Chitosan Microparticles Coupled with MAGE-AX and CpGs as a Treatment for Murine Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Reagents
2.3. Cs Microparticle Manufacture
2.4. Processing for Scanning Electron Microscopy (SEM)
2.5. Bone Marrow-Derived Dendritic Cell Differentiation and Treatment with Cs Microparticles
2.6. Cytotoxicity Test with Calcein Staining and Ethidium Homodimer
2.7. Melanoma Cell Line and MAGE-AX Antigen
2.8. Melanoma Induction in B16-F10 Mice
2.9. Tumor Growth Rate and Survival
2.10. Histopathological Evaluation of Tumor Lesions
2.11. Analysis of Results
3. Results
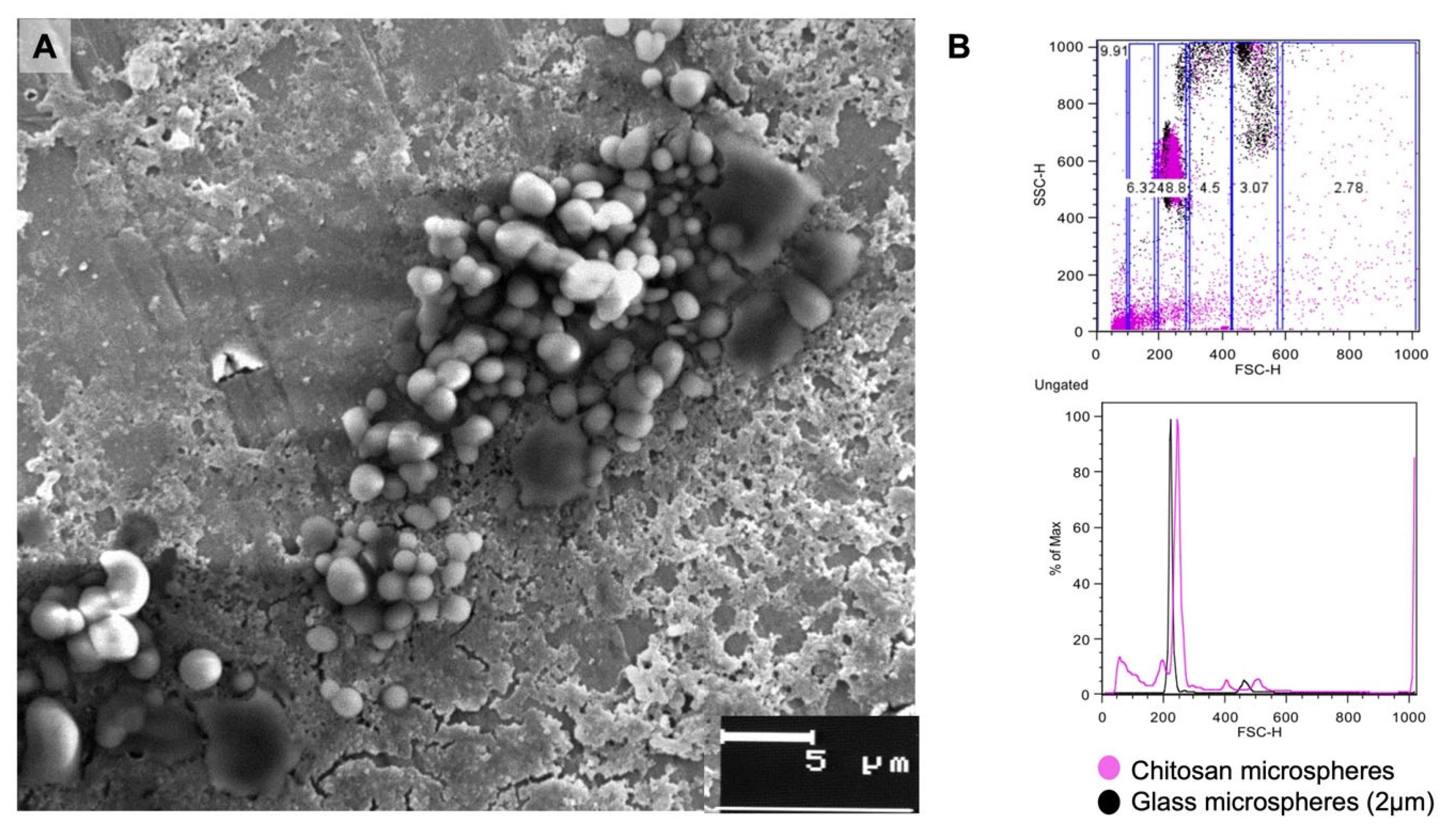
3.1. Size of Cs Microparticles
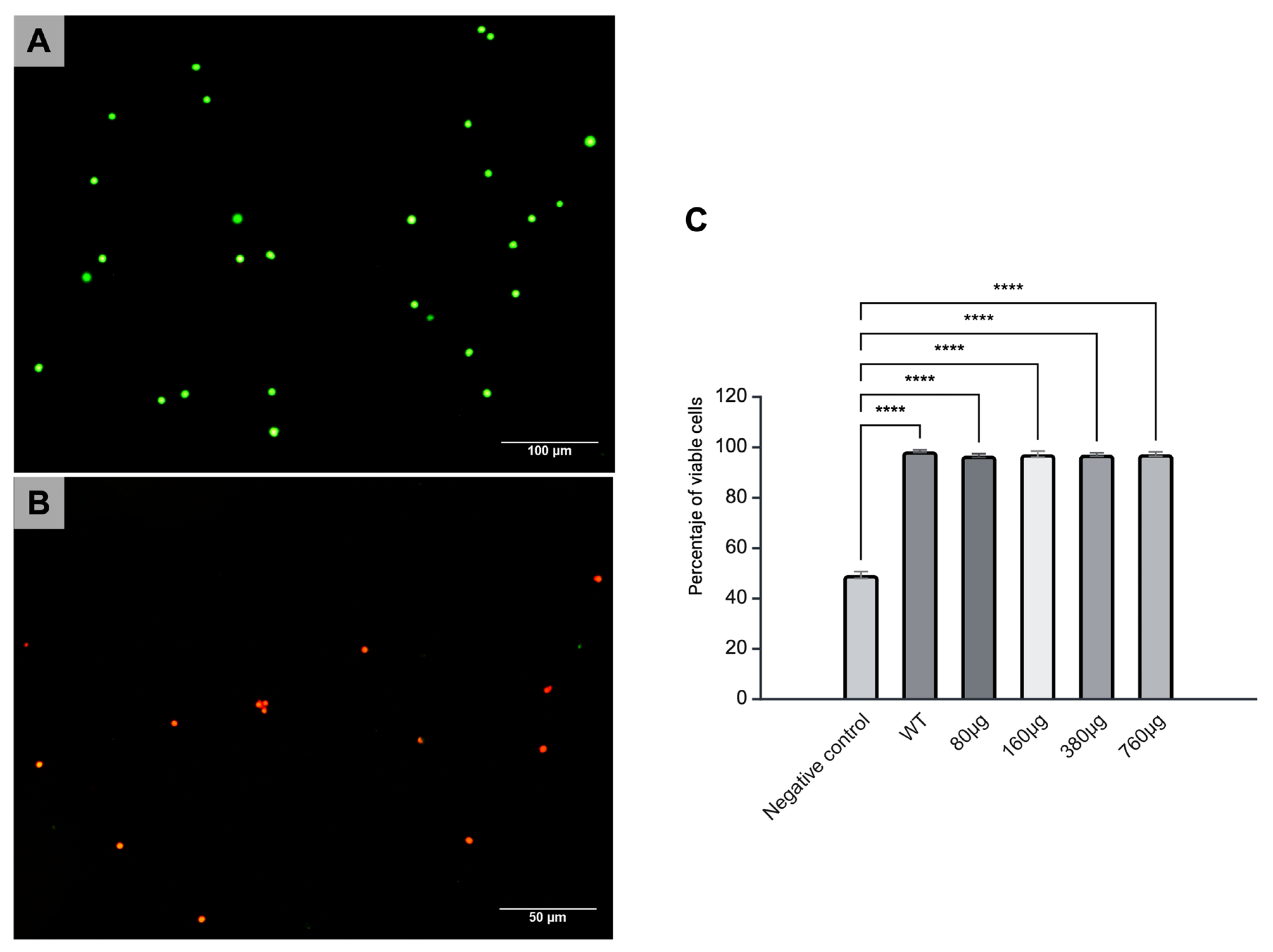
3.2. Cytotoxicity of Cs Microparticles
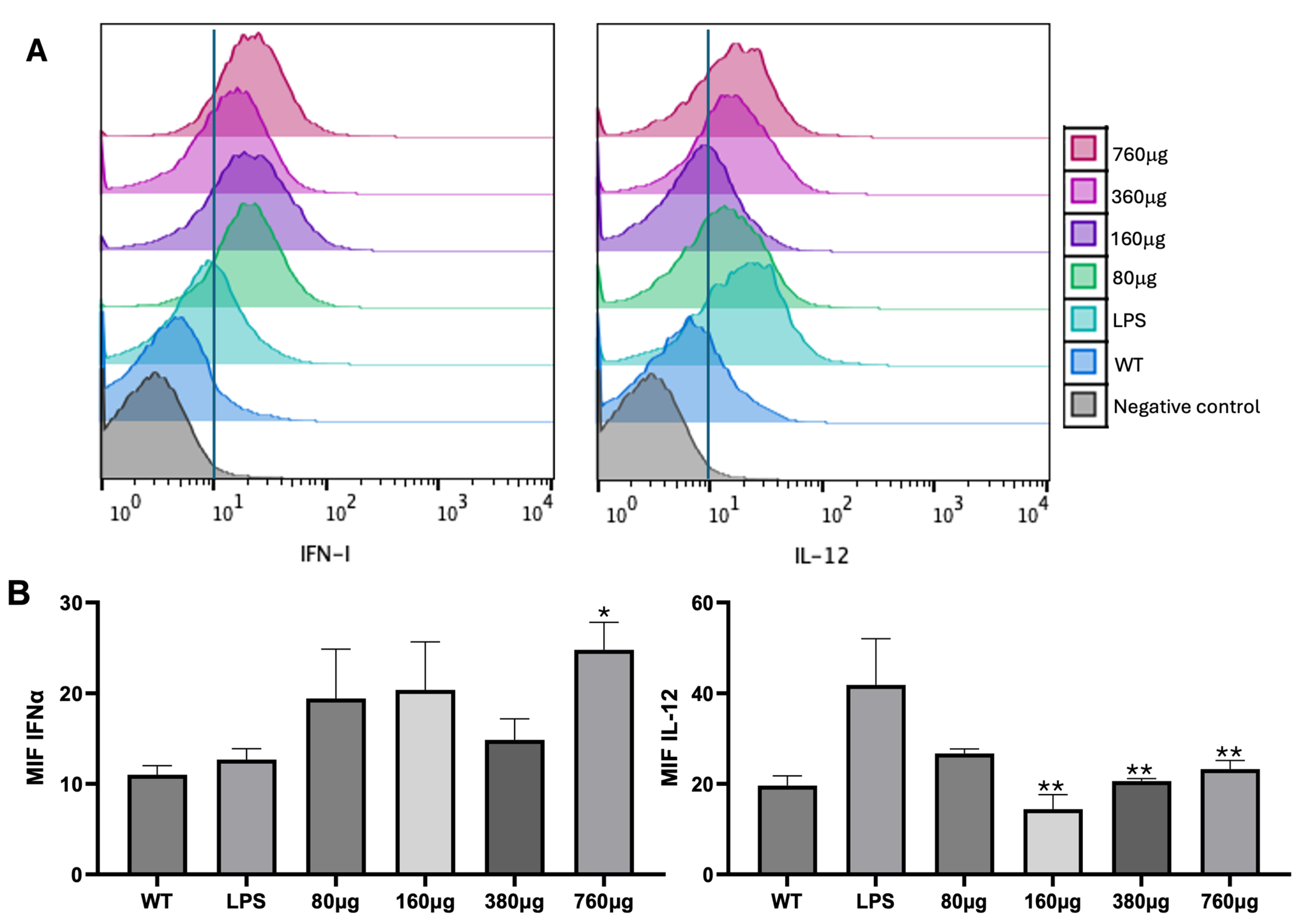
3.3. DC Treatment with Cs Microparticles
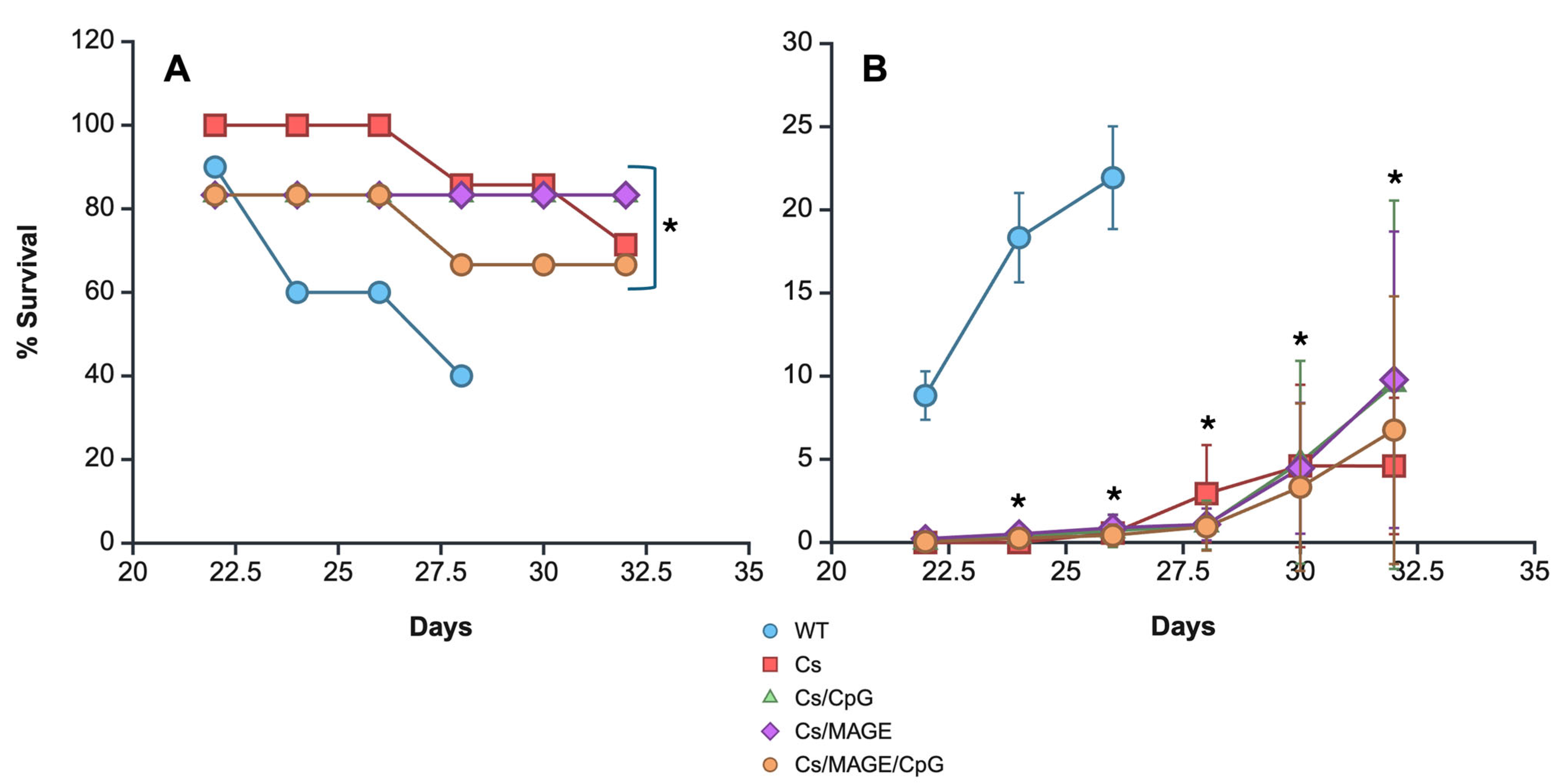
3.4. Survival and Tumor Size
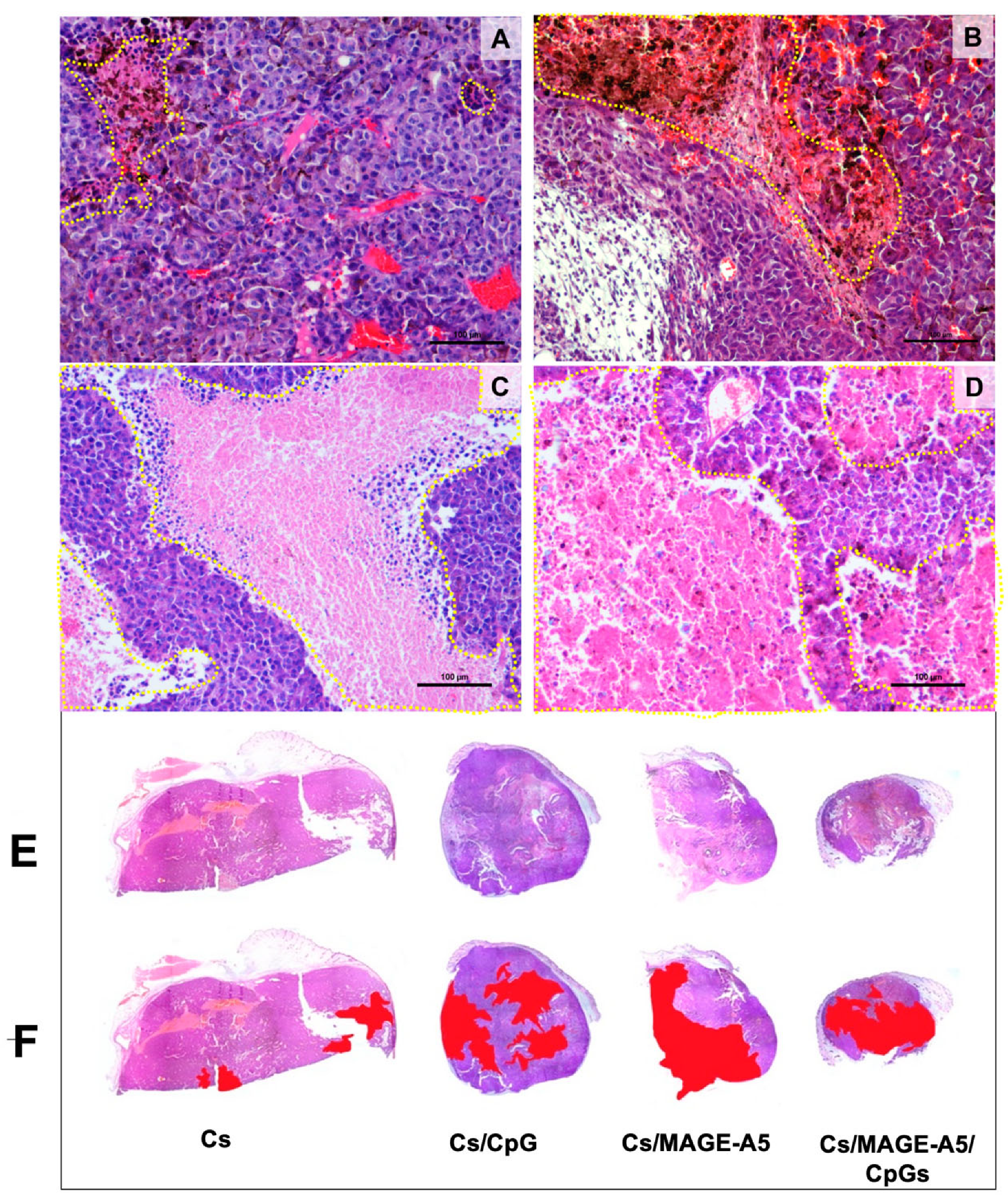
3.5. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CpGs | Unmethylated cytosine–phosphate–guanosine ODNs |
| MAGE | Melanoma antigen |
| Cs | Chitosan |
| DCs | Dendritic cells |
| IFNγ | Interferon gamma |
| NK | Natural killer |
| TLR | Toll-like receptors |
| IL-2 | Interleukin 2 |
| DNA | Deoxyribonucleic acid |
| IFNα | Interferon alpha |
| CD8 | Cluster of Differentiation 8 |
| HBSS | Hank’s Balanced Salt Solution |
| RPMI | Roswell Park Memorial Institute |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| bmDCs | Bone marrow dendritic cells |
| PE | Phycoerythrin |
| ANOVA | Analysis of variance |
| IL-12 | Interleukin 12 |
| SEM | Scanning electron microscopy |
| LPS | Lipopolysaccharide |
| WT | Without treatment |
| RIG I | Retinoic acid-inducible gene I |
References
- Minda, A.G.; Awel, F.S.; Seifudin, K.A.; Gezahegne, M.K. Immunotherapy against Cancer: A Comprehensive Review. J. Cancer Res. Exp. Oncol. 2016, 8, 15–25. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Robinson, B.W.S.; June, C.H.; Lotze, M.T. Principles of Tumor Immunology. In Clinical Immunology: Principles and Practice, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhao, Z.; Zheng, L.; Chen, W.; Weng, W.; Song, J.; Ji, J. Delivery Strategies of Cancer Immunotherapy: Recent Advances and Future Perspectives. J. Hematol. Oncol. 2019, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor CGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Nigar, S.; Shimosato, T. Cooperation of Oligodeoxynucleotides and Synthetic Molecules as Enhanced Immune Modulators. Front. Nutr. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N. Structure-Dependent Immunostimulatory Effect of CpG Oligodeoxynucleotides and Their Delivery System. Int. J. Nanomed. 2012, 7, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Markov OV, M.N.V.V.Z.M. Molecular and Cellular Mechanisms of Antitumor Immune Response Activation by Dendritic Cells. Acta Naturae 2016, 8, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Schooten, E.; Di Maggio, A.; van Bergen en Henegouwen, P.M.P.; Kijanka, M.M. MAGE-A Antigens as Targets for Cancer Immunotherapy. Cancer Treat. Rev. 2018, 67, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Zárate, G.; Herrera-Enríquez, M.Á.; Hernández-Téllez, B.; Jarquín-Yáñez, K.; Castell-Rodríguez, A.E. GK-1 Improves the Immune Response Induced by Bone Marrow Dendritic Cells Loaded with MAGE-AX in Mice with Melanoma. J. Immunol. Res. 2014, 2014, 158980. [Google Scholar] [CrossRef] [PubMed]
- Jarquín-Yáñez, K.; García-Gutierrez, P.; Faustino-Vega, A.; Castell-Rodríguez, A.E.; Piñon-Zárate, G.; Macin-Cabrera, S.A.; Quiri-no-Barreda, C.T.; Miranda-Calderon, J.E. In Vitro Characterisation of Optimised Chitosan Microparticles Loaded with Vancomycin by Factorial Experiment Design. Sist. Inf. Cient. Redalyc 2017, 48, 43–51. [Google Scholar]
- Ito, F.; Chang, A.E. Cancer Immunotherapy. Surg. Oncol. Clin. N. Am. 2013, 22, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Heo, Y.-J.; Han, D.K. New Opportunities for Nanoparticles in Cancer Immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-Cell and NK-Cell Infiltration into Solid Tumors: A Key Limiting Factor for Efficacious Cancer Immunotherapy. Cancer Discov. 2014, 4, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Tran, T.T.P.; Nguyen, H.T.; Phung, C.D.; Jeong, J.-H.; Stenzel, M.H.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Kim, J.O. Nanoparticles for Dendritic Cell-Based Immunotherapy. Int. J. Pharm. 2018, 542, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Poilil Surendran, S.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Liang, J.; Zhang, C.; Wang, X.; Zhang, C.; Ma, G.; Sun, H. Chitosan/Calcium Phosphates Nanosheet as a Vaccine Carrier for Effective Cross-Presentation of Exogenous Antigens. Carbohydr. Polym. 2019, 224, 115172. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.R.J.; Bondet, V.; Rodero, M.P.; Duffy, D. Heterogeneity and Functions of the 13 IFN-α Subtypes—Lucky for Some? Eur. J. Immunol. 2023, 53, 2250307. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, D.; Dai, C.; Cheng, G.; Wang, X.; Zhao, K. Response of Live Newcastle Disease Virus Encapsulated in N-2-Hydroxypropyl Dimethylethyl Ammonium Chloride Chitosan Nanoparticles. Carbohydr. Polym. 2017, 171, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Gao, Y.; Hu, G.; Wang, L.; Cui, S.; Jin, Z. N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows. Vaccines 2021, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Pekcan, M.; Puralı, N.; Esendağlı, G.; Tavukçuoğlu, E.; Rivero-Arredondo, V.; Ontiveros-Padilla, L.; López-Macías, C.; Şenel, S. Development and in Vitro Evaluation of a New Adjuvant System Containing Salmonella Typhi Porins and Chitosan. Int. J. Pharm. 2020, 578, 119129. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan Nanoparticles Act as an Adjuvant to Promote Both Th1 and Th2 Immune Responses Induced by Ovalbumin in Mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, J.; Su, R.; Liu, C.; Guo, X.; Zhou, S. Double Enhancement of Immunogenic Cell Death and Antigen Presentation for Cancer Immunotherapy. Nano Today 2021, 39, 101225. [Google Scholar] [CrossRef]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- Peachman, K.K.; Rao, M.; Alving, C.R.; Burge, R.; Leppla, S.H.; Rao, V.B.; Matyas, G.R. Correlation between Lethal Toxin-Neutralizing Antibody Titers and Protection from Intranasal Challenge with Bacillus Anthracis Ames Strain Spores in Mice after Transcutaneous Immunization with Recombinant Anthrax Protective Antigen. Infect. Immun. 2006, 74, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Chawla-Sarkar, M. Apoptosis and Interferons: Role of Interferon-Stimulated Genes as Mediators of Apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yao, X.; Fu, Y.; Wang, Y. Interferon-α and Its Effects on Cancer Cell Apoptosis (Review). Oncol. Lett. 2022, 24, 235. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma Treatment in Review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Immunotherapeutic Uses of CpG Oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Zárate, G.; Hernández-Téllez, B.; Jarquín-Yáñez, K.; Herrera-Enríquez, M.Á.; Fuerte-Pérez, A.E.; Valencia-Escamilla, E.A.; Castell-Rodríguez, A.E. Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma. Polymers 2022, 14, 4608. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, F.M.; Abd-Rabou, A.A.; Mohamed, M.S. Raloxifene-Encapsulated Hyaluronic Acid-Decorated Chitosan Nanoparticles Selectively Induce Apoptosis in Lung Cancer Cells. Bioorg. Med. Chem. 2019, 27, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, M.S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef] [PubMed]
- Eggert, A.O.; Andersen, M.H.; Voigt, H.; Schrama, D.; Kämpgen, E.; thor Straten, P.; Becker, J.C. Characterization of Mouse MAGE-derived H-2K b-restricted CTL Epitopes. Eur. J. Immunol. 2004, 34, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Zárate, G.; Hernández-Téllez, B.; Ramírez-Cortés, A.; Jarquín-Yáñez, K.; Sampedro-Carrillo, E.A.; Herrera-Enríquez, M.A.; Cárdenas-Monroy, C.A.; Castell-Rodríguez, A.E. Chitosan Microparticles Coupled with MAGE-AX and CpGs as a Treatment for Murine Melanoma. Pharmaceutics 2025, 17, 932. https://doi.org/10.3390/pharmaceutics17070932
Piñón-Zárate G, Hernández-Téllez B, Ramírez-Cortés A, Jarquín-Yáñez K, Sampedro-Carrillo EA, Herrera-Enríquez MA, Cárdenas-Monroy CA, Castell-Rodríguez AE. Chitosan Microparticles Coupled with MAGE-AX and CpGs as a Treatment for Murine Melanoma. Pharmaceutics. 2025; 17(7):932. https://doi.org/10.3390/pharmaceutics17070932
Chicago/Turabian StylePiñón-Zárate, Gabriela, Beatriz Hernández-Téllez, Ariel Ramírez-Cortés, Katia Jarquín-Yáñez, Enrique A. Sampedro-Carrillo, Miguel A. Herrera-Enríquez, Christian A. Cárdenas-Monroy, and Andrés E. Castell-Rodríguez. 2025. "Chitosan Microparticles Coupled with MAGE-AX and CpGs as a Treatment for Murine Melanoma" Pharmaceutics 17, no. 7: 932. https://doi.org/10.3390/pharmaceutics17070932
APA StylePiñón-Zárate, G., Hernández-Téllez, B., Ramírez-Cortés, A., Jarquín-Yáñez, K., Sampedro-Carrillo, E. A., Herrera-Enríquez, M. A., Cárdenas-Monroy, C. A., & Castell-Rodríguez, A. E. (2025). Chitosan Microparticles Coupled with MAGE-AX and CpGs as a Treatment for Murine Melanoma. Pharmaceutics, 17(7), 932. https://doi.org/10.3390/pharmaceutics17070932








