Boosts Oral Bioavailability of the Eye Protective Carotenoid Lutein in Rat Model. Mater. Today Commun. 2021, 28, 102522. [Google Scholar] [CrossRef]
- Nilsson, N.; Storesund, I.; Tho, I.; Nezvalova-Henriksen, K. Co-Administration of Drugs with Parenteral Nutrition in the Neonatal Intensive Care Unit-Physical Compatibility between Three Components. Eur. J. Pediatr. 2022, 181, 2685–2693. [Google Scholar] [CrossRef]
- Klang, M.G. PFAT5 and the Evolution of Lipid Admixture Stability. JPEN J. Parenter. Enter. Nutr. 2015, 39, 67S–71S. [Google Scholar] [CrossRef]
- Driscoll, D.F. The Pharmacopeial Evolution of Intralipid Injectable Emulsion in Plastic Containers: From a Coarse to a Fine Dispersion. Int. J. Pharm. 2009, 368, 193–198. [Google Scholar] [CrossRef]
- Otero-Millán, L.; Lago Rivero, N.; Blanco Rodicio, A.; García Beloso, N.; Legido Soto, J.L.; Piñeiro-Corrales, G. Stability of Lipid Emulsion in Total Parenteral Nutrition: An Overview of Literature. Clin. Nutr. ESPEN 2021, 45, 19–25. [Google Scholar] [CrossRef]
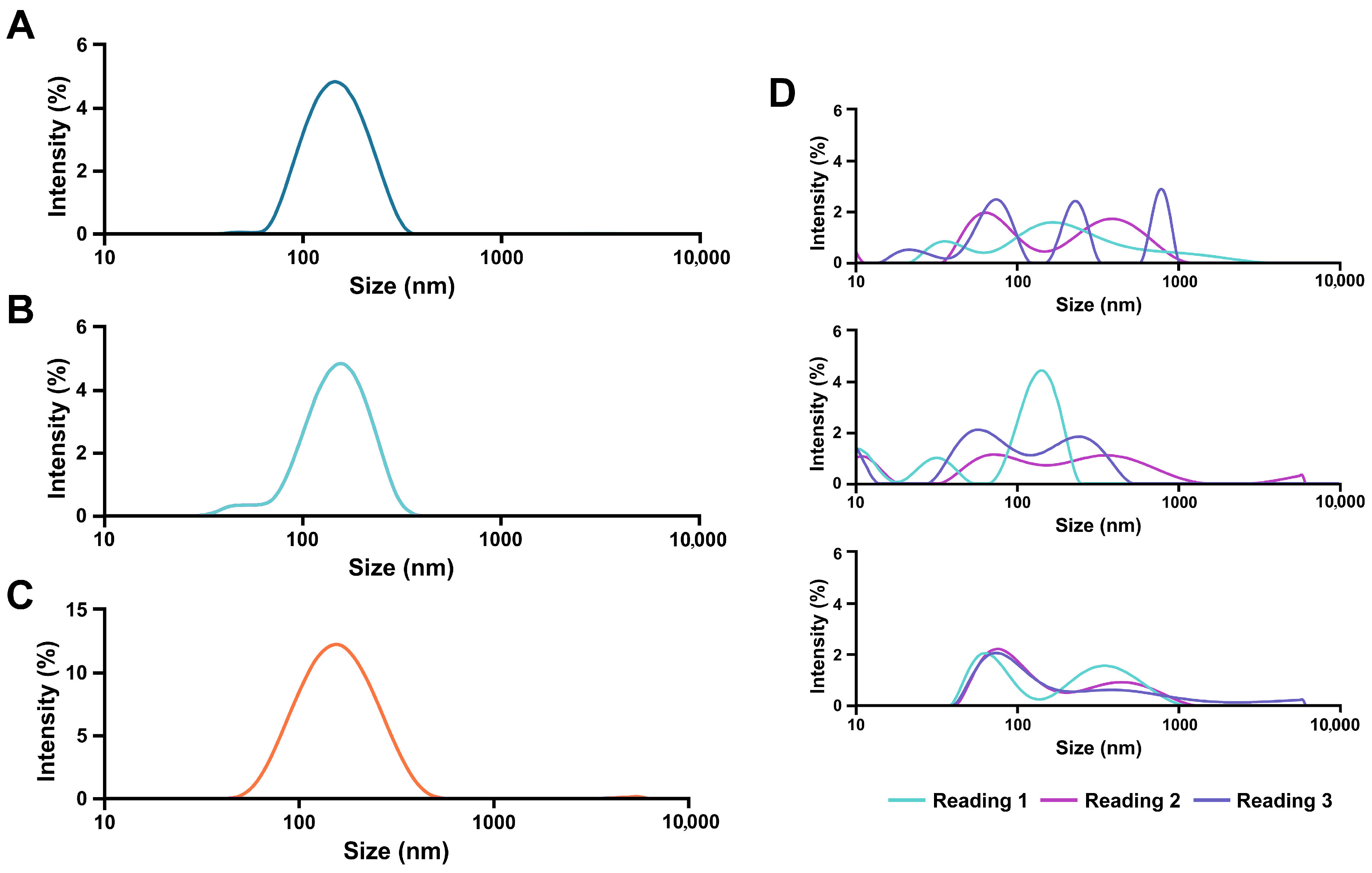
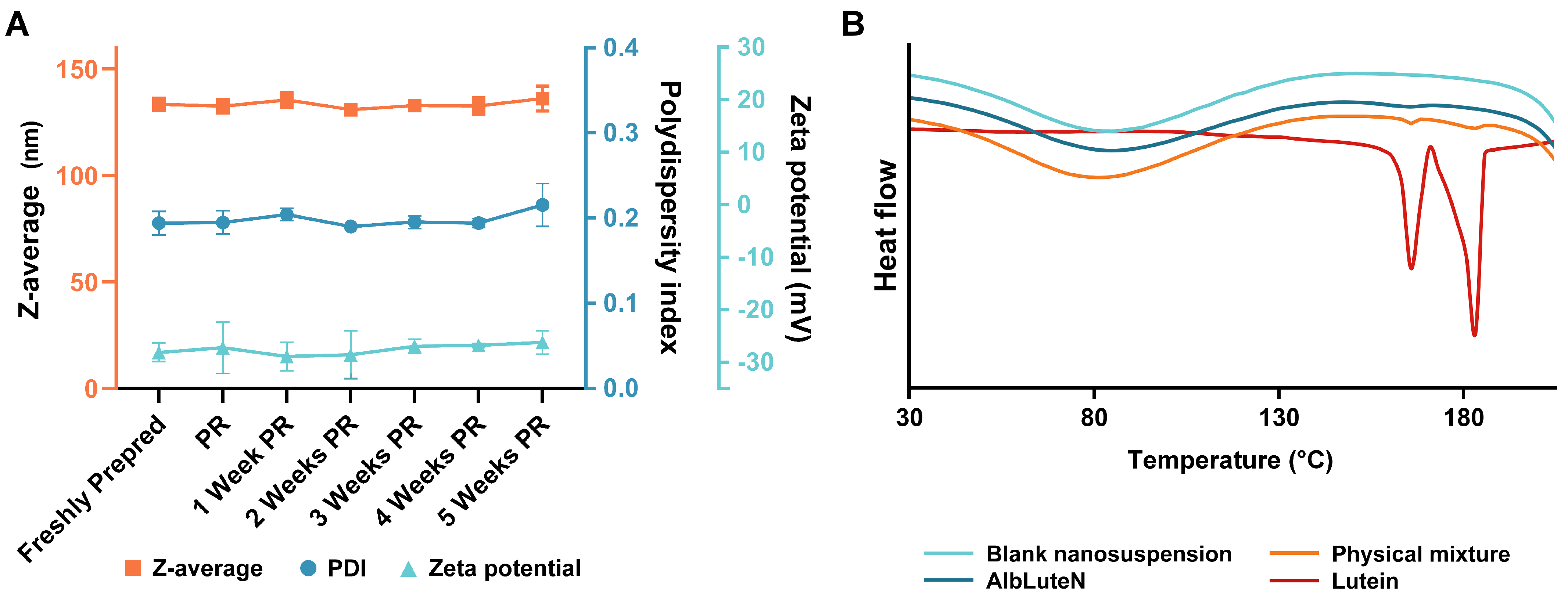
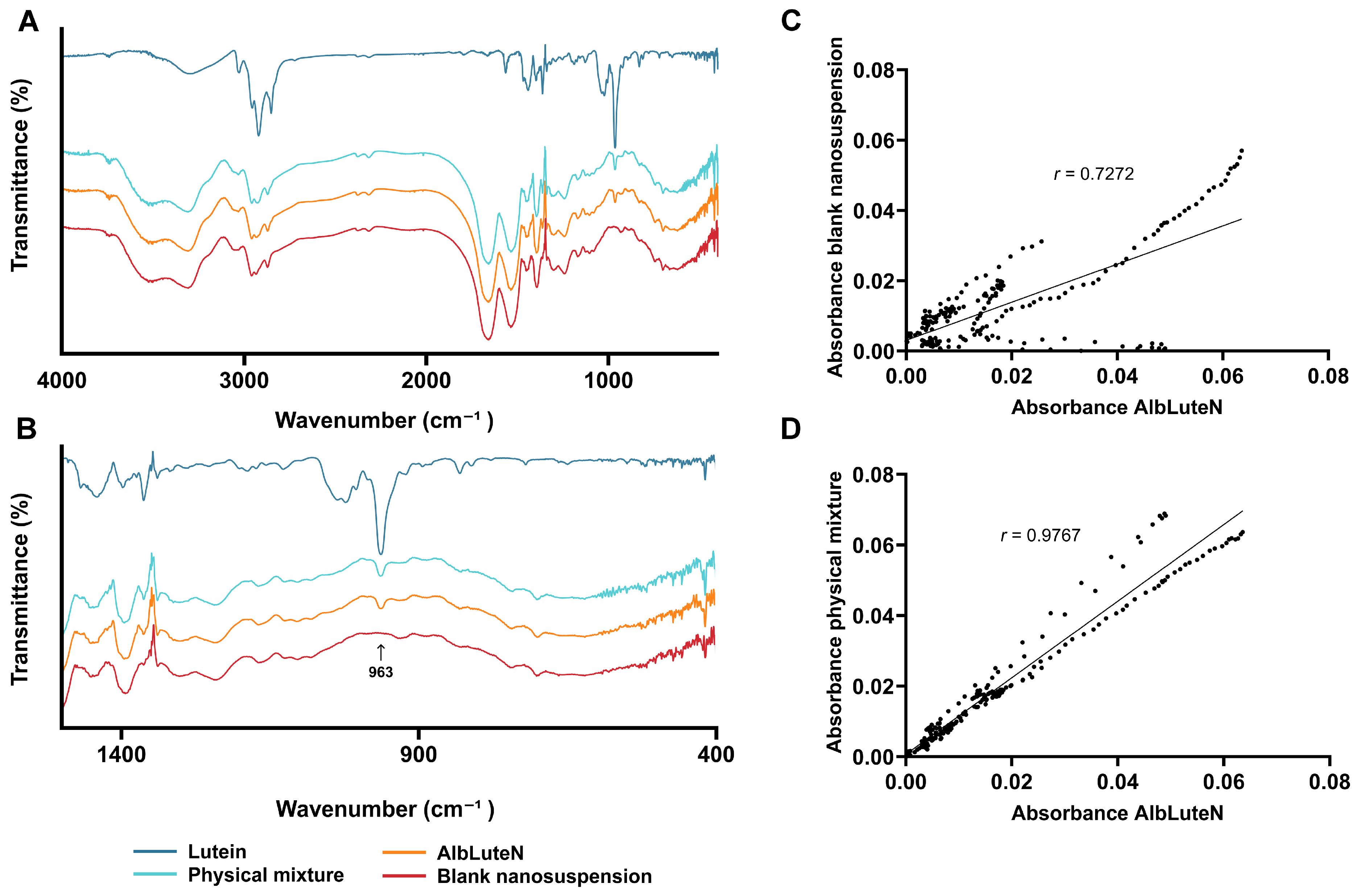
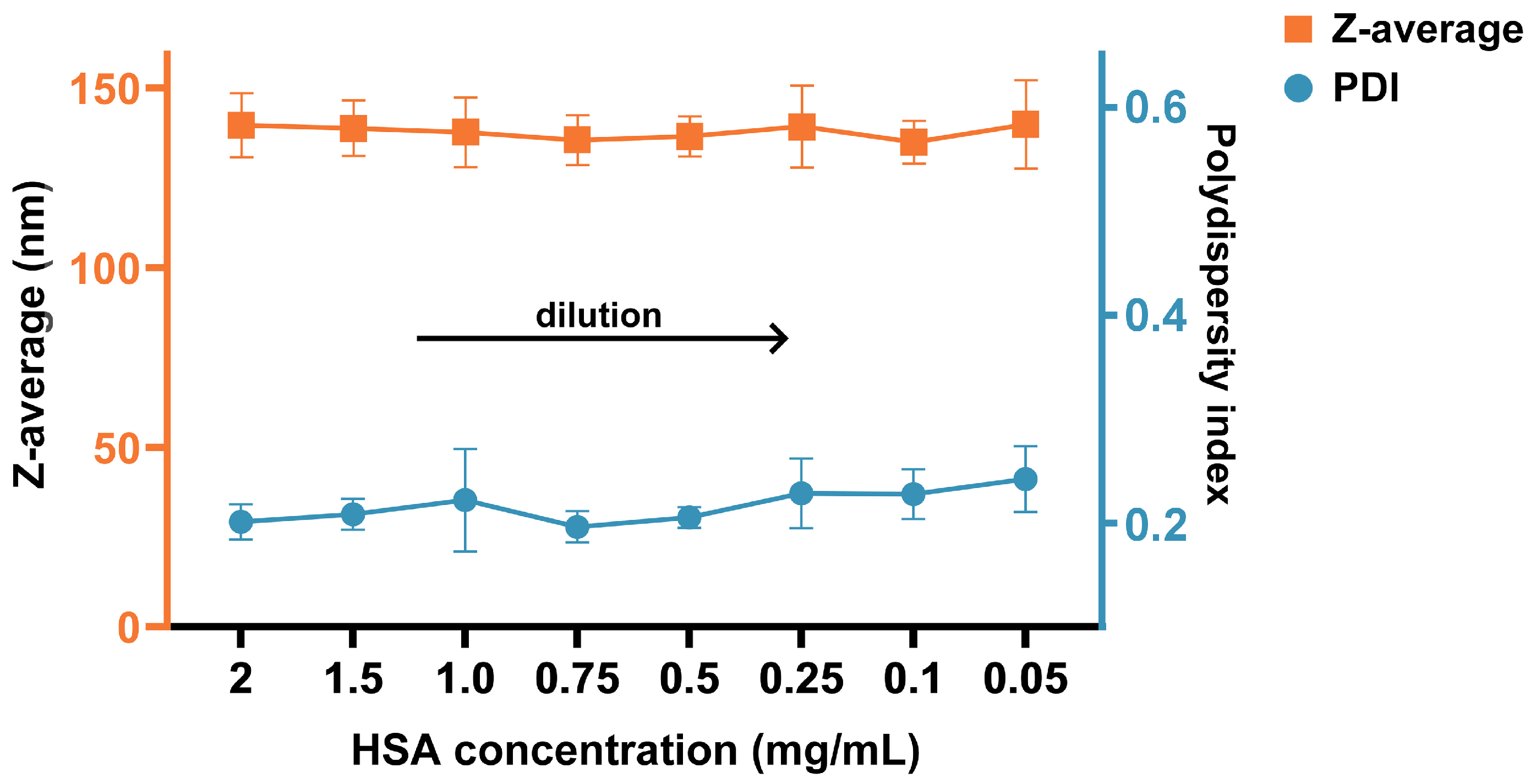
| Parameter/Component | Unit | LPe | OPe | OSp | OPl |
|---|---|---|---|---|---|
| Volume | mL | 1250 | 1250 | 1250 | 1250 |
| Glucose | g | 80 | 80 | 180 | 150 |
| Amino acids | 40 | 40 | 70.1 | 48 | |
| Soybean oil | 25 | 20 | 20 | 20 | |
| MCT | 25 | 25 | 25 | 25 | |
| Omega-3 TG | 0 | 5 | 5 | 5 | |
| Total lipids | 50 | 50 | 50 | 50 | |
| Sodium | mmol | 50 | 50 | 67 | 50 |
| Potassium | 30 | 30 | 47 | 35 | |
| Magnesium | 3.0 | 3.0 | 5.3 | 4.0 | |
| Calcium | 3.0 | 3.0 | 5.3 | 4.0 | |
| Phosphates | 7.5 | 7.5 | 20 | 15 | |
| Total energy | kcal | 955 | 955 | 1475 | 1265 |
| Theoretical osmolarity | mOsm/kg | 840 | 840 | 1545 | 1215 |
| PN Admixture | PFAT5 * (%) | Z-Average (nm) | Zeta Potential (mV) | pH | Osmolality (mOsm/kg) | Lutein Content (%) |
|---|---|---|---|---|---|---|
| LPe control (T0) | 0.002 | 238.9 ± 2.8 | −21.6 ± 0.3 | 5.37 ± 0.01 | 933 ± 9 | N/A |
| LPe + AlbLuteN (T0) | 0.002 | 244.5 ± 0.3 | −21.3 ± 0.5 | 5.36 ± 0.01 | 925 ± 5 | 100.00 ± 0.73 |
| LPe + AlbLuteN (T24) | 0.001 | 245.3 ± 0.5 | −20.8 ± 0.2 | 5.43 ± 0.02 | 924 ± 1 | 97.49 ± 0.61 |
| OPe control (T0) | 0.010 | 239.3 ± 2.9 | −21.6 ± 0.0 | 5.32 ± 0.01 | 918 ± 4 | N/A |
| OPe + AlbLuteN (T0) | 0.008 | 237.3 ± 0.8 | −21.1 ± 0.9 | 5.37 ± 0.01 | 921 ± 4 | 100.00 ± 1.80 |
| OPe + AlbLuteN (T24) | 0.005 | 240.0 ± 0.9 | −20.7 ± 0.0 | 5.42 ± 0.01 | 911 ± 16 | 97.13 ± 6.21 |
| OSp control (T0) | 0.001 | 237.3 ± 2.7 | −15.6 ± 0.6 | 5.42 ± 0.01 | 1871 ± 8 | N/A |
| OSp + AlbLuteN (T0) | 0.001 | 239.9 ± 1.3 | −16.0 ± 0.6 | 5.43 ± 0.02 | 1865 ± 6 | 100.00 ± 6.04 |
| OSp + AlbLuteN (T24) | 0.000 | 241.7 ± 2.0 | −16.0 ± 0.2 | 5.49 ± 0.01 | 1863 ± 7 | 98.06 ± 2.64 |
| OPl control (T0) | 0.004 | 235.9 ± 2.8 | −15.9 ± 0.3 | 5.35 ± 0.01 | 1424 ± 2 | N/A |
| OPl + AlbLuteN (T0) | 0.004 | 239.4 ± 2.4 | −15.8 ± 0.4 | 5.35 ± 0.00 | 1414 ± 6 | 100.00 ± 3.51 |
| OPl + AlbLuteN (T24) | 0.001 | 240.0 ± 1.8 | −15.1 ± 0.3 | 5.42 ± 0.02 | 1412 ± 2 | 101.55 ± 3.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żółnowska, I.; Gostyńska-Stawna, A.; Dominiak, K.; Jadach, B.; Stawny, M. Enhancing Parenteral Nutrition via Supplementation with Antioxidant Lutein in Human Serum Albumin-Based Nanosuspension. Pharmaceutics 2025, 17, 971. https://doi.org/10.3390/pharmaceutics17080971
Żółnowska I, Gostyńska-Stawna A, Dominiak K, Jadach B, Stawny M. Enhancing Parenteral Nutrition via Supplementation with Antioxidant Lutein in Human Serum Albumin-Based Nanosuspension. Pharmaceutics. 2025; 17(8):971. https://doi.org/10.3390/pharmaceutics17080971
Chicago/Turabian StyleŻółnowska, Izabela, Aleksandra Gostyńska-Stawna, Katarzyna Dominiak, Barbara Jadach, and Maciej Stawny. 2025. "Enhancing Parenteral Nutrition via Supplementation with Antioxidant Lutein in Human Serum Albumin-Based Nanosuspension" Pharmaceutics 17, no. 8: 971. https://doi.org/10.3390/pharmaceutics17080971
APA StyleŻółnowska, I., Gostyńska-Stawna, A., Dominiak, K., Jadach, B., & Stawny, M. (2025). Enhancing Parenteral Nutrition via Supplementation with Antioxidant Lutein in Human Serum Albumin-Based Nanosuspension. Pharmaceutics, 17(8), 971. https://doi.org/10.3390/pharmaceutics17080971








