Nanocarriers in Ungual Drug Delivery
Abstract
1. Introduction
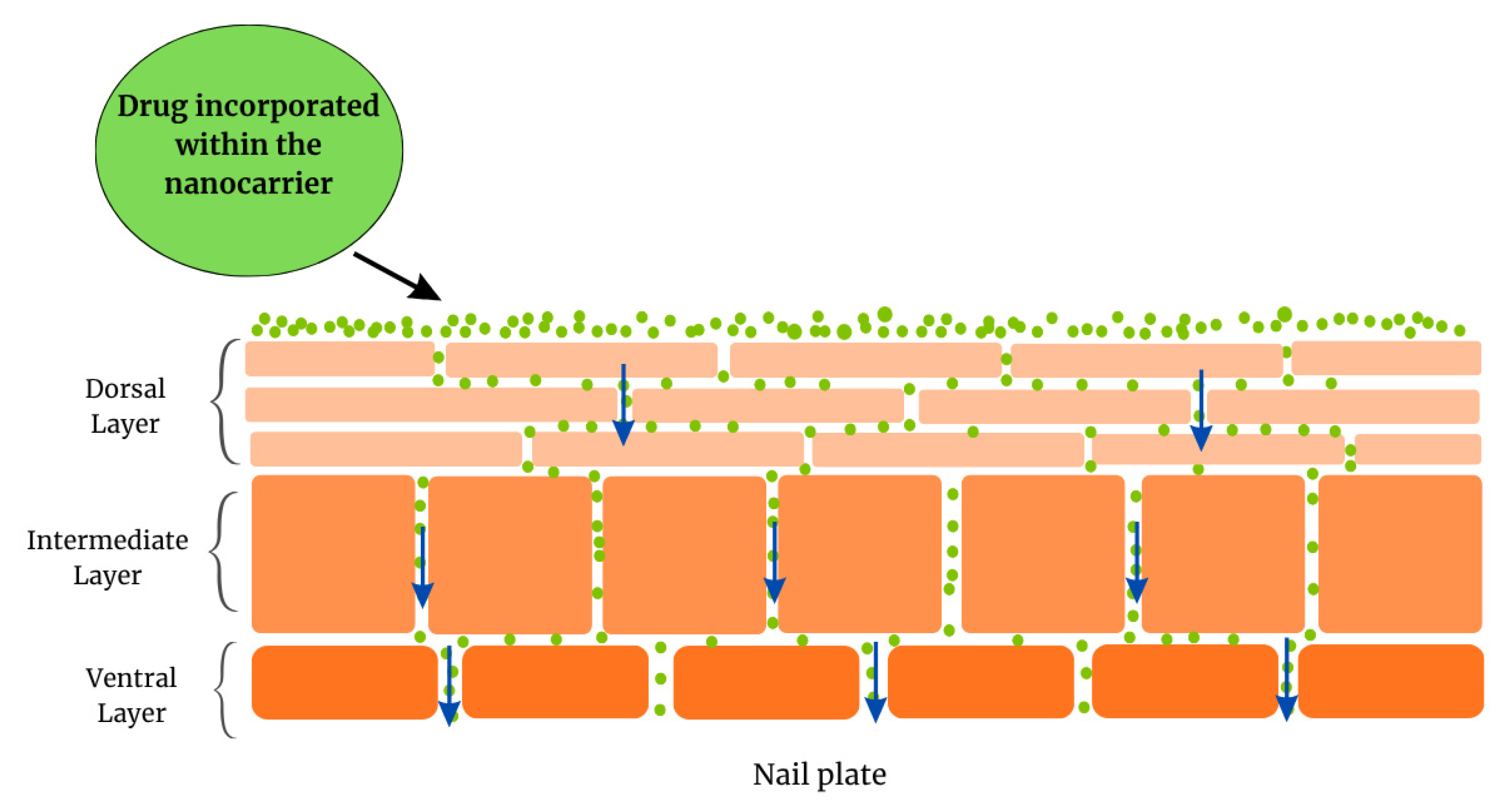
2. Nail Anatomy
- The dorsal layer, a poorly permeable surface, comprising overlapping cells. This layer is just a few cells thick [45].
- The intermediate layer, the thickest layer, is softer and more malleable.
- The ventral layer, a thin layer whose function is to connect the nail plate to the nail bed underneath.
3. Ungual Disorders and Current Therapy
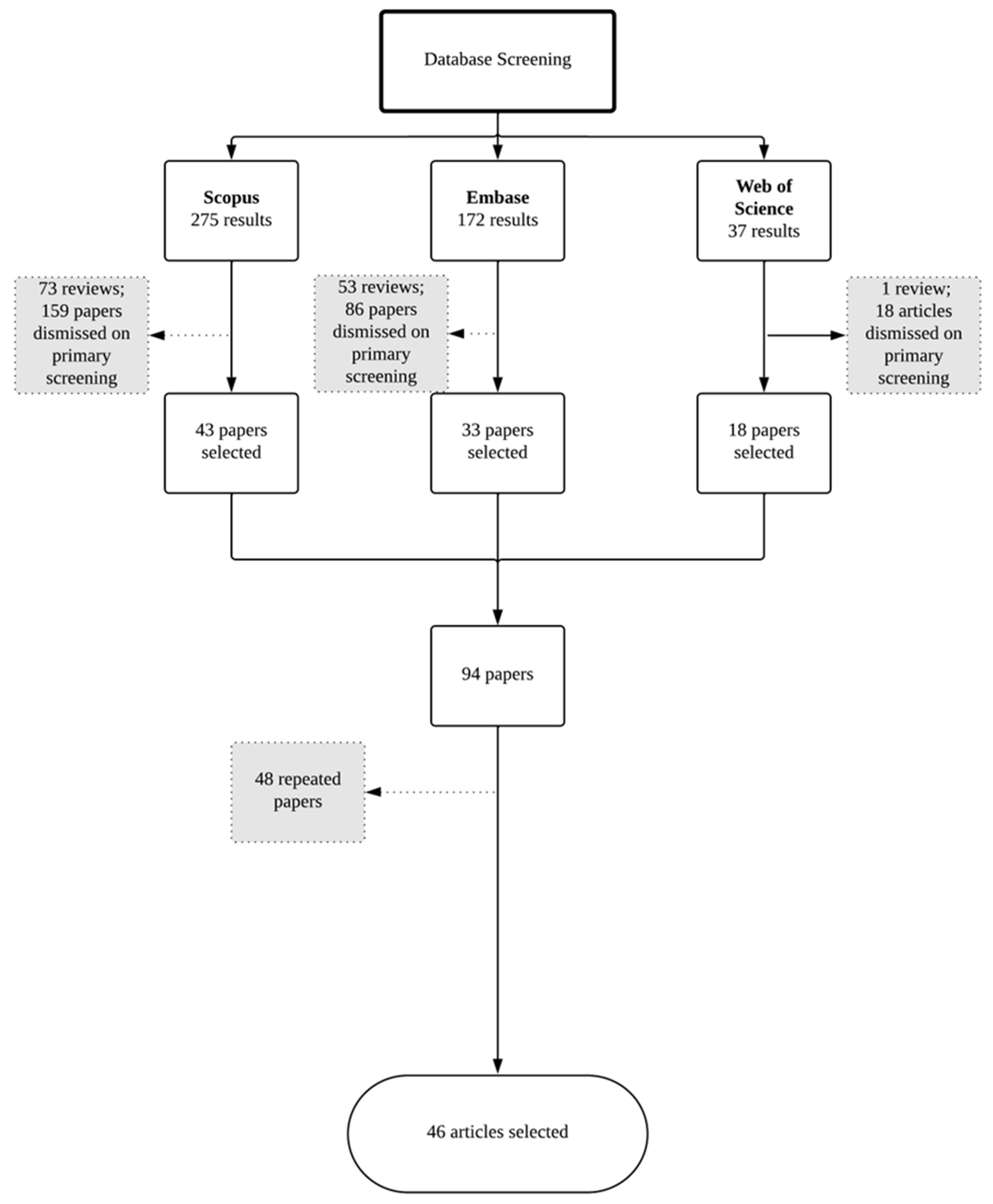
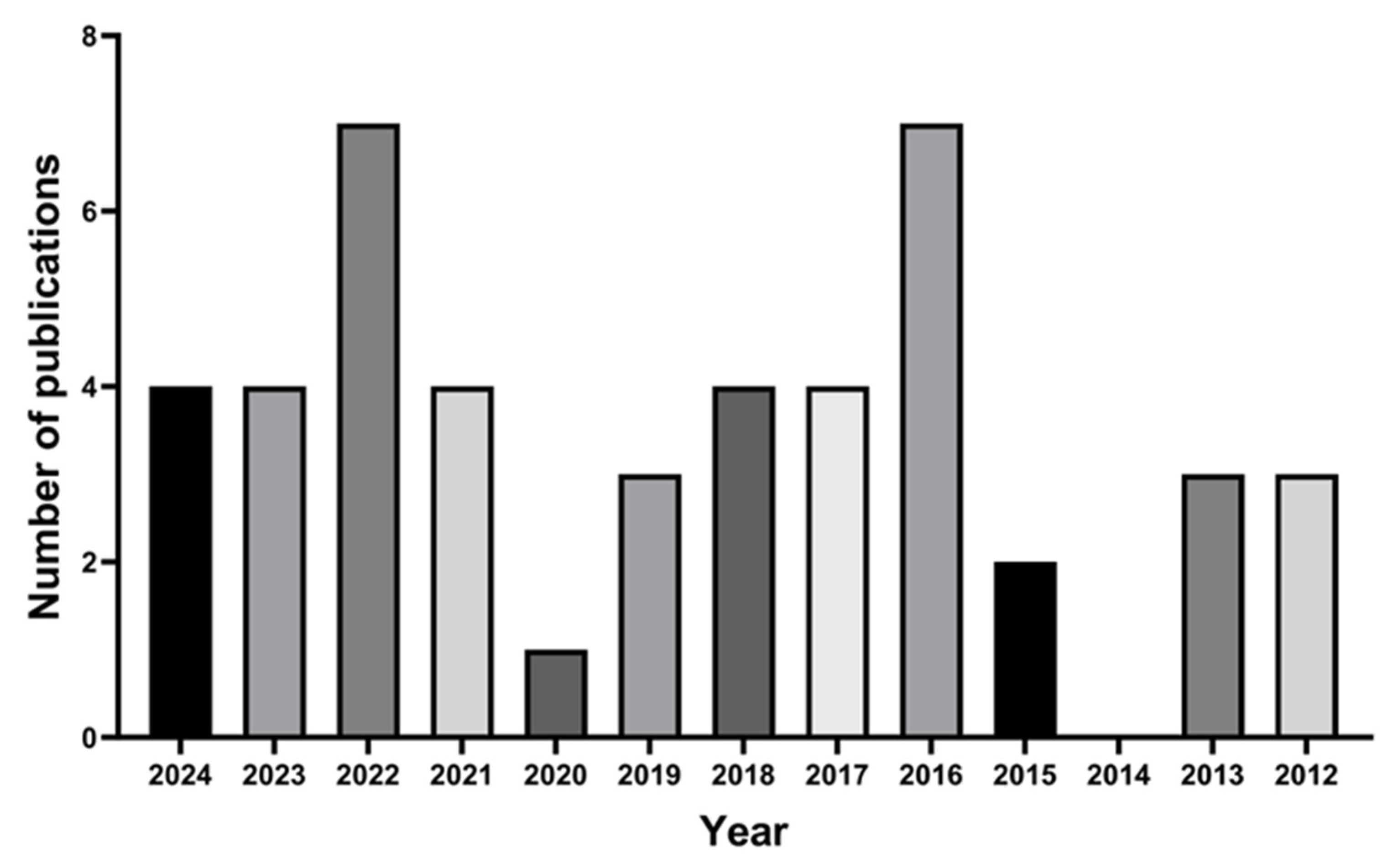
4. Literature Survey
- Original research;
- Written in English;
- Nanostructured systems;
- Formulation designed for topical administration to the nail unit.
5. Nanostructured Systems for Drug Delivery to the Nail
5.1. Vesicular System [78]
- Penetration enhancers containing vesicles (nPEVs): nPEVs are particles designed towards transdermal drug delivery, and have chemical penetration enhancers along with the basic components of LS, providing the ability to permeate biological barriers [23];
5.2. Microemulsions
5.3. Nanoemulsions
5.4. Nanostructured Lipid Carriers and Solid Lipid Nanoparticles
5.5. Polymeric Nanoparticles
5.6. Nanocapsules
5.7. Supramolecular Nanoparticles
5.8. Metal Nanoparticles
6. Pharmaceutical Vehicles
7. Unanswered Questions
8. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murdan, S. Enhancing the Nail Permeability of Topically Applied Drugs. Expert Opin. Drug Deliv. 2008, 5, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, P.P.; Jani, U. Drug Delivery through Nails: Present and Future. New Horiz. Transl. Med. 2017, 3, 252. [Google Scholar]
- Vikas, A.; Rashmin, P.; Mrunali, P.; Chavan, R.B.; Kaushik, T. Mechanistic Insights of Formulation Approaches for the Treatment of Nail Infection: Conventional and Novel Drug Delivery Approaches. AAPS PharmSciTech 2020, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Dhamoon, R.K.; Popli, H.; Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef]
- Tan, E.S.T.; Chong, W.-S.; Tey, H.L. Nail Psoriasis. Am. J. Clin. Dermatol. 2012, 13, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Turner, R.; Wevrett, S.R. Use of in Vitro Performance Models in the Assessment of Drug Delivery across the Human Nail for Nail Disorders. Expert Opin. Drug Deliv. 2018, 15, 983–989. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and Transungual Drug Delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef]
- Narasimha Murthy, S.; Maibach, H.I. Topical Nail Products and Ungual Drug Delivery; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Aulton, M.E.; Taylor, K.M.G. Aulton’s Pharmaceutics E-Book: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Kreutz, T.; de Matos, S.P.; Koester, L.S. Recent Patents on Permeation Enhancers for Drug Delivery Through Nails. Recent Pat. Drug Deliv. Formul. 2019, 13, 203–218. [Google Scholar] [CrossRef]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the Formidable Nail Barrier: A Review of the Nail Microstructure, Composition and Diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef]
- Saner, M.V.; Kulkarni, A.D.; Pardeshi, C.V. Insights into Drug Delivery across the Nail Plate Barrier. J. Drug Target. 2014, 22, 769–789. [Google Scholar] [CrossRef]
- Das Kurmi, B.; Tekchandani, P.; Paliwal, R.; Paliwal, S.R. Transdermal Drug Delivery: Opportunities and Challenges for Controlled Delivery of Therapeutic Agents Using Nanocarriers. Curr. Drug Metab. 2017, 18, 481–495. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Chiu, W.S.; Belsey, N.A.; Garrett, N.L.; Moger, J.; Price, G.J.; Delgado-Charro, M.B.; Guy, R.H. Drug Delivery into Microneedle-Porated Nails from Nanoparticle Reservoirs. J. Control. Release 2015, 220, 98–106. [Google Scholar] [CrossRef]
- Flores, F.C.; Chiu, W.S.; Beck, R.C.R.; da Silva, C.B.; Delgado-Charro, M.B. Enhancement of Tioconazole Ungual Delivery: Combining Nanocapsule Formulation and Nail Poration Approaches. Int. J. Pharm. 2018, 535, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Morgado, L.F.; Trávolo, A.R.F.; Muehlmann, L.A.; Narcizo, P.S.; Nunes, R.B.; Pereira, P.A.G.; Py-Daniel, K.R.; Jiang, C.S.; Gu, J.; Azevedo, R.B.; et al. Photodynamic Therapy Treatment of Onychomycosis with Aluminium-Phthalocyanine Chloride Nanoemulsions: A Proof of Concept Clinical Trial. J. Photochem. Photobiol. B 2017, 173, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.A.; Noaman, I.; El-Elsayyad, H.; El-Mashad, N.; Soliman, M. A Study of the Treatment of Cutaneous Fungal Infection in Animal Model Using Photoactivated Composite of Methylene Blue and Gold Nanoparticle. Photodiagn. Photodyn. Ther. 2016, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Amra, K.; Momin, M. Formulation Evaluation of Ketoconazole Microemulsion-Loaded Hydrogel with Nigella Oil as a Penetration Enhancer. J. Cosmet. Dermatol. 2019, 18, 1742–1750. [Google Scholar] [CrossRef]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, M.C.; Shelat, P.K. Microemulsion-Based Gel of Terbinafine for the Treatment of Onychomycosis: Optimization of Formulation Using D-Optimal Design. AAPS PharmSciTech 2012, 13, 184–192. [Google Scholar] [CrossRef]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Mehta, D.M.; Shelat, P.K. Microemulsion-Based Antifungal Gel Delivery to Nail for the Treatment of Onychomycosis: Formulation, Optimization, and Efficacy Studies. Drug Deliv. Transl. Res. 2012, 2, 463–476. [Google Scholar] [CrossRef]
- Agrawal, V.; Patel, R.; Patel, M. Design, Characterization, and Evaluation of Efinaconazole Loaded Poly(D, L-Lactide-Co-Glycolide) Nanocapsules for Targeted Treatment of Onychomycosis. J. Drug Deliv. Sci. Technol. 2023, 80, 104157. [Google Scholar] [CrossRef]
- Bseiso, E.A.; Nasr, M.; Sammour, O.A.; Abd El Gawad, N.A. Novel Nail Penetration Enhancer Containing Vesicles “NPEVs” for Treatment of Onychomycosis. Drug Deliv. 2016, 23, 2813–2819. [Google Scholar] [CrossRef]
- Chouhan, P.; Saini, T.R. D-Optimal Design and Development of Microemulsion Based Transungual Drug Delivery Formulation of Ciclopirox Olamine for Treatment of Onychomycosis. Indian J. Pharm. Sci. 2016, 78, 498–511. [Google Scholar] [CrossRef]
- Rocha, K.A.D.; Krawczyk-Santos, A.P.; Andrade, L.M.; de Souza, L.C.; Marreto, R.N.; Gratieri, T.; Taveira, S.F. Voriconazole-Loaded Nanostructured Lipid Carriers (NLC) for Drug Delivery in Deeper Regions of the Nail Plate. Int. J. Pharm. 2017, 531, 292–298. [Google Scholar] [CrossRef]
- Tanriverdi, S.T.; Özer, Ö. Novel Topical Formulations of Terbinafine-HCl for Treatment of Onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef]
- Thatai, P.; Sapra, B. Transungual Gel of Terbinafine Hydrochloride for the Management of Onychomycosis: Formulation, Optimization, and Evaluation. AAPS PharmSciTech 2017, 18, 2316–2328. [Google Scholar] [CrossRef]
- Tuncay Tanriverdi, S. Preparation and Characterization of Caffeine Loaded Liposome and Ethosome Formulations for Transungual Application. Turk. J. Pharm. Sci. 2018, 15, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Tuncay Tanriverdi, S.; Hilmioğlu Polat, S.; Yeşim Metin, D.; Kandiloğlu, G.; Özer, Ö. Terbinafine Hydrochloride Loaded Liposome Film Formulation for Treatment of Onychomycosis: In Vitro and In Vivo Evaluation. J. Liposome Res. 2016, 26, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Polla Ravi, S.; Choi, S.Y.; Konda, A.; Cooper, E.A. Strategies for the Enhancement of Nail Plate Permeation of Drugs to Treat Onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 243–255. [Google Scholar] [CrossRef]
- Rabiei, M.; Kashanian, S.; Samavati, S.S.; Jamasb, S.; McInnes, S.J.P. Nanomaterial and Advanced Technologies in Transdermal Drug Delivery. J. Drug Target. 2020, 28, 356–367. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the Transdermal Route: A State of the Art Review and Critical Appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Bajpai, M. Lipid-Based Nanoformulations in Onychomycosis Therapy: Addressing Challenges of Current Therapies and Advancing Treatment. RSC Adv. 2025, 15, 7799–7825. [Google Scholar] [CrossRef]
- Rodriguez-Takeuchi, S.Y.; Villota, V.; Renjifo, M. Anatomy and Pathology of the Nail and Subungual Space: Imaging Evaluation of Benign Lesions. Clin. Imaging 2018, 52, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.R. Harry’s Cosmeticology 9th Edition Volume One, 9th ed.; Chemical Publishing Company: Gloucester, MA, USA, 2015; Volume 1. [Google Scholar]
- Zaias, N. The Nail in Health and Disease; Appleton & Lange: New York, NY, USA, 1980. [Google Scholar]
- Hao, J.; Smith, K.A.; Li, S.K. Iontophoretically Enhanced Ciclopirox Delivery into and across Human Nail Plate. J. Pharm. Sci. 2009, 98, 3608–3616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, Mechanical Properties, Occurrence in Biological Organisms, and Efforts at Bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miyamoto, M.; Sugibayashi, K.; Morimoto, Y. Drug Permeation through the Three Layers of the Human Nail Plate. J. Pharm. Pharmacol. 1999, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fleckman, P. Structure and Function of the Nail Unit. In Nails; Elsevier: Philadelphia, PA, USA, 2005. [Google Scholar]
- Farran, L.; Ennos, A.R.; Eichhorn, S.J. The Effect of Humidity on the Fracture Properties of Human Fingernails. J. Exp. Biol. 2008, 211, 3677–3681. [Google Scholar] [CrossRef]
- Mertin, D.; Lippold, B.C. In-Vitro Permeability of the Human Nail and of a Keratin Membrane from Bovine Hooves: Prediction of the Penetration Rate of Antimycotics through the Nail Plate and Their Efficacy. J. Pharm. Pharmacol. 1997, 49, 866–872. [Google Scholar] [CrossRef]
- Gniadecka, M.; Nielsen, O.F.; Christensen, D.H.; Wulf, H.C. Structure of Water, Proteins, and Lipids in Intact Human Skin, Hair, and Nail. J. Investig. Dermatol. 1998, 110, 393–398. [Google Scholar] [CrossRef]
- Elkeeb, R.; AliKhan, A.; Elkeeb, L.; Hui, X.; Maibach, H.I. Transungual Drug Delivery: Current Status. Int. J. Pharm. 2010, 384, 1–8. [Google Scholar] [CrossRef]
- Koroleva, M.Y.; Yurtov, E.V. Nanoemulsions: The Properties, Methods of Preparation and Promising Applications. Russ. Chem. Rev. 2012, 81, 21–43. [Google Scholar] [CrossRef]
- Repka, M.A.; O’Haver, J.; See, C.H.; Gutta, K.; Munjal, M. Nail Morphology Studies as Assessments for Onychomycosis Treatment Modalities. Int. J. Pharm. 2002, 245, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Targhotra, M.; Sahoo, P.K.; Chauhan, M.K. Onychomycosis: Novel Strategies for Treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101774. [Google Scholar] [CrossRef]
- Maskan Bermudez, N.; Rodríguez-Tamez, G.; Perez, S.; Tosti, A. Onychomycosis: Old and New. J. Fungi 2023, 9, 559. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.M.; Wong, A.H.C. Onychomycosis: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar]
- Garg, T.; Rath, G.; Goyal, A.K. Nanotechnological Approaches for the Effective Management of Psoriasis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1374–1382. [Google Scholar] [CrossRef]
- Thatai, P.; Khan, A.B. Management of Nail Psoriasis by Topical Drug Delivery: A Pharmaceutical Perspective. Int. J. Dermatol. 2020, 59, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Azad, J.; Takwale, A. Management of Nail Psoriasis. Clin. Exp. Dermatol. 2021, 46, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Pasch, M.C. Nail Psoriasis: A Review of Treatment Options. Drugs 2016, 76, 675–705. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Repka, M.A.; Narasimha Murthy, S. Transungual Drug Delivery: An Update. J. Drug Deliv. Sci. Technol. 2014, 24, 301–310. [Google Scholar] [CrossRef]
- Kumar, S.; Talegaonkar, S.; Negi, L.M.; Khan, Z.I. Design and Development of Ciclopirox Topical Nanoemulsion Gel for the Treatment of Subungual Onychomycosis. Indian J. Pharm. Educ. Res. 2012, 46, 303–311. [Google Scholar]
- Oliveira do Nascimento, M.; Lima Guedes, I.; Alves Lopes Junior, C.; Juan Chavez Gutierrez, S.; Medeiros Barreto, H.; Luis Menezes Carvalho, A. Validation of Spectrophotometric Methods for the Simultaneous Determination of Fluconazole and Riparin B in the Development of Lipid Nanoparticles Modified by β-Cyclodextrin: Application for in Vitro Characterization and Ex Vivo Studies of Nail Retention. Microchem. J. 2024, 200, 110387. [Google Scholar] [CrossRef]
- Farheen, F.; Yadav, H.K.; Raizaday, A. Formulation and Evaluation of Nanoparticle Loaded Hydrogel Containing Antifungal Agent for the Treatment of Onychomycosis Using Factorial Design. Int. J. Drug Deliv. Technol. 2024, 14, 1415–1425. [Google Scholar] [CrossRef]
- Al-Suwaytee, S.H.M.; Ben Hadj Ayed, O.; Chaâbane-Banaoues, R.; Kosksi, T.; Shleghm, M.R.; Chekir-Ghedira, L.; Babba, H.; Sfar, S.; Lassoued, M.A. Exploring the Antifungal Effectiveness of a Topical Innovative Formulation Containing Voriconazole Combined with Pinus sylvestris L. Essential Oil for Onychomycosis. Colloids Interfaces 2024, 8, 56. [Google Scholar] [CrossRef]
- Moazeni, M.; Kelidari, H.; Nasirzadehfard, Y.; Shokohi, T.; Roohi, B.; Hajheidari, Z.; Kazeminejad, A.; Parsay, S.; Asare-Addo, K.; Nokhodchi, A. Lesson from Nature: Zataria Multiflora Nanostructured Lipid Carrier Topical Gel Formulation against Candida-Associated Onychomycosis, a Randomized Double-Blind Placebo-Controlled Clinical Trial. Med. Drug Discov. 2024, 22, 100187. [Google Scholar] [CrossRef]
- Bekmukhametova, A.; Antony, A.; Halliday, C.; Chen, S.; Ho, C.H.; Uddin, M.M.N.; Longo, L.; Pedrinazzi, C.; George, L.; Wuhrer, R.; et al. Rose Bengal–Encapsulated Chitosan Nanoparticles for the Photodynamic Treatment of Trichophyton Species. Photochem. Photobiol. 2024, 100, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, S.; Tejas, D.S.; Yamini, M.; Taaha, Z.N.; Monisha, R.L.; Anumita, G.; Logeswaran, K. Fabrication and Characterization of Tolnaftate Loaded Topical Nanoemulgel for the Treatment of Onychomycosis. Int. J. Drug Deliv. Technol. 2023, 13, 461–467. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Rashid, M.A.; Alhamhoom, Y.; Aqil, M.; Mujeeb, M. Linalool-Incorporated Synergistically Engineered Modified Liposomal Nanocarriers for Enhanced Transungual Delivery of Terbinafine against Onychomycosis. Materials 2023, 16, 4424. [Google Scholar] [CrossRef]
- Firooz, A.; Zamani, S.; Ghadrei, A.; Ayatollahi, A.; Tamimi, P.; Khamesipour, A.; Jafari, M.; Fattahi, M. Evaluation of Efficacy and Safety of Topical Nanoliposomal Amphotericin B 0.4% Gel as a Potential Treatment for Onychomycosis: An Interventional Pilot Clinical Study. Dermatol. Ther. 2023, 2023, 9955124. [Google Scholar] [CrossRef]
- Ullah, K.H.; Rasheed, F.; Naz, I.; Ul Haq, N.; Fatima, H.; Kanwal, N.; Ur-Rehman, T. Chitosan Nanoparticles Loaded Poloxamer 407 Gel for Transungual Delivery of Terbinafine HCl. Pharmaceutics 2022, 14, 2353. [Google Scholar] [CrossRef]
- Kesharwani, P.; Fatima, M.; Singh, V.; Sheikh, A.; Almalki, W.H.; Gajbhiye, V.; Sahebkar, A. Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis. Biomimetics 2022, 7, 206. [Google Scholar] [CrossRef]
- Puri, V.; Froelich, A.; Shah, P.; Pringle, S.; Chen, K.; Michniak-Kohn, B. Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation. Pharmaceutics 2022, 14, 2170. [Google Scholar] [CrossRef] [PubMed]
- Almuqbil, R.M.; Sreeharsha, N.; Nair, A.B. Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics 2022, 14, 1419. [Google Scholar] [CrossRef]
- Yasin, G.; Nasr, M.; Abdel Gaber, S.A.; Hüttenberger, D.; Fadel, M. Response Surface Methodological Approach for Optimization of Photodynamic Therapy of Onychomycosis Using Chlorin E6 Loaded Nail Penetration Enhancer Vesicles. J. Photochem. Photobiol. B 2022, 232, 112461. [Google Scholar] [CrossRef]
- Gaballah, E.Y.; Borg, T.M.; Mohamed, E.A. Hydroxypropyl Chitosan Nail Lacquer of Ciclopirox-PLGA Nanocapsules for Augmented in Vitro Nail Plate Absorption and Onychomycosis Treatment. Drug Deliv. 2022, 29, 3304–3316. [Google Scholar] [CrossRef]
- Alqahtani, A.; Raut, B.; Khan, S.; Mohamed, J.M.M.; Al Fatease, A.; Alqahtani, T.; Alamri, A.; Ahmad, F.; Krishnaraju, V. The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers 2022, 14, 325. [Google Scholar] [CrossRef]
- Dantas, K.N.M.; Andrade, L.R.; Lisboa, E.; Santana, V.L.; Santos, A.L.S.; Mello, T.P.; Sangenito, L.S.; Lima, Á.S.; Fricks, A.T.; Begnami, A.F.; et al. Antimycotic Nail Polish Based on Humic Acid-Coated Silver Nanoparticles for Onychomycosis. J. Chem. Technol. Biotechnol. 2021, 96, 2208–2218. [Google Scholar] [CrossRef]
- Kancı Bozoğlan, B.; Duman, O.; Tunç, S. Smart Antifungal Thermosensitive Chitosan/Carboxymethylcellulose/Scleroglucan/Montmorillonite Nanocomposite Hydrogels for Onychomycosis Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125600. [Google Scholar] [CrossRef]
- Abobakr, F.E.; Fayez, S.M.; Elwazzan, V.S.; Sakran, W. Effect of Different Nail Penetration Enhancers in Solid Lipid Nanoparticles Containing Terbinafine Hydrochloride for Treatment of Onychomycosis. AAPS PharmSciTech 2021, 22, 33. [Google Scholar] [CrossRef]
- Krawczyk-Santos, A.P.; da Rocha, P.B.R.; Kloppel, L.L.; Souza, B.d.S.; Anjos, J.L.V.; Alonso, A.; de Faria, D.L.A.; Gil, O.M.; Gratieri, T.; Marreto, R.N.; et al. Enhanced Nail Delivery of Voriconazole-Loaded Nanomicelles by Thioglycolic Acid Pretreatment: A Study of Protein Dynamics and Disulfide Bond Rupture. Int. J. Pharm. 2021, 602, 120597. [Google Scholar] [CrossRef]
- Vörös-Horváth, B.; Das, S.; Salem, A.; Nagy, S.; Böszörményi, A.; Kőszegi, T.; Pál, S.; Széchenyi, A. Formulation of Tioconazole and Melaleuca Alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules 2020, 25, 5544. [Google Scholar] [CrossRef]
- Pereira, R.R.; Testi, M.; Rossi, F.; Silva Junior, J.O.C.; Ribeiro-Costa, R.M.; Bettini, R.; Santi, P.; Padula, C.; Sonvico, F. Ucuùba (Virola Surinamensis) Fat-Based Nanostructured Lipid Carriers for Nail Drug Delivery of Ketoconazole: Development and Optimization Using Box-Behnken Design. Pharmaceutics 2019, 11, 284. [Google Scholar] [CrossRef]
- Lafta, A.K.; Ajah, H.A.; Dakhil, O.A.A.; Ali AL-Wattar, W.M. Biosynthesis of Silver Nanoparticles Using Biomass of Cladosporium Cladosporioidesand Antifungalactivity against Pathogenic Fungi Causing Onychomycosis. Plant Arch. 2019, 19, 4391–4396. [Google Scholar]
- Wang, F.; Yang, P.; Choi, J.S.; Antovski, P.; Zhu, Y.; Xu, X.; Kuo, T.H.; Lin, L.E.; Kim, D.N.H.; Huang, P.C.; et al. Cross-Linked Fluorescent Supramolecular Nanoparticles for Intradermal Controlled Release of Antifungal Drug—A Therapeutic Approach for Onychomycosis. ACS Nano 2018, 12, 6851–6859. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-Ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Flores, F.C.; Rosso, R.S.; Cruz, L.; Beck, R.C.R.; Silva, C.B. An Innovative Polysaccharide Nanobased Nail Formulation for Improvement of Onychomycosis Treatment. Eur. J. Pharm. Sci. 2017, 100, 56–63. [Google Scholar] [CrossRef]
- Shah, V.H.; Jobanputra, A. Enhanced Ungual Permeation of Terbinafine HCl Delivered Through Liposome-Loaded Nail Lacquer Formulation Optimized by QbD Approach. AAPS PharmSciTech 2018, 19, 213–224. [Google Scholar] [CrossRef]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Pandit, R.; Gaikwad, S.; Gade, A.; Rai, M. Biosynthesis of Zinc Oxide Nanoparticles by Petals Extract of Rosa indica L., Its Formulation as Nail Paint and Evaluation of Antifungal Activity against Fungi Causing Onychomycosis. IET Nanobiotechnol. 2017, 11, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ou, R.; Guan, S.; Ye, X.; Hu, B.; Zhang, Y.; Lu, S.; Zhou, Y.; Yuan, Z.; Zhang, J.; et al. A Novel Drug Delivery Gel of Terbinafine Hydrochloride with High Penetration for External Use. Drug Deliv. 2015, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; de Lima, J.A.; Ribeiro, R.F.; Alves, S.H.; Rolim, C.M.B.; Beck, R.C.R.; da Silva, C.B. Antifungal Activity of Nanocapsule Suspensions Containing Tea Tree Oil on the Growth of Trichophyton Rubrum. Mycopathologia 2013, 175, 281–286. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticles-a Historical Perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef]
- Gurny, R.; Boye, T.; Ibrahim, H. Ocular Therapy with Nanoparticulate Systems for Controlled Drug Delivery. J. Control. Release 1985, 2, 353–361. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-Formulations for Transdermal Drug Delivery: A Review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The Definition of Microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Sinico, C.; Fadda, A.M. Vesicular Carriers for Dermal Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 813–825. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2016, 14, 613–633. [Google Scholar] [CrossRef]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Invasomes: Potential Vesicular Systems for Transdermal Delivery of Drug Molecules. J. Drug Deliv. Sci. Technol. 2021, 61, 102166. [Google Scholar] [CrossRef]
- Lakshmi, P.K.; Kalpana, B.; Prasanthi, D. Invasomes-Novel Vesicular Carriers for Enhanced Skin Permeation. Syst. Rev. Pharm. 2013, 4, 26. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.; Khar, R.; Pathan, S.; Khan, Z. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured Lipid Carriers: A Potential Use for Skin Drug Delivery Systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Gratieri, T.; Krawczyk-Santos, A.P.; da Rocha, P.B.R.; Cunha–Filho, M.; Gelfuso, G.M.; Marreto, R.N.; Taveira, S.F. SLN- and NLC-Encapsulating Antifungal Agents: Skin Drug Delivery and Their Unexplored Potential for Treating Onychomycosis. Curr. Pharm. Des. 2018, 23, 6684–6695. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential Enhancement and Targeting Strategies of Polymeric and Lipid-Based Nanocarriers in Dermal Drug Delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable Nanoparticles for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Sala, M.; Elaissari, A.; Fessi, H. Advances in Psoriasis Physiopathology and Treatments: Up to Date of Mechanistic Insights and Perspectives of Novel Therapies Based on Innovative Skin Drug Delivery Systems (ISDDS). J. Control. Release 2016, 239, 182–202. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-Based Supramolecular Nanoparticles for Biomedical Applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef]
- Stoffelen, C.; Huskens, J. Soft Supramolecular Nanoparticles by Noncovalent and Host-Guest Interactions. Small 2016, 12, 96–119. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Shukla, A.K.; Iravani, S. Metallic Nanoparticles: Green Synthesis and Spectroscopic Characterization. Environ. Chem. Lett. 2017, 15, 223–231. [Google Scholar] [CrossRef]
- Monti, D.; Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Galván, J.; Celandroni, F.; Ghelardi, E. Ciclopirox Hydroxypropyl Chitosan (CPX-HPCH) Nail Lacquer and Breathable Cosmetic Nail Polish: In Vitro Evaluation of Drug Transungual Permeation Following the Combined Application. Life 2022, 12, 801. [Google Scholar] [CrossRef]
- Tampucci, S.; Terreni, E.; Zucchetti, E.; Burgalassi, S.; Chetoni, P.; Monti, D. Formulations Based on Natural Ingredients for the Treatment of Nail Diseases. Curr. Pharm. Des. 2020, 26, 556–565. [Google Scholar] [CrossRef]
- Akhtar, N.; Sharma, H.; Pathak, K. Onychomycosis: Potential of Nail Lacquers in Transungual Delivery of Antifungals. Scientifica 2016, 2016, 1387936. [Google Scholar] [CrossRef]
- Elsayed, M.M.A. Development of Topical Therapeutics for Management of Onychomycosis and Other Nail Disorders: A Pharmaceutical Perspective. J. Control. Release 2015, 199, 132–144. [Google Scholar] [CrossRef]
- Kataria, P.; Sharma, G.; Thakur, K.; Bansal, V.; Dogra, S.; Katare, O.P. Emergence of Nail Lacquers as Potential Transungual Delivery System in the Management of Onchomycosis. Expert Opin. Drug Deliv. 2016, 13, 937–952. [Google Scholar] [CrossRef] [PubMed]


| Mechanical | Physical | Chemical |
|---|---|---|
| Abrasion | Iontophoresis | Keratolytic enzymes |
| Nail avulsion | Etching | Solvents |
| Laser therapy | Thiols and sulfites | |
| Electropulsation | Softening agents | |
| Ultrasonic therapy | Penetration enhancers | |
| Photodynamic therapy | Chemical etchants | |
| Microporation | ||
| Hydration | ||
| Occlusion |
| Nanostructured System | API | In Vitro Permeation Assessment | Reference |
|---|---|---|---|
| NLC | Fluconazole and Riparin-B | In vitro permeation study using porcine hoof as diffusion membrane. It was not possible to measure permeated APIs, but authors observed retention of both drugs in hoof membrane. | [56] |
| SLN | Eficonazole and Fluconazole | NA | [57] |
| NE | Voriconazole and Pinus silvestris essential oil | NA | [58] |
| NLC | Zataria multiflora essential oil | NA | [59] |
| NP | Rose Bengal (dye) | NA | [60] |
| NE | Tolnaftate | NA | [61] |
| NC | Efinaconazole | An ex vivo permeation study using bovine hoof membranes demonstrated that the optimized nanocapsule formulation resulted in significantly greater API permeation compared to the reference formulation. | [22] |
| VS | Terbinafine | Linalool-incorporated vesicular systems presented an increase of approximately 2.5x in drug permeation using an in vitro permeation model using goat hooves as diffusion membranes. Additionally, it was observed that formulation containing vesicular systems associated with linalool as permeation enhancer allowed the observation of the drug in deeper regions of the hoof tissue. | [62] |
| VS | Amphotericin B | NA | [63] |
| NP | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human nail clipping as diffusion membrane. Authors compared NP to NP-loaded poloxamer gel containing terbinafine. No significant difference in ungual retention was observed between formulations; however, the presence of permeation enhancer was shown to increase drug permeation. | [64] |
| NP | Itraconazole and difluorated curcumin | In vitro permeation assay in Franz Diffusion Cells using bovine hoof as diffusion membrane. Authors compared the permeation of Itraconazole and Curcumin from NP-loaded gel and plain gel, denoting a sustained drug release profile from NP-loaded gel. | [65] |
| NP | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared the permeation of Terbinafine from NP-loaded gel and control gel. It was noticed that the incorporation of terbinafine in nanoparticles increased the permeation and provided a controlled-release drug profile. | [66] |
| VS | Eficonazole | NA | [67] |
| VS | Chorin e6 | NA | [68] |
| NC | Ciclopirox | NA | [69] |
| VS | Itraconazole | NA | [70] |
| AgNP | NA | NA | [71] |
| Nanocomposites | Oxiconazole nitrate | NA | [72] |
| SLN | Terbinafine | NA | [73] |
| Nanomicelles | Voriconazole | In vitro permeation assay in Franz Diffusion Cells using bovine hoof as diffusion membrane. Authors compared VS, VS-loaded gel, and dispersion containing eficonazole. Incorporation in nanometric system was demonstrated to improve permeation across the barrier, with a slightly better performance when loaded into gel vehicle. | [74] |
| Pickering Emulsions | Tioconazole + tea tree oil | NA | [75] |
| NLC | Ketoconazole | NA | [76] |
| AgNP | NA | NA | [77] |
| ME | Ketoconazole | In vitro permeation assay in Franz Diffusion Cells using porcine skin as diffusion membrane. Authors compared ME-loaded gel containing ketoconazole to a commercial ketoconazole cream. No significant difference in skin retention was observed between formulations. | [19] |
| NC | Tioconazole | In vitro permeation assay in Franz Diffusion Cells using human nail clippings as diffusion membrane. Authors performed the experiment across 7 days, comparing a single administration and daily administration of Tioconazole containing NCs in porated and non-porated nail clippings. This study’s findings suggest that poration of nail had positive effect on tioconazole permeation and that a single administration of the delivery system to porated nail did not present a significant difference from daily administration. | [16] |
| VS | Caffeine (model drug) | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared VSs (liposomes and ethosomes) containing caffeine (model drug) to caffeine dissolved in water and hydroalcoholic solution. Findings suggest that the incorporation of drug in vesicular systems increased drug permeation across the nail, being even more expressive in the presence of ethanol (ethosomes). | [28] |
| NP | Ketoconazole | NA | [78] |
| VS | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared VSs containing terbinafine to commercial formulation (Lamisil® Cream). VSs presented permeation rates up to 2 times higher than the commercial formulation. | [79] |
| NC | Tioconazole | NA | [80] |
| NE | Aluminium- phthalocyanine chloride | NA | [17] |
| NLC | Voriconazole | In vitro permeation assay in Franz Diffusion Cells using animal hoof as diffusion membrane. Authors compared the permeation of unloaded voriconazole with that of NLC containing voriconazole with and without urea as a permeation enhancer. Incorporation of voriconazole in nanostructured system was shown to increase drug retention, but no difference was observed with the presence of urea. | [25] |
| VS | Terbinafine | NA | [81] |
| ME | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using animal hoof as diffusion membrane. Authors compared terbinafine containing ME and ME incorporated into a gel vehicle, with and without the addition of chemical permeation enhancers. Findings show a correlation of use of permeation enhancers as a strategy to improve permeation, the incorporation of ME in gel vehicle with an increase in drug retention, and an increase in permeation with a decrease in particle size. | [27] |
| VS | Sertaconazole | NA | [23] |
| NE | Ketoconazole | In vitro permeation assay in Franz Diffusion Cells using goat hoof as diffusion membrane. Authors compared NE, NE incorporated into a gel vehicle, and a suspension containing ketoconazole. The cumulative amount of ketoconazole permeated from NE-gel was higher than from NE and suspension, which the authors attribute to the presence in the gel in the gel of thioglycolic acid effect as PE. | [82] |
| Au-NP | Au-NP and Methylene blue | NA | [18] |
| ZnO-NP | NA | NA | [83] |
| ME | Ciclopirox olamine | NA | [24] |
| VS | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared VSs containing terbinafine, prepared with different surfactants, loaded in polymeric films. | [29] |
| NP | NA | NA | [15] |
| VS | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared VSs containing terbinafine incorporated into Eudragit® or Pululan® films. Pululan films presented higher cumulative amounts of terbinafine detected in the diffusion membrane. | [84] |
| NE and NC | Tea tree oil | NA | [85] |
| VS | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human cadaver nails as diffusion membrane. Authors compared different types of VSs containing terbinafine. Findings suggest that VSs containing ethanol can increase drug permeation. Additionally, LS formulations incorporated into poloxamer gel presented better retention of drug compared to LS incorporated into chitosan gel. | [26] |
| ME | Itraconazole | In vitro permeation assay in Franz Diffusion Cells using stacked bovine hoof and human skin layers as diffusion membrane. Authors compared ME, ME incorporated into a gel vehicle, and commercial formulation containing itraconazole. ME and ME-gel showed better retention in the membranes, while commercial formulation was found to remain mostly between skin and hoof layers. | [21] |
| ME | Fluconazole | NA | [55] |
| ME | Terbinafine | In vitro permeation assay in Franz Diffusion Cells using human foot skin as diffusion membrane. Authors compared ME, ME incorporated into a gel vehicle, and commercial formulation containing terbinafine. ME presented higher permeation, and ME-gel showed better retention in the skin. | [20] |
| Patent Nº | Title | Year Priority | Purpose | Technology | Invention Summary |
|---|---|---|---|---|---|
| US10201571B2 | Nanoparticle compositions and methods for treating onychomychosis. | 2017 | Onychomycosis treatment | Metallic nanoparticles | Silver metallic nanoparticles, containing or not containing a second metallic entity; in spherical format or “coral-shaped”; can contain or not contain a permeation enhancer; aiming at the treatment of ungual mycoses. |
| WO2011140126A2 | Nail discoloration and fungus treatment. | 2010 | Onychomycosis and nail discoloration treatment | Silver nanoparticles | Silver nanoparticles/colloidal silver dispersed in gel vehicles (hydrogel, hydrogel moisture pads, hydrosol gels); application of nanosilver treatment followed by covering with hydrogel moist pad; |
| WO2015044669A1 | Antifungal topical composition and methods of treatment. | 2013 | Topical treatment of mycosis | Polymeric nanoparticles | Nanoparticles formed by polymers derived from biguanidine capable of forming nanoparticles; containing an antifungal agent for topical administration in form of a cream, ointment, spray, or powder, and/or microneedle array, patch. |
| WO2017163091A1 | Composition and methods of treatment. | 2016 | Treatment of nail and akin mycosis | Polymeric nanoparticles | Nanoparticles formed by polymers derived from biguanidine capable of forming nanoparticles; containing terbinafine or its respective salt and ethanol for topical administration. |
| WO2019002862A1 | Nanoparticles formed of a polymer and terbinafine | 2018 | Treatment of onychomycosis and tinea pedis | Polymeric nanoparticles | Nanoparticles formed by polymers derived from biguanidine capable of forming nanoparticles; containing terbinafine or its respective salt and ethanol for topical administration by a spray device. |
| WO2020092884A2 | Cross-linked supramolecular nanoparticles for controlled release of antifungal drugs and steroids–a new therapeutic approach for onychomycosis and keloid. | 2018 | Treatment of onychomycosis | Self-assembled supramolecular nanoparticles | Self-assembled supramolecular nanoparticles containing antifungal agent; containing reporter molecule (fluorescent reporter); designed for administration by penetration of epidermis layer (tattoo). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Matos, S.P.; de Oliveira Araujo, K.; Kreutz, T.; da Veiga Júnior, V.F.; Teixeira, H.F.; Koester, L.S. Nanocarriers in Ungual Drug Delivery. Pharmaceutics 2025, 17, 1060. https://doi.org/10.3390/pharmaceutics17081060
de Matos SP, de Oliveira Araujo K, Kreutz T, da Veiga Júnior VF, Teixeira HF, Koester LS. Nanocarriers in Ungual Drug Delivery. Pharmaceutics. 2025; 17(8):1060. https://doi.org/10.3390/pharmaceutics17081060
Chicago/Turabian Stylede Matos, Sheila Porto, Karen de Oliveira Araujo, Tainá Kreutz, Valdir Florêncio da Veiga Júnior, Helder Ferreira Teixeira, and Letícia Scherer Koester. 2025. "Nanocarriers in Ungual Drug Delivery" Pharmaceutics 17, no. 8: 1060. https://doi.org/10.3390/pharmaceutics17081060
APA Stylede Matos, S. P., de Oliveira Araujo, K., Kreutz, T., da Veiga Júnior, V. F., Teixeira, H. F., & Koester, L. S. (2025). Nanocarriers in Ungual Drug Delivery. Pharmaceutics, 17(8), 1060. https://doi.org/10.3390/pharmaceutics17081060








