Thermoresponsive and Fluorescent Polymers: From Nanothermometers to Smart Drug Delivery Systems for Theranostics Against Cancer
Abstract
1. Introduction
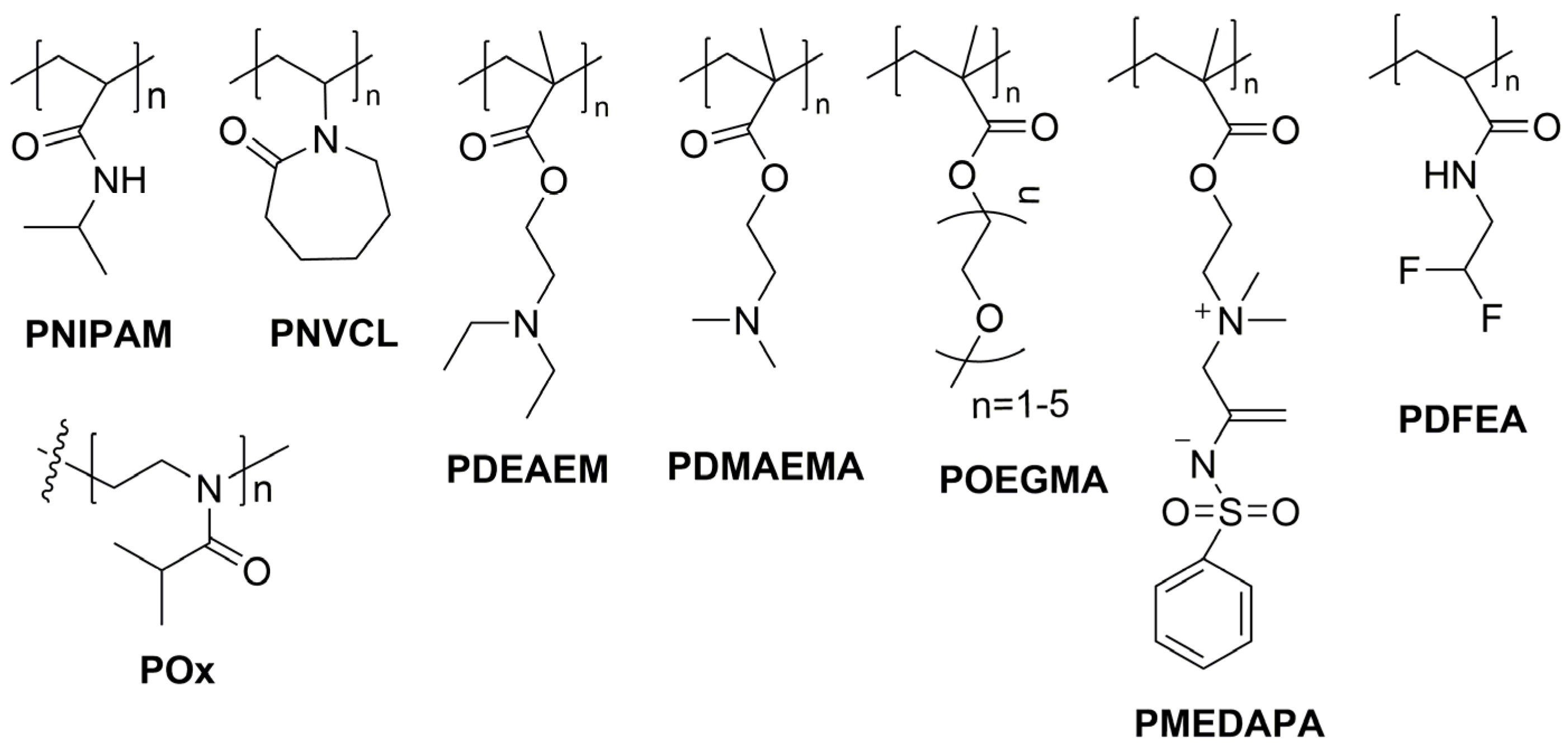
2. Thermoresponsive Polymers
3. Fluorescence in Polymers
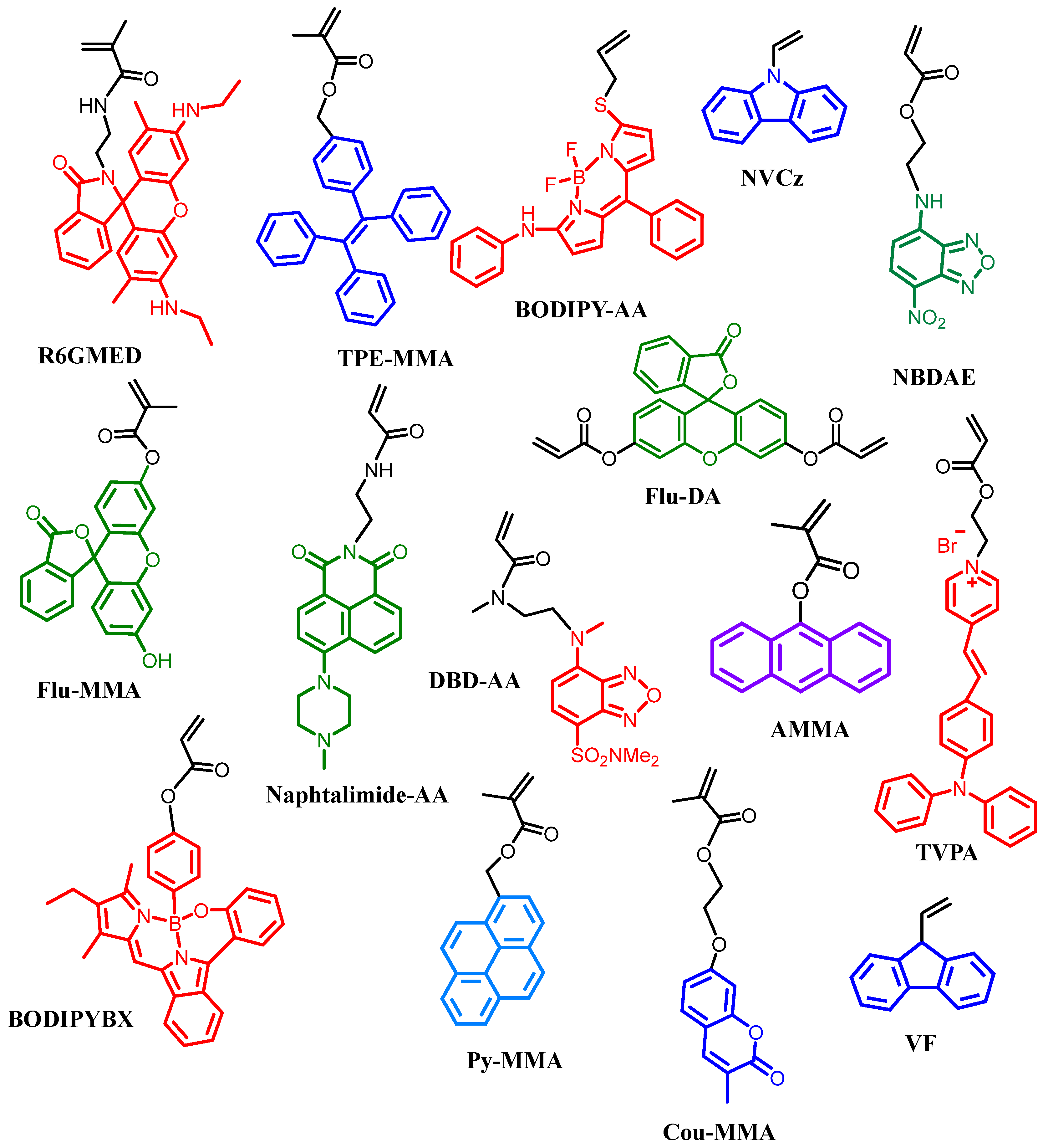
3.1. Fluorescent Polymers
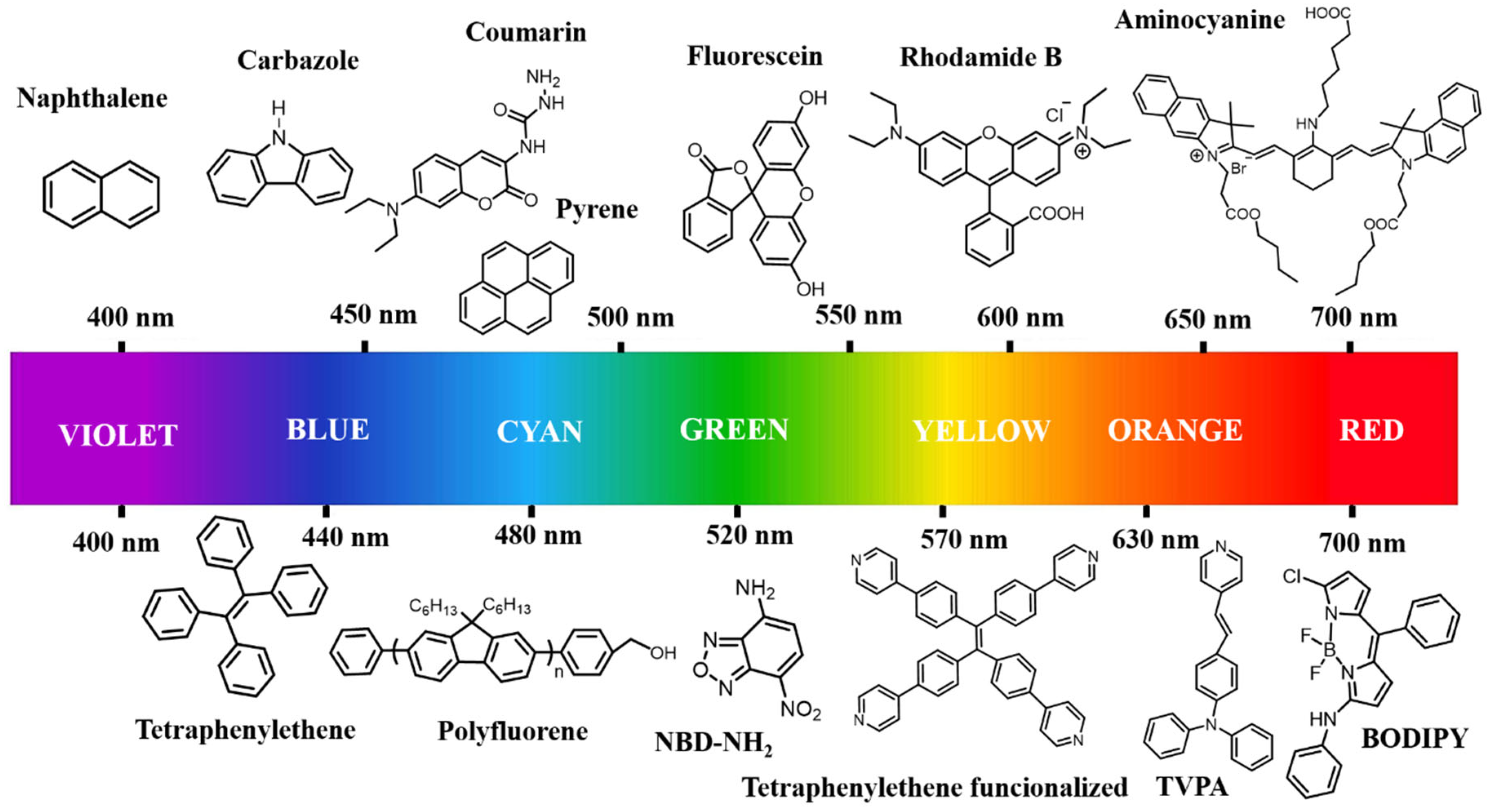
3.2. Organic Fluorophores
3.3. Aggregation-Caused Quenching (ACQ) and Aggregation-Induced Emission (AIE)
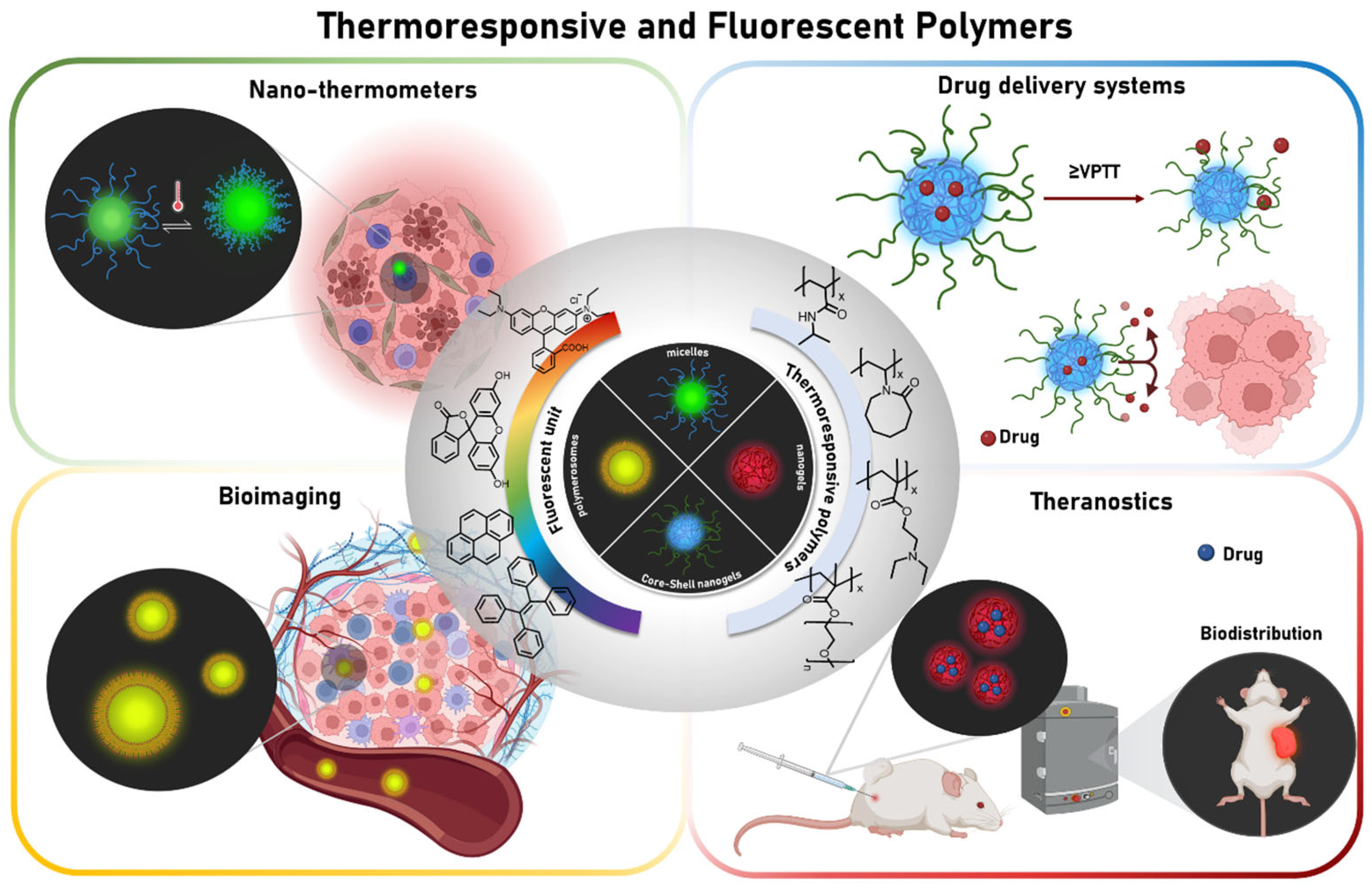
4. Main Morphologies of Thermoresponsive and Fluorescent Polymers in the Nanometric Scale for Biomedical Applications
4.1. Nanogels
4.2. Self-Assembled Block Copolymers
4.2.1. Thermoresponsive and Fluorescent Block Copolymers
4.2.2. Fluorescent Thermoresponsive Aggregates
4.3. Other Thermoresponsive Copolymers and End-Functionalized Fluorescent Polymers
5. Biomedical Applications of Thermo- and Fluorescent Polymeric Nanoparticles
5.1. Nanothermometers
5.2. Drug Delivery Systems
5.3. Bioimaging
5.4. Sensors
5.5. Theranostics
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A431 cells | Human epidermoid carcinoma cell line |
| A549 cells | Human lung adenocarcinoma cell line |
| ACQ | Aggregation-caused quenching |
| AFM | Atomic Force Microscopy |
| AIE | Aggregation-induced emission |
| AIEgens | AIE luminogens |
| AMMA | 9-Anthrylmethyl methacrylate |
| ATRP | Atom transfer radical polymerization |
| B16F10 cells | Mouse melanoma cell line |
| BAC | N,N′ -bis(acryloyl)cystamine |
| BCPs | Block copolymers |
| BHK cells | Fibroblast-like cell line |
| BMPN | N-(n-butyl)-4-(4-[2-methacryloyloxyethyl]-piperazine-1-yl)-1,8-naphthalimide |
| BOBPYBX | Acrylated-dibenzoporphyrin derivative of BODIPY |
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| B-R | Britton–Robinson |
| CMC | Critical Micellar Concentration |
| Cou | Coumarin |
| Cou-MMA | 7-(2-methacryloyloxyethoxy)-4-methylcoumarin |
| CPCs | Colloidal photonic crystals |
| CPNs | Conjugated polymer nanoparticles |
| Cy | Cyanine |
| DA | Dansyl |
| DBD-AA | N-{2-(7-N,N-dimethylaminosulfonyl-2,1,3-benzoxadiazol-4-yl)methylamino}ethyl-N-methylacrylamide |
| Dh | Hydrodynamic diameter |
| DLS | Dynamic Light Scattering |
| DMAA | N,N-dimethylacrylamide |
| DOPE | Dioleoyl |
| DOX | Doxorubicin |
| DP | Dilution polymerization |
| DPA | 2-(Diisopropyl amino)ethyl methacrylate |
| EE | Encapsulation efficiency |
| EP | Emulsion polymerization |
| EPR | Enhanced permeation and retention |
| FIM | N-2-(6-(4-methylpiperazin-1-yl)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl-ethyl)acrylamide |
| FITC | Fluorescein isothiocyanate |
| FL | poly[N-(2,2difluoroethyl)acrylamide] |
| Flu | fluorescein |
| Flu-DA | Fluorescein-O,O’-diacrylate |
| Flu-MMA | Fluorescein methylmethacrylate |
| Flu-NGs | Fluorescein-labeled PNVCL-PHPMA nanogels |
| Fluo-4 AM | Cell permeable calcium ion fluorescent dye |
| FRET | Förster-type resonance energy transfer |
| FRP | Free radical polymerization |
| G361 cells | Human melanoma cell line |
| GSH | Glutathione |
| GTP | Group Transfer Polymerization |
| Hb | Hemoglobin |
| HbO2 | Hemoglobin oxidized |
| HeLa cells | Cervical cancer cells |
| HepG2 cells | Human liver cancer cell line |
| HPMA | N-(2-hydroxypropyl)methacrylamide |
| IC50 | half-maximal inhibitory concentration |
| KS | Potassium fluorescent sensor |
| LAT1 | L-type amino acid transporter 1 |
| LCST | Lower critical solution temperature |
| MAA | Methacrylic Acid |
| MAlpGP | 6-O-Methacryloyl-1,2:3,4-di- O-isopropylidene-D-galactopyranose |
| MCF-7 | Human breast adenocarcinoma |
| MNPs | Magnetic nanoparticles |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Naphtalimide-AA | Acrylamide derivative of naphtalimide |
| NBDAE | 2-Acryloyloxyethylamino-7-nitro-2,1,3-benzoxadiazole |
| NIR | Near-infrared |
| NIR-II | Second near-infrared |
| NMRP | Nitroxide-Mediated Radical Polymerization |
| NVCz | 9-Vinylcarbazole |
| OEGMA | Oligoethyleneglycol methacrylate |
| OXPHOS | Oxidative phosphorylation |
| PC3 cells | Human prostate cancer cell line |
| PCB | Poly(carboxybetaine) |
| PDPA | Poly(diisopropyl amino) ethyl methacrylate |
| PDEAEM | Poly(N,N-diethylaminoethyl methacrylate) |
| PDFEA | Poly[N-(2,2difluoroethyl)acrylamide] |
| PDI | Polydispersity index |
| PDMAEMA | Poly(N,N-dimethylamynoethyl methacrylate) |
| PEGDA | Poly(ethyleneglycol diacrylate) |
| PEGMA | Poly(ethylene glycol) monomethyl ether methacrylate |
| PET | Photoinduced electron transfer |
| PF | Polyfluorene |
| PFV | Poly(fluorene-co-vinylene) |
| PFuMaMA | Furan-protected maleimide-containing methacrylate |
| PGMA | Poly(glycidyl methacrylate) |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PHPMA | Poly[N-(2-hydroxypropyl)-methacrylamide] |
| PL | Photoluminescence |
| P(Llys-co-Asp) | Poly(L-lysine-co-aspartate) |
| PMEDAPA | Poly(2-((2-(methacryloyloxy)ethyl) dimethylammonio) acetyl) (phenylsulfonyl) amide |
| PMeOx | Poly(2-methyl-2-oxazoline) |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PNIPMAM | Poly(N-isopropylmethacrylamide) |
| PNVCL | Poly(N-vinylcaprolactam) |
| POEGMA | Poly(oligo(ethylene glycol) monomethyl ether methacrylate) |
| POx | Poly(2-isopropyl-2-oxazoline) |
| PP | Precipitation polymerization |
| PyMA | 1-Pyrenemethyl methacrylate |
| R6GMED | N-(rhodamine-6G)lactam-N′-methacryloyl ethylenediamine |
| RAFT | Reversible addition–fragmentation chain transfer |
| RH | Rhodamine |
| RIM | Restriction of intramolecular motion |
| RIR | Restriction of intramolecular rotation |
| RIV | Restriction of intramolecular vibration |
| SFEP | Surfactant free emulsion polymerization |
| TAC | 2-triazacryptand [2,2,3]-1-(2-methoxyethoxy) benzene |
| TCMA | 7-[4-(Trifluoromethyl)coumarin]methacrylamide |
| Tcp | Cloud Point Temperature |
| TEM | Transmission Electron Microscopy |
| Tf | Transferrin |
| TFP | Thermoresponsive fluorescent polymer |
| TPE | Tetraphenylethene |
| tPE | Triphenylethene |
| TPE-MMA | Tetraphenylethene methyl methacrylate |
| TPPEBr | Tetraphenylene fluorophore |
| TVPA | Acrylated AIEgen with electron-donating triphenylamine and electron-withdrawing pyridinium unit connected by an ethylene core. |
| UCST | Upper critical solution temperature |
| VF | 9-Vinyl-9H-fluorene |
| VPBA | 4-Vinylphenyl boronic acid |
| VPTT | Volume phase transition temperature |
References
- Wang, C.; Zhao, X.; Wu, K.; Lv, S.; Zhu, C. A Ratiometric Organic Fluorescent Nanogel Thermometer for Highly Sensitive Temperature Sensing. Biosensors 2022, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kawamoto, K.; Inada, N.; Uchiyama, S. Cationic Fluorescent Nanogel Thermometers based on Thermoresponsive Poly(N-isopropylacrylamide) and Environment-Sensitive Benzofurazan. Polymers 2019, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Tsuji, T.; Kawamoto, K.; Okano, K.; Fukatsu, E.; Noro, T.; Ikado, K.; Yamada, S.; Shibata, Y.; Hayashi, T.; et al. A Cell-Targeted Non-Cytotoxic Fluorescent Nanogel Thermometer Created with an Imidazolium-Containing Cationic Radical Initiator. Angew. Chem. Int. Ed. 2018, 57, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Cao, T.; Han, S.-C.; Zhu, X.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. Fluorescence enhancement thermoresponsive polymer luminescent sensors based on BODIPY for intracellular temperature. Sens. Actuators B Chem. 2017, 252, 577–583. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Liu, T.; Zhang, G.; Liu, S. Intracellular Cascade FRET for Temperature Imaging of Living Cells with Polymeric Ratiometric Fluorescent Thermometers. ACS Appl. Mater. Interfaces 2015, 7, 15551–15560. [Google Scholar] [CrossRef]
- González-Urías, A.; Manzanares-Guevara, L.A.; Licea-Claveríe, A.; Ochoa-Terán, A.; Licea-Navarro, A.F.; Bernaldez-Sarabia, J.; Zapata-González, I. Stimuli responsive nanogels with intrinsic fluorescence: Promising nanovehicles for controlled drug delivery and cell internalization detection in diverse cancer cell lines. Eur. Polym. J. 2021, 144, 110200. [Google Scholar] [CrossRef]
- Shakoori, Z.; Ghanbari, H.; Omidi, Y.; Pashaiasl, M.; Akbarzadeh, A.; Jomeh Farsangi, Z.; Rezayat, S.M.; Davaran, S. Fluorescent multi-responsive cross-linked P(N-isopropylacrylamide)-based nanocomposites for cisplatin delivery. Drug Dev. Ind. Pharm. 2017, 43, 1283–1291. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Shen, S.; Jiang, X.; Wang, Y.; Yang, W. Temperature and Redox Dual-Responsive Biodegradable Nanogels for Optimizing Antitumor Drug Delivery. Part. Part. Syst. Charact. 2015, 32, 1092–1101. [Google Scholar] [CrossRef]
- Banerjee, S.L.; Saha, P.; Ganguly, R.; Bhattacharya, K.; Kalita, U.; Pich, A.; Singha, N.K. A dual thermoresponsive and antifouling zwitterionic microgel with pH triggered fluorescent “on-off” core. J. Colloid Interface Sci. 2021, 589, 110–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Chen, S.; Deng, H.; Zhu, X. Building Single-Color AIE-Active Reversible Micelles to Interpret Temperature and pH Stimuli in Both Solutions and Cells. Macromolecules 2018, 51, 5234–5244. [Google Scholar] [CrossRef]
- Wang, Z.; Yong, T.-Y.; Wan, J.; Li, Z.-H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X.-L.; Xu, H.-B.; Zhang, C. Temperature-Sensitive Fluorescent Organic Nanoparticles with Aggregation-Induced Emission for Long-Term Cellular Tracing. ACS Appl. Mater. Interfaces 2015, 7, 3420–3425. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wu, Y.; Lu, X. Temperature-Induced Gelation Enhances Fluorescence in Nanogel Photonic Crystals. ACS Appl. Nano Mater. 2024, 7, 22953–22963. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Jang, G.; Kim, J.; Lee, T.S. Fluorescent, stimuli-responsive, crosslinked PNIPAM-based microgel. Sens. Actuators B Chem. 2015, 207, 623–630. [Google Scholar] [CrossRef]
- Deng, K.; Zhao, X.; Liu, F.; Peng, J.; Meng, C.; Huang, Y.; Ma, L.; Chang, C.; Wei, H. Synthesis of Thermosensitive Conjugated Triblock Copolymers by Sequential Click Couplings for Drug Delivery and Cell Imaging. ACS Biomater. Sci. Eng. 2019, 5, 3419–3428. [Google Scholar] [CrossRef]
- Peng, S.; Wang, H.; Zhao, W.; Xin, Y.; Liu, Y.; Yu, X.; Zhan, M.; Shen, S.; Lu, L. Zwitterionic Polysulfamide Drug Nanogels with Microwave Augmented Tumor Accumulation and On-Demand Drug Release for Enhanced Cancer Therapy. Adv. Funct. Mater. 2020, 30, 2001832. [Google Scholar] [CrossRef]
- Maiti, C.; Dhara, D. Energy-Transfer Phenomena in Thermoresponsive and pH- Switchable Fluorescent Diblock Copolymer Vesicles. Langmuir 2017, 33, 12130–12139. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.A.; Robin, M.P.; Ceric, D.; O’Reilly, R.K.; Marino, S.; Resmini, M. Fluorescent polymeric nanovehicles for neural stem cell modulation. Nanoscale 2016, 8, 17340–17349. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, Y.; Yao, T.; Nguyen, K.T.; Yuan, B. In vivo tumor ultrasound-switchable fluorescence imaging via intravenous injections of size-controlled thermosensitive nanoparticles. Nano Res. 2023, 16, 1009–1020. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- De la Rosa, V.R.; Woisel, P.; Hoogenboom, R. Supramolecular control over thermoresponsive polymers. Mater. Today 2016, 19, 44–55. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Kojima, H. Studies on the phase transition of hydrogels and aqueous solutions of thermosensitive polymers. Polym. J. 2018, 50, 411–418. [Google Scholar] [CrossRef]
- González-Ayón, M.A.; Cerda-Sumbarda, Y.D.; Zizumbo-López, A.; Licea-Claverie, A. Hydrogels with Thermal Responsiveness. In Multifunctional Hydrogels; García-Torres, J., Alemán, C., Gupta, R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 174–213. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef] [PubMed]
- Fatimah; Gurnani, P.; Williams, G.R. Investigating oligo(ethylene glycol) methacrylate thermoresponsive copolymers. Eur. Polym. J. 2024, 216, 113266. [Google Scholar] [CrossRef]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Wang, L.; Wang, S.; Georgiou, T.K. Thermoresponsive block copolymers of increasing architecture complexity: A review on structure–property relationships. Polym. Chem. 2023, 14, 223–247. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Urias, A.; Licea-Claverie, A.; Sañudo-Barajas, J.A.; González-Ayón, M.A. NVCL-Based Hydrogels and Composites for Biomedical Applications: Progress in the Last Ten Years. Int. J. Mol. Sci. 2022, 23, 4722. [Google Scholar] [CrossRef]
- González-Ayón, M.A.; Licea-Rodriguez, J.; Méndez, E.R.; Licea-Claverie, A. NVCL-Based Galacto-Functionalized and Thermosensitive Nanogels with GNRDs for Chemo/Photothermal-Therapy. Pharmaceutics 2022, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Olea, L.A.; González-Urías, M.A.; Licea-Claveríe, A.; González-Ayón, M.A.; Ramírez-Jiménez, A. Thermo- and pH responsive core–shell nanogels: Easy method of synthesis, properties and its use for the loading and releasing of organotin (IV) compounds. Eur. Polym. J. 2025, 228, 113822. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Preparation of a mini-library of thermo-responsive star (NVCL/NVP-VAc) polymers with tailored properties using a hexafunctional xanthate RAFT agent. Polymers 2018, 10, 20. [Google Scholar] [CrossRef]
- Ando, T.; Yamaguchi, K.; Ajiro, H. Thermoresponsive star-shaped polymer with heteroarm type with methacrylates: Preparation by living radical polymerization method and its topological effect. Polym. Chem. 2023, 14, 1027–1035. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Yin, Y.; Zhang, H.; Shi, T.; Zhang, W.; Liao, H.; Li, S.; Tan, X.; Yao, Z.; et al. Thermoresponsive Injectable Microsphere Glycopeptide Hydrogels for Remodeling Dynamic Cell Microenvironments. ACS Appl. Polym. Mater. 2025, 7, 1236–1248. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Colloid Polym. Sci. 2021, 299, 363–383. [Google Scholar] [CrossRef]
- Rasool, A.; Rizwan, M.; Qureshi, A.R.; Rasheed, T.; Bilal, M. Thermo-responsive functionalized polymeric nanocomposites. In Smart Polymer Nanocomposites: Design, Synthesis, Functionalization, Properties, and Applications; Ali, N., Bilal, M., Khan, A., Nguyen, T.A., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–240. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, Y.; Lee, D.; Jang, W.-D. Thermoresponsive Polymer and Fluorescent Dye Hybrids for Tunable Multicolor Emission. Adv. Mater. 2016, 28, 3499–3503. [Google Scholar] [CrossRef]
- Ban, Q.; Li, Y.; Wu, S. Self-fluorescent polymers for bioimaging. View 2022, 3, 20200135. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Qin, A.; Tang, B.Z. AIE polymers in sensing, imaging and theranostic applications. Mater. Chem. Front. 2021, 5, 4073–4088. [Google Scholar] [CrossRef]
- Bu, Q.; Li, P.; Xia, Y.; Hu, D.; Li, W.; Shi, D.; Song, K. Design, Synthesis, and Biomedical Application of Multifunctional Fluorescent Polymer Nanomaterials. Molecules 2023, 28, 3819. [Google Scholar] [CrossRef] [PubMed]
- Bou, S.; Klymchenko, A.S.; Collot, M. Fluorescent labeling of biocompatible block copolymers: Synthetic strategies and applications in bioimaging. Mater. Adv. 2021, 2, 3213–3233. [Google Scholar] [CrossRef]
- Ahumada, G.; Borkowska, M. Fluorescent Polymers Conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef]
- Quintanilla, M.; Henriksen-Lacey, M.; Renero-Lecuna, C.; Liz-Marzán, L.M. Challenges for optical nanothermometry in biological environments. Chem. Soc. Rev. 2022, 51, 4223–4242. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Kong, S.-H.; Seeliger, B.; Andreiuk, B.; Soares, R.V.; Barberio, M.; Diana, M.; Klymchenko, A.S. Near-infrared fluorescent coatings of medical devices for image-guided surgery. Biomaterials 2020, 261, 120306. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, C.; Feng, G.; Zhang, X. Thermo-Responsive Fluorescent Polymers with Diverse LCSTs for Ratiometric Temperature Sensing through FRET. Polymers 2018, 10, 283. [Google Scholar] [CrossRef]
- Trofymchuk, K.; Valanciunaite, J.; Andreiuk, B.; Reisch, A.; Collot, M.; Klymchenko, A.S. BODIPY-loaded polymer nanoparticles: Chemical structure of cargo defines leakage from nanocarrier in living cells. J. Mater. Chem. B 2019, 7, 5199–5210. [Google Scholar] [CrossRef]
- Yang, R.; An, J.; Zhu, H.; Yan, X.; Gao, H. Multipronged design of theranostic nanovehicles with endogenous and exogenous stimuli-responsiveness for precise cancer therapy. J. Mater. Chem. B 2019, 7, 1160–1166. [Google Scholar] [CrossRef]
- Qiao, J.; Mu, X.; Qi, L. Construction of fluorescent polymeric nano-thermometers for intracellular temperature imaging: A review. Biosens. Bioelectron. 2016, 85, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, H.; Liu, H.; Ke, G.; Ren, T.; Xiong, B.; Zhang, X.; Yuan, L. Chemical Approaches to Optimize the Properties of Organic Fluorophores for Imaging and Sensing. Angew. Chem. Int. Ed. 2024, 63, e202315217. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, H.; Zhu, X.; Zhang, J.; Fang, J. Fluorescence thermometers: Intermediation of fundamental temperature and light. Biomater. Sci. 2022, 10, 1855–1882. [Google Scholar] [CrossRef]
- Waghmare, P.S.; Chabukswar, A.R.; Raut, K.G.; Giri, P.T. A Review on Carbazole and Its Derivatives as Anticancer Agents From 2013 to 2024. Chirality 2025, 37, e70021. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ren, T.-B.; Huan, S.; Yuan, L.; Zhang, X.-B. Progress and Perspective of Solid-State Organic Fluorophores for Biomedical Applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef]
- Reineck, P.; Gibson, B.C. Near-Infrared Fluorescent Nanomaterials for Bioimaging and Sensing. Adv. Opt. Mater. 2017, 5, 1600446. [Google Scholar] [CrossRef]
- Schmidt, E.L.; Ou, Z.; Ximendes, E.; Cui, H.; Keck, C.H.C.; Jaque, D.; Hong, G. Near-infrared II fluorescence imaging. Nat. Rev. Methods Primers 2024, 4, 23. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Ping, Q.; Yeo, Y. Quantitative Assessment of Nanoparticle Biodistribution by Fluorescence Imaging, Revisited. ACS Nano 2018, 12, 6458–6468. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X.; et al. Aggregation-Induced Emission (AIE), Life and Health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Koc, K.; Alveroglu, E. Tuning the gel size and LCST of N-isopropylacrylamide nanogels by anionic fluoroprobe. Colloid Polym. Sci. 2016, 294, 285–290. [Google Scholar] [CrossRef]
- Chambre, L.; Degirmenci, A.; Sanyal, R.; Sanyal, A. Multi-Functional Nanogels as Theranostic Platforms: Exploiting Reversible and Nonreversible Linkages for Targeting, Imaging, and Drug Delivery. Bioconjug. Chem. 2018, 29, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Aktan, B.; Chambre, L.; Sanyal, R.; Sanyal, A. “Clickable” Nanogels via Thermally Driven Self-Assembly of Polymers: Facile Access to Targeted Imaging Platforms using Thiol–Maleimide Conjugation. Biomacromolecules 2017, 18, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, R.; Yang, H.; Wang, W.-J.; Zhao, Y.; He, W.; Qiu, Z.; Wang, D.; Xiong, Y.; et al. Multi-Stimuli-Responsive and Cell Membrane Camouflaged Aggregation-Induced Emission Nanogels for Precise Chemo-photothermal Synergistic Therapy of Tumors. ACS Nano 2023, 17, 25205–25221. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yao, T.; Liu, Y.; Yu, S.; Ren, L.; Hong, Y.; Nguyen, K.T.; Yuan, B. Temperature-sensitive polymeric nanogels encapsulating with β-cyclodextrin and ICG complex for high-resolution deep-tissue ultrasound-switchable fluorescence imaging. Nano Res. 2020, 13, 1100–1110. [Google Scholar] [CrossRef]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefał, R.; Janouskova, O.; et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef]
- Shao, G.; Liu, Y.; Cao, R.; Han, G.; Yuan, B.; Zhang, W. Thermo-responsive block copolymers: Assembly and application. Polym. Chem. 2023, 14, 1863–1880. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block copolymer solution self-assembly: Recent advances, emerging trends, and applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Zuo, H.; Yang, F.; Yuan, L.; Zhang, Y.; Zhao, Y. Thermo-responsive polymers with aggregation induced emission. J. Macromol. Sci. A 2021, 58, 317–328. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, X.; Shu, Y.; Wang, J. A “turn-on” polymer nanothermometer based on aggregation induced emission for intracellular temperature sensing. J. Colloid Interface Sci. 2025, 679, 519–528. [Google Scholar] [CrossRef]
- Kong, F.; Lin, M.; Qiu, T. Multi-functional ratiometric fluorescent chemosensors of poly(N-isopropylacrylamide) containing rhodamine 6G and 1,8-naphthalimide moieties. Polymer 2018, 151, 117–124. [Google Scholar] [CrossRef]
- Shen, M.; Pan, T.; Ning, J.; Shi, J.; Liu, H.; Tian, Y. PNIPAM-based extracellular K+ fluorescent sensor for high-throughput analysis. Mater. Today Commun. 2021, 29, 102911. [Google Scholar] [CrossRef]
- Qiao, J.; Hwang, Y.-H.; Kim, D.-P.; Qi, L. Simultaneous Monitoring of Temperature and Ca2+ Concentration Variation by Fluorescent Polymer during Intracellular Heat Production. Anal. Chem. 2020, 92, 8579–8583. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Hiruta, Y.; Wang, J.; Ayano, E.; Kanazawa, H. Design of Environmentally Responsive Fluorescent Polymer Probes for Cellular Imaging. Biomacromolecules 2015, 16, 2356–2362. [Google Scholar] [CrossRef]
- Matsuura, M.; Ohshima, M.; Hiruta, Y.; Nishimura, T.; Nagase, K.; Kanazawa, H. LAT1-Targeting Thermoresponsive Fluorescent Polymer Probes for Cancer Cell Imaging. Int. J. Mol. Sci. 2018, 19, 1646. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Z.; Tang, Y. Conjugated Polymers-Based Thermal-Responsive Nanoparticles for Controlled Drug Delivery, Tracking, and Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Bio Mater. 2019, 2, 4485–4492. [Google Scholar] [CrossRef]
- Fu, L.; Sun, C.; Yan, L. Galactose Targeted pH-Responsive Copolymer Conjugated with Near Infrared Fluorescence Probe for Imaging of Intelligent Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2104–2115. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Manzanares-Guevara, L.A.; Licea-Claverie, A.; Oroz-Parra, I.; Bernaldez-Sarabia, J.; Diaz-Castillo, F.; Licea-Navarro, A.F. Smart Nanoformulation Based on Stimuli-Responsive Nanogels and Curcumin: Promising Therapy against Colon Cancer. ACS Omega 2020, 5, 9171–9184. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Vanaraj, R.; Thirupathi, K.; Ulagesan, S.; Nam, T.-J.; Phan, T.T.V.; Kim, S.-C. L-Lysine-Modified pNIPAm-co-GMA Copolymer Hydrogel for pH- and Temperature-Responsive Drug Delivery and Fluorescence Imaging Applications. Gels 2023, 9, 363. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, K.; Nam, H.Y.; Lee, S.; Kim, K.; Kwon, I.C. Polymers for bioimaging. Prog. Polym. Sci. 2007, 32, 1031–1053. [Google Scholar] [CrossRef]
- Pandey, N.; Menon, J.U.; Takahashi, M.; Hsieh, J.-T.; Yang, J.; Nguyen, K.T.; Wadajkar, A.S. Thermo-responsive Fluorescent Nanoparticles for Multimodal Imaging and Treatment of Cancers. Nanotheranostics 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Qiao, S.L.; Liu, W.J.; Ma, Y.; Wan, D.; Pan, J.; Wang, H. Intracellular construction of topology-controlled polypeptide nanostructures with diverse biological functions. Nat. Commun. 2017, 8, 1276. [Google Scholar] [CrossRef]
- Naha, P.C.; Casey, A.; Tenuta, T.; Lynch, I.; Dawson, K.A.; Byrne, H.J.; Davoren, M. Preparation, characterization of NIPAM and NIPAM/BAM copolymer nanoparticles and their acute toxicity testing using an aquatic test battery. Aquat. Toxicol. 2009, 92, 146–154. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Kiamahalleh, M.V.; Dai, S.; Bi, J.; Jin, B.; Zhang, H. Influence of polymer molecular weight on the in vitro cytotoxicity of poly (N-isopropylacrylamide). Mat. Sci. Eng. C 2016, 59, 509–513. [Google Scholar] [CrossRef]
- Yogev, S.; Shabtay-Orbach, A.; Nyska, A.; Mizrahi, B. Local Toxicity of Topically Administrated Thermoresponsive Systems: In Vitro Studies with In Vivo Correlation. Toxicol. Pathol. 2019, 47, 426–432. [Google Scholar] [CrossRef]

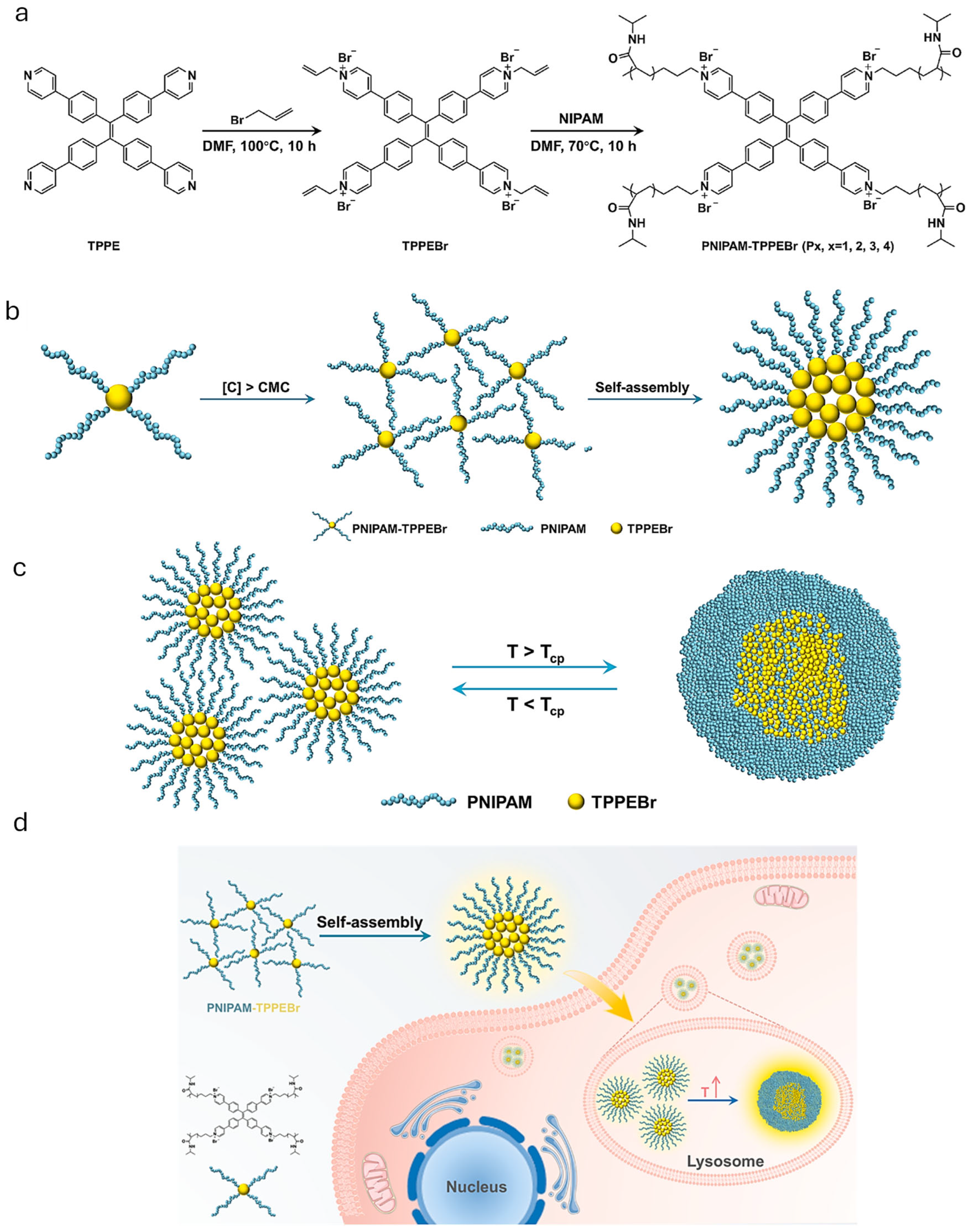

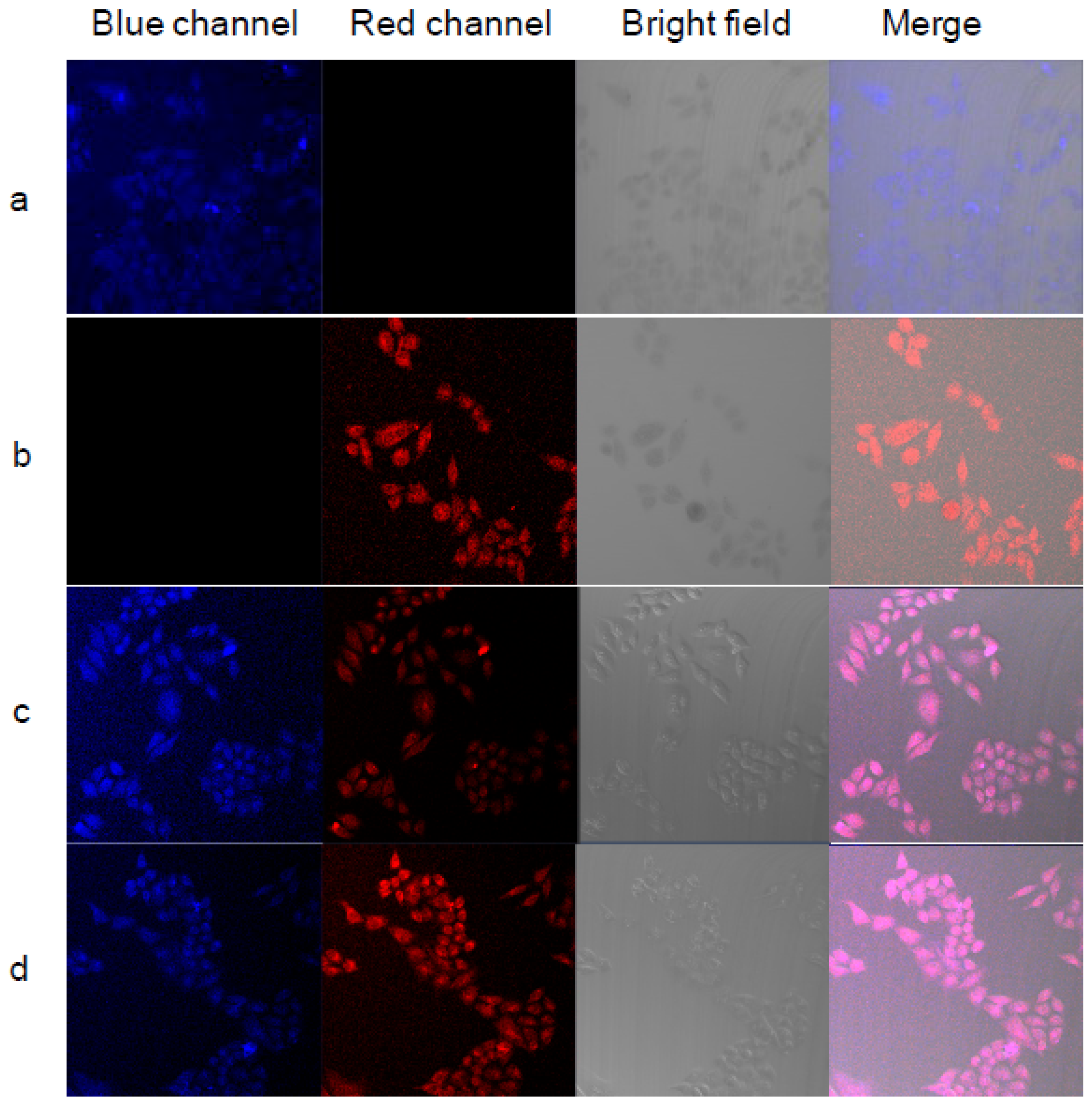

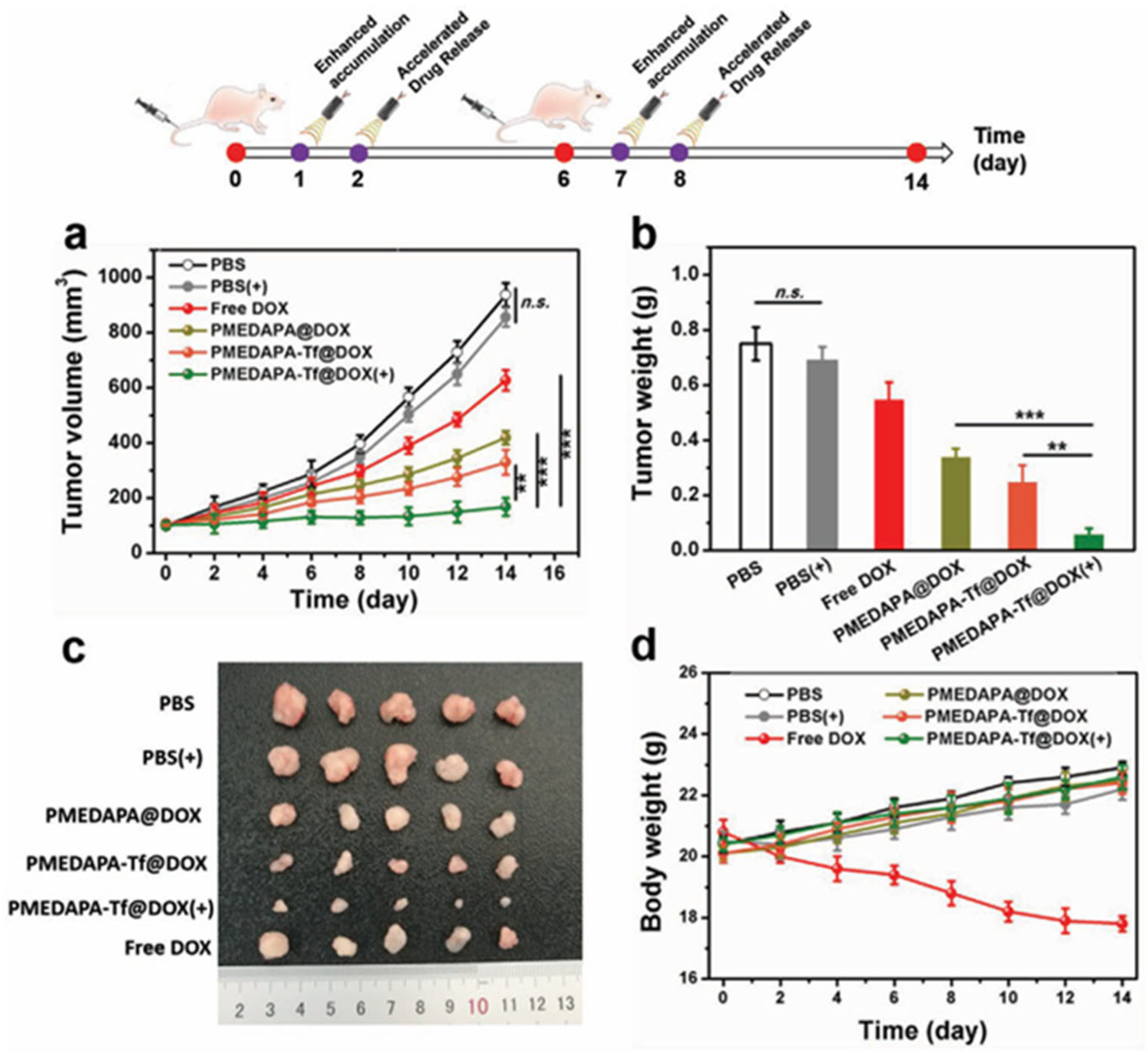
| Thermoresponsive Polymer | Fluorescent Unit | Emission (nm) | Polymerization Technique | Size (nm) | VPTT (°C) a | Application | Ref. |
|---|---|---|---|---|---|---|---|
| PNIPAM | TVPA luminogen | 500–700 | PP | 110 a,f | 32 | Nanothermometer | [1] |
| PNIPAM | DBD-AA monomer | 570–589 | PP with cationic initiator | 157 a,c | 30–35 | Nanothermometer | [2] |
| PNIPAM | Benzothiadiazole | 580 | PP Cationic polymerization | 22.3 d | 32 | Nanothermometer | [3] |
| PNIPAM | Naphtalimide derivative | 450 | EP | 304 a,c | 34–43 | Sensors | [12] |
| PNIPAM | (1) Fluorescein (2) Rhodamine | (1)517 (2) 586 | EP | 173 a,g | 35.5 | Sensors | [13] |
| PNIPAM | Pyranine | 416–432 | PP | 140–550 b | 32 | - | [65] |
| PNVCL-PEGDA PGMA-PCB | Rhodamine B | 586 | RAFT/SFEP | 240–320 a,c | 33–37 | Bioimaging | [9] |
| PDFEA/PHPMA or PMeOx | Fluorine atoms | 600 | RAFT | 120 d | 30–50 e | Bioimaging | [70] |
| PDEAEM-PEGMA | Fluorescein | 520 | SFEP | 183–200 a,c | 40–45 | Drug delivery | [6] |
| PNIPAM | Naphtalimide derivative | 550 | DP | 10–30 a,c | 37 | Drug Delivery | [17] |
| PNIPAM-PDMAEMA | Fluorescein | 517 | EP | 98 a,c | 40 | Drug delivery | [7] |
| PNVCL-PHPMA | (1) Fluorescein (2) Cyanine 7.5 | (1) 517 (2) 800 | PP | 89–196 a,c | 35–44 | Drug delivery | [8] |
| PMEDAPA | Cyanine 5 | 667 | PP | 130–200 a,c | 32–43 | Theranostic | [15] |
| PEGMA/PFuMaMA/PHEMA | (1)BODIPY (2) Cyanine 5 | (1) 605 (2) 715 | RAFT | 92 a,c | 60–70 h | Theranostic | [66] |
| Copolymer | Synthesis Technique | Morphology | Tcp (°C) | Emission Region (nm) | Biological Assay | Application | Ref. |
|---|---|---|---|---|---|---|---|
| P(NIPAM-co-BODIPY-AA) | RAFT | Micelles | 35 | 605 | BHK cells | Nanothermometer | [4] |
| PEGMA-b-P(PNIPAM-co-NBDAE) | RAFT | Micelles | 35 | 536 | HepG2 cells | Nanothermometer | [5] |
| (PNIPAM)x-TPPEBr X = 1,2,3,4 | FRP | Micelles | 31–38 | 560 | A549 cells | Nanothermometer | [75] |
| POx-Pyrene; POx-BODIPY; POx-Porphyrin | Cationic polymerization/Click react. | ------ | 47, 43, 39 | 430, 520, 655 | ------ | Sensors | [40] |
| P(NIPAM-co-BMPN-co-R6GEM) | RAFT | ------ | 25–45 | 520 and 555 | ------ | Sensors | [76] |
| P(NIPAM-co-MAA)-KS | FRP | Aggregates | 38 | 572 | ------ | Sensors | [77] |
| PNIPAM-VPBA-C-Fluo-4AM | RAFT | Micelles | 35 | 738 | HeLa cells | Sensors | [78] |
| P(NIPAM-co-TPE)-b-PDPA | RAFT | Micelles | 36 | 470 | MCF-7 cells | Bioimaging | [10] |
| PNIPAM-TPE-PNIPAM | ATRP | Micelles | 34 | 480 | HeLa cells | Bioimaging | [11] |
| P(NIPAM-co-TCMA) P(NIPMAM-co-BOBPYBX) | FRP | Aggregates | 25–50 | 436 and 628 | HeLa cells | Bioimaging | [48] |
| P(NIPAM-co-FL) | FRP | Aggregates | 30 | 514 | RAW264.7 cells | Bioimaging | [79] |
| Tyr-P(NIPAM-co-DMAA)-FL | RAFT | Aggregates | 34–46 | 517 | HeLa cells | Bioimaging | [80] |
| PEGMA-b-P(NIPAM-r-R6GMED) | RAFT | Vesicles | 25 | 584 | ------ | Drug delivery | [16] |
| PF-b-PNIPAM-b-POEGMA | Sequential click coupling-ATRP | Micelles | 33 | 450 | HeLa cells | Theranostic | [14] |
| PNIPAM-PFV | Heck coupling reaction | Aggregates | 30–35 | 502 | MCF-7 | Theranostic | [81] |
| PMalpGP-b-POEGMA-b-P(Llys-co-Asp) | RAFT-ROP-Click reaction | Micelles | ------ | 597 and 795 | HepG2 and NIH3T3 | Theranostic | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ayón, M.A.; Márquez-Castro, J.E.; Félix-Alcalá, D.V.; Licea-Claverie, A. Thermoresponsive and Fluorescent Polymers: From Nanothermometers to Smart Drug Delivery Systems for Theranostics Against Cancer. Pharmaceutics 2025, 17, 1062. https://doi.org/10.3390/pharmaceutics17081062
González-Ayón MA, Márquez-Castro JE, Félix-Alcalá DV, Licea-Claverie A. Thermoresponsive and Fluorescent Polymers: From Nanothermometers to Smart Drug Delivery Systems for Theranostics Against Cancer. Pharmaceutics. 2025; 17(8):1062. https://doi.org/10.3390/pharmaceutics17081062
Chicago/Turabian StyleGonzález-Ayón, Mirian A., Jesús E. Márquez-Castro, Diana V. Félix-Alcalá, and Angel Licea-Claverie. 2025. "Thermoresponsive and Fluorescent Polymers: From Nanothermometers to Smart Drug Delivery Systems for Theranostics Against Cancer" Pharmaceutics 17, no. 8: 1062. https://doi.org/10.3390/pharmaceutics17081062
APA StyleGonzález-Ayón, M. A., Márquez-Castro, J. E., Félix-Alcalá, D. V., & Licea-Claverie, A. (2025). Thermoresponsive and Fluorescent Polymers: From Nanothermometers to Smart Drug Delivery Systems for Theranostics Against Cancer. Pharmaceutics, 17(8), 1062. https://doi.org/10.3390/pharmaceutics17081062








