Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method
Abstract
1. Introduction
2. Materials and Methods
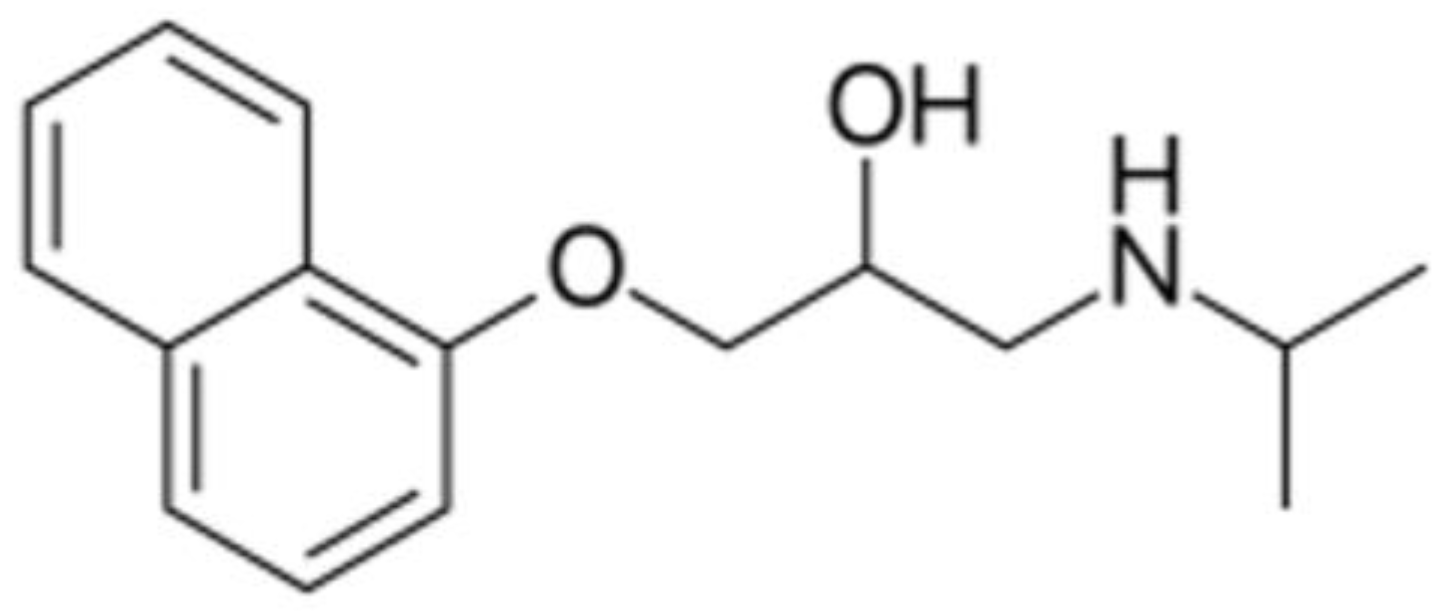
2.1. Materials
2.2. Methods
2.2.1. Viscosity Measurements of Semi-Solid Printing Mixtures
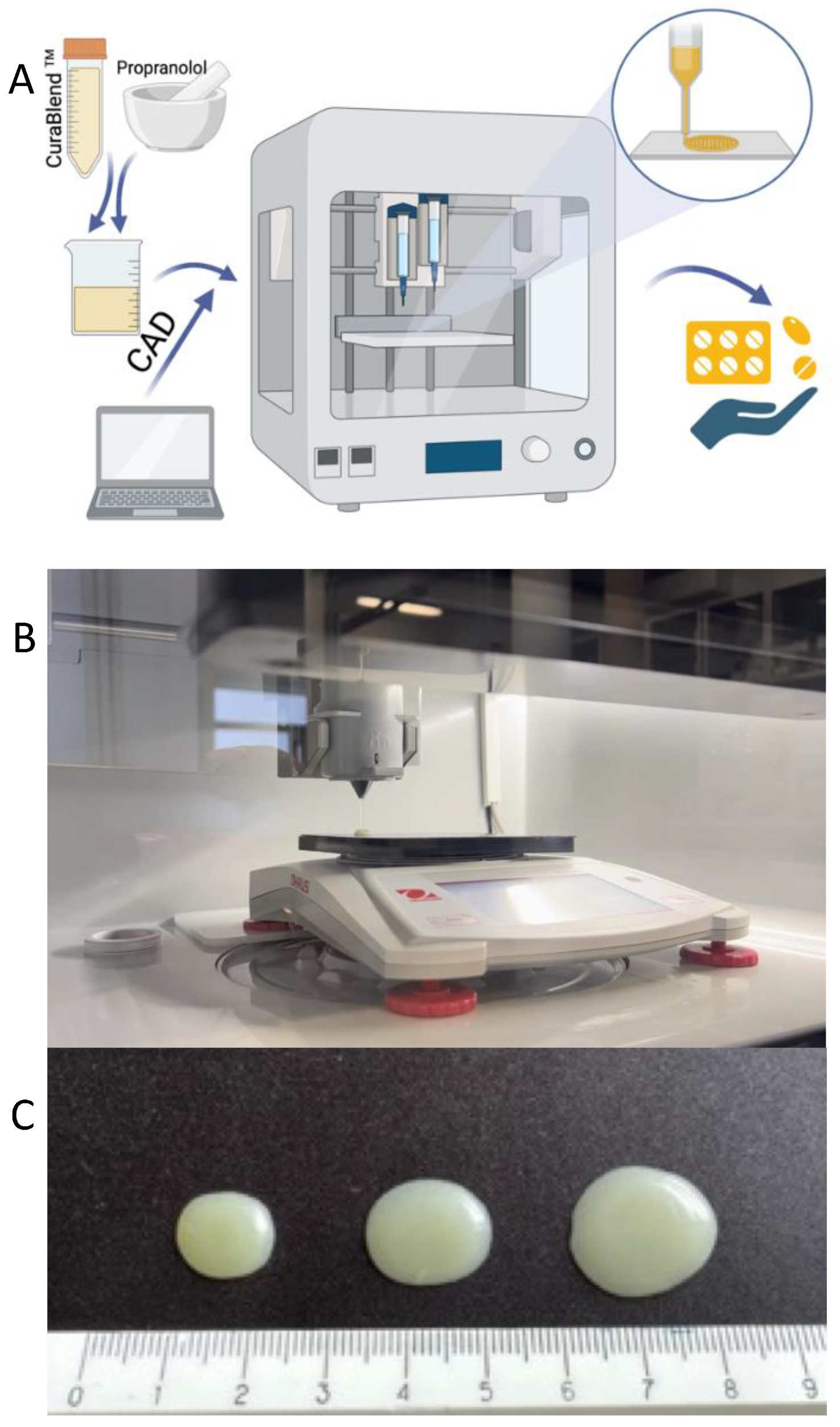
2.2.2. Automated Semi-Solid Extrusion (SSE) Material Deposition
2.2.3. Physical Appearance, Mass, and Dimensions of Gel Tablets
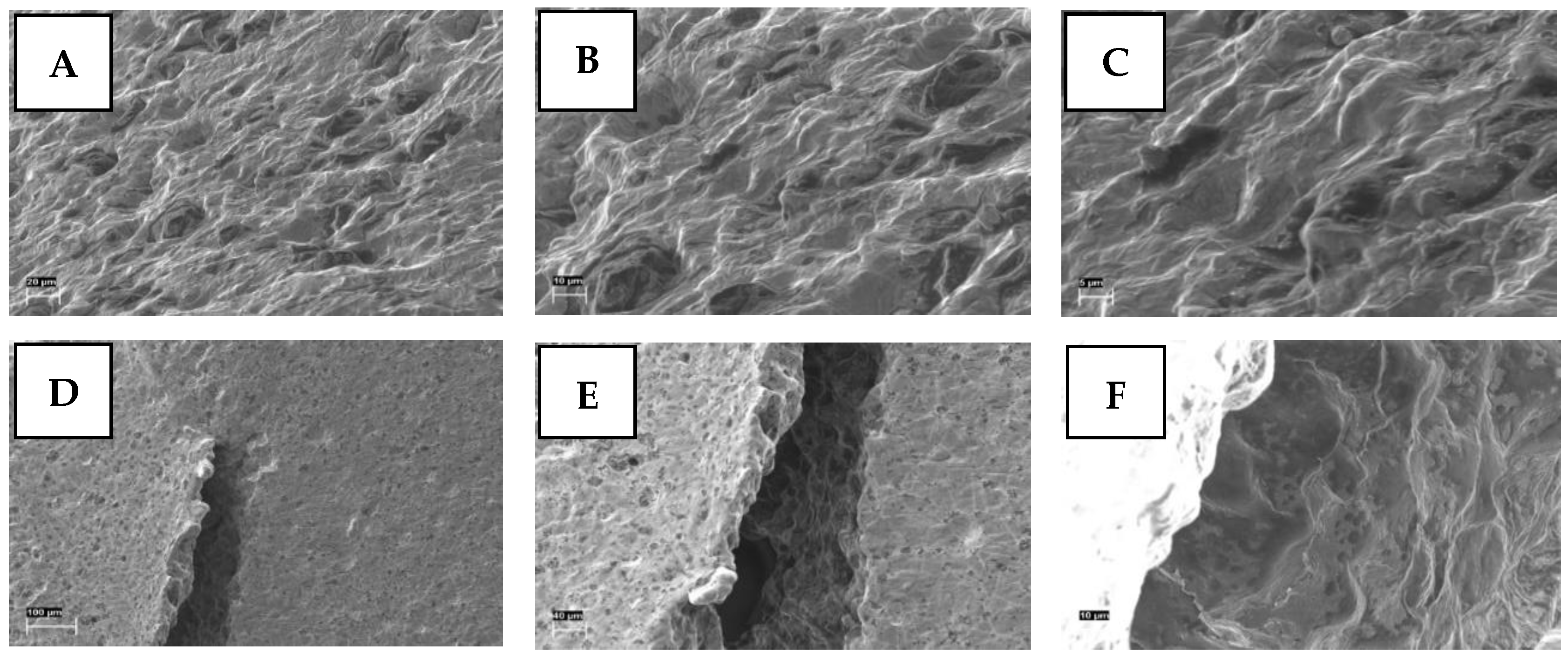
2.2.4. Scanning Electron Microscopy
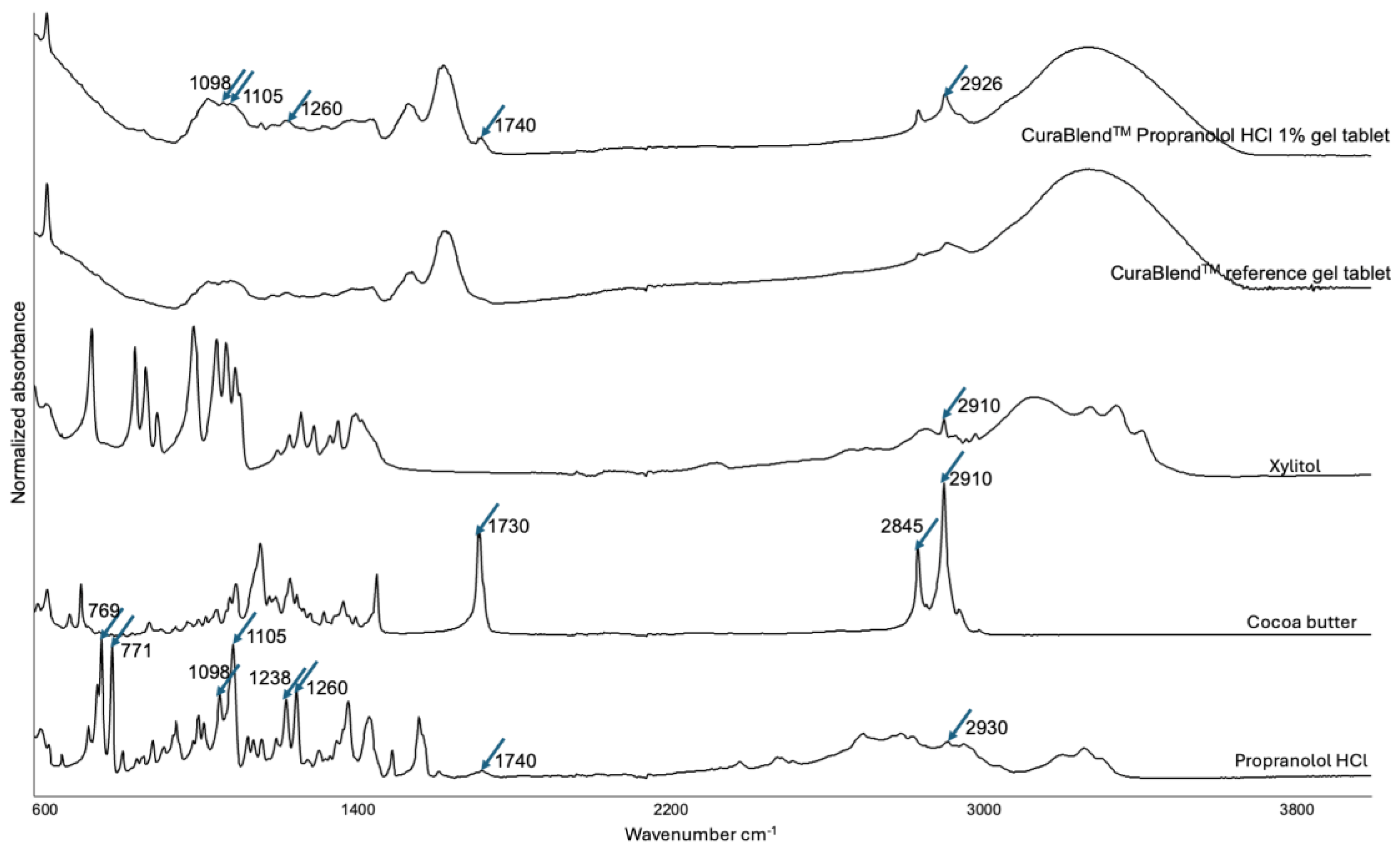
2.2.5. Fourier Transform-Infrared Spectroscopy
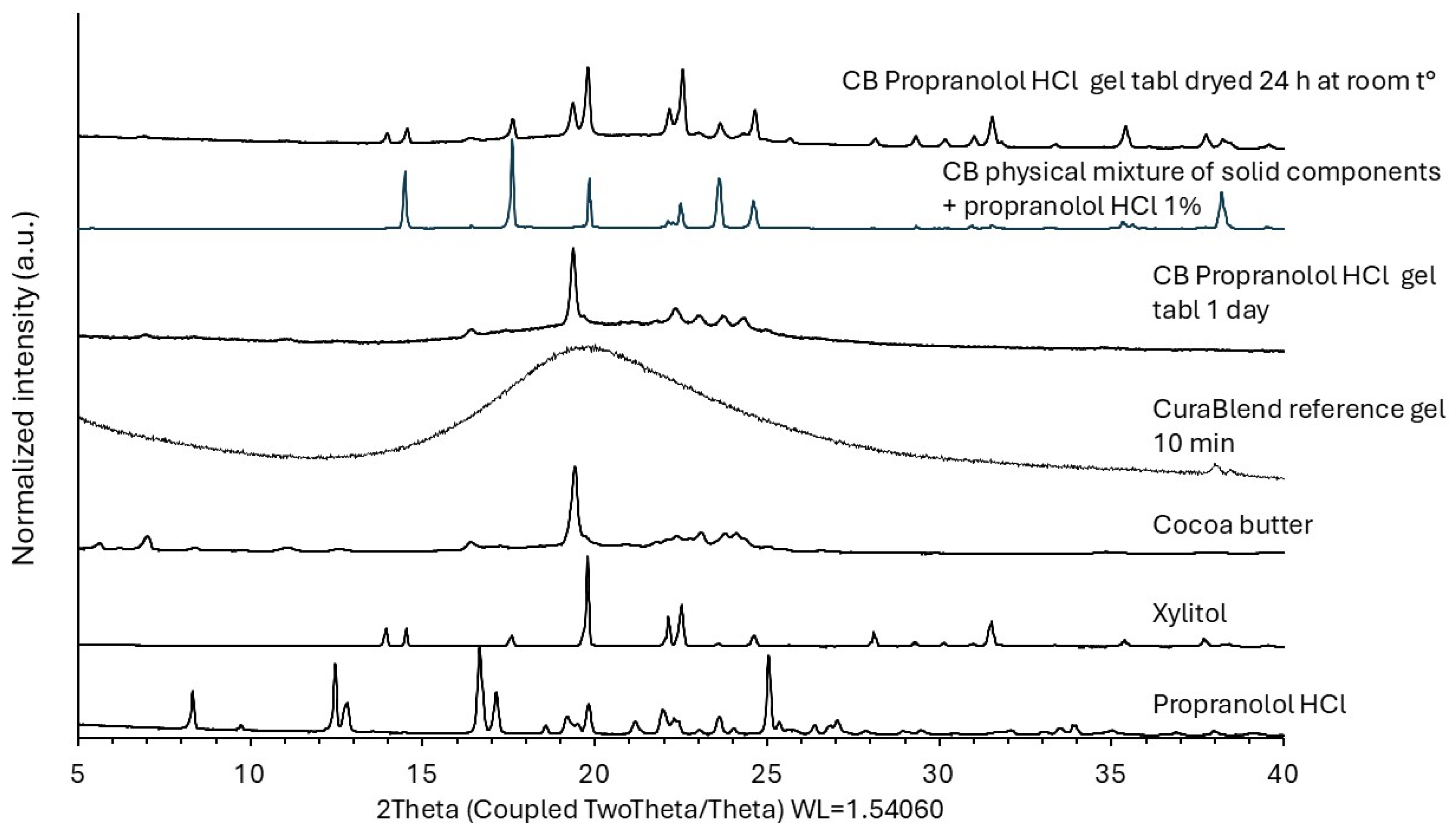
2.2.6. X-Ray Powder Diffraction
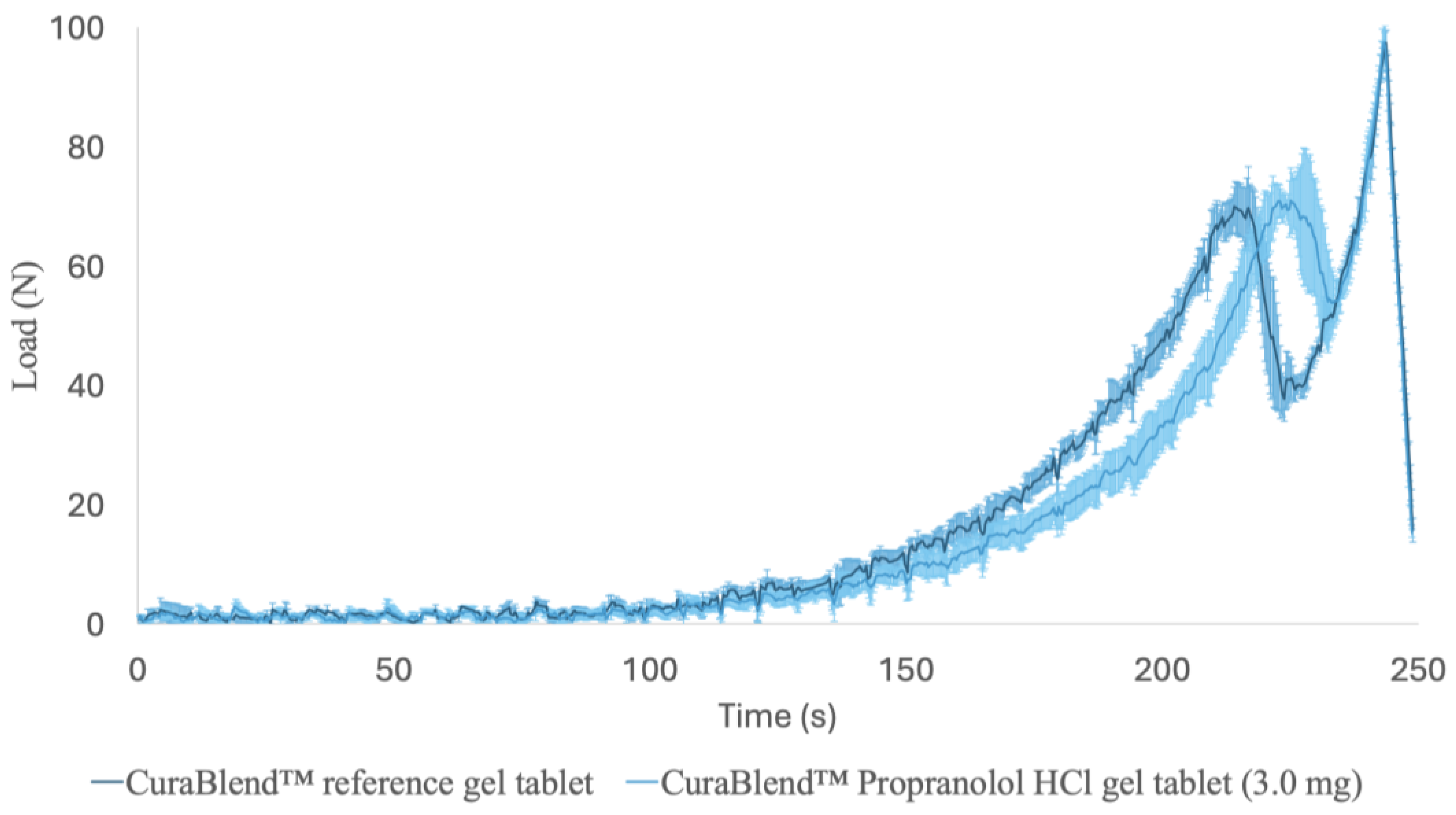
2.2.7. Mechanical Tests
2.2.8. Dissolution Test In Vitro
2.2.9. High-Performance Liquid Chromatography
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Viscosity of Semi-Solid Printing Mixtures
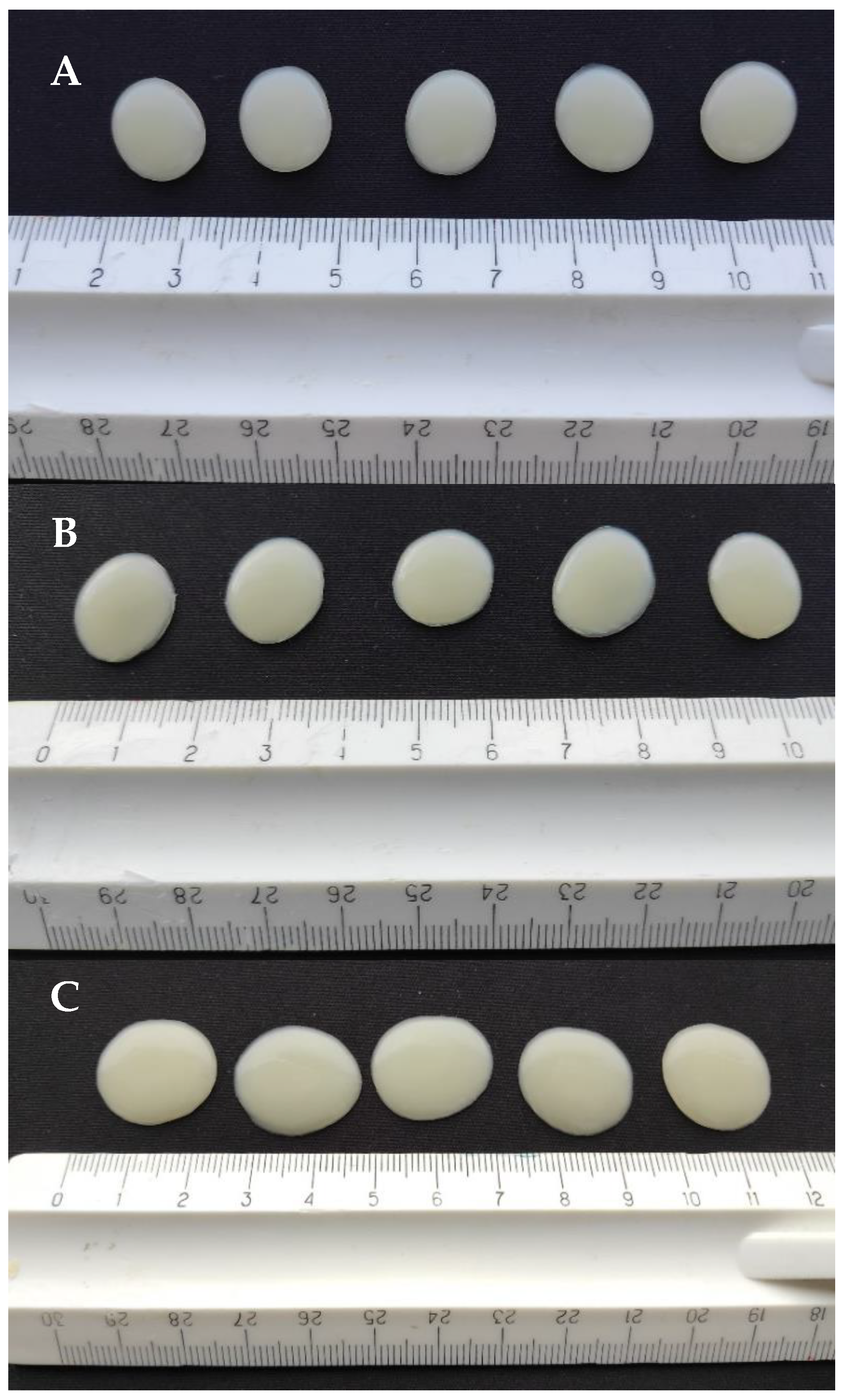
3.2. Physical Appearance of 3D-Printed Gel Tablets
3.3. Weight and Weight Variation of Gel Tablets
3.4. Physicochemical Changes in the API Loaded in the Gel Tablets
3.5. Mechanical Properties
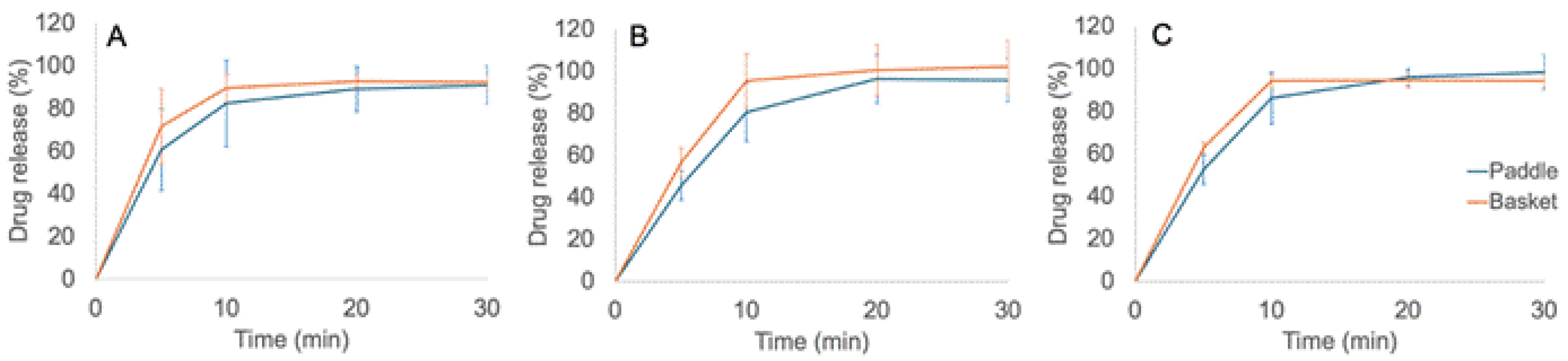
3.6. Dissolution In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Bóveda, M.D.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. Int. J. Pharm. 2024, 657, 124140. [Google Scholar] [CrossRef]
- Ilyes, K. The future of pharmaceutics: 3D-printing? Rom. J. Pharm. Pract. 2020, 13, 138–192. [Google Scholar] [CrossRef]
- Jain, K.; Shukla, R.; Yadav, A.; Ujjwal, R.R.; Singh Flora, S.J. 3D Printing in Development of Nanomedicines. Nanomaterials 2021, 11, 420. [Google Scholar] [CrossRef]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Sjöholm, E.; Manandhar, S.; Lakio, S.; Rosenholm, J.M.; Kaasalainen, M.; Wang, X.; Sandler, N. Personalizing oral delivery of nanoformed piroxicam by semi-solid extrusion 3D printing. Eur. J. Pharm. Sci. 2023, 188, 106497. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Rajan Prakash, D.; Lindfors, L.; Wang, Q.; Wang, X.; Ojala, S.; Sandler, N. 3D-Printed Veterinary Dosage Forms—A Comparative Study of Three Semi-Solid Extrusion 3D Printers. Pharmaceutics 2020, 12, 1239. [Google Scholar] [CrossRef]
- Sandler Topelius, N.; Shokraneh, F.; Bahman, M.; Lahtinen, J.; Hassinen, N.; Airaksinen, S.; Verma, S.; Hrizanovska, L.; Lass, J.; Paaver, U.; et al. Automated Non-Sterile Pharmacy Compounding: A Multi-Site Study in European Hospital and Community Pharmacies with Pediatric Immediate Release Propranolol Hydrochloride Tablets. Pharmaceutics 2024, 16, 678. [Google Scholar] [CrossRef]
- Shokraneh, F.; Filppula, A.M.; Tornio, A.; Aruväli, J.; Paaver, U.; Sandler Topelius, N. Automated extrusion-based dispensing: Personalized dosing and quality control of clopidogrel tablets for pediatric care. Eur. J. Pharm. Sci. 2025, 204, 106967. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef]
- Prasad, A.; Kumar Sinha, A.; Kumar, B.; Prasad, A.; Kumari, M. Individualized dosing of oral propranolol for treatment of infantile hemangioma: A prospective study. Pan Afr. Med. J. 2019, 32, 155. [Google Scholar] [CrossRef]
- Vakili, H.; Nyman, J.O.; Genina, N.; Preis, M.; Sandler, N. Application of a colorimetric technique in quality control for printed pediatric orodispersible drug delivery systems containing propranolol hydrochloride. Int. J. Pharm. 2016, 511, 606–618. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- Jovanović, M.; Petrović, M.; Cvijić, S.; Tomić, N.; Stojanović, D.; Ibrić, S.; Uskoković, P. 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics 2021, 13, 2143. [Google Scholar] [CrossRef]
- Alqahtani, A.A.; Mohammed, A.A.; Fatima, F.; Ahmed, M.M. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol HCl Tablets. Polymers 2023, 15, 3554. [Google Scholar] [CrossRef]
- Suksaeree, J.; Saingam, W.; Monton, C.; Pichayakorn, W. FT-IR Study Between Propranolol Hydrochloride and Pharmaceutically Acceptable Excipients in Physical Mixture. Bull. Health Sci. Technol. 2015, 13, 17–24. [Google Scholar]
- Cirri, M.; Mura, P.; Benedetti, S.; Buratti, S. Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics 2023, 15, 2217. [Google Scholar] [CrossRef]
- Van Malssen, K.F.; Van Langevelde, A.J.; Peschar, R.; Schenk, H. Phase behavior and extended phase scheme of static cocoa-butter investigated with real-time X-ray powder diffraction. J. Am. Oil Chem. Soc. 1999, 76, 669–676. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. The Stability and Nature of the Form IV Polymorph of Cocoa Butter Is Dictated by 1-Palmitoyl-2-Oleoyl-3-Stearoyl-Glycerol. Cryst. Growth Des. 2019, 19, 1488–1493. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Molecular origins of polymorphism in cocoa butter. Annu. Rev. Food Sci. Technol. 2021, 12, 567–590. [Google Scholar] [CrossRef]


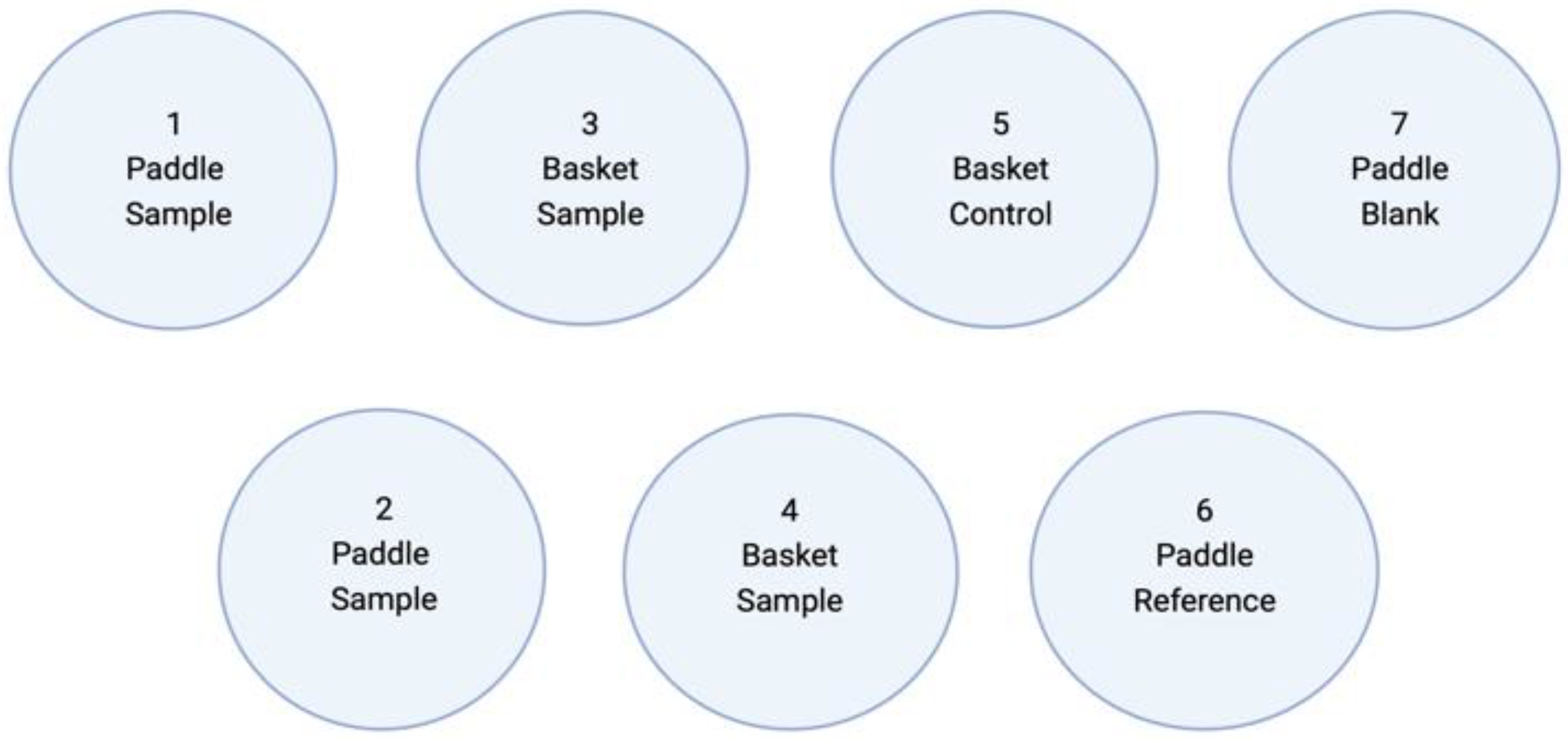
| Parameter | USP 35 | Modified (In-House) |
|---|---|---|
| Apparatus | Basket | Paddle and basket |
| Rotation speed | 100 rpm | 100 rpm |
| Dissolution medium | 0.063 M HCl, 900 mL | 0.063 M HCl, 900 mL |
| Temperature | 36.7 ± 0.2 °C | 36.7 ± 0.2 °C |
| Time | 60 min | 45 min |
| Analytical wavelength (HPLC) | 292 nm | 292 nm |
| Response | Gel Tablet Prepared Using the Automated SSE Material Deposition Method | |||||
|---|---|---|---|---|---|---|
| Tablet Strength 3.0 mg (Mean ± SD) | Tablet Strength 4.0 mg (Mean ± SD) | Tablet Strength 5.0 mg (Mean ± SD) | ||||
| Ref. Gel Tablet (Without API) | CuraBlend™ Gel Tablet Loaded with API (1%) | Ref. | CuraBlend™ Gel Tablet Loaded with API (1%) | Ref. | CuraBlend™ gel Tablet Loaded with API (1%) | |
| Weight (mg) | 280 ± 8.0 | 282 ± 8.3 | 375 ±10.6 | 378 ± 7.6 | 468 ±13.1 | 481 ± 20.8 |
| Diameter (mm) | 12.7 ± 0.2 | 14.8 ± 0.7 | 14.7 ± 0.3 | 17.1 ± 0.8 | 16.5 ± 0.3 | 20.2 ± 1.0 |
| Height (mm) | 2.7 ± 0.1 | 2.1 ± 0.2 | 2.7 ± 0.1 | 2.1 ± 0.1 | 2.7 ± 0.1 | 2.0 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roostar, K.; Meos, A.; Laidmäe, I.; Aruväli, J.; Räikkönen, H.; Peltonen, L.; Airaksinen, S.; Topelius, N.S.; Heinämäki, J.; Paaver, U. Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method. Pharmaceutics 2025, 17, 881. https://doi.org/10.3390/pharmaceutics17070881
Roostar K, Meos A, Laidmäe I, Aruväli J, Räikkönen H, Peltonen L, Airaksinen S, Topelius NS, Heinämäki J, Paaver U. Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method. Pharmaceutics. 2025; 17(7):881. https://doi.org/10.3390/pharmaceutics17070881
Chicago/Turabian StyleRoostar, Kristiine, Andres Meos, Ivo Laidmäe, Jaan Aruväli, Heikki Räikkönen, Leena Peltonen, Sari Airaksinen, Niklas Sandler Topelius, Jyrki Heinämäki, and Urve Paaver. 2025. "Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method" Pharmaceutics 17, no. 7: 881. https://doi.org/10.3390/pharmaceutics17070881
APA StyleRoostar, K., Meos, A., Laidmäe, I., Aruväli, J., Räikkönen, H., Peltonen, L., Airaksinen, S., Topelius, N. S., Heinämäki, J., & Paaver, U. (2025). Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method. Pharmaceutics, 17(7), 881. https://doi.org/10.3390/pharmaceutics17070881






