N-Degron-Based PROTAC Targeting PLK1: A Potential Therapeutic Strategy for Cervical Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Methods
2.1.2. Peptide Synthesis
2.2. MALDI-TOF Mass Spectrometry Analysis of Synthesized Peptides
- NC1: calculated mass for C116H216N57O23P, m/z 2806.72; observed m/z 2807.82 [M + H]+.
- Arg1-K(biotin): calculated mass for C22H41N9O4S, m/z 527.30; observed m/z 528.38 [M + H]+.
- Arg4-K(biotin): calculated mass for C40H77N21O7S, m/z 995.60; observed m/z 996.71 [M + H]+.
- Arg8-K(biotin): calculated mass for C64H125N37O11S, m/z 1620.01; observed m/z 1621.22 [M + H]+.
- Arg10-K(biotin): calculated mass for C76H149N45O13S, m/z 932.21; observed m/z 1933.522 [M + H]+.
- Arg12-K(biotin): calculated mass for C88H173N53O15S, m/z 2244.41; observed m/z 2245.569 [M + H]+.
- Ala4Arg8-ahx-4j: calculated mass for C104H188N45O23S, m/z 2467.87; observed m/z 2468.97 [M + H]+.
2.3. Pull-Down Assay
2.4. Protein Expression and Purification
2.5. Crystallization, Data Collection, Structure Solution, and Refinement
2.6. Isothermal Titration Calorimetry (ITC)
2.7. Compound Treatment and Immunoblotting
2.8. In Vivo Antitumor Activity
2.9. Cell Viability Assay
2.10. Morphological Assessment of NC1 Treated Cancer Cells
2.11. Cellular Uptake of FITC-Conjugated Compounds
2.12. Analysis of G2/M Phase Cell Cycle Arrest by Flow Cytometry
2.13. Evaluation of Cancer Cell Apoptosis Using Fluorescence Apoptosis Assay
2.14. Apoptosis Detection by Flow Cytometry
2.15. Statistical Analysis
3. Results
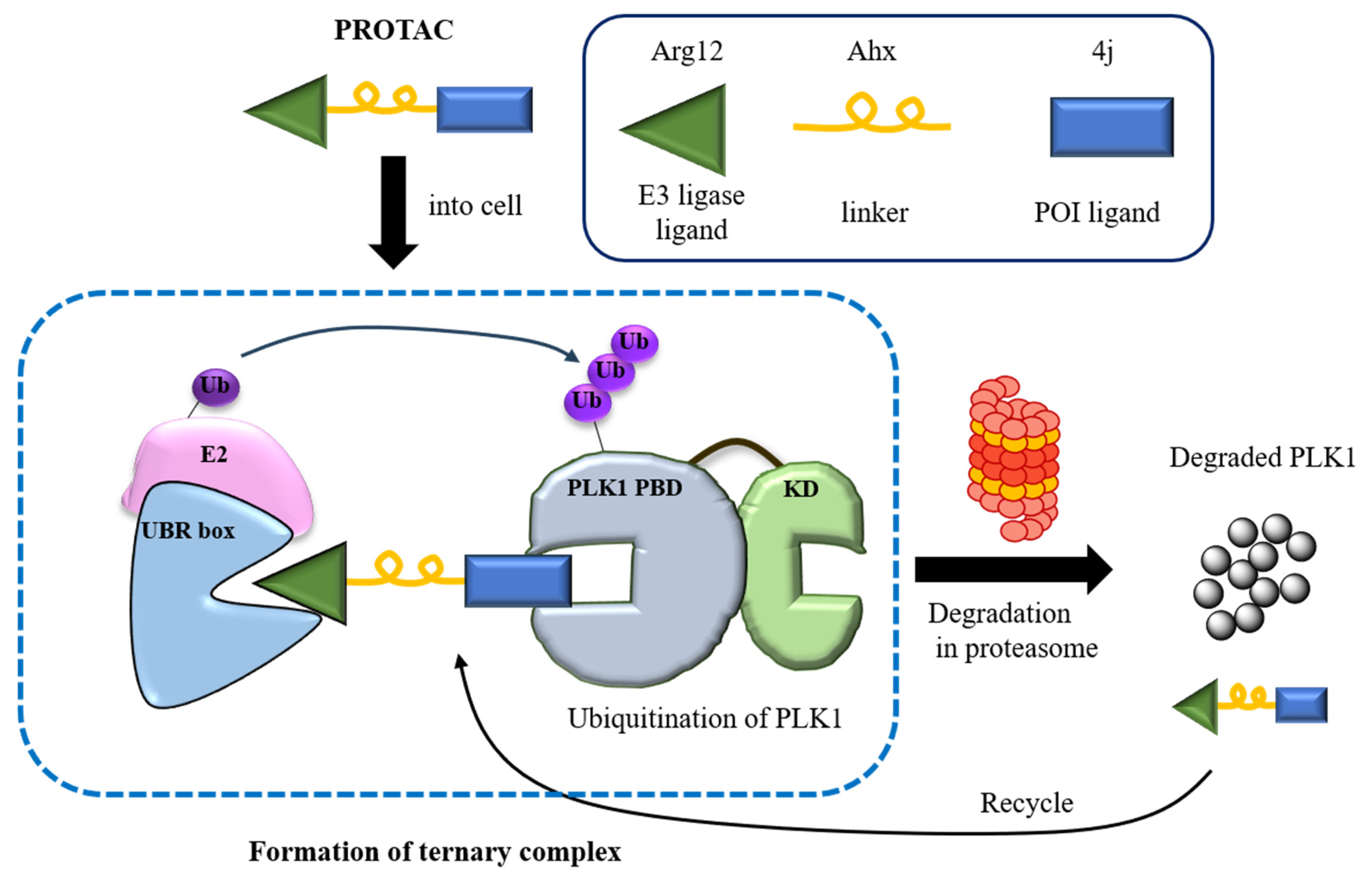
3.1. Design and Synthesis of PROTAC, NC1
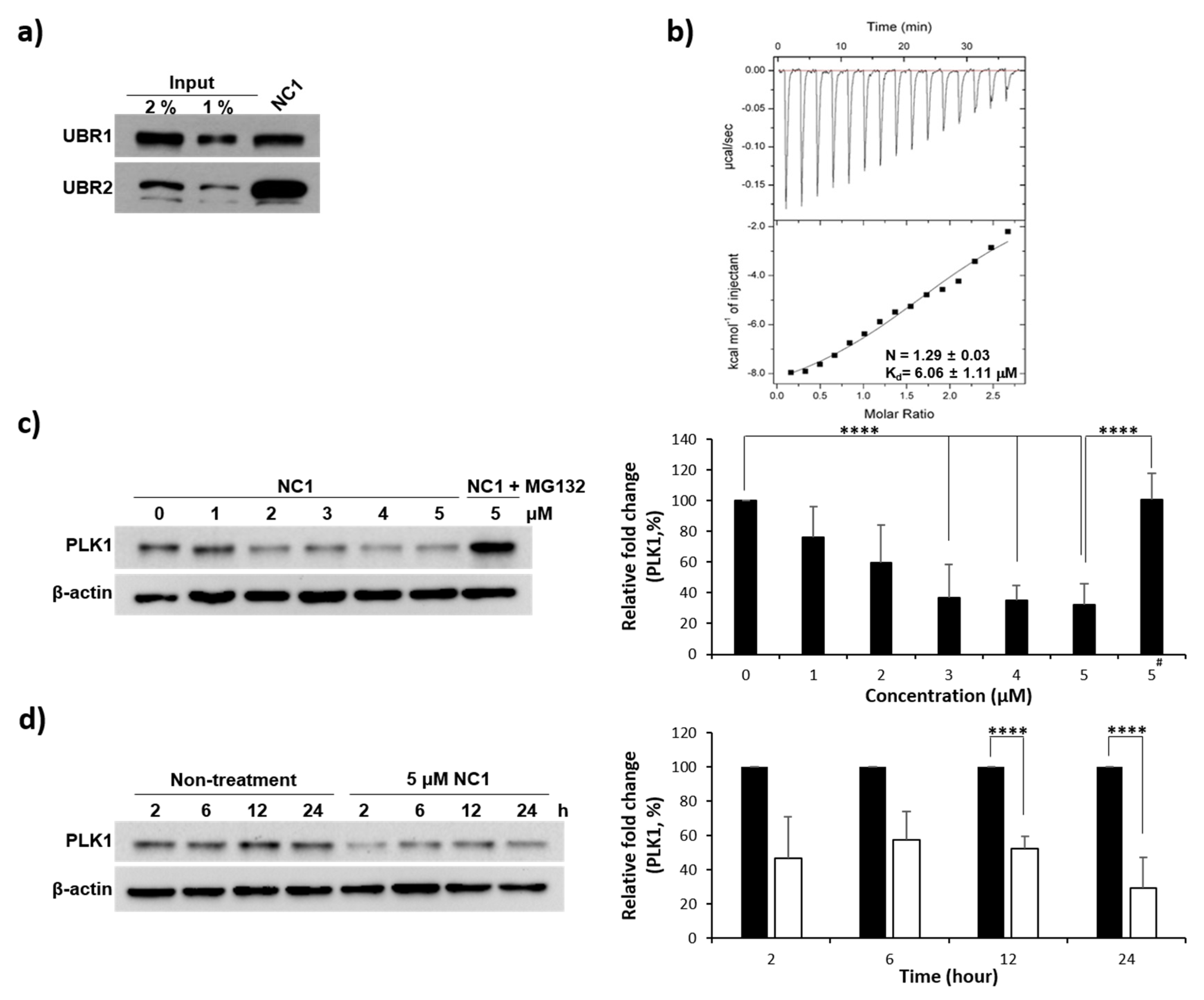
3.2. Validation of Arginine Recognition by UBR1
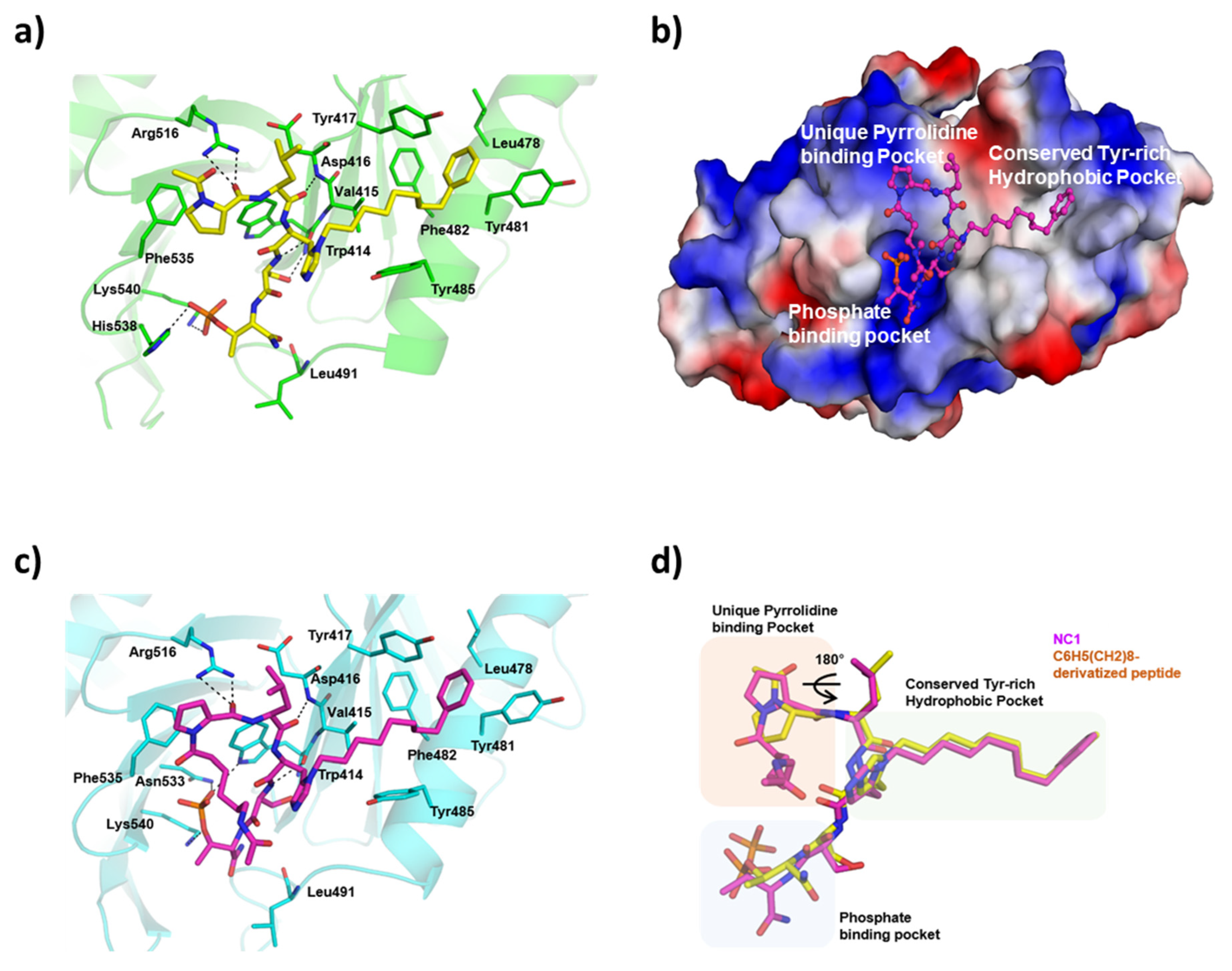
3.3. Recognition of UBR Box by NC1
3.4. Evaluation of PLK1 PBD Binding Affinity
3.5. PLK1 Degradation
3.6. Complex Crystal Structure of PROTAC with PLK1 PBD
3.7. Cell Viability Assay and Cancer Cell Morphology Analysis
3.8. Cell Penetrating Ability of FITC-Conjugated NC1 in Cancer Cells
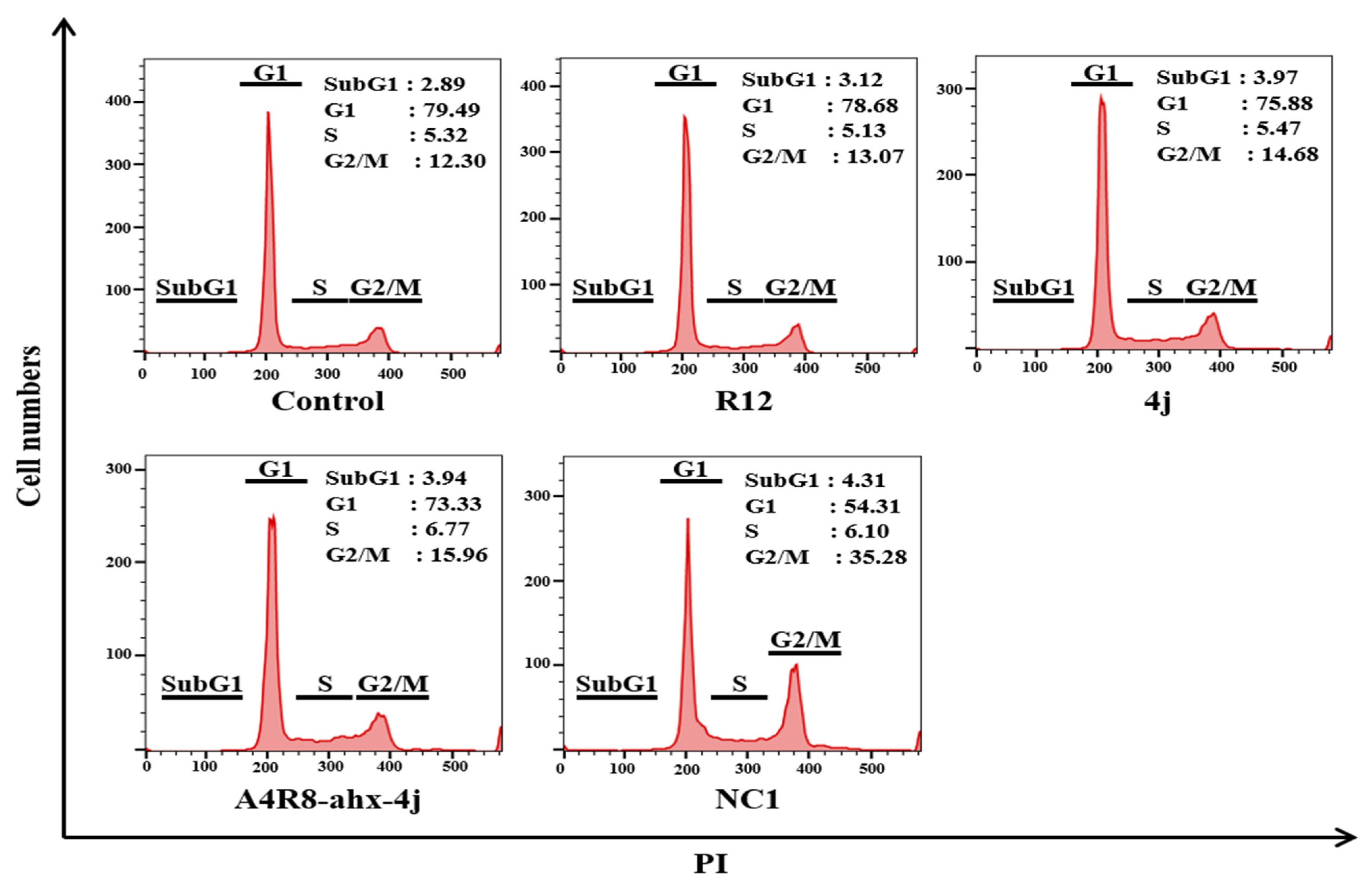
3.9. G2/M Phase Cell Cycle Arrest Using a Fluorescence-Activated Cell Sorter
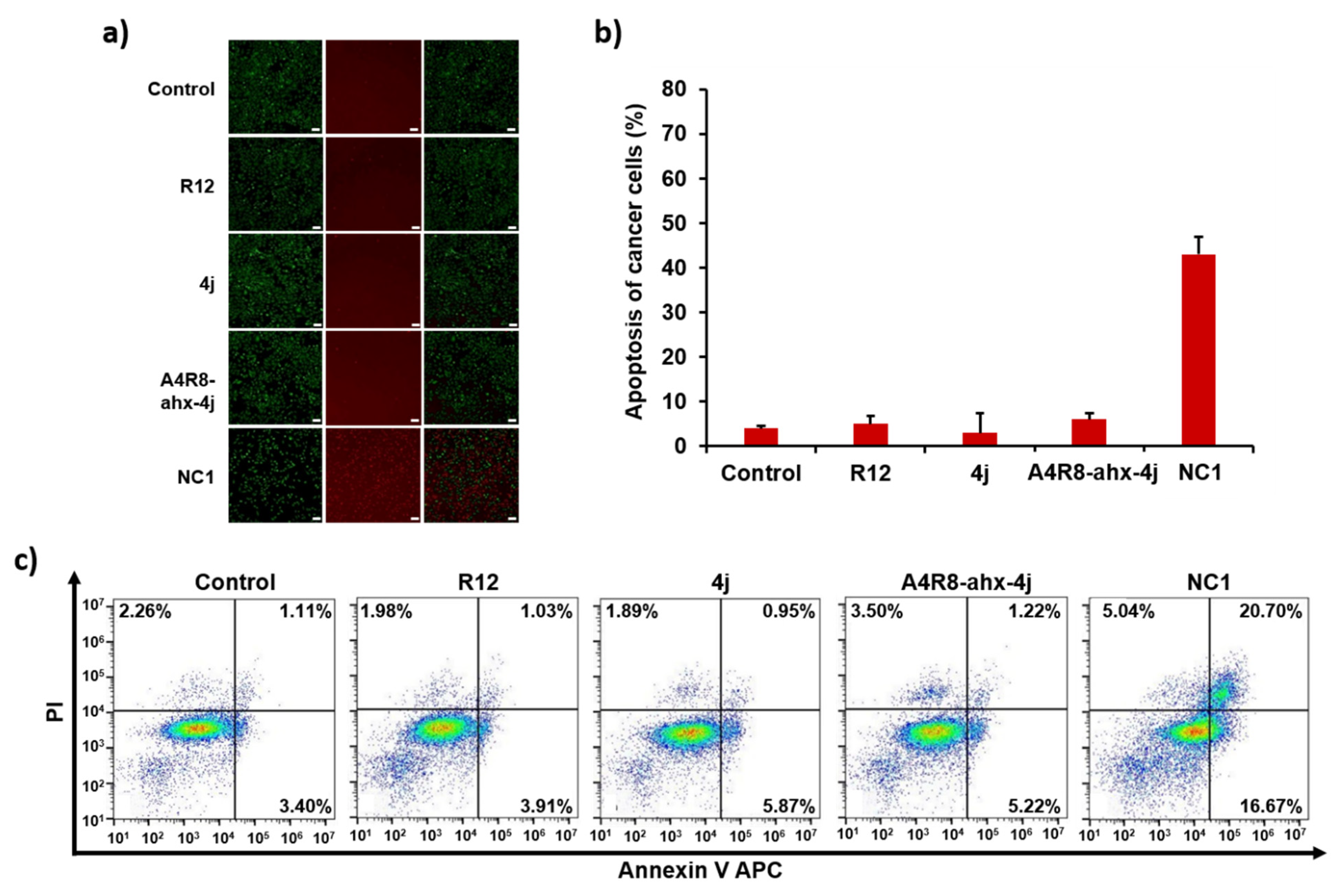
3.10. Apoptosis Effects of NC1 on Cancer Cells
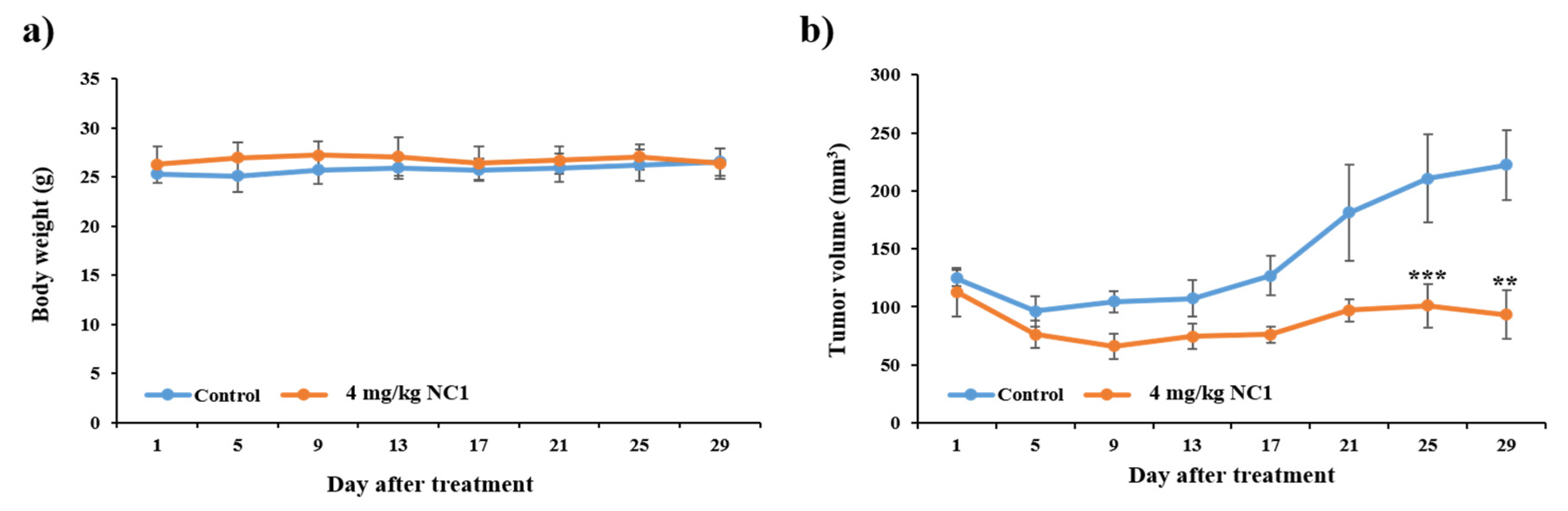
3.11. Anti-Tumorigenic Effect of NC1 on Tumor-Bearing Mice
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 5 March 2024).
- Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2003, 83, 41–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, Y.-X.; Xia, J.-H.; Zhang, H.-X.; Li, H.-B.; Yu, C.-Z. The expression of PLK-1 in cervical carcinoma: A possible target for enhancing chemosensitivity. J. Exp. Clin. Cancer Res. 2009, 28, 130. [Google Scholar] [CrossRef]
- Li, B.H.; Zhou, J.S.; Ye, F.; Cheng, X.D.; Zhou, C.Y.; Lu, W.G.; Xie, X. Reduced miR-100 expression in cervical cancer and precursors and its carcinogenic effect through targeting PLK1 protein. Eur. J. Cancer 2011, 47, 2166–2174. [Google Scholar] [CrossRef]
- Zitouni, S.; Nabais, C.; Jana, S.C.; Guerrero, A.; Bettencourt-Dias, M. Polo-like kinases: Structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 433–452. [Google Scholar] [CrossRef]
- Lee, K.S.; Burke, T.R.; Park, J.-E.; Bang, J.K.; Lee, E. Recent Advances and New Strategies in Targeting Plk1 for Anticancer Therapy. Trends Pharmacol. Sci. 2015, 36, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Strebhardt, K. Multifaceted polo-like kinases: Drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.N.; Park, J.-E.; Kim, E.-H.; Shin, S.Y.; Cheong, C.; Lee, K.S.; Bang, J.K. Plk1-Targeted Small Molecule Inhibitors: Molecular Basis for Their Potency and Specificity. Mol. Cells 2011, 32, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.F.; Pan, S.S.; Ou, R.Y.; Zheng, Z.Z.; Huang, X.X.; Jian, M.T.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Yang, Y.; et al. Volasertib suppresses tumor growth and potentiates the activity of cisplatin in cervical cancer. Am. J. Cancer Res. 2015, 5, 3548–3559. [Google Scholar]
- Gunasekaran, P.; Yim, M.S.; Ahn, M.; Soung, N.-K.; Park, J.-E.; Kim, J.; Bang, G.; Shin, S.C.; Choi, J.; Kim, M.; et al. Development of a Polo-like Kinase-1 Polo-Box Domain Inhibitor as a Tumor Growth Suppressor in Mice Models. J. Med. Chem. 2020, 63, 14905–14920. [Google Scholar] [CrossRef]
- Liu, F.; Park, J.-E.; Qian, W.-J.; Lim, D.; Gräber, M.; Berg, T.; Yaffe, M.B.; Lee, K.S.; Burke, T.R. Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat. Chem. Biol. 2011, 7, 595–601. [Google Scholar] [CrossRef]
- Ahn, M.; Han, Y.-H.; Park, J.-E.; Kim, S.; Lee, W.C.; Lee, S.J.; Gunasekaran, P.; Cheong, C.; Shin, S.Y.; Kim, H.-Y.; et al. A New Class of Peptidomimetics Targeting the Polo-Box Domain of Polo-Like Kinase 1. J. Med. Chem. 2015, 58, 294–304. [Google Scholar] [CrossRef]
- Burslem, G.M.; Smith, B.E.; Lai, A.C.; Jaime-Figueroa, S.; McQuaid, D.C.; Bondeson, D.P.; Toure, M.; Dong, H.; Qian, Y.; Wang, J.; et al. The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell Chem. Biol. 2018, 25, 67–77.e3. [Google Scholar] [CrossRef]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great opportunities for academia and industry. Signal Transduct. Target. Ther. 2019, 4, 64. [Google Scholar] [CrossRef]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. Adv. Sci. Drug Discov. 2020, 26, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Qi, J.; Ronai, Z.E.A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 2018, 18, 69–88. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Hwang, Y.S.; Lee, G.-H.; Park, J.; Kim, J.G.; La, Y.K.; Park, N.Y.; Kothandaraman, R.; Yim, M.S.; Choi, J.; et al. Degradation of Polo-like Kinase 1 by the Novel Poly-Arginine N-Degron Pathway PROTAC Regulates Tumor Growth in Nonsmall Cell Lung Cancer. J. Med. Chem. 2024, 67, 3307–3320. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Fischer, E.S.; Böhm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef]
- Varshavsky, A. The N-end rule pathway and regulation by proteolysis. J. Protein Sci. 2011, 20, 1298–1345. [Google Scholar] [CrossRef]
- Varshavsky, A. N-degron and C-degron pathways of protein degradation. Proc. Natl. Acad. Sci. USA 2019, 116, 358. [Google Scholar] [CrossRef]
- Tasaki, T.; Mulder, L.C.F.; Iwamatsu, A.; Lee, M.J.; Davydov, I.V.; Varshavsky, A.; Muesing, M.; Kwon, Y.T. A Family of Mammalian E3 Ubiquitin Ligases That Contain the UBR Box Motif and Recognize N-Degrons. Mol. Cell. Biol. 2005, 25, 7120–7136. [Google Scholar] [CrossRef]
- Hershko, A.; Heller, H.; Eytan, E.; Reiss, Y. The protein substrate binding site of the ubiquitin-protein ligase system. J. Biol. Chem. 1986, 261, 11992–11999. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Shao, P.; Chen, H.; Campos, B.; McHardy, S.F.; Luo, T.; Rao, H. A modular PROTAC design for target destruction using a degradation signal based on a single amino acid. J. Biol. Chem. 2019, 294, 15172–15175. [Google Scholar] [CrossRef]
- Leboeuf, D.; Pyatkov, M.; Zatsepin, T.S.; Piatkov, K. The Arg/N-Degron Pathway-A Potential Running Back in Fine-Tuning the Inflammatory Response? Biomolecules 2020, 10, 903. [Google Scholar] [CrossRef]
- Cha-Molstad, H.; Yu, J.E.; Feng, Z.; Lee, S.H.; Kim, J.G.; Yang, P.; Han, B.; Sung, K.W.; Yoo, Y.D.; Hwang, J.; et al. p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat. Commun. 2017, 8, 102. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Gao, Y.; Yuan, B.; Qi, X.; Fu, Y.; Zhu, Q.; Cao, T.; Zhang, S.; Yin, L.; et al. USP7 is a novel Deubiquitinase sustaining PLK1 protein stability and regulating chromosome alignment in mitosis. J. Exp. Clin. Cancer Res. 2019, 38, 468. [Google Scholar] [CrossRef]
- Rubner, S.; Scharow, A.; Schubert, S.; Berg, T. Selective Degradation of Polo-like Kinase 1 by a Hydrophobically Tagged Inhibitor of the Polo-Box Domain. Angew. Chem. Int. Ed. 2018, 57, 17043–17047. [Google Scholar] [CrossRef]
- Giráldez, S.; Galindo-Moreno, M.; Limón-Mortés, M.C.; Rivas, A.C.; Herrero-Ruiz, J.; Mora-Santos, M.; Sáez, C.; Japón, M.Á.; Tortolero, M.; Romero, F. G1/S phase progression is regulated by PLK1 degradation through the CDK1/βTrCP axis. FASEB J. 2017, 31, 2925–2936. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, J.; Rong, L.; Wu, S.; Deng, Z.; Fatkhutdinov, N.; Zundell, J.; Fukumoto, T.; Liu, Q.; Kossenkov, A.; et al. ARID1A promotes genomic stability through protecting telomere cohesion. Nat. Commun. 2019, 10, 4067. [Google Scholar] [CrossRef] [PubMed]
- Yim, M.S.; Soung, N.K.; Han, E.H.; Min, J.-Y.; Han, H.; Son, E.-J.; Kim, H.N.; Kim, B.; Bang, J.K.; Ryu, E.K. Vitamin E-Conjugated Phosphopeptide Inhibitor of the Polo-Box Domain of Polo-Like Kinase 1. Mol. Pharm. 2019, 16, 4867–4877. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.D.; Mun, S.R.; Ji, C.H.; Sung, K.W.; Kang, K.Y.; Heo, A.J.; Lee, S.H.; An, J.Y.; Hwang, J.; Xie, X.-Q.; et al. N-terminal arginylation generates a bimodal degron that modulates autophagic proteolysis. Proc. Natl. Acad. Sci. USA 2018, 115, E2716–E2724. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Mastaglia, F.L.; Knuckey, N.W. Cationic Arginine-Rich Peptides (CARPs): A Novel Class of Neuroprotective Agents With a Multimodal Mechanism of Action. Front. Neurol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, X.; Tang, D.; Cao, Y.; Chen, G.; Lu, Y.; Zhuang, L.; Wang, S.; Xu, G.; Zhou, J.; et al. Influence of chk1 and plk1 silencing on radiation- or cisplatin-induced cytotoxicity in human malignant cells. Apoptosis 2006, 11, 1789–1800. [Google Scholar] [CrossRef]
- Yang, X.; Chen, G.; Li, W.; Peng, C.; Zhu, Y.; Yang, X.; Li, T.; Cao, C.; Pei, H. Cervical Cancer Growth Is Regulated by a c-ABL–PLK1 Signaling Axis. Cancer Res. 2017, 77, 1142–1154. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, R.; Wang, Y.; Pan, L.; Luan, F.; Fu, G. PLK1 in cancer therapy: A comprehensive review of immunomodulatory mechanisms and therapeutic opportunities. Front. Immunol. 2025, 16, 1602752. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Mu, X.; Bai, L.; Xu, Y.; Wang, J.; Lu, H. Protein targeting chimeric molecules specific for dual bromodomain 4 (BRD4) and Polo-like kinase 1 (PLK1) proteins in acute myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2020, 521, 833–839. [Google Scholar] [CrossRef]
- Alverez, C.N.; Park, J.-E.; Toti, K.S.; Xia, Y.; Krausz, K.W.; Rai, G.; Bang, J.K.; Gonzalez, F.J.; Jacobson, K.A.; Lee, K.S. Identification of a New Heterocyclic Scaffold for Inhibitors of the Polo-Box Domain of Polo-like Kinase 1. J. Med. Chem. 2020, 63, 14087–14117. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekaran, P.; Shin, S.C.; Hwang, Y.S.; Lee, J.; La, Y.K.; Yim, M.S.; Kim, H.N.; Kim, T.W.; Yang, E.; Lee, S.J.; et al. N-Degron-Based PROTAC Targeting PLK1: A Potential Therapeutic Strategy for Cervical Cancer. Pharmaceutics 2025, 17, 1027. https://doi.org/10.3390/pharmaceutics17081027
Gunasekaran P, Shin SC, Hwang YS, Lee J, La YK, Yim MS, Kim HN, Kim TW, Yang E, Lee SJ, et al. N-Degron-Based PROTAC Targeting PLK1: A Potential Therapeutic Strategy for Cervical Cancer. Pharmaceutics. 2025; 17(8):1027. https://doi.org/10.3390/pharmaceutics17081027
Chicago/Turabian StyleGunasekaran, Pethaiah, Sang Chul Shin, Yeon Sil Hwang, Jihyeon Lee, Yeo Kyung La, Min Su Yim, Hak Nam Kim, Tae Wan Kim, Eunjung Yang, Soo Jae Lee, and et al. 2025. "N-Degron-Based PROTAC Targeting PLK1: A Potential Therapeutic Strategy for Cervical Cancer" Pharmaceutics 17, no. 8: 1027. https://doi.org/10.3390/pharmaceutics17081027
APA StyleGunasekaran, P., Shin, S. C., Hwang, Y. S., Lee, J., La, Y. K., Yim, M. S., Kim, H. N., Kim, T. W., Yang, E., Lee, S. J., Yoon, J. M., Kim, E. E., Jeon, S., Ryu, E. K., & Bang, J. K. (2025). N-Degron-Based PROTAC Targeting PLK1: A Potential Therapeutic Strategy for Cervical Cancer. Pharmaceutics, 17(8), 1027. https://doi.org/10.3390/pharmaceutics17081027







