Abstract
Neuropathic pain is a significant global clinical issue that poses substantial challenges to both public health and the economy due to its complex underlying mechanisms. It has emerged as a serious health concern worldwide. Recent studies involving dorsal root ganglion (DRG) stimulation have provided strong evidence supporting its effectiveness in alleviating chronic pain and its potential for sustaining long-term pain relief. In addition to that, there has been ongoing research with clinical evidence relating to the role of small non-coding ribonucleic acids known as microRNAs in regulating gene expressions affecting pain signals. The signal pathway involves alterations in neuronal excitation, synaptic transmission, dysregulated signaling, and subsequent pro-inflammatory response activation and pain development. When microRNAs are dysregulated in the dorsal root ganglia neurons, they polarize macrophages from anti-inflammatory M2 to inflammatory M1 macrophages causing pain signal generation. By reversing this polarization, a therapeutic activity can be induced. However, the direct delivery of these nucleotides has been challenging due to limitations such as rapid clearance, degradation, and reduction in half-life. Therefore, safe and efficient carrier vehicles are fundamental for microRNA delivery. Here, we present a comprehensive analysis of miRNA-based nano-systems for chronic neuropathic pain, focusing on their impact in dorsal root ganglia. This review provides a critical evaluation of various delivery platforms, including viral, polymeric, lipid-based, and inorganic nanocarriers, emphasizing their therapeutic potential as well as their limitations in the treatment of chronic neuropathic pain. Innovative strategies such as hybrid nanocarriers and stimulus-responsive systems are also proposed to enhance the prospects for clinical translation. Serving as a roadmap for future research, this review aims to guide the development and optimization of miRNA-based therapies for effective and sustained neuropathic pain management.
1. Introduction
Pain is a widespread and burdensome health issue that significantly affects the global population. According to the International Association for the Study of Pain (IASP), pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [1]. It can be classified by duration into acute (short-term) and chronic (long-term) pain, and by cause into nociceptive (tissue damage) and neuropathic (nerve damage) pain. Pain signals originate from receptors in the peripheral neurons, which detect specific nociceptive inputs and transmit them to the spinal neurons, which then relay the signals to the brain for processing. Neuropathic pain occurs as a result of damage to the nerves. According to the IASP, it is defined as pain caused by a lesion or disease affecting the somatosensory nervous system. This type of pain affects approximately 7–10% of the global population and can significantly interfere with daily activities and the overall quality of life. Additionally, around 20–25% of the global population experiences chronic pain, and 20% of adults in Western countries report it as a major health concern [2,3,4,5,6]. Due to the multiple molecular mechanisms involved with neuropathic pain, there are many pre-existing clinical challenges. Furthermore, the currently available pain relief treatments such as surgery, physical therapies, psychological therapies, and medications, face serious challenges along with the extensive usage of medications like opioid or non-opioid drugs, resulting in side effects like drug addiction, abuse, and resistance or tolerance [7,8,9,10,11,12,13].
To overcome these issues, targeting the dorsal root ganglion has demonstrated potential effects on pain alleviation. The dorsal root ganglion (DRG) plays a vital role in neuropathic pain development, transmitting peripheral nociceptive input to the central nervous system. Studies, such as those by Berger et al., support DRG stimulation as a promising neuromodulation strategy for chronic pain management [14]. Pain was once thought to result solely from neuronal activation, but neurons are not the only contributors to pain signaling. In fact, glial cells also play critical roles: peripheral nerve injury activates spinal microglia and satellite glia in the DRG, which then release pro-inflammatory mediators that amplify pain signals [15].
Studies on small non-coding but functional RNAs called microRNAs (miRNAs), indicate miRNA upregulation or downregulation at the DRG following a nerve injury [16]. Molecular mechanisms underlying neuropathic pain involve miRNA dysregulation in DRG neurons, influencing neuronal excitability, synaptic transmission, and macrophage polarization from anti-inflammatory M2 to pro-inflammatory M1 phenotypes, thereby driving pain signaling [16,17,18,19]. At a molecular level, microRNAs act as key regulators of gene expression, orchestrating both immune and neuronal processes in the pain pathway [20]. A few of the miRNAs expressed in neuropathic pain conditions include miR-21, miR-30a, miR-124, miR-125, and miR-183 [21,22,23]. This can be manipulated and used from a therapeutic perspective. A few of the miRNA dysregulations utilized in association with neuropathic pain treatment at the DRG are listed in Table 1. Since miRNA recognition requires binding to a much shorter nucleotide seed sequence than an entire nucleotide as in the case of small interfering RNA (siRNA), the therapeutic index is larger comparatively [24,25].

Table 1.
Neuropathic pain-associated microRNA regulation at DRG.
Current treatments for neuropathic pain, such as opioid medications and surgical interventions, are often limited by issues like poor efficacy and the risk of addiction. In contrast, miRNA-based therapies show considerable potential by directly targeting the underlying molecular mechanisms of pain. However, successful clinical application remains challenging due to difficulties in delivering these therapies effectively, highlighting the need for advanced nanocarrier systems. This review presents a novel and comprehensive perspective by exploring two key areas. First, it investigates the pathophysiological functions of miRNAs in DRG-mediated pain. Second, it examines the development of innovative delivery systems designed to modulate miRNA activity within the DRG. The primary objective is to emphasize the central role of the DRG and miRNA in the onset and persistence of chronic neuropathic pain. Particular attention is given to the complex interactions among neuronal excitability, miRNA dysregulation, synaptic transmission, and inflammatory responses. In addition, the review discusses multiple therapeutic strategies that aim to modulate DRG function through targeted miRNA regulation. Furthermore, the limitations of delivering naked miRNA and an outline of how designing viral and non-viral delivery systems can overcome these barriers were also explored. By highlighting a comprehensive overview of polymeric, inorganic, lipidic, extracellular vesicle, and hydrogel-based delivery vehicles, a possible resolution to the pressing challenges in clinical translation, including immunogenicity, scalability, and long-term safety were addressed. Through these findings, we aim to provide an insight into how engineered miRNA-nano formulations pave the way for new avenues for safe and effective neuropathic pain therapies.
2. Role of the DRG in the Pain Pathway
The DRG or spinal ganglion consists of a cluster of cell bodies, mainly of primary sensory neurons (PSNs) located within a sural sheath surrounded by cerebrospinal fluid (CSF) on the dorsal roots of spinal nerves, just outside the spinal cord (Figure 1A) and is involved in sensory signaling, modulation, and pain transmission [48,49]. Although these structures are approximately the size of a peanut, they contain around 15,000 neurons. They contain various other cell types such as satellite glial cells, endothelial cells, and macrophages along with PSNs [50]. In our human body, there are 31 pairs of spinal nerves of afferent sensory dorsal roots and motor ventral roots which make up the spinal cord. Between adjacent vertebral segments, there are intervertebral foramina from which these spinal nerves emerge. A dorsal root that exits from the intervertebral foramina, when merging with a ventral nerve root, forms the DRG [14]. It accommodates cell bodies of PSNs such “A-neurons” or large-light neurons which transmit non-noxious signals and “C-neurons” or small-dark neurons that transmit noxious signals [51]. Each cell body in the dorsal root ganglia is a modified bipolar neuron known as a pseudo-unipolar neuron. It has a single axonal process that bifurcates into a T-shaped structure, consisting of a central branch that enters the spinal cord and a peripheral branch that connects to somatic and visceral sensory receptors. These pseudo unipolar neurons are associated with the development and maintenance of neuropathic pain since the glial cells in DRGs respond to a nerve injury and produce inflammatory markers [14].
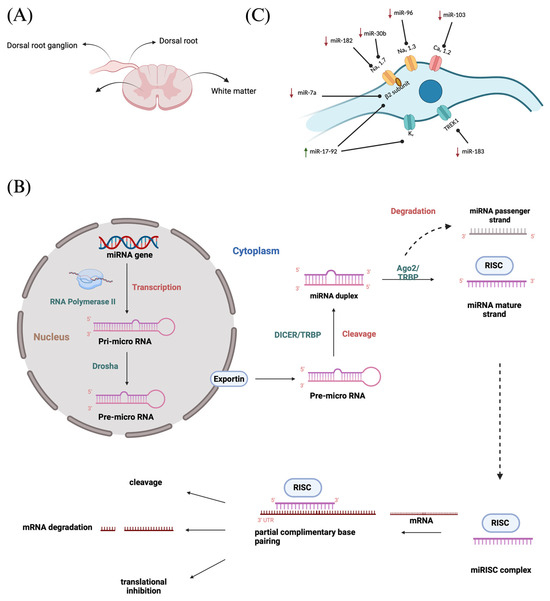
Figure 1.
(A) Spinal cord cross section showing DRG; (B) MiRNA biogenesis—production of miRNAs and mechanism of gene expression regulation followed by miRNA–mRNA interaction resulting in translational repression or degradation of target mRNAs; (C) MiRNA regulation at DRG.
3. MicroRNA Biogenesis and Its Role in the Pain Signal Pathway
3.1. MicroRNA Biogenesis
The miRNAs are a class of small non-coding RNA (ncRNA) having 21 to 25 nucleotides in length. They are partially complementary to one or more messenger RNA (mRNA) molecules and have a pivotal function in post transcriptional gene regulation, translational repression, mRNA cleavage, and deadenylation [51,52,53,54]. The biogenesis of miRNA involves a series of steps such as the transcription of miRNA genes to primary miRNA precursors called pre-miRNAs by RNA polymerase II, which further becomes cleaved by Drosha to form 70-nucleotide hairpin structures known as pre-miRNAs. Then, exportin-5 exports the pre-miRNA out of the nucleus to the cytoplasm where dicer and trans-activation response RNA-binding protein (TRBP) converts it to a 20- to 24-nucleotide double-stranded duplex of mature miRNA strand and a complementary or designated miRNA strand (miRNA-miRNA*) [55,56,57,58]. Subsequently, the mature strand (guide strand) is incorporated into the RNA-induced silencing complex (RISC) by Ago2 and TRBP to form miRISC, and the passenger strand (designated strand) becomes degraded (Figure 1B) [59]. Finally, miRISC binds with target mRNA to induce either protein synthesis interference or degradation of the target mRNA [60]. They act as gene-regulating modulators by post-transcriptionally inhibiting protein expression in targeted mRNAs via 3′-UTR recognition of mRNA in a sequence specific approach [61].
3.2. MicroRNA Regulation of the Pain Signaling Pathway
Understanding the involvement of miRNA in pain pathways enables the identification of novel targets for neuropathic pain. Dynamic gene-regulator-altered miRNA profiles are often implicated in neuropathic pain pathogenesis. For instance, a recent transcriptomic study by Xiong et al. analyzed dorsal root ganglia after chronic constriction injury and found 47 differentially expressed miRNAs; enriched pathways included Rap1, Ras, and Hippo signaling, suggesting broad miRNA-mediated changes in neuropathic pain [62]. These non-coding RNAs that fine-tune gene expression, and alter miRNA profiles are often implicated in the pathogenesis of neuropathic pain. From neuronal excitability and ion channel expression to neuroinflammation and synaptic plasticity, these molecules regulate diverse processes and together shape pain signaling [63]. Following peripheral nerve injury, aberrant miRNA activity can disrupt normal pain transmission, leading to the hyperexcitability of neurons and amplification of pain signals. Based on the signaling pathway, the role of miRNAs can be categorized accordingly.
3.2.1. miRNA Regulation of Ion Channels and Neuronal Excitability
Various ion channels present along the membrane allow the propagation of action potentials via the axon of sensory neurons and transmit electrical impulses from the body to the brain. Following a nerve injury, miRNAs alter the expression in these ion channels, thereby resulting in neuronal excitability and amplified pain perception. Zhao et al. observed that miRNAs play pivotal roles in the development and progression of neuropathic pain by influencing neuronal excitability and plasticity, modulating ion channels, regulating neuroinflammatory responses, and affecting synaptic plasticity, among other critical functions [63]. Ion channels such as voltage-gated sodium channels (VGSCs) and their nine subtypes (Nav 1.1–Nav 1.9), along with calcium channels (Cav), potassium channels (Kv) as well as TREK1 (a mechano-temperature-sensitive potassium channel), play a vital role in neuronal excitability because of miRNA regulation (Figure 1C) [64]. VGSCs critical in sensory neuron firing, are particularly influenced by miRNAs. Golmakani et al. (2024) highlighted that increasing miR-30b (which targets the Nav 1.7 sodium channel) or miR-7a (targeting sodium channel β2 subunits) inhibits pain [65]. MiR-30b overexpression was found to directly downregulate Nav channel expression (including Nav 1.3, Nav 1.6, and Nav 1.7) in injured neurons, thereby reducing neuropathic pain behaviors [30,66,67]. Likewise, miR-7a represses the β2 subunit of VGSCs (an auxiliary subunit encoded by SCN2B), normalizing the hyperexcitability of nociceptive neurons after nerve injury [26]. Consistent with these findings, other miRNAs (miR-96 and miR-182) also suppress Nav channel isoforms to curb repetitive firing [32,41,68]. In addition, a cluster of miRNAs known as miR-17-92 are downregulated in certain neuropathic pain models; when active, this cluster can reduce potassium channel (Kv) currents and thus increase neuronal firing, whereas blocking miR-17-92 alleviates allodynia by restoring the K+ channel function [27]. Similarly, miR-137 inhibition has been shown to normalize Kv1.2 channel levels and DRG neuron firing, reversing pain phenotypes [34,69]. Changes in other miRNA families (for example, the let-7 family) have also been linked to the altered expression of ion channels and heightened sensory neuron activity, further underscoring that dysregulated ion channel expression due to miRNA imbalances is a key mechanism of neuropathic pain.
3.2.2. miRNAs in Neuroinflammation, Glial Signaling, and Microglial Activation
Neuroinflammation is a driving force in chronic pain, and miRNAs are pivotal regulators of the inflammatory pathways in the nervous system. Following nerve injury, activated immune cells (microglia, macrophages) and astrocytes release pro-inflammatory cytokines that sensitize neurons. Certain miRNAs act as breaks on this process by targeting inflammatory signaling molecules. For instance, miR-146a-5p is known to target IRAK1 and TRAF6, adaptor proteins in the Toll-like receptor (TLR) and IL-1 receptor pathways. The upregulation of miR-146a-5p has been shown to dampen these pathways: in a rat chronic constriction injury (CCI) model, the intrathecal delivery of a miR-146a-5p agomir (mimic) significantly alleviated mechanical allodynia and thermal hyperalgesia by suppressing the injury-induced increase of IRAK1 and TRAF6 in the DRG and spinal cord [39]. Conversely, blocking miR-146a worsened pain and elevated IRAK1/TRAF6 levels, confirming that miR-146a-5p’s anti-inflammatory action can prevent neuropathic pain development. In cultured microglial cells, miR-146a overexpression reverses pro-inflammatory markers (Arg-1, IL-10) [70]. Similarly, miR-216a-5p promotes an anti-inflammatory phenotype in microglia via the TLR4 pathway. Upregulating miR-216a-5p was found to inhibit the HMGB1–TLR4–NF-κB signaling cascade, leading to the reduced release of IL-1β, IL-6, and TNF-α and a shift of microglia from the pro-inflammatory M1 state to anti-inflammatory M2 [43,71]. This change corresponds with the prevention of neuropathic pain in spinal cord injury models, positioning miR-216a-5p as a potential therapeutic target for regulating neuroinflammation.
Activated microglia and macrophages themselves are modulated by miRNAs, creating a feedback loop in pain regulation. One notable regulator is miR-124, a microRNA highly enriched in resting (M2-like) microglia. MiR-124 helps keep microglia in an anti-inflammatory state by inhibiting targets such as GRK2 (a kinase that drives pro-inflammatory signaling in glia). Loss of miR-124 after nerve injury has been associated with a skewing of microglia toward the M1 state, exacerbating neuroinflammation and pain. In line with this, restoring miR-124 levels produces analgesic effects: intrathecal administration of miR-124 (or mimetics) reduced neuropathic pain and inflammation by shifting microglial polarization toward the M2 state [33]. For example, here, Willemen et al. found that chronic pain models show reduced miR-124 in spinal microglia and a predominance of M1 markers, whereas intrathecal miR-124 fully prevented the transition to persistent pain and normalized the spinal M1/M2 marker balance. MiR-124 directly suppresses GRK2 expression and thereby modulates the microglial M1/M2 balance. The importance of such non-neuronal regulation is further illustrated by miR-124’s absence leading to unchecked M1 activation and sustained pain. Additionally, miR-146a, miR-216a, and miR-124 are often co-regulated in neuropathic conditions, and their collective downregulation in injured DRGs and spinal cord correlates with heightened cytokine levels and persistent pain [63,72]. Therapeutically boosting these anti-inflammatory miRNAs could thus attenuate the neuroimmune activation underlying chronic pain.
Not only do miRNAs within immune cells matter, but neurons and glia also communicate via miRNA-containing extracellular vesicles, amplifying neuroinflammatory signals. DRG neurons can secrete exosomes or microvesicles that carry specific miRNAs to neighboring immune cells. A striking example is miR-23a: after nerve injury, DRG sensory neurons markedly upregulate miR-23a and package it into exosomes. These miR-23a-rich vesicles are released into the extracellular space and subsequently taken up by macrophages at the injury site [29]. Once inside macrophages, miR-23a binds to and inhibits A20 (TNFAIP3), a negative regulator of NF-κB signaling. The result is enhanced NF-κB activity and a shift of macrophages toward the pro-inflammatory M1 phenotype (with increased Nos2/iNOS and reduced Mrc1/CD206 expression). This neuron-to-immune transfer of miR-23a thereby promotes the release of cytokines that drive neuropathic pain. Importantly, disrupting this pathway has analgesic effects: delivering an exosomal miR-23a antagomir (an inhibitor) in vivo blunted NF-κB activation, reduced M1 macrophage accumulation in the DRG, and attenuated neuropathic hypersensitivity [29]. These findings reveal a novel non-neuronal communication route in pain: injured neurons actively influence immune cells via miRNAs, adding another layer of complexity to pain signaling. Notably, directly inhibiting pro-nociceptive miRNAs (anti-miR therapy) has shown benefits: for example, intrathecal miR-21 antagomir reduced hyperalgesia and shifted macrophages towards an anti-inflammatory phenotype [28,73]. Overall, by controlling cytokine networks, glial activation states (including microglial M1/M2 polarization), and neuron–immune crosstalk, miRNAs serve as critical modulators of neuroinflammation in chronic pain states.
3.2.3. miRNAs in Neuronal Survival and Regeneration
Beyond excitability and inflammation, miRNAs also influence neuron-intrinsic processes like survival, axon regeneration, and synaptic remodeling that can affect chronic pain development. Nerve injury triggers regenerative programs in neurons as well as stress responses (e.g., apoptosis), and miRNAs help govern these outcomes. One well-studied example is miR-21, which is rapidly upregulated in injured DRG neurons and in the surrounding glial cells. MiR-21 is known to promote pro-regenerative signaling in part by targeting the PTEN/Akt/mTOR pathway, a key pathway that, when disinhibited, allows neurons to grow axons and resist apoptosis [74]. Other miRNAs predominantly support the regenerative and survival side. For instance, miR-125b is upregulated in models of nerve injury and has been shown to enhance axon regeneration after spinal cord trauma by suppressing inhibitors of the JAK/STAT3 pathway [75]. The overexpression of miR-125b reduces neuronal apoptosis and inflammation, thereby creating a neuronal protective effect. Similarly, miR-210 is induced as a negative feedback regulator in injured tissues; it targets components of the NF-κB pathway and HIF-1α, thereby reducing inflammatory damage and supporting cell survival [76]. In a spinal injury model, treatment with agomiR-210 decreased levels of IL-1β and TNF-α and protected neurons, indicating its role in counteracting inflammation-related neurotoxicity [77]. Another example is miR-181c-5p, which has been shown to inhibit the pro-apoptotic factor BIM; the upregulation of miR-181c-5p effectively reduced neuron apoptosis and promoted axon regrowth in injured neural tissue [78]. Likewise, miR-138 has been implicated in regenerative mechanisms: an early study found that miR-138 forms a regulatory loop with SIRT1, a deacetylase that promotes axon outgrowth, suggesting that downregulating miR-138 may release pro-regenerative factors and enhance neurite extension [79]. In summary, numerous miRNAs become dysregulated after nerve injury not only in ways that exacerbate pain, but also as part of the attempt to recover neural function.
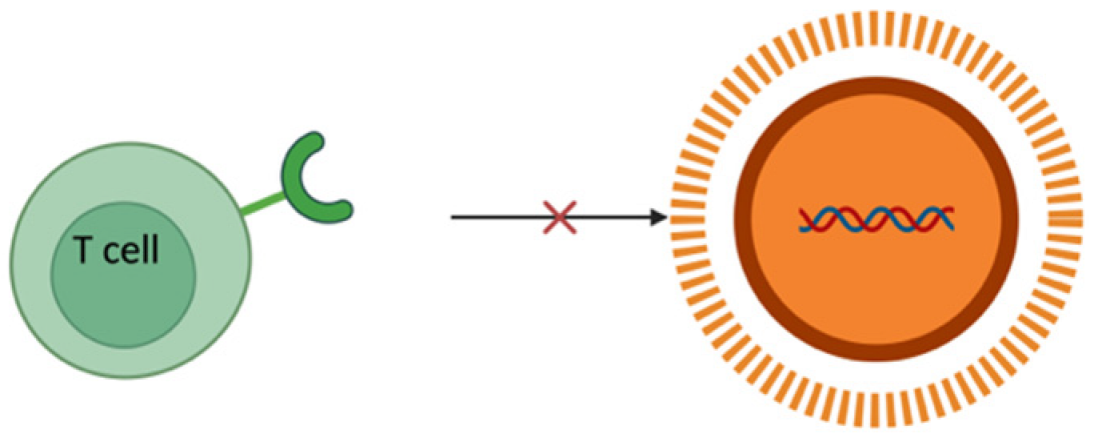
4. MiRNA Delivery Systems
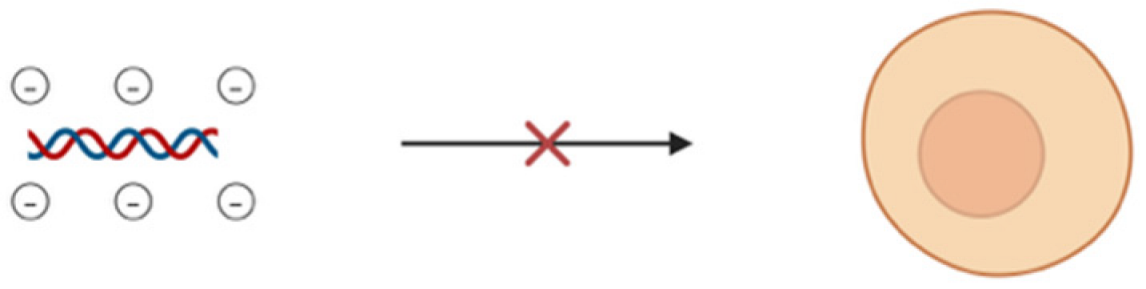
From recent studies, it has been estimated that miRNAs regulate more than 30% of the protein-coding genes associated with various biological processes like cell proliferation, apoptosis, fat metabolism, cell differentiation, and disease progression [80,81]. The recent surge of interest in therapies based on these non-coding RNAs over the past few decades is because of their direct or indirect involvement in various biological anomalies [82,83]. MiRNA mimics or antagomirs (anti-miRNAs) are used as therapeutic agents either to restore or suppress the gene expression [84,85,86,87]. Despite all these factors, there are major limitations or challenges corresponding to the delivery of miRNA. One of the major drawbacks is the inherent instability of miRNA in biological fluids, in which it is susceptible to rapid degradation by nucleases [88]. This degradation in turn results in a significant reduction in the half-life of free miRNAs, thus reducing their therapeutic potential. For an effective therapeutic action, miRNA has to be delivered into the cytoplasm of the target cells in order to interact with the RISC. Navigating through complex biological barriers such as cell membrane and the endosomal compartment is very difficult for a naked miRNA. Therefore, free miRNAs often end up entrapped in endosomes and results in subsequent degradation [89]. Apart from that, rapid clearance from blood, low permeability, and other extracellular barriers such as the reticuloendothelial system (RES) can shorten the half-life of a short ncRNA (Table 2). The negative charge of miRNA poses significant challenges especially in terms of electrostatic repulsion when it comes to cell membrane which is negatively charged due to the presence of phospholipids [90]. This negative charge not only hinders cell membrane penetration but hinders its ability to be internalized by cells and thus reduces the efficiency of delivery. It may also lead to unwanted interactions with positively charged serum proteins leading to miRNA sequestration and reduction in bioavailability as well as therapeutic efficacy. Another challenge is in the form of cations present in biological fluids that may potentially lead to the aggregation of these negatively charged miRNA molecules and reduction in the functional concentration of miRNA, thus complicating its delivery [91]. The ability of miRNAs to bind to multiple mRNA targets may result in unintended interactions or off-target effects and disrupt the expression of genes. Chemically modified or vectorized miRNAs on the other hand face intracellular barriers and intracellular trafficking along with potential issues of diffusion through membranes because of the high molecular weight of the modified miRNAs. Therefore, miRNA needs to be encapsulated and delivered using an optimized delivery vehicle to surpass all the above-mentioned limiting factors.

Table 2.
Key limitations or challenges of delivering naked miRNA vs. encapsulated miRNA delivery using carriers.
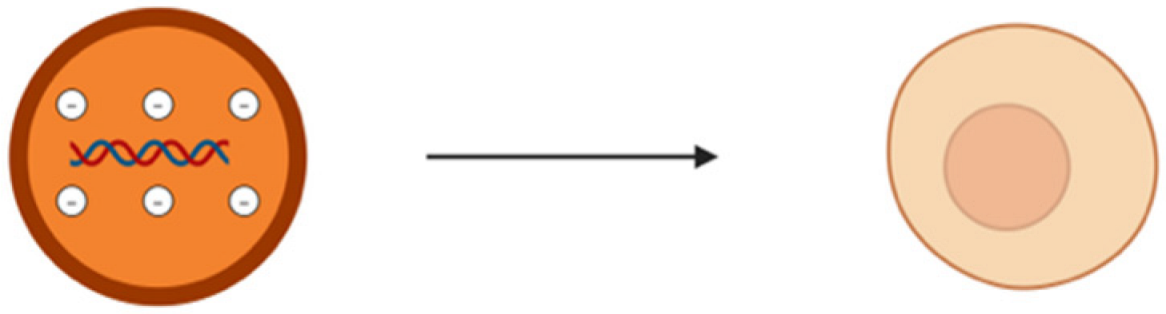
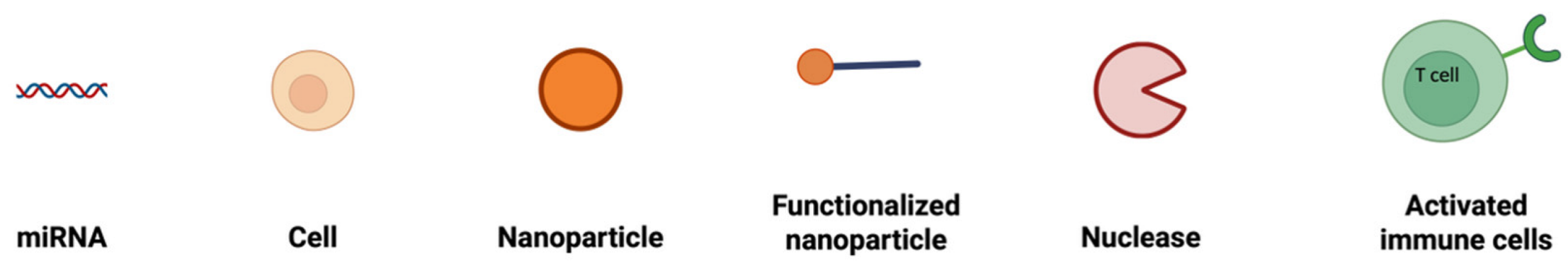
To combat all the limitations associated with the naked delivery of miRNA, a carrier is required which ideally must be biocompatible, biodegradable, non-toxic, non-immunogenic but at the same time protect the genetic material from enzymatic degradation or clearance and display a positive surface charge, along with an optimum size ranging between 50 and 200 nm (Table 2). The positive surface charge helps to overcome the electrostatic repulsion between the negatively charged miRNA and the cell membrane and increase the binding efficiency, thereby protecting it from degradation by nucleases, hindering interactions with serum proteins and allowing endosomal escape via the proton sponge effect [24]. The particle size is often a factor that determines the circulation time, tissue distribution, cellular uptake, and the ability to cross biological barriers. For efficient cellular uptake, particles below 200 nm are effectively internalized by cells via endocytosis, while larger particles often tend to be cleared more quickly by the body [92]. If particles are too small, i.e., less than 10 nm, they may not be able to carry enough cargo or may possibly be excreted too quickly through renal clearance [93]. Along with that, this smaller particle size offers prolonged circulation and better tissue penetration and minimizes aggregation. Furthermore, miRNA must be internalized by the cells and released into the cytoplasm. This is where a carrier system comes into play. There are mainly two types of delivery systems: viral and non-viral carriers (Figure 2). Viral-based systems include the use of retroviruses, lentiviruses, and adenoviruses [94]. A few among the non-viral vectorization materials that can be used as a delivery system includes polymeric, inorganic, lipidic, and extracellular vesicle-based carrier systems [95]. A summary of current miRNA-based delivery systems targeting the DRG for chronic neuropathic pain management is presented in Table 3.
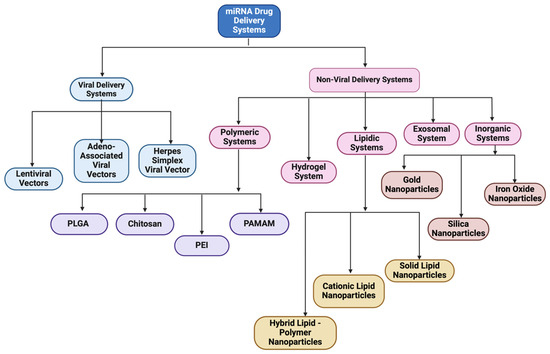
Figure 2.
Classification of miRNA-based drug delivery systems.

Table 3.
Current list of miRNA delivery systems for chronic neuropathic pain treatment at DRG.
4.1. Viral Delivery Systems
Lentivirus, adeno-associated virus, and herpes simplex virus are the most common viral vectors having high transfection efficiency along with a high level of constant expression of miRNA mimics or antagomirs that have modifications in certain specific genomic areas, in order to prevent replication [103].
4.1.1. Lentiviral Vectors
Lentivirus is a subgroup of retroviruses that can transfect both dividing and non-dividing cells and have the ability to transfer and integrate about 8 kb of foreign genetic sequences into the host genome [104]. This viral system offers high transfection efficiency and the long-term stable expression of the incorporated miRNA making them a potential candidate for treating neurologic disorders. A study based on the functional recovery of injured spinal cord in mice using lentivirus-mediated delivery of miR-133b confirmed the role of miRNA in neurite growth enhancement, proving its high therapeutic potential [105]. Lentiviral vector-based delivery of miR-145 in another study demonstrated regulation of human corneal epithelial differentiation [106]. Li H et al. based on a CCI (chronic constriction injury)-induced neuropathic pain model studied the regulatory mechanism of jagged 1 (JAG1) by miR-124-3p and miR-141-3p in neuropathic pain in the spinal cord dorsal horn of rats using recombinant lentivirus [107]. Lentivirus-mediated gene transfer involving the overexpression of miR-142-3p and miR-141 in SNL (spinal nerve ligation) and CCI models, respectively, demonstrated alleviation of neuropathic pain via inhibiting high-mobility group box 1 (HMGB1) [36,37]. Another study based on the upregulation of miR-144 using lentiviral vector in a CCI-induced model confirmed its therapeutic potential via the evaluation of the paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) for neuropathic pain treatment [38]. An experimental study by Shi et al. demonstrated that delivering miR-145 mimics to dorsal root ganglion (DRG) neurons via lentiviral vectors directly targeted Akt3 and inhibited downstream signaling pathways involving nuclear factor kappa B (NF-κB) and the mammalian target of rapamycin (mTOR), resulting in reduced mechanical allodynia and thermal hyperalgesia [96]. Inhibition of X-inactive specific transcript (XIST) by the downregulation of ZEB1 and upregulation miR-150 regulation was studied by Yan et al. on CCI-induced rat models as an axis to provide a therapeutic target for neuropathic pain [97]. In another lentiviral-based delivery of miRNA by Shi et al., alleviation of neuropathic pain was studied on CCI rat models via the inhibition of the TWIK-related potassium channel 1 (TREK-1) channel using miR-183-5p [42]. Another experimental work based on the overexpression of miR-216a-5p using lentiviral vector on CCI rat models attenuated neuropathic pain and improved mechanical allodynia and thermal hyperalgesia [43]. Here, miR-216-5p alleviates neuropathic pain by targeting lysine demethylase 3A (KDM3A) and by inactivating the Wnt/B-catenin signaling pathway (Figure 3).
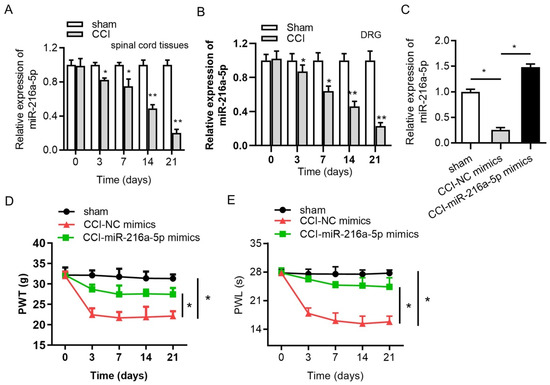
Figure 3.
RT-qPCR analysis of expression of miR-216a-5p in (A) spinal cord, (B) DRG in sham, and CCI rat models (C). Overexpression efficiency (D) effect of overexpression of miRNA on mechanical allodynia. (E) Effect of overexpression of miRNA on thermal hyperalgesia; * p < 0.05, ** p < 0.01 (reprinted from [43] with permission from Elsevier, Amsterdam, The Netherlands).
4.1.2. Adeno-Associated Viral Vectors
Adeno-associated viruses (AAVs) are safe adenoviral vectors due to their deficiency in replication and pathogenicity [108]. Compared to adenoviruses that are double-stranded DNA viruses containing more than 100 serotypes, AAVs are single-stranded DNA parvoviruses from the genus Dependovirus, which consists of 12 primate serotypes (AAV1 to AAV12) [109]. AAVs can cause long-term expression in vivo due to their capability of integrating into a specific site on chromosome 19 without any noticeable effects [94]. The disadvantage of AAVs is associated with their limited transgene capacity of 4.8 kb that limits its applications for large gene delivery, but miRNA, due to its small size, often uses AAVs as a delivery system. They have the ability to transfect both dividing and non-dividing cells.
An investigation on the effects of miR-7a in a spinal SNL rat model using an AAV inhibited the activation of the signal transducer and activator of the transcription 3 (STAT3) signaling pathway and relieved neuropathic pain, proving it to be a significant and potential treatment for neuropathic pain patients (Figure 4) [98]. Another study on miR-7a by Sakai A et al. in an SNL model using male Sprague-Dawley rats demonstrated the alleviation of neuropathic pain via the regulation of neuronal excitation [26]. MiR-17-92, an miRNA cluster based on other experimental research, was found to regulate the voltage-gated potassium channels associated with chronic neuropathic pain [27]. Similarly, Jung et al. delivered an AAV-encoding shRNA targeting GCH1, a pain-related enzyme, to the DRG. They observed that AAV-shGCH1 deactivated microglia and protected against the development of neuropathic pain [110].
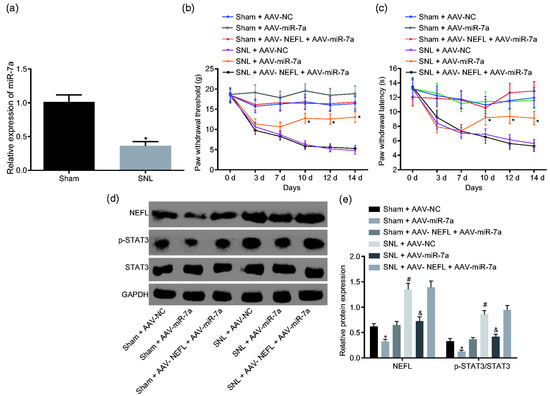
Figure 4.
Overexpression of miR-7a attenuates neuropathic pain by suppressing NEFL expression and the STAT3 signaling pathway. (a) miR-7a expression in sham and SNL-treated rats; * p < 0.05 versus the sham group;. (b) PWT of sham/SNL models post AAV-miR-7a infection after operation. (c) PWT of sham/SNL models in response to infection of AAV-miR-7a after operation (d,e) protein bands and levels of NEFL, STAT3, and extension of STAT3 phosphorylation in SNL-treated rats in response to infection of AAV-miR-7a at the 14th day after operation; * p < 0.05 versus the sham + AAV-NC group; # p < 0.05 versus the SNL + AAV-NC group; & p < 0.05 versus the sham + AAV-NEFL + AAV-miR-7a group (reprinted from [98]).
4.1.3. Herpes Simplex Viral Vectors
Herpes simplex virus (HSV) is a large DNA virus with the capacity to carry multiple therapeutic genes. Its natural ability to infect axonal nerve terminals at peripheral sites makes it an ideal vector for gene delivery to the nervous system. Unlike lentivirus, adeno-associated virus, and retrovirus that only have a small genome-packing capacity of less than 8 kb, HSV can accommodate up to a 150 kb genome [111]. Studies conducted by Chattopadhyay et al. demonstrated that HSV vector-mediated delivery of miRNA into DRG neurons in vivo inhibits voltage-gated sodium channels thereby reducing painful diabetic neuropathy [89]. In this study, streptozotocin (STZ)-induced painful diabetic neuropathy (PDN) male Sprague-Dawley rat models were inoculated with the vector containing the miRNA sequence (QHmiNav) 2 weeks after the onset of diabetes and it resulted in a reduction in Navα subunit levels in DRG neurons as well subsequent reduction in cold allodynia, thermal hyperalgesia, and mechanical hyperalgesia.
4.2. Non-Viral Delivery Systems
Even though viral delivery systems offer high transfection efficiency, often, applications are limited due to potential issues associated with immunogenic and inflammatory responses, low loading capacity, and quality control [112]. On the other hand, non-viral delivery systems offer lower toxicity and reduced immunogenicity, and they are not restricted by transgene size limitations inherent to viral delivery systems [94]. Some of the common non-viral carriers include polymeric, inorganic, lipidic, extracellular vesicle, or hydrogel-based systems.
4.2.1. Polymeric Nano-Systems
Polymeric nanoparticles can be obtained from either naturally occurring polymers such as chitosan and dextran or from synthetic polymers, namely poly(lactic-co-glycolic acid) (PLGA), polyamidoamine (PAMAM), polyethyleneimine (PEI), polylysine (PLL), poly[2-(dimethylamino) ethyl methacrylate] (pDMAEMA), etc. Polymeric nanoparticles are among the most promising nano-delivery systems, owing to their simple synthetic procedures, structure, transfection efficiency, non-immunogenicity, and biocompatible nature [113]. The most common forms include nanospheres and nanocapsules having subclasses such as polyplexes, polymerosomes, and dendrimers.
Polyplexes comprise cationic polymers that can bind with nucleic acids to form compact structures via electrostatic interactions. It is a spontaneous process in which typically, an excess of cationic polymer is used compared to that of the nucleotide (amine to phosphate (N/P) ratio > 1) in order to condense the nucleic acid cargo and have nanoparticles with a positive surface charge and small particle size [114,115]. The negatively charged nucleic acids are well protected within the polymer matrix by the polymer chains from nucleolytic enzymes, and the stability of the formed nanoparticles can be enhanced by the incorporation of covalent cross-linkers or by the addition of an alkyl group [116,117]. The formation of polymerosomes or polymer vesicles on the other hand involves the self-assembly of amphiphilic block co-polymers via the hydrophobic effect. Some of the methods are the direct dissolution of a copolymer, film rehydration, solvent displacement, and probe sonication [118]. Based on the hydrophilic nature which helps in the self-assembling of the block copolymers to form polymerosomes, nucleic acids are well encapsulated in the inner aqueous core of the vesicles. On the other hand, dendrimers can be synthesized by an iterative sequence of growth and activation steps either by divergent or convergent growth approach methods [119]. Every successive reaction leads to the generation of a new branch and thus repeating the number of cycles to form a dendrimer generation. By tuning the dendrimer generation, the mass and the size of the dendrimers can be manipulated, and its surface can be functionalized by the conjugation of different moieties to its reactive terminal groups, causing them to be good gene delivery platforms. The conjugation of hydrophilic polymer chains such as polyethylene glycol (PEG) can improve the stability and biocompatibility of the polyplex and dendrimer systems, by reducing the particle aggregation and preventing coalescence [120,121].
PLGA-Based Nano-System
PLGA is a copolymer produced as a result of catalyzed ring-opening copolymerization of poly-lactic acid (PLA) and poly-glycolic acid (PGA) which decomposes to non-toxic products such as carbon dioxide and water via the Krebs cycle and is eliminated from the body [122]. PLGA has potential drug delivery applications since it is part of formulations that obtained US Food and Drug Administration (FDA) and European Medicine Agency (EMA) approval [123]. The individual monomer unit, PLA, is a hydrophobic polymer with low mechanical strength. On the other hand, PGA is a crystalline and hydrophilic moiety with a fast degradation rate and low water solubility. The properties of PLGA co-polymer depends on the ratio LA/GA of the individual monomer units and the molecular weight of PLGA [124]. An increase in the ratio results in the escalation of overall hydrophobicity, leading to a low degradation rate and slower drug release rate. On the other hand, the lowering of the molecular weight increases the degradation and drug release rates. It has been observed from studies that an increase in the molecular weight also results in nanoparticles with a larger size [125,126]. These parameters based on hydrophobicity, release profile rates, as well as drug loading efficiency, are crucial for formulating a PLGA nanoparticle.
Even though PLGA is biodegradable and non-toxic for a wide variety of clinical applications, its hydrophobic properties along with the subsequent issues of opsonization associated with its intravenous administration make it unsuitable for the effective delivery of miRNA. To prevent this, modifications such as a coating with PEG, which is biocompatible, hydrophilic, non-toxic, and FDA approved, helps to shield the carriers and to enhance permeability and retention [24]. Two main synthesis procedures for PLGA-PEG nano-systems include nanoprecipitation and a double emulsion solvent evaporation method. In the nanoprecipitation method, PLGA and PEG are dissolved in a suitable solvent and added to an aqueous phase for self-assembly, followed by solvent removal via dialysis or volatilization and finally nanoparticle collection. On the other hand, the double emulsion solvent evaporation method involves the addition of PLGA, PEG, and drug in an organic solvent to prepare water in an oil emulsion. The addition of this resultant emulsion into a water phase followed by homogenization and sonication results in a water in oil in water (W/O/W) emulsion [127]. Organic solvent evaporation and subsequent filtration yield drug-loaded PLGA-PEG nanoparticles. Compared to that of a single emulsion solvent evaporation method, this improves the entrapment efficiency as well as sustained release properties of the nano-system [128]. Pham et al., based on a study on miRNA 146a-5p-loaded PLGA nanoparticles in a rat spinal nerve ligation-induced neuropathic pain model, concluded that PLGA nanoparticles were a promising drug delivery platform by improving pain behaviors through the inhibition of multiple inflammatory pathways and the subsequent reduction in proinflammatory cytokine release [129]. In the study, PLGA nanoparticles were prepared via double emulsion through sonication of the primary emulsion containing the miRNA drug in TE7.5 buffer and PLGA dissolved in dichloromethane (DCM), with a secondary water phase containing poly vinyl alcohol (PVA) to form W/O/W. A study based on miRNA-129-5p encapsulated in PLGA nanoparticles by Kalashnikova et al. proved the role of miRNA in the regulation of microglial activation and modulation of innate immune cells as well as inhibited neuroinflammation after CNS injury [130]. Studies were conducted on BV-2 cells, which showed the substantial upregulation of pro-regenerative markers and downregulation of pro-inflammatory markers (Figure 5).
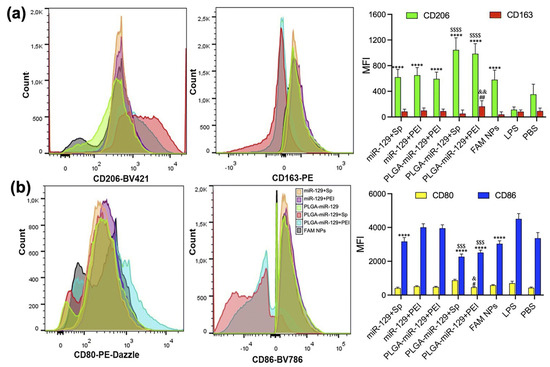
Figure 5.
Flow cytometry analysis, indicating the modulation of activated BV-2 cells. (a) Significant upregulation of pro-regenerative markers CD206 and CD163 in all nanoformulations compared to LPS group. **** p < 0.0001 vs. LPS group and $$$$ p < 0.0001 vs. miR-129+Sp, miR-129+PEI, PLGA-miR-129, and FAM NPs. CD163; ## p < 0.01 vs. LPS group and && p < 0.01 vs. miR-129+Sp, miR-129+PEI, PLGA-miR-129, PLGA-miR-129+Sp, and FAM NPs. (b) Downregulation of pro-inflammatory markers CD80 and CD86 in all nanoformulations. # p < 0.05 vs. LPS group and & p < 0.05 vs. miR-129+Sp, miR-129+PEI, PLGA-miR-129, PLGA-miR-129+Sp, and FAM NPs. CD86; **** p < 0.0001 in response to miR-129+Sp, PLGA-miR-129+Sp, PLGA-miR-129+PEI, and FAM NPs compared to LPS group and $$$ p < 0.001 vs. miR-129+Sp, miR-129+PEI, and PLGA-miR-129 (reprinted from [130]).
Chitosan-Based Nano-System
Chitosan, a derivative of N-deacetylation of chitin, a naturally occurring polymer, is a polysaccharide co-polymer having monomer units, D-glucosamine and N-acetyl-D-glucosamine [131]. It is biocompatible, biodegradable, and has FDA GRAS (generally recognized as safe) status. Recent in vitro and in vivo reports suggest that chitosan-based nanocarriers elevate nitric oxide production and thereby showcase wound healing properties as well [132,133]. A few chitosan-based nanocarriers are undergoing pre-clinical and early clinical development with a minimum of 15 clinical trials, making chitosan-based nano-systems an intervention that can provide safe drug delivery in the near future [134].
Various parameters affect the chitosan-miRNA nanoparticles such as molar mass, degree of deacetylation, and pH. A short-chain-length chitosan with less than a 15 kDa molar mass cannot condense the genetic material and complete the binding to form an intact nanoparticle [95]. On the other hand, chitosan with a molar mass greater than 300 kDa may have a stronger binding affinity with the genetic material that could potentially challenge the gene release [135]. A molar mass 5 to 10 times higher than the nucleotide RNA are required to form compactly structured nanoparticles of a suitable size via electrostatic forces [136]. The commonly used molar mass of chitosan is around 100 kDa, which can formulate nanoparticles of a size less than 200 nm which are safely tolerated by cells in order to have a potential biological effect.
The main chitosan–miRNA-based nanocarrier formulation methodologies involve simple complexation and an ionic gelation method. Mixing of miRNA and chitosan at an appropriate N/P ratio and weight ratios to obtain biologically active nanoparticles forms the basis of simple complexation [137]. On the other hand, ionic gelation is based on the electrostatic interaction between the anionic and cationic charges of miRNA and chitosan, respectively, in the presence of a cross-linking agent such as tripolyphosphate (TPP), which helps in strengthening and stabilizing the chitosan–miRNA interactions [138]. The conjugation of chitosan to tragacanthic acid (TA) and glutathione was used in a study by Shamaeizadeh et al. to deliver miR-219a-5P to treat multiple sclerosis, a chronic neurodegenerative disease [139]. Louw A et al., in a study based on chitosan polyplex-mediated delivery of miR-124, demonstrated an alteration in the inflammatory response in spinal cord injury models (Figure 6) [140].
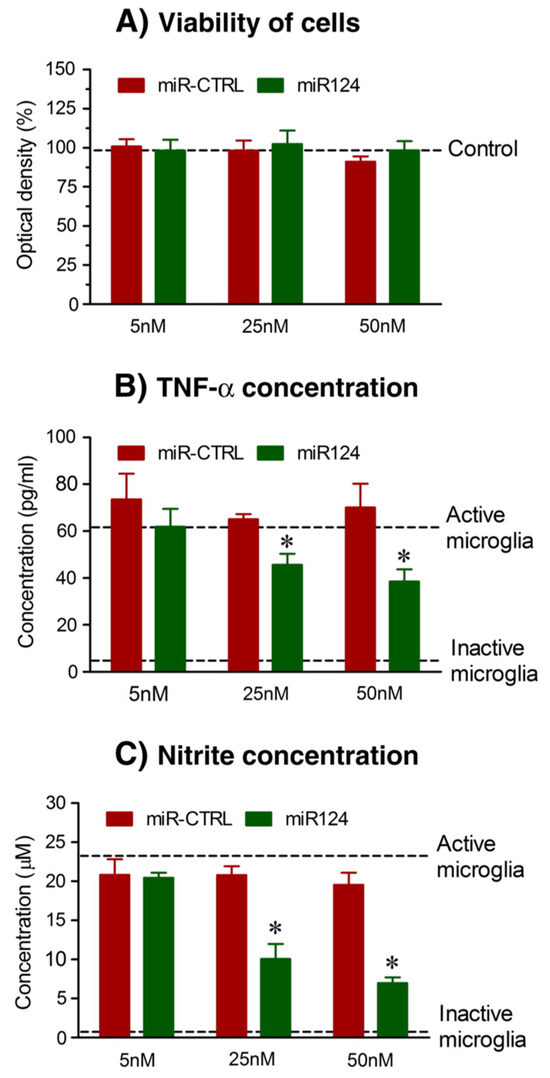
Figure 6.
(A) Cytotoxicity of chitosan/miRNA particles measured by AlamarBlue® viability assay indicate no significant decrease in viability after treatment with chitosan particles. MiR124-loaded chitosan polyplex particles reduce inflammatory factors like (B) TNF-α and (C) nitrite species; * p < 0.01 and 0.05 versus LPS and IFN-γ alone (reprinted from [140] with permission from Elsevier).
PEI-Based Nano-Systems
PEI is a cationic synthetic polymer rich in amine groups, that can condense nucleic acids to form nanoparticles via electrostatic interaction, thereby protecting nucleic acid from degradation and facilitating the cellular uptake of these nucleic acids [112]. Efficient release of the polyplex into the cytoplasm is furthermore enhanced by the proton sponge effect of PEI that helps in endosomal escape [141]. However, the formation of aggregates through intracellular protein interaction is an issue due to its non-biodegradable nature within the cells, as a result of which dose-dependent cytotoxicity is a major concern [142]. In order to reduce the cytotoxicity and enhance transfection efficiency, studies have been conducted on PEI nanocarrier modifications using poly (L-lysine) (PEI-PLL), polyurethane (PU-PEI), poly(1,8-octanediocitric acid)-co-polyethylene glycol grafted with PEI (POCG-PEI), and PLGA/cetylated PEI/hyaluronic acid nanoparticles (PCPH nanoparticles) [143,144,145,146]. In a study by Wang et al. on biodegradable poly(1,8-octanedio-citric acid)-co-polyethylene glycol grafted with PEI (POCG-PEI) for nucleic acid delivery, it was found that this POCG-PEI can bind with siRNA and miRNA as a protective shield from enzymatic degradation, along with enhanced cellular uptake and significant reduction in cytotoxicity as compared to that of unmodified PEI [147]. In another study by Son et al., the introduction of a disulfide (-S-S-) linkage in the branched PEI (SSPEI) enhanced the biodegradability of the polymer and thereby achieved better release of the nucleic acid indirectly with the help of endogenous enzymes [148]. Furthermore, Li et al., by coupling poly (ε-caprolactone) (PCL) to PEI via bio-cleavable disulfide linkage, synthesized an amphiphilic cationic graft polymer called PSSP (polyethyleneimine-cystamine-poly(ε-caprolactone), which showed high transfection efficiencies and low cytotoxicity along with intracellular redox potential to trigger miRNA release [149]. Apart from the PEI modifications, studies based on the use of low-molecular-weight PEI showed better biocompatibility and degradability along with a lower charge density, thus leading to a lower degree of membrane damage and lower cytotoxicity compared to that of the higher-molecular-weight PEI [150]. With regard to pain-related studies conducted using PEI, a study by Cheng et al. using an miR-195 mimic encapsulated in jetPEI, a commercial PEI-based nanoparticle, proved that miR-195-treated stroke rats showed antiapoptotic characteristics via inhibiting Sema3A/Cdc42/JNK signaling and inflammation reduction [151].
Polyamidoamine (PAMAM) Dendrimer-Based Nano-Systems
Dendrimers are hyperbranched polymers with symmetrical structures having a central core surrounded by three or more branches of repeating monomer units that have terminal functional groups at the ends of the branches for conjugation [152,153]. One of the main advantages of dendrimers is that the size, shape, charge, and solubility can be controlled by manipulating the monomer units [154]. PAMAM dendrimers are a group of dendrimers widely used to facilitate miRNA delivery based on their ability to conjugate ligands for targeted cell specificity [155,156,157]. PAMAM has a radial structure with primary amine functional groups on its surface and a diaminobutane core [158,159]. The combination of the surface amines and interior amide linkages offers better biocompatibility for PAMAM dendrimers over other types. However, cytotoxicity and low transfection efficiency are major limiting factors, which can only be overcome by surface modification and alteration of generation [160,161,162]. Studies based on modifying the dendrimer generation and surface functional groups were conducted by Zeng et al.’s group on neuronal differentiation using human progenitor cells, and Wu et al. synthesized aptamer-modified PEGylated PAMAM [163,164]. PAMAM can be used to co-deliver two different molecules such as antagomir-21 and 5-fluorouracil and can also be coupled with other moieties to enable multifaceted therapy and imaging as in the case of gadolinium-functionalized nanographene oxide (Gd-NGO) with PAMAM [165,166].
4.2.2. Inorganic Nano-Systems
The production of structures having a tunable size, morphology, and composition along with the simplicity in formulation procedures makes synthetic or inorganic nanomaterials potential delivery vehicles for miRNA delivery [167,168]. Nucleic acid incorporation can be achieved via encapsulation, adsorption, or covalent adsorption using particle surface modifications with reactive species [169,170]. One of the most commonly used inorganic nanomaterial is silica or mesoporous silica nanoparticles. Other promising inorganic nano-systems include gold nanoparticles and iron oxide nanoparticles; however, currently, there are no studies yet conducted on gold or iron oxide nanoparticle-based delivery of miRNA for neuropathic pain treatment.
Studies on the chemical and mechanical stability of gold nanoparticles demonstrates that they are a suitable delivery system for miRNA or other nucleic acids [171]. The negatively charged miRNA can be loaded onto the surface of the gold nanoparticles upon functionalization with thiol or amino groups [91,172]. Another study by Sukumar et al. shows that polyfunctional gold–iron oxide nanoparticles or polyGION nanoparticles could be surface functionalized with chitosan–cyclodextrin (CD-CS) hybrid polymers, which increased the surface potential from −15 mV to +39 mV due to the excess free positive charges of the amine groups of chitosan, thereby giving it a loading efficiency of 80% [170]. However, gold nanoparticles of 5 nm may disrupt the fibroblast structure after 72 h of exposure, thereby limiting the clinical application due to the potential cytotoxicity [173].
Iron oxide nanoparticles are largely used owing to the magnetic properties of its core which enables it to be used for magnetic resonance imaging (MRI), in addition to its theranostic capabilities of delivering nucleic acids [165]. SPIONs or super paramagnetic iron oxide nanoparticles are often used as contract agents in gene therapy. SPIONs are coated with polymers to stabilize and enhance the biocompatibility, and with lipids to evade RES clearance and the immune response [174,175]. Even though direct studies on iron oxide nanoparticle-based delivery of miRNA for chronic neuropathic pain are scarce, these listed studies highlight the conceptual frameworks indicating the potential applications and pre-clinical approaches that could be extended to chronic pain. The properties of iron oxide nanoparticles such as magnetic targeting and theranostic potential along with high cargo capacity and stability can be utilized for the delivery of miRNA such as miR-146a which can downregulate NF-κB-associated signaling (IRAK1, TRAF6) and reduce pro-inflammatory cytokine release [176]. In the case of miR-124 delivery, they can possibly convert activated M1 macrophages towards the M2 phenotype and alleviate DRG neuroinflammation and attenuate hyperalgesia and allodynia [177].
Silica Nanoparticles
Silica nanoparticles, especially mesoporous silica nanoparticles (MSNs) having a diameter of 100–200 nm are widely used in miRNA delivery due to their simplicity in functionalization, high porous architecture, biocompatibility, and the biodegradable properties [171,178]. Mesoporous silica nanoparticles have the ability to simultaneously deliver and also to have a controlled release of miRNAs and small molecules at the target sites [179]. Large surface areas, high loading capacities, chemically modifiable surfaces along with low toxicity make it a potential delivery system [180]. Pore size plays a major role in both the release rate as well as loading. MSNs with a smaller pore size can provide a tunable release rate for the oligonucleotide, whereas a larger pore size enables it to have a faster release rate and a higher loading capacity [181]. Hosseinpour et al. studied the delivery of miRNA-26a to macrophages to promote M2 polarization via mesoporous core-cone silica nanoparticles [182]. In the study, murine-derived macrophage cell lines and preosteoblastic murine-derived cell lines from a mouse were treated with miRNA-26a-loaded mesoporous. In comparison to polymeric nanoparticles and liposomes, MSNs are more resistant and have the ability of sustained release over time, making it an optimum vehicle for solving problems related to sustained drug release and targeted delivery. Recent studies by Yitian Lu et al. demonstrated that a surface-aminated MSN-mediated miR-26a-5p delivery system exhibited excellent anti-inflammatory effects, effectively reduced microglial activation, and proved to be an effective analgesic for inflammatory- and chemotherapy-induced peripheral neuropathic pain [100].
4.2.3. Lipidic Nano-Systems
Lipid-based nano-delivery systems consisting of a polar head group, hydrophilic tail region, and domain linker along with other lipid components such as cholesterol, phospholipids, and PEG have stable nanostructures in physiological fluids and have the ability to fuse with negatively charged endosomal membranes, thus making it an effective nucleic acid delivery system [183]. Even though several studies have been conducted for miRNA delivery using lipid-based nanoparticles such as liposomes, solid lipid nanoparticles (SLNs) and hybrid lipid–polymer nanoparticles, they are the most widely used methodologies for the in vivo delivery of small interfering RNAs or siRNAs [184,185]. The simplicity and safety features of lipid-based nanocarriers make them one of the most widely used delivery platforms for miRNA [186,187,188,189]. Lipid-based nanoparticles can protect the oligonucleotides from enzyme degradation, promote cellular uptake, and enhance the circulation half-life [190]. In addition to that, SLN and hybrid lipid–polymer nanoparticles have not yet been used in the delivery of miRNA in neuropathic pain treatment.
There are a very few studies based on SLNs used to co-deliver an oligonucleotide and a drug that offer enhanced therapeutic activity due to the nano-structural properties. In one study, anti-miR-21 and pemetrexed were encapsulated into SLNS which helped in higher cellular uptake when compared to that of the free drug [191]. Similarly, in another study, the synergistic effects of miR-34a and paclitaxel were exploited in an SLN co-delivery system [192]. Furthermore, Shi et al. synthesized another cationic SLN composed of dimethyl-dioctadecyl ammonium bromide (DDAB), glyceryl monostearate, polyoxythylene 50 stearate, cholesterol, and soy phosphatidylcholine via film ultrasonic techniques and loaded with miRNA via incubation [193]. Although these studies are primarily on cancer research, SLNs prove to be promising vectors for miRNA delivery, which in turn can be potentially used for chronic pain treatment too.
Lipopolyplexes or hybrid lipid–polymer nanoparticles comprise nucleic acids encapsulated or complexed with a biodegradable polymer that offer protection in a lipid bilayer which allows easy targeting and stealth moiety for a longer half-life in blood [194]. A study by Huang et al. based on lipopolyplexes consisting of PEI and dioleoylphosphatidyl ethanolamine (DOPE)/linoleic acid/1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG) on which transferrin molecules were post inserted for miR-29b delivery showed better efficiency, while the same formulation for miR-1 transferrin-mediated delivery showed 3-fold higher efficiency comparatively [195,196]. Only a few studies have been performed on pain models using lipopolyplexes, but nevertheless, it is a promising carrier. A recent study based on cationic lipid 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) and DOPE together with miR-155 formed a lipoplex that was used in the treatment of intervertebral disc (IVD) degeneration which corresponds to 40% of cases of lower back pain [197].
Cationic Lipid Nanoparticles (LNPs)
A typical cationic lipid-based nanoparticle formulation consists of cationic lipids and helper lipids such as neutral lipids and PEG-lipids. Cationic liposomes or lipid nanoparticles commonly used for miRNA delivery are 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA), DOTAP, DDAB, and 1,2-dioleyloxy-3-dimethylaminopropane (DODMA) [198,199,200,201]. LNPs also possess additional units called helper lipids such as cholesterol and DOPE to increase the stability and reduce the toxicity and PEG to reduce the nanoparticle aggregation and increase the circulation time [202,203,204]. Lipid nanoparticles can also be developed from ionizable cationic lipids having primary, secondary, or tertiary amines as the headgroup domain with pKa values below 7. Based on a study conducted by Yung et al., lipid nanoparticles having tertiary and quaternary amine groups were prepared for therapeutic delivery of miR-21 antagomiR [205]. Even though here, this study relates to cancer treatment, miR-21 antagomiR is commonly used in rat models and blocks miR-21 and alleviates neuropathic pain via the reduction in allodynia and hyperalgesia and lowers neuroinflammatory markers.
Lipofectamine, a common transfection reagent consisting of lipid subunits and forms liposomes in aqueous environment, is often used to entrap nucleic acid payloads such as mRNA, siRNA, miRNA, and plasmid DNA. It is a 3:1 mixture of DOSPA (2,3-dioleoyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propaniminium trifluoroacetate) and DOPE [206]. Li et al. used lipofectamine-based miR-30b-5p transfection to attenuate oxaliplatin-induced peripheral neuropathic pain via Nav 1.6 VGSCs in male Sprague-Dawley rats (Figure 7) [67]. In another study, the upregulation of miR-96 using lipofectamine transfection was found to alleviate neuropathic pain in CCI rat models by inhibiting Nav 1.3 channels in the DRG [32]. Furthermore, studies also highlight the role of potassium voltage-gated channels and miRNA in alleviating neuropathic pain. Zhang et al. studied the influence of lipofectamine-transfected miR-137 agomir/antagomir and Kv1.2 channels on CCI-induced rat models, which demonstrated that the downregulation of miR-137, upregulation of Kcna2, and gene encoding in the Kv1.2 channel alleviate neuropathic pain [34]. Intrathecal injection of lipofectamine/miR-140 on CCI rat models in a study conducted by Li et al. demonstrated the attenuation of neuropathic pain by targeting Sphingosine-1-phosphate receptor 1 (S1PR1) [35]. Studies by Guangyao et al. proved the involvement of the Nav 1.3 channel encoded by the sodium voltage-gated channel alpha subunit 3 (SCN3A) gene, an isoform of tetrodotoxin-sensitive (TTX-S) in CCI rat models and the fact that negative regulation of it using lipofectamine-transfected miR-384-5p ameliorates neuropathic pain both in vivo and in vitro [45]. The regulation of the Nav 1.7 channel encoded by the SCN9A gene in a spared nerve injury (SNI) rat model by using Invivofectamine™ 3.0 (Invitrogen, Carlsbad, CA, USA) transfected miR-182 in neuropathic pain treatment was conducted by Cai et al. [68].
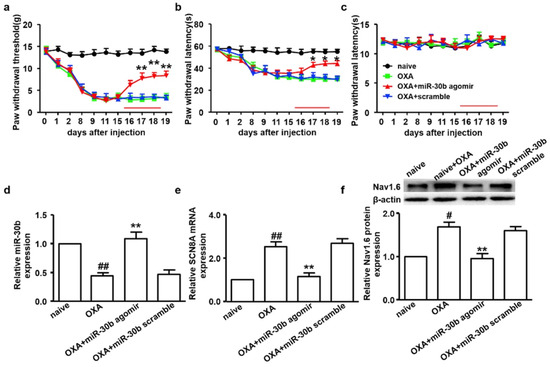
Figure 7.
Intrathecal administration of miR-30b agomir inhibits the expression of Nav 1.6 in DRG neurons and attenuates neuropathy. Figures indicate paw-withdrawal thresholds in response to (a) mechanical stimuli, (b) cold stimuli, and (c) thermal stimuli after over-expression of miR-30b. * p < 0.05 and ** p < 0.01 vs. oxaliplatin group. (d) The relative expression of miR-30b was determined by RT-qPCR in oxaliplatin-injected rats after intrathecal administration of miR-30b agomir. ** p < 0.01 vs. oxaliplatin group, ## p < 0.01 vs. naïve group. Nav 1.6 (e) mRNA and (f) protein expression were decreased in oxaliplatin-injected rats after intrathecal administration of miR-30b agomir. ** p < 0.01 vs. oxaliplatin group. # p < 0.05. (reprinted from [67] with permission from Elsevier).
4.2.4. Extracellular Vesicles-Based Nano-Systems
Extracellular vesicles (EVs) are nanometer-scale carriers composed of phospholipid bilayer-based vesicles that are secreted from all cell types and commonly found in biological fluids such as saliva, blood, breast milk, cerebrospinal fluids, and malignant ascites [207,208]. Owing to the unique biological characteristics along with their role in cell-to-cell communication, EVs have attracted strong interest in therapeutics. Based on their biogenesis, EVs can be classified as exosomes, microvesicles, and apoptotic bodies [209]. The size of an exosome ranges from 40 to 120 nm while that of microvesicles are in the range of 50 to 1000 nm [210,211]. EVs function as a carrier vehicle for infectious particles, oncogenes, and biomolecules such as lipids, proteins, DNA transcription factors, mRNAs, and small or large non-coding RNAs, especially miRNAs between various cell types [212,213]. Over conventional nanocarriers for drugs and therapeutic agents, EVs have advantages such as good biocompatibility, low immunogenicity, capacity for gene delivery, and natural blood–brain barrier penetration [214]. Post peripheral nerve injury, neuro–immune interactions occur in the DRG resulting in the release of EVs containing miRNAs, which become engulfed by macrophages leading to alterations affecting the pain mechanisms [215]. Studies reveal that the inhibition of microRNA23a or miR23a has potential therapeutic intervention for nociceptive hypersensitivity induced as a result of peripheral nerve injury such as an SNI which contributes to enhanced M1 polarization along with the secretion of inflammatory factors [216,217]. Zhang et al. further studied the delivery of extracellular vesicle-encapsulated miR-23a from DRG neurons and its subsequent contribution to the inflammatory response and M1 macrophage polarization in SNI-based neuropathic pain models (Figure 8) [29]. In another investigation by Zhang et al., it was found that exosome-encapsulated miR-181c-5p could attenuate neuropathic pain after sciatic nerve chronic constriction injury [101]. Another study based on the activation of microglia via miR-16-5p knockdown in DRG-derived exosomes by Xing et al. demonstrated the alleviation of neuropathic pain [102].
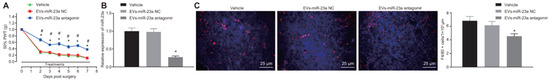
Figure 8.
Delivery of miR-23a antagomir reduced neuropathic hypersensitivity and recruitment of M1 macrophages in vivo (A) mechanical hypersensitivity; * p < 0.05 vs. vehicle mice; # p < 0.05 vs. oligomer-treated mice. (B) RT-qPCR analysis of miRNA expression in DRG. (C) Immunostaining of macrophages (red) and nuclei (blue) in SNI DRG; * p < 0.05 vs. mice treated with EVs-miR-23a-NC or vehicle (reprinted from [29]).
4.2.5. Hydrogel-Based Systems
Hydrogels are a 3-dimensional network with polymer crosslinking that has the ability to absorb water and also retain it [218]. Hydrogels provide a natural aqueous environment for biomolecules by encapsulating them to function in biological systems and provide a diffusion barrier that allows the passage of molecules of a certain threshold only, thus protecting the biological agents from immune rejection or degradation [219]. Due to its ideal injectable characteristics along with controllable degradability, good mechanical properties, and negligible cytotoxicity, hydrogels are often considered as one of the best candidates for drug delivery [220]. In addition to that, the tunability of the stiffness of hydrogels from 0.5 kPa to 5 MPa allows their physical properties to match those of various soft tissues in the human body [221,222,223]. Hydrogels can be classified into three main categories depending on the requirements of the delivery route, such as macroscopic hydrogels, microgels, and nanogels. Microgels and nanogels are smaller hydrogel particles often used as a minimal invasive delivery route owing to its injectable properties, large surface area for bioconjugation, enhanced tissue penetration, and facile natural clearance [224]. Nanohydrogels or nanogels possess a very high surface to volume ratio thereby facilitating cross cellular membrane transfer and efficient cell uptake [225]. Natural polymeric-based nanohydrogels offer advantages such as high biodistribution, non-cytotoxic nature, biodegradability, and high clearance rates. However, they often suffer from uncontrolled structures and unpredictable drug release behavior. These limitations can be addressed by employing synthetic polymeric systems involving materials such as polyamides, PEG, polypeptides, and polyesters [226,227]. Synthetic polymeric-based nanohydrogels demonstrate high stability, controlled architectures, and tunable drug release properties, and are generally designed to exhibit low immunogenicity [228,229]. Nevertheless, minor immune responses can still arise depending on the polymer type, surface modifications, and degradation products. Therefore, by incorporating bioactive probes or by developing hybrid hydrogels that combine both natural and synthetic polymers, it is possible to optimize biocompatibility, minimize immunogenicity, and achieve controlled therapeutic delivery [218].
Hydrogels are often used in gene therapy due to their tunable physical characteristics and controlled release of the nucleic acid drugs. Other advantages of using hydrogels for gene delivery include fewer off-target effects, the maintenance of bioactivity, the possibility of local administration, and the avoidance of multiple administration and on-demand or pulsatile as well as prolonged release of the drugs [230]. Nucleic acids such miRNA can be either loaded directly or by encapsulating it in a nanocarrier system. Nanocarrier loading provides better controllability and specific targeting of cells along with improved bioactivity comparatively. MiRNA-based hydrogels have been studied for cancer therapy, immunomodulation, bone regeneration, cardiovascular disease, spinal cord injury, intervertebral disk degeneration, and more [231,232,233,234,235,236]. Studies by Li et al. on the hydrogel-based controlled delivery of miR-21-5p using mesoporous silica nanoparticles shows an anti-inflammatory response in porcine models by inhibiting the macrophage polarization to M1 [237]. In another study, Wang et al., based on work on curcumin and cholesterol-modified antagomir-21 delivery for intervertebral disc degeneration treatment using an injectable inflammation-responsive gelatin-based hydrogel, demonstrated anti-inflammatory activity by inducing the M2 phenotype polarization of macrophage cells in vitro [238].
5. Conclusions and Future Perspectives
In the recent years, significant scientific contributions have been made in understanding the crucial role of miRNAs in the pathogenesis of chronic neuropathic pain conditions. Studies on miRNA have increased exponentially ever since the demonstration of its use as a therapeutic agent and a biomarker. The regulation of gene expression related to neuroinflammation, neuronal excitability, and synaptic plasticity is only one among the pivotal roles that these short non-coding RNAs play. Nerve injury-induced miRNA dysregulation and subsequent macrophage polarization could be regulated to induce chronic neuropathic pain treatment. Despite these insights, the clinical translation of miRNA-based therapies faces challenges with regard to safe, effective, and successful delivery systems for miRNAs to target tissues.
Drug delivery systems for miRNA-based cancer treatment are relatively more common compared to that for neuropathic pain treatment. Even though recent progress in the formulation of various delivery systems is promising, the formulation of an ideal vector is yet miles away. Limitations and drawbacks from the current data can be used in formulating a better nanocarrier for nucleic acid delivery. Current limitations include miRNA instability in vivo, off-target effects, immune reactions, and lack of DRG-specific targeting. To address these, future research should emphasize stimuli-responsive and multifunctional carriers (e.g., pH- or enzyme-sensitive nanoparticles) and targeted delivery (e.g., with DRG-homing ligands). The co-delivery of miRNA combinations or miRNA plus drugs may enhance the efficacy while minimizing doses. Innovative strategies like hybrid nanocarriers (combining polymer and lipid components), surface engineering, and triggered release systems could help overcome the stability and specificity challenges. In conclusion, while miRNA-based therapies outline and represent a promising frontier in chronic neuropathic pain treatment, the development of efficient and safe systems remains a crucial hurdle. The clinical application and translation face key challenges, including immune response concerns and regulatory approvals. Notably, miR-146a-loaded PLGA nanoparticles were shown to reduce neuroinflammation in rodent pain models; however, translating this into human trials requires addressing nanoparticle stability and precise dosing. Future research should focus on standardized protocols and clinical validation, with miR-146a-loaded PLGA nanoparticles. Currently, no miRNA-based pain therapies have entered Phase III clinical trials. Ongoing research into innovative delivery mechanisms involving advanced nanoparticle platforms is very much essential in harnessing the full therapeutic potential of miRNAs, in order to translate such findings into clinical practice.
Funding
This research was funded by European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Actions (MSCA), Innovative Training Network (ITN) “PIANO”, grant agreement No. 956477.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| AAV | Adeno-associated virus |
| Cav | Calcium channels |
| CD | Cyclodextrin |
| CSF | Cerebrospinal fluid |
| DCM | Dichloromethane |
| DMG-PEG | 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
| DODMA | 1,2-dioleyloxy-3-dimethylaminopropane |
| DOPE | Dioleoylphosphatidyl ethanolamine |
| DOSPA | 2,3-dioleoyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propaniminium trifluoroacetate |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium propane |
| DOTMA | 1,2-di-O-octadecenyl-3-trimethylammonium propane |
| DRG | Dorsal root ganglia |
| EMA | European Medicine Agency |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| Gd-NGO | Gadolinium-functionalized nanographene oxide |
| GRAS | Generally recognized as safe |
| HMGB1 | High-mobility group box 1 |
| HSV | Herpes simplex virus |
| IASP | International Association for the Study of Pain |
| IVD | Intervertebral disc |
| Kv | Potassium channels |
| miRNA | Micro-RNA |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MSNs | Mesoporous silica nanoparticles |
| mTOR | Mechanistic/Mammalian target of rapamycin |
| N/P ratio | Amine to phosphate ratio |
| Nav | Sodium channels |
| ncRNA | Non-coding RNA |
| NF-κB | Nuclear factor kappa B |
| PAMAM | Poly(amidoamine) |
| PCL | Poly (ε-caprolactone) |
| PCPH | PLGA/cetylated PEI/hyaluronic acid |
| pDMAEMA | Poly (2-dimethylamino) ethyl methacrylate |
| PDN | Painful diabetic neuropathy |
| PEG | Poly (ethylene glycol) |
| PEI | Polyethyleneimine |
| PGA | Poly-glycolic acid |
| POCG | Poly(1,8-octanediocitric acid)-co-polyethylene glycol |
| polyGION | Polyfunctional gold-iron oxide nanoparticles |
| PSN | Primary sensory neurons |
| PSSP | Polyethyleneimine-cystamine-poly(ε-caprolactone) |
| PU | Polyurethane |
| PVA | Poly (vinyl alcohol) |
| PWL | Paw withdrawal latency |
| PWT | Paw withdrawal threshold |
| RES | Reticuloendothelial system |
| RISC | RNA-induced silencing complex |
| -S-S- | Disulfide |
| S1PR1 | Sphingosine-1-phosphate receptor 1 |
| SCI | Spinal cord injury |
| SCN3A | Sodium voltage-gated channel alpha subunit 3 |
| siRNA | Small interfering RNA |
| SLNs | Solid lipid nanoparticles |
| SNI | Spared nerve injury |
| SNL | Spared nerve ligation |
| SPIONs | Super paramagnetic iron oxide nanoparticles |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| TA | Tragacanthic acid |
| TPP | Tripolyphosphate |
| TRBP | Trans-activation response RNA-binding protein |
| TREK1 | TWIK-related potassium channel 1 |
| TTX-S | Tetrodotoxin-sensitive |
| VGSC | Voltage-gated sodium channels |
| W/O/W | Water in oil in water |
| XIST | X-inactive specific transcript |
References
- International Association for Study of Pain. IASP Pain Definition. Available online: https://www.iasp-pain.org (accessed on 29 May 2023).
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Chu, H.; Ma, J.; Yan, Y.; Zhang, X.; Liang, Y. The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance. Front. Mol. Neurosci. 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults—United States, 2019–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ducic, I.; Mesbahi, A.N.; Attinger, C.E.; Graw, K. The Role of Peripheral Nerve Surgery in the Treatment of Chronic Pain Associated with Amputation Stumps. Plast. Reconstr. Surg. 2008, 121, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.; Benveniste, H.; McLellan, A.T. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 2018, 69, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, K.R.; Golightly, Y.M. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract. Res. Clin. Rheumatol. 2015, 29, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.d.C.; Eccleston, C.; Morley, S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012, 11, CD007407. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Smith, L. Chronic Pain and Addiction: Challenging Co-occurring Disorders. J. Psychoact. Drugs 2012, 44, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; van der Goes, D.N. Rates of opioid misuse, abuse, and addiction in chronic pain. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Neural Mechanisms Underlying Anxiety–Chronic Pain Interactions. Trends Neurosci. 2016, 39, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Liu, Y.; Possoit, H.; Rogers, A.C.; Moore, W.; Gress, K.; Cornett, E.; Kaye, A.; Imani, F.; Sadegi, K.; et al. Dorsal Root Ganglion (DRG) and Chronic Pain. Anesth. Pain Med. 2021, 11, e113020. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Strickland, I.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.F. Axotomy-Induced miR-21 Promotes Axon Growth in Adult Dorsal Root Ganglion Neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, S.; Qian, T.; Wang, Y.; Ding, F.; Gu, X. Altered microRNA expression following sciatic nerve resection in dorsal root ganglia of rats. Acta Biochim. Biophys. Sin. 2011, 43, 909–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Khayati, S.; Dehnavi, S.; Sadeghi, M.; Tavakol Afshari, J.; Esmaeili, S.A.; Mohammadi, M. The potential role of miRNA in regulating macrophage polarization. Heliyon 2023, 9, e21615. [Google Scholar] [CrossRef] [PubMed]
- Kovanur Sampath, K.; Belcher, S.; Hales, J.; Thomson, O.P.; Farrell, G.; Gisselman, A.S.; Katare, R.; Tumilty, S. The role of micro-RNAs in neuropathic pain-a scoping review. Pain Rep. 2023, 8, e1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Pu, S.; Wu, J.; Lv, Y.; Du, D. Circulating microRNA expression profile: A novel potential predictor for chronic nervous lesions. Acta Biochim. Biophys. Sin. 2014, 46, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Maier, S.F.; Watkins, L.R. MicroRNA-124 and microRNA-146a both attenuate persistent neuropathic pain induced by morphine in male rats. Brain Res. 2018, 1692, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, L.; Zhang, M.D.; Bengtsson Gonzales, C.; Parisien, M.; Belfer, I.; Usoskin, D.; Abdo, H.; Furlan, A.; Häring, M. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science 2017, 356, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kwon, T.H.; Kim, A. Delivery of therapeutic miRNA using polymer-based formulation. Drug Deliv. Transl. Res. 2019, 9, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Saitow, F.; Miyake, N.; Miyake, K.; Shimada, T.; Suzuki, H. miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 2013, 136, 2738–2750. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Saitow, F.; Maruyama, M.; Miyake, N.; Miyake, K.; Shimada, T.; Okada, T.; Suzuki, H. MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat. Commun. 2017, 8, 16079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Guo, J.S.; Li, S.S.; Wu, X.B.; Cao, D.L.; Jiang, B.C.; Jing, P.; Bai, X.; Li, C.; Wu, Z.; et al. TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J. Exp. Med. 2018, 215, 3019–3037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Wang, X.; Zhang, J.; Xie, C. Extracellular vesicle-encapsulated microRNA-23a from dorsal root ganglia neurons binds to A20 and promotes inflammatory macrophage polarization following peripheral nerve injury. Aging 2021, 13, 6752–6764. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Cao, J.; Wang, J.; Ren, X.; Su, S.; Li, M.; Li, Z.; Zhao, Q.; Zang, W. MicroRNA-30b regulates expression of the sodium channel Nav1.7 in nerve injury-induced neuropathic pain in the rat. Mol. Pain 2016, 12, 174480691667152. [Google Scholar] [CrossRef] [PubMed]
- Brandenburger, T.; Johannsen, L.; Prassek, V.; Kuebart, A.; Raile, J.; Wohlfromm, S.; Köhrer, K.; Huhn, R.; Hollmann, M.; Hermanns, H. MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 2019, 708, 134365. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhou, W.; Kang, L.M.; Yan, H.; Zhang, L.; Xu, B.H.; Cai, W. Intrathecal miR-96 Inhibits Nav1.3 Expression and Alleviates Neuropathic Pain in Rat Following Chronic Construction Injury. Neurochem. Res. 2014, 39, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Willemen, H.L.; Huo, X.J.; Mao-Ying, Q.L.; Zijlstra, J.; Heijnen, C.J.; Kavelaars, A. MicroRNA-124 as a novel treatment for persistent hyperalgesia. J. Neuroinflamm. 2012, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rong, L.; Shao, J.; Zhang, Y.; Liu, Y.; Zhao, S.; Li, L.; Yu, W.; Zhang, M.; Ren, X.; et al. Epigenetic restoration of voltage-gated potassium channel Kv1.2 alleviates nerve injury-induced neuropathic pain. J. Neurochem. 2021, 156, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Ma, Z.; Liu, Y.; Sun, Z.; Wu, Y. miR-140 ameliorates neuropathic pain in CCI rats by targeting S1PR1. J. Recept. Signal Transduct. 2021, 41, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Zi, T. Overexpression of microRNA-141 relieves chronic constriction injury-induced neuropathic pain via targeting high-mobility group box 1. Int. J. Mol. Med. 2015, 36, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mou, J.; Cao, L.; Zhen, S.; Huang, H.; Bao, H. MicroRNA-142-3p relieves neuropathic pain by targeting high mobility group box 1. Int. J. Mol. Med. 2018, 41, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Xie, A.; Liao, O.; Ju, F.; Zhou, Y. MicroRNA-144 relieves chronic constriction injury-induced neuropathic pain via targeting RASA1. Biotechnol. Appl. Biochem. 2020, 67, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, F.; Wei, M.; Qiu, Y.; Ma, C.; Shen, L.; Huang, Y. Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J. Neuroinflamm. 2018, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, Y.; Liu, Y.; Liu, H.; Zhang, Z.; Su, Z. Effects of miR-150 on neuropathic pain process via targeting AKT3. Biochem. Biophys. Res. Commun. 2019, 517, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Dong, W.; Zhang, L.; Yang, X. Activating Sirt1 by resveratrol suppresses Nav1.7 expression in DRG through miR-182 and alleviates neuropathic pain in rats. Channels 2020, 14, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.N.; Yuan, Y.T.; Ye, D.; Kang, L.M.; Wen, J.; Chen, H.P. MiR-183-5p Alleviates Chronic Constriction Injury-Induced Neuropathic Pain Through Inhibition of TREK-1. Neurochem. Res. 2018, 43, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, R. MiR-216a-5p alleviates chronic constriction injury-induced neuropathic pain in rats by targeting KDM3A and inactivating Wnt/β-catenin signaling pathway. Neurosci. Res. 2021, 170, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ke, C.; Lu, J. NEAT1 contributes to neuropathic pain development through targeting miR-381/HMGB1 axis in CCI rat models. J. Cell. Physiol. 2018, 233, 7103–7111. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, Y.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. miR-384-5p ameliorates neuropathic pain by targeting SCN3A in a rat model of chronic constriction injury. Neurol. Res. 2020, 42, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, H.; Zhang, A.; Zhao, C.; Mei, Z.; Yao, H.; Fan, Z.; Liang, D. Identifying a novel KLF2/lncRNA SNHG12/miR-494-3p/RAD23B axis in Spare Nerve Injury-induced neuropathic pain. Cell Death Discov. 2022, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, Y.; Shi, B. miR-590-3p Alleviates diabetic peripheral neuropathic pain by targeting RAP1A and suppressing infiltration by the T cells. Acta Biochim. Pol. 2020, 67, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hogan, Q.H. Labat Lecture: The Primary Sensory Neuron. Reg. Anesth. Pain Med. 2010, 35, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.B. THE PENETRATION OF PARTICULATE MATTER FROM THE CEREBROSPINAL FLUID INTO THE SPINAL GANGLIA, PERIPHERAL NERVES, AND PERIVASCULAR SPACES OF THE CENTRAL NERVOUS SYSTEM. J. Neurol. Neurosurg. Psychiatry 1950, 13, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Devor, M. Unexplained peculiarities of the dorsal root ganglion. Pain 1999, 82 (Suppl. 1), S27–S35. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Jaskiewicz, L.; Kolb, F.A.; Pillai, R.S. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr. Opin. Struct. Biol. 2005, 15, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An Abundant Class of Tiny RNAs with Probable Regulatory Roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, E.; Tuschl, T.; Zamore, P.D. A Cellular Function for the RNA-Interference Enzyme Dicer in the Maturation of the let-7 Small Temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005, 436, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional siRNAs and miRNAs Exhibit Strand Bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yekta, S.; Shih, I.H.; Bartel, D.P. MicroRNA-Directed Cleavage of HOXB8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Suzuki, H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem. Biophys. Res. Commun. 2013, 435, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liu, Y.; Ge, X.; Wang, J.; Wang, Z. Transcriptome Analysis of Non-coding RNAs and mRNAs in the Dorsal Root Ganglion of Peripheral Nerve Injury-Induced Neuropathic Pain. Biochem. Genet. 2025; online ahead of print. [Google Scholar]
- Zhao, Y.Y.; Wu, Z.J.; Zhu, L.J.; Niu, T.X.; Liu, B.; Li, J. Emerging roles of miRNAs in neuropathic pain: From new findings to novel mechanisms. Front. Mol. Neurosci. 2023, 16, 1110975. [Google Scholar] [CrossRef] [PubMed]
- Morchio, M.; Sher, E.; Collier, D.A.; Lambert, D.W.; Boissonade, F.M. The Role of miRNAs in Neuropathic Pain. Biomedicines 2023, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, H.; Azimian, A.; Golmakani, E. Newly discovered functions of miRNAs in neuropathic pain: Transitioning from recent discoveries to innovative underlying mechanisms. Mol. Pain 2024, 20, 17448069231225845. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Shao, J.; Zhao, Q.; Ren, X.; Cai, W.; Li, L.; Bai, Q.; Chen, X.; Xu, B.; Wang, J. MiR-30b Attenuates Neuropathic Pain by Regulating Voltage-Gated Sodium Channel Nav1.3 in Rats. Front. Mol. Neurosci. 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, J.; Wang, J.; Liu, Y.; Zhang, Y.; Zhang, M.; Zhang, J.; Ren, X.; Su, S.; Li, Y. MiR-30b-5p attenuates oxaliplatin-induced peripheral neuropathic pain through the voltage-gated sodium channel Nav1.6 in rats. Neuropharmacology 2019, 153, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhao, Q.; Shao, J.; Zhang, J.; Li, L.; Ren, X.; Su, S.; Bai, Q.; Li, M.; Chen, X. MicroRNA-182 Alleviates Neuropathic Pain by Regulating Nav1.7 Following Spared Nerve Injury in Rats. Sci. Rep. 2018, 8, 16750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Dong, H.; Wu, Z.; Jiang, X.; Zou, W. An Overview of the Mechanisms Involved in Neuralgia. J. Inflamm. Res. 2023, 16, 4087–4101. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, Z.; Lin, X.; Wu, X.; Chen, X.; Bai, X.; Zhuang, Y.; Yang, Y.; Zhang, J. Overexpression of miR-146a Might Regulate Polarization Transitions of BV-2 Cells Induced by High Glucose and Glucose Fluctuations. Front. Endocrinol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Zhenzhen, Z.; Fenghao, L.; Meina, M.; Rui, L.; Wenbo, S.; Qi, W. Targeting HMGB1-TLR4 signaling by miR-216a-5p elevation alleviates the inflammatory behavioral hypersensitivity. Neurosci. Lett. 2021, 759, 136043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, F.; Hu, Y.; Qiao, W.; Guan, Y.; Zhang, Z.J.; Liu, S.; Liu, Y. Non-Coding RNAs Regulate Spinal Cord Injury-Related Neuropathic Pain via Neuroinflammation. J. Inflamm. Res. 2023, 16, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Ardizzone, A.; Giosa, D.; Scuderi, S.A.; Calcaterra, E.; Esposito, E.; Capra, A. The Therapeutic Potential of MicroRNA-21 in the Treatment of Spinal Cord Injury. Curr. Issues Mol. Biol. 2025, 47, 70. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xu, L.J.; Han, G.D.; Sun, H.L.; Zhu, G.T.; Jiang, H.T.; Yu, G.; Tang, X. MicroRNA-125b promotes the regeneration and repair of spinal cord injury through regulation of JAK/STAT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 582–589. [Google Scholar] [PubMed]
- Qi, J.; Qiao, Y.; Wang, P.; Li, S.; Zhao, W.; Gao, C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett. 2012, 586, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, T.D.; Wu, H.; Lang, Y.; Li, D.Z.; Ni, S.F.; Lu, H.; Hu, J. Synchrotron radiation micro-CT as a novel tool to evaluate the effect of agomir-210 in a rat spinal cord injury model. Brain Res. 2017, 1655, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Shu, Q.; Yuan, S.; Xing, Z.; Song, J. LncRNA SNHG6 functions as a ceRNA to regulate neuronal cell apoptosis by modulating miR-181c-5p/BIM signalling in ischaemic stroke. J. Cell. Mol. Med. 2019, 23, 6120–6130. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Wang, R.Y.; Saijilafu; Jiao, Z.X.; Zhang, B.Y.; Zhou, F.Q. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013, 27, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Labatut, A.E.; Mattheolabakis, G. Non-viral based miR delivery and recent developments. Eur. J. Pharm. Biopharm. 2018, 128, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Doudna, J.A. Molecular Mechanisms of RNA Interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.C.; Monia, B.P. Therapeutic potential for microRNAs. Adv. Drug Deliv. Rev. 2007, 59, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Brown, D.; Winkler, M. The Promise of MicroRNA Replacement Therapy. Cancer Res. 2010, 70, 7027–7030. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Sáez, E.; Úriz, M.; Sarvide, S.; Urribarri, A.D.; Splinter, P.; Tietz Bogert, P.; Bujanda, L.; Prieto, J.; Medina, J.; et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 2012, 56, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, H.Y.; Hao, N.B.; Tang, B.; Guo, H.; Yong, X.; Dong, H.; Yang, S. microRNA inhibitors: Natural and artificial sequestration of microRNA. Cancer Lett. 2017, 407, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Slack, F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expert. Opin. Drug Discov. 2020, 15, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, J.; Hendriks, A.J.; Nolte, T.M. Clearance of nanoparticles from blood: Effects of hydrodynamic size and surface coatings. Environ. Sci. Nano 2024, 11, 406–417. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Ali, P.; Maedeh, T. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Vandermeulen, G.; Préat, V. Chitosan-based siRNA delivery systems. J. Control. Release 2013, 172, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiang, K.; Li, Z. MiR-145 ameliorates neuropathic pain via inhibiting inflammatory responses and mTOR signaling pathway by targeting Akt3 in a rat model. Neurosci. Res. 2018, 134, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lu, J.; Wang, Y.; Cheng, X.; He, X.; Zheng, W.; Chen, H.; Wang, Y. XIST accelerates neuropathic pain progression through regulation of miR-150 and ZEB1 in CCI rat models. J. Cell. Physiol. 2018, 233, 6098–6106. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.R.; Chen, J.; Yi, H.; Peng, L.Y.; Hu, X.L.; Guo, Q.L. MicroRNA-7a ameliorates neuropathic pain in a rat model of spinal nerve ligation via the neurofilament light polypeptide-dependent signal transducer and activator of transcription signaling pathway. Mol. Pain 2019, 15, 174480691984246. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Zhou, Z.; Hao, S.; Mata, M.; Fink, D.J. Reduction of Voltage Gated Sodium Channel Protein in DRG by Vector Mediated miRNA Reduces Pain in Rats with Painful Diabetic Neuropathy. Mol. Pain 2012, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, S.; Wang, P.; Guo, X.; Qin, Z.; Hou, H.; Tao, T. A novel microglia-targeting strategy based on nanoparticle-mediated delivery of miR-26a-5p for long-lasting analgesia in chronic pain. J. Nanobiotechnol. 2024, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, G.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An. Acad. Bras. Cienc. 2022, 94, e20210564. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, P.; Jia, Y.; Zhang, K.; Liu, M.; Jiang, J. Dorsal root ganglion-derived exosomes deteriorate neuropathic pain by activating microglia via the microRNA-16-5p/HECTD1/HSP90 axis. Biol. Res. 2024, 57, 28. [Google Scholar] [CrossRef] [PubMed]
- Yang, N. An overview of viral and nonviral delivery systems for microRNA. Int. J. Pharm. Investig. 2015, 5, 179. [Google Scholar] [CrossRef] [PubMed]
- Escors, D.; Breckpot, K. Lentiviral Vectors in Gene Therapy: Their Current Status and Future Potential. Arch. Immunol. Ther. Exp. 2010, 58, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.; Yoo, M.; Park, C.S.; Chen, J.; Kügler, S.; Gibbs, K.M.; Schachner, M. Lentiviral Delivery of miR-133b Improves Functional Recovery After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 4659–4671. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.W.; Teng, Y.; Wong, H.K.; Ng, T.K.; Huang, L.; Lei, P.; Choy, K.; Liu, Y.; Zhang, M.; Lam, D.; et al. MicroRNA-145 Regulates Human Corneal Epithelial Differentiation. PLoS ONE 2011, 6, e21249. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, L.; Zhang, Y.; Cao, Y.; Liu, X. SNHG16 aggravates chronic constriction injury-induced neuropathic pain in rats via binding with miR-124-3p and miR-141-3p to upregulate JAG1. Brain Res. Bull. 2020, 165, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Teramato, S.; Ishii, T.; Matsuse, T. Crisis of adenoviruses in human gene therapy. Lancet 2000, 355, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.R.; Chamberlain, J.S. Recombinant Adeno-associated Virus Transduction and Integration. Mol. Ther. 2008, 16, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, W.I.; Lee, Y.S.; Kim, D.H.; Chang, J.W.; Kim, S.W.; Lee, H. Effective relief of neuropathic pain by adeno-associated virus-mediated expression of a small hairpin RNA against GTP cyclohydrolase 1. Mol. Pain 2009, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wei, J.; Yu, C.; Han, X.; Qin, X.; Zhang, C.; Liao, W.; Li, L.; Huang, W. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. J. Mater. Chem. B 2019, 7, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric vehicles for nucleic acid delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Hartman, J.E.M.; Mesquita, B.S.; Haegebaert, R.; Remaut, K.; Neumann, M.; Hak, J.; Censi, R.; Di Martino, P.; Hennik, W.; et al. Effect of Polyplex Size on Penetration into Tumor Spheroids. Mol. Pharm. 2023, 20, 5515–5531. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Mitchell, M.J.; Nie, G. Nanomaterials for Therapeutic RNA Delivery. Matter 2020, 3, 1948–1975. [Google Scholar] [CrossRef]
- Lächelt, U.; Wagner, E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Wu, W.; Bi, Y.; Cai, Z.; Chen, Q.; Li, Y.; Hou, S. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: Systematic study of particle size and drug entrapment efficiency. Int. J. Pharm. 2008, 350, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Phạm, T.L.; Yin, Y.; Kwon, H.H.; Shin, N.; Kim, S.I.; Park, H.; Shin, J.; Shin, H.; Hwang, J.; Song, H. miRNA 146a-5p-loaded poly(D,L-lactic-co-glycolic acid) nanoparticles impair pain behaviors by inhibiting multiple inflammatory pathways in microglia. Nanomedicine 2020, 15, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Cambell, H.; Kolpek, D.; Park, J. Optimization and characterization of miRNA-129-5p-encapsulated poly (lactic-co-glycolic acid) nanoparticles to reprogram activated microglia. Nanoscale Adv. 2023, 5, 3439–3452. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Pattani, A.; Patravale, V.B.; Panicker, L.; Potdar, P.D. Immunological Effects and Membrane Interactions of Chitosan Nanoparticles. Mol. Pharm. 2009, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine (NLM). Clinicaltrails.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 29 May 2023).
- Genedy, H.H.; Delair, T.; Montembault, A. Chitosan Based MicroRNA Nanocarriers. Pharmaceuticals 2022, 15, 1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.Ø.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.Y.; Lesage, F.; Bail, M.; Darras, V.; Chevrier, A.; Buschmann, M. siRNA Delivery with Chitosan: Influence of Chitosan Molecular Weight, Degree of Deacetylation, and Amine to Phosphate Ratio on in Vitro Silencing Efficiency, Hemocompatibility, Biodistribution, and in Vivo Efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef] [PubMed]
- Shamaeizadeh, N.; Varshosaz, J.; Mirian, M.; Aliomrani, M. Glutathione targeted tragacanthic acid-chitosan as a non-viral vector for brain delivery of miRNA-219a-5P: An in vitro/in vivo study. Int. J. Biol. Macromol. 2022, 200, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.M.; Kolar, M.K.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Kjems, J.; Novikov, L. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomedicine 2016, 12, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Son, S.; Jang, J.; Youn, H.; Lee, S.; Lee, D.; Lee, Y.; Jeong, J.; Kim, W.; Lee, D. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials 2011, 32, 4968–4975. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.Y.; Cherng, J.Y.; Hsu, H.S.; Wang, M.L.; Tsai, C.M.; Lu, K.H.; Chien, Y.; Hung, S.; Chen, Y.; Wong, C.; et al. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial–mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J. Control. Release 2012, 159, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Chang, Y.L.; Li, H.Y.; Larsson, M.; Wu, W.W.; Chien, C.S.; Wang, C.; Chu, P.; Chen, K.; Lo, W. Synergistic effects of carboxymethyl-hexanoyl chitosan, cationic polyurethane-short branch PEI in miR122 gene delivery: Accelerated differentiation of iPSCs into mature hepatocyte-like cells and improved stem cell therapy in a hepatic failure model. Acta Biomater. 2015, 13, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. miRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, G.; Sun, B.; Tian, T.; Hu, F.; Xiao, Z. A novel type of self-assembled nanoparticles as targeted gene carriers: An application for plasmid DNA and antimicroRNA oligonucleotide delivery. Int. J. Nanomedicine. 2016, 11, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, Y.; Yu, M.; Ma, P.X.; Mao, C.; Lei, B. Photoluminescent and biodegradable polycitrate-polyethylene glycol-polyethyleneimine polymers as highly biocompatible and efficient vectors for bioimaging-guided siRNA and miRNA delivery. Acta Biomater. 2017, 54, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Hwang, D.W.; Singha, K.; Jeong, J.H.; Park, T.G.; Lee, D.S.; Kim, W. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release 2011, 155, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, H.; Zhao, M.; Fu, Y.; Sun, X. Intracellular redox potential-responsive micelles based on polyethylenimine-cystamine-poly(ε-caprolactone) block copolymer for enhanced miR-34a delivery. Polym. Chem. 2015, 6, 1952–1960. [Google Scholar] [CrossRef]
- Gebhart, C.L.; Kabanov, A.V. Evaluation of polyplexes as gene transfer agents. J. Control. Release 2001, 73, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Wang, Y.S.; Hsu, P.Y.; Chen, C.Y.; Liao, Y.C.; Juo, S.H.H. miR-195 Has a Potential to Treat Ischemic and Hemorrhagic Stroke through Neurovascular Protection and Neurogenesis. Mol. Ther. Methods Clin. Dev. 2019, 13, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xing, Z.; Yang, J.; Wang, Y.; Chen, J.; Zhang, Y.; Shi, W.; Li, Q. Chondroitin sulfate-functionalized polyamidoamine-mediated miR-34a delivery for inhibiting the proliferation and migration of pancreatic cancer. RSC Adv. 2016, 6, 70870–70876. [Google Scholar] [CrossRef]
- LIU, X.; LI, G.; SU, Z.; JIANG, Z.; CHEN, L.; WANG, J.; YU, S.; LIU, Z. Poly(amido amine) is an ideal carrier of miR-7 for enhancing gene silencing effects on the EGFR pathway in U251 glioma cells. Oncol. Rep. 2013, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.H.; Luo, B.; Dang, M.N.; Irvin-Choy, N.; Valcourt, D.M.; Day, E.S. Polymer nanocarriers for MicroRNA delivery. J. Appl. Polym. Sci. 2020, 137, 48651. [Google Scholar] [CrossRef] [PubMed]
- Bolu, B.; Sanyal, R.; Sanyal, A. Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †. Molecules 2018, 23, 1570. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Santos, S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Wang, S.; Qian, X.; Fan, J.; Wang, Z.; Song, P.; Zhang, X.; Lu, W.; Ju, D. Interplay of Oxidative Stress and Autophagy in PAMAM Dendrimers-Induced Neuronal Cell Death. Theranostics 2015, 5, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Trent Magruder, J.; Lin, Y.A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.; Blue, M.; Kannan, S.; Johnston, M.; et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 249, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Berényi, S.; Mihály, J.; Wacha, A.; Tőke, O.; Bóta, A. A mechanistic view of lipid membrane disrupting effect of PAMAM dendrimers. Colloids Surf. B Biointerfaces 2014, 118, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wu, X.; Tai, Z.; Zhu, Q.; Fan, W.; Ding, B.; Zhang, L.; Yao, C.; Wang, X.; Zhang, W.; et al. Study on the prostate cancer-targeting mechanism of aptamer-modified nanoparticles and their potential anticancer effect in vivo. Int. J. Nanomed. 2014, 9, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Huang, C.Y.; Lin, C.W.; Liu, H.L.; Huang, C.W.; Liao, S.S.; Chen, P.; Lu, Y.; Wei, K.; Ma, C. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials 2014, 35, 6534–6542. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kang, C.S.; Yuan, X.B.; Zhou, X.; Xu, P.; Han, L.; Wang, G.; Jia, Z.; Zhong, Y.; Yu, S. Co-delivery of as-miR-21 and 5-FU by Poly(amidoamine) Dendrimer Attenuates Human Glioma Cell Growth in Vitro. J. Biomater. Sci. Polym. Ed. 2010, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J. Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Ambrosone, A.; Hernandez, Y.; Tian, F.; McCully, M.; Berry, C.C.; Baptista, P.; Tortiglione, C.; de la Fuente, J. 15 years on siRNA delivery: Beyond the State-of-the-Art on inorganic nanoparticles for RNAi therapeutics. Nano Today 2015, 10, 421–450. [Google Scholar] [CrossRef]
- Titze de Almeida, S.; Horst, C.; Soto-Sánchez, C.; Fernandez, E.; Titze de Almeida, R. Delivery of miRNA-Targeted Oligonucleotides in the Rat Striatum by Magnetofection with Neuromag®. Molecules 2018, 23, 1825. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Piñeiro, I.; Badiola, I.; Sanchez, A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol. Adv. 2017, 35, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.; Gulei, D.; Zimta, A.A.; Onaciu, A.; Magdo, L.; Tigu, A.B.; Ionescu, C.; Irimie, A.; Buiga, R.; Berindan-Neagoe, I. Nanoscale delivery systems for microRNAs in cancer therapy. Cell. Mol. Life Sci. 2020, 77, 1059–1086. [Google Scholar] [CrossRef] [PubMed]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Imai, Y.; Koumoto, K.; Kaiden, T.; Kono, K.; Aoshima, S. Magnetoresponsive On-Demand Release of Hybrid Liposomes Formed from Fe 3 O 4 Nanoparticles and Thermosensitive Block Copolymers. Small 2011, 7, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L.; et al. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, Y.; Wang, L.; Liu, Y.; Wang, X.; Li, Y.; Chi, G. miR-124: A Promising Therapeutic Target for Central Nervous System Injuries and Diseases. Cell. Mol. Neurobiol. 2022, 42, 2031–2053. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Park, I.K.; Cho, C.S. Nanoparticle-mediated delivery of therapeutic genes: Focus on miRNA therapeutics. Expert. Opin. Drug Deliv. 2013, 10, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.M. Getting miRNA Therapeutics into the Target Cells for Neurodegenerative Diseases: A Mini-Review. Front. Mol. Neurosci. 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Qian, L.; Uttamchandani, M.; Li, L.; Yao, S.Q. Single-Vehicular Delivery of Antagomir and Small Molecules to Inhibit miR-122 Function in Hepatocellular Carcinoma Cells by using “Smart” Mesoporous Silica Nanoparticles. Angew. Chem. Int. Ed. Engl. 2015, 54, 10574–10578. [Google Scholar] [CrossRef] [PubMed]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.W.; Nagaraj, H.; Rotello, V. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Dai, H.; Walsh, L.J.; Xu, C. Mesoporous Core–Cone Silica Nanoparticles Can Deliver miRNA-26a to Macrophages to Exert Immunomodulatory Effects on Osteogenesis In Vitro. Nanomaterials 2023, 13, 1755. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.; Stebbing, D.; Crosley, E.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Zappavigna, S.; Scotti, L.; Abate, M.; Porru, M.; Leonetti, C.; Caraglia, M.; De Rosa, G. Hybrid lipid self-assembling nanoparticles for brain delivery of microRNA. Int. J. Pharm. 2020, 588, 119693. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hatakeyama, H.; Sakurai, Y.; Hyodo, M.; Akita, H.; Harashima, H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 2012, 163, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, R.; Samanta, S.; Chaudhuri, A. Cationic amphiphiles: Promising carriers of genetic materials in gene therapy. Chem. Soc. Rev. 2009, 38, 3326. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The Headgroup Evolution of Cationic Lipids for Gene Delivery. Bioconjug. Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, L.; Racie, T.; Frank-Kamenetsky, M.; Yip, K.; et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Küçüktürkmen, B.; Bozkır, A. Development and characterization of cationic solid lipid nanoparticles for co-delivery of pemetrexed and miR-21 antisense oligonucleotide to glioblastoma cells. Drug Dev. Ind. Pharm. 2018, 44, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control. Release 2014, 194, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, L.; Gong, T.; Zhang, Z.; Sun, X. Systemic Delivery of microRNA-34a for Cancer Stem Cell Therapy. Angew. Chem. Int. Ed. 2013, 52, 3901–3905. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.L.; Persano, S.; Persano, F. Lipid-Based Vectors for Therapeutic mRNA-Based Anti-Cancer Vaccines. Curr. Pharm. Des. 2019, 25, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X.; Yang, Z.; Gallego-Perez, D.; Ma, J.; Zhao, X.; Xie, J.; Nakano, I.; Lee, L. Targeted Delivery of Tumor Suppressor microRNA-1 by Transferrin-Conjugated Lipopolyplex Nanoparticles to Patient-Derived Glioblastoma Stem Cells. Curr. Pharm. Biotechnol. 2014, 15, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.; Chan, K.; et al. Targeted Delivery of microRNA-29b by Transferrin-Conjugated Anionic Lipopolyplex Nanoparticles: A Novel Therapeutic Strategy in Acute Myeloid Leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, B.; Lepeltier, É.; Rouleau, D.; Le Visage, C.; Benoit, J.P.; Passirani, C.; Guicheux, J.; Fusellier, M.; Clouet, J. Lipid nanocapsules for intracellular delivery of microRNA: A first step towards intervertebral disc degeneration therapy. Int. J. Pharm. 2022, 624, 121941. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Yu, B.; Mao, Y.; Nana-Sinkam, S.P.; Lee, L.J. MicroRNA Delivery by Cationic Lipoplexes for Lung Cancer Therapy. Mol. Pharm. 2011, 8, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, D.; Campbell, N.R.; Karikari, C.; Chivukula, R.; Kent, O.A.; Mendell, J.T.; Maitra, A. Restitution of Tumor Suppressor MicroRNAs Using a Systemic Nanovector Inhibits Pancreatic Cancer Growth in Mice. Mol. Cancer Ther. 2011, 10, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Zhang, M.; Datta, J.; Xie, X.; Su, T.; Li, H.; Teknos, T.; Pan, Q. Lipid-based Nanoparticle Delivery of Pre-miR-107 Inhibits the Tumorigenicity of Head and Neck Squamous Cell Carcinoma. Mol. Ther. 2012, 20, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.; Jacob, S.; Ghoshal, K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine 2013, 9, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Granot, Y.; Peer, D. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—An innate immune system standpoint. Semin Immunol. 2017, 34, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [PubMed]
- Koltover, I.; Salditt, T.; Rädler, J.O.; Safinya, C.R. An Inverted Hexagonal Phase of Cationic Liposome-DNA Complexes Related to DNA Release and Delivery. Science 1998, 281, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Yung, B.C.; Li, J.; Zhang, M.; Cheng, X.; Li, H.; Yung, E.M.; Kang, C.; Cosby, L.; Liu, Y.; Teng, L.; et al. Lipid Nanoparticles Composed of Quaternary Amine–Tertiary Amine Cationic Lipid Combination (QTsome) for Therapeutic Delivery of AntimiR-21 for Lung Cancer. Mol. Pharm. 2016, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Huang, L. Time-dependent maturation of cationic liposome–DNA complex for serum resistance. Gene Ther. 1998, 5, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Igami, K.; Uchiumi, T.; Ueda, S.; Kamioka, K.; Setoyama, D.; Gotoh, K.; Akimoto, M.; Matsumoto, S.; Kang, D. Characterization and function of medium and large extracellular vesicles from plasma and urine by surface antigens and Annexin, V. PeerJ Anal. Chem. 2020, 2, e4. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Shan, Q.; Gu, P.; Wang, X.M.; Tai, L.W.; Sun, M.; Luo, X.; Sun, L.; Cheung, C. miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J. Neuroinflammation 2018, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tang, X.H.; Pan, W.; Xie, Z.M.; Zhang, G.F.; Ji, M.H.; Yang, J.; Zhou, M.; Zhou, Z. Spared Nerve Injury Increases the Expression of Microglia M1 Markers in the Prefrontal Cortex of Rats and Provokes Depression-Like Behaviors. Front. Neurosci. 2017, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Narain, R.; Zeng, H. Hydrogels. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. [Google Scholar]
- Pérez-Luna, V.; González-Reynoso, O. Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Lin, Z.; Lei, Q.; Liu, Y.; Li, X.; Liu, M.; Wu, G.; Luo, S.; Wang, H.; et al. Injectable halloysite-g-chitosan hydrogels as drug carriers to inhibit breast cancer recurrence. Compos. B Eng. 2021, 221, 109031. [Google Scholar] [CrossRef]
- Bodugoz-Senturk, H.; Macias, C.E.; Kung, J.H.; Muratoglu, O.K. Poly(vinyl alcohol)–acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials 2009, 30, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro. Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, K.; Kitamura, N.; Fujiki, H.; Kurokawa, T.; Iwamoto, M.; Ueno, M.; Kanaya, F.; Osada, Y.; Gong, J.; Yasuda, K. Artificial cartilage made from a novel double-network hydrogel: In vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J. Biomed. Mater. Res. A 2010, 93A, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Xu, X.; Duncan, R.L.; Jia, X. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials 2011, 32, 2466–2478. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Rostamizadeh, K.; Salari, D.; Hamidi, M. Preparation and characterization of tri-block poly(lactide)–poly(ethylene glycol)–poly(lactide) nanogels for controlled release of naltrexone. Int. J. Pharm. 2011, 416, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Trimaille, T.; Pertici, V.; Gigmes, D. Recent advances in synthetic polymer based hydrogels for spinal cord repair. Comptes Rendus Chim. 2016, 19, 157–166. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2016, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Liu, S.; Liu, W.; Zhang, H.; Zhou, T.; Wu, D.; Yang, P.; Shen, L.; Chen, J.; et al. The promotion of bone regeneration through positive regulation of angiogenic–osteogenic coupling using microRNA-26a. Biomaterials 2013, 34, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Liu, Y.; Chung, J.J.; Wang, T.; Gaffey, A.C.; Lu, M.; Cavanaugh, C.; Zhou, S.; Kande, R.; Atluri, P.; et al. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Eng. 2017, 1, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, H.; Zheng, D.; Zhang, H.; Deng, L.; Cui, W.; Zhang, Y.; Santos, H.; Shen, H. Gene-Hydrogel Microenvironment Regulates Extracellular Matrix Metabolism Balance in Nucleus Pulposus. Adv. Sci. 2020, 7, 1902099. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, e1902232. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Jin, R.; Chen, L.; Dang, M.; Cao, H.; Dong, Y.; Cai, B.; Bai, G.; Gooding, J.; et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, eabd6740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, M.; Wu, Y.; Hu, C.; Zhang, B.; Guo, C.; Song, H.; Kong, Q.; Wang, Y. Sustained gene delivery from inflammation-responsive anti-inflammatory hydrogels promotes extracellular matrix metabolism balance in degenerative nucleus pulposus. Compos. B Eng. 2022, 236, 109806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).