Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Nanostructured Formulations
2.2.1. Dynamic Light Scattering (DLS) and Zeta Potential
2.2.2. Atomic Force Microscopy (AFM)
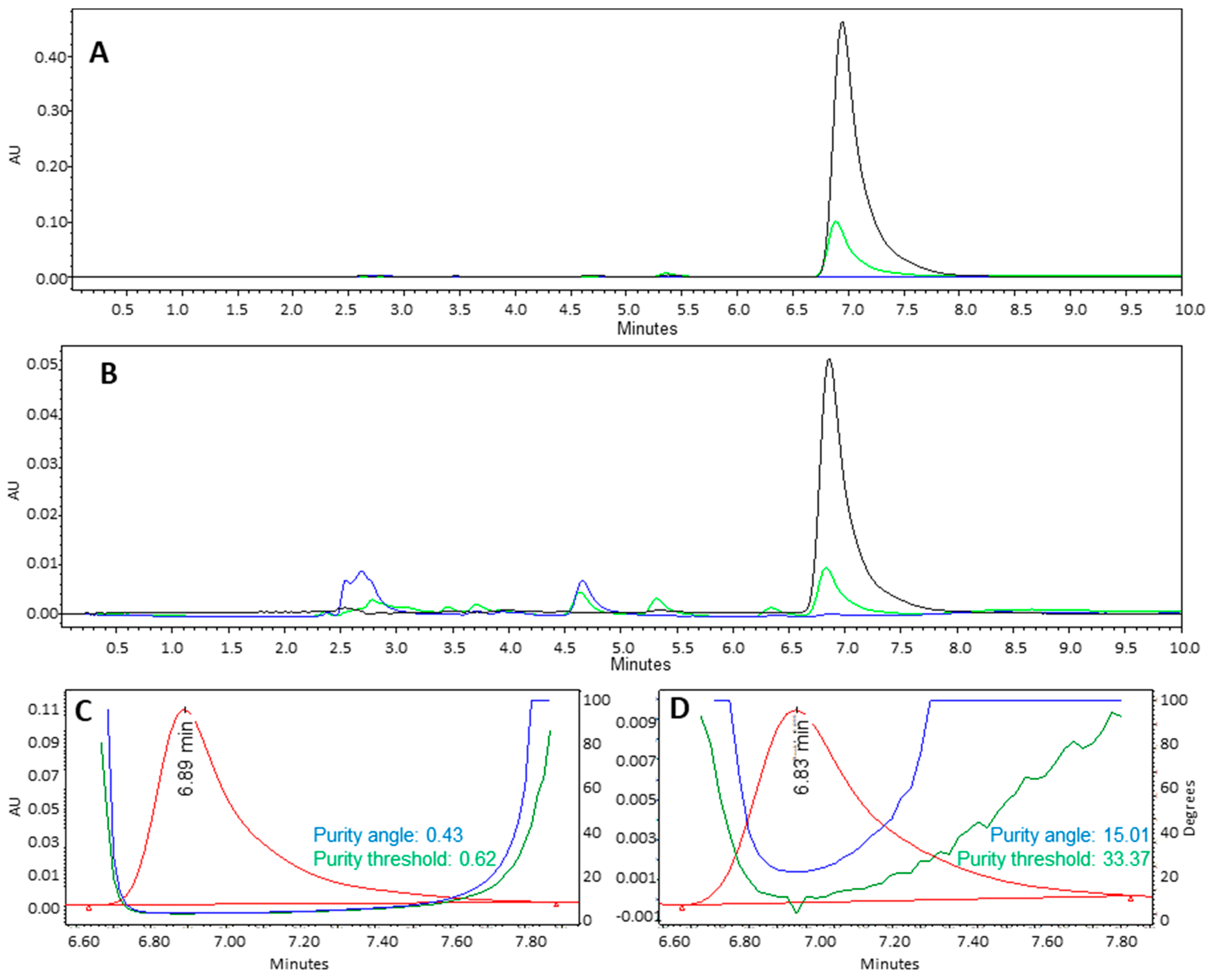
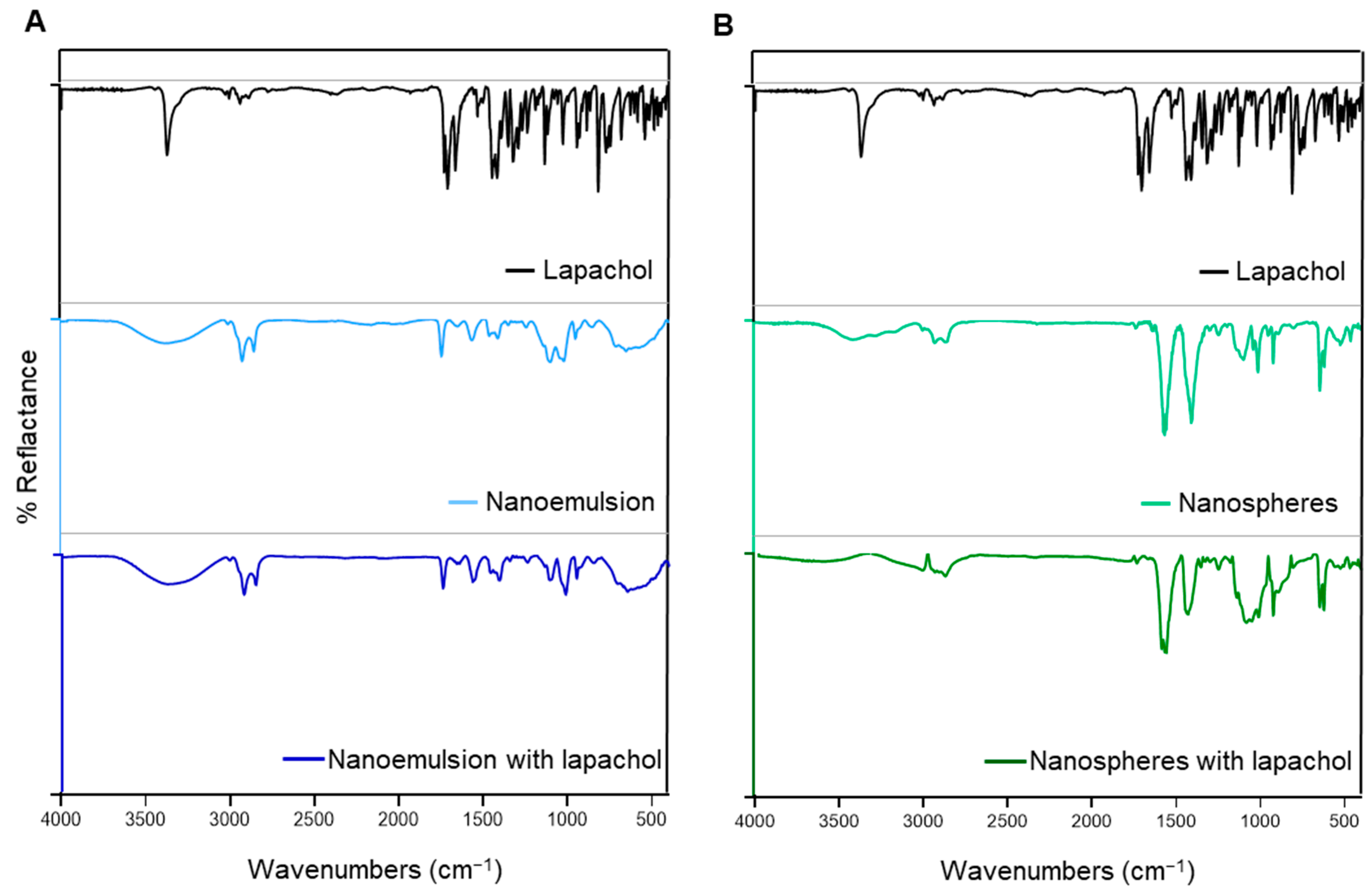
2.2.3. Fourier Transform Infrared (FTIR) Spectroscopy and High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD)
2.2.4. Encapsulation Efficiency and Drug Release
2.3. Cytotoxicity Assay
2.4. Cell Uptake
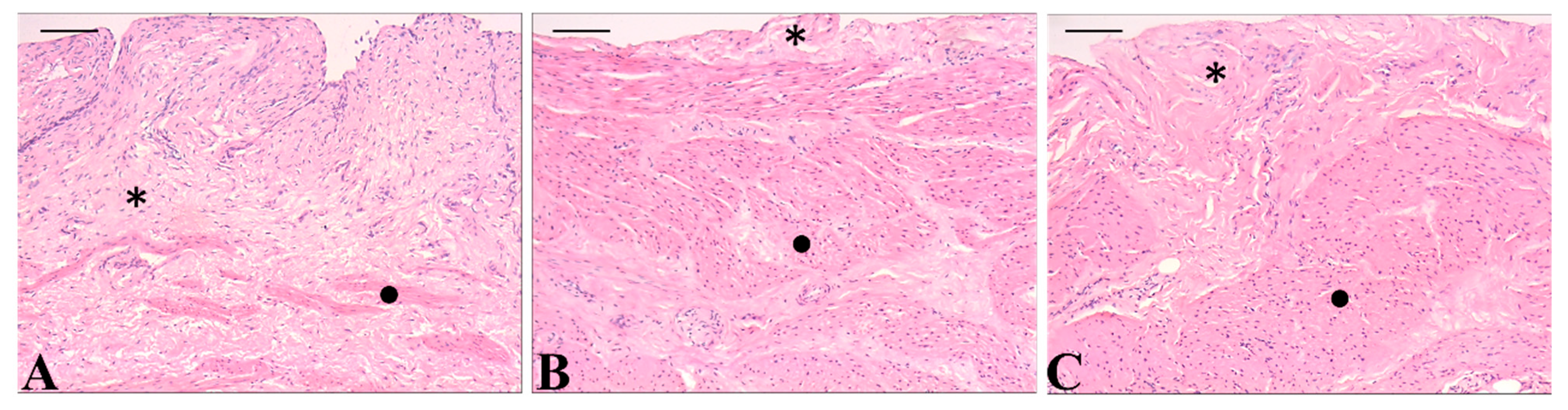
2.5. Ex Vivo Toxicity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Morphological Characterization of the Nanoemulsion and Nanospheres
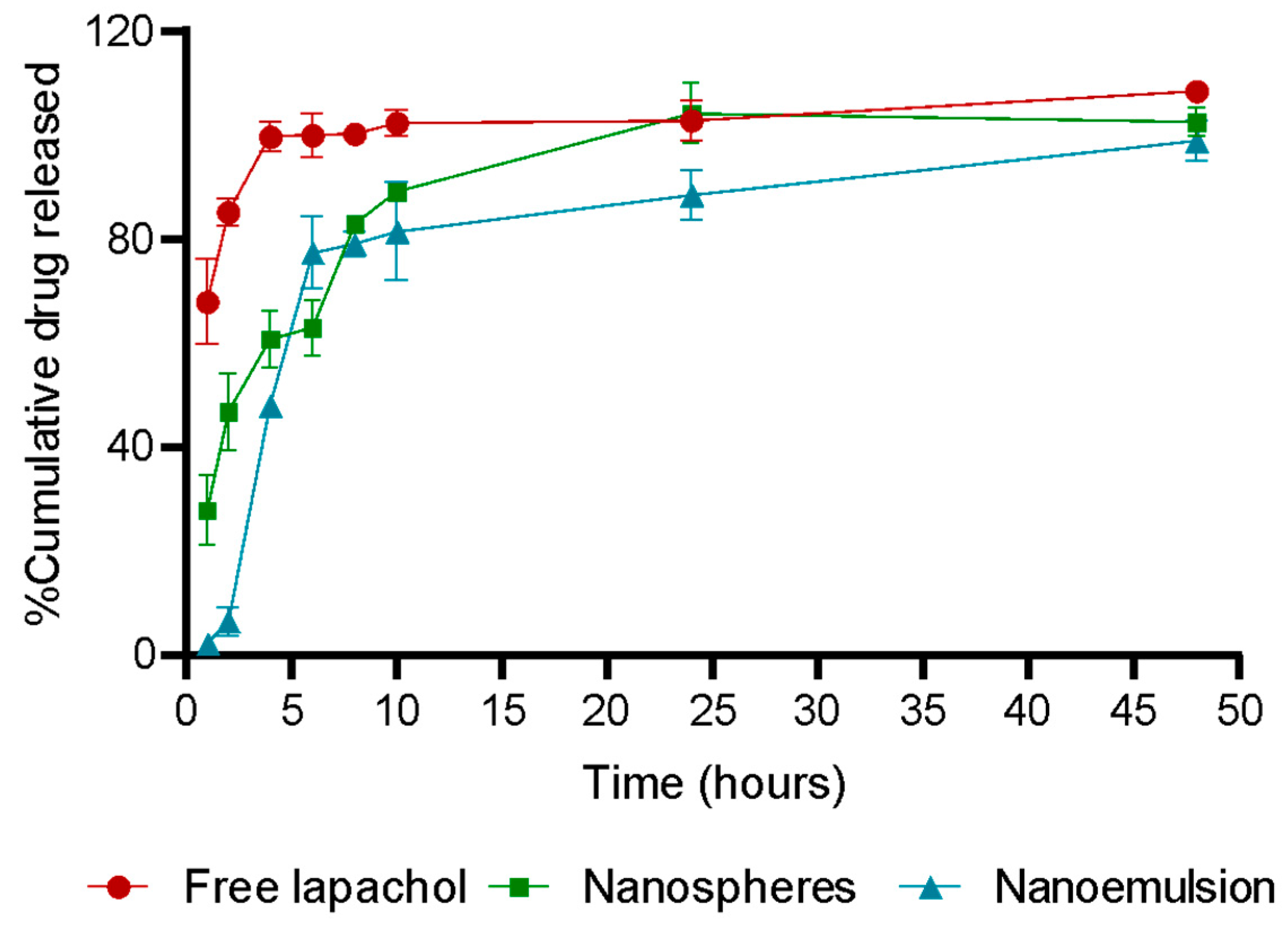
3.2. Encapsulation Efficiency and Drug Release
3.3. Chemical Stability of Lapachol in Formulation Obtention Process
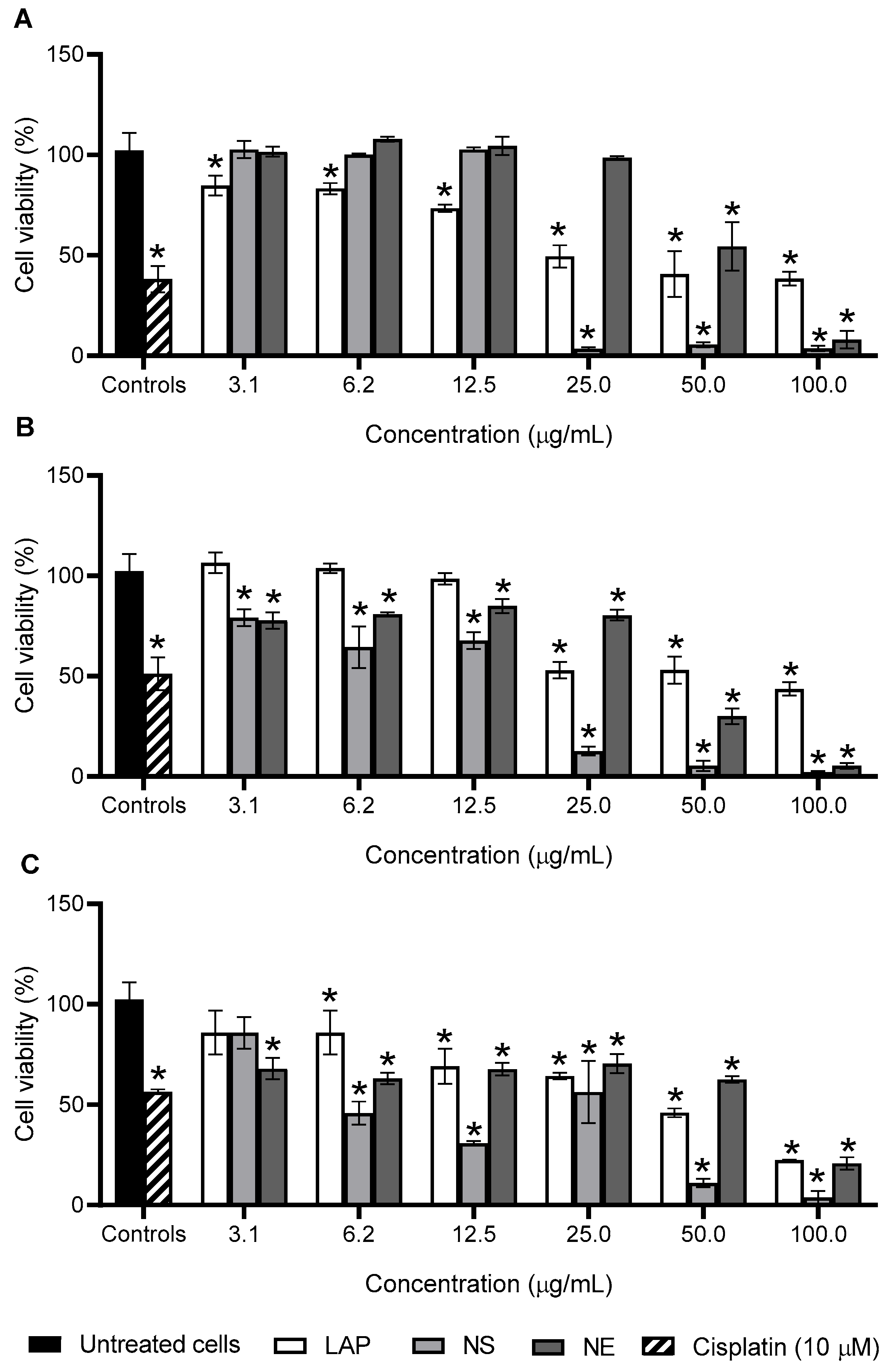
3.4. Antiproliferative Activity
3.5. In Vitro Selectivity and Ex Vivo Toxicity
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapoor, N.; Kandwal, P.; Sharma, G.; Gambhir, L. Redox ticklers and beyond: Naphthoquinone repository in the spotlight against inflammation and associated maladies. Pharmacol. Res. 2021, 174, 105968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hunto, S.T.; Yang, Y.; Lee, J.; Cho, J.Y. Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules 2020, 25, 4294. [Google Scholar] [CrossRef] [PubMed]
- Gómez Castellanos, J.R.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brandão, G.C.; Missias, F.C.R.; Arantes, L.M.; Soares, L.F.; Roy, K.K.; Doerksen, R.J.; de Oliveira, A.B.; Pereira, G.R. Antimalarial naphthoquinones. Synthesis via click chemistry, in vitro activity, docking to PfDHODH and SAR of lapachol-based compounds. Eur. J. Med. Chem. 2018, 145, 191–205. [Google Scholar] [CrossRef]
- da Sousa, L.d.S.; Correia, T.S.; Dos Farias, F.d.S.; Santana, M.D.F.; Lara, T.S. Influence of arbuscular mycorrhizal fungi density on growth and metabolism of Handroanthus serratifolius (Vahl) S.O. Grose seedlings. Physiol. Plant. 2023, 175, e14067. [Google Scholar] [CrossRef]
- da Silva Júnior, E.N.; Jardim, G.A.M.; Jacob, C.; Dhawa, U.; Ackermann, L.; de Castro, S.L. Synthesis of quinones with highlighted biological applications: A critical update on the strategies towards bioactive compounds with emphasis on lapachones. Eur. J. Med. Chem. 2019, 179, 863–915. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R. Lapachol and lapachone analogs: A journey of two decades of patent research (1997–2016). Expert Opin. Ther. Patents 2017, 27, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini-Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef]
- Zu, X.; Xie, X.; Zhang, Y.; Liu, K.; Bode, A.M.; Dong, Z.; Kim, D.J. Lapachol is a novel ribosomal protein S6 kinase 2 inhibitor that suppresses growth and induces intrinsic apoptosis in esophageal squamous cell carcinoma cells. Phytother. Res. 2019, 33, 2337–2346. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, L.; Lu, C.; Li, X.; Wu, Y.; Zhang, X.; Shen, Y. Lapachol inhibits the growth of lung cancer by reversing M2-like macrophage polarization via activating NF-κB signaling pathway. Cell. Signal. 2023, 112, 110902. [Google Scholar] [CrossRef]
- Mendes Miranda, S.E.; Lemos, J.d.A.; Fernandes, R.S.; Silva, J.d.O.; Ottoni, F.M.; Townsend, D.M.; Rubello, D.; Alves, R.J.; Cassali, G.D.; Ferreira, L.A.M.; et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed. Pharmacother. 2021, 133, 110936. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.-M. Effect of Lapachol on the Inhibition of Matrix Metalloproteinase Related to the Invasion of Human Fibrosarcoma Cells. Curr. Mol. Pharmacol. 2021, 14, 620–626. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Q.; Wang, H.; Xu, P.; Yuan, R.; Li, X.; Bai, L.; Xue, M. Inhibitory effects of lapachol on rat C6 glioma in vitro and in vivo by targeting DNA topoisomerase I and topoisomerase II. J. Exp. Clin. Cancer Res. 2016, 35, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Bai, L.; Zhou, X.; Xu, P.; Li, X.; Xu, H.; Zheng, Y.; Zhao, Y.; Lu, S.; Xue, M. Development of long-circulating lapachol nanoparticles: Formation, characterization, pharmacokinetics, distribution and cytotoxicity. RSC Adv. 2020, 10, 30025–30034. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, A.; Manchanda, R.; Kumari, M. Lipid-engineered nanotherapeutics for cancer management. Front. Pharmacol. 2023, 14, 1125093. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Fang, C.-L.; Chen, C.-H.; Fang, J.-Y. Permeation enhancer-containing water-in-oil nanoemulsions as carriers for intravesical cisplatin delivery. Pharm. Res. 2009, 26, 2314–2323. [Google Scholar] [CrossRef]
- Rinaldi, F.; Maurizi, L.; Forte, J.; Marazzato, M.; Hanieh, P.N.; Conte, A.L.; Ammendolia, M.G.; Marianecci, C.; Carafa, M.; Longhi, C. Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells. Nanomaterials 2021, 11, 1569. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.-S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Min, K.H.; Min, D.S.; Kim, A.Y.; Lee, C.-M.; Cho, Y.W.; Lee, S.C.; Kim, K.; Jeong, S.Y.; Park, K.; et al. Hydrotropic oligomer-conjugated glycol chitosan as a carrier of paclitaxel: Synthesis, characterization, and in vivo biodistribution. J. Control. Release 2009, 140, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Junges, A.; Monteiro, W.F.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; et al. Chitosan and chitosan/PEG nanoparticles loaded with indole-3-carbinol: Characterization, computational study and potential effect on human bladder cancer cells. Mater. Sci. Eng. C 2021, 124, 112089. [Google Scholar] [CrossRef]
- Winnicka, A.; Brzeszczyńska, J.; Saluk, J.; Wigner-Jeziorska, P. Nanomedicine in Bladder Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 10388. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, J.; Yu, Z.; Lu, J.; Sun, Z.; Chen, Z. Redefining bladder cancer treatment: Innovations in overcoming drug resistance and immune evasion. Front. Immunol. 2025, 16, 1537808. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, S.; Lai, W.-F.; Zhang, D. The Progress of Chitosan-Based Nanoparticles for Intravesical Bladder Cancer Treatment. Pharmaceutics 2023, 15, 211. [Google Scholar] [CrossRef]
- Lu, S.; Xu, L.; Kang, E.T.; Mahendran, R.; Chiong, E.; Neoh, K.G. Co-delivery of peptide-modified cisplatin and doxorubicin via mucoadhesive nanocapsules for potential synergistic intravesical chemotherapy of non-muscle-invasive bladder cancer. Eur. J. Pharm. Sci. 2016, 84, 103–115. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Hou, J.; Sun, B.; Zhu, B.; Qiao, Z.; Su, Y.; Zhu, X. Paclitaxel/Chitosan Nanosupensions Provide Enhanced Intravesical Bladder Cancer Therapy with Sustained and Prolonged Delivery of Paclitaxel. ACS Appl. Bio Mater. 2018, 1, 1992–2001. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Shan, X.; Xu, T.; Liu, Z.; Hu, X.; Zhang, Y.-D.; Wang, B. Safety and toxicology of the intravenous administration of Ang2-siRNA plasmid chitosan magnetic nanoparticles. Mol. Med. Rep. 2017, 15, 736–742. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef]
- Masalova, O.; Kulikouskaya, V.; Shutava, T.; Agabekov, V. Alginate and Chitosan Gel Nanoparticles for Efficient Protein Entrapment. Phys. Procedia 2013, 40, 69–75. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Amparo, T.R.; Sousa, L.R.D.; Xavier, V.F.; Seibert, J.B.; Paiva, D.L.; Silva, D.d.S.d.; Teixeira, L.F.d.M.; dos Santos, O.D.H.; Vieira, P.M.d.A.; de Souza, G.H.B.; et al. Protium spruceanum Extract Enhances Mupirocin Activity When Combined with Nanoemulsion-Based Hydrogel: A Multi-Target Strategy for Treating Skin and Soft Tissue Infections. Pharmaceutics 2024, 16, 700. [Google Scholar] [CrossRef]
- Špaglová, M.; Čuchorová, M.; Čierna, M.; Poništ, S.; Bauerová, K. Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels. Gels 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Q2A Guideline Validation of Analytical Methods Definitions and Terminology; (CPMP/ICH/381/95); Validation of Analytical Procedures: Definitions and Terminology; European Medicines Agency: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Vaz, L.B.A.; Amparo, T.R.; Reis, A.C.C.; Silva, B.d.M.; Magalhães, C.L.d.B.; Kohlhoff, M.; Brandão, G.C. Identification, characterization and quantification of xanthones from Fridericia formosa leaves extract with antiviral activity. Sci. Rep. 2024, 14, 2258. [Google Scholar] [CrossRef]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel—A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Jonjaroen, V.; Jitrakorn, S.; Charoonnart, P.; Kaewsaengon, P.; Thinkohkaew, K.; Payongsri, P.; Surarit, R.; Saksmerprome, V.; Niamsiri, N. Optimizing chitosan nanoparticles for oral delivery of double-stranded RNA in treating white spot disease in shrimp: Key insights and practical implications. Int. J. Biol. Macromol. 2025, 290, 138970. [Google Scholar] [CrossRef]
- Filho, I.K.; Machado, C.S.; Diedrich, C.; Karam, T.K.; Nakamura, C.V.; Khalil, N.M.; Mainardes, R.M. Optimized Chitosan-Coated Gliadin Nanoparticles Improved the Hesperidin Cytotoxicity over Tumor Cells. Braz. Arch. Biol. Technol. 2021, 64, e21200795. [Google Scholar] [CrossRef]
- Dadashi, H.; Vandghanooni, S.; Karamnejad-Faragheh, S.; Karimian-Shaddel, A.; Eskandani, M.; Jahanban-Esfahlan, R. A rapid protocol for synthesis of chitosan nanoparticles with ideal physicochemical features. Heliyon 2024, 10, e32228. [Google Scholar] [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A.; Navarro-Marchal, S.A.; Galisteo-González, F. Lipid-core nanoparticles: Classification, preparation methods, routes of administration and recent advances in cancer treatment. Adv. Colloid Interface Sci. 2023, 314, 102871. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- Simpson, E.; Sarwar, H.; Jack, I.; Lowry, D. Evaluation of the Potential of Chitosan Nanoparticles as a Delivery Vehicle for Gentamicin for the Treatment of Osteomyelitis. Antibiotics 2024, 13, 208. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Tsai, M.-J.; Chang, L.-C.; Wu, P.-C. Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy. Pharmaceutics 2020, 12, 949. [Google Scholar] [CrossRef]
- Rodrigues, F.; Diniz, L.; Sousa, R.; Honorato, T.; Simão, D.; Araújo, C.; Gonçalves, T.; Rolim, L.; Goto, P.; Tedesco, A.; et al. preparation and characterization of nanoemulsion containing a natural naphthoquinone. Quim. Nova 2018, 41, 756–761. [Google Scholar] [CrossRef]
- Segoloni, E.; Di Maria, F. UV–VIS spectral and GC–MS characterization of Handroanthus serratifolius (Vahl.) Grose (a.k.a. Tabebuia serratifolia (Vahl.) Nichols/Lapacho) heartwood main extractives: A comparison of protocols aimed at a practical evaluation of Lapachol and Dehydro-α-Lapachone content. Eur. J. Wood Wood Prod. 2018, 76, 1547–1561. [Google Scholar] [CrossRef]
- Montazeri, S.; Rastegari, A.; Mohammadi, Z.; Nazari, M.; Yousefi, M.; Samadi, F.Y.; Najafzadeh, S.; Aghsami, M. Chitosan nanoparticle loaded by epidermal growth factor as a potential protein carrier for wound healing: In vitro and in vivo studies. IET Nanobiotechnology 2023, 17, 204–211. [Google Scholar] [CrossRef]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-A.; Wen, K.-C.; Liu, J.-H.; Zhang, C.; Zhang, F.; Li, F.-Q. Strategies for intravesical drug delivery: From bladder physiological barriers and potential transport mechanisms. Acta Pharm. Sin. B 2024, 14, 4738–4755. [Google Scholar] [CrossRef] [PubMed]
- Amparo, T.R.; Almeida, T.C.; Sousa, L.R.D.; Xavier, V.F.; da Silva, G.N.; Brandão, G.C.; dos Santos, O.D.H. Nanostructured Formulations for a Local Treatment of Cancer: A Mini Review About Challenges and Possibilities. Pharmaceutics 2025, 17, 205. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Hassanpour, M.; Jafari, H.; Sharifi, S.; Rezaie, J.; Lighvan, Z.M.; Mahdavinia, G.R.; Gohari, G.; Akbari, A. Salicylic acid-loaded chitosan nanoparticles (SA/CTS NPs) for breast cancer targeting: Synthesis, characterization and controlled release kinetics. J. Mol. Struct. 2021, 1245, 131040. [Google Scholar] [CrossRef]
- de Oliveira, T.D.; Cabeza, N.A.; da Silva, G.T.S.T.; Ruiz, A.L.T.G.; Caires, A.R.L.; da Silveira, R.G.; Rodrigues, D.C.M.; Fiorucci, A.R.; dos Anjos, A. Coordination of the natural ligand lapachol to iron(II): Synthesis, theoretical study and antiproliferative activity. Transit. Met. Chem. 2021, 46, 111–120. [Google Scholar] [CrossRef]
- Maurizii, G.; Moroni, S.; Núnez, J.V.J.; Curzi, G.; Tiboni, M.; Aluigi, A.; Casettari, L. Non-invasive peptides delivery using chitosan nanoparticles assembled via scalable microfluidic technology. Carbohydr. Polym. Technol. Appl. 2024, 7, 100424. [Google Scholar] [CrossRef]
- Apollo, A.; Ortenzi, V.; Scatena, C.; Zavaglia, K.; Aretini, P.; Lessi, F.; Franceschi, S.; Tomei, S.; Sepich, C.A.; Viacava, P.; et al. Molecular characterization of low grade and high grade bladder cancer. PLoS ONE 2019, 14, e0210635. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Z.; Chen, W.; Li, D.; Zhang, H.; Zhu, Y.; Mo, Q.; Zhao, X.; Fan, Q.; Deng, F.; et al. Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist. Updates 2023, 68, 100938. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Pajaniradje, S.; Nair, A.S.; Chandramohan, S.; Bhat, S.A.; Manikandan, E.; Rajagopalan, R. An in-vitro study of active targeting & anti-cancer effect of folic acid conjugated chitosan encapsulated indole curcumin analogue nanoparticles. Int. J. Biol. Macromol. 2024, 282, 136990. [Google Scholar] [CrossRef]
- Duarte, J.L.; Di Filippo, L.D.; Vilella, K.J.A.; Dutra, J.A.P.; Ribeiro, D.M.; da Silva, M.F.; de Medeiros, A.I.; Chorilli, M. Chitosan-coated nanoemulsion for intranasal administration increases temozolomide mucosal permeation, cellular uptake, and In vitro cytotoxicity in glioblastoma multiforme cells. J. Drug Deliv. Sci. Technol. 2024, 102, 106390. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Bhirud, D.; Bhattacharya, S.; Raval, H.; Sangave, P.C.; Gupta, G.L.; Paraskar, G.; Jha, M.; Sharma, S.; Belemkar, S.; Kumar, D.; et al. Chitosan nanoparticles of imatinib mesylate coated with TPGS for the treatment of colon cancer: In-vivo & in-vitro studies. Carbohydr. Polym. 2025, 348, 122935. [Google Scholar] [CrossRef]
- Barbosa, F.; Araújo, J.; Gonçalves, V.M.F.; Palmeira, A.; Cunha, A.; Silva, P.M.A.; Fernandes, C.; Pinto, M.; Bousbaa, H.; Queirós, O.; et al. Evaluation of Antitumor Activity of Xanthones Conjugated with Amino Acids. Int. J. Mol. Sci. 2024, 25, 2121. [Google Scholar] [CrossRef]
- Pereira, D.I.; Amparo, T.R.; Almeida, T.C.; Costa, F.S.F.; Brandão, G.C.; dos Santos, O.D.H.; da Silva, G.N.; de Souza, G.H.B. Cytotoxic activity of butanolic extract from Sambucus nigra L. flowers in natura and vehiculated in micelles in bladder cancer cells and fibroblasts. Nat. Prod. Res. 2020, 36, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; McNeil, A.K.; Novak, I.; McNeil, P.L.; Gehl, J. Difference in Membrane Repair Capacity Between Cancer Cell Lines and a Normal Cell Line. J. Membr. Biol. 2016, 249, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Şenyiğit, Z.A.; Karavana, S.Y.; Ozdemir, D.I.; Caliskan, C.; Waldner, C.; Sen, S.; Bernkop-Schnürch, A.; Baloglu, E. Design and evaluation of an intravesical delivery system for superficial bladder cancer: Preparation of gemcitabine HCl-loaded chitosan–thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers. Int. J. Nanomed. 2015, 10, 6493–6507. [Google Scholar] [CrossRef] [PubMed]

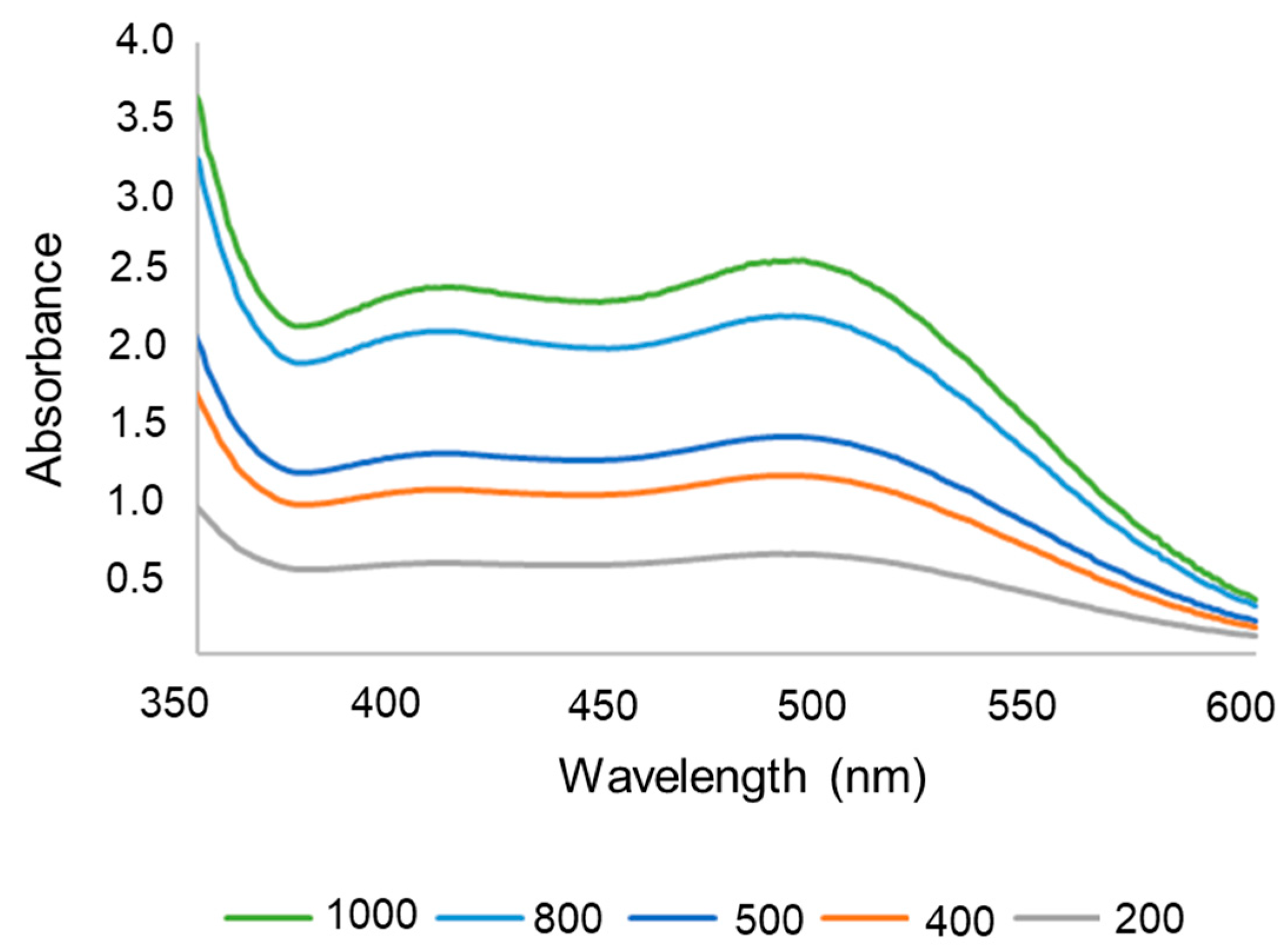

| Linearity | |
| r2 | 0.9992 |
| Equation | y = 0.0024x + 0.1075 |
| Significance | p = < 0.0001 (a ≠ 0) |
| Linearity | p = 1.0000 (linear) |
| Precision (repeatability) | |
| Concentrations (µg mL−1) | RSD |
| 100 | 2.99 |
| 200 | 1.81 |
| 400 | 1.68 |
| 500 | 2.04 |
| 800 | 1.56 |
| 1000 | 1.13 |
| Accuracy | |
| Concentrations (µg mL−1) | RSD |
| 100 | 2.99 |
| 500 | 2.04 |
| 1000 | 1.13 |
| Sample | Kinetic Models (R2) | |||
|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |
| Nanospheres | 0.5709 | 0.4760 | 0.7672 | 0.8820 |
| Nanoemulsion | 0.4454 | 0.2715 | 0.6381 | 0.7200 |
| Cell Lines (CC50 µg/mL) | |||
|---|---|---|---|
| T24 | J82 | RT4 | |
| Free lapachol | 42.9 ± 6.1 a | 35.9 ± 2.0 a | 32.7 ± 3.8 a |
| Nanospheres | 6.5 ± 1.4 b | 10.1 ± 1.3 b | 14.7 ± 1.8 b |
| Nanoemulsion | 9.4 ± 1.4 b | 29.0 ± 3.7 b | 38.4 ± 9.1 a |
| MRC-5 CC50 (µg/mL) | Selectivity Index | |||
|---|---|---|---|---|
| T24 | J82 | RT4 | ||
| Free lapachol | 15.7 ± 0.7 a | 0.37 ± 0.05 a | 0.44 ± 0.02 a | 0.48 ± 0.06 a |
| Nanospheres | 6.2 ± 0.9 b | 0.99 ± 0.20 b | 0.62 ± 0.08 b | 0.43 ± 0.05 b |
| Nanoemulsion | 5.5 ± 0.2 b | 0.60 ± 0.09 a | 0.19 ± 0.03 b | 0.15 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amparo, T.R.; Anunciação, K.d.F.d.; Almeida, T.C.; Sousa, L.R.D.; Xavier, V.F.; Seibert, J.B.; Barboza, A.P.M.; Vieira, P.M.d.A.; dos Santos, O.D.H.; da Silva, G.N.; et al. Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines. Pharmaceutics 2025, 17, 868. https://doi.org/10.3390/pharmaceutics17070868
Amparo TR, Anunciação KdFd, Almeida TC, Sousa LRD, Xavier VF, Seibert JB, Barboza APM, Vieira PMdA, dos Santos ODH, da Silva GN, et al. Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines. Pharmaceutics. 2025; 17(7):868. https://doi.org/10.3390/pharmaceutics17070868
Chicago/Turabian StyleAmparo, Tatiane Roquete, Kamila de Fátima da Anunciação, Tamires Cunha Almeida, Lucas Resende Dutra Sousa, Viviane Flores Xavier, Janaína Brandão Seibert, Ana Paula Moreira Barboza, Paula Melo de Abreu Vieira, Orlando David Henrique dos Santos, Glenda Nicioli da Silva, and et al. 2025. "Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines" Pharmaceutics 17, no. 7: 868. https://doi.org/10.3390/pharmaceutics17070868
APA StyleAmparo, T. R., Anunciação, K. d. F. d., Almeida, T. C., Sousa, L. R. D., Xavier, V. F., Seibert, J. B., Barboza, A. P. M., Vieira, P. M. d. A., dos Santos, O. D. H., da Silva, G. N., & Brandão, G. C. (2025). Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines. Pharmaceutics, 17(7), 868. https://doi.org/10.3390/pharmaceutics17070868






