Nanocarriers Containing Curcumin and Derivatives for Arthritis Treatment: Mapping the Evidence in a Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Eligibility Criteria
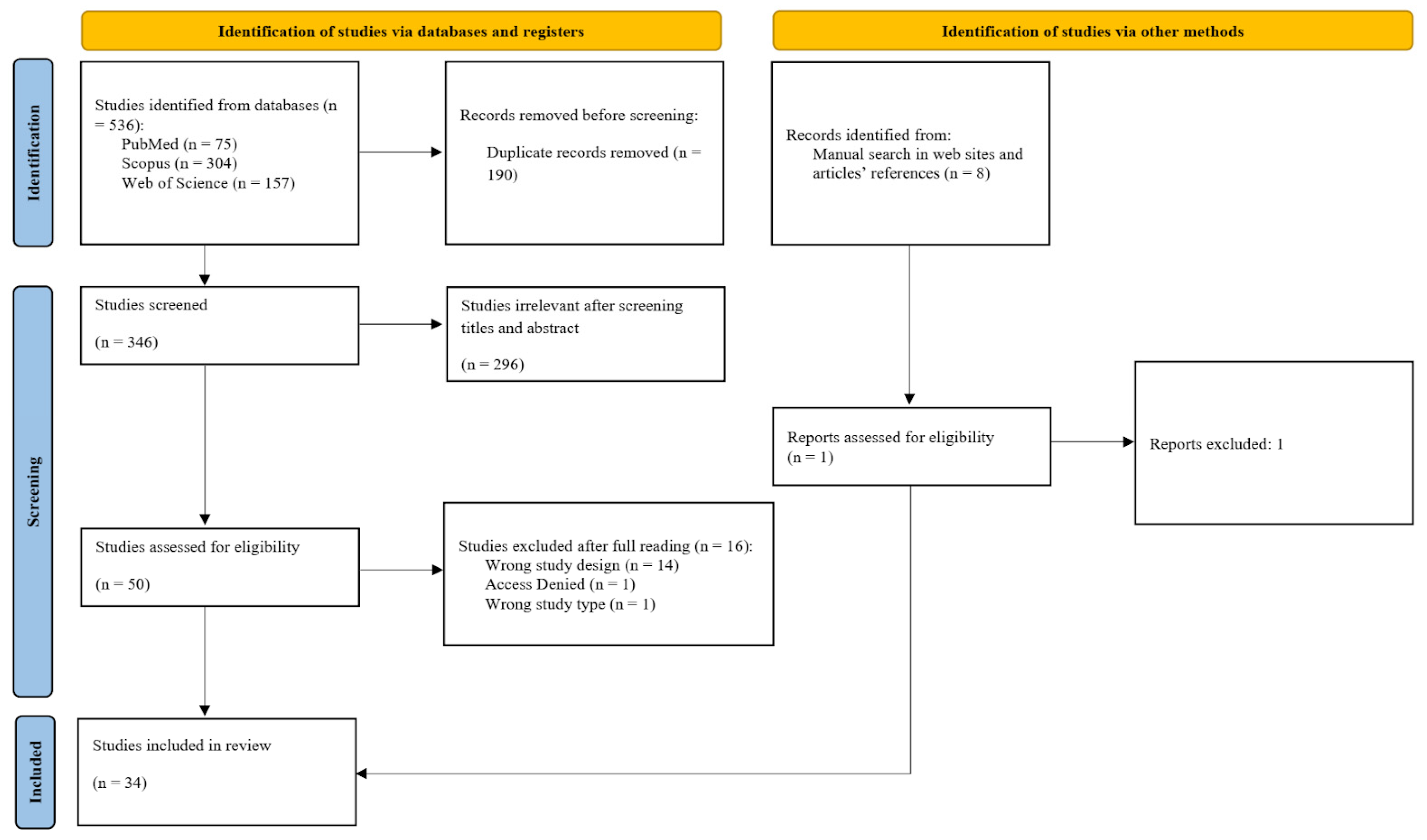
2.3. Study Selection
2.4. Data Extraction and Synthesis
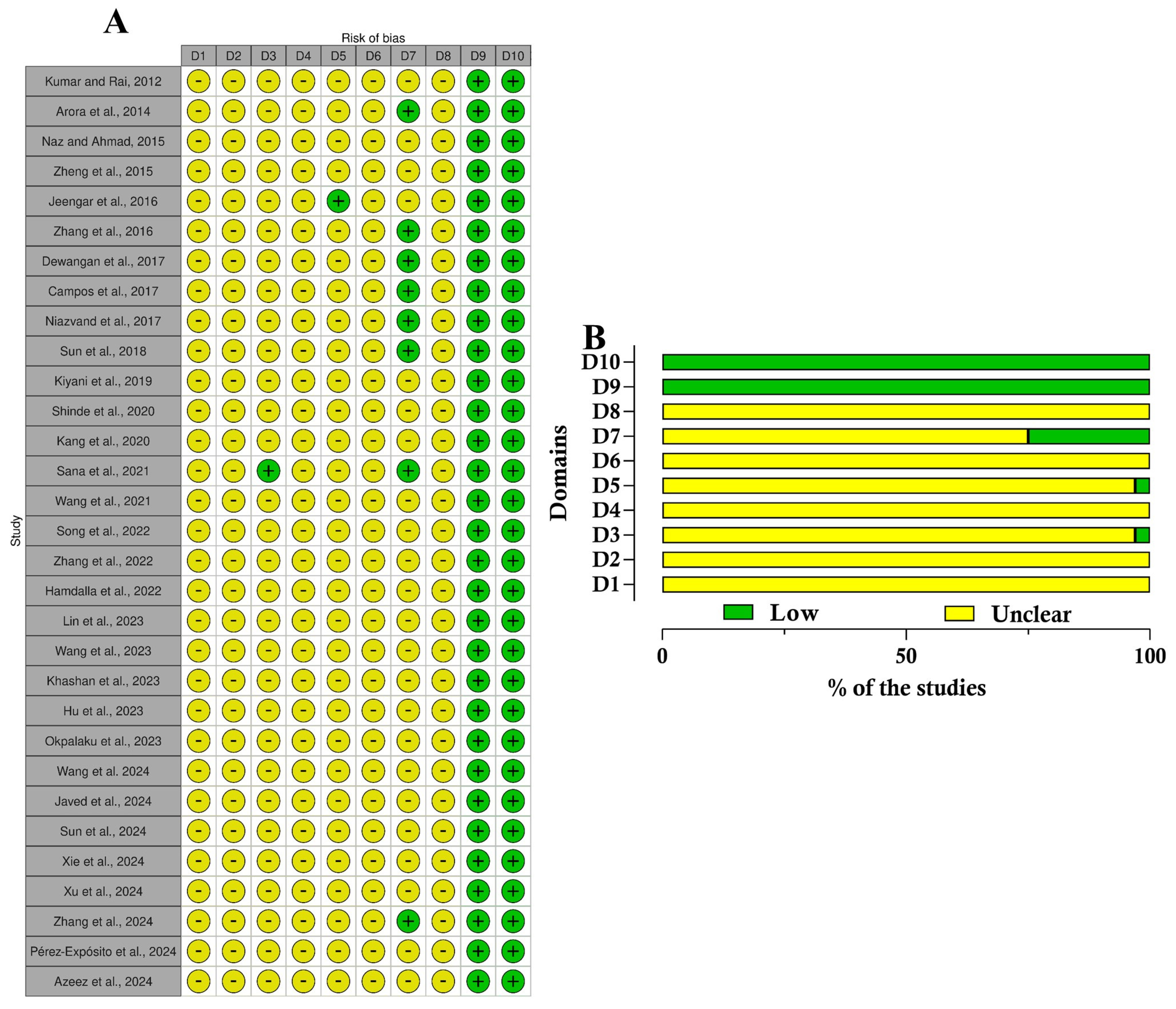
2.5. Risk of Bias Evaluation
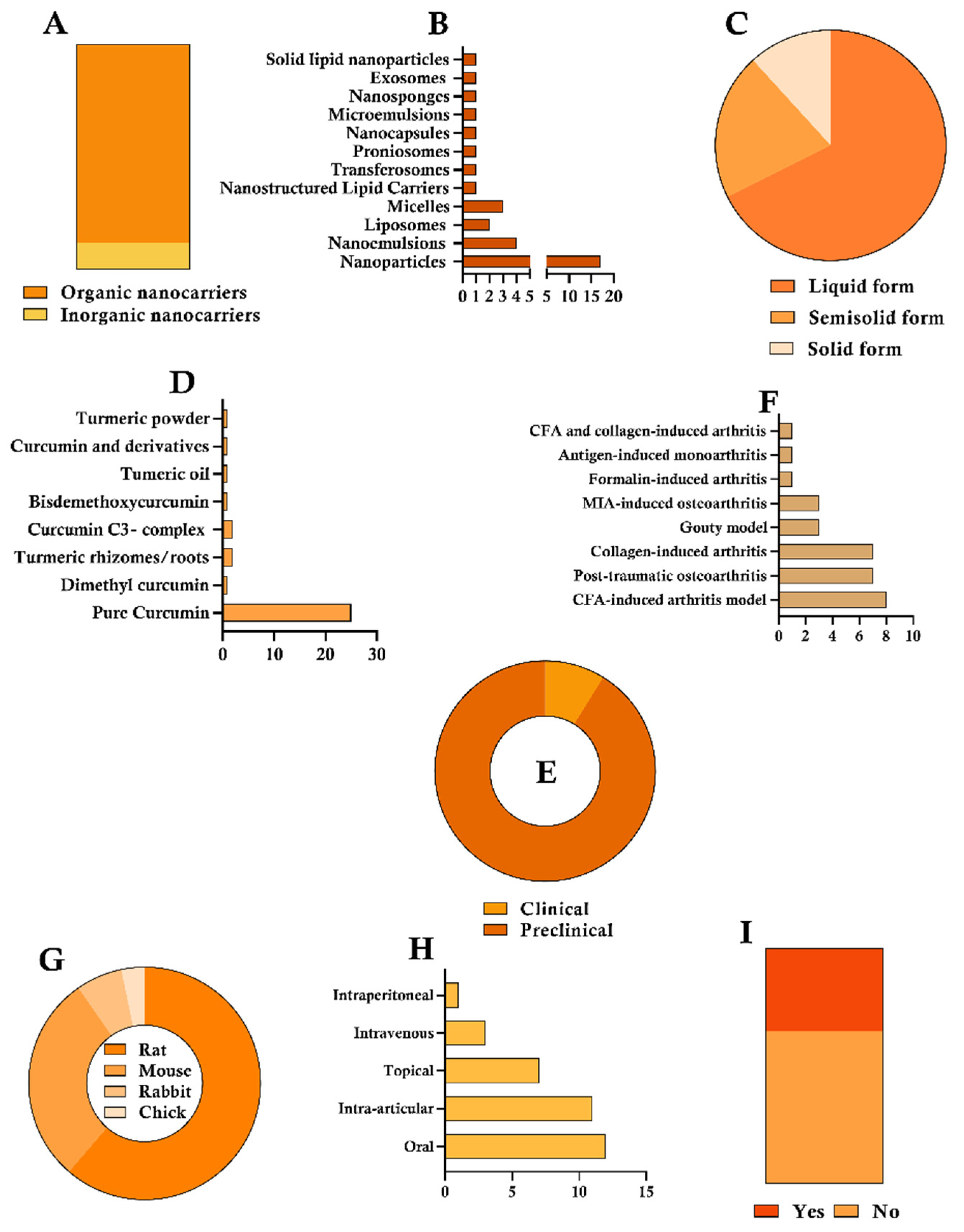
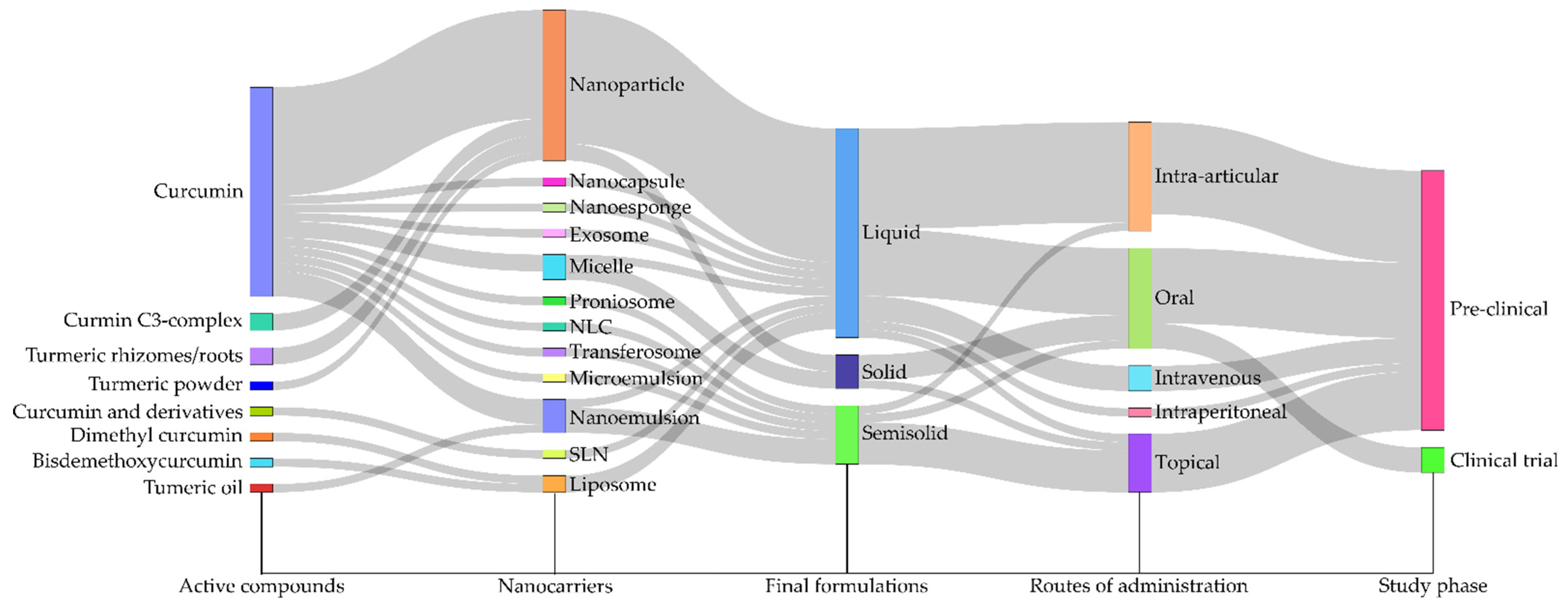
3. Results
4. Discussion
4.1. Formulation
4.1.1. Nanoparticles
4.1.2. Nanoemulsions
4.1.3. Other CUR-Loading Nanocarriers
4.2. Curcumin Source
4.3. Experimental Design of Preclinical and Clinical Studies
4.3.1. Preclinical Studies
4.3.2. Clinical Studies
4.4. Efficacy Outcomes from Preclinical Studies of Nano-Based CUR
4.4.1. Protocol and Administration Schedule
4.4.2. Experimental Approaches for Assessing Pharmacological Outcomes
4.5. Toxicological and Safety Investigations
4.6. Risk of Bias Evaluation
4.7. Expert Opinion and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACP | Acid-activatable curcumin polymer |
| AgNPs | Silver nanoparticles |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| AuNP | Gold nanoparticle |
| AuNP-PAH-CUR | AuNP with poly(allylamine hydrochloride) |
| BDMC | Bisdemethoxycurcumin |
| CFA | Complete Freund’s Adjuvant |
| CIA | Collagen-induced arthritis |
| CMCAB | Carboxymethycellulose acetate butyrate |
| COX-2 | Cyclooxygenase-2 |
| CUR | Curcumin |
| DAS-28 | Disease Activity Score of 28 joints |
| DiMC | Dimethyl curcumin |
| FoxP3 | Forkhead Box P3 |
| GA/CUR | Glycyrrhizic acid nanocomplex with curcumin |
| HA | Hyaluronic acid |
| H&E | Hematoxylin and Eosin |
| HDL | High-Density Lipoprotein |
| IL-10 | Interleukin 10 |
| IL-1 β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| ISO | International Organization for Standardization |
| LDL | Low-Density Lipoprotein |
| Lipo-DiMC | Liposome encapsulating dimethyl curcumin |
| MIA | Monoidoacetic acid or mono-iodoacetate |
| Micro-CT | Micro-computed tomography |
| MMD | Medial meniscus destabilization |
| MSU | Monosodium urate |
| Nano–CUR | Curcumin nanocarrier |
| NF-κB | Nuclear factor kappa B |
| NLCs | Nanostructured lipid carriers |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OARSI | Osteoarthritis Research Society International |
| OA | Osteoarthritis |
| PAH | Polyallylamine hydrochloride |
| PBS | Phosphate-buffered saline |
| PCC | Population, Concept, and Context |
| PDI | Polydispersity index |
| PLGA | Poly lactic-co-glycolic acid |
| RA | Rheumatoid arthritis |
| RCT | Randomized controlled trial |
| RoB 2 | Risk of Bias 2 |
| ROS | Reactive oxygen species |
| SJC | Swollen Joint Count |
| SLNs | Solid lipid nanoparticles |
| TGF-β | Transforming Growth Factor Beta |
| TJT | Tender Joint Count |
| TNF-α | Tumor Necrosis Factor Alpha |
| TPGS | D-α-tocopherol polyethylene glycol 1000 succinate |
| WHO | World Health Organization |
References
- Sudoł-Szopińska, I.; Schueller-Weidekamm, C.; Plagou, A.; Teh, J. Ultrasound in Arthritis. Radiol. Clin. N. Am. 2017, 55, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Doherty, J.B. Arthritis Pain. J. Gerontol. Soc. Work. 2008, 50 (Suppl. S1), 79–103. [Google Scholar] [CrossRef]
- Bessis, N.; Decker, P.; Assier, E.; Semerano, L.; Boissier, M.C. Arthritis Models: Usefulness and Interpretation. Semin. Immunopathol. 2017, 39, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Goldring, S.R. Pathogenesis of Bone and Cartilage Destruction in Rheumatoid Arthritis. Rheumatology 2003, 42, ii11–ii16. [Google Scholar] [CrossRef]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis Year in Review 2024: Epidemiology and Therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, I.J.; Cho, M.-S.; Lee, J. A Case of Rheumatoid Vasculitis Involving Hepatic Artery in Early Rheumatoid Arthritis. J. Korean Med. Sci. 2017, 32, 1207–1210. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Rheumatoid Arthritis. Available online: https://www.who.int/news-room/fact-sheets/detail/rheumatoid-arthritis (accessed on 30 June 2025).
- Johnson, V.L.; Hunter, D.J. The Epidemiology of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Tan, Y.; Buch, M.H. “Difficult to Treat” Rheumatoid Arthritis: Current Position and Considerations for next Steps. RMD Open 2022, e002387. [Google Scholar] [CrossRef]
- Hofman, Z.L.M.; Roodenrijs, N.M.T.; Nikiphorou, E.; Kent, A.L.; Nagy, G.; Welsing, P.M.J.; van Laar, J.M. Difficult-to-Treat Rheumatoid Arthritis: What Have We Learned and What Do We Still Need to Learn? Rheumatology 2025, 64, 65–73. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and Carcinogenic Properties of Curcumin: Considerations for Its Clinical Development as a Cancer Chemopreventive and Chemotherapeutic Agent. Mol. Nutr. Food Res. 2008, 52, S103–S127. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Curcumin and Curcuma Longa Extract in the Treatment of 10 Types of Autoimmune Diseases: A Systematic Review and Meta-Analysis of 31 Randomized Controlled Trials. Front. Immunol. 2022, 13, 896476. [Google Scholar] [CrossRef] [PubMed]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and Safety of Curcuma Domestica Extracts in Patients with Knee Osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Liu, J.; Thant, Y.; Weng, W.; Wei, C.; Bao, R.; Adu-Frimpong, M.; Yu, Q.; Deng, W.; et al. Bisdemethoxycurcumin-Conjugated Vitamin E TPGS Liposomes Ameliorate Poor Bioavailability of Free Form and Evaluation of Its Analgesic and Hypouricemic Activity in Oxonate-Treated Rats. J. Nanopart. Res. 2021, 23, 122. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Dong, L.; Chen, P.; Liu, W.; Yang, L. Cartilage-Targeting and Inflammatory-Responsive Nanocarriers for Effective Osteoarthritis Treatment via Reactive Oxygen Species Scavenging and Anti-Angiogenesis. J. Mater. Sci. Technol. 2023, 143, 30–42. [Google Scholar] [CrossRef]
- Dewangan, A.K.; Mazumder, S.; Perumal, Y.; Chopra, K.; Mazumder, S. Preparation, Characterization and Anti-Inflammatory Effects of Curcumin Loaded Carboxymethyl Cellulose Acetate Butyrate Nanoparticles on Adjuvant Induced Arthritis in Rats. J. Drug Deliv. Sci. Technol. 2017, 41, 269–279. [Google Scholar] [CrossRef]
- Sana, E.; Zeeshan, M.; Ain, Q.U.; Khan, A.U.; Hussain, I.; Khan, S.; Lepeltier, E.; Ali, H. Topical Delivery of Curcumin-Loaded Transfersomes Gel Ameliorated Rheumatoid Arthritis by Inhibiting NF-Κβ Pathway. Nanomedicine 2021, 16, 819–837. [Google Scholar] [CrossRef]
- Arora, R.; Kuhad, A.; Kaur, I.P.; Chopra, K. Curcumin Loaded Solid Lipid Nanoparticles Ameliorate Adjuvant-Induced Arthritis in Rats. Eur. J. Pain 2015, 19, 940–952. [Google Scholar] [CrossRef]
- Jeengar, M.K.; Rompicharla, S.V.K.; Shrivastava, S.; Chella, N.; Shastri, N.R.; Naidu, V.G.M.; Sistla, R. Emu Oil Based Nano-Emulgel for Topical Delivery of Curcumin. Int. J. Pharm. 2016, 506, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Kumar, K.; Rai, A.K. Proniosomal Formulation of Curcumin Having Anti-Inflammatory and Anti-Arthritic Activity in Different Experimental Animal Models. Pharmazie 2012, 67, 852–857. [Google Scholar] [CrossRef]
- Naz, Z.; Ahmad, F.J. Curcumin-Loaded Colloidal Carrier System: Formulation Optimization, Mechanistic Insight, Ex Vivo and in Vivo Evaluation. Int. J. Nanomed. 2015, 10, 4293–4307. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The Effect of Curcumin and Its Nanoformulation on Adjuvant-Induced Arthritis in Rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin Slows Osteoarthritis Progression and Relieves Osteoarthritis-Associated Pain Symptoms in a Post-Traumatic Osteoarthritis Mouse Model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef]
- da Silva Campos, W.N.; Leite, A.E.T.; Sonego, D.A.; de Andrade, M.A.; Pizzinatto, F.D.; Marangoni, V.S.; Zucolotto, V.; Nakazato, L.; Colodel, E.M.; de Souza, R.L. Síntese e Caracterização de Nanopartículas de Ouro Conjugadas Com Curcumina e Seus Efeitos Na Osteoartrite Experimental Induzida. Ciênc. Rural 2017, 47, e20161001. [Google Scholar] [CrossRef]
- Niazvand, F.; Khorsandi, L.; Abbaspour, M.; Orazizadeh, M.; Varaa, N.; Maghzi, M.; Ahmadi, K. Curcumin-Loaded Poly Lactic-Co-Glycolic Acid Nanoparticles Effects on Mono-Iodoacetate -Induced Osteoarthritis in Rats. Vet. Res. Forum 2017, 8, 155. [Google Scholar] [PubMed]
- Sun, Z.; Wei, T.; Zhou, X. Liposomes Encapsulated Dimethyl Curcumin Regulates Dipeptidyl Peptidase I Activity, Gelatinase Release and Cell Cycle of Spleen Lymphocytes in-Vivo to Attenuate Collagen Induced Arthritis in Rats. Int. Immunopharmacol. 2018, 65, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Kiyani, M.M.; Sohail, M.F.; Shahnaz, G.; Rehman, H.; Akhtar, M.F.; Nawaz, I.; Mahmood, T.; Manzoor, M.; Bokhari, S.A.I. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina 2019, 55, 10. [Google Scholar] [CrossRef]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of Curcumin Nanomicelle on the Clinical Symptoms of Patients with Rheumatoid Arthritis: A Randomized, Double-Blind, Controlled Trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Shinde, C.; Venkatesh, M.P.; Pramod Kumar, T.; Pai, D.R. Nanostructured Lipid Carrier-Based Smart Gel: A Delivery Platform for Intra-Articular Therapeutics. Autoimmunity 2021, 54, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jung, E.; Hyeon, H.; Seon, S.; Lee, D. Acid-Activatable Polymeric Curcumin Nanoparticles as Therapeutic Agents for Osteoarthritis. Nanomedicine 2020, 23, 102104. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hajialilo, M.; Dolati, S.; Eghbal-Fard, S.; Heydarlou, H.; Ghaebi, M.; Ghassembaglou, A.; Aghebati-Maleki, L.; Samadi Kafil, H.; Kamrani, A.; et al. The Effects of Nanocurcumin on Treg Cell Responses and Treatment of Ankylosing Spondylitis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Cell Biochem. 2020, 121, 103–110. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.Y.; You, G.; Kang, Y.Y.; Yang, J.; Mok, H. Formulation of Glycyrrhizic Acid-Based Nanocomplexes for Enhanced Anti-Cancer and Anti-Inflammatory Effects of Curcumin. Biotechnol. Bioprocess Eng. 2022, 27, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Liao, M.; Li, S.; Feng, Q.; Cao, X. Curcumin-Laden Amphiphilic Chitosan Microemulsion with Enhanced Transdermal Delivery, Skin Compatibility and Anti-Arthritic Activity. J. Drug Deliv. Sci. Technol. 2022, 78, 103997. [Google Scholar] [CrossRef]
- Hamdalla, H.M.; Ahmed, R.R.; Galaly, S.R.; Naguib, I.A.; Alghamdi, B.S.; Ahmed, O.M.; Farghali, A.; Abdul-Hamid, M. Ameliorative Effect of Curcumin Nanoparticles against Monosodium Iodoacetate-Induced Knee Osteoarthritis in Rats. Mediat. Inflamm. 2022, 2022, 8353472. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, S.; Ye, X.; Dai, M.; Yang, G.; Liu, L. Antimicrobial Curcumin Nanoparticles Downregulate Joint Inflammation and Improve Osteoarthritis. Macromol. Res. 2023, 31, 1179–1187. [Google Scholar] [CrossRef]
- Khashan, A.A.; Dawood, Y.; Khalaf, Y.H. Green Chemistry and Anti-Inflammatory Activity of Silver Nanoparticles Using an Aqueous Curcumin Extract. Results Chem. 2023, 5, 100913. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, F.; Wei, Y.; Liu, J.; Nie, Y.; Xie, J.; Yang, L.; Luo, R.; Shen, B.; Wang, Y. Drug-Embedded Nanovesicles Assembled from Peptide-Decorated Hyaluronic Acid for Rheumatoid Arthritis Synergistic Therapy. Biomacromolecules 2023, 24, 3532–3544. [Google Scholar] [CrossRef] [PubMed]
- Okpalaku, O.; Uronnachi, E.; Okoye, E.; Umeyor, C.; Nwakile, C.; Okeke, T.; Attama, A. Evaluating Some Essential Oils-Based and Coconut Oil Nanoemulgels for the Management of Rheumatoid Arthritis. Lett. Appl. NanoBioSci. 2023, 12, 75. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, R.; Zou, M.; Jiang, L.; Kong, L.; Zhao, S.; Zhang, X.; Wang, W.; Xu, B. Combined ROS Sensitive Folate Receptor Targeted Micellar Formulations of Curcumin Effective Against Rheumatoid Arthritis in Rat Model. Int. J. Nanomed. 2024, 19, 4217–4234. [Google Scholar] [CrossRef]
- Javed, C.; Noreen, R.; Niazi, S.g.; Kiyani, M.M.; Ul Ain, Q. Anti-Gouty Arthritis and Anti-Inflammatory Effects of Curcumin Nanoparticles in Monosodium Urate Crystals Induced Balb/C Mice. Inflammopharmacology 2024, 32, 1929–1940. [Google Scholar] [CrossRef]
- Lustberg, M.; Fan-Havard, P.; Wong, F.L.; Hill, K.; Phelps, M.A.; Herrera, K.W.; Tsai, N.C.; Synold, T.; Feng, Y.; Kalu, C.; et al. Randomized Placebo-Controlled, Double-Blind Clinical Trial of Nanoemulsion Curcumin in Women with Aromatase Inhibitor-Induced Arthropathy: An Alliance/NCORP Pilot Trial. Breast Cancer Res. Treat. 2024, 205, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, J.; Liu, X.; An, J.; Hu, Y.; Wang, J.; Zhu, F.; Feng, H.; Cheng, S.; Tian, H.; et al. Chondroitin Sulfate-Modified Tragacanth Gum–Gelatin Composite Nanocapsules Loaded with Curcumin Nanocrystals for the Treatment of Arthritis. J. Nanobiotechnol. 2024, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, Y.; Yang, X.; Yu, P.; Ban, D. Green Synthesis of Gold Nanoparticles Mediated by Extract of Curcuma Longa under Ultrasonic Condition: Investigation of Its Application for Reduction of Dye Pollutants and Repairing the Articular Cartilage in an Animal Model of Osteoarthritis of the Knee. Inorg. Chem. Commun. 2024, 162, 112169. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Wei, Y.; Cao, S.; Deng, J.; Li, G.; Sheng, W.; Qi, T.; Zhang, P.; Lin, J.; et al. Curcumin-Loaded Biomimetic Nanosponges for Osteoarthritis Alleviation by Synergistically Suppressing Inflammation and Ferroptosis. Chem. Eng. J. 2024, 491, 152132. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Wang, Z.; Wang, D.; Lu, Y.; Zhang, K.; Yuan, Y.; Mei, X.; Chen, S. M1 Type Macrophage Targeted Anti-Inflammatory Exosomes Derived from BMSC for the Treatment of Acute and Chronic Inflammatory Diseases. Mater. Des. 2024, 240, 112844. [Google Scholar] [CrossRef]
- Pérez Expósito, R.E.; Ortega Núñez, M.A.; Buján Varela, M.J.; Vega Rodríguez, R.M.; Ortíz Chércoles, A.I.; De La Torre Escuredo, B.J. Efficacy of New Active Viscosupplements on the Behavior of an Experimental Model of Osteoarthritis. Rev. Esp. Cir. Ortop. Traumatol. 2024, 69, 150–157. [Google Scholar] [CrossRef]
- Azeez, S.; Fatima, M.; Gul, O.; Rehman, H.; Shad, M.A.; Nawaz, H. Zinc Oxide Nanoparticles-Doped Curcumin-Assisted Recovery of Rheumatoid Arthritis and Antioxidant Status in Experimental Rabbits. BioMedicine 2024, 14, 49–59. [Google Scholar] [CrossRef]
- Mulvaney, P. Nanoscience vs Nanotechnology-Defining the Field. ACS Nano 2015, 9, 2215–2217. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of Curcumin and Curcumin Glucuronide in the Central Nervous System of Mice after Oral Delivery of Nano-Curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, Structure, and Physical Properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-Thickened Nanoemulsion System for Topical Delivery of Lipophilic Drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Henrique Marcondes Sari, M.; Mota Ferreira, L.; Cruz, L. The Use of Natural Gums to Produce Nano-Based Hydrogels and Films for Topical Application. Int. J. Pharm. 2022, 626, 122166. [Google Scholar] [CrossRef]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in Nanotechnology-Based Delivery Systems for Curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin Loaded Chitin Nanogels for Skin Cancer Treatment via the Transdermal Route. Nanoscale 2011, 4, 239–250. [Google Scholar] [CrossRef]
- Pegoraro, C.; MacNeil, S.; Battaglia, G. Transdermal Drug Delivery: From Micro to Nano. Nanoscale 2012, 4, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Guccione, C.; Manconi, M.; Fadda, A.M.; Sinico, C. Vesicles and Micelles: Two Versatile Vectors for the Delivery of Natural Products. J. Drug Deliv. Sci. Technol. 2016, 32, 241–255. [Google Scholar] [CrossRef]
- Johnson, S.M.; Bangham, A.D.; Hill, M.W.; Korn, E.D. Single Bilayer Liposomes. Biochim. Biophys. Acta Biomembr. 1971, 233, 820–826. [Google Scholar] [CrossRef]
- Fenske, D.B. Structural and Motional Properties of Vesicles as Revealed by Nuclear Magnetic Resonance. Chem. Phys. Lipids 1993, 64, 143–162. [Google Scholar] [CrossRef]
- Fenske, D.B.; Chonn, A.; Cullis, P.R. Liposomal Nanomedicines: An Emerging Field. Toxicol. Pathol. 2008, 36, 21–29. [Google Scholar] [CrossRef]
- Bayat, F.; Pourmadadi, M.; Eshaghi, M.M.; Yazdian, F.; Rashedi, H. Improving Release Profile and Anticancer Activity of 5-Fluorouracil for Breast Cancer Therapy Using a Double Drug Delivery System: Chitosan/Agarose/γ-Alumina Nanocomposite@Double Emulsion. J. Clust. Sci. 2023, 34, 2565–2577. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2016, 14, 613–633. [Google Scholar] [CrossRef]
- Oyarzún, P.; Gallardo-Toledo, E.; Morales, J.; Arriagada, F. Transfersomes as Alternative Topical Nanodosage Forms for the Treatment of Skin Disorders. Nanomedicine 2021, 16, 2465–2489. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. New, Highly Efficient Formulation of Diclofenac for the Topical, Transdermal Administration in Ultradeformable Drug Carriers, Transfersomes. Biochim. Biophys. Acta Biomembr. 2001, 1514, 191–205. [Google Scholar] [CrossRef]
- Hofer, C.; Hartung, R.; Göbel, R.; Deering, P.; Lehmer, A.; Breul, J. New Ultradeformable Drug Carriers for Potential Transdermal Application of Interleukin-2 and Interferon-α: Theoretic and Practical Aspects. World J. Surg. 2000, 24, 1187–1189. [Google Scholar] [CrossRef]
- Chaurasiya, P.; Ganju, E.; Upmanyu, N.; Ray, S.K.; Jain, P. Transfersomes: A Novel Technique for Transdermal Drug Delivery. J. Drug Deliv. Ther. 2019, 9, 279–285. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Exploring Therapeutic Potential of Invasomes, Transfersomes, Transethosomes, Oleic Acid Vesicles, and Cubosomes Adopting Topical/Transdermal. Micro Nanosyst. 2021, 14, 3–20. [Google Scholar] [CrossRef]
- Niu, Z.; Conejos-Sánchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-Based Nanocarriers for Oral Peptide Delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-Based Nanocapsules for Drug Delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2016, 9, 8. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, E.; Zhang, B.; Murphy, C.J.; Sui, M.; Zhao, J.; Wang, J.; Tang, J.; Fan, M.; Van Kirk, E.; et al. Prodrugs Forming High Drug Loading Multifunctional Nanocapsules for Intracellular Cancer Drug Delivery. J. Am. Chem. Soc. 2010, 132, 4259–4265. [Google Scholar] [CrossRef]
- Gradzielski, M.; Duvail, M.; De Molina, P.M.; Simon, M.; Talmon, Y.; Zemb, T. Using Microemulsions: Formulation Based on Knowledge of Their Mesostructure. Chem. Rev. 2021, 121, 5671–5740. [Google Scholar] [CrossRef]
- Luna-Canales, I.C.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Nava-Arzaluz, G.; Piñón-Segundo, E.; Martínez-Cruz, G.; Ganem-Rondero, A. Curcumin-Loaded Microemulsion: Formulation, Characterization, and In Vitro Skin Penetration. Drug Dev. Ind. Pharm. 2023, 49, 42–51. [Google Scholar] [CrossRef]
- Szumała, P.; Macierzanka, A. Topical Delivery of Pharmaceutical and Cosmetic Macromolecules Using Microemulsion Systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- More, S.K.; Pawar, A.P. Preparation, Optimization and Preliminary Pharmacokinetic Study of Curcumin Encapsulated Turmeric Oil Microemulsion in Zebra Fish. Eur. J. Pharm. Sci. 2020, 155, 105539. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R.; Tumbiolo, S.; Bertinetti, L.; Coluccia, S. Structural Evidence of Differential Forms of Nanosponges of Beta-Cyclodextrin and Its Effect on Solubilization of a Model Drug. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 201–211. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.P.; Cavalli, R. Cyclodextrin-Based Nanosponges Encapsulating Camptothecin: Physicochemical Characterization, Stability and Cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid Lipid Nanoparticles for Targeted Brain Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid Lipid Based Nanocarriers: An Overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Kouhsoltani, M.; Hamishehkar, H. Proniosomes in Transdermal Drug Delivery. Curr. Pharm. Des. 2015, 21, 2883–2891. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal Drug Delivery: Overcoming the Skin’s Barrier Function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Mazdaei, M.; Asare-Addo, K.; Mazdaei, M.; Asare-Addo, K. A Mini-Review of Nanocarriers in Drug Delivery Systems. Br. J. Pharm. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Koh, E.J.; Kim, R.N.; Byun, J.W.; Phi, J.H.; Yang, J.; Wang, K.C.; Park, A.K.; Hwang, D.W.; Lee, J.Y.; et al. Extracellular Vesicle-Associated MiR-135b and -135a Regulate Stemness in Group 4 Medulloblastoma Cells by Targeting Angiomotin-like 2. Cancer Cell Int. 2020, 20, 558. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Liu, S.; Wang, T.; Ianni, A.; Bober, E.; Braun, T.; Xiang, R.; Yue, S.; Lu, J.; et al. Exosomal Tetraspanins Mediate Cancer Metastasis by Altering Host Microenvironment. Oncotarget 2017, 8, 62803–62815. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Deshmukh, S.K.; Chavva, S.R.; Tyagi, N.; Srivastava, S.K.; Patel, G.K.; Singh, A.P.; Singh, S. Drug-Loaded Exosomal Preparations from Different Cell Types Exhibit Distinctive Loading Capability, Yield, and Antitumor Efficacies: A Comparative Analysis. Int. J. Nanomed. 2019, 14, 531–541. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From Ancient Medicine to Current Clinical Trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Teymouri, M.; Barati, N.; Pirro, M.; Sahebkar, A. Biological and Pharmacological Evaluation of Dimethoxycurcumin: A Metabolically Stable Curcumin Analogue with a Promising Therapeutic Potential. J. Cell Physiol. 2018, 233, 124–140. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Sandur, S.K.; Priyadarsini, I.K. Differential Antioxidant/pro-Oxidant Activity of Dimethoxycurcumin, a Synthetic Analogue of Curcumin. Free Radic. Res. 2011, 45, 959–965. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Gupta, P.K.; Bhandari, N.; Shah, H.N.; Khanchandani, V.; Keerthana, R.; Nagarajan, V.; Hiremath, L.; Gupta, P.K.; Bhandari, N.; Shah, H.N.; et al. An Update on Nanoemulsions Using Nanosized Liquid in Liquid Colloidal Systems. In Nanoemulsions-Properties, Fabrications and Applications; IntechOpen: Fontana, CA, USA, 2019. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A Promising Phase in Drug Delivery. Curr. Pharm. Des. 2019, 26, 279–291. [Google Scholar] [CrossRef]
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A Promising Novel Formulation for Treatment of Skin Ailments. Polym. Bull. 2022, 79, 4441–4465. [Google Scholar] [CrossRef]
- Kollias, G.; Papadaki, P.; Apparailly, F.; Vervoordeldonk, M.J.; Holmdahl, R.; Baumans, V.; Desaintes, C.; Di Santo, J.; Distler, J.; Garside, P.; et al. Animal Models for Arthritis: Innovative Tools for Prevention and Treatment. Ann. Rheum. Dis. 2011, 70, 1357–1362. [Google Scholar] [CrossRef]
- Mobasheri, A.; Batt, M. An Update on the Pathophysiology of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight Bearing as a Measure of Disease Progression and Efficacy of Anti-Inflammatory Compounds in a Model of Monosodium Iodoacetate-Induced Osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, G.M.; Siebelt, M.; Leijs, M.J.C.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; Van Osch, G.J.V.M. Mesenchymal Stem Cells Reduce Pain but Not Degenerative Changes in a Mono-Iodoacetate Rat Model of Osteoarthritis. J. Orthop. Res. 2014, 32, 1167–1174. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like Synoviocytes: Key Effector Cells in Rheumatoid Arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, S.; Knedla, A.; Tennie, C.; Kampmann, A.; Wunrau, C.; Dinser, R.; Korb, A.; Schnäker, E.M.; Tarner, I.H.; Robbins, P.D.; et al. Synovial Fibroblasts Spread Rheumatoid Arthritis to Unaffected Joints. Nat. Med. 2009, 15, 1414–1420. [Google Scholar] [CrossRef]
- Cho, Y.G.; Cho, M.L.; Min, S.Y.; Kim, H.Y. Type II Collagen Autoimmunity in a Mouse Model of Human Rheumatoid Arthritis. Autoimmun. Rev. 2007, 7, 65–70. [Google Scholar] [CrossRef]
- Bolon, B.; Stolina, M.; King, C.; Middleton, S.; Gasser, J.; Zack, D.; Feige, U. Rodent Preclinical Models for Developing Novel Antiarthritic Molecules: Comparative Biology and Preferred Methods for Evaluating Efficacy. Biomed. Res. Int. 2011, 2011, 569068. [Google Scholar] [CrossRef] [PubMed]
- Buglak, N.E.; Bahnson, E.S.M. A Rat Carotid Artery Pressure-Controlled Segmental Balloon Injury with Periadventitial Therapeutic Application. J. Vis. Exp. 2020, 161, 10-3791. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-Induced Arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Holmdahl, R.; Jansson, L.; Larsson, E.; Rubin, K.; Klareskog, L. Homologous Type II Collagen Induces Chronic and Progressive Arthritis in Mice. Arthritis Rheum. 1986, 29, 106–113. [Google Scholar] [CrossRef]
- Boissier, M.C.; Feng, X.Z.; Carlioz, A.; Roudier, R.; Fournier, C. Experimental Autoimmune Arthritis in Mice. I. Homologous Type II Collagen Is Responsible for Self-Perpetuating Chronic Polyarthritis. Ann. Rheum. Dis. 1987, 46, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.L. Hormones and Autoimmunity: Animal Models of Arthritis. Baillieres Clin. Rheumatol. 1996, 10, 259–271. [Google Scholar] [CrossRef]
- Patil, T.; Soni, A.; Acharya, S. A Brief Review on in Vivo Models for Gouty Arthritis. Metabol. Open 2021, 11, 100100. [Google Scholar] [CrossRef]
- Terkeltaub, R. Update on Gout: New Therapeutic Strategies and Options. Nat. Rev. Rheumatol. 2010, 6, 30–38. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Fattori, V.; Zaninelli, T.H.; Badaro-Garcia, S.; Borghi, S.M.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, T.M.; et al. Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice. Front. Pharmacol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O.; et al. Monosodium Urate Crystals Induce Oxidative Stress in Human Synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef]
- van den Berg, W.B.; Joosten, L.A.B.; van Lent, P.L.E.M. Murine Antigen-Induced Arthritis. Methods Mol. Med. 2007, 136, 243–253. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Subramaniyan, V.; Chandiran, S.; Kayarohanam, S.; Kanniyan, D.C.; Velaga, V.S.S.R.; Muhammad, S. Antiarthritic Activity of Achyranthes Aspera on Formaldehyde-Induced Arthritis in Rats. Open Access Maced. J. Med. Sci. 2019, 7, 2709. [Google Scholar] [CrossRef]
- Keeble, J.E.; Brain, S.D. A Role for Substance P in Arthritis? Neurosci. Lett. 2004, 361, 176–179. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Moore-Medlin, T.; Ekshyyan, O.; Gu, X.; Abreo, F.; Nathan, C.-A.O. Local and Systemic Curcumin C3 Complex Inhibits 4NQO-Induced Oral Tumorigenesis via Modulating FGF-2/FGFR-2 Activation. Am. J. Cancer Res. 2018, 8, 2538. [Google Scholar]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The Anti-Inflammatory Activity of Specific-Sized Hyaluronic Acid Oligosaccharides. Carbohydr. Polym. 2022, 276, 118699. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic Acid: Its Function and Degradation in In Vivo Systems. Stud. Nat. Prod. Chem. 2008, 34, 789–882. [Google Scholar] [CrossRef]
- Maynard, R.L. Nano-Technology and Nano-Toxicology. Emerg. Health Threats J. 2012, 5, 17508. [Google Scholar] [CrossRef] [PubMed]

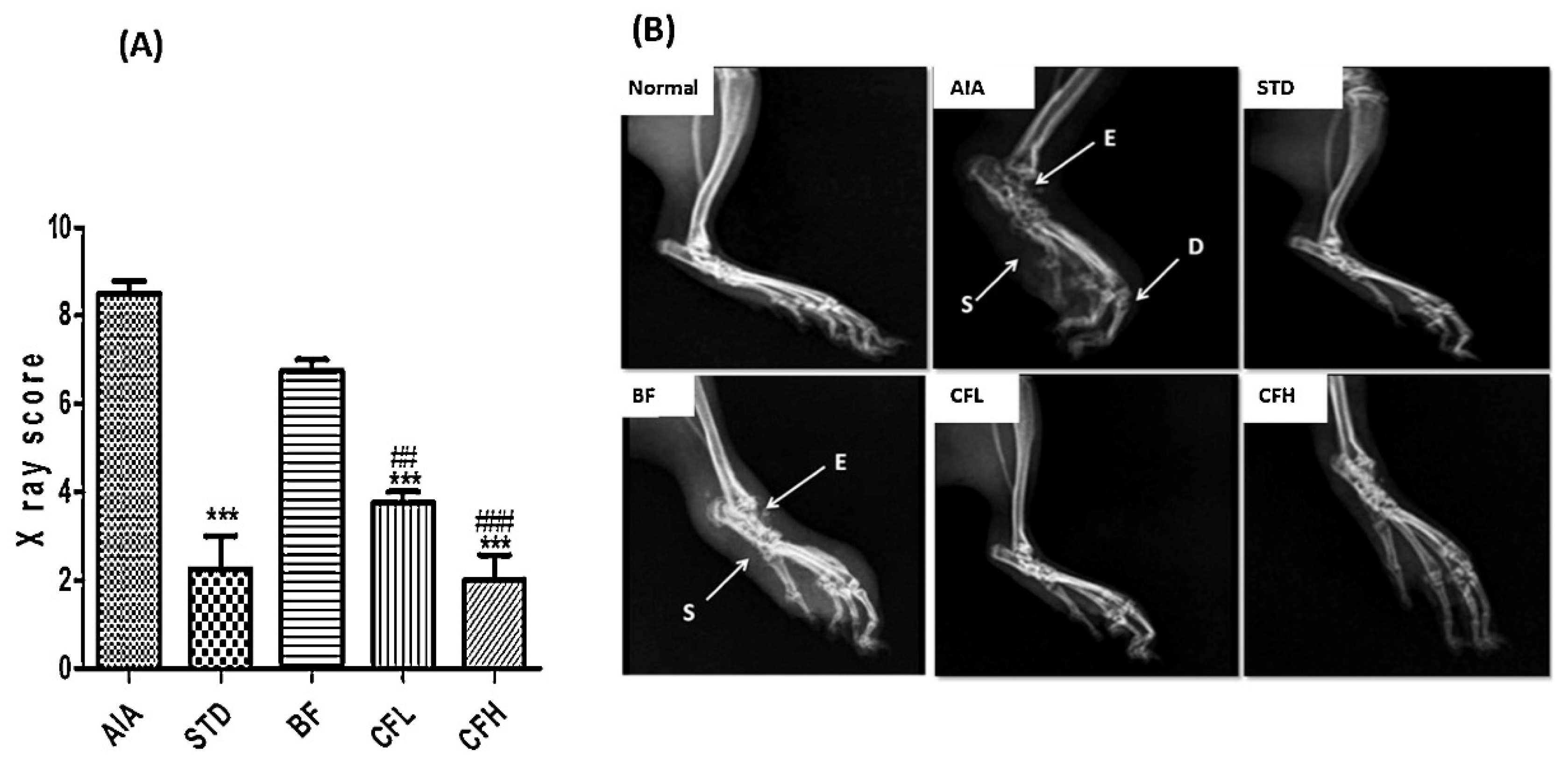
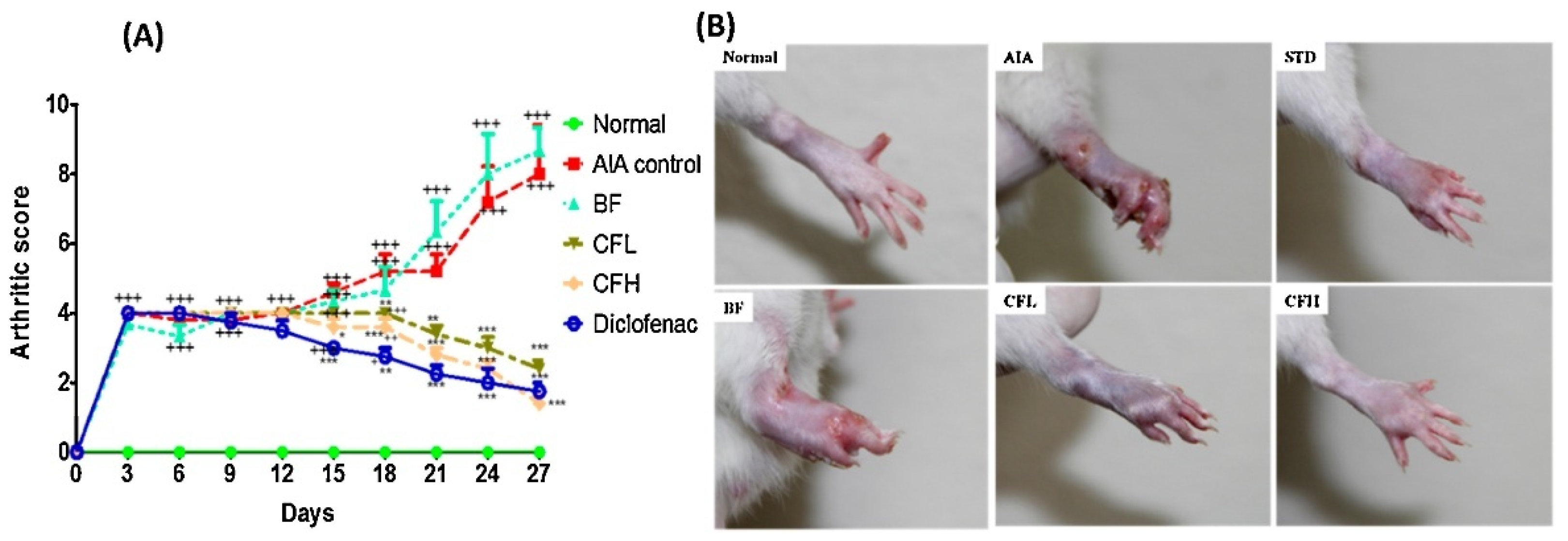
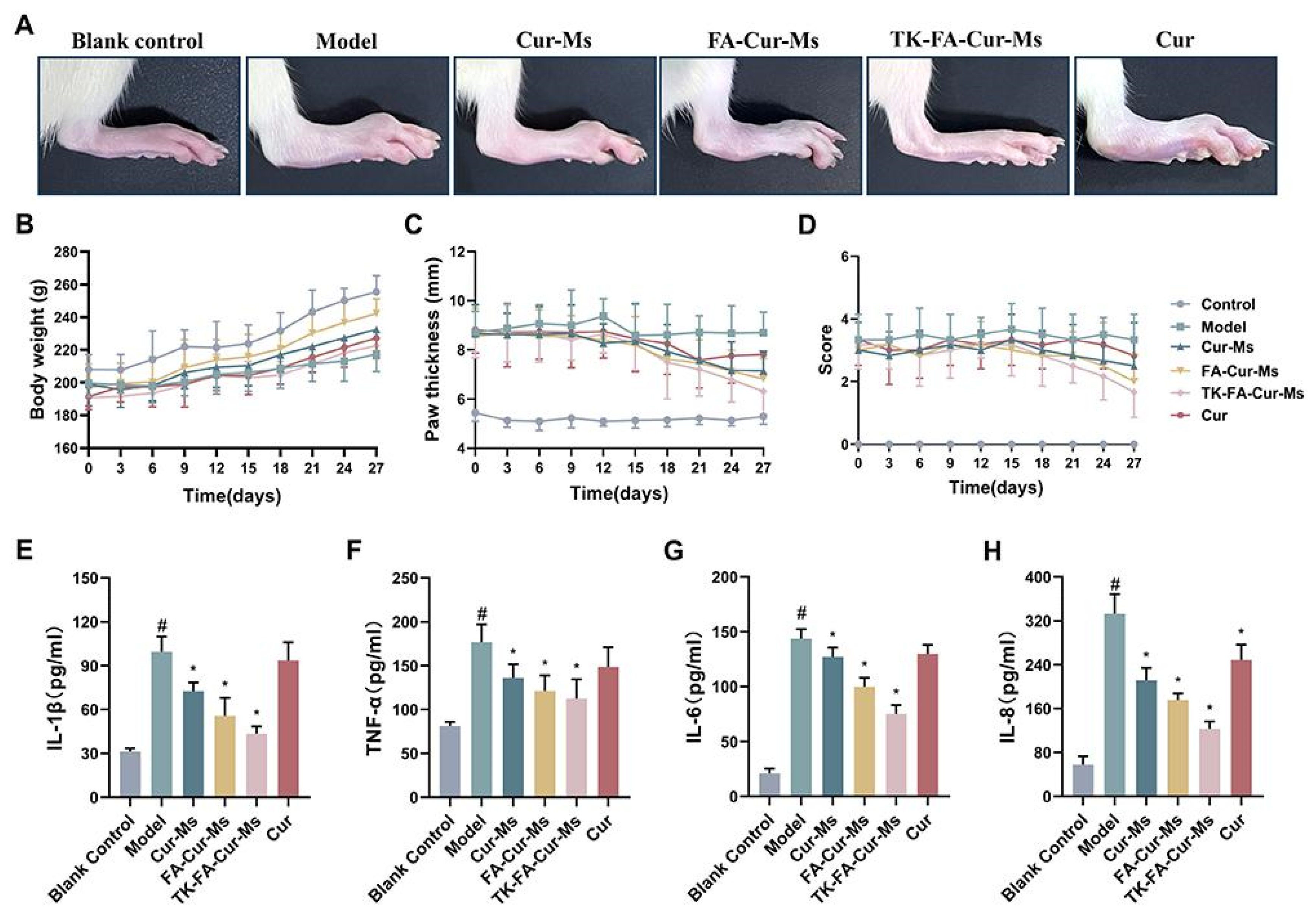
| Author | Nanocarrier | Composition | Preparation Method | Final Form |
|---|---|---|---|---|
| Kumar and Rai, 2012 [32] | Proniosomes | Organic | Ether injection method | Semisolid |
| Arora et al., 2014 [24] | Solid lipid nanoparticle | Organic | Hot homogenization and a melt ultrasonication | Liquid |
| Naz and Ahmad, 2015 [33] | Nanoemulsion | Organic | Spontaneous emulsification | Semisolid |
| Zheng et al., 2015 [34] | Nanoemulsion | Organic | High-pressure homogenization method | Semisolid |
| Jeengar et al., 2016 [25] | Nanoemulsion | Organic | Spontaneous emulsification | Semisolid |
| Zhang et al., 2016 [35] | Nanoparticle | Organic | Ultrasonification | Solid |
| Dewangan et al., 2017 [22] | Nanoparticle | Organic | Nanoprecipitation | Liquid |
| Campos et al., 2017 [36] | Nanoparticle | Inorganic | Conjugation | Liquid |
| Niazvand et al., 2017 [37] | Nanoparticle | Organic | Solvent solid-in-oil-in-water emulsion evaporation technique | Liquid |
| Sun et al., 2018 [38] | Liposome | Organic | Thin-film method | Liquid |
| Kiyani et al., 2019 [39] | Nanoparticle | Organic | Ultrasonification | Liquid |
| Javadi et al., 2019 [40] | Micelle | Organic | Patent | Solid |
| Shinde et al., 2020 [41] | Nanostructured lipid carrier | Organic | Hot homogenization and a subsequent melt ultrasonication | Semisolid |
| Kang et al., 2020 [42] | Nanoparticle | Organic | Self-emulsification | Liquid |
| Ahmadi et al., 2020 [43] | Micelle | Organic | Patent | Solid |
| Sana et al., 2021 [23] | Transferosome | Organic | Thin-film hydration | Semisolid |
| Wang et al., 2021 [20] | Liposome | Organic | Thin-film method | Liquid |
| Song et al., 2022 [44] | Nanoparticle | Organic | Sonication and reversible noncovalent interactions | Liquid |
| Zhang et al., 2022 [45] | Microemulsion | Organic | Spontaneous emulsification | Semisolid |
| Hamdalla et al., 2022 [46] | Nanoparticle | Organic | Nanoprecipitation | Liquid |
| Lin et al., 2023 [47] | Nanoparticle | Organic | Nanoprecipitation | Liquid |
| Wang et al., 2023 [21] | Nanoparticle | Organic | Nanoprecipitation | Liquid |
| Khashan et al., 2023 [48] | Nanoparticle | Inorganic | Green chemistry | Liquid |
| Hu et al., 2023 [49] | Nanoparticle | Organic | Sonication and reversible noncovalent interactions | Liquid |
| Okpalaku et al., 2023 [50] | Nanoemulsion | Organic | Spontaneous emulsification | Liquid |
| Wang et al., 2024 [51] | Micelle | Organic | Thin-film dispersion | Liquid |
| Javed et al., 2024 [52] | Nanoparticle | Organic | Nanoprecipitation | Liquid |
| Lustberg et al., 2024 [53] | Nanoparticle | Organic | Patent | Solid |
| Sun et al., 2024 [54] | Nanocapsules | Organic | Inborn microcrystallization method | Liquid |
| Xie et al., 2024 [55] | Nanoparticle | Inorganic | Ultrasound assisted synthesis | Liquid |
| Xu et al., 2024 [56] | Nanosponge | Organic | Nanoprecipitation | Liquid |
| Zhang et al., 2024 [57] | Exosome | Organic | Ultrasound encapsulation | Liquid |
| Pérez-Expósito et al., 2024 [58] | Nanoparticle | Organic | - | Liquid |
| Azeez et al., 2024 [59] | Nanoparticle | Inorganic | Co-precipitation | Liquid |
| Author | CUR Form and Administration Route | Study Design | Safety Assessment |
|---|---|---|---|
| Kumar and Rai, 2012 [32] | Pure drug, topical administration | CFA-induced arthritis rat model | Skin irritancy test on male albino rabbits |
| Arora et al., 2014 [24] | 95% cur and 5% methoxycurcumin + bis-methoxycurcumin, oral administration | CFA-induced arthritis rat model | - |
| Naz and Ahmad, 2015 [33] | Pure drug, intra-articular administration | CFA-induced arthritis rat model | - |
| Zheng et al., 2015 [34] | Pure drug, oral administration | CFA-induced arthritis rat model | - |
| Jeengar et al., 2016 [25] | Pure drug, topical administration | CFA-induced arthritis rat model | - |
| Zhang et al., 2016 [35] | Pure drug, topical administration | Post-traumatic osteoarthritis mouse model | - |
| Dewangan et al., 2017 [22] | Pure drug, oral administration | CFA-induced arthritis rat model | - |
| Campos et al., 2017 [36] | Pure drug, intra-articular administration | Post-traumatic osteoarthritis mouse model | - |
| Niazvand et al., 2017 [37] | Pure drug, oral administration | Monoiodoacetate-induced osteoarthritis in rats | - |
| Sun et al., 2018 [38] | Dimethyl curcumin (DiMC), intra-articular administration | Collagen-induced arthritis rat model | - |
| Kiyani et al., 2019 [39] | Turmeric powder, oral administration | Monosodium urate-induced Gouty mouse model | Clinical parameters, biochemical analyses, and histopathological evaluation |
| Javadi et al., 2019 [40] | C3-complex form of curcumin, oral administration | Randomized, double-blind, controlled trial with RA patients | - |
| Shinde et al., 2020 [41] | Pure drug, intra-articular administration | Antigen-induced monoarthritis model in rats | Knee histopathological studies |
| Kang et al., 2020 [42] | Pure drug, intra-articular administration | Monoidoacetic acid (MIA)-induced knee osteoarthritis—mouse | Hepatic markers |
| Ahmadi et al., 2020 [43] | C3-complex form of curcumin, oral administration | Randomized, double-blind, placebo-controlled clinical trial with patients clinically diagnosed with AS | - |
| Sana et al., 2021 [23] | Pure drug, topical administration | CFA-induced arthritis mice model | In vivo Draize skin irritation and histopathological studies |
| Wang et al., 2021 [20] | BDMC, oral administration | Potassium oxonate induced Gouty rat model | - |
| Song et al., 2022 [44] | Pure drug, intraperitoneal administration | Collagen-induced arthritis rat model | - |
| Zhang et al., 2022 [45] | Pure drug, topical administration | Collagen-induced arthritis mouse model | Skin irritation test in mice |
| Hamdalla et al., 2022 [46] | Pure drug, intra-articular administration | Monoidoacetic acid (MIA)-induced knee osteoarthritis | - |
| Lin et al., 2023 [47] | Pure drug, intra-articular administration | Post-traumatic osteoarthritis mouse model | Hemolysis assay |
| Wang et al., 2023 [21] | Pure drug, intra-articular administration | Post-traumatic osteoarthritis mouse model | - |
| Khashan et al., 2023 [48] | Turmeric rhizomes in dry form, oral administration | CFA-induced arthritis rat model | - |
| Hu et al., 2023 [49] | Pure drug, intra-articular administration | Collagen-induced arthritis rat model | - |
| Okpalaku et al., 2023 [50] | Turmeric oil, topical administration | Formalin-induced arthritis rat model | Skin irritation test in mice |
| Wang et al., 2024 [51] | Pure drug, intravenous administration | Collagen-induced arthritis (CIA) rat model | Histopathological evaluation |
| Javed et al., 2024 [52] | Pure drug, oral administration | Monosodium urate-induced Gouty mouse model | - |
| Lustberg et al., 2024 [53] | Curcumin C3-complex, oral administration | Randomized placebo-controlled, double-blind clinical trial—Aromatase inhibitor-induced arthralgia | Gastrointestinal adverse effects were commonly reported |
| Sun et al., 2024 [54] | Pure drug, intravenous administration | Collagen-induced arthritis mouse model | Histopathological evaluation |
| Xie et al., 2024 [55] | Fresh Curcuma longa roots, intra-articular administration | Post-traumatic osteoarthritis Chick model | - |
| Xu et al., 2024 [56] | Pure drug, intra-articular administration | Post-traumatic osteoarthritis rat model | - |
| Zhang et al., 2024 [57] | Pure curcumin, intravenous administration | Collagen-induced arthritis mouse model | - |
| Pérez-Expósito et al., 2024 [58] | Pure drug, intra-articular administration | Post-traumatic osteoarthritis rabbit model | - |
| Azeez et al., 2024 [59] | Pure drug, oral administration | CompleteFreund’s adjuvant and collagen-induced arthritis rabbit model | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, B.Y.S.; Chong, S.I.; Souza, N.M.P.; Lazo, R.E.L.; Pontarolo, R.; Rego, F.G.d.M.; Ferreira, L.M.; Sari, M.H.M. Nanocarriers Containing Curcumin and Derivatives for Arthritis Treatment: Mapping the Evidence in a Scoping Review. Pharmaceutics 2025, 17, 1022. https://doi.org/10.3390/pharmaceutics17081022
Sato BYS, Chong SI, Souza NMP, Lazo REL, Pontarolo R, Rego FGdM, Ferreira LM, Sari MHM. Nanocarriers Containing Curcumin and Derivatives for Arthritis Treatment: Mapping the Evidence in a Scoping Review. Pharmaceutics. 2025; 17(8):1022. https://doi.org/10.3390/pharmaceutics17081022
Chicago/Turabian StyleSato, Beatriz Yurie Sugisawa, Susan Iida Chong, Nathalia Marçallo Peixoto Souza, Raul Edison Luna Lazo, Roberto Pontarolo, Fabiane Gomes de Moraes Rego, Luana Mota Ferreira, and Marcel Henrique Marcondes Sari. 2025. "Nanocarriers Containing Curcumin and Derivatives for Arthritis Treatment: Mapping the Evidence in a Scoping Review" Pharmaceutics 17, no. 8: 1022. https://doi.org/10.3390/pharmaceutics17081022
APA StyleSato, B. Y. S., Chong, S. I., Souza, N. M. P., Lazo, R. E. L., Pontarolo, R., Rego, F. G. d. M., Ferreira, L. M., & Sari, M. H. M. (2025). Nanocarriers Containing Curcumin and Derivatives for Arthritis Treatment: Mapping the Evidence in a Scoping Review. Pharmaceutics, 17(8), 1022. https://doi.org/10.3390/pharmaceutics17081022










