Inhibition of Human Coronavirus 229E by Lactoferrin-Derived Peptidomimetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.1.1. Synthesis of N-Methyl Peptides
2.1.2. Synthesis of Peptoids
2.1.3. Purification and Characterization
2.2. Biological Procedures
2.2.1. Cells
2.2.2. Virus
2.2.3. Virus Titration
2.2.4. MTT (3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2htetrazolium Bromide) Assay of Cell Viability
2.2.5. Antiviral Assay
2.3. Direct Binding Assay
2.3.1. Microscale Thermophoresis (MST)
2.3.2. Surface Plasmon Resonance (SPR)
2.3.3. Nanoscale Differential Scanning Fluorometry
2.4. Computational Studies
3. Results
3.1. Antiviral Assay
3.2. Biophysical Characterization of Spike-Binding Peptidomimetics
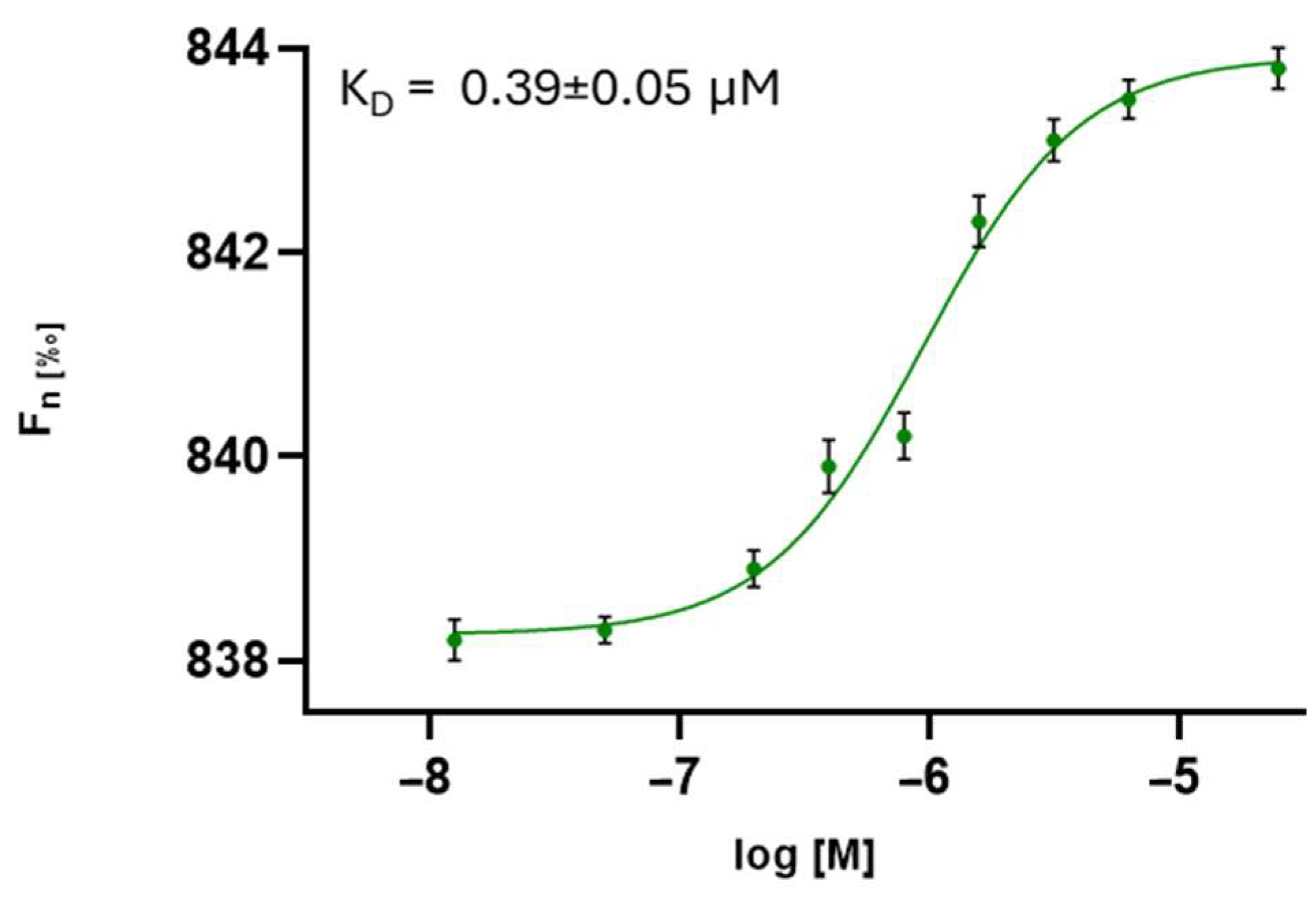
3.2.1. Peptidomimetic–Spike Interactions Determined by MST
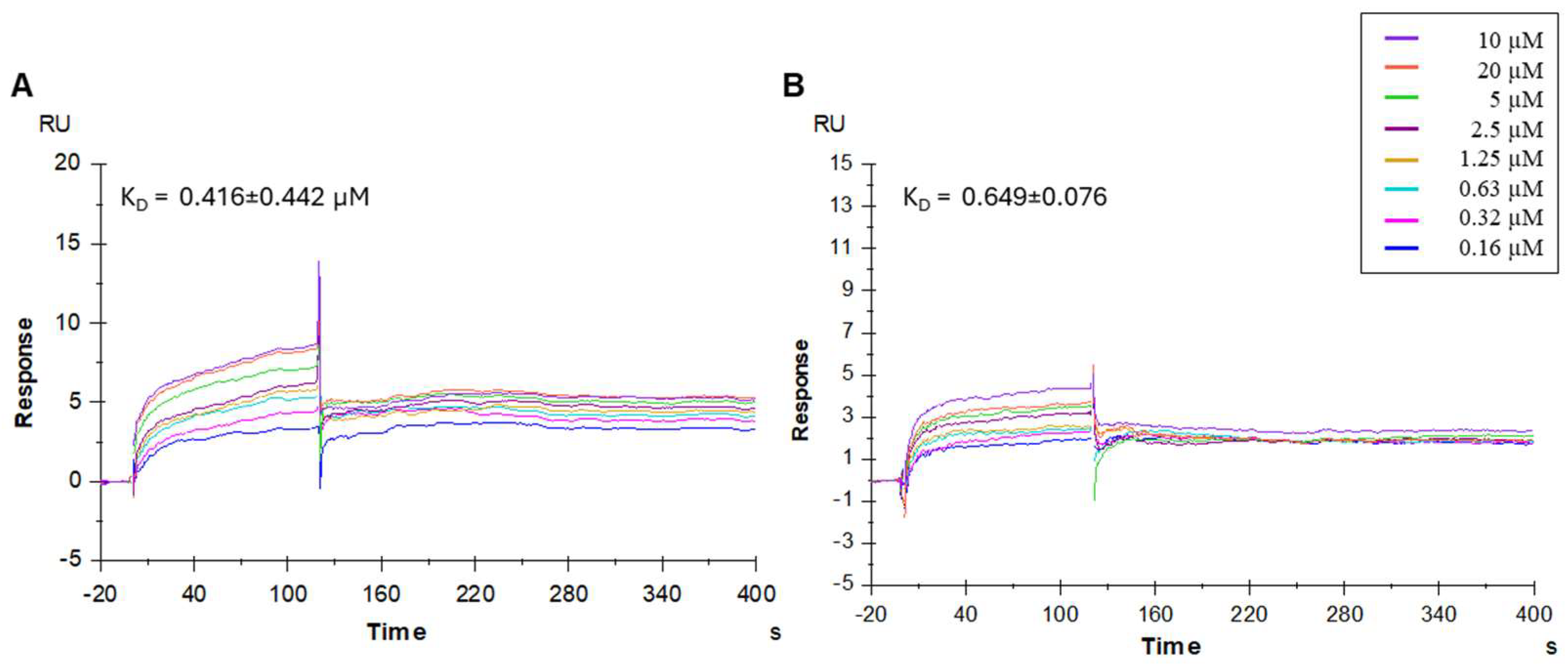
3.2.2. Binding Affinity of Compounds to Spike Protein Determined by SPR
3.2.3. NanoDSF of Spike Protein–Peptidomimetics
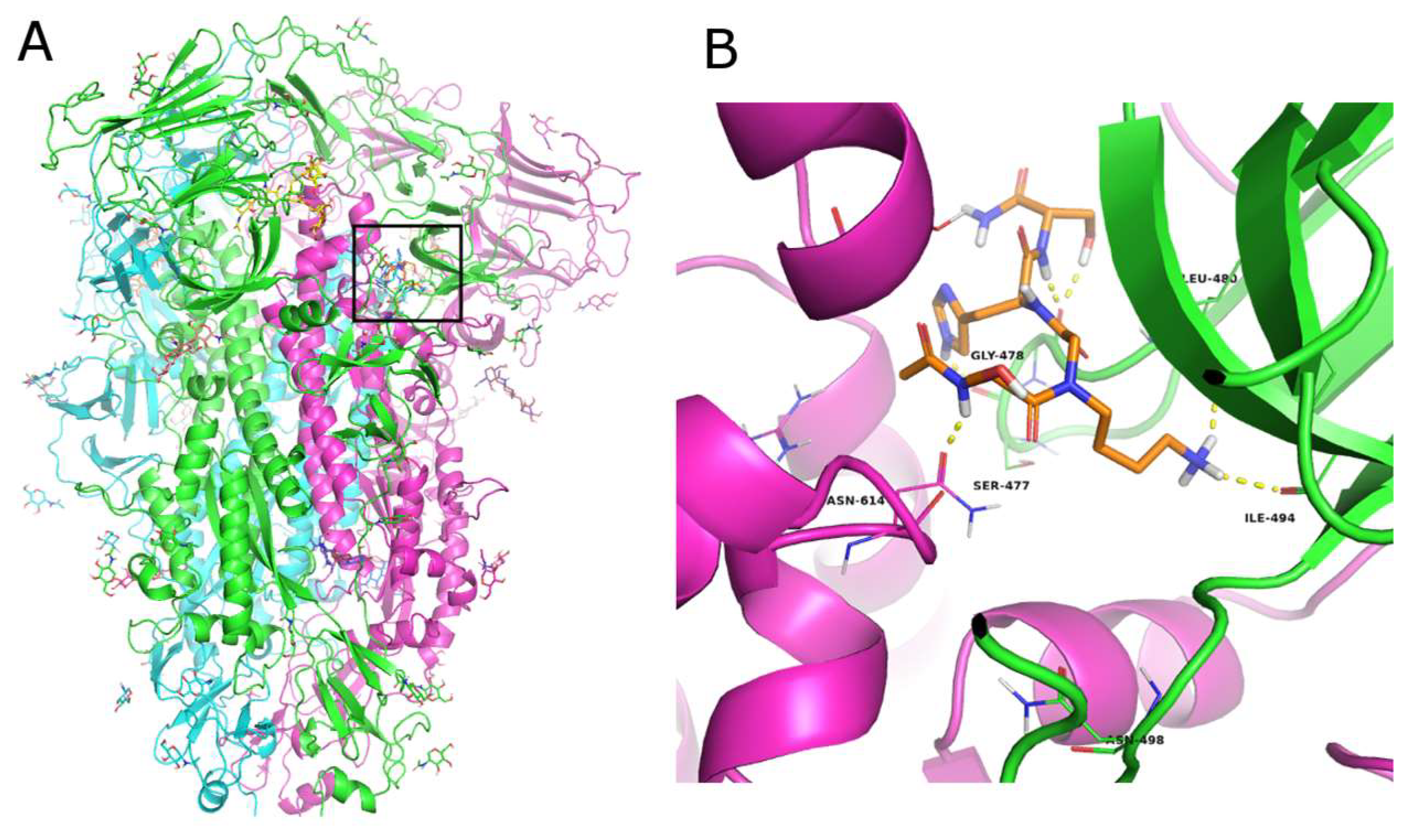
3.3. Computational Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirota, S.B.; Doxey, M.C.; Dominguez, R.-M.V.; Bender, R.G.; Vongpradith, A.; Albertson, S.B.; Novotney, A.; Burkart, K.; Carter, A.; Abdi, P.; et al. Global, regional, and national burden of upper respiratory infections and otitis media, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2025, 25, 36–51. [Google Scholar] [CrossRef]
- Johnston, S. Impact of viruses on airway diseases. Eur. Respir. Rev. 2005, 14, 57–61. [Google Scholar] [CrossRef]
- Eccles, R. Common cold. Front. Allergy 2023, 4, 1224988. [Google Scholar] [CrossRef]
- Smith, A.M. Host-pathogen kinetics during influenza infection and coinfection: Insights from predictive modeling. Immunol. Rev. 2018, 285, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Liposomal lactoferrin exerts antiviral activity against HCoV-229E and SARS-CoV-2 pseudoviruses in vitro. Viruses 2023, 15, 972. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, Y.; Michelow, I.C.; Choe, Y.J. Global seasonality of human coronaviruses: A systematic review. Open Forum Infect. Dis. 2020, 7, ofaa443. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Bosis, S.; Esposito, S. Effects of coronavirus infections in children. Emerg. Infect. Dis. 2010, 16, 183–188. [Google Scholar] [CrossRef]
- Lu, R.; Yu, X.; Wang, W.; Duan, X.; Zhang, L.; Zhou, W.; Xu, J.; Xu, L.; Hu, Q.; Lu, J.; et al. Characterization of human coronavirus etiology in Chinese adults with acute upper respiratory tract infection by real-time RT-PCR assays. PLoS ONE 2012, 7, e38638. [Google Scholar] [CrossRef]
- Marcone, D.N.; Ricarte, C.; Videla, C.; Ekstrom, J.; Carballal, G.; Vidaurreta, S.; Echavarría, M. Rinovirus. Frecuencia en niños con infección respiratoria aguda, no internados [Rhinoviruses. Frequency in nonhospitalized children with acute respiratory infection]. Medicina (B Aires) 2012, 72, 28–32. (In Spanish) [Google Scholar]
- Ottogalli, M.E.; Rodríguez, P.E.; Frutos, M.C.; Moreno, L.B.; Ghietto, L.M.; Cuffini, C.G.; Cámara, J.A.; Adamo, M.P.; Valinotto, L.E.; Cámara, A. Circulation of human coronaviruses OC43 and 229E in Córdoba, Argentina. Arch. Virol. 2021, 166, 929–933. [Google Scholar] [CrossRef]
- Dowell, S.F. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg. Infect. Dis. 2001, 7, 369–374. [Google Scholar] [CrossRef]
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encycl. Virol. 2021, 2, 428–440. [Google Scholar] [CrossRef]
- Choi, E.S.; Han, S.; Son, J.W.; Song, G.B.; Ha, S.D. Inactivation methods for human coronavirus 229E on various food-contact surfaces and foods. Food Control. 2022, 142, 109271. [Google Scholar] [CrossRef] [PubMed]
- Superti, F. Lactoferrin from bovine milk: A protective companion for life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Superti, F. Antiviral activity of lactoferrin. In Recent Developments in Antiviral Research; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2001; Volume 1, pp. 193–203. [Google Scholar]
- Sano, H.; Nagai, K.; Tsutsumi, H.; Kuroki, Y. Lactoferrin and surfactant protein A exhibit distinct binding specificity to F protein and differently modulate respiratory syncytial virus infection. Eur. J. Immunol. 2003, 33, 2894–2902. [Google Scholar] [CrossRef]
- Di Biase, A.M.; Pietrantoni, A.; Tinari, A.; Siciliano, R.; Valenti, P.; Antonini, G.; Seganti, L.; Superti, F. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 2003, 69, 495–502. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Di Biase, A.M.; Tinari, A.; Marchetti, M.; Valenti, P.; Seganti, L.; Superti, F. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 2003, 47, 2688–2691. [Google Scholar] [CrossRef]
- Seganti, L.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral activity of lactoferrin towards naked viruses. Biometals 2004, 17, 295–299. [Google Scholar] [CrossRef]
- Superti, F.; Berlutti, F.; Paesano, R.; Valenti, P. Structure and activity of lactoferrin-a multi functional protective agent for human health. In Iron Metabolism and Disease; Fuchs, H., Ed.; Research Signpost: Trivandrum, India, 2008; Volume 8, pp. 1–32. [Google Scholar]
- Yamamoto, H.; Ura, Y.; Tanemura, M.; Koyama, A.; Takano, S.; Uematsu, J.; Kawano, M.; Tsurudome, M.; O’Brien, M.; Komada, H. Inhibitory effect of bovine lactoferrin on human parainfluenza virus type 2 infection. J. Health Sci. 2010, 54, 613–617. [Google Scholar] [CrossRef]
- Superti, F.; Agamennone, M.; Pietrantoni, A.; Ammendolia, M.G. Bovine lactoferrin prevents influenza A virus infection by interfering with the fusogenic function of viral hemagglutinin. Viruses 2019, 11, 51. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Sugita, C.; Yoshida, H.; Abe, F.; Sonoda, T.; Kurokawa, M. Effects of lactoferrin on infectious diseases in Japanese summer: A randomized, double-blinded, placebo-controlled trial. J. Microbiol. Immunol. Infect. 2021, 54, 566–574. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal lactoferrin as potential preventative and cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Navarro, R.; Paredes, J.L.; Tucto, L.; Medina, C.; Angles-Yanqui, E.; Nario, J.C.; Ruiz-Cabrejos, J.; Quintana, J.L.; Turpo-Espinoza, K.; Mejia-Cordero, F.; et al. Bovine lactoferrin for the prevention of COVID-19 infection in health care personnel: A double-blinded randomized clinical trial (LF-COVID). Biometals 2023, 36, 463–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef]
- Superti, F.; Agamennone, M.; Ammendolia, M.G.; Pietrantoni, A.; Lannutti, F. Lactoferrin Derived Peptides for Use as Broad-Spectrum Inhibitors of Influenza Virus Infection. WO2013072946, 23 May 2013. [Google Scholar]
- Scala, M.C.; Sala, M.; Pietrantoni, A.; Spensiero, A.; Di Micco, S.; Agamennone, M.; Bertamino, A.; Novellino, E.; Bifulco, G.; Gomez-Monterrey, I.M.; et al. Lactoferrin-derived peptides active towards influenza: Identification of three potent tetrapeptide inhibitors. Sci. Rep. 2017, 7, 10593. [Google Scholar] [CrossRef]
- Scala, M.C.; Marchetti, M.; Superti, F.; Agamennone, M.; Campiglia, P.; Sala, M. Rational design of novel peptidomimetics against influenza A virus: Biological and computational studies. Int. J. Mol. Sci. 2023, 24, 14268. [Google Scholar] [CrossRef]
- Mariano, A.; Bigioni, I.; Marchetti, M.; Scotto d’Abusco, A.; Superti, F. Repositioned natural compounds and nanoformulations: A promising combination to counteract cell damage and inflammation in respiratory viral infections. Molecules 2023, 28, 4045. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H.A. Simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Superti, F.; Marchetti, M.; Rapetti Mogol, G. Inactivation of influenza virus by a blend of essential plant oil vapour and aerosol. J.Biol. Regul. Homeost. Agents. 2021, 35, 1667–1675. [Google Scholar] [CrossRef]
- Scala, M.C.; Agamennone, M.; Pietrantoni, A.; Di Sarno, V.; Bertamino, A.; Superti, F.; Campiglia, P.; Sala, M. Discovery of a novel tetrapeptide against influenza a virus: Rational design, synthesis, bioactivity evaluation and computational studies. Pharmaceuticals 2021, 14, 959. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Schrödinger Release 2025-1: Maestro, Epik, Glide, MacroModel, Prime, Protein Preparation Workflow, LigPrep, SiteMap; Schrödinger, LLC: New York, NY, USA, 2025. [Google Scholar]
- Mohamadi, F.; Richard, N.G.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. MacroModel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Halgren, T.A. New Method for Fast and Accurate Binding-Site Identification and Analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Halgren, T.A. Identifying and Characterizing Binding Sites and Assessing Druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Tubert-Brohman, I.; Sherman, W.; Repasky, M.; Beuming, T. Improved Docking of Polypeptides with Glide. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Holdgate, G.; Embrey, K.; Milbradt, A.; Davies, G. Biophysical methods in early drug discovery. ADMET DMPK 2019, 7, 222–241. [Google Scholar] [CrossRef]
- Navratilova, I.; Hopkins, A.L. Fragment screening by surface plasmon resonance. ACS Med. Chem. Lett. 2010, 1, 44–48. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tomlinson, A.C.; Wong, A.H.; Zhou, D.; Desforges, M.; Talbot, P.J.; Benlekbir, S.; Rubinstein, J.L.; Rini, J.M. The Human Coronavirus HCoV-229E S-Protein Structure and Receptor Binding. eLife 2019, 8, e51230. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shi, Y.; Ding, W.; Niu, T.; Sun, L.; Tan, Y.; Chen, Y.; Shi, J.; Xiong, Q.; Huang, X.; et al. Cryo-EM Analysis of the HCoV-229E Spike Glycoprotein Reveals Dynamic Prefusion Conformational Changes. Nat. Commun. 2021, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Agamennone, M.; Superti, F. Broad-Spectrum Activity of Small Molecules Acting against Influenza a Virus: Biological and Computational Studies. Pharmaceuticals 2022, 15, 301. [Google Scholar] [CrossRef]
- Hamre, D.; Procknow, J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef]
- Lachance, C.; Arbour, N.; Cashman, N.R.; Talbot, P.J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J. Virol. 1998, 72, 6511–6519. [Google Scholar] [CrossRef]
- Shirato, K.; Imada, Y.; Kawase, M.; Nakagaki, K.; Matsuyama, S.; Taguchi, F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014, 86, 2146–2153. [Google Scholar] [CrossRef]
- Xia, S.; Xu, W.; Wang, Q.; Wang, C.; Hua, C.; Li, W.; Lu, L.; Jiang, S. Peptide-Based Membrane Fusion Inhibitors Targeting HCoV-229E Spike Protein HR1 and HR2 Domains. Int. J. Mol. Sci. 2018, 19, 487. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; di Patti, M.C.B.; Iacovelli, F.; Conte, M.P.; Ianiro, G.; Romeo, A.; Campione, E.; Bianchi, L.; Valenti, P.; et al. Lactoferrin Binding to SARS-CoV-2 Spike Glycoprotein Blocks Pseudoviral Entry and Relieves Iron Protein Dysregulation in Several In Vitro Models. Pharmaceutics 2022, 14, 2111. [Google Scholar] [CrossRef]
- Rajeswaran, W.G.; Hocart, S.J.; Murphy, W.A.; Taylor, J.E.; Coy, D.H. Highly potent and subtype selective ligands derived by N-methyl scan of a somatostatin antagonist. J. Med. Chem. 2001, 44, 1305–1311. [Google Scholar] [CrossRef]
- Dechantsreiter, M.A.; Planker, E.; Mathä, B.; Lohof, E.; Hölzemann, G.; Jonczyk, A.; Goodman, S.L.; Kessler, H. N-methylated cyclic RGD peptides as highly active and selective αvβ3 integrin antagonists. J. Med. Chem. 1999, 42, 3033–3040. [Google Scholar] [CrossRef]
- Yeager, C.L.; Ashmun, R.A.; Williams, R.K.; Cardellichio, C.B.; Shapiro, L.H.; Look, A.T.; Holmes, K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992, 357, 420–422. [Google Scholar] [CrossRef]
- Wang, Q.C.; Wang, L.; Zhang, Y.; Zhang, X.; Zhang, L.; Shang, W.; Bai, F. Probing the Allosteric Inhibition Mechanism of a Spike Protein Using Molecular Dynamics Simulations and Active Compound Identifications. J. Med. Chem. 2022, 65, 2827–2835. [Google Scholar] [CrossRef]
- Gupta, G.; Verkhivker, G. Exploring Binding Pockets in the Conformational States of the SARS-CoV-2 Spike Trimers for the Screening of Allosteric Inhibitors Using Molecular Simulations and Ensemble-Based Ligand Docking. Int. J. Mol. Sci. 2024, 25, 4955. [Google Scholar] [CrossRef]

| Compound | Sequence | Influenza A H1N1 Virus a,b | 229E Coronavirus | ||
|---|---|---|---|---|---|
| EC50 c (nM) | SI d | EC50 c (nM) | SI d | ||
| 1 | SLDC | 4.6 ± 0.05 × 10−3 | >5.4 × 106 | 50 ± 0.4 | >5 × 102 |
| 2 | SKHS | 4.8 ± 0.12 × 10−5 | >5.2 × 108 | 500 ± 2 | >5 × 101 |
| 3 | SK(N-Me)HS | 0.142 ± 0.002 | >1.78 × 103 | 0.031 ± 0.007 | >8 × 105 |
| 4 | S(N-Me)LDC | 0.085 ± 0.0004 | >2.9 × 105 | -- | -- |
| 5 | SNKHS e | 0.470 ± 0.002 | >5.3 × 104 | 0.0177 ± 0.002 | >14.1 × 105 |
| 6 | SKHNhS e | 0.500 ± 0.004 | >5 × 105 | -- | -- |
| Compound | Sequence | Adsorption | Infection Cycle |
|---|---|---|---|
| 3 | SK(N-Me)HS | EC25 a (nM) 4.3 ± 0.6 × 10−2 | EC50 b (nM) 4.3 ± 0.6 × 10−2 |
| 5 | SNKHS | EC50 b (nM) 2.5 ± 0.4 × 10−2 | EC50 b (nM) 2.0 ± 0.2 × 10−2 |
| Compound | Sequence a | MST KD (µM) | SPR KD (µM) | ∆Tm (°C) |
|---|---|---|---|---|
| 1 | SLDC | 4.92 ± 0.7 | >50 | −0.9 |
| 2 | SKHS | 5.12 ± 0.6 | 41.44 ± 0.87 | −0.7 |
| 3 | SK(N-Me)HS | 0.39 ± 0.05 | 0.416 ± 0.442 | 0.3 |
| 4 | S(N-Me)LDC | 2.6 ± 1.3 | 1.153 ± 0.182 | 0.1 |
| 5 | SNKHS | 3.3 ± 1.7 | 0.649 ± 0.076 | 0.9 |
| 6 | SKHNhS | 11 ± 0.8 | 1.507 ± 0.465 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, M.C.; Marchetti, M.; Landi, M.; Fantacuzzi, M.; Superti, F.; Agamennone, M.; Campiglia, P.; Sala, M. Inhibition of Human Coronavirus 229E by Lactoferrin-Derived Peptidomimetics. Pharmaceutics 2025, 17, 1006. https://doi.org/10.3390/pharmaceutics17081006
Scala MC, Marchetti M, Landi M, Fantacuzzi M, Superti F, Agamennone M, Campiglia P, Sala M. Inhibition of Human Coronavirus 229E by Lactoferrin-Derived Peptidomimetics. Pharmaceutics. 2025; 17(8):1006. https://doi.org/10.3390/pharmaceutics17081006
Chicago/Turabian StyleScala, Maria Carmina, Magda Marchetti, Martina Landi, Marialuigia Fantacuzzi, Fabiana Superti, Mariangela Agamennone, Pietro Campiglia, and Marina Sala. 2025. "Inhibition of Human Coronavirus 229E by Lactoferrin-Derived Peptidomimetics" Pharmaceutics 17, no. 8: 1006. https://doi.org/10.3390/pharmaceutics17081006
APA StyleScala, M. C., Marchetti, M., Landi, M., Fantacuzzi, M., Superti, F., Agamennone, M., Campiglia, P., & Sala, M. (2025). Inhibition of Human Coronavirus 229E by Lactoferrin-Derived Peptidomimetics. Pharmaceutics, 17(8), 1006. https://doi.org/10.3390/pharmaceutics17081006








