Synthesis and Evaluation of [18F]AlF-NOTA-iPD-L1 as a Potential Theranostic Pair for [177Lu]Lu-DOTA-iPD-L1
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Modeling
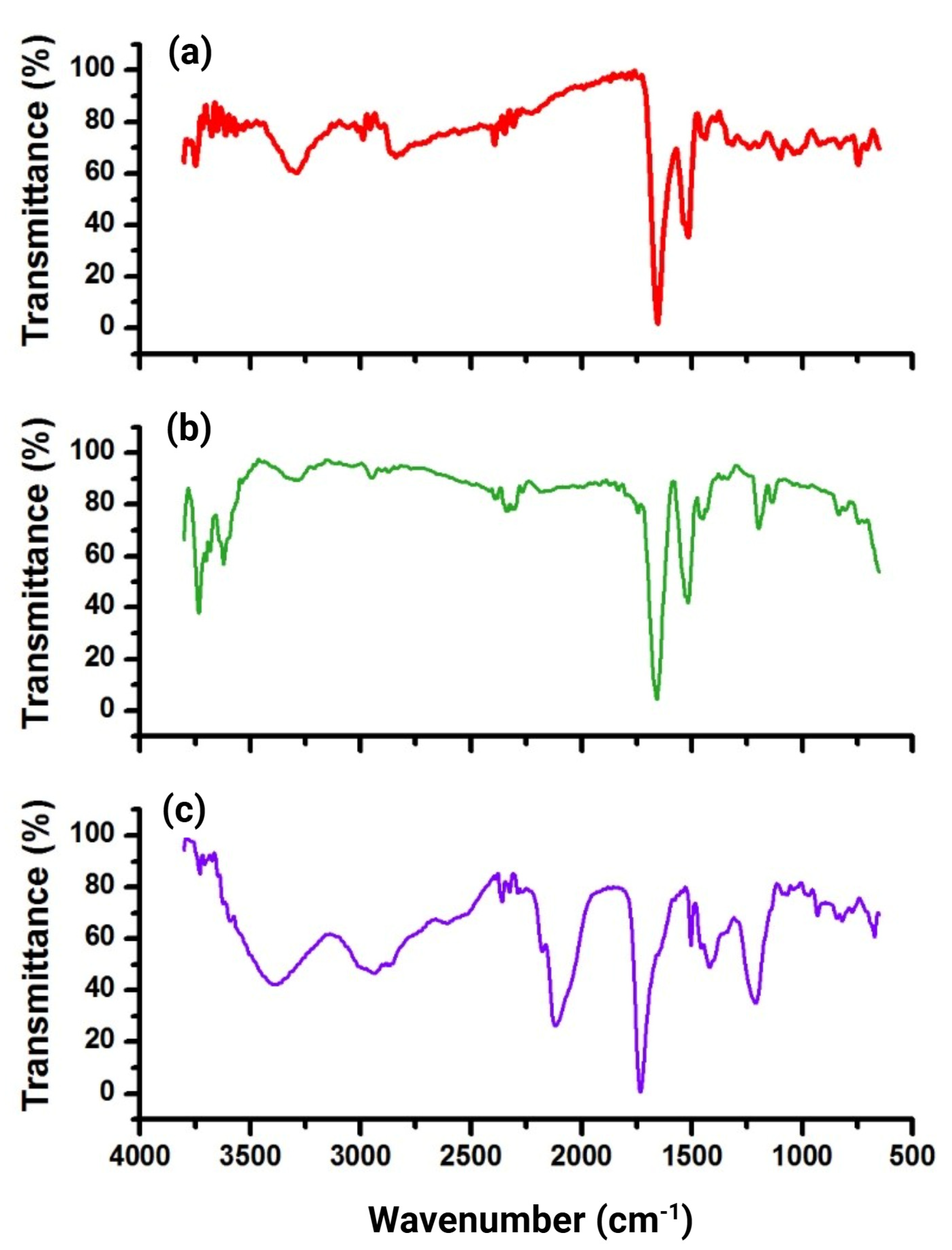
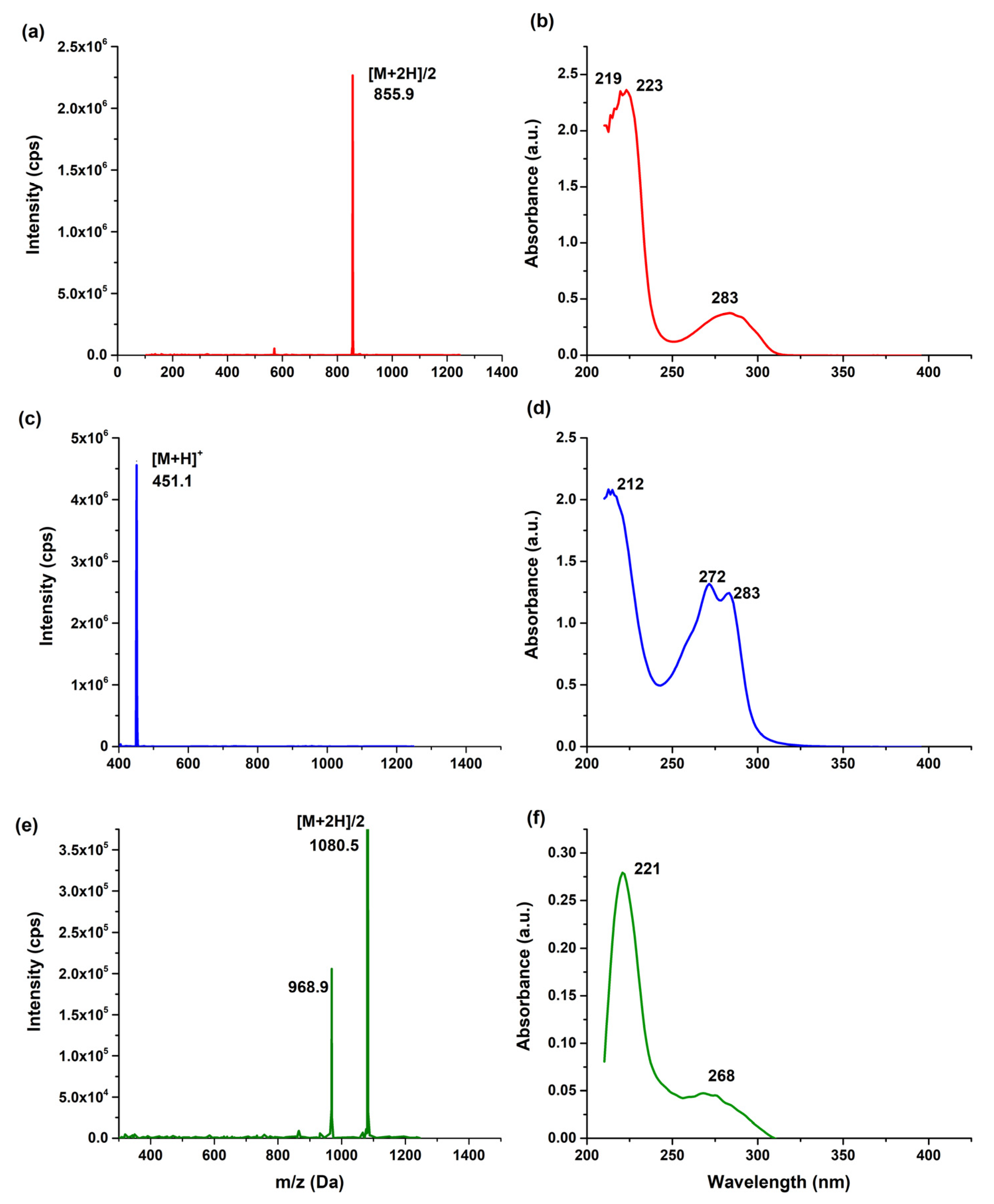
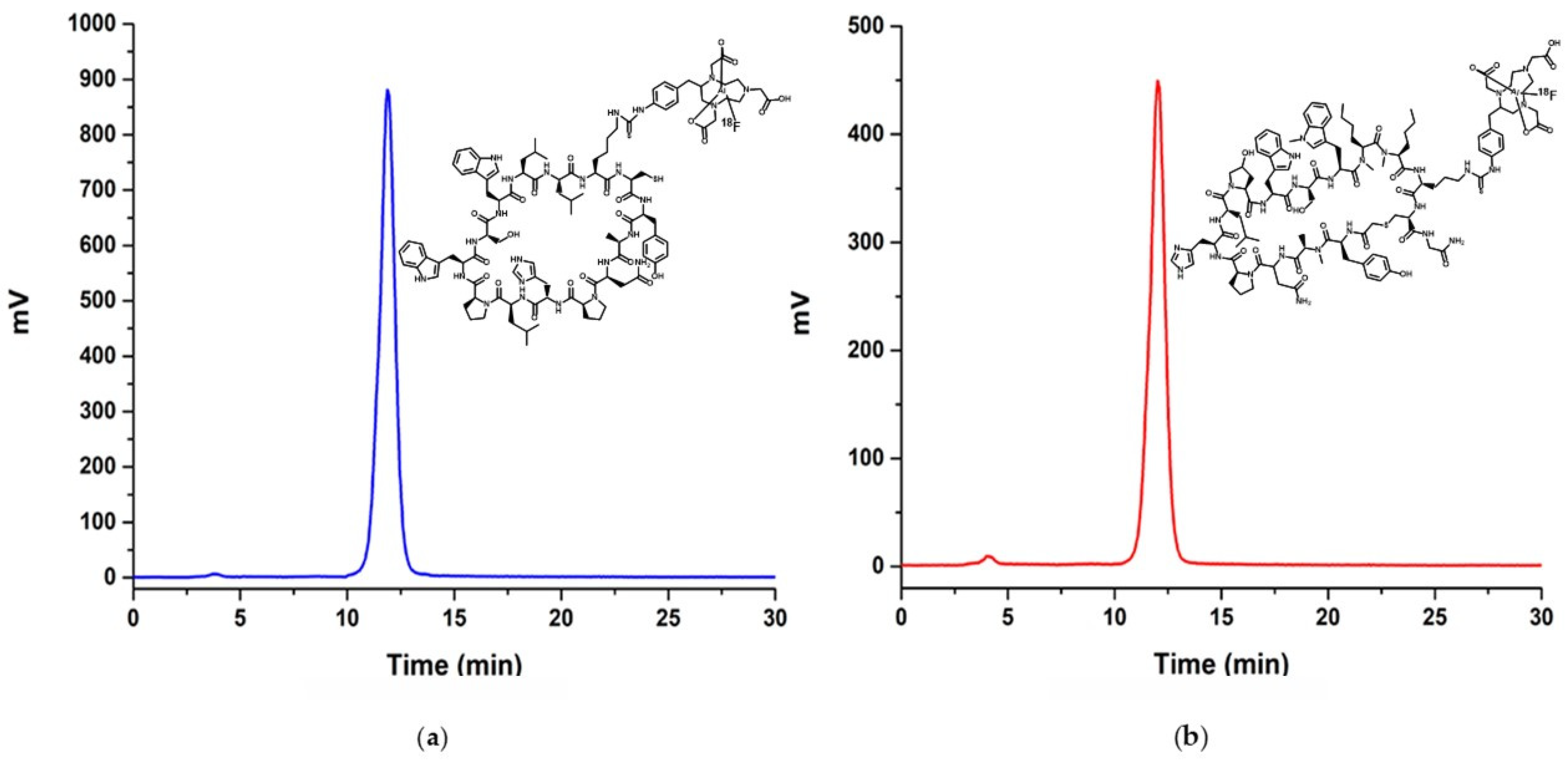
2.2. Preparation and Characterization of NOTA-iPD-L1
2.3. Radioactive Labeling
2.4. Radiochemical Purity Assessment
2.5. Serum Stability
2.6. Partition Coefficient
2.7. Cell Culture
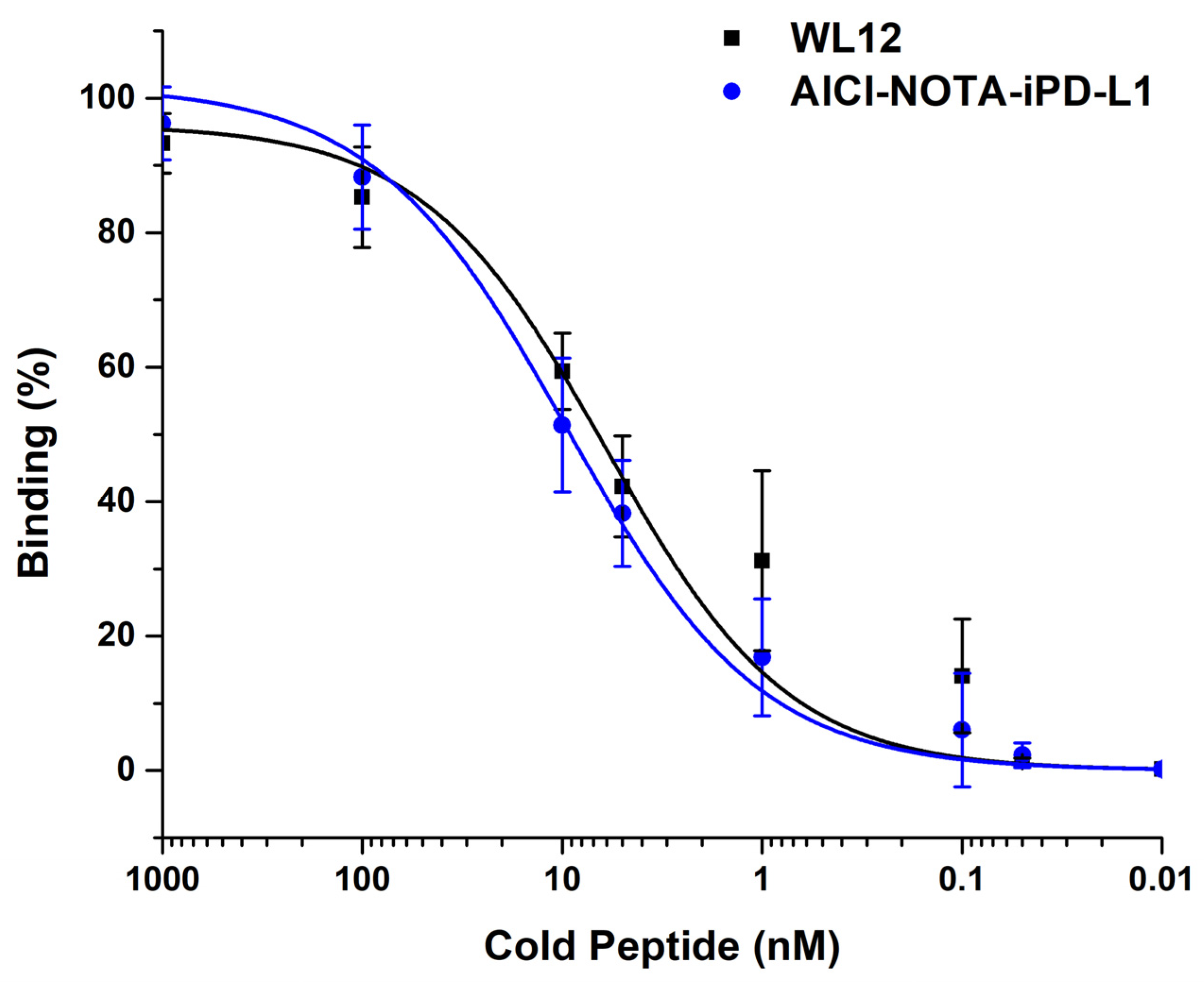
2.8. Affinity Assay
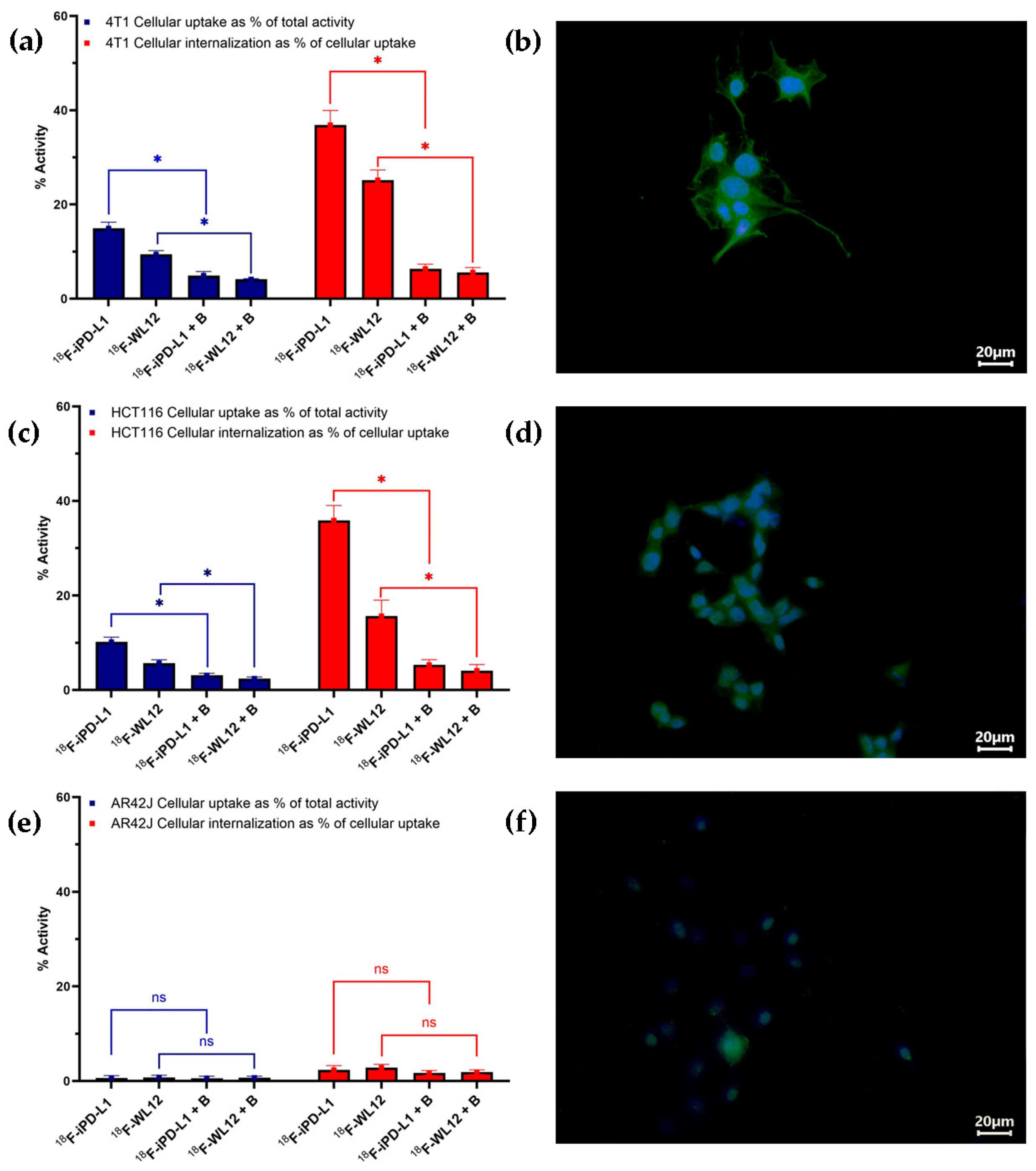
2.9. Cell Internalization and Uptake
2.10. Immunofluorescence Tests
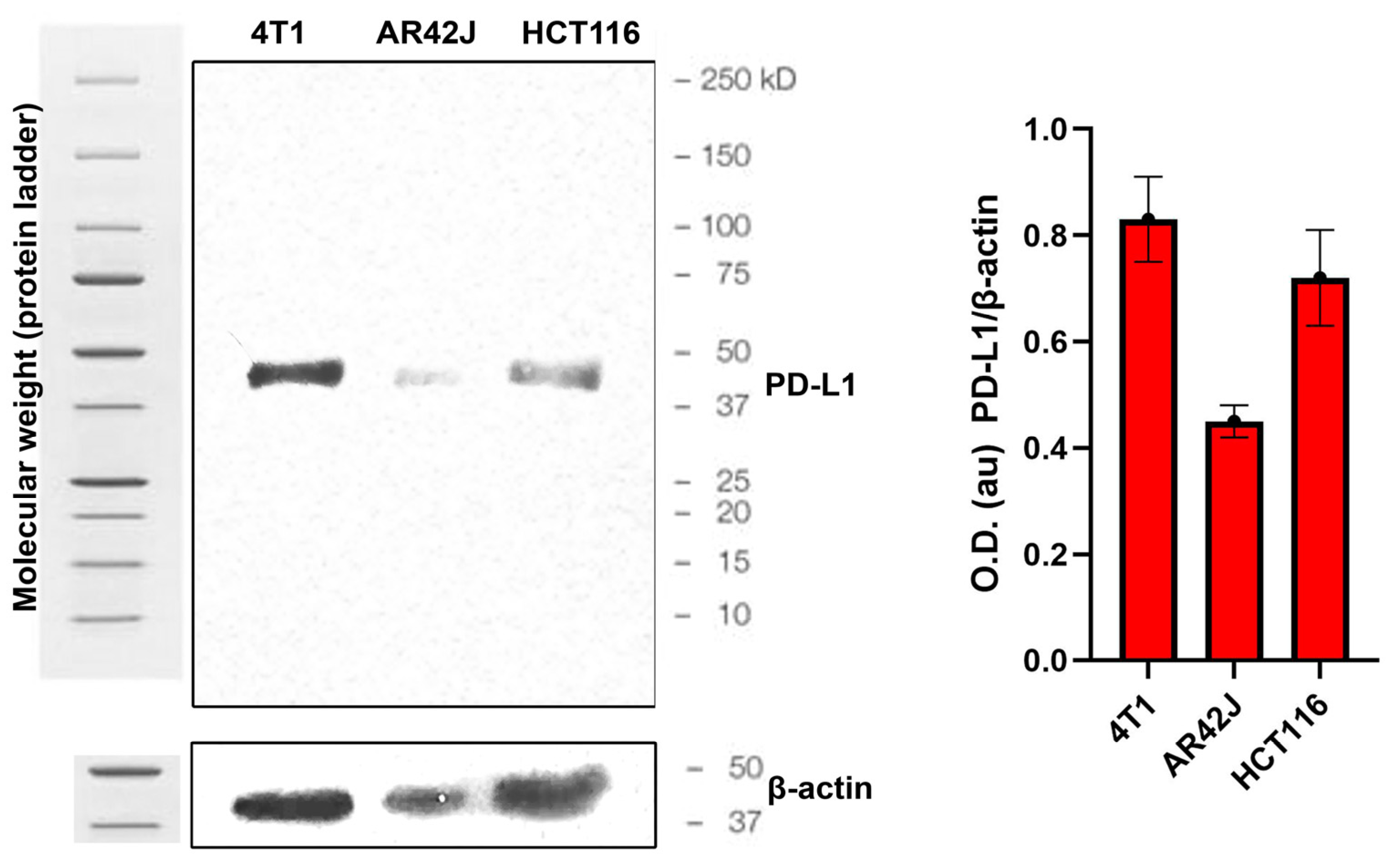
2.11. Western Blot Tests
2.12. Biodistribution
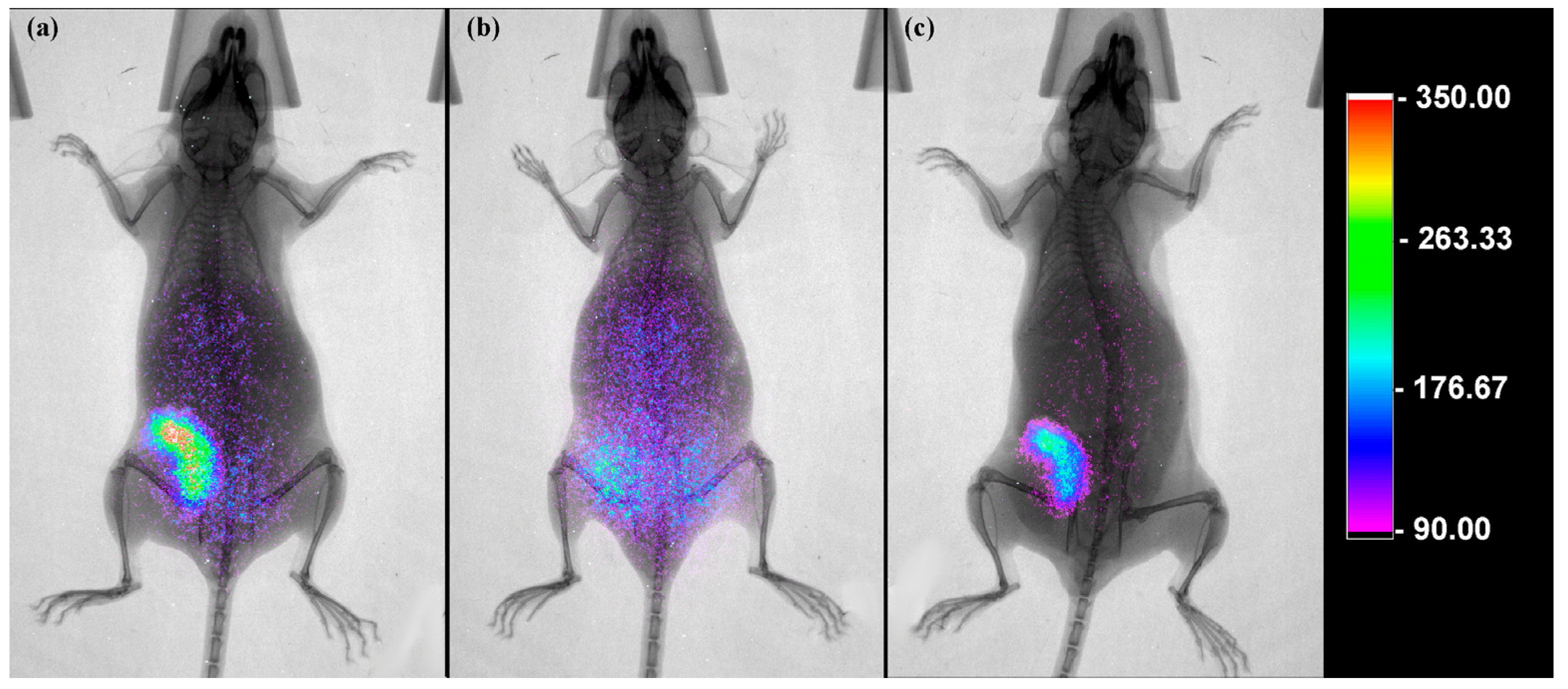
2.13. Molecular Imaging
3. Results
3.1. Molecular Modeling
3.2. Chemical Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
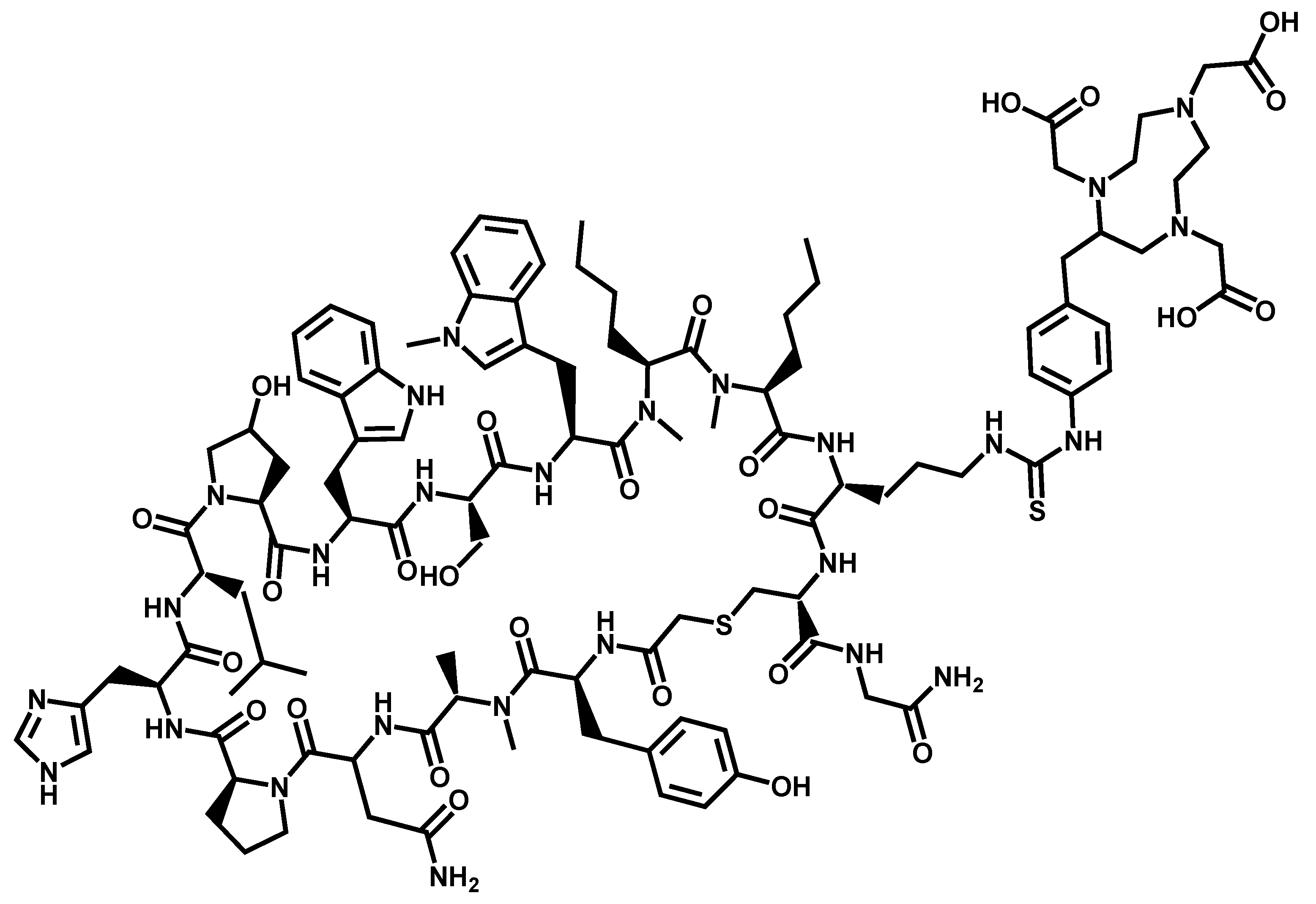
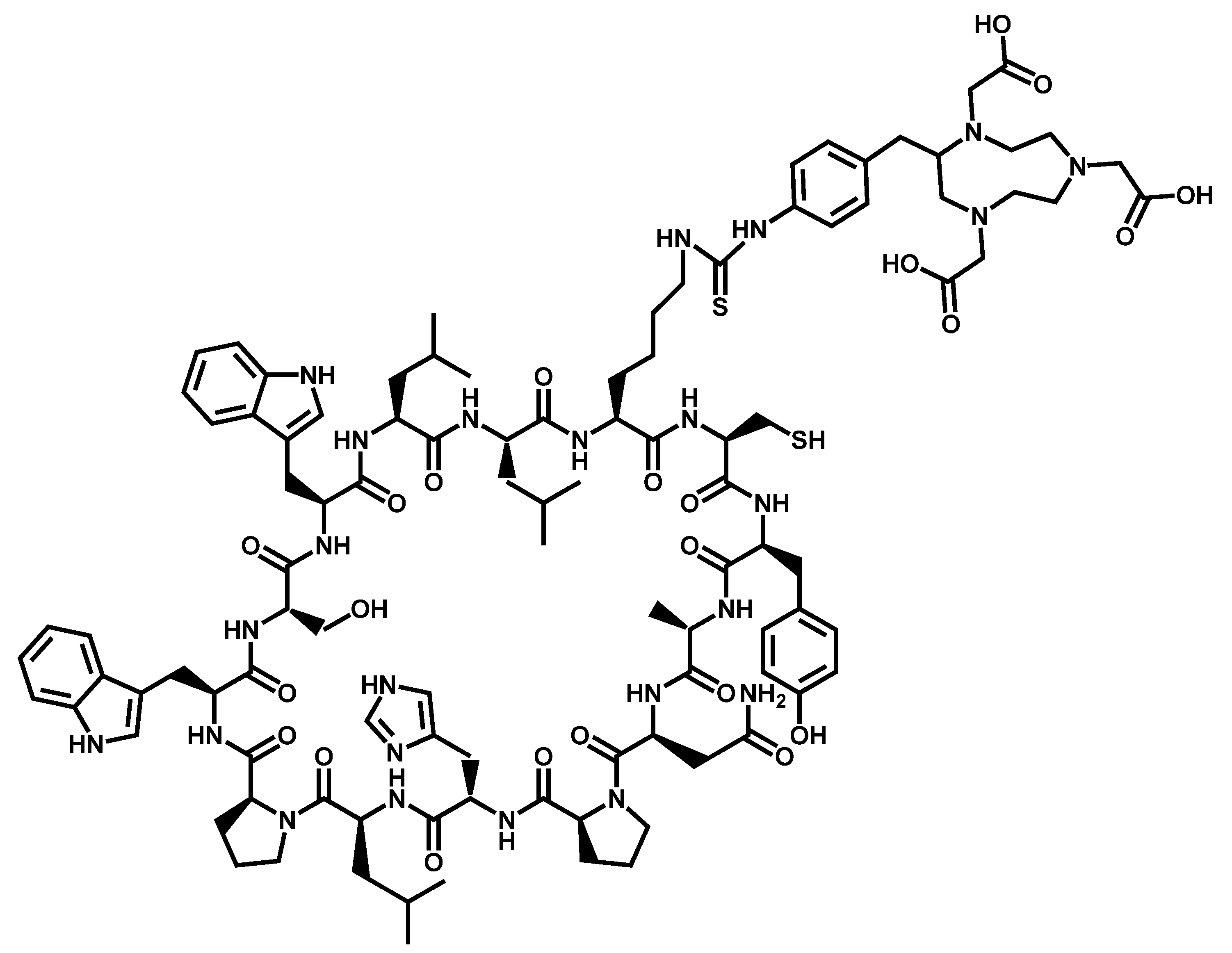

References
- Gutic, B.; Bozanovic, T.; Mandic, A.; Dugalic, S.; Todorovic, J.; Stanisavljevic, D.; Dugalic, M.G.; Sengul, D.; Detanac, D.A.; Sengul, I.; et al. Programmed cell death-1 and its ligands: Current knowledge and possibilities in immunotherapy. Clinics 2023, 78, 100177. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Liu, Y.; Liu, L.; Gao, S.; Gao, X.; Feng, Y.; Sun, Z.; Zhang, Y.; Wang, C. Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer 2023, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Noma, K.; Kato, T.; Ohara, T.; Tanabe, S.; Takeda, Y.; Matsumoto, H.; Nishimura, S.; Kunitomo, T.; Akai, M.; et al. PD-L1-expressing cancer-associated fibroblasts induce tumor immunosuppression and contribute to poor clinical outcome in esophageal cancer. Cancer Immunol. Immunother. 2023, 72, 3787–3802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, Y.; Wang, W.; Cai, Q.; Ge, F.; Chen, Z.; Zheng, J.; Zhang, Y.; Deng, H.; Chen, Y.; et al. Efficacy and safety of immune checkpoint inhibitors for individuals with advanced EGFR-mutated non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitors: A systematic review, meta-analysis, and network meta-analysis. Lancet Oncol. 2024, 25, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Maddiboyina, B.; Arora, S.; Riadi, Y.; Md, S.; Alhakamy, N.A.; Kesharwani, P. The emerging role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Drug Discov. Today 2021, 26, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Jagodinsky, J.C.; Harari, P.M.; Morris, Z.S. The Promise of Combining Radiation Therapy with Immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tong, F.; Zhang, R.; Chen, L.; Huang, Y.; Dong, X. The Research Progress of PD-1/PD-L1 Inhibitors Enhancing Radiotherapy Efficacy. Front. Oncol. 2021, 11, 799957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Zboralski, D.; Osterkamp, F.; Christensen, E.; Bredenbeck, A.; Schumann, A.; Hoehne, A.; Schneider, E.; Paschke, M.; Ungewiss, J.; Haase, C.; et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Clark, S.D.; Guo, B.; Zhang, T.; Jeansonne, D.; Jeyaseelan, S.J.; Francis, J.; Huang, W. Challenges in the combination of radiotherapy and immunotherapy for breast cancer. Expert Rev. Anticancer Ther. 2023, 23, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.; de Jong, M.C.; Baas, P. Synergizing systemic responses by combining immunotherapy with radiotherapy in metastatic non-small cell lung cancer: The potential of the abscopal effect. Lung Cancer 2020, 142, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Luna-Gutiérrez, M.; Azorín-Vega, E.; Oros-Pantoja, R.; Ocampo-García, B.; Cruz-Nova, P.; Jiménez-Mancilla, N.; Bravo-Villegas, G.; Santos-Cuevas, C.; Meléndez-Alafort, L.; Ferro-Flores, G. Lutetium-177 labeled iPD-L1 as a novel immunomodulator for cancer-targeted radiotherapy. EJNMMI Radiopharm. Chem. 2025, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, L.; Rajagopal, S.; Gajendran, C.; Sadhu, N.M.; Mohd, Z.; Gosu, R.; Friedmann-Morvinski, D.; Kandan, S.; Birudukota, S.; V, K. Novel, small molecule inhibitors of PD-1/PD-L1 pathway. J. Clin. Oncol. 2022, 40, 2597. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X.; Chen, H.; Zhang, H.; Ma, T.; Zhang, Y.; Zhang, C.; Zhang, G.; Liu, X.; Yan, D. BPI-371153, an orally bioavailable small molecule PD-L1 inhibitor. Cancer Res. 2022, 82, 5444. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Jin, X.; Huang, J.; Zhou, X. 458 Characterization of HZ-G206. A potent and oral small molecule PD-L1 inhibitor. J. ImmunoTher. Cancer 2022, 10 (Suppl. S2), A478. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lesniak, W.G.; Nimmagadda, S. Non-invasive Imaging of Immune Checkpoint Ligand PD-L1 in Tumors and Metastases for Guiding Immunotherapy. Mol. Imaging 2017, 16, 1536012117718459. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Kumar, D.; Lisok, A.; Chatterjee, S.; Wharram, B.; Venkateswara Rao, K.; Mease, R.; Dannals, R.F.; Pomper, M.G.; Nimmagadda, S. Peptide-Based 68Ga-PET Radiotracer for Imaging PD-L1 Expression in Cancer. Mol. Pharm. 2018, 15, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, X.; Zhou, M.; Rao, W.; Ji, X.; Wang, X.; Xiao, X.; Hu, S. Non-invasive Monitoring of Programmed Death-Ligand 2 Expression with Positron Emission Tomography using 68Ga-labeled Peptide Antagonist in Preclinical and Exploratory Human Studies. Research 2024, 7, 0523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pang, Y.; Ding, Y.; Chen, J.; Fang, J.; Yu, L.; Ruan, D.; Dai, Y.; Zhou, H.; Fu, H.; et al. 68Ga-NK224 PET/CT for Non-invasive Evaluation of PD-L1 Expression and Inter-Tumor Heterogeneity: A Translational Exploratory Study. Clin. Cancer Res. 2025, 31, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Liu, X.; Pan, X.; He, S.; Zhang, J.; Shen, H.; Tang, S.; Song, S. Radiosynthesis and Evaluation of a Novel 68Ga-Labeled Peptide for PD-L1-Targeted PET Imaging. Mol. Pharm. 2025, 22, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Peng, L.; Yang, T.; Chen, J.; Xie, C.; Xue, L.; Zhang, D.; Wu, R.; Zhang, X.; Zha, Z. 68Ga-Labeled Peptide for Non-invasive Quantifying Tumor Exposure of PD-L1 Therapeutics. ACS Omega 2025, 10, 12495–12504. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhang, B.; Li, J.; Shi, J.; Jia, T.; Wang, Y.; Chen, Z.; Sang, S.; Deng, S. A novel 68Ga-labeled cyclic peptide molecular probe based on the computer-aided design for non-invasive imaging of PD-L1 expression in tumors. Bioorg. Chem. 2023, 140, 106785. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Ranganathan, D.; Wagh, N.; Shafie, A.; Gaber, A.; Abbasi, A.; Kjaer, A.; Tworowska, I.; Núñez, R. 64Cu-DOTATATE PET/CT for Imaging Patients with Known or Suspected Somatostatin Receptor-Positive Neuroendocrine Tumors: Results of the First U.S. Prospective, Reader-Masked Clinical Trial. J. Nucl. Med. 2020, 61, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Oehme, L.; Freudenberg, R.; Hofheinz, F.; van den Hoff, J.; Kotzerke, J.; Hoberück, S. Comparison of image quality and spatial resolution between 18F, 68Ga, and 64Cu phantom measurements using a digital Biograph Vision PET/CT. EJNMMI Phys. 2022, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, J.; Kang, F.; Wang, J.; Niu, M.; Ou, H.; Ye, J.; Zhang, M.; Dong, J.; Li, G.; et al. Exploration of Bicyclic Peptide Ligands for Immune-Specific PET Imaging: Targeting Tumor PD-L1 with [18F]AlF-BCY10959. Mol. Pharm. 2025, 22, 3456–3467. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Zhang, N.; Zhu, J.; Hu, X.; Wang, Q.; Qiu, B.; Liu, Q.; Qiu, L.; Lin, J. Synthesis and preclinical evaluation of small molecule-based radiotracers for PET imaging of PD-L1 expression and dynamics. Eur. J. Nucl. Med. Mol. Imaging 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, K.; Zhang, Y.; Chen, Y.; Wang, S.; Zhao, K.; Liu, Z.; Hu, M. Peptide-based immuno-PET/CT monitoring of dynamic PD-L1 expression during glioblastoma radiotherapy. J. Pharm. Anal. 2025, 15, 101082. [Google Scholar] [CrossRef] [PubMed]
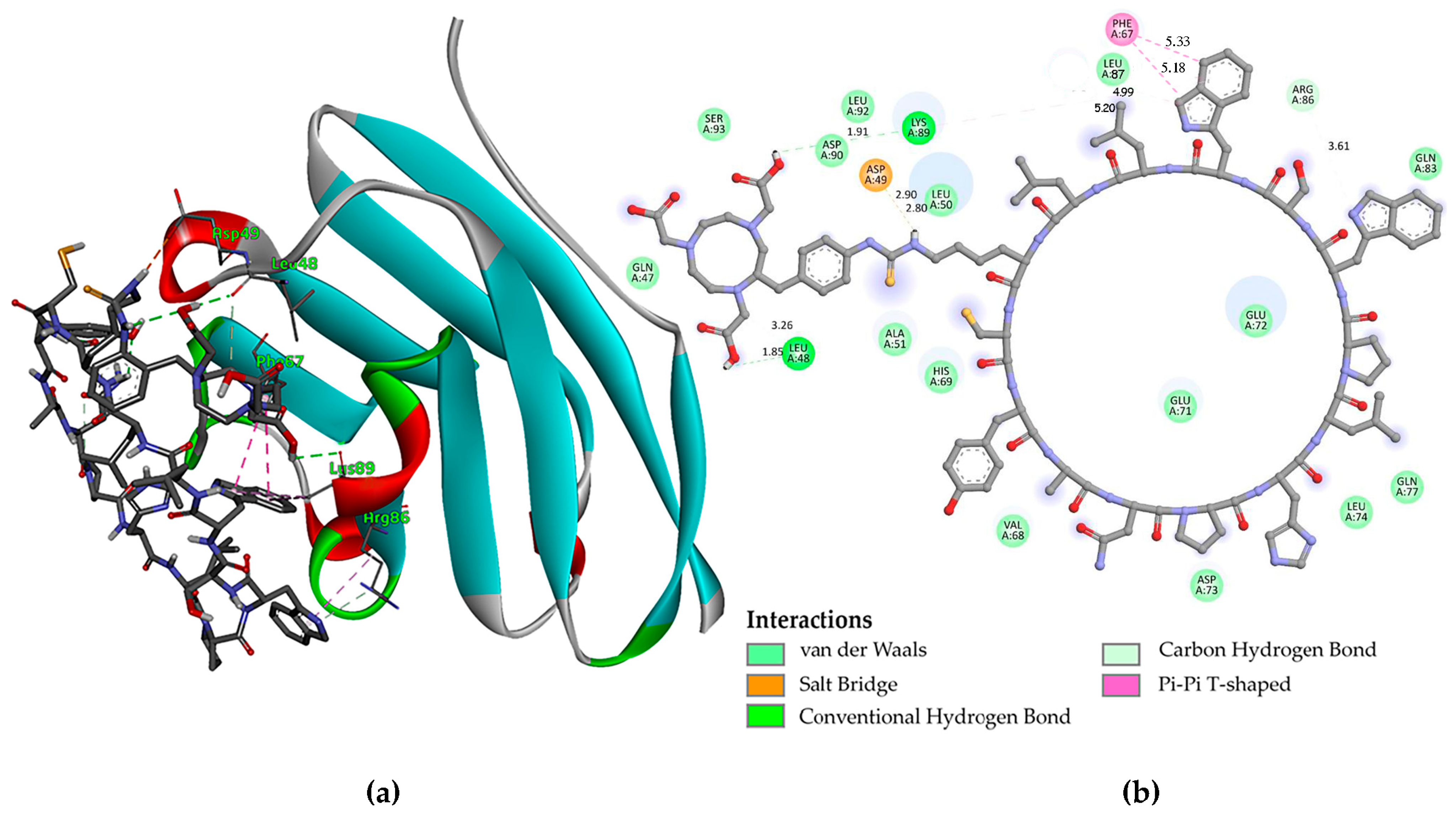
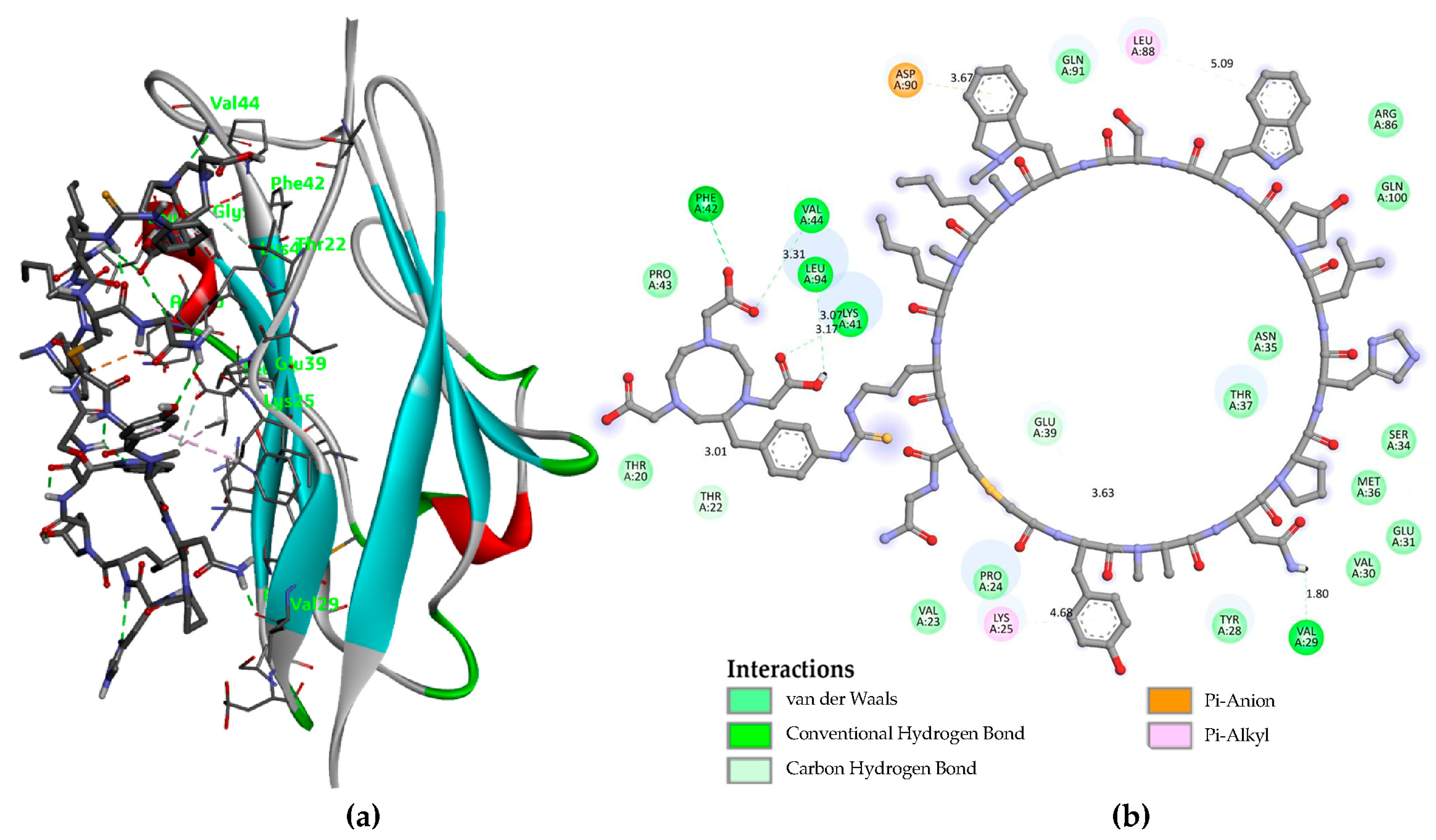
| Organ/Tissue | Time (h) | % ID/g |
|---|---|---|
| Kidney | 1 2 3 | 47.43 ± 5.38 52.31 ± 4.73 35.84 ± 3.91 |
| Liver | 1 2 3 | 22.66 ± 3.13 19.15 ± 3.34 15.52 ± 2.38 |
| Heart | 1 2 3 | 1.62 ± 0.47 0.91 ± 0.55 0.47 ± 0.11 |
| Spleen | 1 2 3 | 1.69 ± 0.24 1.26 ± 0.25 0.84 ± 0.13 |
| Lung | 1 2 3 | 1.81 ± 0.35 0.78 ± 0.23 0.37 ± 0.11 |
| Intestine | 1 2 3 | 0.22 ± 0.17 0.61 ± 0.35 0.83 ± 0.37 |
| Unblocking tumor (4T1) | 1 2 3 | 6.49 ± 0.98 * 6.54 ± 1.23 5.97 ± 1.31 |
| Blocking tumor (4T1) | 1 | 1.81 ± 1.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Jiménez-Mancilla, N.; Lara-Almazán, N.; Oros-Pantoja, R.; Santos-Cuevas, C.; Azorín-Vega, E.; Meléndez-Alafort, L. Synthesis and Evaluation of [18F]AlF-NOTA-iPD-L1 as a Potential Theranostic Pair for [177Lu]Lu-DOTA-iPD-L1. Pharmaceutics 2025, 17, 920. https://doi.org/10.3390/pharmaceutics17070920
Ferro-Flores G, Luna-Gutiérrez M, Ocampo-García B, Jiménez-Mancilla N, Lara-Almazán N, Oros-Pantoja R, Santos-Cuevas C, Azorín-Vega E, Meléndez-Alafort L. Synthesis and Evaluation of [18F]AlF-NOTA-iPD-L1 as a Potential Theranostic Pair for [177Lu]Lu-DOTA-iPD-L1. Pharmaceutics. 2025; 17(7):920. https://doi.org/10.3390/pharmaceutics17070920
Chicago/Turabian StyleFerro-Flores, Guillermina, Myrna Luna-Gutiérrez, Blanca Ocampo-García, Nallely Jiménez-Mancilla, Nancy Lara-Almazán, Rigoberto Oros-Pantoja, Clara Santos-Cuevas, Erika Azorín-Vega, and Laura Meléndez-Alafort. 2025. "Synthesis and Evaluation of [18F]AlF-NOTA-iPD-L1 as a Potential Theranostic Pair for [177Lu]Lu-DOTA-iPD-L1" Pharmaceutics 17, no. 7: 920. https://doi.org/10.3390/pharmaceutics17070920
APA StyleFerro-Flores, G., Luna-Gutiérrez, M., Ocampo-García, B., Jiménez-Mancilla, N., Lara-Almazán, N., Oros-Pantoja, R., Santos-Cuevas, C., Azorín-Vega, E., & Meléndez-Alafort, L. (2025). Synthesis and Evaluation of [18F]AlF-NOTA-iPD-L1 as a Potential Theranostic Pair for [177Lu]Lu-DOTA-iPD-L1. Pharmaceutics, 17(7), 920. https://doi.org/10.3390/pharmaceutics17070920










