Extemporaneous Compounding, Pharmacy Preparations and Related Product Care in the Netherlands
Abstract
1. Introduction and Aim
2. Rationale of Pharmacy Preparations
2.1. What to Dispense
2.2. Types of Pharmacy Preparations
2.3. Justification of Pharmacy Preparations
3. The Dutch Healthcare System and Pharmacy Preparations
3.1. Demographic Data
3.2. Professional Organisations and Support
3.3. Community Pharmacy
3.4. Hospital Pharmacy
3.5. Pharmacy Technicians and Pharmacy Assistants
4. The Dutch Regulatory and Legal Framework for Pharmacy Preparations
4.1. The Position and Qualification of the Dutch Pharmacist
4.2. Extemporaneous Pharmacy Preparations
4.2.1. Medicines Prepared in a Pharmacy for Own Patients
- The preparation takes place in the pharmacy, based on a medical prescription for an individual patient or as stock for patients yet to be determined by that pharmacy;
- The preparation complies with the Ph. Eur.;
- The preparation is intended for dispensing on a small scale.
- Dispensing to up to approximately 50 unique patients per month for long-term use of the medication;
- Dispensing to approximately 150 patients per month for short-term use.
4.2.2. Pharmacy-to-Pharmacy Delivery and Outsourcing
4.3. Enforcement
4.4. International Context
5. Quality Assurance of Pharmacy Preparations
5.1. Quality by Design (QbD)
5.2. Standardisation of Formulations
5.3. Product File and Production Batch Record
5.4. Preparation for Administration and Reconstitution
5.5. Raw Materials
6. Pharmacy Education in the Netherlands
6.1. Universities and Pharmacy (-Related) Programmes
6.2. Alignment with the Professional Field
6.3. Bachelor’s Pharmacy Programme
6.4. Master’s Pharmacy Programme
6.5. Post-Master’s Specialisation Programmes and Post-Academic Education
6.5.1. Community Pharmacy Specialist
6.5.2. Hospital Pharmacy Specialist
6.5.3. Post-Academic Education
6.6. Textbook
7. Special Topics Linked to Pharmacy Preparations
7.1. Optimising Individual Treatment
7.1.1. Paediatric Dosage Forms
7.1.2. Swallowing Difficulties and Feeding Tubes
7.1.3. Hospital-at-Home Care
7.2. Specialist Product Groups
7.2.1. Reconstitution of Oncolytic Medicinal Products and Biologicals
7.2.2. Total Parenteral Nutrition (TPN)
7.2.3. Advanced Therapy Medicinal Products (ATMPs)
7.2.4. Radiopharmaceuticals and Optical Tracers
7.2.5. Clinical Trial Medication
- Re-labelling and encapsulation of licensed medicines for clinical trial use;
- Small-scale production of non-sterile medicines such as capsules, tablets, and dermal preparations;
- Sterile preparations, including reconstitution of trial medication produced by the pharmaceutical industry and small-scale in-house production of sterile products (e.g., biologicals, ATMPs, radiopharmaceuticals, other imaging agents, conjugated photodynamic therapy agents, and small-molecule products).
7.3. Technological Innovations
7.3.1. Robotisation in Reconstitution
7.3.2. Patient-Centric Solid Oral Dosage Forms
8. Conclusions, Perspectives and Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning | English Translation of Dutch (If Applicable) |
| AI | Artificial intelligence | |
| API | Active pharmaceutical ingredient | |
| ATMP | Advanced therapy medicinal product | |
| BP | British Pharmacopoeia | |
| BSc | Bachelor of Science | |
| CBG | College ter Beoordeling van Geneesmiddelen | Medicines Evaluation Board (MEB) |
| CEP | Certificate of suitability to the monographs of the European Pharmacopoeia | |
| CJI | Charlotte Jacobs Institute | |
| DAC/NRF | Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium | German Drug Codex/New German Formulary |
| EAHP | European Association of Hospital Pharmacists | |
| ECTS | European Credit Transfer System | |
| EDQM | European Directorate for the Quality of Medicines and HealthCare | |
| EMA | European Medicines Agency | |
| EU | European Union | |
| FDA | US Food and Drug Administration | |
| FNA | Formularium der Nederlandse Apothekers | Formulary of Dutch Pharmacists |
| GCP | Good Clinical Practice | |
| GMO | Genetically modified organism | |
| GMP | Good Manufacturing Practice | - |
| GMP-Z | GMP-Ziekenhuisfarmacie | GMP-Hospital pharmacy |
| GW | Geneesmiddelenwet | Medicines Act |
| ICH | International Conference on Harmonisation | |
| IGJ | Inspectie Gezondheidszorg en Jeugd | Health and Youth Care Inspectorate |
| IMP | Investigational medicinal product | |
| KNMP | Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie | Royal Dutch Pharmacists Association |
| LNA | Laboratorium der Nederlandse Apothekers | Laboratory of Dutch Pharmacists |
| LU | Universiteit Leiden (UL) | Leiden University |
| MAA | Marketing Authorisation Application | |
| MAH | Marketing Authorisation Holder | |
| MIA | Manufacturing and Importation Authorisation | |
| MSc | Master of Science | |
| NVZA | Nederlandse Vereniging voor Ziekenhuisapothekers | Dutch Association of Hospital Pharmacists |
| OPAT | Outpatient parenteral antimicrobial therapy | |
| PET | Positron emission tomography | |
| PFSS | Prefilled sterilised syringe | |
| Ph. Eur. | European Pharmacopoeia | - |
| PIC/S | Pharmaceutical Inspection Convention/Pharmaceutical Inspection Co-operation Scheme | |
| PQS | Pharmaceutical quality system | |
| QA | Quality assurance | |
| QbD | Quality by design | |
| QP | Qualified person | |
| RiFaS | Risico instrument Farmaceutische Stoffen | Risk Instrument for Pharmaceutical Substances |
| RTA | Ready to administer | |
| RTU | Ready to use | |
| SIG | Special Interest Group | |
| SmPC | Summary of Product Characteristics | |
| SPECT | Single-photon emission computed tomography | |
| TPN | Total parenteral nutrition | |
| US(A) | United States (of America) | |
| USP | United States Pharmacopeia | |
| UG | Rijksuniversiteit Groningen (RUG) | University of Groningen |
| UU | Universiteit Utrecht (UU) | Utrecht University |
| VTGM | Voor toediening gereedmaken | Reconstitution (literally: Preparation for administration) |
| VU | Vrije Universiteit Amsterdam | |
| Wet BIG | Wet op de Beroepen in de Individuele Gezondheidszorg | Professions in Individual Health Care Act |
| WGBO | Wet op de Geneeskundige Behandelingsovereenkomst | Medical Treatment Contracts Act |
References
- Schalekamp, T.; Haisma, H.J. Domeinspecifiek Referentiekader & Raamplan Farmacie 2016. [2016 Pharmacist Competency Framework & Domain-Specific Frame of Reference for the Netherlands]; KNMP Medicijn Media: Den Haag, The Netherlands, 2016; Available online: https://www.knmp.nl/sites/default/files/2021-11/Referentiekader%20en%20Raamplan%202016%20def.pdf (accessed on 28 October 2024).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/homepage (accessed on 12 December 2024).
- College ter Beoordeling van Geneesmiddelen (CBG) (Dutch Medicines Evaluation Board). Available online: https://www.cbg-meb.nl (accessed on 12 December 2024).
- Stichting Farmaceutische Kengetallen (SFK) (Foundation for Pharmaceutical Statistics). Data en Feiten 2021. Available online: https://www.sfk.nl/sites/sfk-nl/files/2025-01/Data_en_feiten_2021.pdf (accessed on 26 May 2025).
- Stichting Farmaceutische Kengetallen (SFK). Aandeel generiek verder toegenomen. Pharm. Weekbl. 2024, 159, 7. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Pharmaceutical Preparations. 07/2024: 2619. In European Pharmacopoeia, 11.5th ed.; EDQM Council of Europe: Strasbourg, France, 2023; Available online: https://pheur-online.edqm.eu/content/2619/en/current/ (accessed on 11 December 2024).
- European Association of Hospital Pharmacists (EAHP). EAHP Position Paper on Pharmacy Preparations and Compounding. Making a Difference in Medication by Delivering Tailor-Made Medicines for the Benefit of Patients. October 2020. Available online: https://eahp.eu/wp-content/uploads/2024/03/eahp_position_paper_on_pharmacy_preparations_and_compounding_october_2020.pdf (accessed on 12 December 2024).
- United States Food and Drug Administration (FDA). Human Drug Compounding. Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/human-drug-compounding (accessed on 28 October 2024).
- Scheepers, H.P.A.; Langedijk, J.; Neerup Handlos, V.; Walser, S.; Schutjens, M.D.B.; Neef, C. Legislation on the preparation of medicinal products in European pharmacies and the Council of Europe Resolution. Eur. J. Hosp. Pharm. 2017, 24, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Resolution CM/Res(2016)1 on Quality and Safety Assurance Requirements for Medicinal Products Prepared in Pharmacies for the Special Needs of Patients. Available online: https://search.coe.int/cm/Pages/result_details.aspx?ObjectID=090000168065c132 (accessed on 12 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). KNMP Kennisbank. LNA-Mededeling Productzorg (January 2020). Available online: https://kennisbank.knmp.nl (upon subscription, accessed on 17 June 2025).
- European Commission. The Rules Governing Medicinal Products in the European Union. EudraLex. Volume 4. Good Manufacturing Practice (GMP) Guidelines. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 11 December 2024).
- U.S. Food and Drugs Administration (FDA). Current Good Manufacturing Practice (CGMP) Regulations. Available online: https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations (accessed on 12 December 2024).
- World Health Organization (WHO). Health Products Policy and Standards. Good Manufacturing Practices. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/gmp (accessed on 12 December 2024).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Quality Guidelines. Available online: https://www.ich.org/page/quality-guidelines (accessed on 11 December 2024).
- Pharmaceutical Inspection Convention. PIC/S Guide to Good Practices for the Preparation of Medicinal Products in Healthcare Establishments. PE 010-4. Available online: https://picscheme.org/docview/3443 (accessed on 11 December 2024).
- Koster, A.S.; Mantel-Teeuwisse, A.K.; Woerdenbag, H.J.; Mulder, W.M.C.; Wilffert, B.; Schalekamp, T.; Buurma, H.; Wilting, I.; Westein, M.P.D. Alignment of CanMEDS-Based undergraduate and postgraduate Pharmacy curricula in The Netherlands. Pharmacy 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Professionale Standaard Farmaceutische Zorg. Available online: https://www.knmp.nl/richtlijnen/professionele-standaard-farmaceutische-zorg (accessed on 29 November 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Charter Professionalism of the Pharmacist. Foundation for Acting Professionally and Ethically. [Translation of ‘Handvest van de Apotheker. Grondslag voor Professioneel en Ethisch Handelen’ (2017)]; KNMP: Den Haag, The Netherlands, 2018; Available online: https://www.knmp.nl/sites/default/files/2021-12/Charter%20Professionalism%20of%20the%20Pharmacist.pdf (accessed on 29 November 2024).
- Nederlandse Vereniging voor Ziekenhuisapothekers (NVZA) (Dutch Association of Hospital Pharmacists). NVZA. Available online: https://nvza.nl (accessed on 16 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). KNMP Kennisbank. Available online: https://kennisbank.knmp.nl/ (upon subscription, accessed on 23 January 2025).
- Nederlandse Vereniging voor Ziekenhuisapothekers (NVZA) (Dutch Association of Hospital Pharmacists). GMP-Z Richtlijn; NVZA: Utrecht, The Netherlands. Available online: https://nvza.nl/voor-professionals/gmp/ (accessed on 29 October 2024).
- Kennisinstituut V&VN. Voor Toediening Gereed Maken (VTGM); V&VN: Utrecht, The Netherlands. Available online: https://kennisplatform.venvn.nl/onderwerp/vtgm/ (accessed on 23 January 2025).
- Council of Europe. Resolution CM/Res(2016)2 on Good Reconstitution Practices in Health Care Establishments for Medicinal Products for Parenteral Use. Available online: https://www.edqm.eu/en/d/162941 (accessed on 28 November 2024).
- Le Brun, P.; Crauste-Manciet, S.; Krämer, I.; Smith, J.; Woerdenbag, H. Chapter 1. Introduction. In Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Le Brun, P., Crauste-Manciet, S., Krämer, I., Smith, J., Woerdenbag, H., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 1–5. [Google Scholar]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical compounding: A history, regulatory overview, and systematic review of compounding errors. J. Med. Toxicol. 2021, 17, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.R.; Steadman, K.J. Extemporaneously compounded medicines. Aust. Prescr. 2017, 40, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J.; Boos, J. Pediatric and geriatric drug delivery. Exp. Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Bouwman-Boer, Y. Pharmacy preparations: Back in the limelight? Pharmacists make up your mind! Int. J. Pharm. 2016, 514, 11–14. [Google Scholar] [CrossRef]
- Ofei, K. The role of compounding pharmacists during drug shortages. Int. J. Pharm. Compd. 2022, 26, 298–301. [Google Scholar] [PubMed]
- Centraal Bureau voor de Statistiek (CBS) (Statistics Netherlands). Available online: https://opendata.cbs.nl/#/CBS/nl/dataset/03759ned/table?dl=39E0B (accessed on 11 June 2025).
- Statista. Netherlands: Median Age of the Population 1950 to 2100. Available online: https://www.statista.com/statistics/276734/median-age-of-the-netherlands-population/ (accessed on 17 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Producten en Diensten. Available online: www.knmp.nl/over-de-knmp/producten-en-diensten/ (accessed on 16 December 2024).
- Risico Instrument Farmaceutische Stoffen (RiFaS). Available online: https://www.rifas.nl (accessed on 16 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Richtlijn Bereiden. (Directive Pharmacy Preparations); KNMP: Den Haag, The Netherlands, 2016; Available online: https://www.knmp.nl/richtlijnen/bereiden (accessed on 29 October 2024).
- European Association of Hospital Pharmacists (EAHP). Available online: https://eahp.eu (accessed on 16 December 2024).
- Inspectie Gezondheidszorg en Jeugd (IGJ) (Health and Youth Care Inspectorate). Geneesmiddelen. Available online: https://www.igj.nl/zorgsectoren/geneesmiddelen (accessed on 13 March 2025).
- Stichting Farmaceutische Kengetallen (SFK) (Foundation for Pharmaceutical Statistics). Data en Feiten 2024. Available online: https://www.sfk.nl/publicaties/data-en-feiten/Dataenfeiten2024def.pdf (accessed on 11 December 2024).
- Stichting Farmaceutische Kengetallen (SFK). Bereidingen: Aandeel orale preparaten daalt naar 33%. Pharm. Weekbl. 2024, 159, 6. [Google Scholar]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association) (Alexanderstraat 11, 2514 JL Den Haag, The Netherlands). Personal communication, 7 January 2025.
- Zorginstituut Nederland. Geneesmiddelen. Available online: https://www.zorginstituutnederland.nl/Verzekerde+zorg/geneesmiddelen-zvw (accessed on 17 June 2025).
- Informatie over Volksgezondheid en Zorg (VZinfo). Ziekenhuiszorg. Aanbod. Instellingen. Available online: https://www.vzinfo.nl/ziekenhuiszorg/aanbod/instellingen (accessed on 20 June 2025).
- Le Brun, P.P.J.; de Boer, A.H.; Frijlink, H.W.; Heijerman, H.G.M. A review of the technical aspects of drug nebulization. Pharm. World. Sci. 2000, 22, 75–81. [Google Scholar] [CrossRef]
- Inspectie Gezondheidszorg en Jeugd (IGJ). Collegiaal Doorleveren van Eigen Bereidingen. Available online: https://www.igj.nl/zorgsectoren/geneesmiddelen/beschikbaarheid-van-geneesmiddelen/collegiaal-doorleveren (accessed on 28 April 2025).
- CIBG. Ministry of Health, Welfare and Sport. Fabrikantenvergunning. Available online: https://www.farmatec.nl/vergunningen/fabrikantenvergunning#:~:text=De%20fabrikantenvergunning%20is%20verplicht%20voor,Noorwegen%2C%20IJsland%20en%20Liechtenstein (accessed on 12 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). KNMP Kennisbank. LNA-Mededeling Organisatie Apotheekbereiding (October 2022). Available online: https://kennisbank.knmp.nl (upon subscription, accessed on 30 May 2025).
- European Commission. The Rules Governing Medicinal Products in the European Union. Annex 1. Manufacture of Sterile Medicinal Products; Directorate-General for Health and Food Safety: Brussels, Belgium. Available online: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf (accessed on 16 December 2024).
- Larmené-Beld, K.H.M.; Frijlink, H.W.; Taxis, K. A systematic review and meta-analysis of microbial contamination of parenteral medication prepared in a clinical versus pharmacy environment. Eur. J. Clin. Pharmacol. 2019, 75, 609–617. [Google Scholar] [CrossRef]
- Hedlund, N.; Beer, I.; Hoppe-Tichy, T.; Trbovich, P. Systematic evidence review of rates and burden of harm of intravenous admixture drug preparation errors in healthcare settings. BMJ Open 2017, 7, e015912. [Google Scholar] [CrossRef] [PubMed]
- Larmené-Beld, K.H.M.; Spronk, J.T.; Luttjeboer, J.; Taxis, K.; Postma, M.J. A cost minimization analysis of ready-to-administer prefilled sterilized syringes in a Dutch hospital. Clin. Ther. 2019, 41, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Wettenbank. Besluit Opleiding en Deskundigheidsgebied Apothekersassistent. Available online: https://wetten.overheid.nl/BWBR0008927/2023-01-01 (accessed on 2 April 2025).
- Kwaliteitsregistratie en Accreditatie Beroepsbeoefenaren in de Zorg (Kabiz). Apothekersassistent. Available online: https://www.kabiz.nl/beroepen/beroep.aspx?onderwerp=apothekersassistent (accessed on 2 April 2025).
- CIBG; Ministerie van Volksgezondheid en Sport (Ministry of Health, Welfare and Sport). BIG Register. Available online: https://bigregister.nl (accessed on 11 December 2024).
- EduFarma. Available online: https://edufarma.nl/fv/ (accessed on 18 December 2024).
- MediaVision. Available online: www.mediavision.nl (accessed on 18 December 2024).
- Pharmatech. Available online: https://www.farmaceutische-educatie.nl/cursus/ (accessed on 18 December 2024).
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). KNMP Kennisbank. LNA-Procedure P-06: Persoonsgebonden Kwalificatie bij Aseptische Handelingen (April 2012). Available online: https://kennisbank.knmp.nl (upon subscription, accessed on 2 April 2025).
- Aleman, J.; Crul, M. Blijven werken aan bekwaamheid. Pharm. Weekbl. 2013, 148, 28–31. [Google Scholar]
- Optima Farma. HBO Opleiding Farmakunde. Available online: https://optimafarma.nl/hbo-opleiding-farmaceutisch-consulent-en-farmakunde (accessed on 17 April 2025).
- Wettenbank. Wet op de Beroepen in de Individuele Gezondheidszorg. Available online: https://wetten.overheid.nl/BWBR0006251/2024-07-01 (accessed on 12 December 2024).
- European Commission. Reform of the EU Pharmaceutical Legislation. Available online: https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe/reform-eu-pharmaceutical-legislation_en?prefLang=nl (accessed on 24 January 2025).
- Wettenbank. Geneesmiddelenwet. Available online: https://wetten.overheid.nl/BWBR0021505/2024-01-01 (accessed on 12 December 2024).
- Wettenbank. Wet op de Geneeskundige Behandelingsovereenkomst. Available online: https://wetten.overheid.nl/BWBR0007021/2006-02-01 (accessed on 12 December 2024).
- European Union. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32001L0083 (accessed on 29 October 2024).
- European Commission. A pharmaceutical Strategy for Europe. Available online: https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe_en (accessed on 12 December 2024).
- Bruins, B.J. Letter of the Minister of Healthcare to the Parliament on April 8, 2019. Kamerstuk 29477, nr. 569. Available online: https://www.tweedekamer.nl/kamerstukken/brieven_regering/detail?id=2019Z07030&did=2019D14445 (accessed on 2 April 2025).
- Bos, E. Doorleveren bereidingen: Alle voorwaarden zijn uitgewerkt. Pharm. Weekbl. 2017, 152, 8–10. [Google Scholar]
- Inspectie Gezondheidszorg en Jeugd (IGJ) (Health and Youth Care Inspectorate). Circulaire Handhavend Optreden bij Collegiaal Doorleveren van Eigen Bereidingen Door Apothekers. Available online: https://www.igj.nl/publicaties/circulairen/2023/07/25/circulaire-handhavend-optreden-bij-collegiaal-doorleveren-van-eigen-bereidingen-door-apothekers-kopie (accessed on 29 October 2024).
- Z-Index. G-Standaard; Z-Index BV: Utrecht, The Netherlands. Available online: https://www.z-index.nl/g-standaard (accessed on 29 October 2024).
- Bijwerkingencentrum Lareb (Netherlands Pharmacovigilance Centre Lareb). Available online: https://www.lareb.nl (accessed on 13 December 2024).
- Ministerie van Volksgezondheid en Sport (Ministry of Health, Welfare and Sport). Beleidsregel Collegiaal Doorleveren en ter Hand Stellen van Apotheekbereidingen. Staatscourant van het Koninkrijk der Nederlanden, 2024, 39917. 6 December 2024. Available online: https://officielebekendmakingen.nl/stcrt-2024-39917 (accessed on 24 March 2025).
- Inspectie Gezondheidszorg en Jeugd (IGJ) (Health and Youth Care Inspectorate). Toezichtsdocument bij de Beleidsregel ‘Collegiaal Doorleveren en ter Hand Stellen van Apotheekbereidingen’. Available online: https://www.igj.nl/publicaties/toetsingskaders/2024/12/09/toezichtsdocument-bij-de-beleidsregel-collegiaal-doorleveren-en-ter-hand-stellen-van-apotheekbereidingen (accessed on 24 March 2025).
- Wettenbank. Beleidsregel Collegiaal Doorleveren en ter Hand Stellen van Apotheekbereidingen. Available online: https://wetten.overheid.nl/BWBR0050656/2025-02-01 (accessed on 24 March 2025).
- European Medicines Agency (EMA). Reform of the EU Pharmaceutical Legislation. Available online: https://health.ec.europa.eu/medicinal-products/legal-framework-governing-medicinal-products-human-use-eu/reform-eu-pharmaceutical-legislation_en (accessed on 24 March 2025).
- The New York Times. U.S. Races to Replenish Storm-Battered Supplies of iv Fluids at Hospitals. 10 October 2024. Available online: https://www.nytimes.com/2024/10/09/health/hurricane-helene-iv-shortages.html (accessed on 17 June 2025).
- Medisch Contact. Tekort aan Infuuszakken Door Orkaan Helene nog niet Voorbij. 26 November 2024. Available online: https://www.medischcontact.nl/actueel/laatste-nieuws/artikel/tekort-aan-infuuszakken-door-orkaan-helene-nog-niet-voorbij (accessed on 18 June 2025).
- Kiseļova, O.; Mauriņa, B.; Šidlovska, V.; Zvejnieks, J. The extent of extemporaneous preparation and regulatory framework of extemporaneous compounding in Latvia. Medicina 2019, 55, 531. [Google Scholar] [CrossRef]
- Scheepers, H.P.A.; Neerup Handlos, V.; Walser, S.; Schutjens, M.D.B.; Neef, C. Impact of the Council of Europe resolution on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients. Eur. J. Hosp. Pharm. 2017, 24, 218–223. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Legal and Regulatory Framework for Community Pharmacies in the WHO European Region; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2019; Available online: https://iris.who.int/bitstream/handle/10665/326394/9789289054249-eng.pdf (accessed on 2 April 2025).
- Scope of DAC/NRF. Available online: https://dacnrf.pharmazeutische-zeitung.de/ueber-uns/english-version (accessed on 12 June 2025).
- The FDA Group. 503A vs. 503B: A Quick-Guide to Compounding Pharmacy Designations & Regulations. 16 November 2021. Available online: https://www.thefdagroup.com/blog/503a-vs-503b-compounding-pharmacies (accessed on 2 April 2025).
- U.S. Food and Drug Administration (FDA). Drugs. Available online: https://www.fda.gov/drugs (accessed on 17 June 2025).[Green Version]
- European Medicines Agency (EMA). Quality by Design. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/quality-design (accessed on 3 February 2025).[Green Version]
- US Food & Drug Administration (FDA). Building Quality into the Design and Conduct of Clinical Studies: Integrating Quality by Design (QbD) and Risk-Based Monitoring (RBM) Approaches. Available online: https://www.fda.gov/drugs/news-events-human-drugs/building-quality-design-and-conduct-clinical-studies-integrating-quality-design-qbd-and-risk-based (accessed on 3 February 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association) (Alexanderstraat 11, 2514 JL Den Haag, The Netherlands). Personal communication, 27 May 2025.[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). KNMP Kennisbank. LNA-Procedure Productdossier (December 2012). Available online: https://kennisbank.knmp.nl (upon subscription, accessed on 26 March 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Oralia.nl. Available online: https://www.oralia.nl (accessed on 26 March 2025).[Green Version]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Substances for pharmaceutical use. 01/1024: 2034. In European Pharmacopoeia, 11.5th ed.; EDQM Council of Europe: Strasbourg, France, 2023; Available online: https://pheur-online.edqm.eu/content/2034/en/current/ (accessed on 5 January 2025).[Green Version]
- European Medicines Agency (EMA). Guideline on Active Substance Master File Procedure. CHMP/QWP/227/02 Rev 4/ Corr. 8. EMA, Amsterdam, The Netherlands, 8 November 2018. Available online: https://www.ema.europa.eu/en/documents/report/final-guideline-active-substance-master-file-procedure-revision-4_en.pdf (accessed on 5 January 2025).[Green Version]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Certification of Suitability to the Monographs of the European Pharmacopoeia. How to Read CEP; EDQM Certification of Substances Department: Strasbourg, France, 2018; Available online: https://www.edqm.eu/documents/52006/156629/Guidelines+-+Certification+of+suitability+to+the+Monographs+of+the+European+Pharmacopoeia+-+How+to+read+a+CEP.pdf/7461fefc-12cb-ee0f-8100-de16bee28a4b?t=1639142423648 (accessed on 5 January 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Beoordeling leveranciers (LNA). Available online: https://www.knmp.nl/over-de-knmp/producten-en-diensten/productzorg-bereiding-en-toediening/beoordeling-leveranciers (accessed on 30 May 2025).[Green Version]
- Stichting Farmaceutische Kengetallen (SFK). Kwart meer Eerstejaars aan Farmaceutische Opleidingen. Available online: https://www.sfk.nl/publicatie/2025/pw-artikel/kwart-meer-eerstejaars-aan-farmaceutische-opleidingen (accessed on 3 March 2025).[Green Version]
- Wettenbank. Besluit Opleidingseisen Apotheker. Available online: https://wetten.overheid.nl/BWBR0008895/2016-10-06 (accessed on 3 March 2025).[Green Version]
- Royal College of Physicians and Surgeons of Canada. The CanMEDS Framework. Available online: https://www.royalcollege.ca/en/standards-and-accreditation/canmeds.html (accessed on 22 April 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Specialisten Registratie Commissie—Openbare Farmacie. Available online: https://www.knmp.nl/beroepsontwikkeling/colleges-en-commissies/specialisten-registratie-commissie-openbare-farmacie (accessed on 3 March 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Inhoud Opleiding Openbaar Apotheker. Available online: https://www.knmp.nl/beroepsontwikkeling/opleidingen/inhoud-opleiding-openbaar-apotheker (accessed on 3 March 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Inhoud Opleiding Ziekenhuisapotheker. Available online: https://www.knmp.nl/beroepsontwikkeling/opleidingen/inhoud-opleiding-ziekenhuis-apotheker (accessed on 3 March 2025).[Green Version]
- Koninklijke Nederlandse Maatschappij ter Bevordering der Pharmacie (KNMP) (Royal Dutch Pharmacists Association). Nascholingen en Accreditatie. Available online: https://www.knmp.nl/beroepsontwikkeling/nascholingen-en-accreditatie (accessed on 3 March 2025).[Green Version]
- Bouwman-Boer, Y.; Fenton-May, V.; Le Brun, P. Preface. In Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. v–vi. [Google Scholar][Green Version]
- Le Brun, P.; Crauste-Manciet, S.; Krämer, I.; Smith, J.; Woerdenbag, H. (Eds.) Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Springer Nature: Cham, Switzerland, 2023. [Google Scholar][Green Version]
- Van Riet-Nales, D.A.; De Jager, K.E.; Schobben, A.F.A.M.; Egberts, T.C.G.; Rademaker, C.M.A. The availability and age-appropriateness of medicines authorized for children in the Netherlands. Br. J. Clin. Pharmacol. 2011, 72, 1365–2125. [Google Scholar] [CrossRef]
- Fadda, H.M.; Weiler, H.; Carvalho, M.; Lee, Y.Z.; Dassouki, H.; AbuBlan, R.; Iurian, S.; Hamid, A.; Şeremet, G.; Li, Z.; et al. Pediatric oral extemporaneous preparations and practices: International Pharmaceutical Federation (FIP) global study. Pediatric oral extemporaneous preparations and practices: International Pharmaceutical Federation (FIP) global study. Eur. J. Pharm. Biopharm. 2024, 204, 114483. [Google Scholar] [CrossRef]
- Khan, D.; Kirby, D.; Bryson, S.; Shah, A.M.; Rahman Mohammed, R. Paediatric specific dosage forms: Patient and formulation considerations. Int. J. Pharm. 2022, 616, 121501. [Google Scholar] [CrossRef]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the paediatric population: A review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Reflection Paper: Formulations of Choice for the Paediatric Population; EMEA: London, UK, 2006; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 24 January 2025).
- Kinderformularium. Available online: www.kinderformularium.nl (accessed on 25 May 2025).
- Woerdenbag, H.J.; Visser, J.C.; Leferink op Reinink, M.P.A.M.; van Orsoy, R.R.; Eissens, A.C.; Hagedoorn, P.; Dijkstra, H.; Allersma, D.P.; Ng, S.W.; Smeets, O.S.N.M.; et al. Performance of tablet splitters, crushers, and grinders in relation to personalised medication with tablets. Pharmaceutics 2022, 14, 320. [Google Scholar] [CrossRef]
- Hamad, Y.; Dodda, S.; Frank, A.; Beggs, J.; Sleckman, C.; Kleinschmidt, G.; Lane, M.A.; Burnett, Y. Perspectives of patients on outpatient parenteral antimicrobial therapy: Experiences and adherence. Open Forum Infect. Dis. 2020, 7, ofaa205. [Google Scholar] [CrossRef]
- Psaltikidis, E.M.; Silva, E.N.D.; Bustorff-Silva, J.M.; Moretti, M.L.; Resende, M.R. Economic evaluation of outpatient parenteral antimicrobial therapy: A systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.; Roberts, J.A.; Cotta, M.O.; Rogers, B.; Pollard, J.; Assefa, G.M.; Erku, D.; Sime, F.B. Safety and efficacy of outpatient parenteral antimicrobial therapy: A systematic review and meta-analysis of randomized clinical trials. Int. J. Antimicrob. Agents 2024, 64, 107263. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.N.; Baker, C.A.; Kind, A.C.; Sannes, M.R. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int. J. Antimicrob. Agents 2015, 46, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wolie, Z.T.; Roberts, J.A.; Gilchrist, M.; McCarthy, K.; Sime, F.B. Current practices and challenges of outpatient parenteral antimicrobial therapy: A narrative review. J. Antimicrob. Chemother. 2024, 79, 2083–2102. [Google Scholar] [CrossRef]
- Stichting Werkgroep Antibioticabeleid (SWAB). Praktijkgids. Implementatie van een OPAT-Programma in Het Ziekenhuis. Available online: https://swab.nl/nl/exec/file/download/263 (accessed on 9 June 2025).
- European Union. Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004L0037 (accessed on 12 December 2024).
- Nederlandse Vereniging van Ziekenhuis Apothekers (NVZA) (Dutch Association of Hospital Pharmacists). GMP-Z Herziening 2013 Z4: Handelingen Met Risicovolle Stoffen en Preparaten. Available online: https://nvza.nl/wp-content/uploads/gmpz_z4_herziening_2013_handelingen_met_risicovolle_stoffen_en_preparaten.pdf (accessed on 12 December 2024).
- Carrez, L.; Bouchoud, L.; Fleury-Souverain, S.; Combescure, C.; Falaschi, L.; Sadeghipour, F.; Bonnabry, P. Reliability of chemotherapy preparation processes: Evaluating independent double-checking and computer-assisted gravimetric control. J. Oncol. Pharm. Pract. 2017, 23, 83–92. [Google Scholar] [CrossRef]
- Terkola, R.; Czejka, M.; Bérubé, J. Evaluation of real-time data obtained from gravimetric preparation of antineoplastic agents shows medication errors with possible critical therapeutic impact: Results of a large-scale, multicentre, multinational, retrospective study. J. Clin. Pharm. Ther. 2017, 42, 446–453. [Google Scholar] [CrossRef]
- Benizri, F.; Dalifard, B.; Zemmour, C.; Henriquet, M.; Fougereau, E.; Le Franc, B. DrugCam®—An intelligent video camera system to make safe cytotoxic drug preparations. Int. J. Pharm. 2016, 502, 198–207. [Google Scholar] [CrossRef]
- Geersing, T.H.; Klous, M.G.; Franssen, E.J.F.; van den Heuvel, J.J.G.; Crul, M. Robotic compounding versus manual compounding of chemotherapy: Comparing dosing accuracy and precision. Eur. J. Pharm. Sci. 2020, 155, 105536. [Google Scholar] [CrossRef]
- Integraal Kankercentrum Nederland (IKNL) (Netherlands Comprehensive Cancer Organisation). Available online: https://iknl.nl (accessed on 12 December 2024).
- Crul, M.; Breukels, O. Safe handling of cytostatic drugs: Recommendations from independent science. Eur. J. Hosp. Pharm. 2024, 31, 191–196. [Google Scholar] [CrossRef]
- Crul, M.; Hilhorst, S.; Breukels, O.; Bouman-d’Onofrio, J.R.C.; Stubbs, P.; van Rooij, J.G. Occupational exposure of pharmacy technicians and cleaning staff in Dutch hospitals. J. Occup. Environ. Hyg. 2020, 17, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hilliquin, D.; Tanguay, C.; Bussières, J.F. External contamination of commercial containers by antineoplastic agents: A literature review. Eur. J. Hosp. Pharm. 2020, 27, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Ministerie van Sociale Zaken en Werkgelegenheid. Arboportaal, Cytostatica. Available online: https://www.arboportaal.nl/onderwerpen/cytostatica (accessed on 14 May 2025).
- Van den Berg, R.B.; de Poot, S.; Swart, E.L.; Crul, M. Assessment of occupational exposure to nebulized isopropyl alcohol as disinfectant during aseptic compounding of parenteral cytotoxic drugs in cleanrooms. J. Occup. Environ. Hyg. 2021, 18, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Nederlandse Vereniging van Ziekenhuis Apothekers (NVZA) (Dutch Association of Hospital Pharmacists). Crashkaart Oncolytica. Available online: https://nvza.nl/wp-content/uploads/Crashkaart-NVZA-versie-8_26112023-final.xls (accessed on 2 April 2025).
- Gonnermann, C.; Maier, L. Computer-assisted compounding of TPN-solutions. Krankenhauspharmazie 1998, 19, 67–70. [Google Scholar]
- Pichard, C.; Schwarz, G.; Frei, A.; Kyle, U.; Jolliet, P.; Morel, P.; Romand, J.A.; Sierro, C. Economic investigation of the use of three-compartment total parenteral nutrition bag: Prospective randomized unblinded controlled study. Clin. Nutr. 2000, 19, 245–251. [Google Scholar] [CrossRef]
- Mays, A.; Ayers, P.; Monczka, R.D.; Cober, M.P. Safety in parenteral nutrition compounding. Nutr. Clin. Pract. 2023, 38, 1253–1262. [Google Scholar] [CrossRef]
- Berlana, D. Parenteral nutrition overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Riskin, A.; Picaud, J.C.; Shamir, R. ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Standard Versus Individualized Parenteral Nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Advanced Therapy Medicinal Products: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/advanced-therapy-medicinal-products-overview (accessed on 6 February 2025).
- European Commission. The Rules Governing Medicinal Products in the European Union. Volume 4. Guidelines on Good Manufacturing Practice Specific to Advanced Therapy Medicinal Products. Available online: https://health.ec.europa.eu/document/download/ad33d9dd-03f0-4bef-af53-21308ce2187d_en?filename=2017_11_22_guidelines_gmp_for_atmps.pdf (accessed on 6 February 2025).
- Mastrobatista, E.; Doevendans, E.; van Tol, N.P.; Kemp, V.; de Vrij, J.; Hoogendoorn, K. Pharmaceutical Biotechnology, 6th ed.; Crommelin, D.J.A., Sindelar, R.D., Meiboom, B., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 323–399. [Google Scholar]
- DARE-NL. Available online: https://www.dare-nl.nl (accessed on 18 June 2025).
- European Clinical Trial Database. HOVON 161: A Phase II Non-Inferiority Study Comparing Point-of-Care Produced CAR T-Cell to Commercial CAR T-Cells in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Available online: https://euclinicaltrials.eu/search-for-clinical-trials/?lang=en&EUCT=2024-511979-15-00 (accessed on 6 February 2025).
- Eerkens, A.L.; Esajas, M.D.; Brummel, K.; Vledder, A.; van Rooij, N.; Plat, A.; Avalos Haro, S.B.; Paijens, S.T.; Slagter-Menkema, L.; Schuuring, E.; et al. Vvax001, a therapeutic vaccine for patients with HPV16-positive high-grade cervical intraepithelial neoplasia: A phase II trial. Clin. Cancer Res. 2025, 31, 1016–1026. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Creemers, J.H.A.; van Oort, I.M.; Schreibelt, G.; Gorris, M.A.J.; Mehra, N.; Simons, M.; de Goede, A.L.; van Rossum, M.M.; Croockewit, A.J.; et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with vlinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunother. Cancer 2019, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, P.K.J.D.; van Hauten, P.M.M.; Janssen, L.D.; de Goede, A.L.; Berrien-Elliott, M.M.; van der Meer, J.M.R.; Mousset, C.M.; Roeven, M.W.H.; Foster, M.; Blijlevens, N.; et al. Good manufacturing practice production of CD34+ progenitor-derived NK cells for adoptive immunotherapy in acute myeloid leukemia. Cancer Immunol. Immunother. 2023, 72, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C.; et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef]
- Yonezawa Ogusuku, I.E.; Herbel, V.; Lennartz, S.; Brandes, C.; Argiro, E.; Fabian, C.; Hauck, C.; Hoogstraten, C.; Veld, S.; Hageman, L.; et al. Automated manufacture of ΔNPM1 TCR-engineered T cells for AML therapy. Mol. Ther. Methods Clin. Dev. 2024, 32, 101224. [Google Scholar] [CrossRef]
- European Clinical Trial Database. A Study of the Safety and Efficacy of MB-dNPM-1-TCR.1 in Patients with Relapsed or Refractory AML. Available online: https://euclinicaltrials.eu/search-for-clinical-trials/?lang=en&EUCT=2023-505806-41-00 (accessed on 6 February 2025).
- Straetemans, T.; Kierkels, G.J.J.; Doorn, R.; Jansen, K.; Heijhuurs, S.; dos Santos, J.M.; van Muyden, A.D.D.; Vie, H.; Clemenceau, B.; Raymakers, R.; et al. GMP-grade manufacturing of T cells engineered to express a defined ΓδTCR. Front. Immunol. 2018, 9, 1062. [Google Scholar] [CrossRef]
- De Witte, M.; Scheepstra, J.; Weertman, N.; Daudeij, A.; van der Wagen, L.; Oostvogels, R.; de Haar, C.; Prins, H.-J.; Dohmen, W.; Bartels-Wilmer, C.; et al. First in human clinical responses and persistence data on TEG001: A next generation of engineered Aβ T cells targeting AML and MM with a high affinity Γ9δ2TCR. Blood 2022, 140 (Suppl. S1), 12737–12739. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Bloemendal, M.; van Willigen, W.W.; Hins-de Bree, S.; de Goede, A.L.; de Boer, A.J.; Bos, K.J.H.; Duiveman-de Boer, T.; Olde Nordkamp, M.A.M.; et al. Adjuvant dendritic cell therapy in stage IIIB/C melanoma: The MIND-DC randomized phase III trial. Nat. Commun. 2024, 15, 1632. [Google Scholar] [CrossRef]
- Aerts, J.G.; Belderbos, R.; Baas, P.; Scherpereel, A.; Bezemer, K.; Enninga, I.; Meijer, R.; Willemsen, M.; Berardi, R.; Fennell, D.; et al. Dendritic cells loaded with allogeneic tumour cell lysate plus best supportive care versus best supportive care alone in patients with pleural mesothelioma as maintenance therapy after chemotherapy (DENIM): A multicentre, open-label, randomised, phase 2/3 study. Lancet Oncol. 2024, 25, 865–878. [Google Scholar] [CrossRef]
- Weatherman, K.D.; Kowalsky, R.J. Radiopharmaceuticals, nuclear medicine, and nuclear pharmacy: An overview. In Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine, 4th ed.; Kowalsky, R.J., Weatherman, K.D., Eds.; American Pharmacists Association: Washington, DC, USA, 2020; pp. 3–18. [Google Scholar]
- Nederlandse Vereniging van Ziekenhuisapothekers (NVZA) (Dutch Association of Hospital Pharmacists). GMP-Z Annex 3. Bereiding van Radiofarmaca, Herziening 2021. Available online: https://nvza.nl/wp-content/uploads/GMP-Z-herziening-2021-Annex-3-Bereiding-van-radiofarmaca.pdf (accessed on 8 April 2025).
- Lange, R.; Schreuder, N.; Hendrikse, H. Radiopharmaceuticals. In Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Le Brun, P., Crauste-Manciet, S., Krämer, I., Smith, J., Woerdenbag, H., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 531–550. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Radiopharmaceutical preparations. (0125). In European Pharmacopoeia, 11.5th ed.; EDQM Council of Europe: Strasbourg, France, 2023; Available online: https://pheur-online.edqm.eu/content/0125/en/current/ (accessed on 21 October 2024).
- European Commission. Eudralex. The Rules Governing Medicinal Products in the European Union. Volume 4. EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use. Annex 3. Manufacture of Radiopharmaceuticals. Available online: https://health.ec.europa.eu/document/download/bf281e1f-4897-469a-ba60-18d867b14a94_en?filename=2008_09_annex3_en.pdf (accessed on 21 October 2024).
- United Nations. Agreement Concerning the International Carriage of Dangerous Goods by Road. Available online: https://unece.org/transport/standards/transport/dangerous-goods/adr-2023-agreement-concerning-international-carriage (accessed on 21 October 2024).
- Schreuder, N.; Jacobs, N.A.; Jager, P.L.; Kosterink, J.G.W.; van Puijenbroek, E.P. Patient-reported adverse events of radiopharmaceuticals: A prospective study of 1002 patients. Drug Saf. 2021, 44, 211–222. [Google Scholar] [CrossRef]
- Baum, R.P.; Rösch, F. (Eds.) Theranostics, Gallium-68, and Other Radionuclides: A Pathway to Personalized Diagnosis and Treatment, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- European Medicines Association (EMA). Clinical Trials Regulation. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/clinical-trials-human-medicines/clinical-trials-regulation (accessed on 24 January 2025).
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.G.E.; Kist de Ruijter, L.; Lub-de Hooge, M.N.; Dierckx, R.A.; Elias, S.G.; Oosting, S.F. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 241–255. [Google Scholar] [CrossRef] [PubMed]
- de Groot, D.J.A.; Lub-de Hooge, M.N.; van Meerten, T.; Brouwers, A.H.; de Vries, E.G.E. Facts and hopes for PET imaging-derived immunotherapy biomarkers. Clin. Cancer Res. 2024, 30, 5252–5259. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nature Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Schouw, H.M.; Huisman, L.A.; Janssen, Y.F.; Slart, R.H.J.A.; Borra, R.J.H.; Willemsen, A.T.M.; Brouwers, A.H.; van Dijl, J.M.; Dierckx, R.A.; van Dam, G.M.; et al. Targeted optical fluorescence imaging: A meta-narrative review and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4272–4292. [Google Scholar] [CrossRef]
- Linssen, M.D.; ter Weele, E.J.; Allersma, D.P.; Lub-de Hooge, M.N.; van Dam, G.M.; Jorritsma-Smit, A.; Nagengast, W.B. Roadmap for the development and clinical translation of optical tracers cetuximab-800CW and trastuzumab-800CW. J. Nucl. Med. 2019, 60, 418–423. [Google Scholar] [CrossRef]
- Allen, L.V., Jr. Compounding for investigational and clinical studies. Int. J. Pharm. Compd. 2022, 26, 201–209. [Google Scholar] [PubMed]
- Hoppentocht, M.; Akkerman, O.W.; Hagedoorn, P.; Alffenaar, J.W.; van der Werf, T.S.; Kerstjens, H.A.; Frijlink, H.W.; de Boer, A.H. Tolerability and pharmacokinetic evaluation of inhaled dry powder tobramycin free base in non-cystic fibrosis bronchiectasis patients. PLoS ONE 2016, 11, e0149768. [Google Scholar] [CrossRef]
- Maurer, J.M.; Schellekens, R.C.A.; van Rieke, H.M.; Stellaard, F.; Wutzke, K.D.; Buurman, D.J.; Dijkstra, G.; Woerdenbag, H.J.; Frijlink, H.W.; Kosterink, J.G.W. ColoPulse tablets perform comparably in healthy volunteers and Crohn’s patients and show no influence of food and time of food intake on bioavailability. J. Control. Release 2013, 172, 618–624. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Otten, A.T.; Gareb, B.; Huijmans, J.E.; Eissens, A.C.; Rehman, A.; Dijkstra, G.; Kosterink, J.G.W.; Frijlink, H.W.; Schellekens, R.C.A. Capsules with ileocolonic-targeted release of vitamin B2, B3, and C (ColoVit) intended for optimization of gut health: Development and validation of the production process. Pharmaceutics 2023, 15, 1354. [Google Scholar] [CrossRef]
- Batson, S.; Mitchell, S.A.; Lau, D.; Canobbio, M.; de Goede, A.; Singh, I.; Loesch, U. Automated compounding technology and workflow solutions for the preparation of chemotherapy: A systematic review. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 330–336. [Google Scholar] [CrossRef]
- Zamani, M.; Chan, K.; Wilcox, J. Pharmacy technicians’ perceptions of risk reduction strategies implemented in response to the repetitive strain injury associated with sterile compounding. Int. J. Pharm. Compd. 2021, 25, 182–186. [Google Scholar] [PubMed]
- European Commission. Guidance for the Safe Management of Hazardous Medicinal Products at Work. Available online: https://osha.europa.eu/sites/default/files/documents/guidance%20for%20the%20safe%20management%20of%20hazardous%20medicinal-KE0723274ENN.pdf (accessed on 15 November 2024).
- Cerutti, A.; Ledoux, T.; Vantard, N.; Cerfon, M.A.; Kimbidima, R.; Larbre, V.; Herledan, C.; Lattard, C.; Baudouin, A.; Caffin, A.G.; et al. Comparing different robots available in the European market for the preparation of injectable chemotherapy and recommendations to users. J. Oncol. Pharm. Pract. 2023, 29, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Masini, C.; Nanni, O.; Antaridi, S.; Gallegati, D.; Marri, M.; Paolucci, D.; Minguzzi, M.; Altini, M. Automated preparation of chemotherapy: Quality improvement and economic sustainability. Am. J. Health Syst. Pharm. 2014, 7, 579–585. [Google Scholar] [CrossRef]
- Deljehier, T.; Bouguéon, G.; Heloury, J.; Moreno, V.; Berroneau, A.; Crauste-Manciet, S. Simulation program of a cytotoxic compounding robot for monoclonal antibodies and anti-infectious sterile drug preparation. J. Oncol. Pharm. Pract. 2019, 25, 1873–1890. [Google Scholar] [CrossRef]
- Jobard, M.; Brandely-Piat, M.L.; Chast, F.; Batista, R. Qualification of a chemotherapy-compounding robot. J. Oncol. Pharm. Pract. 2020, 26, 312–324. [Google Scholar] [CrossRef]
- Geersing, T.H.; Franssen, E.J.F.; Pilesi, F.; Crul, M. Microbiological performance of a robotic system for aseptic compounding of cytostatic drugs. Eur. J. Pharm. Sci. 2019, 130, 181–185. [Google Scholar] [CrossRef]
- Krämer, I.; Federici, M.; Schierl, R. Environmental and product contamination during the preparation of antineoplastic drugs with robotic systems. Pharm. Technol. Hosp. Pharm. 2018, 3, 153–164. [Google Scholar] [CrossRef]
- Schierl, R.; Masini, C.; Groeneveld, S.; Fischer, E.; Böhlandt, A.; Rosini, V.; Paolucci, D. Environmental contamination by cyclophosphamide preparation: Comparison of conventional manual production in biological safety cabinet and robot-assisted production by APOTECAchemo. J. Oncol. Pharm. Pract. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Werumeus Buning, A.; Geersing, T.H.; Crul, M. The assessment of environmental and external cross-contamination in preparing ready-to-administer cytotoxic drugs: A comparison between a robotic system and conventional manual production. Int. J. Pharm. Pract. 2020, 28, 66–74. [Google Scholar] [CrossRef]
- Lyousoufi, M.; Lafeber, I.; Kweekel, D.; de Winter, B.C.M.; Swen, J.J.; Le Brun, P.P.H.; Bijleveld-Olierook, E.C.M.; van Gelder, T.; Guchelaar, H.J.; Moes, D.J.A.R.; et al. Development and bioequivalence of 3D-printed medication at the point-of-care: Bridging the gap toward personalized medicine. Clin. Pharmacol. Ther. 2023, 113, 1125–1131. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Develop. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Breitkreutz, J.; Quodbach, J. 3D printed mini-floating-polypill for Parkinson’s disease: Combination of levodopa, benserazide, and pramipexole in various dosing for personalized therapy. Pharmaceutics 2022, 14, 931. [Google Scholar] [CrossRef]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, L.I.; Ayyoubi, S.; Tajqurishi, M.; Quodbach, J.; Vermonden, T.; Kok, R.J. 3D-printed prednisolone phosphate suppositories with tunable dose and rapid release for the treatment of inflammatory bowel disease. Int. J. Pharm. 2024, 649, 123639. [Google Scholar] [CrossRef]
- Levine, V.R.; Paulsson, M.; Strømme, M.; Quodbach, J.; Lindh, J. Off-the-shelf medication transformed: Custom-dosed metoprolol tartrate tablets via semisolid extrusion additive manufacturing and the perception of this technique in a hospital context. Int. J. Pharm. X 2024, 8, 100277. [Google Scholar] [CrossRef]
- Bos, E. Apotheker kan met 3D-printer maatwerk leveren voor kind of kwetsbare oudere. Pharm. Weekbl. 2025, 160, 8–9. [Google Scholar]
- Meruva, S.; Singaraju, A.B.; Vinjamuri, B.P.; Ternik, R.; Stagner, W.C. Current state of minitablet product design: Review. J. Pharm. Sci. 2024, 113, 1123–1154. [Google Scholar] [CrossRef]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef]



| Product Type | Specification and Examples |
|---|---|
| Topical preparations | Creams, ointments, and gels are compounded for patients who require specific formulations for skin disorders (e.g., eczema, psoriasis, or fungal infections). Lotions are used for conditions like dandruff, seborrheic dermatitis, and acne. |
| Oral preparations | Liquid formulations can be prepared as alternatives to medication typically available as tablet or capsule for patients who have difficulty swallowing solid medication, such as children and the elderly. Capsules can be either compounded from raw materials or from ground commercially available tablets, for example, to allow dose adaptation. |
| Paediatric formulations | Medicines may be compounded with flavours to make them more palatable for children. Dosages can be adjusted to suit the child’s weight or age. |
| Suppositories | Rectal or vaginal suppositories are compounded for medications that need to be administered via these routes, often for treating conditions such as haemorrhoids and vaginal infections. |
| Sterile solutions | Intravenously administered medications include antibiotic solutions dispensed in disposable infusion pumps or infusion bags for home treatment. Subcutaneous medications, such as morphine or lidocaine, are prepared for pain management when precise dosing is required or when commercial formulations are unavailable. |
| Condition | Explanation |
|---|---|
| Unmet need | The compounded product may only be outsourced, compounded, and delivered if the patient’s condition cannot be adequately treated with a product that is licensed (registered) in the Netherlands. Both the dispensing and compounding pharmacies are responsible for verifying this requirement. |
| Notification | The compounding pharmacy is obligated to register its product in the national medicinal products database used by all healthcare stakeholders (the G-Standard, maintained and updated by Z-Index [69]). This ensures transparency regarding the products available and the pharmacy involved in pharmacy-to-pharmacy delivery. |
| Product file | For each product, the compounding pharmacy must maintain a file that justifies the product and its design. This file should include the medical application (pharmacotherapy), as well as the chemical and pharmaceutical aspects, such as composition, preparation method, quality controls, stability studies and expiration date, microbiological controls, and other relevant data. |
| GMP | The compounding pharmacy is required to comply with the EU-GMP standards, which necessitates the implementation of a fully functioning pharmaceutical quality system. Compliance is monitored by the IGJ through its on-site inspection programme. Typically, regulatory inspections are conducted every three years. |
| Pharmacovigilance | Compounding pharmacies must implement a pharmacovigilance system to document and assess (potential) adverse reactions on severity and causality, which should be reported to the national pharmacovigilance centre Lareb [70]. |
| Promotion | The promotion, advertisement and/or offering of incentives for compounded products is prohibited. It is permitted to send a price list to interested dispensing pharmacies and to respond to information requests. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| University | Level | Programme | Duration (ECTS) (1) | Access to MSc Pharmacy? (2) | Qualifies as Pharmacist? |
|---|---|---|---|---|---|
| Leiden University | Bachelor | Bio-Pharmaceutical Sciences | 180 | No (3) | - |
| Master | Bio-Pharmaceutical Sciences | 120 | - | No | |
| Master | Pharmacy | 180 | - | Yes | |
| University of Groningen | Bachelor | Pharmacy | 180 | Yes (4) | - |
| Master | Medical Pharmaceutical Sciences | 120 | - | No | |
| Master | Molecular Medicine and Innovative Treatment | 120 | - | No | |
| Master | Pharmacy | 180 | - | Yes | |
| Utrecht University | Bachelor | College of Pharmaceutical Sciences | 180 | Yes (5) | - |
| Bachelor | Pharmacy | 180 | Yes | - | |
| Master | Drug Innovation | 120 | - | No | |
| Master | Pharmacy | 180 | - | Yes | |
| Vrije Universiteit Amsterdam | Bachelor | Pharmaceutical Sciences | 180 | No | - |
| Master | Drug Discovery Sciences | 120 | - | No |
| (A) |
| Bachelor’s Pharmacy Programme |
| Students who complete a Bachelor of Pharmacy degree programme possess knowledge and understanding of: |
|
| (B) |
| Master’s Pharmacy Programme |
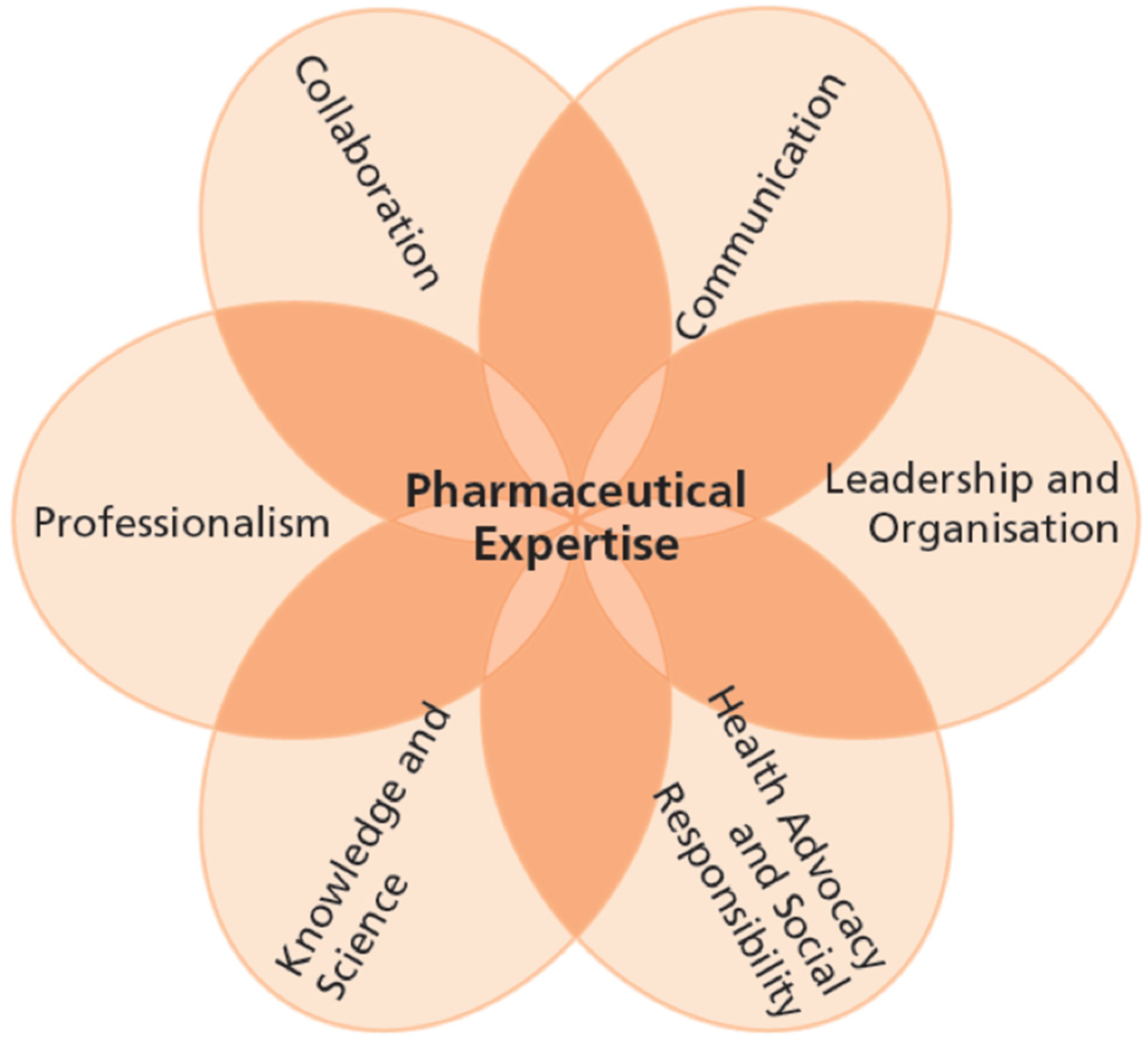
| Pharmaceutical Expertise |
| Pharmacists are able to: |
|
| Communication |
| Pharmacists are able to: |
|
| Knowledge and Science |
| Pharmacists are able to: |
|
| Health Advocacy and Social Responsibility |
| Pharmacists are able to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ATMP | Indication | Institution | Manufacturing Process | Reference |
|---|---|---|---|---|
| Anti-CD19 CAR-T cells | Diffuse large B cell lymphoma | University Medical Center Groningen | CD4+ and CD8+ cells are enriched from leukapheresis material and ex vivo activated, transduced by a lentiviral vector, expanded, harvested, and formulated into the drug product. | [136] |
| Vvax-001 | HPV-induced cancers | University Medical Center Groningen | RNA is produced from plasmid DNA and transfected into Vero cells that produce the Vvax-001 replicon particles, which, after purification, are formulated into the drug product. | [137] |
| Tumour-infiltrating lymphocytes (TIL) | Melanoma | Netherlands Cancer Institute | Melanoma lesion is surgically resected and enzymatic digested to harvest TILs, which are ex vivo expanded for 2–4 weeks, harvested, and formulated into the drug product. | [138] |
| Dendritic cell vaccination | Melanoma; prostate cancer | Radboud University Medical Center | Dendritic cells are isolated from apheresis material, matured, loaded with antigen, harvested, and formulated into the drug product. | [139,140] |
| Natural killer (NK) cells | Acute myeloid leukemia | Radboud University Medical Center | NK cells are ex vivo generated from umbilical cord blood-derived CD34+ progenitor cells using a mixture of cytokines and growth factors and are subsequently expanded, harvested, and formulated into the drug product. | [141,142] |
| ΔNPM1 T cell receptor (TCR)-engineered T cells | NPM1 mutated acute myeloid leukemia | Leiden University Medical Center | CD8+ cells are enriched from leukapheresis material and ex vivo activated, transduced by a lentiviral vector, expanded, harvested, and formulated into the drug product. | [143,144] |
| Vγ9Vδ2T cell receptor engineered T cells (TEG001) | Acute myeloid leukemia; multiple myeloma | University Medical Center Utrecht | T cells from leukapheresis material are ex vivo activated, transduced by a retroviral vector, expanded, purified, harvested, and formulated into the drug product. | [145,146] |
| MesoPher | Mesothelioma | Erasmus Medical Center | Monocytes are enriched from leukapheresis material and ex vivo cultured and differentiated into dendritic cells after which these dendritic cells are cultured in the presence of allogenic tumour lysate. Subsequently, the dendritic cells are matured, harvested, and formulated into the drug product. | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woerdenbag, H.J.; van Basten, B.; Oussoren, C.; Smeets, O.S.N.M.; Annaciri-Donkers, A.; Crul, M.; Maurer, J.M.; Schimmel, K.J.M.; Kemper, E.M.; Hooge, M.N.L.-d.; et al. Extemporaneous Compounding, Pharmacy Preparations and Related Product Care in the Netherlands. Pharmaceutics 2025, 17, 1005. https://doi.org/10.3390/pharmaceutics17081005
Woerdenbag HJ, van Basten B, Oussoren C, Smeets OSNM, Annaciri-Donkers A, Crul M, Maurer JM, Schimmel KJM, Kemper EM, Hooge MNL-d, et al. Extemporaneous Compounding, Pharmacy Preparations and Related Product Care in the Netherlands. Pharmaceutics. 2025; 17(8):1005. https://doi.org/10.3390/pharmaceutics17081005
Chicago/Turabian StyleWoerdenbag, Herman J., Boy van Basten, Christien Oussoren, Oscar S. N. M. Smeets, Astrid Annaciri-Donkers, Mirjam Crul, J. Marina Maurer, Kirsten J. M. Schimmel, E. Marleen Kemper, Marjolijn N. Lub-de Hooge, and et al. 2025. "Extemporaneous Compounding, Pharmacy Preparations and Related Product Care in the Netherlands" Pharmaceutics 17, no. 8: 1005. https://doi.org/10.3390/pharmaceutics17081005
APA StyleWoerdenbag, H. J., van Basten, B., Oussoren, C., Smeets, O. S. N. M., Annaciri-Donkers, A., Crul, M., Maurer, J. M., Schimmel, K. J. M., Kemper, E. M., Hooge, M. N. L.-d., Schreuder, N., Eikmann, M., Ramcharan, A. S., Lantink, R. B., Quodbach, J., Boersma, H. H., Kelder, O., Larmené-Beld, K. H. M., Le Brun, P. P. H., ... Gareb, B. (2025). Extemporaneous Compounding, Pharmacy Preparations and Related Product Care in the Netherlands. Pharmaceutics, 17(8), 1005. https://doi.org/10.3390/pharmaceutics17081005











