Drug Metabolism and Pharmacokinetic Evaluation of a Novel RNase H2 Inhibitor for the Treatment of Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Vitro Studies
2.2.1. R14 Incubation in Rat Whole Blood
2.2.2. Phase I Metabolism in Rat Liver Microsomes
2.2.3. CYP Isoform Identification
2.2.4. Metabolic Kinetic Studies
2.2.5. R14 Metabolism in Human Hepatocytes
2.2.6. R14 Permeability in the Caco-2 Cell Culture Model
2.3. Pharmacokinetic Studies of R14 in Rats
2.3.1. Animals
2.3.2. Surgical Procedures
2.3.3. Dosing and Sampling
2.4. R14 Quantification Using UHPLC-MS/MS
2.4.1. Preparation of Stock Solutions
2.4.2. Sample Preparation
2.4.3. UHPLC-MS/MS Conditions
2.5. Quantification of R14 and Metabolites Using UPLC
2.6. Identification of Unknown Metabolites of R14 Using UHPLC-QTOF MS/MS
2.6.1. Urine Sample Preparation
2.6.2. Plasma, Blood, and Microsomal Sample Preparation
2.6.3. Instrumentation and Analytical Conditions
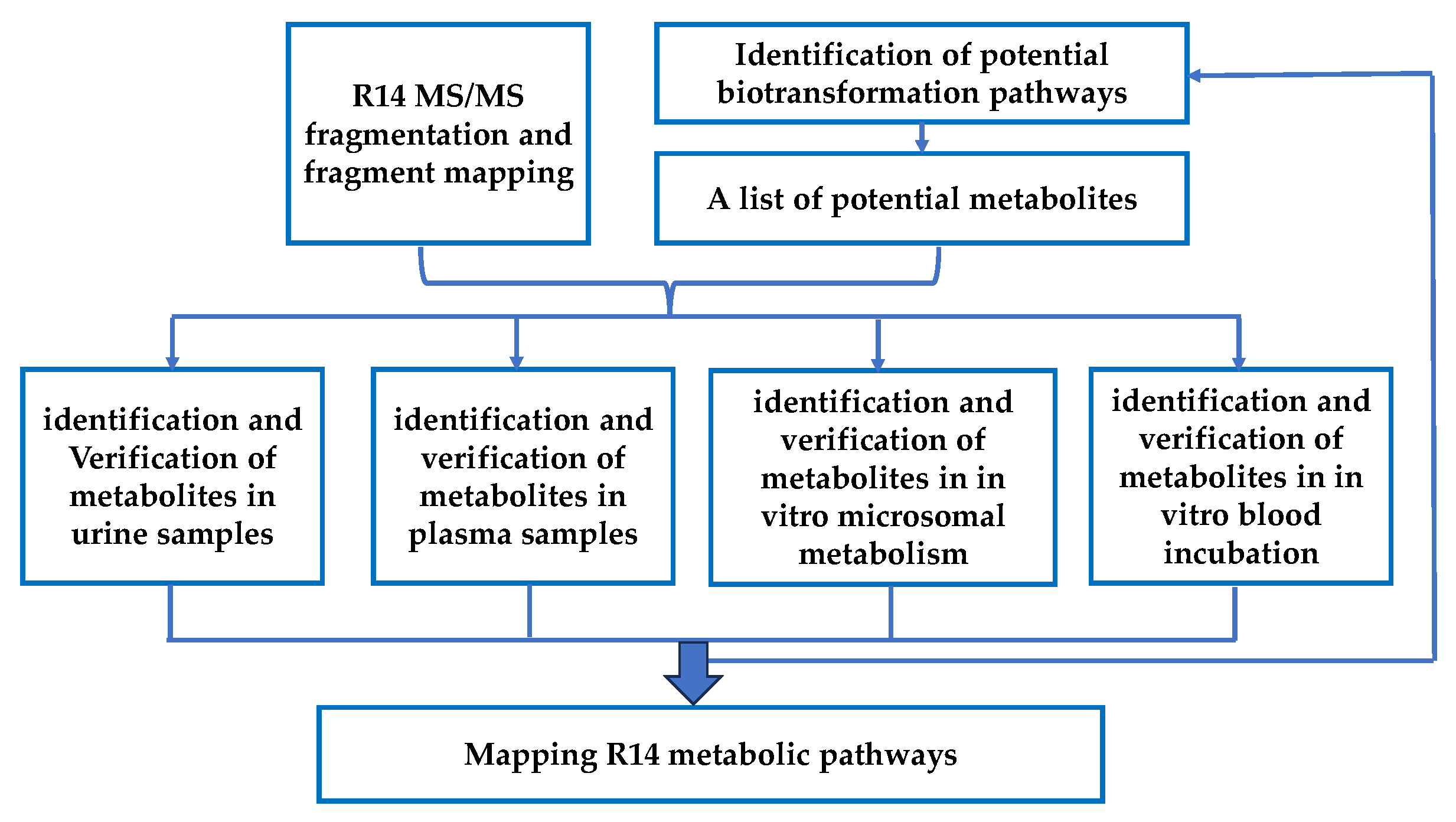
2.6.4. Strategies for R14 Unknown Metabolites’ Identification
2.7. Pharmacokinetic Analysis
2.8. Use of Generative AI Tools
3. Results
3.1. Pharmacokinetics of R14
3.2. Fragmentation of the Parent Drug R14
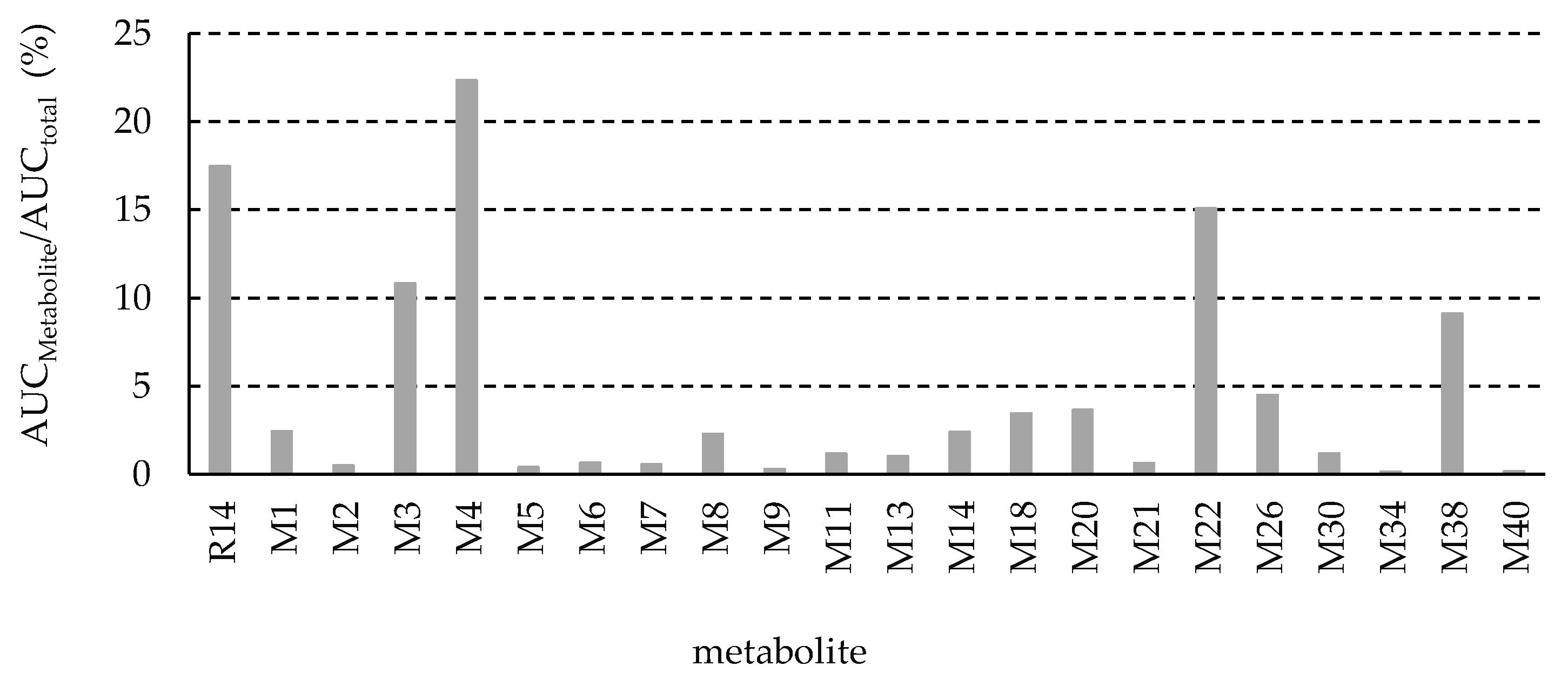
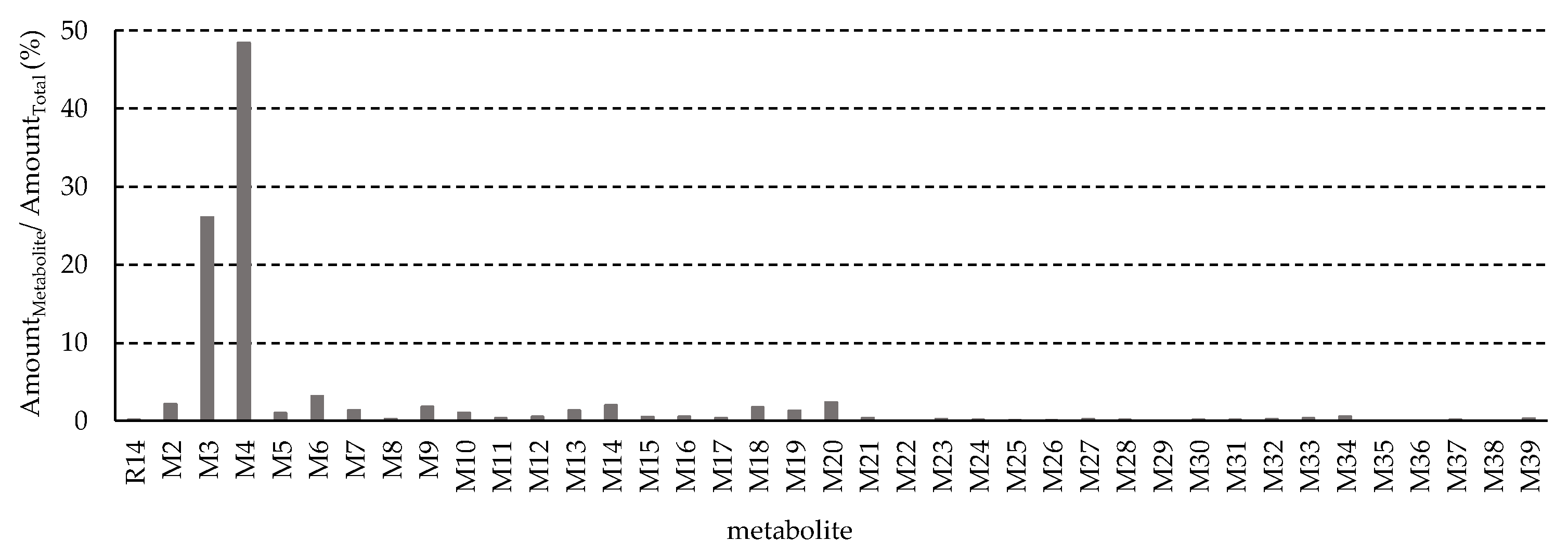
3.3. Identification of R14 Metabolites
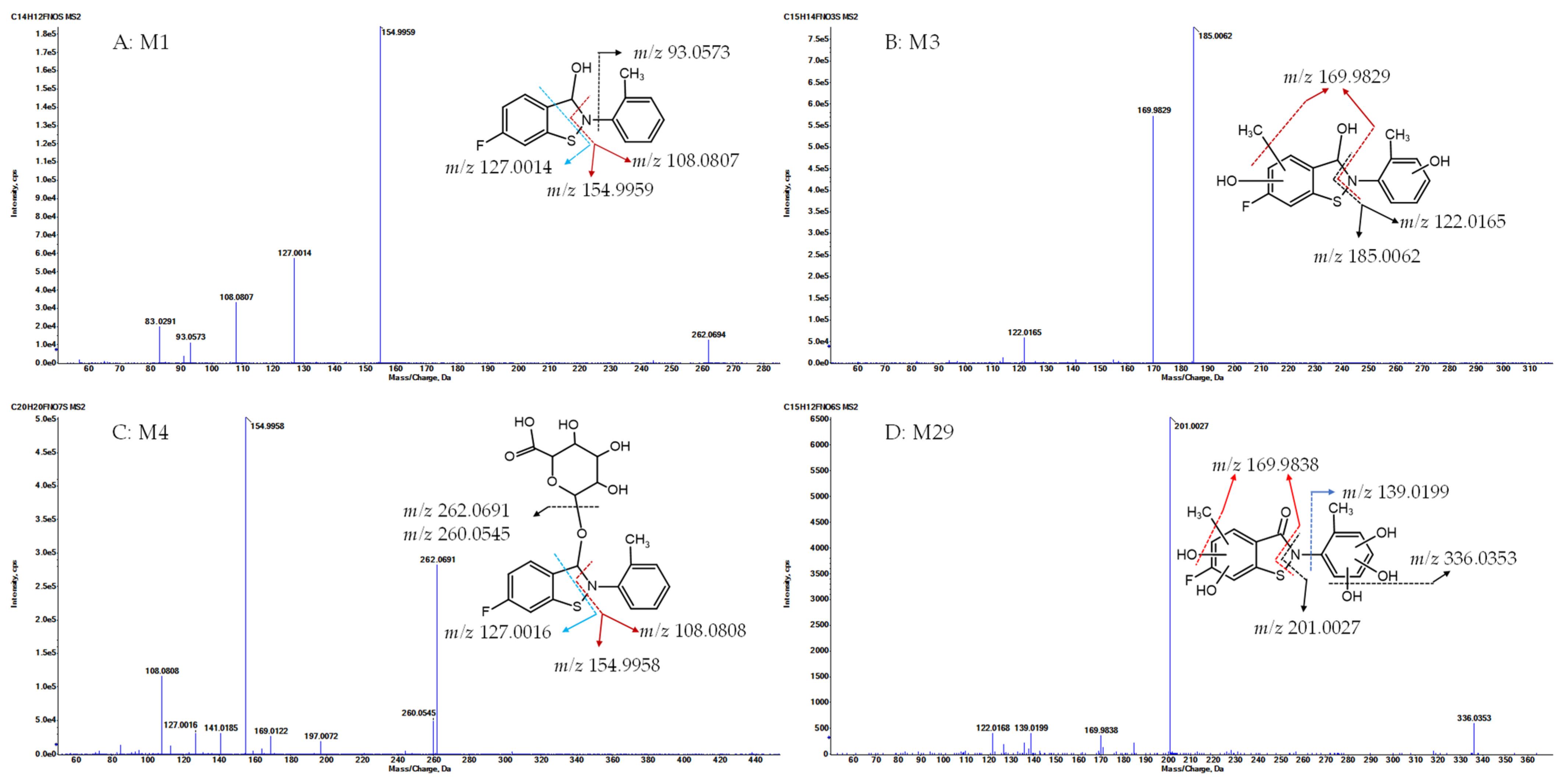
- Metabolite M1, with a protonated ion at m/z 262.0695, was eluted at 23.1 min in the UHPLC-QTOF MS/MS analysis and was identified as the sole major metabolic product following the incubation of R14 in blank rat whole blood for 3 days at room temperature (Figure 4A). In contrast, M1 was detected at relatively low levels in the in vivo plasma samples. The proposed chemical formula for M1 is C14H12FNOS, corresponding to a calculated [M + H]+ m/z of 262.0696, with a mass error of −0.5 ppm. M1 was identified as a hydrogenation product of R14, resulting from the reduction of the carbonyl functional group. MS/MS fragmentation supported this structural assignment, showing characteristic fragment ions at m/z 154.9959, 127.0014 ([FC6H3S+H]+, calculated m/z 127.0012), and 108.0807 ([C6H4CH3NH2+H]+, calculated m/z 108.0808) (Figure 6A).
- Metabolite M3, with a protonated ion at m/z 308.0750, was identified at a retention time of 12 min in the urine samples analyzed by UHPLC-QTOF MS/MS. The proposed molecular formula for M3 is C15H14FNO3S, corresponding to a calculated [M + H]+ m/z of 308.0751, with a mass error of –0.4 ppm. This composition suggests that M3 is a mono-methylated and di-hydroxylated derivative of M1. In the MS/MS spectrum, key fragment ions were observed at m/z 185.0062 and 169.9829, indicating that mono-methylation and mono-hydroxylation occurred on ring A, while the additional hydroxylation likely took place on ring C (Figure 6B).
- Metabolite M4, with a protonated ion at m/z 438.1017, was eluted at 12.3 min in the UHPLC-QTOF MS/MS analysis of rat urine samples. The proposed molecular formula for M4 is C20H20FNO7S, corresponding to a calculated [M + H]+ m/z of 438.1017, with a mass error of –0.1 ppm. The presence of an additional C6H8O6 moiety (176.0315 Da) compared to M1 suggests that M4 is a glucuronide conjugate of M1. MS/MS fragmentation further supported this structure, with characteristic ions observed at m/z 262.0691 ([M1 + H]+), 154.9958, 127.0016, and 108.0808, confirming M4 as the glucuronide conjugate of M1 (Figure 6C).
- Metabolite M29, with a protonated ion at m/z 354.0442, was eluted at 12.7 min in the UHPLC-QTOF MS/MS analysis of rat urine samples. The proposed molecular formula for M29 is C15H12FNO6S, corresponding to a calculated [M + H]+ m/z of 354.0442, with a mass error within acceptable limits. This composition indicates the addition of a CH2O5 moiety relative to R14, suggesting that M29 may be a mono-methylated and penta-hydroxylated derivative of R14, or alternatively, a mono-hydroxylation product of either M31 or M34. MS/MS fragmentation of M29 revealed key product ions at m/z 201.0027, 336.0353, and 169.9838. These fragments support the presence of a mono-methyl group and two hydroxyl substitutions on ring A, with the remaining three hydroxyl groups located on ring C. Based on this fragmentation pattern, M29 is proposed to be a mono-hydroxylation product of M31 (on ring A) or M34 (on ring C) (Figure 6D).
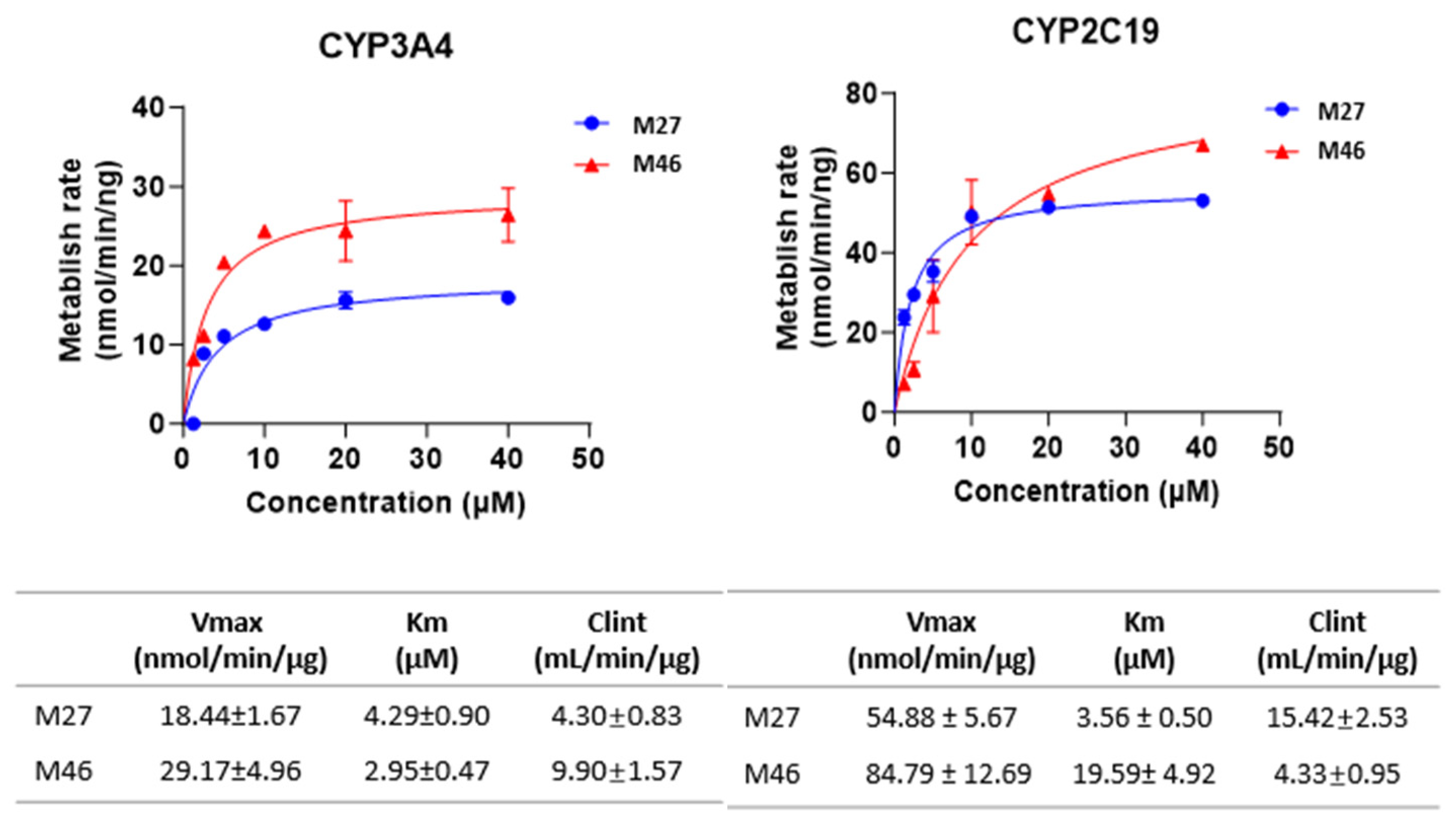
3.4. In Vitro Metabolism of the Two Major Phase I Metabolites, M27 and M46
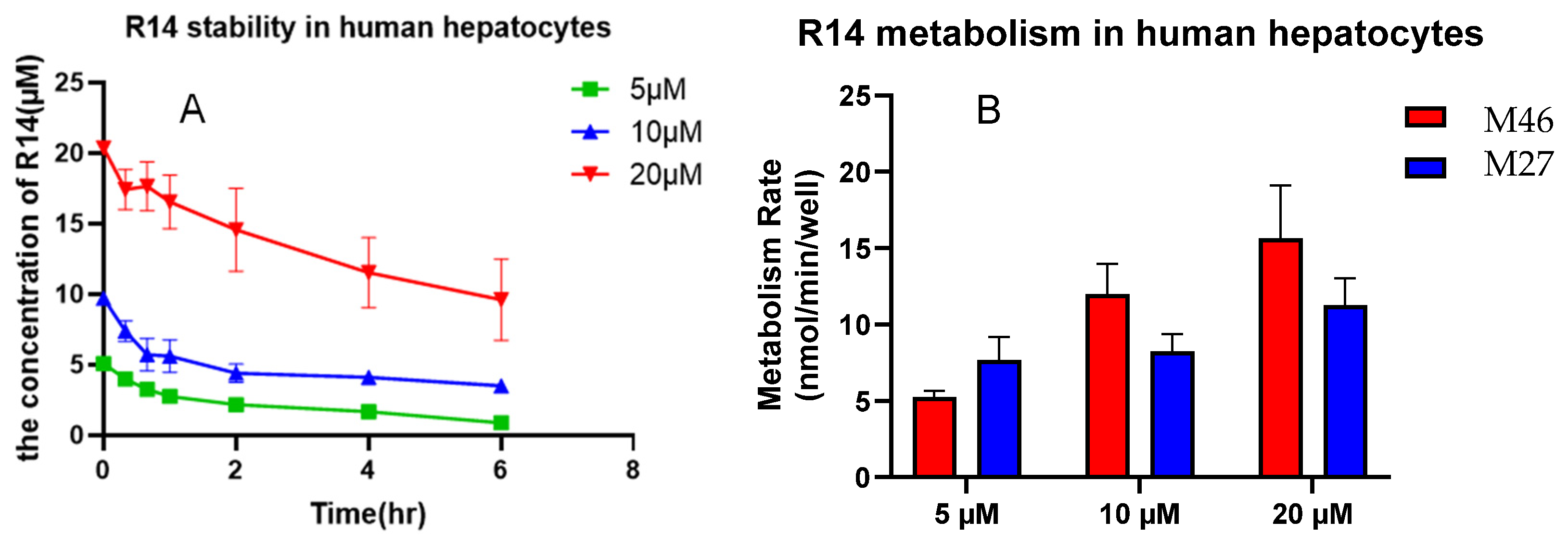
3.4.1. Metabolism of M27 and M46 in Human Hepatocytes
3.4.2. CYP Isoforms Involvement in M27 and M46 Formation
3.4.3. Enzyme Kinetics of M27 and M46 Formation
3.5. R14 Permeability in the Caco-2 Cell Culture Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CAD | collision gas |
| Cl | clearance |
| Clint | intrinsic clearance |
| Cmax | maximum plasma concentration |
| CE | collision energy |
| DP | declustering potential |
| F | oral bioavailability |
| FDA | The Food and Drug Administration |
| HR | High resolution |
| i.v. | intravenous |
| ICH | the International Council for Harmonisation |
| IDA | Information-dependent-acquisition |
| IS | internal standard |
| Km | Michaelis-Menten constant |
| MRT | mean residence time |
| MS | mass spectrometry |
| PBS | phosphate-buffered saline |
| R14 | 6-Fluoro-2-(2-methylphenyl)-1,2-benzothiazol-3(2H)-one |
| RNase | ribonuclease |
| RT | retention time |
| SD | Sprague Dawley |
| T½ | half-life |
| TEER | transepithelial electrical resistance |
| Tmax | time to reach maximum plasma drug concentration |
| TNBC | triple-negative breast cancer |
| UHPLC-MS/MS | ultra-high-performance liquid chromatography tandem mass spectrometry |
| UHPLC-QTOF MS/MS | ultra-high-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry |
| Vmax | maximum reaction velocity |
| Vd | volume of distribution |
References
- Hsu, J.Y.; Chang, C.J.; Cheng, J.S. Survival, Treatment Regimens and Medical Costs of Women Newly Diagnosed with Metastatic Triple-Negative Breast Cancer. Sci. Rep. 2022, 12, 729. [Google Scholar] [CrossRef]
- Kesireddy, M.; Elsayed, L.; Shostrom, V.K.; Agarwal, P.; Asif, S.; Yellala, A.; Krishnamurthy, J. Overall Survival and Prognostic Factors in Metastatic Triple-Negative Breast Cancer: A National Cancer Database Analysis. Cancers 2024, 16, 1791. [Google Scholar] [CrossRef]
- Mandapati, A.; Lukong, K.E. Triple-Negative Breast Cancer: Approved Treatment Options and Their Mechanisms of Action. J. Cancer Res. Clin. Oncol. 2023, 149, 3701–3719. [Google Scholar] [CrossRef]
- Nguyen, T.Q.A.; McGrail, D.J.; Dai, H.; Cifci, A.; Du, Y.; Meric-Bernstam, F.; Lin, S.Y. Targeting RNase H2: A Dual Mechanism Strategy to Elevate Replication Stress, DNA Damage, and Antitumor Immunity in Triple-Negative Breast Cancer. Cancer Res. 2025, 85 (8_Suppl. 1), 4209. [Google Scholar] [CrossRef]
- Smith, S.M.E.; Min, J.; Ganesh, T.; Diebold, B.; Kawahara, T.; Zhu, Y.; McCoy, J.; Sun, A.; Snyder, J.P.; Fu, H.; et al. Ebselen and Congeners Inhibit NADPH Oxidase 2-Dependent Superoxide Generation by Interrupting the Binding of Regulatory Subunits. Chem. Biol. 2012, 19, 752–763. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Lin, S.Y.; Xie, H.; Liang, D. A Stability-Indicating LC-MS/MS Method for Quantification of a NOX Inhibitor R14 in Its Bisulfite Adduct Form for Pharmacokinetic Studies. J. Pharm. Biomed. Anal. 2023, 228, 115326. [Google Scholar] [CrossRef]
- Issa, N.T.; Wathieu, H.; Ojo, A.; Byers, S.W.; Dakshanamurthy, S. Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools. Curr. Drug Metab. 2017, 18, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, H.; Di, C.; Xu, F.; Sun, H.; Xu, Y.; Liu, H.; Wu, L.; Ding, K.; Zhang, T.; et al. Identification and Validation of Active Ingredient in Cerebrotein Hydrolysate-I Based on Pharmacokinetic and Pharmacodynamic Studies. Drug Metab. Dispos. 2023, 51, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, L.; Chen, Z.; Zheng, Y.; Diao, X.; Zhong, D. Metabolite Identification in the Preclinical and Clinical Phase of Drug Development. Curr. Drug Metab. 2021, 22, 838–857. [Google Scholar] [CrossRef] [PubMed]
- FDA. Safety Testing of Drug Metabolites: Guidance for Industry, Revision 2; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/72279/download (accessed on 29 June 2025).
- International Council for Harmonisation. ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; European Medicines Agency: Amsterdam, The Netherlands, 2009; Available online: https://database.ich.org/sites/default/files/M3_R2__Guideline.pdf (accessed on 15 July 2025).
- FDA. Guidance for Industry: Nonclinical Safety Evaluation of Drug or Biologic Combinations; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2006. Available online: https://www.fda.gov/media/119657/download (accessed on 15 July 2025).
- Liu, Y.; Hu, M. Absorption and Metabolism of Flavonoids in the Caco-2 Cell Culture Model and a Perfused Rat Intestinal Model. Drug Metab. Dispos. 2002, 30, 370–377. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Caceres-Cortes, J.; Huang, R.Y.; Tymiak, A.A.; Zhang, Y. Chromatography-Based Methods for Determining Molar Extinction Coefficients of Cytotoxic Payload Drugs and Drug-Antibody Ratios of Antibody Drug Conjugates. J. Chromatogr. A 2016, 1455, 133–139. [Google Scholar] [CrossRef]
- Hall, C.; Lueshen, E.; Mošat’, A.; Linninger, A.A. Interspecies Scaling in Pharmacokinetics: A Novel Whole-Body Physiologically Based Modeling Framework to Discover Drug Biodistribution Mechanisms In Vivo. J. Pharm. Sci. 2012, 101, 1221–1241. [Google Scholar] [CrossRef]
- Benet, L.Z.; Zia-Amirhosseini, P. Basic Principles of Pharmacokinetics. Toxicol. Pathol. 1995, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Bowman, C.M.; Liu, S.; Sodhi, J.K. The Extended Clearance Concept Following Oral and Intravenous Dosing: Theory and Critical Analyses. Pharm. Res. 2018, 22,35, 242. [Google Scholar] [CrossRef]
- Lin, J.H.; Lu, A.Y. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol. Rev. 1997, 49, 403–449. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The Solubility–Permeability Interplay and Oral Drug Formulation Design: Two Heads Are Better Than One. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Grañana-Castillo, S.; Williams, A.; Pham, T.; Khoo, S.; Hodge, D.; Akpan, A.; Bearon, R.; Siccardi, M. General Framework to Quantitatively Predict Pharmacokinetic Induction Drug-Drug Interactions Using In Vitro Data. Clin. Pharmacokinet. 2023, 62, 737–748. [Google Scholar] [CrossRef]
- Peng, Y.; Cheng, Z.; Xie, F. Evaluation of Pharmacokinetic Drug-Drug Interactions: A Review of the Mechanisms, In Vitro and In Silico Approaches. Metabolites 2021, 11, 75. [Google Scholar] [CrossRef]
- Bauman, J.N.; Doran, A.C.; Gualtieri, G.M.; Hee, B.; Strelevitz, T.; Cerny, M.A.; Banfield, C.; Plotka, A.; Wang, X.; Purohit, V.S.; et al. The Pharmacokinetics, Metabolism, and Clearance Mechanisms of Ritlecitinib, a Janus Kinase 3 and Tyrosine-Protein Kinase Family Inhibitor, in Humans. Drug Metab. Dispos. 2024, 52, 1124–1136. [Google Scholar] [CrossRef]
- Derdau, V.; Elmore, C.S.; Hartung, T.; McKillican, B.; Mejuch, T.; Rosenbaum, C.; Wiebe, C. The Future of (Radio)-Labeled Compounds in Research and Development within the Life Science Industry. Angew. Chem. Int. Ed. 2023, 62, e202306019. [Google Scholar] [CrossRef]
- Penner, N.; Xu, L.; Prakash, C. Radiolabeled Absorption, Distribution, Metabolism, and Excretion Studies in Drug Development: Why, When, and How? Chem. Res. Toxicol. 2012, 25, 513–531. [Google Scholar] [CrossRef]
- Reddy, G.N.; Laltanpuii, C.; Sonti, R. Review on In Vivo Profiling of Drug Metabolites with LC-MS/MS in the Past Decade. Bioanalysis 2021, 13, 1697–1722. [Google Scholar] [CrossRef]
- Goey, A.K.; Mukashyaka, M.C.; Patel, Y.; Rodino-Klapac, L.R.; East, L. Characterization of Nonclinical Drug Metabolism and Pharmacokinetic Properties of Phosphorodiamidate Morpholino Oligonucleotides, a Novel Drug Class for Duchenne Muscular Dystrophy. Drug Metab. Dispos. 2024, 52, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Montilla, C.A.; Moroni, S.; Moscatelli, G.; Rocco, D.M.; González, N.; Altcheh, J.M.; Bournissen, F.G. Major Benznidazole Metabolites in Patients Treated for Chagas Disease: Mass Spectrometry-Based Identification, Structural Analysis and Detoxification Pathways. Toxicol. Lett. 2023, 377, 71–82. [Google Scholar] [CrossRef]
- Rashid, M.M.; Oh, H.A.; Lee, H.; Jung, B.H. Metabolite Identification of AZD8055 in Sprague-Dawley Rats after a Single Oral Administration Using Ultra-Performance Liquid Chromatography and Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 145, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rincon Nigro, M.E.; Du, T.; Gao, S.; Kaur, M.; Xie, H.; Olaleye, O.A.; Liang, D. Metabolite Identification of a Novel Anti-Leishmanial Agent OJT007 in Rat Liver Microsomes Using LC-MS/MS. Molecules 2022, 27, 2854. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, S.; Yerramilli, U.; Bai, A.; Dalvie, D.; Brooks, J.; Wang, X.; Selkirk, J.V.; Yan, Y.G.; Zhang, P.; Hargreaves, R.; et al. Absorption, Metabolism, and Excretion, In Vitro Pharmacology, and Clinical Pharmacokinetics of Ozanimod, a Novel Sphingosine 1-Phosphate Receptor Modulator. Drug Metab. Dispos. 2021, 49, 405–419. [Google Scholar] [CrossRef]
- Rasheed, S.; Fatima, M.; Rehman, K.; Kamal, S.; Hussain, I.; Akash, M.S. Bioanalytical Techniques for Prediction of Metabolic Activity of Drug-Metabolizing Enzymes. In Biochemistry of Drug Metabolizing Enzymes; Akash, M.S.H., Rehman, K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 399–425. [Google Scholar]
- Kraemer, M.; Broecker, S.; Madea, B.; Hess, C. Decarbonylation: A Metabolic Pathway of Cannabidiol in Humans. Drug Test. Anal. 2019, 11, 957–967. [Google Scholar] [CrossRef]
- Watanabe, S.; Vikingsson, S.; Roman, M.; Green, H.; Kronstrand, R.; Wohlfarth, A. In Vitro and In Vivo Metabolite Identification Studies for the New Synthetic Opioids Acetylfentanyl, Acrylfentanyl, Furanylfentanyl, and 4-Fluoro-Isobutyrylfentanyl. AAPS J. 2017, 19, 1102–1122. [Google Scholar] [CrossRef]
- Sahu, A.; Balhara, A.; Raju, N.; Kumar, B.K.; Sharma, P.; Singh, D.K.; Singh, S. Characterization of Degradation Products of Celiprolol Hydrochloride Using Hyphenated Mass and NMR Techniques. J. Pharm. Biomed. Anal. 2021, 197, 113953. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, D.; Reddy, A.; Jadav, T.; Sahu, A.K.; Tekade, R.K.; Sengupta, P. Advancements in Practical and Scientific Bioanalytical Approaches to Metabolism Studies in Drug Development. Bioanalysis 2021, 13, 913–930. [Google Scholar] [CrossRef] [PubMed]
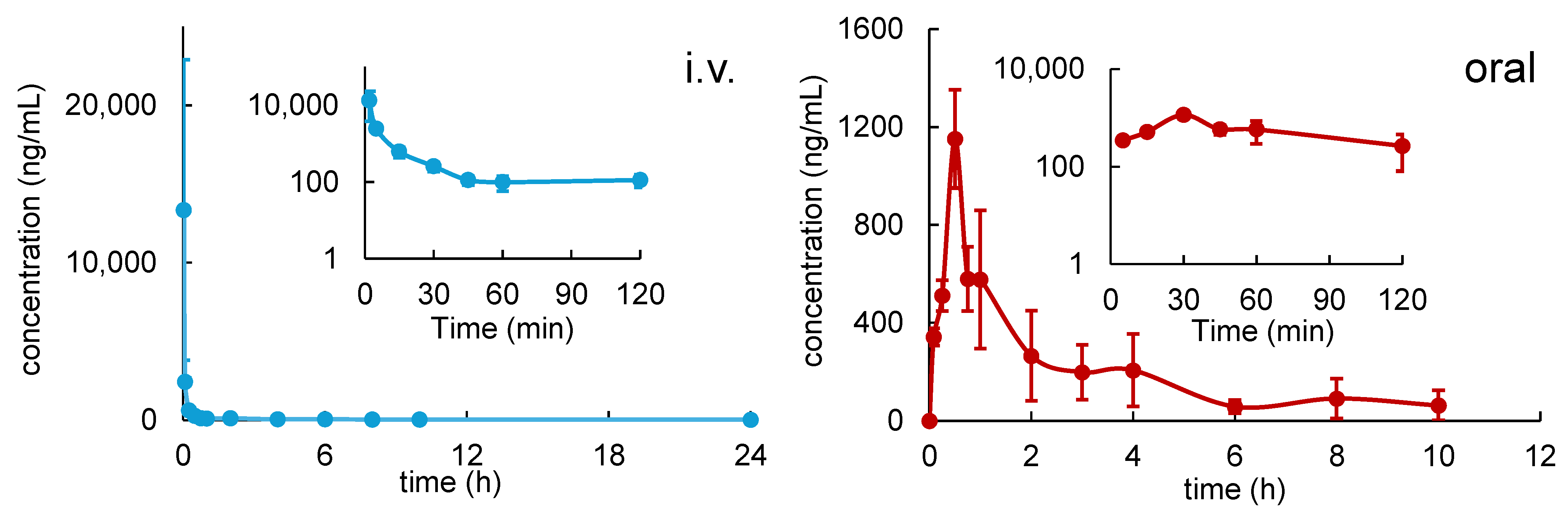






| Parameters | MEAN ± SD | |
|---|---|---|
| i.v. Administration (n = 3) | Oral Administration (n = 3) | |
| Dose (mg/kg) | 5 | 50 |
| Cmax (ng/mL) | - | 1152 ± 201 |
| Tmax (h) | - | 0.5 |
| AUC0→10h (h∙ng/mL) | 1582 ± 477 | 2076 ± 255 |
| AUC0→inf (h∙ng/mL) | 1813 ± 444 | 2426 ± 344 |
| Half-life (h) | 5.39 ± 0.94 | 2.99 ± 1.41 |
| CL(/F) (L/h/kg) | 2.63 ± 0.43 | 22.04 ± 2.18 |
| Vd(/F) (L/kg) | 20.70 ± 6.11 | 93.35 ± 38.08 |
| MRT (h) | 1.63 ± 0.39 | 2.82 ± 0.50 |
| F% | - | 13.38 |
| % R14 excreted unchanged in 24 h urine | 0.035 ± 0.006 | 0.021 ± 0.008 |
| Metabolite | RT (min) | Formula | Calculated m/z | Observed m/z | Mass Error (ppm) | Characteristic Fragment Ions (m/z) | Reactions | Source | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U * | P | M | B | ||||||||
| R14 | 19.6 | C14H10FNOS | 260.054 | 260.0539 | −0.3 | 167.9916, 164.0166, 106.0650, 91.0541 | Parent | ||||
| M1 | 23.1 | C14H12FNOS | 262.0696 | 262.0695 | −0.5 | 154.9959, 127.0014, 108.0807 | Carbonyl reduction | √ | √ | ||
| M2 | 9.9 | C15H12FNO3S | 306.0595 | 306.0598 | 1.1 | 276.0493, 185.0072, 169.9836, 288.0493 | R14 mono- methylation and di-hydroxylation | √ | √ | ||
| M3 | 12.0 | C15H14FNO3S | 308.0751 | 308.075 | −0.4 | 185.0062, 169.9829, 122.0165 | M1 mono- methylation, di-hydroxylation | √ | √ | ||
| M4 | 12.3 | C20H20FNO7S | 438.1017 | 438.1017 | −0.1 | 262.0691, 154.9958, 127.0016, 108.0808 | M1 glucuronidation | √ | √ | ||
| M5 | 13.5 | C15H14FNO3S | 308.0751 | 308.0754 | 0.9 | 169.0122, 141.0182, 153.9886 | M1 mono- methylation, bi-hydroxylation | √ | √ | ||
| M6 | 13.7 | C16H16FNO2S2 | 338.0679 | 338.0682 | 0.8 | 169.0121, 141.0182 | M30 methanethiol addition | √ | √ | ||
| M7 | 15.4 | C16H16FNO2S2 | 338.0679 | 338.0683 | 1.1 | 169.0121, 141.0172 | M30 methanethiol addition | √ | √ | ||
| M8 | 20.6 | C15H12FNO3S | 306.0595 | 306.0596 | 0.4 | 169.0120, 141.0171, 153.9886 | R14 mono- methylation and bi-hydroxylation | √ | √ | ||
| M9 | 9.5 | C21H22FNO9S | 484.1072 | 484.1075 | 0.6 | 185.0076, 169.9842 | M3 glucuronidation | √ | √ | ||
| M10 | 9.8 | C15H14FNO4S | 324.0700 | 324.0703 | 0.8 | 276.0497, 185.0071, 169.9840 | M1 mono-methylation tri-hydroxylation | √ | |||
| M11 | 10.9 | C15H14FNO4S | 324.0700 | 324.0703 | 0.8 | 201.0027, 185.0047, 306.0603 | M1 mono-methylation tri-hydroxylation | √ | √ | ||
| M12 | 11.4 | C15H14FNO6S2 | 388.0319 | 388.0319 | −0.1 | 185.0071, 169.9839 | M3 sulfation | √ | |||
| M13 | 11.8 | C15H14FNO2S | 292.0802 | 292.0804 | 0.7 | 169.0121, 153.9885, 141.0172 | M1 mono- methylation, mono-hydroxylation | √ | √ | ||
| M14 | 11.9 | C21H22FNO8S | 468.1123 | 468.1124 | 0.2 | 292.0812, 169.0122, 141.0182 | M13 glucuronidation | √ | √ | ||
| M15 | 12.5 | C21H22FNO9S | 484.1072 | 484.1077 | 1.0 | 308.0769, 169.0128, 141.0184 | M15 glucuronidation | √ | |||
| M16 | 12.6 | C16H16FNO4S | 338.0857 | 338.0858 | 0.3 | 185.0072, 169.9840, 122.0168 | M1 di-methylation tri-hydroxylation | √ | |||
| M17 | 13.2 | C16H16FNO3S | 322.0908 | 322.0908 | 0.1 | 169.0132, 185.0072, 141.0183 | M1 di- methylation, di-hydroxylation | √ | |||
| M18 | 13.5 | C15H14FNO6S2 | 388.0319 | 388.0319 | −0.1 | 169.0127, 141.0175 | M5 sulfation | √ | √ | ||
| M19 | 13.7 | C22H24FNO8S2 | 514.1 | 514.1002 | 0.4 | 338.0689, 169.0123, 141.0184 | M6 or M7 glucuronidation | √ | |||
| M20 | 13.9 | C15H14FNO5S2 | 372.037 | 372.0369 | −0.3 | 169.0121, 141.0178 | M13 sulfation | √ | √ | ||
| M21 | 14.9 | C16H16FNO3S | 322.0908 | 322.0908 | 0.1 | 169.0129, 141.0170 | M1 di- methylation, di-hydroxylation | √ | √ | ||
| M22 | 15.3 | C15H14FNO2S | 292.0802 | 292.0803 | 0.3 | 185.0072, 169.9836 | M1 mono- methylation, mono-hydroxylation | √ | √ | ||
| M23 | 15.4 | C22H24FNO8S2 | 514.1 | 514.1001 | 0.2 | 338.0697, 169.0128, 141.0184 | M6 or M7 glucuronidation | √ | |||
| M24 | 7.7 | C15H12FNO4S | 322.0544 | 322.0547 | 1.0 | 201.0019, 187.0226 | R14 mono- methylation and tri-hydroxylation | √ | |||
| M25 | 9.9 | C15H14FNO7S2 | 404.0268 | 404.0271 | 0.6 | 185.0066, 169.9833 | M10 sulfation | √ | |||
| M26 | 10.3 | C15H12FNO2S | 290.0646 | 290.0646 | 0.2 | 258.0399, 185.0090, 169.9839, 106.0655, 91.0548 | R14 mono- methylation and mono-hydroxylation | √ | √ | ||
| M27 | 12.1 | C14H10FNO2S | 276.0489 | 276.0490 | 0.3 | 106.0652, 79.0545, 77.0387 | R14 mono-hydroxylation | √ | √ | ||
| M28 | 12.4 | C22H24FNO9S | 498.1229 | 498.1232 | 0.7 | 169.0128, 322.0924, 141.0181 | M21 glucuronidation | √ | |||
| M29 | 12.7 | C15H12FNO6S | 354.0442 | 354.0442 | 0 | 201.0027, 336.0353, 169.9838 | R14 mono- methylation and penta-hydroxylation | √ | |||
| M30 | 13.1 | C15H12FNO2S | 290.0646 | 290.0647 | 0.5 | 148.0401, 169.0129, 155.0335 | R14 mono- methylation and mono-hydroxylation | √ | √ | ||
| M31 | 13.7 | C15H12FNO5S | 338.0493 | 338.0492 | −0.3 | 185.0076, 169.9843, 122.0171 | R14 mono- methylation and tetra-hydroxylation | √ | |||
| M32 | 14.9 | C16H16FNO6S2 | 402.0476 | 402.0479 | 0.8 | 169.0120, 141.0176 | M21 sulfation | √ | |||
| M33 | 15.5 | C16H16FNO5S3 | 418.0247 | 418.0246 | −0.3 | 169.0127, 141.0178 | M6 or M7 sulfation | √ | |||
| M34 | 16.1 | C15H12FNO5S | 338.0493 | 338.0497 | 1.2 | 201.0029, 169.9844, 320.0410 | R14 mono- methylation and tetra-hydroxylation | √ | √ | ||
| M35 | 16.6 | C21H20FNO9S | 482.0916 | 482.0919 | 0.7 | 306.0597, 169.0129, 288.0513 | M8 glucuronidation | √ | |||
| M36 | 17.0 | C16H16FNO5S3 | 418.0247 | 418.0246 | −0.3 | 169.0129, 141.0181 | M6 or M7 sulfation | √ | |||
| M37 | 17.7 | C15H12FNO4S | 322.0544 | 322.0545 | 0.4 | 185.0074, 169.9842, 122.0171 | R14 mono- methylation and tri-hydroxylation | √ | |||
| M38 | 18.1 | C15H14FNOS | 276.0853 | 276.0856 | 1.1 | 169.0129, 153.9898, 141.0188 | M1 mono- methylation | √ | √ | ||
| M39 | 19.2 | C15H12FNO4S | 322.0544 | 322.0548 | 1.3 | 169.0128, 141.0178 | R14 mono- methylation and tri-hydroxylation | √ | |||
| M40 | 14.9 | C15H14FNO3S | 308.0751 | 308.0752 | 0.2 | 201.0025, 169.9841 | M1 mono- methylation, bi-hydroxylation | √ | |||
| M41 | 8.6 | C14H10FNO3S | 292.0438 | 292.0441 | 1.0 | 122.0609, 106.0653 | R14 di-hydroxylation | √ | |||
| M42 | 9.2 | C14H10FNO4S | 308.0387 | 308.0389 | 0.5 | 290.0313, 202.9830, 106.0653 | R14 tri-hydroxylation | √ | |||
| M43 | 9.6 | C14H10FNO3S | 292.0438 | 292.0440 | 0.6 | 106.0659 | R14 di-hydroxylation | √ | |||
| M44 | 14.0 | C14H10FNO3S | 292.0438 | 292.0441 | 1.0 | 122.0608, 154.9968 | R14 di-hydroxylation | √ | |||
| M45 | 16.7 | C14H10FNO4S | 308.0387 | 308.0389 | 0.5 | 290.0308, 170.9922, 138.0558 | R14 tri-hydroxylation | √ | |||
| M46 | 17.5 | C14H10FNO2S | 276.0489 | 276.0492 | 1.1 | 122.0607, 154.9973 | R14 mono-hydroxylation | √ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, H.; Ma, J.; Du, T.; Gao, S.; Chen, Y.; Lin, S.-Y.; Liang, D. Drug Metabolism and Pharmacokinetic Evaluation of a Novel RNase H2 Inhibitor for the Treatment of Triple-Negative Breast Cancer. Pharmaceutics 2025, 17, 1052. https://doi.org/10.3390/pharmaceutics17081052
Wang Y, Xie H, Ma J, Du T, Gao S, Chen Y, Lin S-Y, Liang D. Drug Metabolism and Pharmacokinetic Evaluation of a Novel RNase H2 Inhibitor for the Treatment of Triple-Negative Breast Cancer. Pharmaceutics. 2025; 17(8):1052. https://doi.org/10.3390/pharmaceutics17081052
Chicago/Turabian StyleWang, Yang, Huan Xie, Jing Ma, Ting Du, Song Gao, Yuan Chen, Shiaw-Yih Lin, and Dong Liang. 2025. "Drug Metabolism and Pharmacokinetic Evaluation of a Novel RNase H2 Inhibitor for the Treatment of Triple-Negative Breast Cancer" Pharmaceutics 17, no. 8: 1052. https://doi.org/10.3390/pharmaceutics17081052
APA StyleWang, Y., Xie, H., Ma, J., Du, T., Gao, S., Chen, Y., Lin, S.-Y., & Liang, D. (2025). Drug Metabolism and Pharmacokinetic Evaluation of a Novel RNase H2 Inhibitor for the Treatment of Triple-Negative Breast Cancer. Pharmaceutics, 17(8), 1052. https://doi.org/10.3390/pharmaceutics17081052










