In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Cell Lines, and Mice
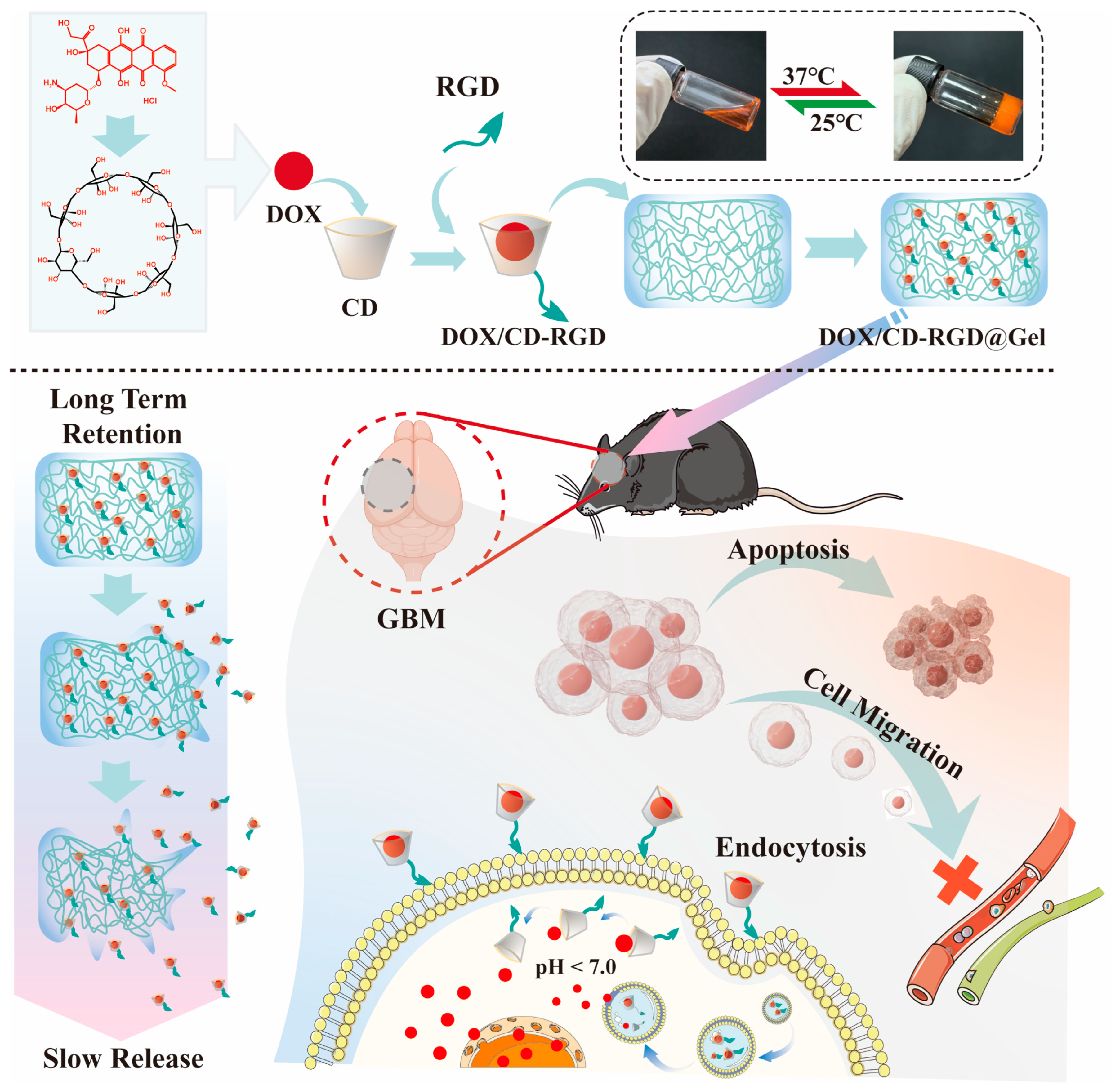
2.2. Preparation and Characterization of DOX/RGD-CD
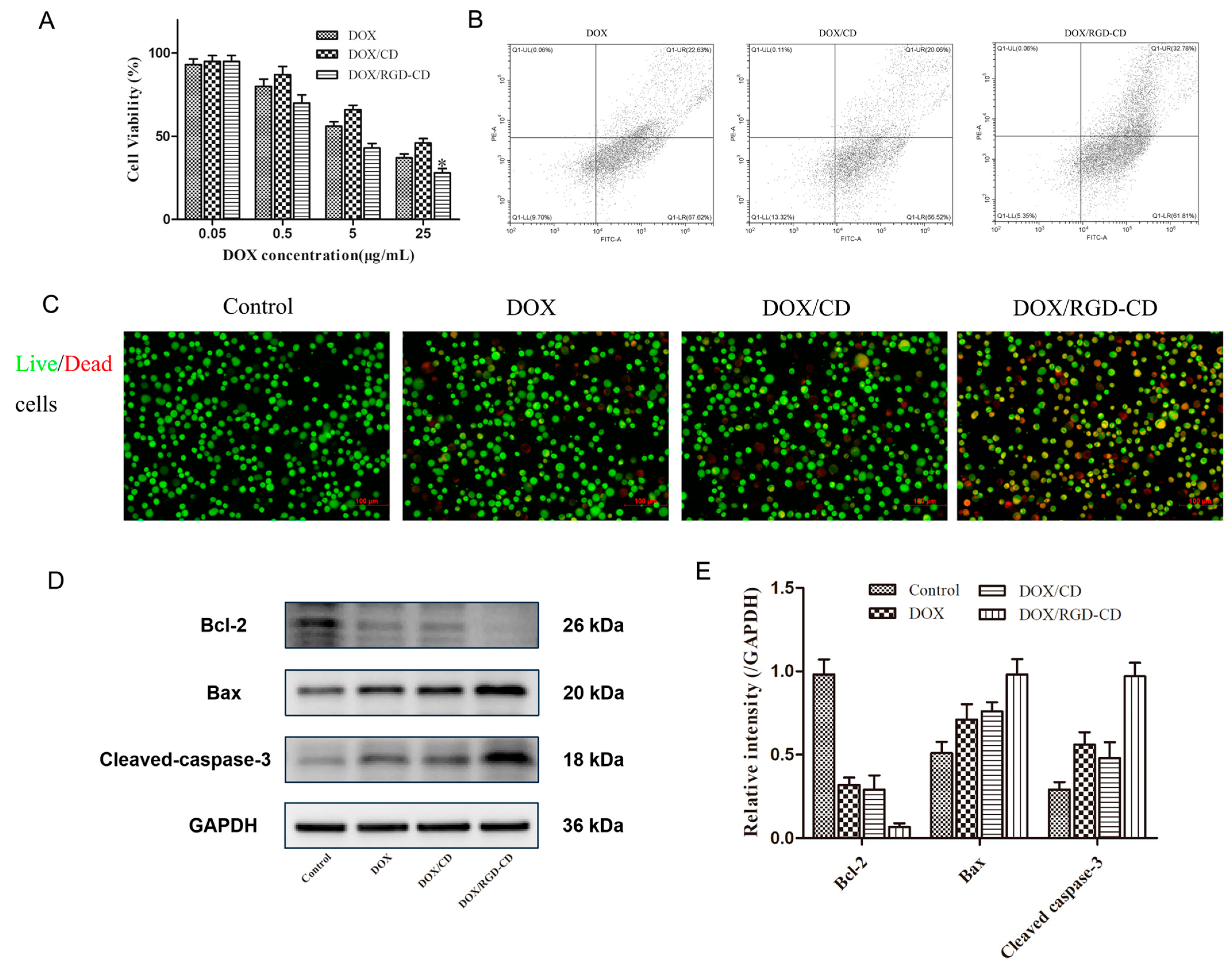
2.3. In Vitro Cytotoxicity Study
2.4. Live/Dead Cell Assay
2.5. Apoptosis Assay
2.6. Expression of Apoptosis-Related Proteins in GL261 Cells
2.7. Cell Migration and Invasion Assay
2.8. Expression of Invasion-Related Proteins in GL261 Cells
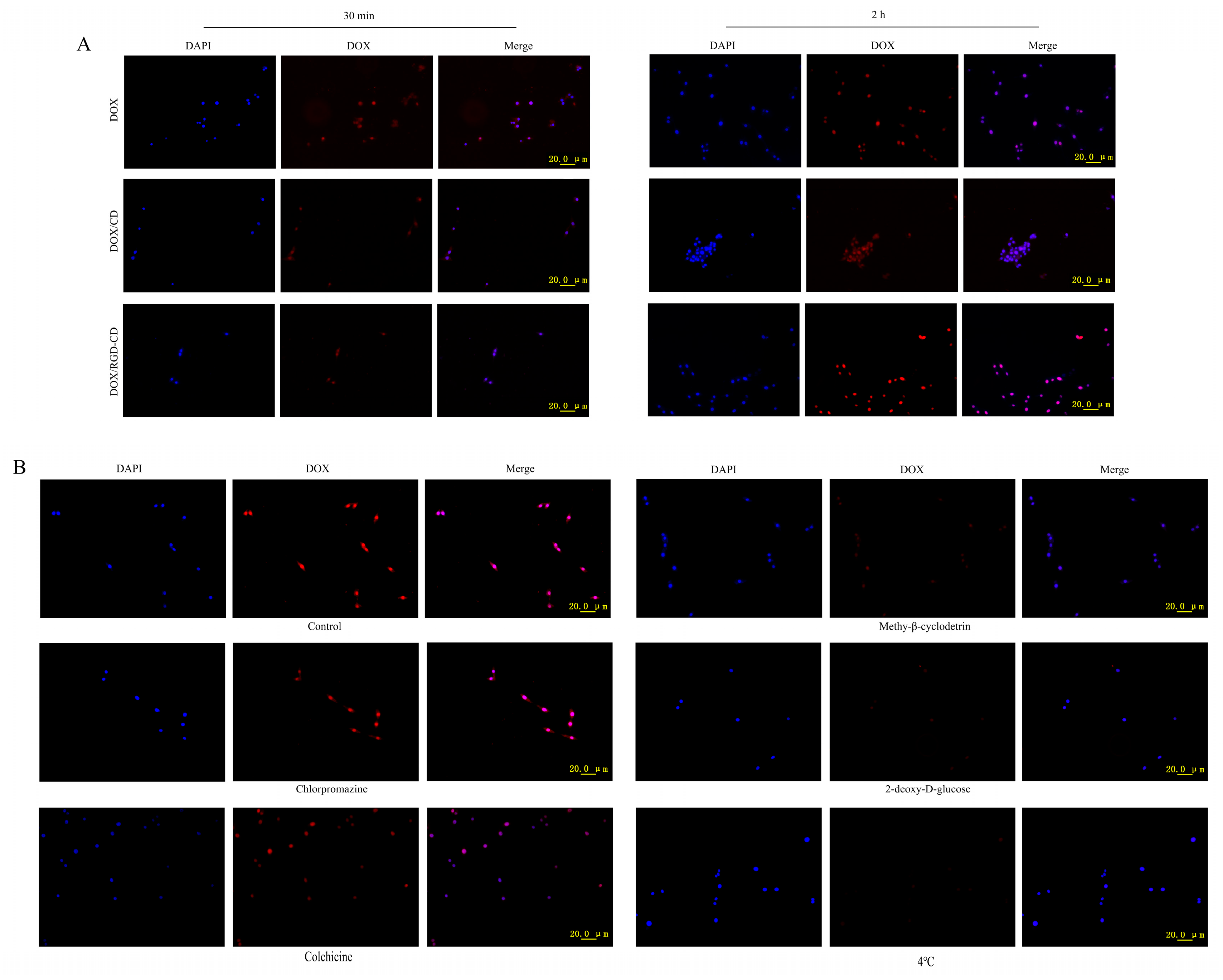
2.9. Evaluation of In Vitro Uptake
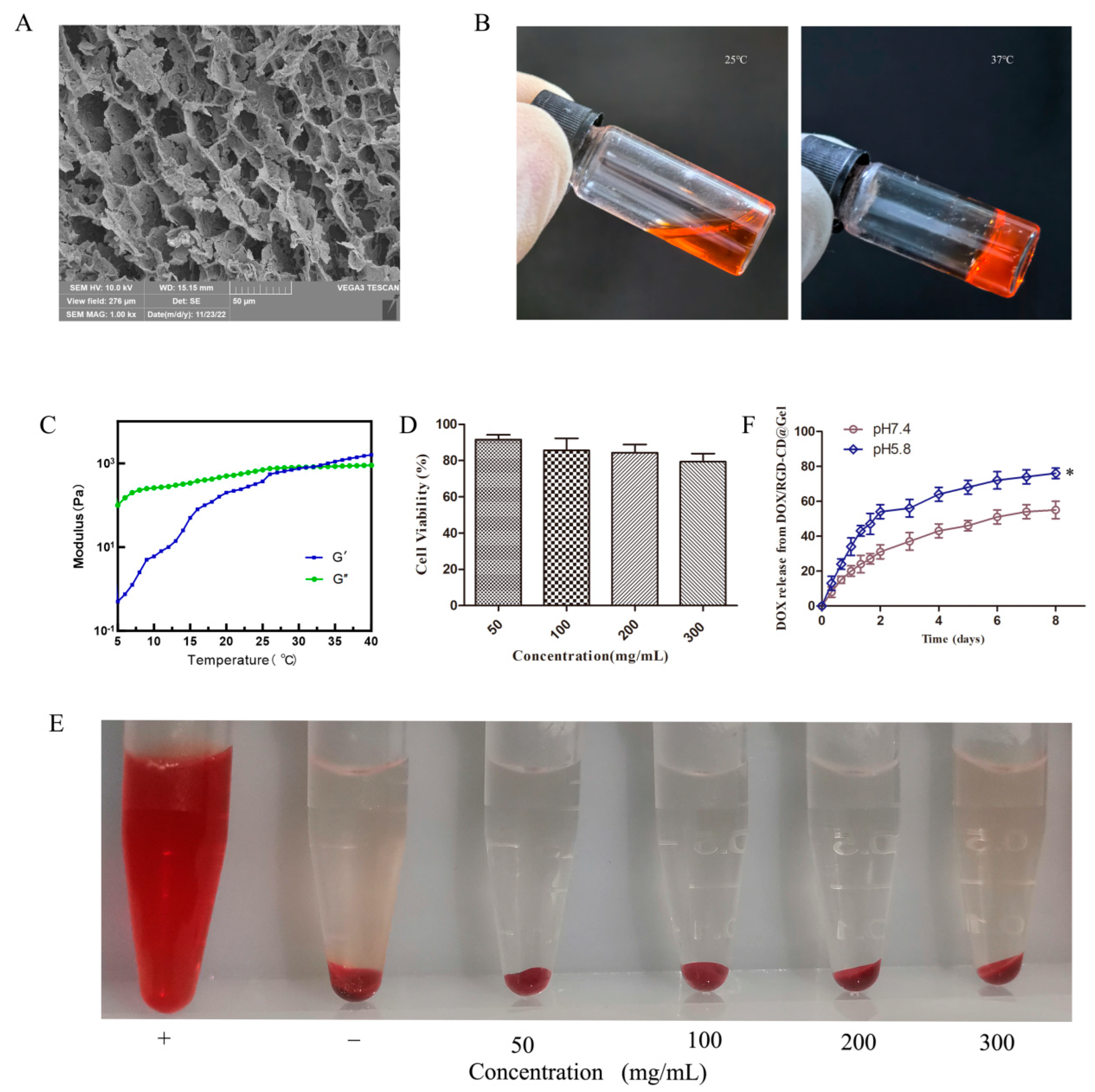
2.10. Preparation and Characterization of DOX/RGD-CD@Gel
2.11. In Vitro Drug Release Assay
2.12. Establishment of the Glioblastoma Tumor Model
2.13. In Vivo Retention of DOX/RGD-CD@Gel
2.14. Tumor Treatment with DOX/RGD-CD@Gel In Vivo
2.15. In Vivo Safety Evaluation of DOX/RGD-CD@Gel
2.16. Statistical Analysis
3. Results and Discussion
3.1. Characterization of DOX/RGD-CD
3.2. Cytotoxicity of DOX/RGD-CD
3.3. Effects of DOX/RGD-CD on GL261 Cell Migration and Invasion
3.4. Uptake of DOX/RGD-CD by GL261 Cells
3.5. Characterization of DOX/RGD-CD@Gel
3.6. Antitumor Efficacy of DOX/RGD-CD@Gel In Vivo
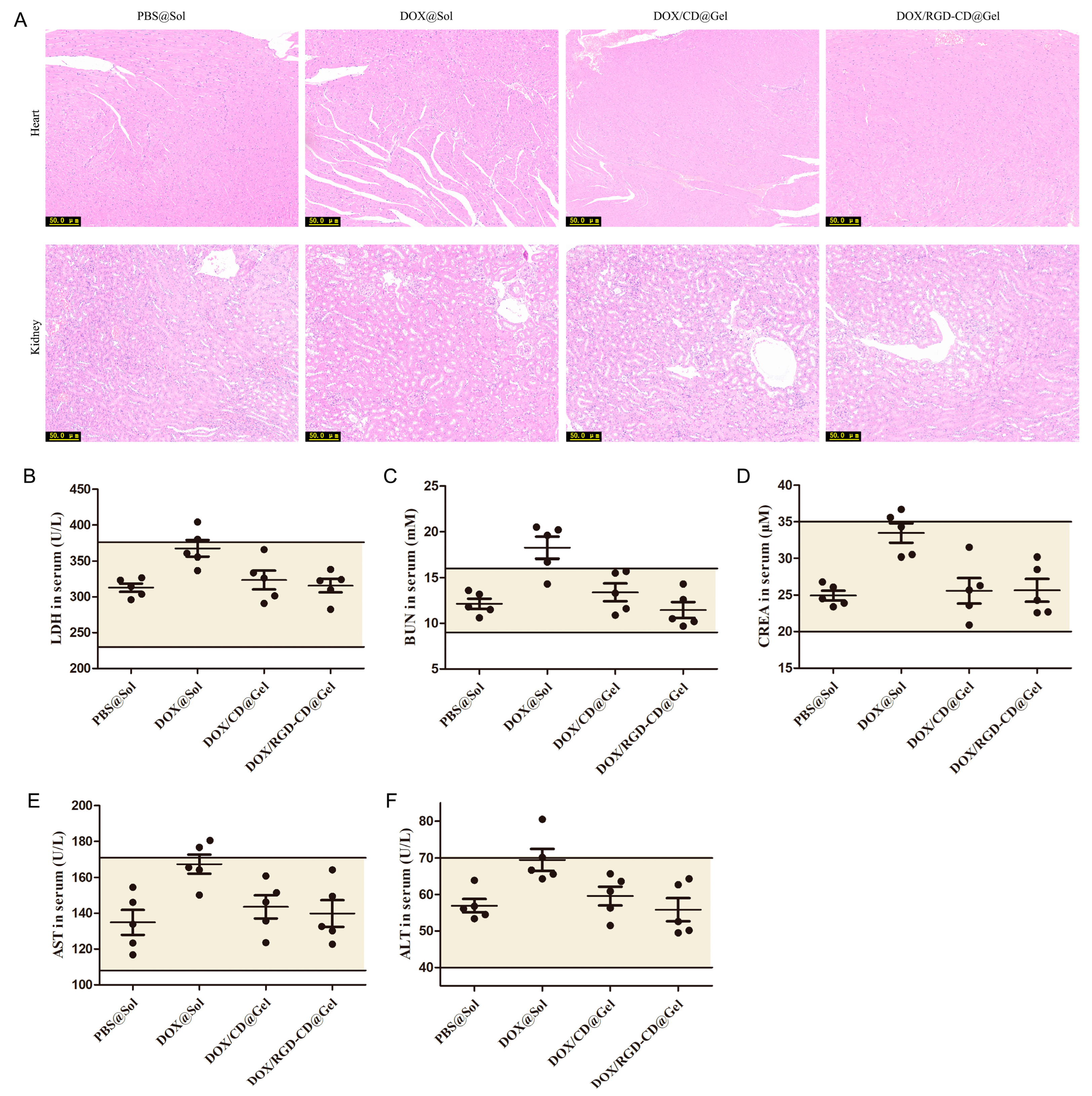
3.7. Preliminary Safety Evaluation of DOX/RGD-CD@Gel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fine, H.A. Glioblastoma: Not Just Another Cancer. Cancer Discov. 2024, 14, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Andreieva, S.; Korets, K.; Mykytenko, D.; Huleyuk, N.; Vassetzky, Y.; Kavsan, V. Systemic and local immunosuppression in glioblastoma and its prognostic significance. Front. Immunol. 2024, 15, 1326753. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.; Henoch, I.; Smits, A.; Rydenhag, B.; Ozanne, A. Quality of life in patients with glioblastoma and their relatives. Acta Neurol. Scand. 2022, 146, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; Lopez-Blanch, R.; Pineda, B.; Gutierrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef] [PubMed]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, H.; Zhang, Y.; Lu, D.; Zheng, L.; Wang, Y.; Chen, S.; Liu, T. Preparation and evaluation of the PD0721-DOX antibody-drug conjugate targeting EGFRvIII to inhibit glioblastoma. Exp. Ther. Med. 2024, 27, 254. [Google Scholar] [CrossRef] [PubMed]
- Shimia, M.; Amini, M.; Ravari, A.O.; Tabnak, P.; Valizadeh, A.; Ghaheri, M.; Yousefi, B. Thymoquinone reversed doxorubicin resistance in U87 glioblastoma cells via targeting PI3K/Akt/mTOR signaling. Chem. Biol. Drug Des. 2024, 104, e14587. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Khoee, S.; Mafakheri, F.; Sadri, E.; Mahabadi, V.P.; Karimi, M.R.; Shirvalilou, S.; Khoei, S. Active targeted delivery of theranostic thermo/pH dual-responsive magnetic Janus nanoparticles functionalized with folic acid/fluorescein ligands for enhanced DOX combination therapy of rat glioblastoma. J. Mater. Chem. B 2024, 12, 5957–5973. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.S.; Lu, Y.J.; Huang, Y.S.; Chen, J.P. Chitosan-coated magnetic graphene oxide for targeted delivery of doxorubicin as a nanomedicine approach to treat glioblastoma. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129401. [Google Scholar] [CrossRef] [PubMed]
- Koula, G.; Yakati, V.; Rachamalla, H.K.; Bhamidipati, K.; Kathirvel, M.; Banerjee, R.; Puvvada, N. Integrin receptor-targeted, doxorubicin-loaded cerium oxide nanoparticles delivery to combat glioblastoma. Nanomedicine 2024, 19, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and non-covalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wei, R.; Wu, J.; Chen, X.; Wen, Y.; Chen, J. Cyclodextrin Drugs in Liposomes: Preparation and Application of Anticancer Drug Carriers. AAPS PharmSciTech 2024, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Nie, T.; Fang, Y.; You, X.; Huang, H.; Wu, J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J. Mater. Chem. B 2022, 10, 2077–2096. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Tosto, R.; Giglio, V.; Pappalardo, G.; Oliveri, V.; Maric, I.; Mariggio, M.A.; Vecchio, G. Cyclodextrin polymers decorated with RGD peptide as delivery systems for targeted anti-cancer chemotherapy. Investig. New Drugs 2019, 37, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tamura, A.; Yui, N. Enhanced Tumor Targeting and Antitumor Activity of Methylated beta-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides. Biomolecules 2024, 14, 223. [Google Scholar]
- Kebebe, D.; Wu, Y.; Zhang, B.; Yang, J.; Liu, Y.; Li, X.; Ma, Z.; Lu, P.; Liu, Z.; Li, J. Dimeric c(RGD) peptide conjugated nanostructured lipid carriers for efficient delivery of Gambogic acid to breast cancer. Int. J. Nanomed. 2019, 14, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, C.; Wang, F.; Liu, H.; Dong, G.; Zhang, S.; Liu, T. Functional drug carriers formed by RGD-modified beta-CD-HPG for the delivery of docetaxel for targeted inhibition of nasopharyngeal carcinoma cells. RSC Adv. 2022, 12, 18004–18011. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, V.; Kolpeja, E.; Baumkotter, G.; Clauder, F.; Bokel, A.; Armbruster, F.P.; Drees, P.; Gercek, E.; Ritz, U. Bone sialoprotein stimulates cancer cell adhesion through the RGD motif and the alphavbeta3 and alphavbeta5 integrin receptors. Oncol. Lett. 2024, 28, 542. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Kondaiah, P.; Bhattacharya, S.; Boturyn, D.; Dumy, P. Co-liposomes comprising a lipidated multivalent RGD-peptide and a cationic gemini cholesterol induce selective gene transfection in alphavbeta3 and alphavbeta5 integrin receptor-rich cancer cells. J. Mater. Chem. B 2014, 2, 5758–5767. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, X.; Guo, B.; Han, Y. Preparation, thermal response mechanisms and biomedical applications of thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2023, 20, 641–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, J.; Sun, J.; Jiang, Y.; Zheng, W.; Hu, W.; Qian, H. Recent advances on thermosensitive hydrogels-mediated precision therapy. Asian J. Pharm. Sci. 2024, 19, 100911. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Firouzi, J.; Sodeifi, N.; Ebrahimi, M.; Miller, D.W. Salinomycin-loaded injectable thermosensitive hydrogels for glioblastoma therapy. Int. J. Pharm. 2021, 598, 120316. [Google Scholar] [CrossRef] [PubMed]
- Anggelia, M.R.; Cheng, H.Y.; Lin, C.H. Thermosensitive Hydrogels as Targeted and Controlled Drug Delivery Systems: Potential Applications in Transplantation. Macromol. Biosci. 2024, 24, e2400064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, K.; Weng, W.; Chen, L.; Wei, C.; Bao, R.; Adu-Frimpong, M.; Cao, X.; Yu, Q.; Shi, F.; et al. Liquiritin-Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex: Preparation, Characterization, Bioavailability and Antitumor Activity Evaluation. J. Pharm. Sci. 2022, 111, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, Y.; Qiao, S.; Liu, M.; Ji, Q.; Zhang, B.L.; Mei, Q.B.; Zhou, S. Nano-Codelivery of Temozolomide and siPD-L1 to Reprogram the Drug-Resistant and Immunosuppressive Microenvironment in Orthotopic Glioblastoma. ACS Nano 2022, 16, 7409–7427. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zheng, C.; Fan, Z. Cardamonin suppressed the migration, invasion, epithelial mesenchymal transition (EMT) and lung metastasis of colorectal cancer cells by down-regulating ADRB2 expression. Pharm. Biol. 2022, 60, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, B.; Dan, W.; Wei, Y.; Li, M.; Guo, C.; Zhang, Y.; Xie, H. Liquiritigenin inhibits the migration, invasion, and EMT of prostate cancer through activating ER stress. Arch. Biochem. Biophys. 2024, 761, 110184. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Gagliano, N. E-Cadherin in Pancreatic Ductal Adenocarcinoma: A Multifaceted Actor during EMT. Cells 2020, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cao, L.L.; Ballout, F.; Belkhiri, A.; Peng, D.; Chen, L.; Chen, Z.; Soutto, M.; Wang, T.C.; Que, J.; et al. Reflux conditions induce E-cadherin cleavage and EMT via APE1 redox function in oesophageal adenocarcinoma. Gut 2023, 73, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.; Kadri, M.; Arosio, D.; Dal Corso, A.; Coll, J.L.; Gennari, C.; Boturyn, D. Multimeric Presentation of RGD Peptidomimetics Enhances Integrin Binding and Tumor Cell Uptake. Chemistry 2020, 26, 7492–7496. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Wang, X.; Wang, Q.; Dai, Q.; Peng, Y.; Yuan, Q.; Mou, N.; Lv, S.; Weng, B.; Wang, Y.; et al. Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics 2023, 15, 1827. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Z.; Hao, W.; Yang, Z.; Farag, M.; Vong, C.T.; Wang, Y.; Wang, S. Berberine and magnolol exert cooperative effects on ulcerative colitis in mice by self-assembling into carrier-free nanostructures. J. Nanobiotechnol. 2024, 22, 538. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, H.; Xu, S.; Kong, J.; Gao, F.; Wang, Z.; Li, Y.; Dai, Z.; Jiang, X.; Ding, X.; et al. Novel Natural Carrier-Free Self-Assembled Nanoparticles for Treatment of Ulcerative Colitis by Balancing Immune Microenvironment and Intestinal Barrier. Adv. Healthc. Mater. 2023, 12, e2301826. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yi, M.; Wei, H.; Zhang, S. Construction of Folate-Conjugated and pH-Responsive Cell Membrane Mimetic Mixed Micelles for Desirable DOX Release and Enhanced Tumor-Cellular Target. Langmuir 2022, 38, 9546–9555. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Lu, Q.; Wei, H.; Zhang, S. Phosphorylcholine zwitterionic shell-detachable mixed micelles for enhanced cancerous cellular uptakes and increased DOX release. J. Mater. Chem. B 2022, 10, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, S.; Rajendran, A.K.; Moon, Y.G.; Hang, N.S. Stimuli-responsive dynamic hydrogels: Design, properties and tissue engineering applications. Mater. Horiz. 2023, 10, 3325–3350. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Inda, M.E.; Lin, S.; Wu, J.; Kim, Y.; Chen, X.; Ma, D.; Lu, T.K.; Zhao, X. Magnetic Living Hydrogels for Intestinal Localization, Retention, and Diagnosis. Adv. Funct. Mater. 2021, 31, 2010918. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, B.; Jia, Y.; Lei, M.; Li, Z.; Liu, H.; Huang, H.; Xu, F.; Li, J.; Wei, Z. Hyaluronate- and gelatin-based hydrogels encapsulating doxycycline as a wound dressing for burn injury therapy. Acta Biomater. 2023, 164, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Poudel, K.; Emami, F.; Ku, S.K.; Jeong, J.H.; Kim, J.O.; Yook, S. Localized therapy using anti-PD-L1 anchored and NIR-responsive hollow gold nanoshell (HGNS) loaded with doxorubicin (DOX) for the treatment of locally advanced melanoma. Nanomedicine 2021, 33, 102349. [Google Scholar] [CrossRef] [PubMed]
- Biezunska-Kusiak, K.; Kulbacka, J.; Choromanska, A.; Rembialkowska, N.; Michel, O.; Saczko, J. Evaluation of the Anticancer Activity of Calcium Ions Introduced into Human Breast Adenocarcinoma Cells MCF-7/WT and MCF-7/DOX by Electroporation. Pharmaceuticals 2023, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Sabokrouh, A.; Atabi, F.; Jassem, R.M.; Mohammadi, R. The Anticancer Efficacy of Platinum Azidothymidin on Hepatocellular Carcinoma Via Affecting the Telomerase and the BcL-2 Genes Expression. J. Gastrointest. Cancer 2020, 51, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, L.; Zhao, B.; Lu, J.; Zhao, Y. E-cadherin is downregulated by microenvironmental changes in pancreatic cancer and induces EMT. Oncol. Rep. 2018, 40, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.I.; Hoffmann, M.; Rinis, N.; Bartels, M.F.; Winterhalter, P.R.; Hoelscher, C.; Hennig, R.; Himmelreich, N.; Thiel, C.; Ruppert, T.; et al. Glycosyltransferase POMGNT1 deficiency strengthens N-cadherin-mediated cell-cell adhesion. J. Biol. Chem. 2021, 296, 100433. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, T.; Wang, R.; Sun, Y.; Chu, L.; Lu, X.; Sun, K. Homotypic targeted nanoplatform enable efficient chemoimmunotherapy and reduced DOX cardiotoxicity in chemoresistant cancer via TGF-beta1 blockade. J. Control. Release 2023, 361, 147–160. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Wang, Z.; Qiu, P.; Tong, Z.; Wang, B.; Sun, Y.; Sun, X.; Sui, L.; Jia, H.; Wang, J.; et al. In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy. Pharmaceutics 2025, 17, 938. https://doi.org/10.3390/pharmaceutics17070938
Yuan X, Wang Z, Qiu P, Tong Z, Wang B, Sun Y, Sun X, Sui L, Jia H, Wang J, et al. In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy. Pharmaceutics. 2025; 17(7):938. https://doi.org/10.3390/pharmaceutics17070938
Chicago/Turabian StyleYuan, Xiaofeng, Zhenhua Wang, Pengcheng Qiu, Zhenhua Tong, Bingwen Wang, Yingjian Sun, Xue Sun, Lu Sui, Haiqiang Jia, Jiajun Wang, and et al. 2025. "In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy" Pharmaceutics 17, no. 7: 938. https://doi.org/10.3390/pharmaceutics17070938
APA StyleYuan, X., Wang, Z., Qiu, P., Tong, Z., Wang, B., Sun, Y., Sun, X., Sui, L., Jia, H., Wang, J., Tang, H., & Ye, W. (2025). In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy. Pharmaceutics, 17(7), 938. https://doi.org/10.3390/pharmaceutics17070938





