Design of Clofazimine-Loaded Lipid Nanoparticles Using Smart Pharmaceutical Technology Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clofazimine-Loaded NLCs Formulation
2.3. Clofazimine-Loaded NLCs Characterization
2.3.1. Particle Size, Surface Charge, and Polydispersity
2.3.2. Encapsulation Efficiency and Drug-Loading Quantification
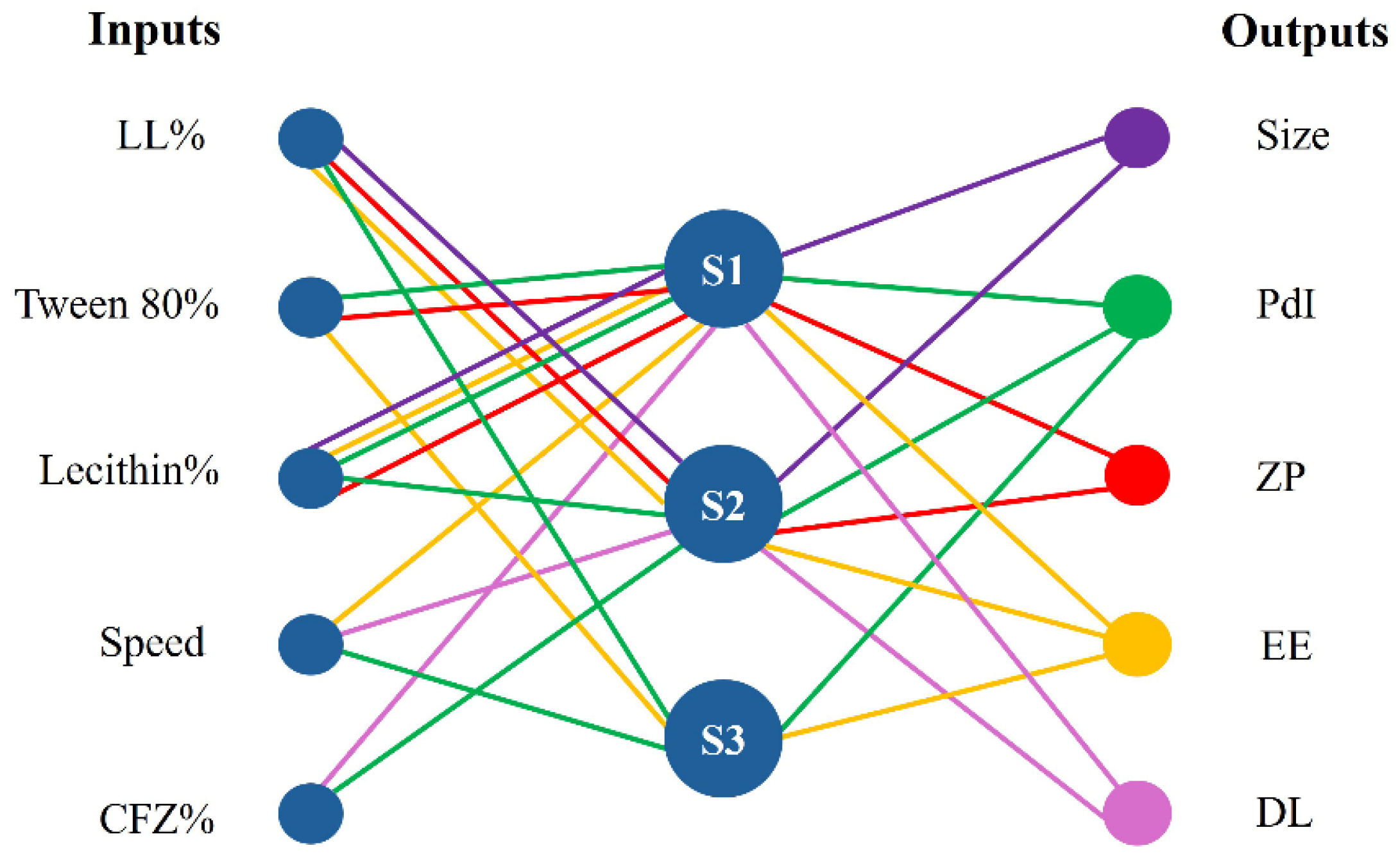
2.4. Database Modeling by Artificial Intelligence Tools
2.5. In Silico Docking Analysis
2.6. Cell Viability Studies
2.7. Microbiology Studies
3. Results and Discussion
3.1. Clofazimine-Loaded NLC Characterization
3.2. Database Modeling by Artificial Intelligence Tools
3.3. Analysis of NFL Model Performance
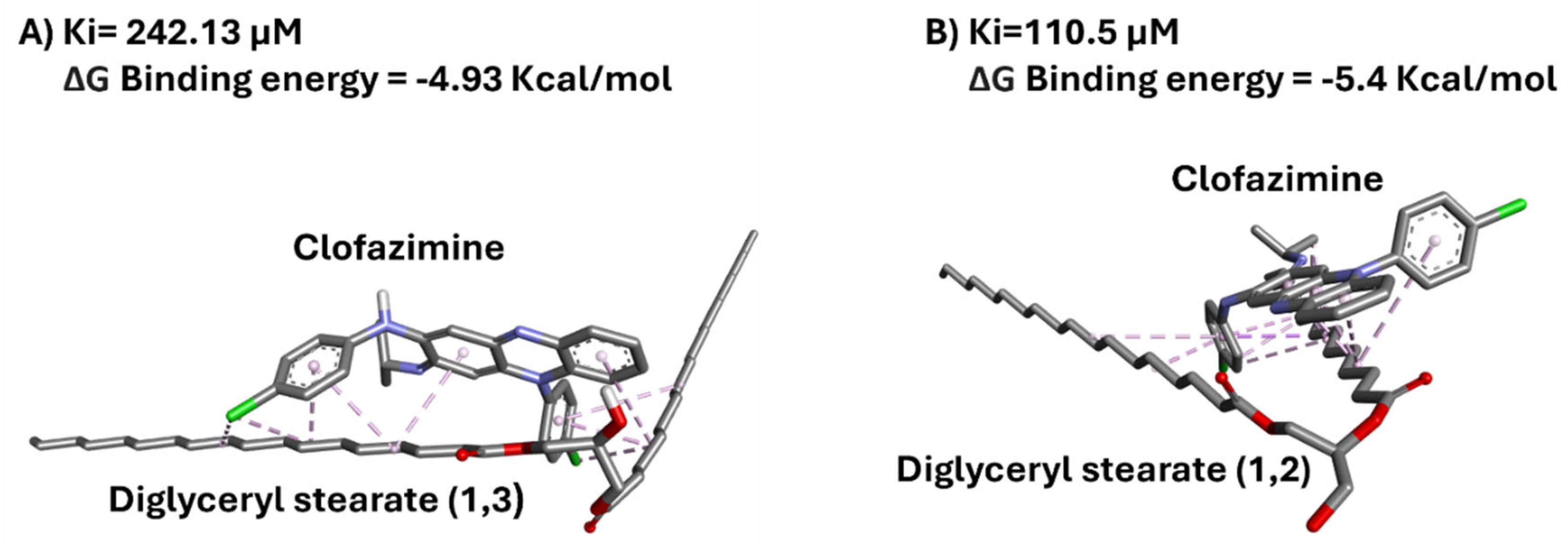
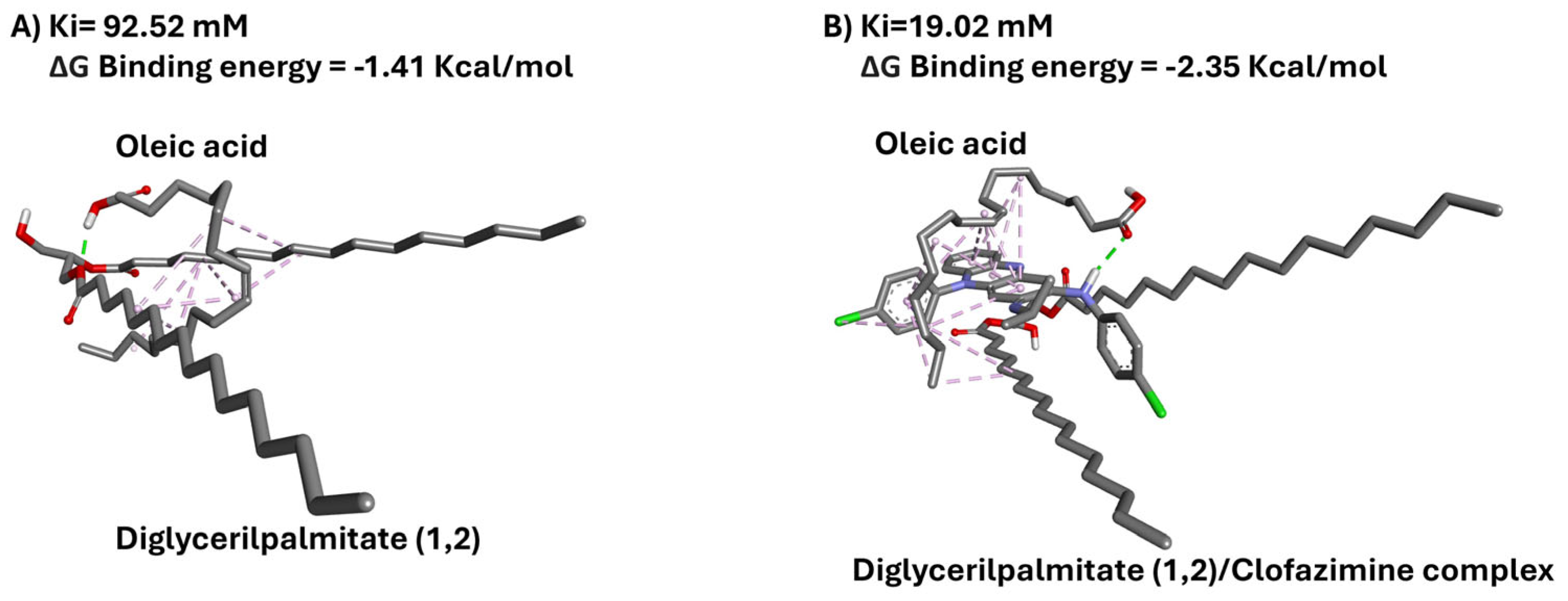
3.4. In Silico Docking Analysis
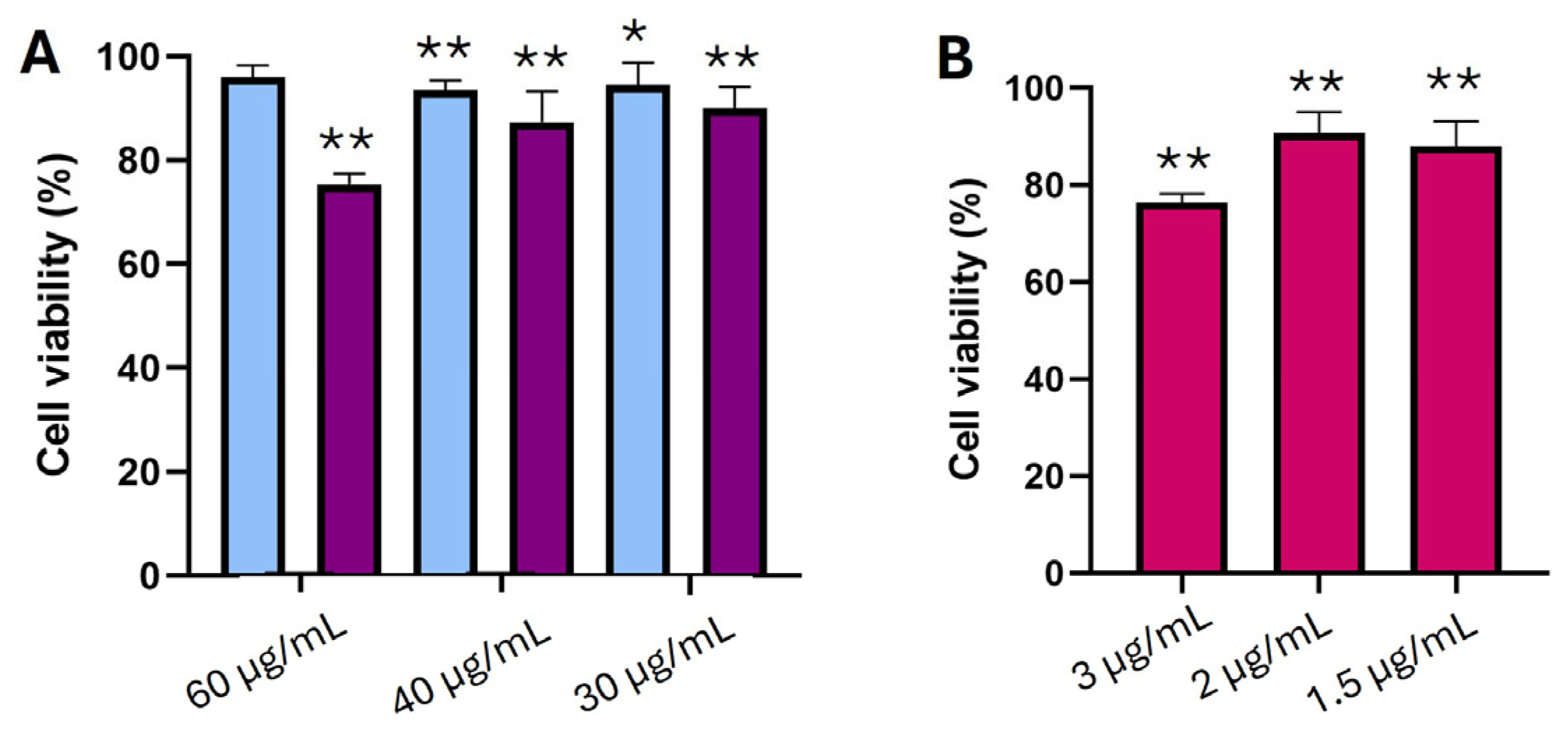
3.5. In Vitro Cell Viability Studies
3.6. Microbiology Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFZ | Clofazimine |
| WHO | World health organization |
| MRSA | Methicillin resistant Staphylococcus aureus |
| NLCs | Nanostructured lipid carriers |
| LNs | Lipid nanoparticles |
| GRAS | Generally recognized as safe |
| AI | Artificial intelligence |
| NFL | Neurofuzzy logic |
| ANN | Artificial neural networks |
| LL | Liquid lipid |
| DMF | Dymethil formamide |
| SL | Solid lipid |
| PdI | Polydispersity index |
| ZP | Zeta potential |
| DLS | Dynamic light scattering |
| EE | Encapsulation efficiency |
| DL | Drug loading |
| MWCO | Molecular weight cut off |
| ASMOD | Adaptative spline modeling of data |
| ANOVA | Analysis of variance |
| RFB | Rifabutin |
| MW | Molecular weight |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Foetal bovine serum |
References
- Gao, M.; Tagami, T.; Ogawa, K.; Ozeki, T. Preparation and in vitro characterization of clofazimine-loaded albumin nanocomposite microparticles for inhalation therapy. J. Drug Deliv. Sci. Technol. 2024, 93, 105364. [Google Scholar] [CrossRef]
- Shah, R.A.; Hsu, J.I.; Patel, R.R.; Mui, U.N.; Tyring, S.K. Antibiotic resistance in dermatology: The scope of the problem and strategies to address it. J. Am. Acad. Dermatol. 2022, 86, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Offman, E.M.; Leestemaker-Palmer, A.; Fathi, R.; Keefe, B.; Bibliowicz, A.; Raday, G.; Bermudez, L.E. Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model. Antibiotics 2024, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Oliva, B.; O’Neill, A.J.; Miller, K.; Stubbings, W.; Chopra, I. Anti-staphylococcal activity and mode of action of clofazimine. J. Antimicrob. Chemother. 2004, 53, 435–440. [Google Scholar] [CrossRef]
- Shimanovich, U.; Gedanken, A. Nanotechnology solutions to restore antibiotic activity. J. Mater. Chem. B 2016, 4, 824–833. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the diagnosis and treatment of antibiotic-resistant infections. Antibiotics 2024, 13, 121. [Google Scholar] [CrossRef]
- Virzì, N.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Consoli, V.; Salerno, L.; Vanella, L.; Pittalà, V.; Diaz-Rodriguez, P. Heme oxygenase 1 inhibitor discovery and formulation into nanostructured lipid carriers as potent and selective treatment against triple negative metastatic breast cancer. Int. J. Pharm. 2025, 668, 124997. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers–a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Rouco, H.; Diaz-Rodriguez, P.; Rama-Molinos, S.; Remuñán-López, C.; Landin, M. Delimiting the knowledge space and the design space of nanostructured lipid carriers through Artificial Intelligence tools. Int. J. Pharm. 2018, 553, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Rouco, H.; Diaz-Rodriguez, P.; Gaspar, D.P.; Gonçalves, L.M.; Cuerva, M.; Remuñán-López, C.; Almeida, A.J.; Landin, M. Rifabutin-loaded nanostructured lipid carriers as a tool in oral anti-mycobacterial treatment of Crohn’s disease. Nanomaterials 2020, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Colbourn, E.A.; Rowe, R.C. Novel approaches to neural and evolutionary computing in pharmaceutical formulation: Challenges and new possibilities. Future Med. Chem. 2009, 1, 713–726. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; Landín, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A novel method for the production of core-shell microparticles by inverse gelation optimized with artificial intelligent tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef]
- Garcia-del Rio, L.; Diaz-Rodriguez, P.; Landin, M. Design of novel orotransmucosal vaccine-delivery platforms using artificial intelligence. Eur. J. Pharm. Biopharm. 2021, 159, 36–43. [Google Scholar] [CrossRef]
- Jara, M.O.; Catalan-Figueroa, J.; Landin, M.; Morales, J.O. Finding key nanoprecipitation variables for achieving uniform polymeric nanoparticles using neurofuzzy logic technology. Drug Deliv. Transl. Res. 2018, 8, 1797–1806. [Google Scholar] [CrossRef]
- Grillo, A.; Kane, M.; Perkins, M.; Penn, N. Characterizing the Formulation Design Space. BioPharm International. 2010, 23, 30–39. [Google Scholar]
- von Stosch, M.; Schenkendorf, R.; Geldhof, G.; Varsakelis, C.; Mariti, M.; Dessoy, S.; Vandercammen, A.; Pysik, A.; Sanders, M. Working within the design space: Do our static process characterization methods suffice? Pharmaceutics 2020, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Landin, M.; Rowe, R.C. Artificial neural networks technology to model, understand, and optimize drug formulations. In Formulation Tools for Pharmaceutical Development; Woodhead Publishing: Sawston, UK, 2013; pp. 7–37. [Google Scholar]
- Diaz-Rodriguez, P.; Landin, M. Smart design of intratumoral thermosensitive beta-lapachone hydrogels by Artificial Neural Networks. Int. J. Pharm. 2012, 433, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Rowe, R.C.; York, P. Comparison of neurofuzzy logic and neural networks in modelling experimental data of an immediate release tablet formulation. Eur. J. Pharm. Sci. 2006, 28, 394–404. [Google Scholar] [CrossRef]
- Colbourn, E.; Rowe, R. Neural computing and formulation optimization. In Encyclopedia of Pharmaceutical Science and Technology; CRC Press: Boca Raton, FL, USA, 2013; p. 13. [Google Scholar]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-eluting contact lenses: Effects of molecular imprinting and sterilization on drug loading and release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef]
- Martínez-Borrajo, R.; Rouco, H.; Virzì, N.F.; Diaz-Rodriguez, P.; Landin, M. Modulation of IFN-γ induced macrophage inflammatory responses via indomethacin-loaded NLCs for OA management. Int. J. Pharm. 2024, 666, 124823. [Google Scholar] [CrossRef]
- Longhin, E.M.; El Yamani, N.; Rundén-Pran, E.; Dusinska, M. The alamar blue assay in the context of safety testing of nanomaterials. Front. Toxicol. 2022, 4, 981701. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Alliance, T. Clofazimine. Tuberculosis 2008, 88, 96–99. [Google Scholar]
- Verbić, T.Ž.; Tam, K.Y.; Veljković, D.Ž.; Serajuddin, A.T.; Avdeef, A. Clofazimine pK a Determination by Potentiometry and Spectrophotometry: Reverse Cosolvent Dependence as an Indicator of the Presence of Dimers in Aqueous Solutions. Mol. Pharm. 2023, 20, 3160–3169. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, D.; Duan, C.; Jia, L.; Wang, Y.; Feng, F.; Zhang, Q. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J. Microencapsul. 2010, 27, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Jazuli, I.; Nabi, B.; Alam, T.; Baboota, S.; Ali, J. Optimization of nanostructured lipid carriers of lurasidone hydrochloride using box-behnken design for brain targeting: In vitro and in vivo studies. J. Pharm. Sci. 2019, 108, 3082–3090. [Google Scholar] [CrossRef]
- Mishra, A.; Vuddanda, P.R.; Singh, S. Intestinal lymphatic delivery of praziquantel by solid lipid nanoparticles: Formulation design, in vitro and in vivo studies. J. Nanotechnol. 2014, 2014, 351693. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Vitorino, C.; Carvalho, F.A.; Almeida, A.J.; Sousa, J.J.; Pais, A.A. The size of solid lipid nanoparticles: An interpretation from experimental design. Colloids Surf. B Biointerfaces 2011, 84, 117–130. [Google Scholar] [CrossRef]
- Karavas, E.; Georgarakis, E.; Sigalas, M.P.; Avgoustakis, K.; Bikiaris, D. Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug–polymer interactions. Eur. J. Pharm. Biopharm. 2007, 66, 334–347. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Preparation of ondansetron hydrochloride-loaded nanostructured lipid carriers using solvent injection method for enhancement of pharmacokinetic properties. Pharm. Res. 2019, 36, 138. [Google Scholar] [CrossRef]
- Lala, R.R.; Shinde, A.S. Development, optimization, and in vitro evaluation of atorvastatin calcium and vinpocetine codelivery by solid lipid nanoparticles for cancer therapy. Future J. Pharm. Sci. 2021, 7, 202. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanoparticle Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Wang, X.; Yu, B.; Ren, W.; Mo, X.; Zhou, C.; He, H.; Jia, H.; Wang, L.; Jacob, S.T.; Lee, R.J. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J. Control. Release 2013, 172, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; McClements, D.J.; He, X.; Xu, X.; Tan, F.; Yang, D.; Sun, Q.; Dai, L. Interfacial properties and structure of Pickering emulsions co-stabilized by different charge emulsifiers and zein nanoparticles. Food Hydrocoll. 2024, 146, 109285. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.H. Electrolyte-and pH-stabilities of aqueous solid lipid nanoparticle (SLN™) dispersions in artificial gastrointestinal media. Eur. J. Pharm. Biopharm. 2001, 52, 203–210. [Google Scholar] [CrossRef]
- Jódar-Reyes, A.; Torcello-Gómez, A.; Wulff-Pérez, M.; Gálvez-Ruiz, M.; Martín-Rodríguez, A. Different stability regimes of oil-in-water emulsions in the presence of bile salts. Food Res. Int. 2010, 43, 1634–1641. [Google Scholar] [CrossRef]
- Mitrović, J.R.; Divović-Matović, B.; Knutson, D.E.; Petković, M.; Djorović, D.; Randjelović, D.V.; Dobričić, V.D.; Đoković, J.B.; Lunter, D.J.; Cook, J.M. High amount of lecithin facilitates oral delivery of a poorly soluble pyrazoloquinolinone ligand formulated in lipid nanoparticles: Physicochemical, structural and pharmacokinetic performances. Int. J. Pharm. 2023, 633, 122613. [Google Scholar] [CrossRef]
- Mancini, G.; Lopes, R.M.; Clemente, P.; Raposo, S.; Gonçalves, L.M.; Bica, A.; Ribeiro, H.M.; Almeida, A.J. Lecithin and parabens play a crucial role in tripalmitin-based lipid nanoparticle stabilization throughout moist heat sterilization and freeze-drying. Eur. J. Lipid Sci. Technol. 2015, 117, 1947–1959. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Evaluation of biocompatibility and cytotoxicity using keratinocyte and fibroblast cultures. Ski. Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef]
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical impact of Staphylococcus aureus skin and soft tissue infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for Cytotoxicity: In Vitro Methods. International Organization for Standardization: Geneva, Switzerland, 1999.
- Niu, Y.; Yang, C.; Zhou, J.; Huang, S.; Liu, J. Two new compounds with antimicrobial activities from the seeds of Voacanga africana. Phytochem. Lett. 2016, 18, 208–212. [Google Scholar] [CrossRef]

| Formulation | * LL% | Tween 80% (w/v) | * Lecithin% | Speed (rpm) | * CFZ % |
|---|---|---|---|---|---|
| 1 | 40 | 1.5 | 0.5 | 13,400 | 5 |
| 2 | 57.5 | 3 | 1 | 16,800 | 3.75 |
| 3 | 75 | 1 | 0 | 20,400 | 2.5 |
| 4 | 40 | 1 | 0 | 20,400 | 5 |
| 5 | 75 | 3 | 0.5 | 13,400 | 3.75 |
| 6 | 57.5 | 1.5 | 1 | 16,800 | 2.5 |
| 7 | 40 | 1.5 | 0 | 13,400 | 5 |
| 8 | 57.5 | 1 | 1 | 16,800 | 2.5 |
| 9 | 75 | 3 | 0.5 | 20,400 | 3.75 |
| 10 | 75 | 1.5 | 1 | 13,400 | 5 |
| 11 | 40 | 3 | 0 | 20,400 | 2.5 |
| 12 | 57.5 | 1 | 0.5 | 16,800 | 3.75 |
| 13 | 57.5 | 1 | 0.5 | 13,400 | 3.75 |
| 14 | 75 | 3 | 0 | 20,400 | 2.5 |
| 15 | 40 | 1.5 | 1 | 16,800 | 5 |
| Formulation | Size (nm) | PdI | ZP (mV) | EE% | DL% |
|---|---|---|---|---|---|
| 1 | 1503 ± 267 | 0.54 ± 0.22 | −30 ± 0 | 89.7 ± 3.2 | 4.6 ± 0.3 |
| 2 | 630 ± 31 | 0.58 ± 0 | −28 ± 0 | 99.6 ± 36.4 | 3.7 ± 1.3 |
| 3 | 353 ± 44 | 0.52 ± 0.09 | −39 ± 1 | 93.9 ± 3.3 | 2.4 ± 0.1 |
| 4 | 621 ± 86 | 0.64 ± 0.02 | −31 ± 2 | 89.3 ± 24.7 | 4.5 ± 1.2 |
| 5 | 321 ± 134 | 0.31 ± 0.10 | −34 ± 1 | 84.5 ± 0.9 | 3.2 ± 0.1 |
| 6 | 594 ± 189 | 0.54 ± 0.01 | −36 ± 1 | 93.4 ± 1.8 | 2.6 ± 0.4 |
| 7 | 395 ± 83 | 0.52 ± 0.14 | −31 ± 0 | 98.2 ± 3.1 | 5.0 ± 0.0 |
| 8 | 1230 ± 28 | 0.58 ± 0.05 | −38 ± 0 | 96.4 ± 6.1 | 2.3 ± 0.2 |
| 9 | 724 ± 619 | 0.65 ± 0.40 | −34 ± 3 | 105.3 ± 16.8 | 2.7 ± 0.2 |
| 10 | 127 ± 11 | 0.17 ± 0.01 | −39 ± 1 | 101.4 ± 10.2 | 4.9 ± 0.6 |
| 11 | 145 ± 2 | 0.16 ± 0.01 | −31 ± 0 | 91.3 ± 12.3 | 2.3 ± 0.3 |
| 12 | 1254 ± 231 | 0.49 ± 0.44 | −40 ± 2 | 92.1 ± 4.2 | 3.5 ± 0.2 |
| 13 | 1333 ± 279 | 0.46 ± 0.51 | −36 ± 2 | 80.2 ± 15.4 | 3.0 ± 0.7 |
| 14 | 297 ± 102 | 0.35 ± 0.12 | −32 ± 1 | 86.7 ± 3.6 | 2.2. ± 0.1 |
| 15 | 211 ± 7 | 0.24 ± 0.01 | −35 ± 0 | 100.0 ± 0.5 | 5.0 ± 0.0 |
| Output | Submodels | Inputs | R2 | Calculated f Value | Degrees of Freedom | f Critical (p < 0.05) |
|---|---|---|---|---|---|---|
| Size | Submodel 1 | Lecithin% | 70.34 | 4.27 | 5 and 9 | 3.48 |
| Submodel 2 | LL% | |||||
| PdI | Submodel 1 | Tween% × Lecithin% | 92.67 | 5.06 | 10 and 4 | 5.96 |
| Submodel 2 | CFZ% × Lecithin% | |||||
| Submodel 3 | LL% × speed | |||||
| ZP | Submodel 1 | Tween% × Lecithin% | 90.45 | 7.26 | 5 and 9 | 3.48 |
| Submodel 2 | LL% | |||||
| EE% | Submodel 1 | Speed × Lecithin% | 90.45 | 5.26 | 9 and 5 | 4.77 |
| Submodel 2 | LL% | |||||
| Submodel 3 | Tween% | |||||
| DL% | Submodel 1 | CFZ% | 97.06 | 59.42 | 5 and 9 | 3.48 |
| Submodel 2 | Speed |
| Formulation | * LL% | Tween 80% (w/v) | * Lecithin% | Speed (rpm) | * CFZ % |
|---|---|---|---|---|---|
| 16 | 75 | 1.5 | 1 | 15,200 | 5 |
| 17 | 55 | 1 | 0.4 | 13,400 | 5 |
| Formulation | Size (nm) | PdI | ZP (mV) | EE% | DL% |
|---|---|---|---|---|---|
| 16 | 132 ± 4 | 0.17 ± 0.01 | −22 ± 1 | 92.6 ± 0.2 | 4.6 ± 0.2 |
| 17 | 570 ± 1 | 0.62 ± 0.05 | −24 ± 0 | 84.1 ± 13.1 | 4.3 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouco, H.; Virzì, N.F.; Menéndez-Rodríguez, C.; Potel, C.; Diaz-Rodriguez, P.; Landin, M. Design of Clofazimine-Loaded Lipid Nanoparticles Using Smart Pharmaceutical Technology Approaches. Pharmaceutics 2025, 17, 873. https://doi.org/10.3390/pharmaceutics17070873
Rouco H, Virzì NF, Menéndez-Rodríguez C, Potel C, Diaz-Rodriguez P, Landin M. Design of Clofazimine-Loaded Lipid Nanoparticles Using Smart Pharmaceutical Technology Approaches. Pharmaceutics. 2025; 17(7):873. https://doi.org/10.3390/pharmaceutics17070873
Chicago/Turabian StyleRouco, Helena, Nicola Filippo Virzì, Carolina Menéndez-Rodríguez, Carmen Potel, Patricia Diaz-Rodriguez, and Mariana Landin. 2025. "Design of Clofazimine-Loaded Lipid Nanoparticles Using Smart Pharmaceutical Technology Approaches" Pharmaceutics 17, no. 7: 873. https://doi.org/10.3390/pharmaceutics17070873
APA StyleRouco, H., Virzì, N. F., Menéndez-Rodríguez, C., Potel, C., Diaz-Rodriguez, P., & Landin, M. (2025). Design of Clofazimine-Loaded Lipid Nanoparticles Using Smart Pharmaceutical Technology Approaches. Pharmaceutics, 17(7), 873. https://doi.org/10.3390/pharmaceutics17070873







