Application of Gene Therapy to Oral Diseases
Abstract
1. Introduction
1.1. Background and Rationale
1.2. Global Disease Burden
1.3. Review Structure and Roadmap
2. Gene Therapy Fundamentals
2.1. Definition and Principles
2.2. Gene Transfer Technologies
- Biological methods: Introducing genes via genetically modified viral vectors such as adenovirus, retrovirus, and AAV.
- Chemical methods: Using non-viral compound vectors like cationic lipids, polymers, and calcium phosphate to neutralize or provide positive charge to negatively charged nucleic acids.
- Physical methods: Directly delivering nucleic acids into the cell cytoplasm or nucleus, with electroporation being a representative technique.
2.2.1. Viral Vector Systems
2.2.2. Non-Viral Vector Systems
2.2.3. Physical Delivery Methods
2.3. Delivery Approaches
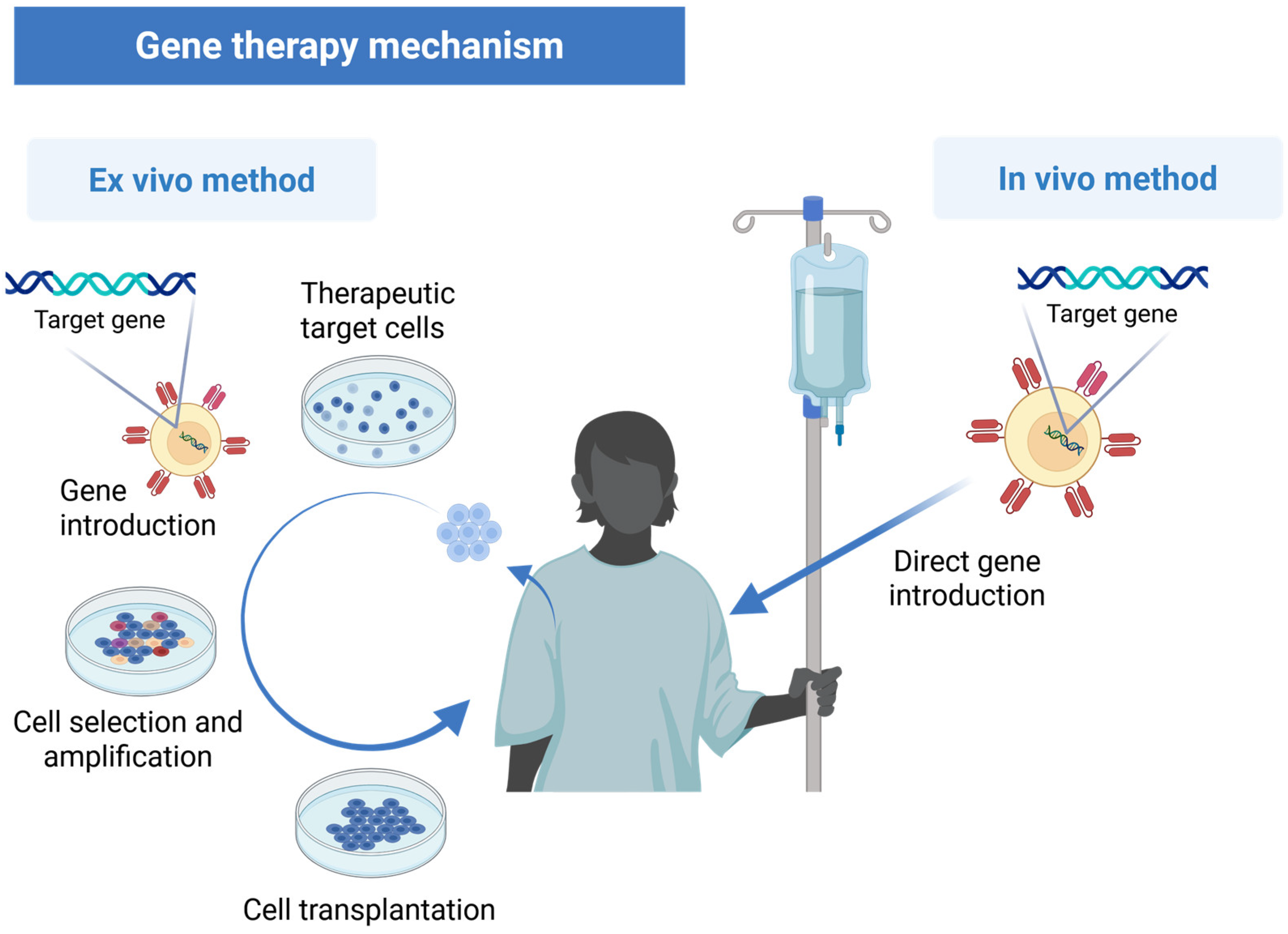
2.3.1. In Vivo vs. Ex Vivo Methods
2.3.2. Vector Selection for Specific Oral Applications
Decision Framework for Vector Selection
- Target tissue characteristics: Epithelial vs. mesenchymal vs. neural tissues.
- Expression requirements: Transient (days–weeks) vs. sustained (months–years).
- Accessibility: Surface-accessible vs. deep tissue targeting.
- Safety profile: Local vs. systemic exposure tolerance.
- Manufacturing feasibility: Clinical-grade production capabilities [38].
2.3.3. Oral-Specific Delivery Considerations
- Nuclease activity: Saliva contains DNases and RNases that rapidly degrade nucleic acids [52].
- Dilution effects: Continuous salivary flow (0.5–1.5 mL/min) dilutes locally administered vectors [37].
- pH variability: Fluctuations between 5.5 and 7.5 affect vector stability and cellular uptake.
- Solution strategies: Protective formulations, mucoadhesive carriers, and enzyme inhibitors.
- Epithelial tight junctions: Barrier to paracellular transport of large vectors.
- Mucus layer: Physical obstruction requiring penetration-enhancing strategies.
- Cellular turnover: Rapid epithelial renewal (7–14 days) limits sustained expression.
2.4. Historical Development and Current Status
3. Cancer Gene Therapy
3.1. Advances in Targeting Mechanisms
- Tumor-specific promoters: Using promoters that are active only in cancer cells to drive the expression of therapeutic genes, thereby limiting gene expression to the tumor microenvironment [77].
- Cancer-specific microRNA (miRNA) targeting: Incorporating miRNA target sequences into therapeutic constructs to enable post-transcriptional regulation of gene expression specifically in cancer cells [78].
- Dual-targeting strategies: Combining multiple targeting approaches, such as cell surface receptors and intracellular factors, to improve the specificity and efficacy of gene therapy [79].
3.2. RNA Interference and CRISPR Applications
- RNAi-based approaches: Small interfering RNAs (siRNAs) and short hairpin RNAs (shRNAs) have been used to silence oncogenes and cancer-promoting factors. Recent clinical trials have shown promising results in using RNAi to target previously “undruggable” oncogenes in head and neck cancers [80].
- CRISPR-Cas9 applications: This technology allows for precise genome editing, enabling the correction of oncogenic mutations, knockout of oncogenes, or enhancement of tumor suppressor genes. Preclinical studies have demonstrated the potential of CRISPR-Cas9 for targeting oral cancer cells with specific genetic alterations [81].
3.3. Combination Approaches
- Chemovirotherapy: Combining oncolytic viruses with chemotherapy to achieve synergistic effects. This approach has shown promising results in clinical trials for head and neck squamous cell carcinoma [82].
- Radiovirotherapy: Using gene therapy in conjunction with radiation therapy to enhance radiosensitization of cancer cells. This strategy has demonstrated improved outcomes in preclinical studies of oral cancer [83].
- Immunogene therapy: Combining gene therapy with immunotherapy approaches, such as immune checkpoint inhibitors, to enhance anti-tumor immune responses. Recent clinical trials have reported improved outcomes with this combination approach [42].
3.4. mRNA Therapeutics for Oral Cancer
3.4.1. Current Approaches
- Tumor antigen vaccines: Lipid nanoparticle-encapsulated mRNA encoding tumor-specific antigens (such as human papillomavirus [HPV] E6/E7 in HPV-positive oral cancers) has shown promise in preclinical studies. This approach stimulates tumor-specific immune responses without the safety concerns associated with viral vectors. Recent Phase I trials have demonstrated the safety and immunogenicity of these approaches in head and neck cancers [69].
- Cytokine mRNA delivery: Local delivery of mRNAs encoding immunostimulatory cytokines (interleukin 12 [IL-12] and interferon γ [IFN-γ]) can reshape the tumor microenvironment from immunosuppressive to immunostimulatory. Intratumoral administration of IL-12 mRNA in preclinical oral cancer models demonstrated significant tumor regression and increased CD8+ T-cell infiltration [84].
- Tumor suppressor replacement: Transient expression of tumor suppressor proteins through mRNA delivery offers a potential alternative to viral-mediated gene replacement therapy. This approach is particularly relevant for p53-deficient oral cancers, where even temporary restoration of p53 function may sensitize cells to conventional treatments.
3.4.2. Delivery Innovations
3.4.3. Clinical Translation
3.5. Resistance Mechanisms and Strategies to Overcome Them
3.5.1. Vector-Related Resistance Mechanisms
- Use of alternative viral serotypes with lower seroprevalence;
- Immunosuppressive protocols during treatment;
- Vector modification to evade immune recognition;
- Switching to non-viral delivery systems for subsequent treatments.
- Protective formulations using polymer coatings or encapsulation;
- Co-administration of enzyme inhibitors;
- Modified delivery schedules to overwhelm clearance mechanisms;
- Local delivery techniques that bypass salivary exposure.
3.5.2. Cellular Resistance Mechanisms
- Receptor-independent delivery methods (electroporation, ultrasound);
- Vector retargeting using alternative cellular receptors;
- Cell-penetrating peptides to enhance membrane permeability;
- Combination with permeabilization agents.
- Combination therapy targeting multiple pathways simultaneously;
- DNA repair inhibitors to sensitize cells to therapeutic genes;
- Alternative therapeutic targets independent of p53 pathways;
- Personalized approaches based on genetic profiling [88].
3.5.3. Tumor Microenvironment Resistance
- Combination with immune checkpoint inhibitors;
- Local delivery of immunostimulatory cytokines (IL-12, IFN-γ);
- Depletion of regulatory immune cells;
- Microenvironment reprogramming using multiple therapeutic modalities [89].
4. Gene Therapy for Oral Cancer Pain
4.1. Gene Therapy Approaches for Cancer Pain
- Endothelin B receptor gene therapy: High methylation of endothelin B receptor genes in oral cancer tissues has been identified as a contributing factor to cancer pain. In mouse models of oral cancer, gene transfer techniques to restore endothelin B receptor expression have shown promising results in reducing pain behaviors through the induction of β-endorphin secretion [99,100,101].
- μ-opioid receptor gene therapy: Research has demonstrated that the μ-opioid receptor (OPRM1) gene is often hypermethylated in oral cancer lesions, contributing to increased pain sensitivity. Non-viral vector-mediated delivery of the OPRM1 gene to cancer tissues has shown efficacy in preclinical models, reducing cancer-related pain without the systemic side effects associated with traditional opioid medications [43].
- RNAi approaches: siRNA and shRNA technologies targeting pain-mediating factors such as transient receptor potential vanilloid 1, Nav1.7, and inflammatory cytokines have shown promise in preclinical studies of cancer pain [100]. These approaches allow for specific silencing of pain-promoting genes, offering a more targeted approach to pain management.
- CRISPR-based interventions: Emerging research is exploring the potential of CRISPR-Cas9 technology to modify genes involved in cancer pain signaling pathways. This approach offers the advantage of permanent genetic modifications that could provide long-term pain relief [101].
4.2. Delivery Methods for Oral Cancer Pain Gene Therapy
- Viral vectors: AAV vectors have shown particular promise for delivering pain-modulating genes to sensory neurons and cancer microenvironments. Recent modifications to AAV capsids have improved their tropism for sensory neurons involved in pain transmission [45].
- Non-viral vectors: Advances in non-viral delivery systems, such as lipid nanoparticles, dendrimers, and cell-penetrating peptides, have improved the efficiency of gene delivery while reducing the risks associated with viral vectors [44].
- Local delivery approaches: Localized delivery techniques, including direct intratumoral injection, peritumoral administration, and intraganglionic delivery, have been refined to enhance therapeutic efficacy while minimizing systemic exposure [15].
4.3. Clinical Translation and Future Directions
- Early-phase clinical trials: Several Phase I/II clinical trials are underway to evaluate the safety and preliminary efficacy of gene therapy approaches for cancer pain, including those targeting oral cancer pain [102].
- Personalized approaches: Advances in genetic profiling and biomarker identification are enabling more personalized gene therapy approaches for cancer pain, taking into account individual genetic variations in pain sensitivity and response to therapy [88].
- Combination strategies: Emerging research supports the use of gene therapy in combination with conventional pain management approaches, potentially allowing for reduced dosages of traditional analgesics while maintaining or improving pain control [87].
5. Gene Therapy for Xerostomia
5.1. Advances in AQP1 Gene Therapy
- Clinical trial successes: The first-in-human clinical trial using adenoviral-mediated AQP1 gene transfer (AdhAQP1) for radiation-induced xerostomia showed positive results, with increased parotid flow rates in a subset of treated patients. Long-term follow-up data confirmed both the safety and durability of this approach [14].
- Vector improvements: Next-generation vectors, including serotype 2 AAV (AAV2) vectors carrying the AQP1 gene, have shown enhanced safety profiles and prolonged gene expression compared to adenoviral vectors [18].
- Delivery optimization: Ultrasound-assisted non-viral gene transfer techniques have improved the efficiency of AQP1 delivery to salivary glands, offering a potentially safer alternative to viral vectors [106].
5.2. Alternative Gene Therapy Approaches for Xerostomia
- Anti-inflammatory cytokine gene therapy: For Sjögren’s syndrome mouse models, successful treatments include anti-inflammatory cytokine IL-10 gene delivery via AAV, which helps to modulate the autoimmune response and reduce inflammatory damage to salivary glands [107].
- Vasoactive intestinal peptide (VIP) gene therapy: VIP gene transfer has shown efficacy in murine models of Sjögren’s syndrome, with improvements in salivary flow and reductions in lymphocytic infiltration of salivary glands [108].
- Neurotrophic factor gene therapy: Delivery of genes encoding neurotrophic factors, such as neurturin and glial cell line-derived neurotrophic factor, has demonstrated potential for protecting salivary gland innervation from radiation damage [109].
5.3. Stem Cell-Based Approaches
- Gene-modified mesenchymal stem cells: Mesenchymal stem cells (MSCs) engineered to express therapeutic genes, such as AQP1, IL-10, or growth factors, have shown enhanced efficacy in preclinical models of xerostomia [110].
- Induced pluripotent stem cells (iPSCs): iPSC-derived salivary gland cells, combined with gene modification techniques, offer a promising approach for personalized regenerative therapy for damaged salivary glands [111].
- Salivary gland organoids: Recent advances in organoid technology have enabled the generation of salivary gland organoids from stem cells, providing a valuable platform for testing gene therapy approaches and studying salivary gland development and function [112].
5.4. Future Directions in Xerostomia Gene Therapy
- Temporospatial control of gene expression: Advanced gene delivery systems that allow for controlled expression of therapeutic genes in response to specific stimuli or in targeted cell populations are being developed [113].
- Combination therapies: Approaches combining gene therapy with conventional treatments, such as pilocarpine or cevimeline, show potential for enhanced therapeutic outcomes [114].
- Preventive strategies: Gene therapy approaches applied before radiation treatment to protect salivary glands from damage are being explored, potentially offering a more effective approach than post-treatment interventions [115].
5.5. Complementary Lubrication Approaches in Xerostomia Management
5.5.1. Advanced Lubricant Formulations
5.5.2. Smart Hydrogel Systems
5.5.3. Integrated Approaches
6. Gene Therapy for Dental Caries and Periodontal Diseases
6.1. Advances in Gene Therapy for Dental Caries
- Antimicrobial peptide gene therapy: Delivery of genes encoding antimicrobial peptides, such as defensins and cathelicidins, has shown promise in reducing cariogenic bacterial loads and preventing dental caries in preclinical models [51].
- Salivary enzyme gene therapy: Gene therapy approaches targeting salivary enzymes involved in maintaining oral pH and remineralization processes have been investigated for their potential to prevent dental caries [119].
- Bacteriophage-based approaches: Engineered bacteriophages carrying CRISPR/Cas systems have been developed to specifically target and eliminate Streptococcus mutans, the primary cariogenic bacterium, while preserving beneficial oral microbiota [120].
6.2. Advances in Gene Therapy for Periodontal Diseases
6.2.1. Regenerative Approaches
- Growth factor gene therapy: Studies report success using non-viral vectors and electroporation to deliver genes encoding platelet-derived growth factor (PDGF) and bone morphogenetic proteins (BMPs) for hard tissue regeneration around teeth and implants [46,47,48]. These approaches have shown enhanced periodontal tissue regeneration compared to direct protein delivery, with prolonged local expression of growth factors.
- Scaffold-based gene delivery: Advanced biomaterial scaffolds incorporating gene delivery systems have been developed to provide both structural support and sustained release of therapeutic genes at the site of periodontal defects [123]. These systems enable spatiotemporal control of gene expression, facilitating the regeneration of complex periodontal structures.
- Cell-based gene delivery: Genetically modified MSCs overexpressing regenerative factors have shown enhanced potential for periodontal tissue regeneration. Recent studies have demonstrated that MSCs engineered to express BMP-7 or PDGF-B can significantly improve bone and periodontal ligament regeneration in animal models [49].
6.2.2. Immunomodulatory Approaches
- Anti-inflammatory cytokine gene therapy: Delivery of genes encoding anti-inflammatory cytokines, such as IL-4, IL-10, and IL-1 receptor antagonist, has shown efficacy in reducing periodontal inflammation and bone loss in preclinical models [124].
- RNA interference strategies: siRNA and miRNA approaches targeting inflammatory mediators have demonstrated potential for controlling periodontal inflammation and preventing tissue destruction [31].
6.3. Biomaterial Advances for Periodontal Gene Therapy
- Smart hydrogels: Stimuli-responsive hydrogels that can control the release of gene therapy vectors in response to specific environmental cues, such as pH changes or the presence of bacterial products, have been developed for periodontal applications [125].
- Three-dimensional-printed scaffolds: Computer-aided design and 3D printing technologies have enabled the production of patient-specific scaffolds incorporating gene delivery systems, optimized for individual periodontal defects [126].
- Nanoparticle-based delivery systems: Advanced nanoparticle formulations, including lipid nanoparticles, polymeric nanoparticles, and inorganic nanoparticles, have improved the stability and transfection efficiency of gene therapy vectors for periodontal applications [33].
6.4. Clinical Translation and Future Prospects
- Regulatory considerations: The regulatory pathway for gene therapy products for periodontal applications is becoming more defined, with several products entering early-phase clinical trials [127].
- Scale-up and manufacturing: Advances in biomanufacturing technologies are addressing challenges in the scale-up and production of gene therapy vectors for dental applications [58].
- Combination approaches: Integrating gene therapy with other treatment modalities, such as guided tissue regeneration, bioactive materials, and conventional pharmacotherapy, offers promising strategies for enhancing therapeutic outcomes [128].
7. Future Directions and Challenges in Oral Gene Therapy
7.1. Emerging Technologies and Approaches
7.1.1. CRISPR-Cas9 Applications in Oral Gene Therapy
7.1.2. Artificial Intelligence in Oral Gene Therapy
7.1.3. Exosome-Based Delivery Systems
7.2. Implementation Challenges
7.2.1. Oral-Specific Delivery Barriers
- Nuclease activity: Saliva contains DNases and RNases that rapidly degrade nucleic acids.
- Dilution effects: Continuous salivary flow (0.5–1.5 mL/min) dilutes locally administered vectors.
- pH variability: Fluctuations between 5.5 and 7.5 affect vector stability and cellular uptake.
- Solution strategies: Protective formulations, mucoadhesive carriers, and enzyme inhibitors.
- Mucosal immunity: Active immune surveillance in oral tissues.
- Previous viral exposure: Pre-existing immunity to common viral vectors.
- Inflammatory responses: Risk of exacerbating existing oral inflammatory conditions.
- Mitigation strategies: Immunosuppressive co-delivery, novel vector serotypes, and immunomodulatory approaches.
7.2.2. Manufacturing and Scalability Challenges
7.2.3. Economic Considerations and Healthcare Integration
7.2.4. Regulatory Pathways
- Vector optimization: Developing vectors with improved targeting specificity, reduced immunogenicity, and enhanced transduction efficiency for oral tissues remains a significant challenge [23].
- Delivery barriers: Overcoming biological barriers to gene delivery in the oral environment, including the mucosal barrier, salivary flow, and microbial biofilms, requires innovative approaches [36].
- Safety concerns: Addressing safety issues, such as off-target effects, insertional mutagenesis, and immune responses, remains a critical consideration for clinical translation [57].
- Regulatory hurdles: Navigating the complex regulatory landscape for gene therapy products presents challenges for clinical development and commercialization [133].
- Cost and accessibility: Ensuring the affordability and accessibility of gene therapy approaches, particularly in resource-limited settings, remains a significant challenge [132].
7.2.5. Patient-Reported Outcomes and Quality of Life Considerations
- Xerostomia Inventory (XI) for subjective dry mouth assessment;
- Oral Health Impact Profile (OHIP) for functional limitations;
- Summated Xerostomia Inventory (SXI) for symptom severity;
- Patient-reported eating, drinking, and speech difficulties.
- Brief Pain Inventory (BPI) for pain interference with daily activities;
- Oral Health-Related Quality of Life (OHRQoL) measures;
- Functional Assessment of Cancer Therapy—Head and Neck (FACT-H&N);
- Sleep quality and mood assessments related to pain management.
- Oral Health Impact Profile-14 (OHIP-14)
- Patient-reported masticatory function improvements;
- Aesthetic satisfaction scores;
- Social confidence and professional impact measures.
- Include baseline and longitudinal QOL measurements;
- Use validated instruments specific to oral conditions;
- Assess both disease-specific and general health-related quality of life;
- Incorporate the patient’s global impression of change scales;
- Evaluate the durability of QOL improvements beyond biological endpoints.
7.3. Ethical Considerations
- Informed consent: Ensuring that patients fully understand the risks, benefits, and limitations of gene therapy interventions is essential for ethical clinical practice [134].
- Equity and access: Addressing disparities in access to gene therapy treatments, particularly for marginalized and underserved populations, is a critical ethical consideration [135].
- Germline modifications: Distinguishing between somatic and germline genetic modifications and establishing appropriate boundaries for clinical applications remains an important ethical discussion [136].
- Long-term monitoring: Establishing protocols for long-term monitoring of patients receiving gene therapy treatments to assess delayed effects and outcomes is essential [137].
8. Conclusions
8.1. Current State Assessment
8.2. Translational Outlook
8.3. Future Integration with Precision Medicine
Author Contributions
Funding
Conflicts of Interest
References
- Blaese, R.M.; Culver, K.W.; Miller, A.D.; Carter, C.S.; Fleisher, T.; Clerici, M.; Shearer, G.; Chang, L.; Chiang, Y.; Tolstoshev, P.; et al. T lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after 4 years. Science 1995, 270, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Mendell, J.R.; Schultz, M.; Sproule, D.M.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Ragni, M.V.; Rasko, J.E.J.; Raffini, L.J.; Samelson-Jones, B.J.; Ozelo, M.; Hazbon, M.; Runte, A.; Recht, M.; Zhou, S.; et al. Long-term follow-up of AAV for gene transfer: AAV2-hFIX16 for severe hemophilia B. Mol. Ther. 2020, 28, 2073–2082. [Google Scholar] [CrossRef]
- Muramatsu, S.; Fujimoto, K.; Kato, S.; Mizukami, H.; Asari, S.; Ikeguchi, K.; Kawakami, T.; Urabe, M.; Kume, A.; Sato, T.; et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol. Ther. 2010, 18, 1731–1735. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.P.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Jin, S.; Zhu, M.; Yan, J.; Fang, X.; Zhang, H.; Shi, J. Gene therapy for periodontal tissue engineering: Current approaches and future perspectives. Front. Genet. 2022, 13, 1070131. [Google Scholar]
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene editing and CRISPR in the clinic: Current and future perspectives. Biosci. Rep. 2020, 40, BSR20200127. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- Wiley Database. Gene Therapy Clinical Trials Worldwide. Available online: https://www.genetherapynet.com/clinical-trials.html (accessed on 11 April 2025).
- Walther, W.; Stein, U. Viral vectors for gene transfer: A review of their use in the treatment of human diseases. Drugs 2000, 60, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral vectors for gene therapy: Translational and clinical outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [PubMed]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Billings, M.E.; Goldsmith, C.M.; Baum, B.J. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2016, 24, 176–186. [Google Scholar] [CrossRef]
- Keeler, A.M.; Flotte, T.R. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): Where are we, and how did we get here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Lai, Z.; Yin, H.; Cabrera-Pérez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.; Nogee, D.; et al. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef]
- Lombaert, I.; Poduval, P.; Jensen, D.H.; Moss, J.; Baum, B.J.; Pringle, S. Advances in salivary gland gene therapy-from bench to bedside. Nat. Rev. Drug Discov. 2023, 22, 629–647. [Google Scholar]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Dorr, U.; Benvenuto, M.; Belot, S.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; Basile, G.D.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.L.; et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D. Genetically Engineered Vaccinia Viruses As Agents for Cancer Treatment, Imaging, and Transgene Delivery. Front. Oncol. 2017, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Dai, J.; Hanatani, S.; Haku, K.; Yamanaka, T.; Ishioka, M.; Takayama, T.; Yuvienco, C.; Khapli, S.; Moursi, A.M.; et al. Long-term efficient gene delivery using polyethylenimine with modified Tat peptide. Biomaterials 2014, 35, 1707–1715. [Google Scholar] [CrossRef]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Yamano, S.; Dai, J.; Hanatani, S.; Haku, K.; Yamanaka, T.; Ishioka, M.; Takayama, T.; Yuvienco, C.; Khapli, S.; Moursi, A.M.; et al. Efficient in vivo gene delivery using modified Tat peptide with cationic lipids. Biotechnol. Lett. 2014, 36, 1447–1452. [Google Scholar] [CrossRef]
- Kasai, H.; Inoue, K.; Imamura, K.; Yuvienco, C.; Montclare, J.K.; Yamano, S. Efficient siRNA delivery and gene silencing using a lipopolypeptide hybrid vector mediated by a caveolae-mediated and temperature-dependent endocytic pathway. J. Nanobiotechnology 2019, 17, 11. [Google Scholar] [CrossRef]
- Yamano, S.; Dai, J.; Yuvienco, C.; Khapli, S.; Moursi, A.M.; Montclare, J.K. Modified Tat peptide with cationic lipids enhances gene transfection efficiency via temperature-dependent and caveolae-mediated endocytosis. J. Control. Release 2011, 152, 278–285. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Guo, J.; Li, Y.; Qu, Y.; Liu, J.; Chen, Y.; Bao, X.; Li, L.; Sun, Y. Effect of local hIL-10 gene therapy on experimental periodontitis in ovariectomized rats. Acta Odontol. Scand. 2017, 75, 268–277. [Google Scholar] [CrossRef]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, R.; Fang, Y.; Gu, H.; Wang, Y. Developing functional hydrogels for treatment of oral diseases. Smart Med. 2024, 3, e20240020. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, L.; Liu, B.; Tian, G.; Li, C.; Zhang, L.; Sang, X.; Wang, L.; Li, Y.; Chen, Z.; et al. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing. J. Nanobiotechnology 2022, 20, 291. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy- an overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- High, K.A.; Roncarolo, M.G. Gene therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Touchot, N.; Flume, M. Early insights from commercialization of gene therapies. Nat. Biotechnol. 2017, 35, 381–383. [Google Scholar]
- Chisholm, E.; Bapat, U.; Chisholm, C.; Alusi, G.; Vassaux, G. Gene therapy in head and neck cancer: A review. Postgrad. Med. J. 2007, 83, 731–737. [Google Scholar] [CrossRef]
- Gleich, L.L.; Salamone, F.N. Molecular genetics of head and neck cancer. Cancer Control 2002, 9, 369–378. [Google Scholar] [CrossRef]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: Principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Zhang, Y.; Wang, S.; Liu, Z.; Yang, L.; Liu, J.; Xiu, M. Recent advances in mRNA delivery systems for the treatment of oral and maxillofacial diseases. Exploration 2023, 3, 20210146. [Google Scholar] [CrossRef]
- Yamano, S.; Viet, C.T.; Dang, D.; Dai, J.; Hanatani, S.; Takayama, T.; Kasai, H.; Inoue, K.; Imamura, K.; Yuvienco, C.; et al. Ex vivo nonviral gene delivery of μ-opioid receptor to attenuate cancer-induced pain. Pain 2017, 158, 240–251. [Google Scholar] [CrossRef]
- Guedon, J.M.; Wu, S.; Zheng, X.; Churchill, C.C.; Glorioso, J.C.; Liu, C.H.; McArdle, J.J.; Fink, D.J. Current gene therapy using viral vectors for chronic pain. Mol. Pain 2015, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Ehlert, E.M.; Eggers, R.; Pool, C.W.; Hermening, S.; Huseinovic, A.; Timmermans, E.; Blits, B.; Verhaagen, J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol. Ther. 2010, 18, 715–724. [Google Scholar] [CrossRef]
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2017, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Chiba, M.; Kishimoto, K.N.; Nakamura, M.; Abe, M.; Itoi, E. Transfer of the bone morphogenetic protein 4 gene into rat periodontal ligament by in vivo electroporation. Arch. Oral Biol. 2017, 74, 123–132. [Google Scholar] [CrossRef]
- Kawai, M.; Kataoka, Y.H.; Sonobe, J.; Yamamoto, H.; Inubushi, T.; Ozeki, N.; Muneta, T.; Matsumoto, K.; Sekiya, I.; Koga, H. Non-surgical model for alveolar bone regeneration by bone morphogenetic protein-2/7 gene therapy. J. Periodontol. 2018, 89, 85–94. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Jiang, J.; Zhang, Q.; Yan, F. Inhibiting PHD2 in bone marrow mesenchymal stem cells via lentiviral vector-mediated RNA interference facilitates the repair of periodontal tissue defects in SD rats. Oncotarget 2017, 8, 72676–72699. [Google Scholar] [CrossRef]
- Huang, G.T.; Zhang, H.B.; Yin, C.; Shi, S.C.; Dang, D. Human β-defensin-2 gene transduction of dental pulp cells: A model for pulp antimicrobial gene therapy. Int. J. Oral Biol. 2004, 29, 7–12. [Google Scholar]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Kubo, S.; Kubota, H.; Ohnishi, Y.; Yamada, K.; Kitanaka, M.; Okahashi, N.; Kohsaki, T.; Yamashita, Y.; Koga, T. Expression and secretion of an Arthrobacter dextranase in the oral bacterium Streptococcus gordonii. Infect. Immun. 1993, 61, 4375–4381. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Lehner, T. Mechanism of oral tolerance induction to therapeutic proteins. Adv. Drug Deliv. Rev. 1998, 34, 175–193. [Google Scholar]
- Zhang, Y.; Chen, X.; Wang, H.; Jin, L.J.; Song, S. Novel gene delivery systems for periodontal tissue engineering. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Kulkarni, C.; Kinney, J.S. Gene delivery to oral tissues: Challenges and therapeutic opportunities. Periodontol. 2000 2021, 86, 194–207. [Google Scholar]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Jafari, M.; Soltani, M.; Naahidi, S.; Karunaratne, D.N.; Chen, P. Nonviral approach for targeted nucleic acid delivery. Curr. Med. Chem. 2012, 19, 197–208. [Google Scholar] [CrossRef]
- ARM. Advanced Manufacturing Strategies to Increase the Reach of Cell and Gene Therapies. 2024. Available online: https://alliancerm.org/advanced-manufacturing-strategies/ (accessed on 10 April 2025).
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Mueller, K.T.; Waldron, E.; Grupp, S.A.; Levine, J.E.; Laetsch, T.W.; Pulsipher, M.A.; Boyer, M.W.; August, K.J.; Hamilton, J.; Awasthi, R.; et al. Clinical Pharmacology of Tisagenlecleucel in B-cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2018, 24, 6175–6184. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Jean, G.W.; Huynh, C.; Alzghari, S.K.; Lowe, D.B. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy 2015, 35, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L. Mechanism of action of immunotherapy. Semin. Oncol. 2014, 41, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Kanada, M.; Kim, B.D.; Hardy, J.W.; Ronald, J.A.; Bachmann, M.H.; Bernard, M.P.; Perez, G.I.; Zarea, A.A.; Ge, T.J.; Withrow, A.; et al. Microvesicle-mediated delivery of minicircle DNA results in effective gene-directed enzyme prodrug cancer therapy. Mol. Cancer Ther. 2019, 18, 2331–2342. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Chen, W.H.; Lecaros, R.L.G.; Tseng, Y.C.; Huang, L.; Hsu, Y.C. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett. 2015, 359, 65–74. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Huang, L.; Lee, T.C.; Hsu, Y.C. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol. Ther. 2016, 24, 106–116. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Chen, Y.; Wang, Y.; Min, G.; Zhao, Y.; Zhang, H.; Liu, J.; Chen, X.; Wei, Q.; et al. Advanced biomaterials and technologies for oral cancer therapy and diagnosis. Bioact. Mater. 2024, 31, 139–160. [Google Scholar]
- Wang, Y.; Liu, R.; Sun, X.; Guo, L.; Wang, S.; Shi, P.; Zhang, H.; Chen, X.; Li, J.; Yang, F.; et al. Gene therapy strategies for head and neck cancer treatment. J. Control. Release 2023, 353, 791–806. [Google Scholar]
- Chan, N.; Chunling, Y. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar]
- Omori, H.; Nishio, M.; Masuda, M.; Miyachi, Y.; Ueda, F.; Nakano, T.; Sato, K.; Mimori, K.; Yamamoto, K.; Aikou, S.; et al. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci. Adv. 2020, 6, eaay3324. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wang, P.Y.; Hutzen, B.; Sprague, L.; Swain, H.M.; Love, J.K.; Stanek, J.R.; Boon, L.; Santiago, D.N.; Cronk, R.J.; et al. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci. Rep. 2017, 7, 2396. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hutzen, B.; Wedekind, M.F.; Cripe, T.P. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virother. 2018, 7, 65–77. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Dolan, J.C.; Hameed, A.; Ye, Y. Emerging molecular targets for cancer pain therapy. Nat. Rev. Cancer 2023, 23, 209–225. [Google Scholar]
- Yang, N.; Li, J.; Li, J.; Wei, X.; Zhu, Y.; Fan, S.; Zhang, H.; Zhang, L.; Wang, Y.; Gao, Y.; et al. MicroRNAs: Pleiotropic regulators in the tumor microenvironment and their implications for HNC therapy. Front. Oncol. 2022, 12, 819430. [Google Scholar]
- Tong, S.; Moyo, B.; Lee, C.M.; Leong, K.; Bao, G. Engineered materials for in vivo delivery of genome-editing machinery. Nat. Rev. Mater. 2019, 4, 726–737. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Xu, K.; Gao, J.; Li, J.; Zhang, Y.; Wang, L.; Chen, X.; Liu, Y.; Zhou, M.; et al. Oncolytic virus and PD-1/PD-L1 blockade combination therapy for head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2023, 72, 2435–2447. [Google Scholar]
- Liu, J.; Jia, L.; Hu, J.; Wang, X.; Liu, H.; Zhu, L.; Chen, Y.; Zhang, H.; Li, W.; Wang, Y.; et al. Radiovirotherapy: A novel strategy for the treatment of oral squamous cell carcinoma. Front. Oncol. 2022, 12, 940389. [Google Scholar]
- Tan, Y.; Li, J.; Wang, H.; Tan, H.; Zou, T.; Tao, Y.; Chen, X.; Liu, Y.; Zhang, W.; Gao, F.; et al. Recent progress in cancer immunotherapies combining oncolytic virotherapy and immune checkpoint inhibitors. Adv. Drug Deliv. Rev. 2023, 197, 114807. [Google Scholar]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.; Ke, C. Gene therapy vectors based on nanomaterials for the treatment of oral cancer: Current status and future perspectives. Exploration 2023, 3, 20210058. [Google Scholar]
- Nayak, S.; Herzog, R.W. Progress and prospects: Immune responses to viral vectors. Gene Ther. 2010, 17, 295–304. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Sumitani, M.; Nishizawa, D.; Hozumi, J.; Yamada, Y.; Nagakura, Y.; Sumitani, M.; Ikeda, K.; Nishiyama, A.; Kato, A.; Ueda, H. Genetic implications in quality palliative care and preventing opioid crisis in cancer-related pain management. J. Neurosci. Res. 2020, 98, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, H.; Li, J.; Liu, S.; Jin, Y. Gene therapy approaches for cancer pain management. Pain 2023, 164, 1327–1340. [Google Scholar]
- Gorsky, M.; Epstein, J.B.; Oakley, C.; Le, N.D.; Hay, J.; Stevenson-Moore, P. Carcinoma of the tongue: A case series analysis of clinical presentation, risk factors, staging, and outcome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 546–552. [Google Scholar] [CrossRef]
- Ye, Y.; Dang, D.; Zhang, J.; Viet, C.T.; Lam, D.K.; Dolan, J.C.; Gibbs, J.L.; Schmidt, B.L. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Cancer Ther. 2011, 10, 1667–1676. [Google Scholar] [CrossRef]
- Ye, Y.; Ono, K.; Bernabé, D.G.; Viet, C.T.; Pickering, V.; Dolan, J.C.; Ono, K.; Forsthuber, L.; Patil, P.G.; Schmidt, B.L. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol. Commun. 2014, 2, 62. [Google Scholar] [CrossRef]
- Schmidt, B.L. What pain tells us about cancer. Pain 2015, 156, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr. Opin. Anaesthesiol. 2010, 23, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Cuffari, L.; Siqueira, J.T.T.; Nemr, K.; Rapaport, A. Pain complaint as the first symptom of oral cancer: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 56–61. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef]
- Barta, A.; Lewis, S.D.; Cortes, C.; Pacheco, M.; Heinz, B.; West, D.; Gregory, K.; Sanchez, E.P.; Manjarrez, J.; Sibbald, C.; et al. Gene therapy approaches to pain management. J. Transl. Genet. Genom. 2021, 5, 319–338. [Google Scholar]
- Pickering, V.; Gupta, R.J.; Quang, P.; Jordan, R.C.; Schmidt, B.L. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur. J. Pain 2008, 12, 293–300. [Google Scholar] [CrossRef]
- Handy, C.R.; Krudy, C.; Boulis, N. Gene therapy: A potential approach for cancer pain. Pain Res. Treat. 2011, 2011, 987597. [Google Scholar] [CrossRef]
- Viet, C.T.; Ye, Y.; Dang, D.; Lam, D.K.; Achdjian, S.; Zhang, J.; Schmidt, B.L. Re-expression of the methylated EDNRB gene in oral squamous cell carcinoma attenuates cancer-induced pain. Pain 2011, 152, 2323–2332. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Z.; Zhang, H.; Atianjoh, F.E.; Zhao, J.Y.; Liang, L.; Wang, W.; Guan, X.; Kao, S.C.; Tiwari, V.; et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 2013, 16, 1024–1031. [Google Scholar] [CrossRef]
- Collins, K.H.; Lenz, K.L.; Pollitt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Eastman, W.M.; Mosiman, E.; Xie, Z.; Chubinskaya, S.; et al. Application of CRISPR-Cas9 to the treatment of osteoarthritis. Mol. Ther. 2021, 29, 3221–3230. [Google Scholar]
- Fink, D.J.; Wechuck, J.; Mata, M.; Glorioso, J.C.; Goss, J.; Krisky, D.; Wolfe, D. Gene therapy for pain: Results of a phase I clinical trial. Ann. Neurol. 2011, 70, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M.J. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef]
- Kok, M.R.; Yamano, S.; Lodde, B.M.; Wang, J.; Couwenhoven, R.I.; Yakar, S.; Voutetakis, A.; Langer, J.A.; Chiorini, J.A.; Baum, B.J. Local adeno-associated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjögren’s syndrome. Hum. Gene Ther. 2003, 14, 1605–1618. [Google Scholar] [CrossRef]
- Lodde, B.M.; Mineshiba, F.; Wang, J.; Cotrim, A.P.; Afione, S.; Tak, P.P.; Baum, B.J. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjögren’s syndrome. Ann. Rheum. Dis. 2006, 65, 195–200. [Google Scholar] [CrossRef]
- Zheng, C.; Cotrim, A.P.; Rowzee, A.; Swaim, W.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin. Cancer Res. 2011, 17, 2842–2851. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current strategies to enhance adipose stem cell function: An update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takarada, T.; Yoneda, Y.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef] [PubMed]
- Pringle, S.; Maimets, M.; van der Zwaag, M.; Stokman, M.A.; van Gosliga, D.; Zwart, E.; Witjes, M.J.; de Haan, G.; van Os, R.; Coppes, R.P. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Lynggaard, C.D.; Therkildsen, J.R.; Eriksen, J.G.; Pedersen, A.M.; Sørensen, J.A.; Overgaard, J.; Johansen, J.; Andersen, E.; Herlev, O.; Hansen, H.S.; et al. A phase 1 first-in-human trial of human adipose-derived mesenchymal stromal cells in patients with radiation-induced xerostomia. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 639–648. [Google Scholar]
- Hai, B.; Yang, Z.; Shangguan, L.; Zhao, Y.; Boyer, A.; Liu, F. Concurrent transient activation of Wnt/β-catenin pathway prevents radiation damage to salivary glands. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the secrets behind liquid superlubricity: A state-of-the-art review on phenomena and mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Austin, W.; Hdeib, M.; Fraser, P.; Goldchtaub, M.; Shams, E.; Han, T.; Zhang, S.; Saad, A.; Kaur, K.; Haran, N.; et al. Oral Lubrication, Xerostomia, and Advanced Macromolecular Lubricants for Treatment of Dry Mouth. Lubricants 2024, 12, 126. [Google Scholar] [CrossRef]
- Cirelli, J.A.; Park, C.H.; MacKool, K.; Taba, M.; Lustig, K.H.; Burstein, H.; Giannobile, W.V. AAV2/1-TNFR gene delivery prevents periodontal disease progression. Gene Ther. 2009, 16, 426–436. [Google Scholar] [CrossRef]
- Jin, L.J.; Zhang, Y.; Chen, M.; Wang, H. Gene therapy in periodontal regeneration: Current status and future perspectives. J. Dent. Res. 2023, 102, 1089–1098. [Google Scholar]
- Jung, Y.H.; Park, J.Y.; Kim, H.J.; Lee, S.M.; Kim, S.H.; Yun, J.H. Regenerative potential of bone morphogenetic protein 7-engineered mesenchymal stem cells in ligature-induced periodontitis. Tissue Eng. Part A 2023, 29, 200–210. [Google Scholar] [CrossRef]
- Daghrery, A.; Syed, S.N.; Almymany, F.; Aouida, M.; Mustafa, K.; Fristad, I.; Leknes, K.N. Advanced biomaterials for periodontal tissue regeneration. Genesis 2022, 60, e23501. [Google Scholar] [CrossRef]
- Dang, D.; Taheri, S.; Das, S.; Ghosh, P.; Prince, L.S.; Saif, T. Computational perspectives of cellular gene regulatory networks with multiplexed single-cell data. Annu. Rev. Biomed. Data Sci. 2020, 3, 195–225. [Google Scholar]
- Theodoridis, K.; Arampatzis, A.S.; Liasi, G.; Tsalikis, L.; Barmpalexis, P.; Christofilos, D.; Chatzinikolaidou, M.; Fatouros, D.G. Development of an interpenetrating network of chitosan/gelatin hybrid hydrogel incorporating plicalocenol. Int. J. Mol. Sci. 2023, 24, 16754. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Kanai, R.; Hasegawa, K.; Ogaeri, T.; Tran, S.D.; Sumita, Y. Recent progress in regenerative therapy for damaged salivary glands: From bench to bedside. Oral Dis. 2024, 30, 38–49. [Google Scholar]
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wei, Q.; Li, J.; Zhang, H.; Wang, Y.; et al. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.N.; Rungarunlert, S.; Urkasemsin, G.; Adine, C.; Souza, G.R. Three-dimensional bioprinting nanotechnologies towards clinical application of stem cells. Stem Cells Int. 2016, 2016, 7564689. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Wang, H.; Liu, L. The application of artificial intelligence in oral gene therapy: Challenges and opportunities. Innov. Life 2023, 2, 100016. [Google Scholar]
- Marsden, G.; Towse, A.; Pearson, S.D.; Dreitlein, B.; Henshall, C. Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value; ICER: Boston, MA, USA, 2017. [Google Scholar]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Kimmelman, J. Recent developments in gene transfer: Risk and ethics. BMJ 2005, 330, 79–82. [Google Scholar] [CrossRef]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health 2011, 28, 12–20. [Google Scholar]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore 1994, 23, 129–138. [Google Scholar]
- Slade, G.D.; Spencer, A.J. Development and evaluation of the Oral Health Impact Profile. Community Dent. Health 1994, 11, 3–11. [Google Scholar]
- Ormond, K.E.; Mortlock, D.P.; Scholes, D.T.; Bombard, Y.; Brody, L.C.; Faucett, W.A.; Garrison, N.A.; Hercher, L.; Isasi, R.; Middleton, A.; et al. Human germline genome editing. Am. J. Hum. Genet. 2017, 101, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal stem cells extract (MSCsE)-based therapy alleviates xerostomia and keratoconjunctivitis sicca in Sjogren’s syndrome-like disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Huh, S.; D’Souza, V.; Arkin, S.; Roberts, E.; McIntosh, A. Practical and Statistical Considerations for the Long Term Follow-Up of Gene Therapy Trial Participants. Clin. Pharmacol. Ther. 2024, 115, 139–146. [Google Scholar] [CrossRef]

| Vector Type | Genome | Packaging Capacity | Integration | Duration of Expression | Main Advantages | Main Limitations | Oral Applications | Key References |
|---|---|---|---|---|---|---|---|---|
| Adenovirus | dsDNA | 8–36 kb | Non-integrating | Transient (days to weeks) | High transduction efficiency; Transduces dividing and non-dividing cells; Easy to produce in high titers | High immunogenicity; Pre-existing immunity common | Oral cancer; Xerostomia; Periodontal diseases | [12,13,14,15] |
| Adeno-associated virus (AAV) | ssDNA | 4.7 kb | Rarely integrating | Long-term (months to years) | Low immunogenicity; Transduces dividing and non-dividing cells; Multiple serotypes for tissue targeting | Limited packaging capacity; Slow onset of expression | Salivary gland disorders; Periodontal regeneration; Cancer pain | [16,17,18,19] |
| Retrovirus | ssRNA | 8 kb | Integrating | Long-term (years) | Stable gene expression due to integration | Only transduces dividing cells; Risk of insertional mutagenesis | Primarily ex vivo approaches for oral cancer | [20,21] |
| Lentivirus | ssRNA | 8 kb | Integrating | Long-term (years) | Transduces dividing and non-dividing cells; Lower immunogenicity than adenovirus | Risk of insertional mutagenesis; Complex production | Ex vivo modification of cells for periodontal regeneration | [22,23] |
| Herpes simplex virus | dsDNA | >30 kb | Non-integrating | Transient | Large packaging capacity; Natural neurotropism | Cytotoxicity; Complex genome | Oral cancer pain; Oncolytic therapy for oral cancer | [24] |
| Vaccinia virus | dsDNA | >25 kb | Non-integrating | Transient | Large packaging capacity; Strong immunogenicity | Limited targeting specificity; Safety concerns | Cancer immunotherapy | [25] |
| Vector Type | Composition | Packaging Capacity | Transfection Efficiency | Duration of Expression | Main Advantages | Main Limitations | Oral Applications | Key References |
|---|---|---|---|---|---|---|---|---|
| Lipid-based systems | Cationic lipids, helper lipids, PEG-lipids | Unlimited | Moderate | Short-term (days) | Low immunogenicity; Easy to produce; Suitable for various nucleic acids | Serum instability; Cytotoxicity at high concentrations | Oral cancer; Mucosal delivery | [28,29,30] |
| Polymer-based systems | PEI, PLL, PAMAM, chitosan | Unlimited | Low to moderate | Short-term (days) | Low cost; Versatility; Stability | Lower efficiency than viral vectors; Potential cytotoxicity | Periodontal diseases; Controlled release applications | [26,28,31] |
| Lipopoly-plex | Combination of lipids and polymers | Unlimited | Moderate to high | Short-term (days) | Improved efficiency compared to individual components; Reduced toxicity | Complex formulation; Batch-to-batch variability | Cancer pain; Periodontal regeneration | [28,29] |
| Cell-penetrating peptides (CPP) | Short peptides with membrane-penetrating properties | Limited by complexation | Moderate | Short-term (days) | Enhanced cellular uptake; Low immunogenicity | Limited stability; High cost | Targeting oral cancer cells; Salivary gland delivery | [28,32] |
| Inorganic nanoparticles | Gold, silica, calcium phosphate | Variable | Low to moderate | Short-term (days) | Stability; Surface functionalization options | Potential toxicity; Variable efficiency | Dental hard tissue regeneration; Antimicrobial applications | [33] |
| Exosomes | Natural membrane vesicles | Limited by vesicle size | Variable | Short to medium term | Low immunogenicity; Natural targeting | Complex isolation; Standardization challenges | Emerging applications in oral inflammatory diseases | [34] |
| Oral Application | Preferred Vector Types | Key Considerations | Key References |
|---|---|---|---|
| Oral Cancer | Adenoviral, Oncolytic viral vectors | High transduction efficiency, tumor selectivity | [39,40,41,42] |
| Cancer Pain | AAV, Non-viral | Neurotropism, localized delivery, safety | [43,44,45] |
| Xerostomia | AAV, Adenoviral | Duration of expression, immunogenicity | [14,15,18,19] |
| Periodontal Diseases | Non-viral, AAV with biomaterials | Scaffold integration, spatial control | [46,47,48,49] |
| Dental Caries | Non-viral, Bacteriophage | Mucosal delivery, microbiome targeting | [50,51] |
| Disease | Vector | Therapeutic Gene | Phase | Trial ID | Status | Primary Outcomes | Key Findings | Key References |
|---|---|---|---|---|---|---|---|---|
| Radiation-induced xerostomia | Adenovirus | Aquaporin-1 (AQP1) | I/II | NCT02446249 | Completed | Safety; Change in parotid salivary flow rate | Increased parotid flow rate in 5/11 patients; No serious adverse events | [14,105] |
| Radiation-induced xerostomia | AAV2 | Aquaporin-1 (AQP1) | I | NCT02749448 | Active | Safety; Vector shedding; Preliminary efficacy | Ongoing; Preliminary results show improved safety profile compared to adenoviral vectors | [18,19] |
| Head and neck squamous cell carcinoma | Oncolytic adenovirus (ONCOS-102) | GM-CSF | I | NCT01598129 | Completed | Maximum tolerated dose; Safety | Acceptable safety profile; Evidence of immune activation in tumor microenvironment | [76,77,78] |
| Head and neck squamous cell carcinoma | Oncolytic herpes simplex virus (HSV-1716) | - | I | NCT00931931 | Completed | Safety; Virus replication in tumor | Well-tolerated; Evidence of selective replication in tumor tissues | [24,25] |
| Oral cancer pain | Non-viral CPP/lipid | μ-opioid receptor | Pre-clinical | - | - | Safety; Change in pain scores | Preliminary data indicates significant reduction in pain scores; No serious adverse events | [43,44] |
| Head and neck cancer | mRNA lipid nanoparticles | HPV E6/E7 antigens | I | NCT03418480 | Active | Safety; Immunogenicity | Ongoing; Early results show induction of antigen-specific T cell responses | [69,85] |
| Sjögren’s syndrome | AAV | VIP | Pre-clinical | - | - | Reduction in inflammatory markers; Restoration of salivary flow | 60% improvement in salivary flow in mouse models; Reduction in lymphocytic infiltration | [108] |
| Periodontal diseases | Plasmid/polymer | PDGF-B | I/II | NCT00540423 | Completed | Safety; Clinical attachment level gain | Safe with no significant adverse events; 1.3–2.3 mm mean clinical attachment level gain | [46,47] |
| Advanced head and neck cancer | Retrovirus | Wild-type p53 | II | NCT00004224 | Completed | Tumor response rate | 26% objective response rate; Median survival of 7.5 months vs. 4.5 months in control group | [66,67] |
| Recurrent head and neck cancer | Adenovirus (INGN 201) | p53 | II/III | NCT00041626 | Completed | Locoregional tumor control | 40% local tumor control rate when combined with chemotherapy vs. 20% with chemotherapy alone | [66,67,82] |
| Dental caries | Plasmid DNA/cationic liposome | Antimicrobial peptide LL-37 | Pre-clinical | - | - | Reduction in S. mutans colonization | 85% reduction in S. mutans biofilm formation in ex vivo models | [118] |
| Peri-implantitis | Adenovirus | BMP-7 | Pre-clinical | - | - | Bone regeneration around implants | 40% greater bone fill in animal models compared to control treatments | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamano, S.; Inoue, K.; Taguchi, Y. Application of Gene Therapy to Oral Diseases. Pharmaceutics 2025, 17, 859. https://doi.org/10.3390/pharmaceutics17070859
Yamano S, Inoue K, Taguchi Y. Application of Gene Therapy to Oral Diseases. Pharmaceutics. 2025; 17(7):859. https://doi.org/10.3390/pharmaceutics17070859
Chicago/Turabian StyleYamano, Seiichi, Kenji Inoue, and Yoichiro Taguchi. 2025. "Application of Gene Therapy to Oral Diseases" Pharmaceutics 17, no. 7: 859. https://doi.org/10.3390/pharmaceutics17070859
APA StyleYamano, S., Inoue, K., & Taguchi, Y. (2025). Application of Gene Therapy to Oral Diseases. Pharmaceutics, 17(7), 859. https://doi.org/10.3390/pharmaceutics17070859







