Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation
Abstract
1. Introduction
1.1. Background and Clinical Relevance of Transdermal Drug Delivery
1.2. Limitations on the Use of TDD
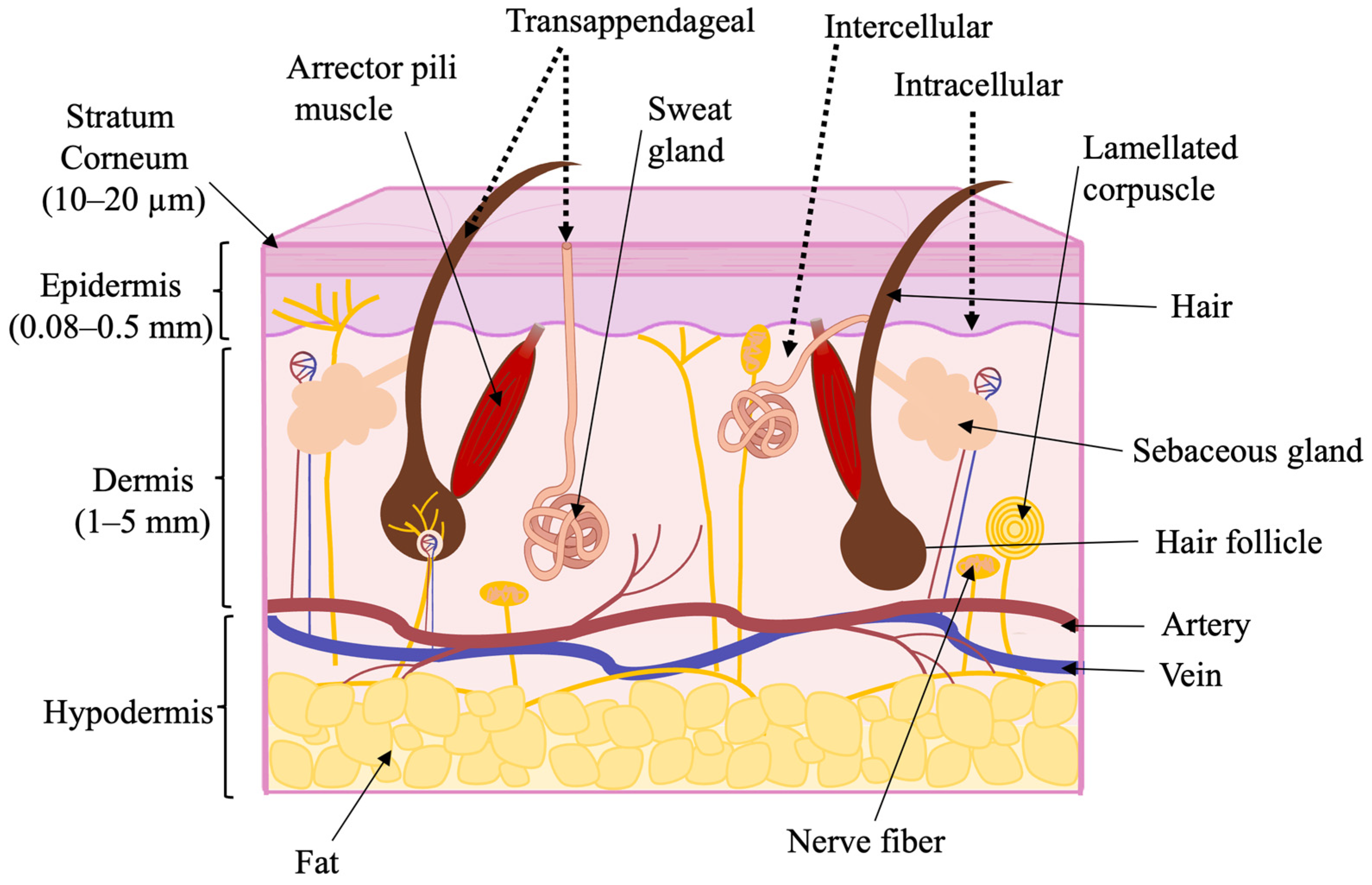
1.3. Skin as a Biological Barrier
1.4. The Influence of Physical and Chemical Properties on Skin Permeability
2. Methods for Enhancing Skin Permeability
| Enhancer/Method | Example Enhancer(s) | Example of API | Application Form | Improved Permeation Data | Reference |
|---|---|---|---|---|---|
| Fatty acid | Oleic acid 5% w/w | Olanzapine (OLZ) | Suspension applied to stratum corneum in the donor chamber of Franz cell. | 3.3-fold increase in enhancement ratio vs. a control formulation of OLZ in propylene glycol | [41] |
| Essential oil | Perilla-ketone (PEK) (3 and 5% w/v) from Perilla frutescens (L.) | Puerarin | Essential oil used on rat skin in horizontal dual-chamber diffusion cells. | PEK significantly enhanced puerarin penetration, with enhancement ratios of 2.96 ± 0.07 at 3% and 3.39 ± 0.21 at 5% (w/v). | [42] |
| Microneedles | Solid microneedle roller (173 ± 26.94 µm micro-channels) | Potassium chloride (KCl) | Aqueous KCl solution applied to porcine skin in Franz diffusion cells after MN pretreatment. | ≈10-fold increase in transdermal flux (6.33 ± 18.70 mg cm−2 h−1 via microneedle-enhanced permeation vs. 0.637 ± 0.02 mg cm−2 h−1 via passive diffusion) | [17] |
| Iontophoresis | 0.2, 0.5, and 1 mA/cm2 current density iontophoresis via Ag/AgCl electrodes | Tetracycline HCl | Niosomal Tetracycline-HCl gel applied to full-thickness porcine skin in Franz diffusion cells. | At 1 mA cm−2, 2.2-fold increase in cumulative permeation versus passive diffusion; ~20% of the loaded dose delivered within the first 30 min | [43] |
| Liposome | 1,2-dioleoyloxy-3-trimethylammoniumpropan (DOTAP)/Span 80/cholesterol liposomes (F-07) incorporated into Carbopol-940 hydrogel | Simvastatin | Optimised liposomal gel, applied topically to full-thickness dorsal-skin wounds in rats. | The optimized liposome gel achieved a 97% reduction in wound area after 16 days, compared to the posistive control gel with 59.67% reduction rate. | [44] |
| Ethosome | Soybean-lecithin ethosomes (30% v/v ethanol) dispersed in Carbomer-940 gel | Huperzine A | Ethosomal gel applied to full-thickness mouse abdominal skin in Franz diffusion cells. | The Huperzine A ethosome gel showed enhanced permeation, with a Q24 of 40.99 ± 4.83 μg/cm2, outperforming the ordinary gel (21.49 ± 1.99 μg/cm2) and cream (16.80 ± 1.57 μg/cm2) (p < 0.01). | [45] |
2.1. Chemical Methods of Permeability Enhancement
2.1.1. Water
2.1.2. Hydrocarbons
2.1.3. Alcohols
2.1.4. Fatty Acids
2.1.5. Esters of Fatty Acids
2.1.6. Amines
2.1.7. Amides
2.1.8. Surfactants
2.1.9. Terpenes
2.1.10. Essential Oils
2.1.11. Sulfoxides
| Type | Mechanism | Advantages | Disadvantages | Examples | Reference |
|---|---|---|---|---|---|
| Water | Swelling of the stratum corneum and loosening of its compact structure facilitate drug permeation | The most natural penetrator increases penetration of both hydrophobic and hydrophilic drugs | Limited action | Water | [31,46] |
| Hydrocarbons | Disruption of the lipid structure of the stratum corneum | Good solvents for lipophilic drugs; occlusive properties improve skin hydration | Low enhancement for hydrophilic drugs; greasy residue may affect patient compliance | Alkanes, alkenes, halogenated alkanes, squalane, squalene, mineral oil | [31] |
| Alcohols | Lipid and protein extraction; Swelling of the stratum corneum; Improving the distribution of drugs in the skin; Drug supersaturation | Increasing diffusion rate due to drug supersaturation; Cyclic polyols can interact with biological barriers; The use of propylene glycols minimizes drug contact time with tissues | Isopropanol and n-propanol cause significant disruption of the stratum corneum and keratinocytes; Fatty alcohols increase transepidermal water loss, | Ethanol, glycerol, fatty alcohols, cyclic polyols, Isopropyl alcohol | [31,34,47,51] |
| Fatty acids | Disruption of lipid structures of the stratum corneum; Enhanced spreading of drugs in the stratum corneum; Formation of lipophilic complexes with drugs | Good skin compatibility; effective for both hydrophilic and lipophilic drugs, low cost | Less efficient trans-configurations and unsaturated compounds | Linoleic, Lauric, oleic, caprylic, Palmitoleic, and other fatty acids | [26,27,30] |
| Esters of fatty acids | Disruption of the lipid organization within the stratum corneum | Enhanced transdermal penetration of a wide range of drugs has been found | At high concentrations, Transcutol® can dehydrate the stratum corneum, thereby reducing transdermal drug penetration | Isopropyl myristate, Isopropyl palmitate, Transcutol®, Ethyl oleate | [31,48,52] |
| Amines | Improving the distribution of drugs in the skin; Separation of the lamellar lipid bilayers of the stratum corneum | Disrupt lipid packing; enhance delivery of a wide range of actives | Potential for irritation or allergic response with prolonged use | Primary, secondary and tertiary, cyclic and acyclic amines | [31] |
| Amides | Reducing the diffusion resistance of the drug substance in the stratum corneum; Integrating into the region of the lipid bilayer; Increased fluidity of stratum corneum lipids; Disruption of lipid structures of the stratum corneum | Enhance the penetration of hydrophilic and hydrophobic compounds and some peptides | Adverse effects associated with the use of pyrrolidones; Insufficient chemical stability of urea | Azone (laurocapram), pyrrolidone, urea | [31,46] |
| Surfactants | Denaturation or binding to skin proteins; increased fluidity of intercellular lipids in the stratum corneum; direct penetration through the stratum corneum or interaction with corneocytes | Improve solubilization and diffusion of drugs; widely used in formulations, a broad spectrum of surfactants is commercially available on the market | Cationic surfactants cause dermal irritation | Anionic (Sodium lauryl sulfate), cationic amines, alkyl imidazolines, alkoxylated amines and quaternary ammonium compounds), zwitter-ionic, non-ionic, Cetyltrimethylammonium bromide | [31,46] |
| Terpenes | Influence on the polar hydrophilic end of the lipid bilayer; Disruption of the hydrogen bonding network; Formation of new polar channels in the skin | Temporary and relatively low skin irritation; Higher penetration characteristics; The combination of terpenes improves the penetration effect; Enhanced membrane lipid fluidity | Can cause mild to moderate irritation at high concentrations | Oxygen-containing sesquiterpenes, menthol, Eugenol, 1,4-cineole, 1,8-cineole, Thymol, Limonene | [49,50] |
| Essential oils | Disintegration of highly ordered intercellular lipid structure between corneocytes in the stratum corneum; Interaction with intercellular protein leading to conformational modifications | Safety; Quickly metabolized; Not accumulated in the body; Quickly excreted from the body after application to the skin; Enhanced drug distribution in the stratum corneum” | Potential for allergic reactions with repeated use | Perilla-ketone (PEK), Peppermint Oil, Turpentine Oil | [32,50,52] |
| Sulfoxides | Formation of solvent-filled free spaces in the stratum corneum | High-efficacy | Locally irritating effect | Dimethyl sulfoxide | [31,48] |
2.2. Physical Methods of Permeability Enhancement
2.2.1. Iontophoresis
2.2.2. Sonophoresis
2.2.3. Microneedles
2.2.4. Elongated Microparticles
2.2.5. Electroporation
2.2.6. Needle-Free Jet Injection
| Method | Basis of Method | Advantages | Limitations | Examples | Reference |
|---|---|---|---|---|---|
| Iontophoresis | The use of a pair of electrodes placed on the skin to create an electrical potential between the skin surface and capillaries | Does not disturb the structure of the skin; Easy integration of conductive bases with iontophoretic systems | The delivery of negatively charged molecules is hampered; Low amperage can limit transport efficacy; High amperage can increase the risk of skin irritation; Requirement for the use of electrically conductive bases | LidoSite® (lidocaine and epinephrine for anesthesia) IONSYS™ (fentanyl iontophoretic system for acute postoperative) | [53,54,55] |
| Sonophoresis | Use of mechanical force generated by ultrasound that increases skin permeability to drugs through hyperthermia or cavitation | Ability to deliver large and hydrophilic molecules; | High-frequency sonophoresis can cause damage to deep skin tissues; Low-frequency sonophoresis often requires an appropriate environment; The need for sophisticated devices | SonoPrep® (ultrasonic skin permeation system and topical anesthetic kit) | [54,58,61] |
| Microneedles | Creation of micro-sized pathways for transporting molecules | Less invasive than parenteral routes of administration; Low level of pain and discomfort during use; Self-administration by patients; Ability to deliver macromolecules | Risk of skin irritation or infection; Limited drug loading per microneedle; Requires specialized manufacturing | MicronJet™ (dissolving hyaluronic acid microneedles) | [16,17,62] |
| Elongated microparticles | When applied, elongated microparticles that may be mixed with the drug pass through the epidermis carrying the drug | Unlimited area of application on the skin; Penetrates primarily into the epidermis, minimizing damage to the dermis; Natural elimination (transepidermal) | More suitable for use with low-viscosity creams, gels, and lotions | Elongated silica microparticles with hyaluronic acid | [36,38,63] |
| Electroporation | The use of high-voltage electrical pulses of a millisecond or microsecond duration, under which pores are formed in the skin | Facilitates the penetration of hydrophilic macromolecules as well as biomolecules; Formation of reversible pores in the skin | May cause discomfort or skin irritation; Requires electronic devices and energy sources; Not suitable for all drug types | Nanocomposite hydrogel system serving as both drug reservoir and skin electrode for electric-pulse delivery | [57,59,60,64] |
| Needle-free jet injection | A high-pressure device is used to achieve a high rate of liquid drug injection, allowing the therapeutic agents to penetrate the epidermis and spread into the subcutaneous fatty tissue without the use of a needle. | Ability to deliver macromolecules; Ability to modify the delivery area by flow rate and orifice diameter; | Injection depth may vary; Potential for bruising or local pain; High device cost and maintenance | ZETAJET Needle-Free Injection Therapy System | [33,35,65] |
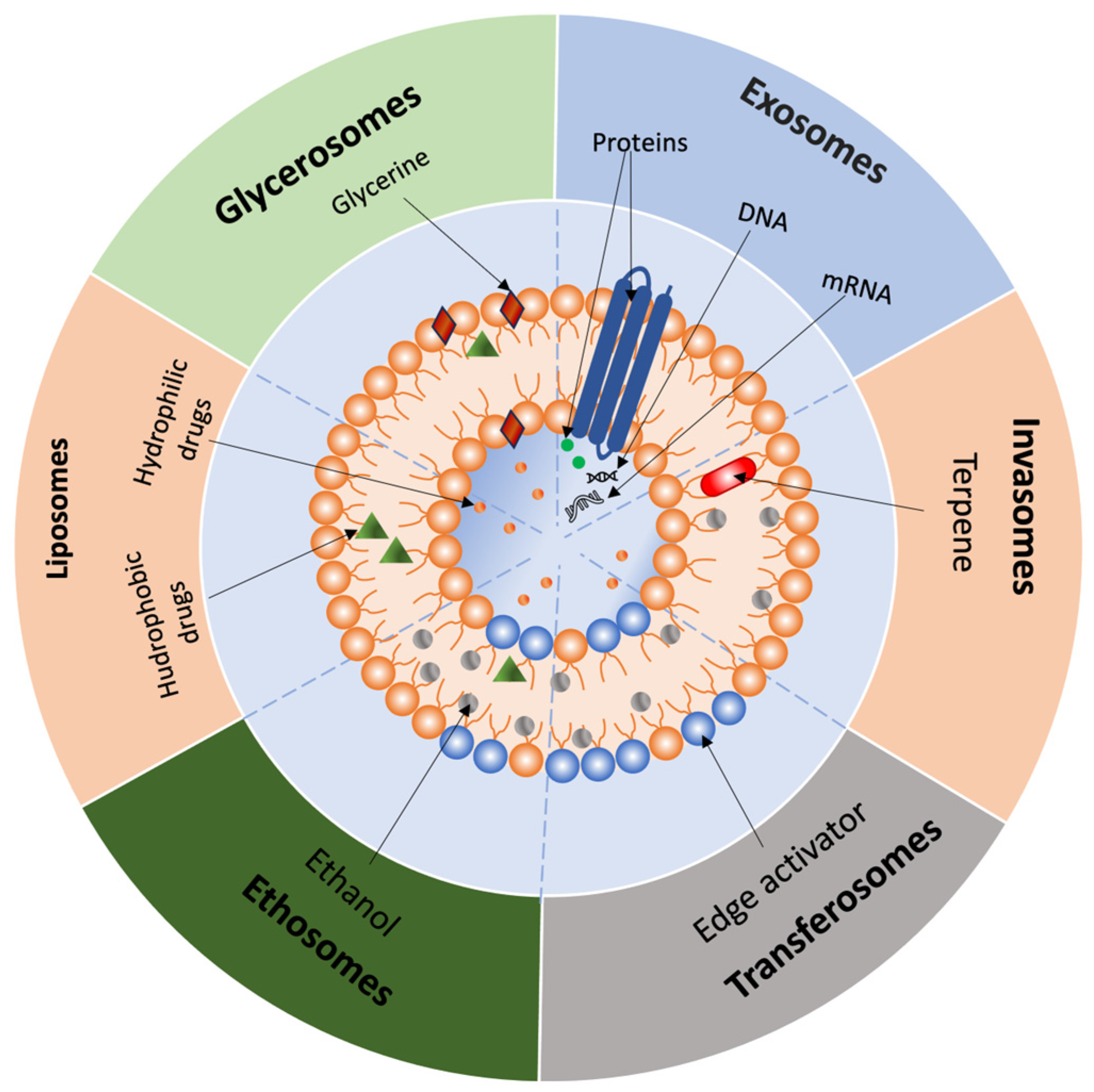
2.3. Nanotechnology Methods of Permeability Enhancement
2.3.1. Nanoemulsion and Microemulsion
2.3.2. Liposomes
2.3.3. Invasomes
2.3.4. Transferosomes
2.3.5. Ethosomes
2.3.6. Glycerosomes
2.3.7. Polymeric Nanoparticles
2.3.8. Solid Lipid Nanoparticles (SLNs)
2.3.9. Nanostructured Lipid Carriers (NLCs)
2.3.10. Self-Emulsifying Systems
2.3.11. Exosomes
3. Assessment Methods for Transdermal Drug Delivery Systems
3.1. In Vitro/Ex Vivo Methods
3.1.1. Vertical Diffusion Cells (VDCs)
3.1.2. Flow Cells
3.1.3. Organ-on-a-Chip Devices
3.1.4. Tape Stripping
3.2. In Vivo Models
4. Visualization and Readout Methods for Evaluating Skin Penetration
4.1. Confocal Laser Scanning Microscopy (CLSM)
4.2. Confocal Raman Spectroscopy (CRS)
5. Membranes and Skin Models
5.1. Human Skin
5.2. Animal Skin
5.3. Artificial Membrane
5.4. Human Skin Equivalents
5.4.1. MIVO® (Multi In Vitro Organ) Systems
5.4.2. Skin-PAMPA (Parallel Artificial Membrane Permeability Assay) System
6. Regulatory Aspects of Transdermal Drug Delivery Systems
6.1. Regulatory and Safety Framework for Transdermal Systems
6.2. Bioequivalence Assessment of Transdermal Drug Delivery Systems
6.3. IVRT and IVPT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDD | Transdermal drug delivery |
| NSAI | Non-steroidal aromatase inhibitor |
| APIs | Active pharmaceutical ingredients |
| OLZ | Olanzapine |
| PEK | Perilla-ketone |
| DOTAR | 1,2-dioleoyloxy-3-trimethylammoniumpropan |
| ATRA | All-trans retinoic acid |
| PA | Phosphatidic acid |
| PEA | Phosphatidylethanol |
| PC | Phosphatidylcholine |
| PG | Phosphatidylglycerol |
| PEG | Polyethylene glycol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| PDI | Polydispersity index |
| PLGA | Poly (lactic-co-glycolic) acid |
| mPEG | Methoxypolyethylene glycol |
| PBA-G5D | Phenylboronic acid-PAMAM dendrimer |
| PTX | Paclitaxel |
| PEC | Polyelectrolyte complex |
| SLNs | Solid Lipid Nanoparticles |
| NLCs | Nanostructured Lipid Carriers |
| PELNVs | Plant exosome-like vesicles |
| ROS | Reactive oxygen species |
| VDCs | Vertical Diffusion Cells |
| MIVO® | Multi In Vitro Organ |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| PSU | Polysulfone |
| PES | Polyethersulfone |
| RHE | Reconstructed human epidermis |
| FT | Full-thickness |
| HPLC | High-Performance Liquid Chromatography |
| CLSM | Confocal Laser Scanning Microscopy |
| CRS | Confocal Raman Spectroscopy |
| DOFM | Dermal Open-Flow Microperfusion |
| Cmax | Maximum plasma concentration |
| AUC | Area Under the Curve |
| AUC0−t | Area Under the Curve from time zero to the last measurable concentration |
| AUC0−∞ | Area Under the Curve from time zero extrapolated to infinity—gives the complete systemic exposure |
| FDA | The United States Food and Drug Administration |
| EAEU | The Eurasian Economic Union |
| EEU | The Eurasian Economic Union |
| EMA | The European Medicines Agency |
| IVRT | In vitro release test |
| IVPT | In vitro permeation test |
References
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal Patches: History, Development and Pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Zadymova, N.M. Colloidochemical Aspects of Transdermal Drug Delivery (Review). Colloid J. 2013, 75, 491–503. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Vora, D.; Banga, A.K. Development and Evaluation of a Drug-in-Adhesive Transdermal Delivery System for Delivery of Olanzapine. Expert Opin. Drug Deliv. 2022, 19, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Cullen, B.D.; Tang, M.; Fang, Y. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 2020, 21, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Hulihan, J.; Messenheimer, J.; Ali, S.; Keenan, N.; Griesser, J.; Gutterman, D.L.; Sebree, T.; Sadleir, L.G. Safety and Tolerability of Transdermal Cannabidiol Gel in Children With Developmental and Epileptic Encephalopathies: A Nonrandomized Controlled Trial. JAMA Netw. Open 2021, 4, e2123930. [Google Scholar] [CrossRef] [PubMed]
- Heussler, H.; Cohen, J.; Silove, N.; Tich, N.; Bonn-Miller, M.O.; Du, W.; O’Neill, C.; Sebree, T. A Phase 1/2, Open-Label Assessment of the Safety, Tolerability, and Efficacy of Transdermal Cannabidiol (ZYN002) for the Treatment of Pediatric Fragile X Syndrome. J. Neurodev. Disord. 2019, 11, 16. [Google Scholar] [CrossRef]
- Steffen, R.; Cramer, J.P.; Burchard, G.; Jelinek, T.; Schwarz, U.; Ramdas, P.; Chatterjee, S.; Jiang, Z.-D.; DuPont, H.L.; Dewasthaly, S.; et al. Efficacy of a Travelers’ Diarrhea Vaccine System in Travelers to India. J. Travel Med. 2013, 20, 374–379. [Google Scholar] [CrossRef]
- Eypper, E.H.; Johnson, P.V.; Purro, E.I.; Hohmann, E.L. Transcutaneous Immunization of Healthy Volunteers with an Attenuated Listeria Monocytogenes Vaccine Strain and Cholera Toxin Adjuvant. Vaccine 2013, 31, 3257–3261. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, J.U.; Kim, S.H.; Noh, J.Y.; Kim, H.R.; Lee, J.; Chu, H.; Jeong, K.Y.; Park, K.H.; Kim, J.D.; et al. Successful Transdermal Allergen Delivery and Allergen-Specific Immunotherapy Using Biodegradable Microneedle Patches. Biomaterials 2018, 150, 38–48. [Google Scholar] [CrossRef]
- Walczak, A.; Siger, M.; Ciach, A.; Szczepanik, M.; Selmaj, K. Transdermal Application of Myelin Peptides in Multiple Sclerosis Treatment. JAMA Neurol. 2013, 70, 1105–1109. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B.; Aspiro, A.; Di Cerbo, A. A Preliminary Study of Painless and Effective Transdermal Botulinum Toxin A Delivery by Jet Nebulization for Treatment of Primary Hyperhidrosis. Drug Des. Devel. Ther. 2014, 8, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Mehrsai, A.R.; Namdari, F.; Salavati, A.; Dehghani, S.; Allameh, F.; Pourmand, G. Comparison of Transdermal Electromotive Administration of Verapamil and Dexamethasone versus Intra-Lesional Injection for Peyronie’s Disease. Andrology 2013, 1, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.Y.; Shih, G.; Ilani, N.; Wang, C.; Page, S.T.; Bremner, W.J.; Swerdloff, R.S.; Sitruk-Ware, R.; Blithe, D.L.; Amory, J.K. Acceptability of a Transdermal Gel-Based Male Hormonal Contraceptive in a Randomized Controlled Trial. Contraception 2014, 90, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.S.; D’Souza, A.; Aibani, N.; Nair, S.S.; Sandbhor, P.; Kumari, D.; Banerjee, R. Stable Liposome in Cosmetic Platforms for Transdermal Folic Acid Delivery for Fortification and Treatment of Micronutrient Deficiencies. Sci. Rep. 2018, 8, 16122. [Google Scholar] [CrossRef]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. An Overview of Microneedle Applications, Materials, and Fabrication Methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar] [CrossRef]
- Abiandu, I.; Ita, K. Transdermal Delivery of Potassium Chloride with Solid Microneedles. J. Drug Deliv. Sci. Technol. 2019, 53, 101216. [Google Scholar] [CrossRef]
- Losquadro, W.D. Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289. [Google Scholar] [CrossRef]
- Pandey, P.C.; Shukla, S.; Skoog, S.A.; Boehm, R.D.; Narayan, R.J. Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors 2019, 19, 1028. [Google Scholar] [CrossRef]
- Murphrey, M.B.; Miao, J.H.; Zito, P.M. Histology, Stratum Corneum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Benson, H.A.E. Transfersomes for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Brito, S.; Baek, M.; Bin, B.-H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1403. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation Enhancer Strategies in Transdermal Drug Delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and Opportunities in Dermal/Transdermal Delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Mathur, V.; Satrawala, Y.; Rajput, M. Physical and Chemical Penetration Enhancers in Transdermal Drug Delivery System. Asian J. Pharm. 2010, 4, 173–183. [Google Scholar] [CrossRef]
- Patel, H.; Trivedi, D.; Bhandari, A.; Shah, D. Penetration Enhancers for Transdermal Drug Delivery System: A Review. Int. J. Pharm. Innov. J. Pharm. Cosmetol. 2011, 1, 67–80. [Google Scholar]
- Parhi, R.; Swain, S. Transdermal Evaporation Drug Delivery System: Concept to Commercial Products. Adv. Pharm. Bull. 2018, 8, 535–550. [Google Scholar] [CrossRef]
- Lim, D.-J.; Kim, H.-J. Microneedles in Action: Microneedling and Microneedles-Assisted Transdermal Delivery. Polymers 2022, 14, 1608. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton Rule for the Skin Penetration of Chemical Compounds and Drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of Transdermal Drug Delivery via Synergistic Action of Chemicals. Biochim. Biophys. Acta BBA—Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Long, L.; Zhang, J.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Transdermal Delivery of Peptide and Protein Drugs: Strategies, Advantages and Disadvantages. J. Drug Deliv. Sci. Technol. 2020, 60, 102007. [Google Scholar] [CrossRef]
- Kanikkannan, N.; Singh, M. Skin Permeation Enhancement Effect and Skin Irritation of Saturated Fatty Alcohols. Int. J. Pharm. 2002, 248, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Alberti, I.; Lapteva, M.; Kalia, Y.N. Next Generation Intra- and Transdermal Therapeutic Systems: Using Non- and Minimally-Invasive Technologies to Increase Drug Delivery into and across the Skin. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tayeb, H.; Wang, H.; Dang, N.; Mohammed, Y.H.; Osseiran, S.; Belt, P.J.; Roberts, M.S.; Evans, C.L.; Sainsbury, F.; et al. Using Elongated Microparticles to Enhance Tailorable Nanoemulsion Delivery in Excised Human Skin and Volunteers. J. Control. Release Off. J. Control. Release Soc. 2018, 288, 264–276. [Google Scholar] [CrossRef]
- Liu, S.; Deng, T.; Cheng, H.; Lu, J.; Wu, J. Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases. Pharmaceutics 2025, 17, 746. [Google Scholar] [CrossRef]
- Raphael, A.P.; Garrastazu, G.; Sonvico, F.; Prow, T.W. Formulation Design for Topical Drug and Nanoparticle Treatment of Skin Disease. Ther. Deliv. 2015, 6, 197–216. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Mirtaleb, M.S.; Shahraky, M.K.; Ekrami, E.; Mirtaleb, A. Advances in Biological Nano-Phospholipid Vesicles for Transdermal Delivery: A Review on Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102331. [Google Scholar] [CrossRef]
- Virani, A.; Dholaria, N.; Mohd, H.; Albayati, N.; Michniak-Kohn, B. Effect of Chemical Penetration Enhancers on the Transdermal Delivery of Olanzapine in Human Skin in Vitro. AAPS Open 2024, 10, 4. [Google Scholar] [CrossRef]
- Zhao, N.; Hao, J.; Zhao, Y.; Zhao, B.; Lin, J.; Song, J.; Wang, M.; Luo, Z. A Novel Natural Penetration Enhancer for Transdermal Drug Delivery: In Vitro/In Vivo Evaluation and Penetration Enhancement Mechanism. Pharmaceutics 2025, 17, 254. [Google Scholar] [CrossRef]
- Duman, G.; Gucu, E.; Utku, F.S.; Uner, B.; Macit, M.; Sarialtin, S.; Ozilgen, M. Kinetic Assessment of Iontophoretic Delivery Efficiency of Niosomal Tetracycline Hydrochloride Incorporated in Electroconductive Gel. Drug Deliv. Transl. Res. 2024, 14, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Rahamathulla, M.; Pokale, R.; Al-Ebini, Y.; Osmani, R.A.M.; Thajudeen, K.Y.; Gundawar, R.; Ahmed, M.M.; Farhana, S.A.; Shivanandappa, T.B. Simvastatin-Encapsulated Topical Liposomal Gel for Augmented Wound Healing: Optimization Using the Box-Behnken Model, Evaluations, and In Vivo Studies. Pharmaceuticals 2024, 17, 697. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, R.; Xu, X.; Ye, S.; Huang, A. Preparation and Evaluation of Transdermal Permeation of Huperzine A Ethosomes Gel in Vitro. BMC Pharmacol. Toxicol. 2024, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Vasyuchenko, E.P.; Orekhov, P.S.; Armeev, G.A.; Bozdaganyan, M.E. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics 2021, 13, 66. [Google Scholar] [CrossRef]
- Cartner, T.; Brand, N.; Tian, K.; Saud, A.; Carr, T.; Stapleton, P.; Lane, M.E.; Rawlings, A.V. Effect of Different Alcohols on Stratum Corneum Kallikrein 5 and Phospholipase A2 Together with Epidermal Keratinocytes and Skin Irritation. Int. J. Cosmet. Sci. 2017, 39, 188–196. [Google Scholar] [CrossRef]
- Gildeeva, G.N.; Ejova, E.A.; Zakaliukina, E.V.; Ivanova, A.A. The trans-dermal therapeutic systems as a convenient alternative of traditional medicinal forms. Probl. Sotsialnoi Gig. Zdr. Istor. Meditsiny 2019, 27, 997–1002. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of Terpene Alcohols as Highly Potent, Reversible, and Low Toxic Skin Penetration Enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of Essential Oils as Skin Permeation Enhancers: Penetration Enhancement Effect and Mechanism of Action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef]
- Sim, Y.S.; Wong, L.C.; Yeoh, S.C.; Almashhadani, A.; Alrimawi, B.H.; Goh, C.F. Skin Penetration Enhancers: Mechanistic Understanding and Their Selection for Formulation and Design. Drug Deliv. Transl. Res. 2025, 224. [Google Scholar] [CrossRef]
- Ruan, J.; Wan, X.; Quan, P.; Liu, C.; Fang, L. Investigation of Effect of Isopropyl Palmitate on Drug Release from Transdermal Patch and Molecular Dynamics Study. AAPS PharmSciTech 2019, 20, 174. [Google Scholar] [CrossRef]
- Blue Cross Blue Shield of Massachusetts. Medical Policy: Iontophoresis and Phonophoresis as a Transdermal Technique for Drug Delivery. Available online: https://www.bluecrossma.org/medical-policies/sites/g/files/csphws2091/files/acquiadam-assets/095%20Iontophoresis%20and%20Phonophoresis%20as%20a%20Transdermal%20Technique%20for%20Drug%20Delivery%20prn.pdf (accessed on 19 July 2025).
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in Transdermal Insulin Delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). LidoSite® (Lidocaine and Epinephrine Iontophoretic Patch) Labeling Information; NDA 021504. 2004. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21504_lidoSite_lbl.pdf (accessed on 19 July 2025).
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Controlled Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-Enhanced Transdermal Drug Delivery: Effects of logP, pKa, Solubility and Penetration Time. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 151, 105410. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in Transdermal Drug Deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kougkolos, G.; Laudebat, L.; Dinculescu, S.; Simon, J.; Golzio, M.; Valdez-Nava, Z.; Flahaut, E. Skin Electroporation for Transdermal Drug Delivery: Electrical Measurements, Numerical Model and Molecule Delivery. J. Control. Release Off. J. Control. Release Soc. 2024, 367, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Blagus, T.; Markelc, B.; Cemazar, M.; Kosjek, T.; Preat, V.; Miklavcic, D.; Sersa, G. In Vivo Real-Time Monitoring System of Electroporation Mediated Control of Transdermal and Topical Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 862–871. [Google Scholar] [CrossRef]
- 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=14526 (accessed on 27 June 2025).
- 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=k092746 (accessed on 27 June 2025).
- Yamada, M.; Dang, N.; Lin, L.L.; Flewell-Smith, R.; Jane L Espartero, L.; Bramono, D.; Grégoire, S.; Belt, P.J.; Prow, T.W. Elongated Microparticles Tuned for Targeting Hyaluronic Acid Delivery to Specific Skin Strata. Int. J. Cosmet. Sci. 2021, 43, 738–747. [Google Scholar] [CrossRef]
- Wong, T.W. Electrical, Magnetic, Photomechanical and Cavitational Waves to Overcome Skin Barrier for Transdermal Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 193, 257–269. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). 510(k) Summary: ZETAJET Needle-Free Injection Therapy System, K090003. Silver Spring, MD: FDA. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf9/K090003.pdf (accessed on 19 July 2025).
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical Applications of Microemulsion through Dermal and Transdermal Route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Meng, X.; Li, C.; Wang, H.; Zhang, S. Enhancement of Transdermal Delivery of Artemisinin Using Microemulsion Vehicle Based on Ionic Liquid and Lidocaine Ibuprofen. Colloids Surf. B Biointerfaces 2020, 189, 110886. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lin, H.; Wang, Y.; Wang, X.; Yao, J.; Fu, X.; Yu, X. Design, Optimization and Evaluation of a Microemulsion-Based Hydrogel with High Malleability for Enhanced Transdermal Delivery of Levamisole. Int. J. Pharm. 2021, 605, 120829. [Google Scholar] [CrossRef] [PubMed]
- Souza de Araujo, G.R.; Mendonça da Cruz Macieira, G.; Xavier de Oliveira, D.; Santos Matos, S.; Nery Dos Santos, Q.; Otubo, L.; Antunes de Souza Araújo, A.; Cavalcante Duarte, M.; Moreira Lira, A.A.; de Souza Nunes, R.; et al. Microemulsions Formed by PPG-5-CETETH-20 at Low Concentrations for Transdermal Delivery of Nifedipine: Structural and in Vitro Study. Colloids Surf. B Biointerfaces 2022, 214, 112474. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, S.; Du, Y.; Li, D.; Pan, N.; Chai, J.; Li, D. A New Application of Surfactant-Free Microemulsion: Solubilization and Transport of Drugs and Its Transdermal Release Properties. J. Taiwan Inst. Chem. Eng. 2022, 138, 104473. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.-A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Lipid Cell Biology. Polyunsaturated Phospholipids Facilitate Membrane Deformation and Fission by Endocytic Proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef]
- Tsuji, T.; Morita, S.-Y.; Ikeda, Y.; Terada, T. Enzymatic Fluorometric Assays for Quantifying All Major Phospholipid Classes in Cells and Intracellular Organelles. Sci. Rep. 2019, 9, 8607. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Shashi, K.; Satinder, K.; Parashar, B. A Complete Review on: Liposomes. Int. Res. J. Pharm. 2012, 3, 10–16. [Google Scholar]
- Nandi, U.; Onyesom, I.; Douroumis, D. Transferrin Conjugated Stealth Liposomes for Sirolimus Active Targeting in Breast Cancer. J. Drug Deliv. Sci. Technol. 2021, 66, 102900. [Google Scholar] [CrossRef]
- Huang, M.; Pu, Y.; Peng, Y.; Fu, Q.; Guo, L.; Wu, Y.; Zheng, Y. Biotin and Glucose Dual-Targeting, Ligand-Modified Liposomes Promote Breast Tumor-Specific Drug Delivery. Bioorg. Med. Chem. Lett. 2020, 30, 127151. [Google Scholar] [CrossRef] [PubMed]
- El-Tokhy, F.S.; Abdel-Mottaleb, M.M.A.; El-Ghany, E.A.; Geneidi, A.S. Design of Long Acting Invasomal Nanovesicles for Improved Transdermal Permeation and Bioavailability of Asenapine Maleate for the Chronic Treatment of Schizophrenia. Int. J. Pharm. 2021, 608, 121080. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Yasir, M.; Singh, L.; Jafar, M.; Warsi, M.H.; Panda, D.S. Luteolin-Loaded Invasomes Gel for Transdermal Delivery: Development, Optimization, in-Vitro, and Preclinical Evaluation. J. Oleo Sci. 2024, 73, 1221–1240. [Google Scholar] [CrossRef] [PubMed]
- El-Kayal, M.; Hatem, S. A Comparative Study between Nanostructured Lipid Carriers and Invasomes for the Topical Delivery of Luteolin: Design, Optimization and Pre-Clinical Investigations for Psoriasis Treatment. J. Drug Deliv. Sci. Technol. 2024, 97, 105740. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as Nanocarriers for Drugs across the Skin: Quality by Design from Lab to Industrial Scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Khan, M.I.; Yaqoob, S.; Madni, A.; Akhtar, M.F.; Sohail, M.F.; Saleem, A.; Tahir, N.; Khan, K.-R.; Qureshi, O.S. Development and In Vitro/Ex Vivo Evaluation of Lecithin-Based Deformable Transfersomes and Transfersome-Based Gels for Combined Dermal Delivery of Meloxicam and Dexamethasone. BioMed Res. Int. 2022, 2022, 8170318. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal Nanocarriers: The Impact of Constituents and Formulation Techniques on Ethosomal Properties, in Vivo Studies, and Clinical Trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Malviya, N.; Prabakaran, A.; Alexander, A. Comparative Study on Ethosomes and Transferosomes for Enhancing Skin Permeability of Sinapic Acid. J. Mol. Liq. 2023, 383, 122098. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wu, T.; He, Z.; Guo, T.; Feng, N. Optimizing Glycerosome Formulations via an Orthogonal Experimental Design to Enhance Transdermal Triptolide Delivery. Acta Pharm. Zagreb Croat. 2022, 72, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Glycerosomes and Use Thereof in Pharmaceutical and Cosmetic Preparations for Topical Application—Patent WO-2010102770-A1—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/WO-2010102770-A1 (accessed on 13 April 2025).
- Shahien, M.M.; Alshammari, A.; Ibrahim, S.; Ahmed, E.H.; Atia, H.A.; Elariny, H.A.; Abdallah, M.H. Development of Glycerosomal pH Triggered In Situ Gelling System to Ameliorate the Nasal Delivery of Sulpiride for Pediatric Psychosis. Gels 2024, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A New Tool for Effective Dermal and Transdermal Drug Delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Younes, N.F.; Habib, B.A. Augmented Local Skin Accumulation Efficiency of Sertaconazole Nitrate via Glycerosomal Hydrogel: Formulation, Statistical Optimization, Ex Vivo Performance and in Vivo Penetration. J. Drug Deliv. Sci. Technol. 2022, 72, 103364. [Google Scholar] [CrossRef]
- Sharma, D.; Rani, A.; Singh, V.D.; Shah, P.; Sharma, S.; Kumar, S. Glycerosomes: Novel Nano-Vesicles for Efficient Delivery of Therapeutics. Recent Adv. Drug Deliv. Formul. 2023, 17, 173–182. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef]
- Ruan, H.; Shen, L.; Hou, X.; Li, J.; Guo, T.; Zhu, C.; Feng, N.; Zhang, Y. Phytosterol-Mediated Glycerosomes Combined with Peppermint Oil Enhance Transdermal Delivery of Lappaconitine by Modulating the Lipid Composition of the Stratum Corneum. Drug Deliv. Transl. Res. 2023, 13, 3014–3029. [Google Scholar] [CrossRef]
- Gupta, P.; Mazumder, R.; Padhi, S. Glycerosomes: Advanced Liposomal Drug Delivery System. Indian J. Pharm. Sci. 2020, 82, 385–397. [Google Scholar] [CrossRef]
- Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9, 752. [Google Scholar] [CrossRef]
- Ahmed, S.; Attia, H.; Saher, O.; Fahmy, A.M. Augmented Glycerosomes as a Promising Approach against Fungal Ear Infection: Optimization and Microbiological, Ex Vivo and in Vivo Assessments. Int. J. Pharm. X 2024, 8, 100295. [Google Scholar] [CrossRef] [PubMed]
- Yasser, M.; El Naggar, E.E.; Elfar, N.; Teaima, M.H.; El-Nabarawi, M.A.; Elhabal, S.F. Formulation, Optimization and Evaluation of Ocular Gel Containing Nebivolol Hcl-Loaded Ultradeformable Spanlastics Nanovesicles: In Vitro and in Vivo Studies. Int. J. Pharm. X 2024, 7, 100228. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and Optimization of Fisetin Loaded Glycerol Based Soft Nanovesicles by Box-Behnken Design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, R.; Liu, C.; Yang, C. Improved Stability and Skin Penetration through Glycethosomes Loaded with Glycyrrhetinic Acid. Int. J. Cosmet. Sci. 2022, 44, 249–261. [Google Scholar] [CrossRef]
- Naguib, M.J.; Salah, S.; Abdel Halim, S.A.; Badr-Eldin, S.M. Investigating the Potential of Utilizing Glycerosomes as a Novel Vesicular Platform for Enhancing Intranasal Delivery of Lacidipine. Int. J. Pharm. 2020, 582, 119302. [Google Scholar] [CrossRef]
- Zaki, R.M.; Alfadhel, M.M.; Alossaimi, M.A.; Elsawaf, L.A.; Devanathadesikan Seshadri, V.; Almurshedi, A.S.; Yusif, R.M.; Said, M. Central Composite Optimization of Glycerosomes for the Enhanced Oral Bioavailability and Brain Delivery of Quetiapine Fumarate. Pharmaceuticals 2022, 15, 940. [Google Scholar] [CrossRef]
- AbouSamra, M.M.; Farouk, F.; Abdelhamed, F.M.; Emam, K.A.F.; Abdeltawab, N.F.; Salama, A.H. Synergistic Approach for Acne Vulgaris Treatment Using Glycerosomes Loaded with Lincomycin and Lauric Acid: Formulation, in Silico, in Vitro, LC-MS/MS Skin Deposition Assay and in Vivo Evaluation. Int. J. Pharm. 2023, 646, 123487. [Google Scholar] [CrossRef]
- Casula, E.; Manca, M.L.; Perra, M.; Pedraz, J.L.; Lopez-Mendez, T.B.; Lozano, A.; Calvo, E.; Zaru, M.; Manconi, M. Nasal Spray Formulations Based on Combined Hyalurosomes and Glycerosomes Loading Zingiber Officinalis Extract as Green and Natural Strategy for the Treatment of Rhinitis and Rhinosinusitis. Antioxidants 2021, 10, 1109. [Google Scholar] [CrossRef]
- Aati, S.; Farouk, H.O.; Elkarmalawy, M.H.; Aati, H.Y.; Tolba, N.S.; Hassan, H.M.; Rateb, M.E.; Hamad, D.S. Intratracheal Administration of Itraconazole-Loaded Hyaluronated Glycerosomes as a Promising Nanoplatform for the Treatment of Lung Cancer: Formulation, Physiochemical, and In Vivo Distribution. Pharmaceutics 2024, 16, 1432. [Google Scholar] [CrossRef]
- Patil, P.; Rahangdale, M.; Sawant, K. Atorvastatin Loaded Glycerosomal Patch as an Effective Transdermal Drug Delivery: Optimization and Evaluation. Ther. Deliv. 2024, 15, 957–976. [Google Scholar] [CrossRef]
- Vanti, G.; Ntallis, S.G.; Panagiotidis, C.A.; Dourdouni, V.; Patsoura, C.; Bergonzi, M.C.; Lazari, D.; Bilia, A.R. Glycerosome of Melissa Officinalis L. Essential Oil for Effective Anti-HSV Type 1. Molecules 2020, 25, 3111. [Google Scholar] [CrossRef]
- Emelyanova, I.V.; Lopatina, G.P.П. Method for Producing Tysole Complex of Tetracoptane hydrotetrakis(oxy -3,4-dihydroxypropyl)titanium with Decane -1,23-trihydroxypropane Having Transcutaneous Conductivity of Medicamentous Additives. Available online: https://goo.su/sqsGm (accessed on 13 April 2025).
- Ron, G.I.; Akmalova, G.M.; Emel’yanova, I.V. Evaluation of the clinical efficacy of a new composition of tizol with triamcinolon in complex treatment of patients with erosive ulcerous form of lichen planus of the oral mucosa. Stomatologiia 2015, 94, 13–15. [Google Scholar] [CrossRef]
- Mokhova, O.; Glukhov, A. Characteristics of the Wound Process in Regional Applications Aquacomplexes Glitserosolvata Titanium and Oxytocin in Experiment. J. Exp. Clin. Surg. 2014, 7, 419–423. [Google Scholar]
- Glukhov, A.; Mokhova, O.; Mikulich, E. Assessment of Efficiency of Complex Treatment of Soft Tissue Wounds by Means of Oxytocin and Titanium Aquacomplex Glycerosolvate in Experiment. J. New Med. Technol. EJournal 2014, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, A.G.; Zimin, Y.V.; Peretyagin, S.P.; Didenko, N.V.; Martusevich, A.; Larionova, K.D. Status of Liver Enzymes as an Indicator of Local Treatment of Burn Trauma in Experiment. Sovrem. Tehnol. V Med. 2013, 5, 20–23. [Google Scholar]
- Sadikova, A. Clinical-morphological, immunological and immunohistochemical characteristics of lichen red planus. Int. Multidiscip. J. Res. Dev. 2024, 11, 354–355. [Google Scholar]
- Firsova, I.V.; Fedotova, Y.M.; Porojskij, S.V.; Makedonova, Y.A.; Mihalchenko, V.F. Efficiency of topical application of mucoadhesive drugs for treating red flat lichen of the oral cavity. J. Volgogr. State Med. Univ. 2019, 16, 59–64. [Google Scholar] [CrossRef]
- Sharonova, N.A.; Temkin, E.S.; Poroshin, A.V. Clinical trial of natural combination therapies in the treatment of chronic generalized periodontitis of moderate severity. J. Volgogr. State Med. Univ. 2019, 16, 65–68. [Google Scholar] [CrossRef]
- Silicon-Titanium-Containing Derivatives of Polymers and Hydrogels Based on Them. Available online: https://patents.google.com/patent/RU2382046C1/ru (accessed on 13 April 2025).
- RU2255939C2—Silicon Glycerates Eliciting Transcutaneous Conductivity of Medicinal Agents and Glycerohydrogels Based on Thereof. Available online: https://yandex.ru/patents/doc/RU2255939C2_20050710 (accessed on 13 April 2025).
- RU2322448C2—Solvate Complexes of Silicon and Titanium Glycerates Manifesting Transcutan Activity and Hydrogels Based Thereon. Available online: https://yandex.ru/patents/doc/RU2322448C2_20080420 (accessed on 13 April 2025).
- Zabokritskiy, N.A. Pharmacological assessment of immunotropic activity of new gel metabiotic on cellular and humoral immunity in experimentally modeled thermal skin burns. Russ. J. Immunol. 2020, 23, 125–132. [Google Scholar] [CrossRef]
- Gackowski, M.; Osmałek, T.; Froelich, A.; Otto, F.; Schneider, R.; Lulek, J. Phototoxic or Photoprotective?-Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations-A Review. Int. J. Mol. Sci. 2023, 24, 8159. [Google Scholar] [CrossRef]
- Fernandes, D. Theranostic Polymeric Nanoparticles for Cancer. BioNanoScience 2023, 13, 1609–1644. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Otero-Espinar, F.J. Mucoadhesive PLGA Nanospheres and Nanocapsules for Lactoferrin Controlled Ocular Delivery. Pharmaceutics 2022, 14, 799. [Google Scholar] [CrossRef]
- Brunato, S.; Mastrotto, F.; Bellato, F.; Bastiancich, C.; Travanut, A.; Garofalo, M.; Mantovani, G.; Alexander, C.; Preat, V.; Salmaso, S.; et al. PEG-Polyaminoacid Based Micelles for Controlled Release of Doxorubicin: Rational Design, Safety and Efficacy Study. J. Control. Release 2021, 335, 21–37. [Google Scholar] [CrossRef]
- Essien, E.N.; Revi, N.; Khatri, V.; Liu, S.; Van Thiel, G.; Bijukumar, D. Methotrexate and Sulforaphane Loaded PBA-G5-PAMAM Dendrimers as a Combination Therapy for Anti-Inflammatory Response in an Intra-Articular Joint Arthritic Animal Model. Int. J. Pharm. 2023, 642, 123150. [Google Scholar] [CrossRef] [PubMed]
- A Facile Method for Anti-Cancer Drug Encapsulation into Polymersomes with a Core-Satellite Structure—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35904177/ (accessed on 27 June 2025).
- Unagolla, J.M.; Jayasuriya, A.C. Drug Transport Mechanisms and in Vitro Release Kinetics of Vancomycin Encapsulated Chitosan-Alginate Polyelectrolyte Microparticles as a Controlled Drug Delivery System. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and Characterization of Chitosan Nanoparticles Loaded Nanofiber Hybrid System for Vaginal Controlled Release of Benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef] [PubMed]
- Chantaburanan, T.; Teeranachaideekul, V.; Jintapattanakit, A.; Chantasart, D.; Junyaprasert, V.B. Enhanced Stability and Skin Permeation of Ibuprofen-Loaded Solid Lipid Nanoparticles Based Binary Solid Lipid Matrix: Effect of Surfactant and Lipid Compositions. Int. J. Pharm. X 2023, 6, 100205. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Salehi, H.; Entezar-Almahdi, E.; Masjedi, M. Preparation and Characterization of Sumatriptan Loaded Solid Lipid Nanoparticles for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2020, 57, 101719. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B. Solid Lipid Nanoparticles for Hydrophilic Drugs. J. Controlled Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, X.; Yang, M.; Yang, D.; Liu, J. Transdermal Drug Delivery of Triptolide-Loaded Nanostructured Lipid Carriers: Preparation, Pharmacokinetic, and Evaluation for Rheumatoid Arthritis. Int. J. Pharm. 2019, 554, 235–244. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured Lipid Carriers (NLCs) as Drug Delivery Platform: Advances in Formulation and Delivery Strategies. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahmood, S.; Danish Ansari, M.; Gull, A.; Sharma, N.; Sultana, Y. Nanostructured Lipid Carrier to Overcome Stratum Corneum Barrier for the Delivery of Agomelatine in Rat Brain; Formula Optimization, Characterization and Brain Distribution Study. Int. J. Pharm. 2021, 607, 121006. [Google Scholar] [CrossRef] [PubMed]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured Lipid Carrier-Embedded Polyacrylic Acid Transdermal Patches for Improved Transdermal Delivery of Capsaicin. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-Coated Nanostructured Lipid Carriers for Transdermal Delivery of Tetrahydrocurcumin for Breast Cancer Therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef] [PubMed]
- van Staden, D.; Plessis, J.d.; Viljoen, J. Development of Topical/Transdermal Self-Emulsifying Drug Delivery Systems, Not as Simple as Expected. Sci. Pharm. 2020, 88, 17. [Google Scholar] [CrossRef]
- Ponto, T.; Latter, G.; Luna, G.; Leite-Silva, V.R.; Wright, A.; Benson, H.A.E. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics 2021, 13, 649. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Q.; Ma, C.; Xia, Q. Non-Aqueous Self-Double-Emulsifying Drug Delivery System: A New Approach to Enhance Resveratrol Solubility for Effective Transdermal Delivery. Colloids Surf. Physicochem. Eng. Asp. 2016, 489, 360–369. [Google Scholar] [CrossRef]
- El Maghraby, G.M. Self-Microemulsifying and Microemulsion Systems for Transdermal Delivery of Indomethacin: Effect of Phase Transition. Colloids Surf. B Biointerfaces 2010, 75, 595–600. [Google Scholar] [CrossRef]
- Badran, M.; Taha, E.; Tayel, M.; Al-Suwayeh, S. Ultra-Fine Self Nanoemulsifying Drug Delivery System for Transdermal Delivery of Meloxicam: Dependency on the Type of Surfactants. J. Mol. Liq. 2014, 190, 16–22. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Liao, H.; Fu, H.; Yang, X.; Xiang, Q.; Zhang, S. Plant Exosome-like Nanoparticles as Biological Shuttles for Transdermal Drug Delivery. Bioengineering 2023, 10, 104. [Google Scholar] [CrossRef]
- Mahdipour, E. Beta Vulgaris Juice Contains Biologically Active Exosome-like Nanoparticles. Tissue Cell 2022, 76, 101800. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.M.; Wiemann, S.; Ambreen, G.; Zhou, J.; Engelhardt, K.; Brüßler, J.; Bakowsky, U.; Li, S.-M.; Mandic, R.; Pocsfalvi, G.; et al. Cucumber-Derived Exosome-like Vesicles and PlantCrystals for Improved Dermal Drug Delivery. Pharmaceutics 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, H.; Xie, Z. METTL3-Modified Exosomes from Adipose-Derived Stem Cells Enhance the Proliferation and Migration of Dermal Fibroblasts by Mediating m6A Modification of CCNB1 mRNA. Arch. Dermatol. Res. 2025, 317, 418. [Google Scholar] [CrossRef]
- Wu, C.; Yu, Q.; Huang, C.; Li, F.; Zhang, L.; Zhu, D. Microneedles as Transdermal Drug Delivery System for Enhancing Skin Disease Treatment. Acta Pharm. Sin. B 2024, 14, 5161–5180. [Google Scholar] [CrossRef]
- Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, W.; Ma, Q.; Gao, Y.; Wang, W.; Zhang, X.; Dong, Y.; Zhang, T.; Liang, Y.; Han, S.; et al. Functional Nano-Systems for Transdermal Drug Delivery and Skin Therapy. Nanoscale Adv. 2023, 5, 1527–1558. [Google Scholar] [CrossRef]
- Gupta, D.K.; Ahad, A.; Aqil, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Chapter 18—Iontophoretic Drug Delivery: Concepts, Approaches, and Applications. In Advanced and Modern Approaches for Drug Delivery; Nayak, A.K., Hasnain, M.S., Laha, B., Maiti, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 515–546. ISBN 978-0-323-91668-4. [Google Scholar]
- Franz, T.J. Percutaneous Absorption on the Relevance of in Vitro Data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Lane, M.E. In Vitro Permeation Testing for the Evaluation of Drug Delivery to the Skin. Eur. J. Pharm. Sci. 2024, 201, 106873. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Namjoshi, S.N.; Telaprolu, K.C.; Jung, N.; Shewan, H.M.; Stokes, J.R.; Benson, H.A.E.; Grice, J.E.; Raney, S.G.; Rantou, E.; et al. Impact of Different Packaging Configurations on A Topical Cream Product. Pharm. Res. 2024, 41, 2043–2056. [Google Scholar] [CrossRef]
- Pensado, A.; Hattam, L.; White, K.A.J.; McGrogan, A.; Bunge, A.L.; Guy, R.H.; Delgado-Charro, M.B. Skin Pharmacokinetics of Transdermal Scopolamine: Measurements and Modeling. Mol. Pharm. 2021, 18, 2714–2723. [Google Scholar] [CrossRef]
- Brighenti, M.d.S.; Montanheri, L.R.d.S.; Duque, M.D.; Andreo-Filho, N.; Lopes, P.S.; Garcia, M.T.J.; Mackenzie, L.; Leite-Silva, V.R. In Vitro Drug Release and Ex Vivo Dermal Drug Permeation Studies of Selected Commercial Benzoyl Peroxide Topical Formulations: Correlation Between Human and Porcine Skin Models. Mol. Pharm. 2025, 22, 1365–1372. [Google Scholar] [CrossRef]
- Zhang, Q.; Murawsky, M.; LaCount, T.D.; Hao, J.; Ghosh, P.; Raney, S.G.; Kasting, G.B.; Li, S.K. Evaluation of Heat Effects on Fentanyl Transdermal Delivery Systems Using In Vitro Permeation and In Vitro Release Methods. J. Pharm. Sci. 2020, 109, 3095–3104. [Google Scholar] [CrossRef]
- In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-permeation-test-studies-topical-drug-products-submitted-andas (accessed on 23 April 2025).
- Decision of the EEC Council No. 85 Dated 3 November 2016 on the Approval of the Rules for Conducting Bioequivalence Studies of Medicinal Products within the Eurasian Economic Union. Available online: https://docs.eaeunion.org/documents/306/2592/ (accessed on 19 May 2025).
- Quality and Equivalence of Locally Applied, Locally Acting Cutaneous Products—Scientific Guideline | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/quality-equivalence-locally-applied-locally-acting-cutaneous-products-scientific-guideline (accessed on 24 April 2025).
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document for the Conduct of Skin Absorption Studies. Available online: https://www.oecd.org/en/publications/guidance-document-for-the-conduct-of-skin-absorption-studies_9789264078796-en.html (accessed on 19 May 2025).
- Shin, S.H.; Srivilai, J.; Ibrahim, S.A.; Strasinger, C.; Hammell, D.C.; Hassan, H.E.; Stinchcomb, A.L. The Sensitivity of In Vitro Permeation Tests to Chemical Penetration Enhancer Concentration Changes in Fentanyl Transdermal Delivery Systems. AAPS PharmSciTech 2018, 19, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Ossowicz-Rupniewska, P.; Nowak, A.; Klebeko, J.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N.; et al. Assessment of the Effect of Structural Modification of Ibuprofen on the Penetration of Ibuprofen from Pentravan® (Semisolid) Formulation Using Human Skin and a Transdermal Diffusion Test Model. Materials 2021, 14, 6808. [Google Scholar] [CrossRef] [PubMed]
- Krumpholz, L.; Polak, S.; Wiśniowska, B. Towards the Understanding of the IVPT Results Variability-Development, Verification and Validation of the PBPK Model of Caffeine in Vitro Human Skin Permeation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2025, 204, 106943. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Namjoshi, S.N.; Jung, N.; Windbergs, M.; Benson, H.A.E.; Grice, J.E.; Raney, S.G.; Roberts, M.S. Topical Semisolid Drug Product Critical Quality Attributes with Relevance to Cutaneous Bioavailability and Pharmacokinetics: Part I-Bioequivalence of Acyclovir Topical Creams. Pharm. Res. 2024, 41, 1507–1520. [Google Scholar] [CrossRef]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and Transdermal Drug Delivery Using Microneedles—Fabrication, Performance Evaluation and Application to Lymphatic Delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef]
- Pulsoni, I.; Lubda, M.; Aiello, M.; Fedi, A.; Marzagalli, M.; von Hagen, J.; Scaglione, S. Comparison Between Franz Diffusion Cell and a Novel Micro-Physiological System for In Vitro Penetration Assay Using Different Skin Models. SLAS Technol. 2022, 27, 161–171. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Varga-Medveczky, Z.; Kocsis, D.; Naszlady, M.B.; Fónagy, K.; Erdő, F. Skin-on-a-Chip Technology for Testing Transdermal Drug Delivery-Starting Points and Recent Developments. Pharmaceutics 2021, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.M.; Kim, K.; Choi, K.C.; Sung, G.Y. Side-Effect Test of Sorafenib Using 3-D Skin Equivalent Based on Microfluidic Skin-on-a-Chip. J. Ind. Eng. Chem. Seoul Korea 2020, 82, 71–80. [Google Scholar] [CrossRef]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs-Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 Efficacy Test for Human Skin Equivalents Using a Pumpless Skin-On-A-Chip System. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma Longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef]
- Mohamadali, M.; Ghiaseddin, A.; Irani, S.; Amirkhani, M.A.; Dahmardehei, M. Design and Evaluation of a Skin-on-a-Chip Pumpless Microfluidic Device. Sci. Rep. 2023, 13, 8861. [Google Scholar] [CrossRef]
- Jonsdottir, F.; Snorradottir, B.S.; Gunnarsson, S.; Georgsdottir, E.; Sigurdsson, S. Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics 2022, 14, 1880. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Garg, A. An Insight of Techniques for the Assessment of Permeation Flux across the Skin for Optimization of Topical and Transdermal Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2021, 62, 102355. [Google Scholar] [CrossRef]
- Bodenlenz, M.; Tiffner, K.I.; Raml, R.; Augustin, T.; Dragatin, C.; Birngruber, T.; Schimek, D.; Schwagerle, G.; Pieber, T.R.; Raney, S.G.; et al. Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Clin. Pharmacokinet. 2017, 56, 91–98. [Google Scholar] [CrossRef]
- Schwagerle, G.; Sharp, M.J.; Parr, A.; Schimek, D.; Mautner, S.I.; Birngruber, T. Detailed Pharmacokinetic Characterization of Advanced Topical Acyclovir Formulations with IVPT and in Vivo Open Flow Microperfusion. Int. J. Pharm. 2023, 643, 123269. [Google Scholar] [CrossRef]
- Shinkai, N.; Korenaga, K.; Okumura, Y.; Mizu, H.; Yamauchi, H. Microdialysis Assessment of Percutaneous Penetration of Ketoprofen after Transdermal Administration to Hairless Rats and Domestic Pigs. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2011, 78, 415–421. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, Y.; Cheng, B.; Wei, Y.; Wei, Y.; Piao, J.; Li, F.; Zheng, H. Correlation between in Vivo Microdialysis Pharmacokinetics and Ex Vivo Permeation for Sinomenine Hydrochloride Transfersomes with Enhanced Skin Absorption. Int. J. Pharm. 2022, 621, 121789. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.N.; Braeckman, R.; Oh, C. Comparison of Steady-State Pharmacokinetics of Donepezil Transdermal Delivery System with Oral Donepezil. J. Alzheimers Dis. JAD 2022, 90, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Tracking the Transdermal Penetration Pathways of Optimized Curcumin-Loaded Chitosan Nanoparticles via Confocal Laser Scanning Microscopy. Int. J. Biol. Macromol. 2018, 108, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Krombholz, R.; Liu, Y.; Lunter, D.J. In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy. Pharmaceutics 2021, 13, 67. [Google Scholar] [CrossRef]
- Dos Santos, L.; Sousa, M.P.J.; Azoia, N.G.; Cavaco-Paulo, A.M.; Martin, A.A.; Favero, P.P. In Vivo Confocal Raman Spectroscopy and Molecular Dynamics Analysis of Penetration of Retinyl Acetate into Stratum Corneum. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2017, 174, 279–285. [Google Scholar] [CrossRef]
- Botelho, M.A.; Queiroz, D.B.; Barros, G.; Guerreiro, S.; Fechine, P.; Umbelino, S.; Lyra, A.; Borges, B.; Freitas, A.; Queiroz, D.C.d.; et al. Nanostructured Transdermal Hormone Replacement Therapy for Relieving Menopausal Symptoms: A Confocal Raman Spectroscopy Study. Clin. Sao Paulo Braz. 2014, 69, 75–82. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In Vitro Skin Models as a Tool in Optimization of Drug Formulation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef]
- Lehman, P.A.; Franz, T.J. Observations on the Tritiated Water and TEWL Skin Integrity Tests: Relevance to In Vitro Permeation Testing (IVPT). Pharm. Res. 2024, 41, 1149–1161. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Meseguer, S.; Fuguet, E.; Port, A.; Rosés, M. Suitability of Skin-PAMPA and Chromatographic Systems to Emulate Skin Permeation. Influence of pH on Skin-PAMPA Permeability. Microchem. J. 2023, 190, 108567. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Rahma, A.; Lane, M.E.; Sinkó, B. A Comparative Study of the in Vitro Permeation of 2-Phenoxyethanol in the Skin PAMPA Model and Mammalian Skin. Int. J. Pharm. 2023, 635, 122692. [Google Scholar] [CrossRef]
- Sinkó, B.; Bárdos, V.; Vesztergombi, D.; Kádár, S.; Malcsiner, P.; Moustie, A.; Jouy, C.; Takács-Novák, K.; Grégoire, S. Use of an In Vitro Skin Parallel Artificial Membrane Assay (Skin-PAMPA) as a Screening Tool to Compare Transdermal Permeability of Model Compound 4-Phenylethyl-Resorcinol Dissolved in Different Solvents. Pharmaceutics 2021, 13, 1758. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Novel In Vitro Investigational Methods for Modeling Skin Permeation: Skin PAMPA, Raman Mapping. Pharmaceutics 2020, 12, 803. [Google Scholar] [CrossRef]
- ISO 10993-1:2018. Available online: https://www.iso.org/ru/standard/68936.html (accessed on 27 June 2025).
- ISO 14971:2019. Available online: https://www.iso.org/ru/standard/72704.html (accessed on 27 June 2025).

| Parameter | Limits |
|---|---|
| Water solubility | >1 mg/mL |
| Lipophilicity (Po/w) | 10–1000 |
| Molecular weight | <500 Da |
| Melting temperature | <200 °C |
| pH of saturated water solution | 5–9 |
| Dose | <10 mg/day |
| Carrier Type | Key Features | Advantages | Disadvantage | Reference |
|---|---|---|---|---|
| Nanoemulsions | Submicron o/w, w/o, or bicontinuous systems; stabilized by surfactants; nanometer droplet size | High drug solubilization; enhanced skin permeation; encapsulates both hydrophilic and lipophilic drugs; kinetic stability | High surfactant content (>20%) can irritate skin; surfactant reduction needed | [36,66,71] |
| Microemulsions | Transparent, thermodynamically stable systems; droplet sizes typically below 0.1 μm (100 nm); surfactant-stabilized o/w or w/o phases | Excellent solvent capacity; increased permeability; easy to prepare; surfactant and lipid-based skin disruption | High surfactant content (>20%) may cause skin irritation | [66,67,68,70] |
| Liposomes | Spherical vesicles with phospholipid bilayers; encapsulate hydrophilic, lipophilic, and amphiphilic drugs | Versatile drug loading; biocompatible; modifiable with PEG, ligands for targeting | Short half-life, rapid clearance, instability in plasma | [72,73,77] |
| Invasomes | Modified liposomes with phospholipids, ethanol, and terpenes; enhance penetration via synergistic effects | Improved drug penetration; utilize natural enhancers; effective for various APIs | Potential skin irritation due to terpene and ethanol content | [81,82,83] |
| Transferosomes | Highly elastic, ultra-deformable vesicles with phospholipids, surfactants, and ethanol | Enhanced skin penetration through deformation; suitable for diverse solubility drugs | Requires surfactants | [84,85] |
| Ethosomes | Transferosomes with high ethanol content; ethanol disrupts lipid packing in the stratum corneum | Deep skin layer penetration; improved delivery via vesicle fusion with membranes | High ethanol content may cause dryness and irritation | [86] |
| Glycerosomes | Flexible vesicles with 10–30% glycerol; high encapsulation efficiency and deformability | High stability; moisturizing effect; effective for dermal and mucosal delivery | Too much glycerol reduces homogeneity and encapsulation efficiency | [88,90,91,93] |
| Polymeric Nanoparticles | Composed of polymers (e.g., PLGA, PEG); includes nanospheres, micelles, dendrimers, etc. | Controlled release; high drug loading; biocompatible; diverse structural options | Scaling complexity, potential polymer toxicity, costly synthesis | [122,123,124,131] |
| Solid Lipid Nanoparticles (SLNs) | Solid lipid matrix stabilized by surfactants; forms occlusive film enhancing hydration | Enhanced skin hydration; sustained release; physical stability; biocompatibility | Limited loading of hydrophilic drugs, potential stability issues | [129,131] |
| Nanostructured Lipid Carriers (NLCs) | Blend of solid and liquid lipids; overcomes SLN limitations, improves drug loading and stability | Higher drug loading; improved stability; controlled release; deeper skin penetration | Risk of crystallization; production complexity | [132,133,134] |
| Self-Emulsifying Systems | Isotropic mixtures forming emulsions upon contact with aqueous phase; high solubilizing capacity | Ease of formulation; increased solubility; spontaneous emulsification; occlusive action | High surfactant content may cause irritation; needs optimization | [137,138,140] |
| Exosomes | Extracellular vesicles (30–150 nm); mediate intercellular communication; penetrate skin via endocytosis, phagocytosis, or membrane fusion | Biocompatibility, low immunogenicity, high skin permeability | Challenges in standardization | [142,143,144,145,146,147] |
| Strategy Type | Examples | Mechanism | Key Features | Reference |
|---|---|---|---|---|
| Chemical | Ethanol, fatty acids, terpenes, sulfoxides | Disrupt lipid bilayers, increase hydration | Low or moderate cost of production, difficult to use and register the drug | [26,27,31,34,46,47,48,49] |
| Physical | Microneedles, iontophoresis, sonophoresis, electroporation | Create microchannels or drive drug using energy | High cost of production, difficult to use and register the drug, often refers to a combination of a medical device and a medicinal product | [16,17,54,57,58,59,60,64,150] |
| Nanocarrier-based | Liposomes, ethosomes, transferosomes, SLNs, NLCs | Enhance solubility, flexibility, and targeting | Moderate cost of production, difficult to use and register the drug | [72,73,84,85,86,129,131,132,133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhrushina, E.O.; Shumkova, M.M.; Avdonina, Y.V.; Ananian, A.A.; Babazadeh, M.; Pouya, G.; Grikh, V.V.; Zubareva, I.M.; Kosenkova, S.I.; Krasnyuk, I.I., Jr.; et al. Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation. Pharmaceutics 2025, 17, 936. https://doi.org/10.3390/pharmaceutics17070936
Bakhrushina EO, Shumkova MM, Avdonina YV, Ananian AA, Babazadeh M, Pouya G, Grikh VV, Zubareva IM, Kosenkova SI, Krasnyuk II Jr., et al. Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation. Pharmaceutics. 2025; 17(7):936. https://doi.org/10.3390/pharmaceutics17070936
Chicago/Turabian StyleBakhrushina, Elena O., Marina M. Shumkova, Yana V. Avdonina, Arsen A. Ananian, Mina Babazadeh, Ghazaleh Pouya, Viktoria V. Grikh, Irina M. Zubareva, Svetlana I. Kosenkova, Ivan I. Krasnyuk, Jr., and et al. 2025. "Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation" Pharmaceutics 17, no. 7: 936. https://doi.org/10.3390/pharmaceutics17070936
APA StyleBakhrushina, E. O., Shumkova, M. M., Avdonina, Y. V., Ananian, A. A., Babazadeh, M., Pouya, G., Grikh, V. V., Zubareva, I. M., Kosenkova, S. I., Krasnyuk, I. I., Jr., & Krasnyuk, I. I. (2025). Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation. Pharmaceutics, 17(7), 936. https://doi.org/10.3390/pharmaceutics17070936







