AQbD Approach Applied to NIR in a Complex Topical Formulation: Bifonazole as Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bifonazole Cream Formulation
2.3. Analytical Methods Development
2.3.1. Risk Assessment
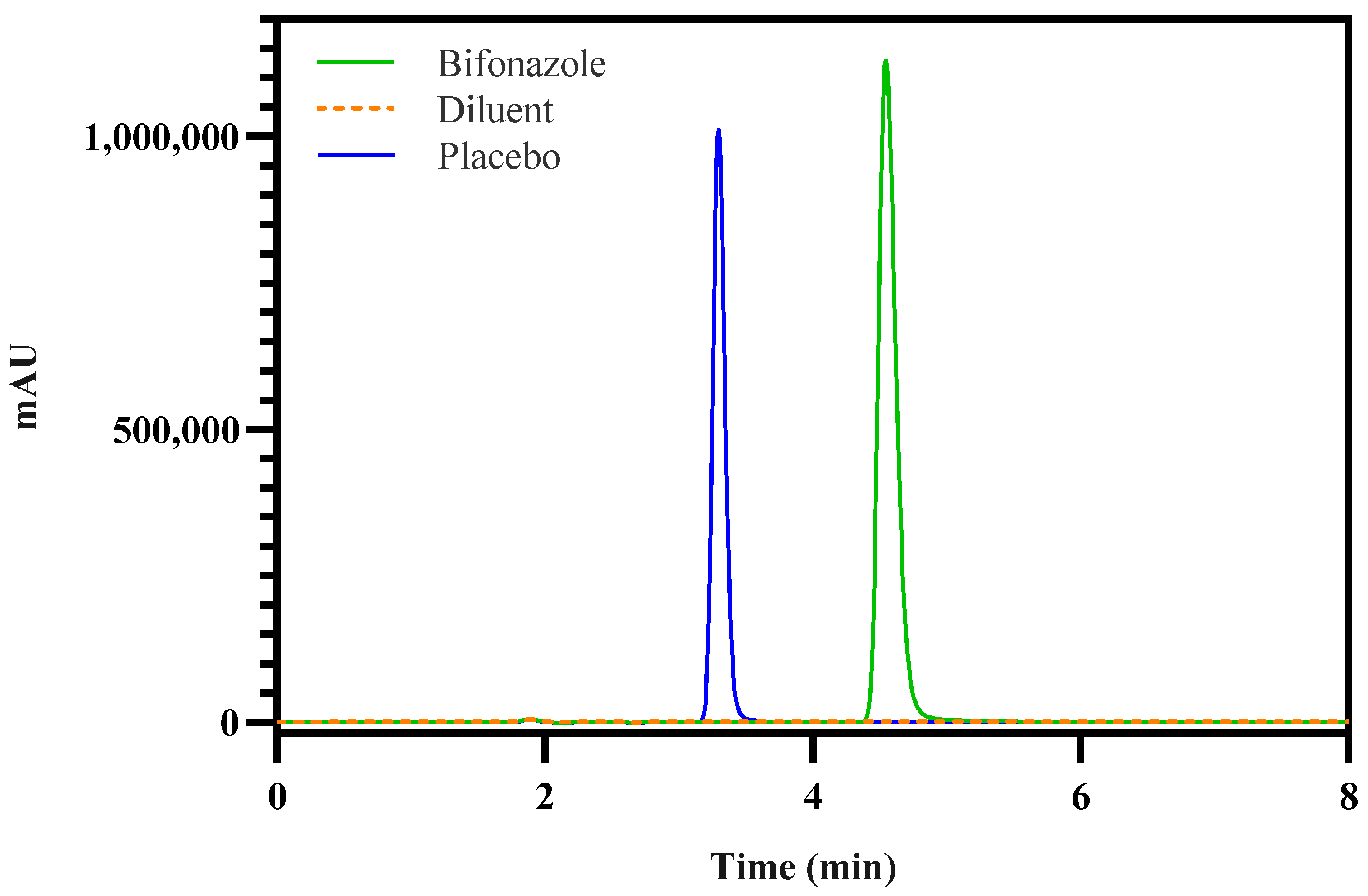
2.3.2. RP-HPLC
Instrumentation and Chromatographic Conditions
Preparation of Standard Solutions for Drug Formulations
Preparation of Sample Solutions for Drug Formulation
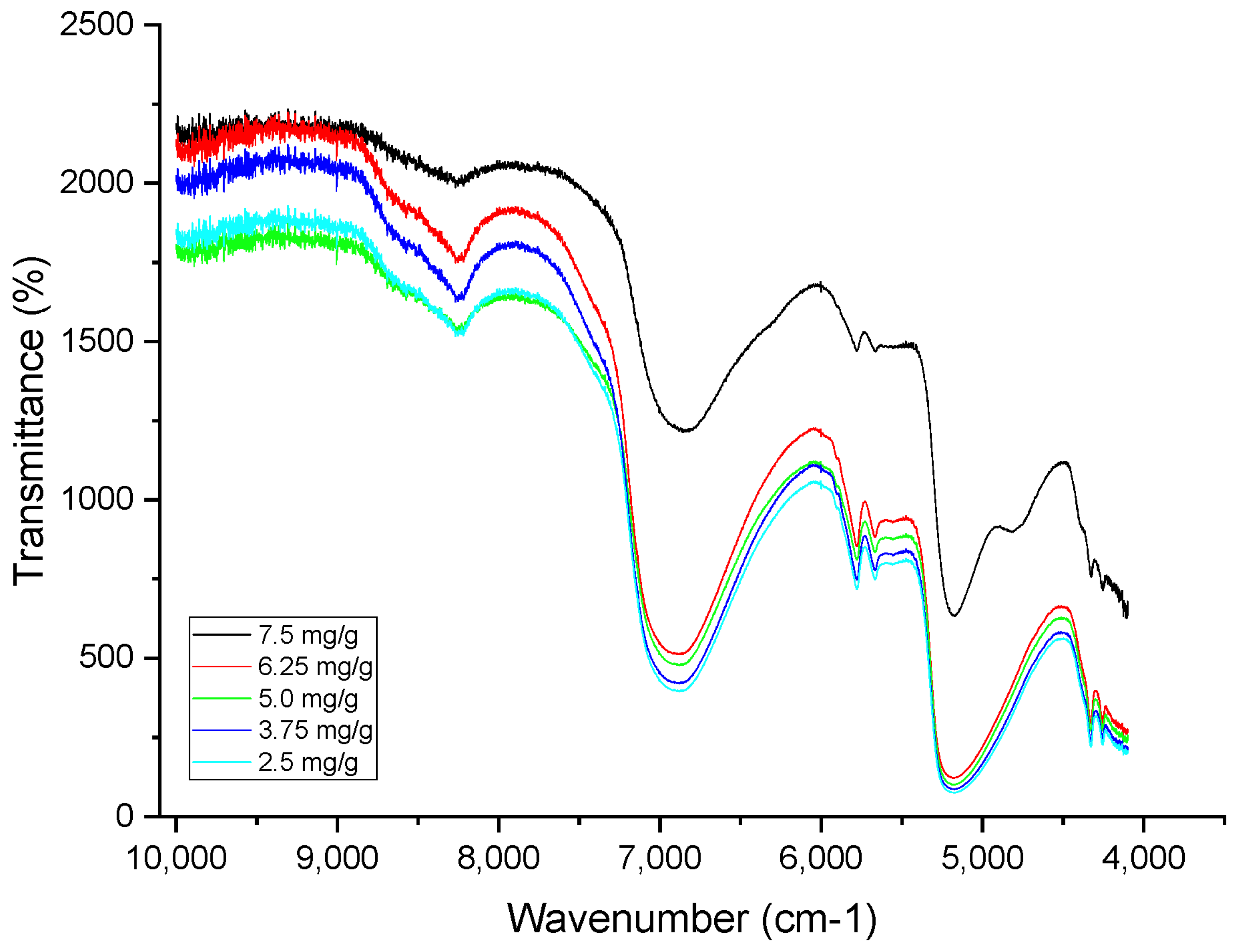
2.3.3. NIR
Instrumentation and NIR Conditions
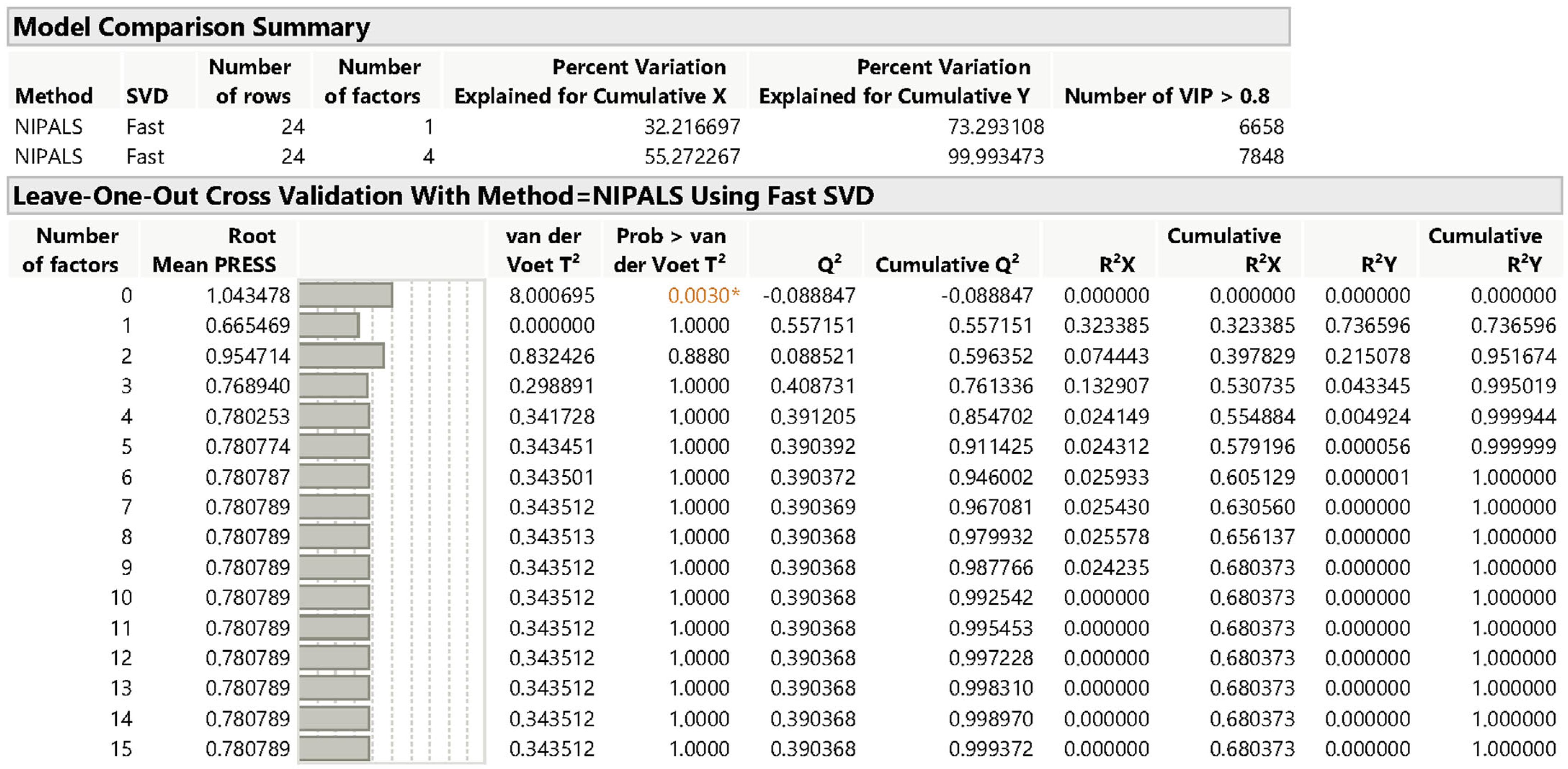
Multivariate Data Treatment
3. Results and Discussion
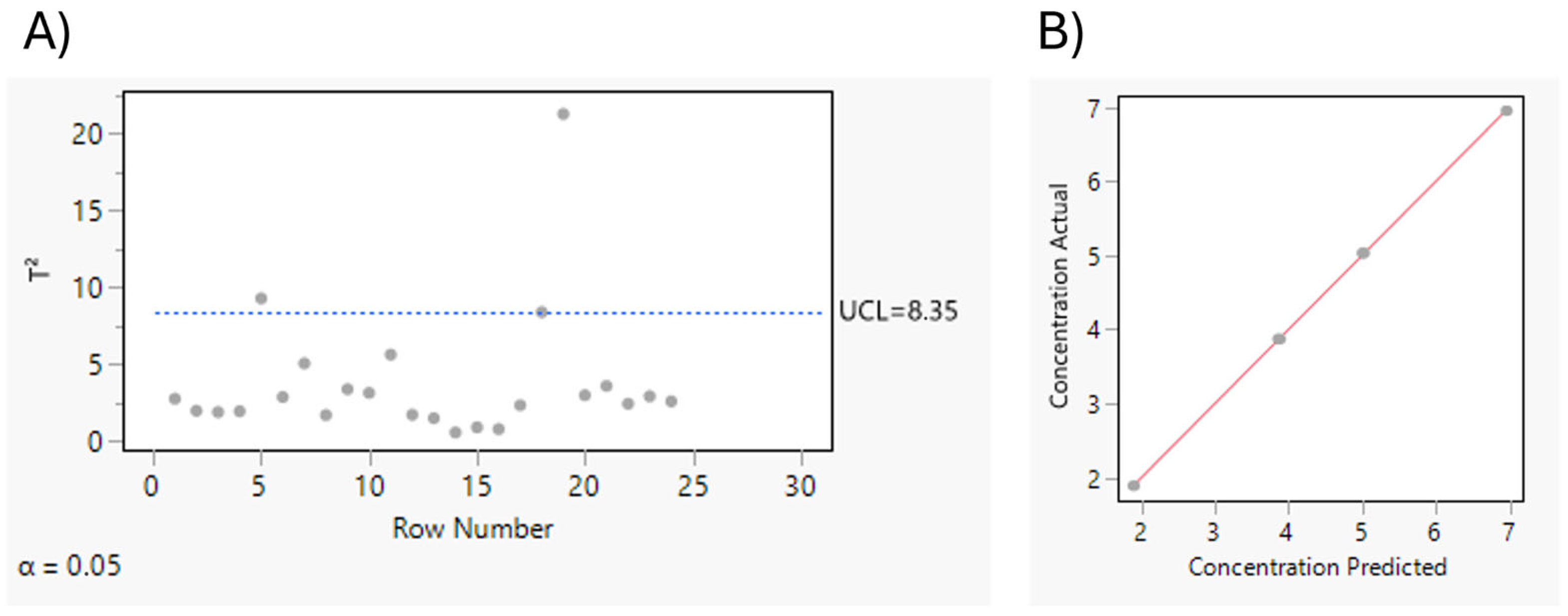
Scrutinizing Drug Product Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Shah, D.S.; Gurram, S.; Gadlawar, V.N.; Jha, D.K.; Kamble, S.P.; Amin, P.D. Co-Amorphous System of Bifonazole for Improved in-Vitro Permeation and Antifungal Activity. JCIS Open 2024, 14, 100108. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Hosny, K.M. Nano-Vesicular Delivery System Loaded by Bifonazole: Preparation, Optimization, and Assessment of Pharmacokinetic and Antifungal Activity. J. Drug Deliv. Sci. Technol. 2019, 49, 316–322. [Google Scholar] [CrossRef]
- Lackner, T.E.; Clissold, S.P. Bifonazole: A Review of Its Antimicrobial Activity and Therapeutic Use in Superficial Mycoses. Drugs 1989, 38, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Kumar, M.; Pahwa, R.; Gupta, S.; Bhatt, S.; Kumari, B.; Kumar Upadhyay, P.; Pandurangan, A. Formulation and in Vivo Pharmacodynamics Studies of Nanostructured Lipid Carriers for Topical Delivery of Bifonazole Running Head: Nanostructured Lipid Carriers as Vesicles for Topical Delivery of Bifonazole. Acta Pharm. Sci. 2021, 59, 559–580. [Google Scholar] [CrossRef]
- Miranda, M.; Veloso, C.; Brown, M.; Alberto, A.A.C.; Cardoso, C.; Vitorino, C. Topical Bioequivalence: Experimental and Regulatory Considerations Following Formulation Complexity. Int. J. Pharm. 2022, 620, 121705. [Google Scholar] [CrossRef]
- Patel, D.; Patel, D.; Prajapati, J.; Patel, U.; Patel, V. Formulation of Thermoresponsive and Buccal Adhesive in Situ Gel for Treatment of Oral Thrush Containing Poorly Water Soluble Drug Bifonazole. J. Pharm. Bioallied Sci. 2012, 4, 116–117. [Google Scholar] [CrossRef]
- Manian, M.; Jain, P.; Vora, D.; Banga, A.K. Formulation and Evaluation of the In Vitro Performance of Topical Dermatological Products Containing Diclofenac Sodium. Pharmaceutics 2022, 14, 1892. [Google Scholar] [CrossRef]
- Calvo, N.L.; Alvarez, V.A.; Lamas, M.C.; Leonardi, D. New Approaches to Identification and Characterization of Tioconazole in Raw Material and in Pharmaceutical Dosage Forms. J. Pharm. Anal. 2019, 9, 40–48. [Google Scholar] [CrossRef]
- Popović, G.; Čakar, M.; Agbaba, D. Determination of Bifonazole in Creams Containing Methyl- and Propyl p-Hydroxybenzoate by Derivative Spectrophotometric Method. J. Pharm. Biomed. Anal. 2003, 33, 131–136. [Google Scholar] [CrossRef]
- Council of Europe. Bifonazole Monograph. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; pp. 1977–1979. [Google Scholar]
- The Stationery Office. Bifonazole Monograph. In British Pharmacopoeia 2023; The Stationery Office: London, UK, 2023. [Google Scholar]
- EMA. Guideline on the Use of Near Infrared Spectroscopy (NIRS) by the Pharmaceutical Industry and the Data Requirements for New Submissions and Variations; EMA: Amsterdam, The Netherlands, 2014; Volume 44, pp. 1–28. [Google Scholar]
- Chiarentin, L.; Cardoso, C.; Miranda, M.; Vitorino, C. Rheology of Complex Topical Formulations: An Analytical Quality by Design Approach to Method Optimization and Validation. Pharmaceutics 2023, 15, 1810. [Google Scholar] [CrossRef] [PubMed]
- Puchert, T.; Holzhauer, C.V.; Menezes, J.C.; Lochmann, D.; Reich, G. A New PAT/QbD Approach for the Determination of Blend Homogeneity: Combination of on-Line NIRS Analysis with PC Scores Distance Analysis (PC-SDA). Eur. J. Pharm. Biopharm. 2011, 78, 173–182. [Google Scholar] [CrossRef]
- Nobari Moghaddam, H.; Tamiji, Z.; Akbari Lakeh, M.; Khoshayand, M.R.; Haji Mahmoodi, M. Multivariate Analysis of Food Fraud: A Review of NIR Based Instruments in Tandem with Chemometrics. J. Food Compos. Anal. 2022, 107, 104343. [Google Scholar] [CrossRef]
- Wahl, P.R.; Fruhmann, G.; Sacher, S.; Straka, G.; Sowinski, S.; Khinast, J.G. PAT for Tableting: Inline Monitoring of API and Excipients via NIR Spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87, 271–278. [Google Scholar] [CrossRef]
- Hakemeyer, C.; Strauss, U.; Werz, S.; Jose, G.E.; Folque, F.; Menezes, J.C. At-Line NIR Spectroscopy as Effective PAT Monitoring Technique in Mab Cultivations during Process Development and Manufacturing. Talanta 2012, 90, 12–21. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC-Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Blanco, M.; Cruz, J.; Bautista, M. Development of a Univariate Calibration Model for Pharmaceutical Analysis Based on NIR Spectra. Anal. Bioanal. Chem. 2008, 392, 1367–1372. [Google Scholar] [CrossRef]
- Atanaskova, E.; Angelovska, V.; Chachorovska, M.; Anevska Stojanovska, N.; Petrushevski, G.; Makreski, P.; Geskovski, N. Development of Novel Portable NIR Spectroscopy Process Analytical Technology (PAT) Tool for Monitoring the Transition of Ibuprofen to Ibuprofen Sodium during Wet Granulation Process. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2024, 317, 124369. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsidou, E.; Karavasili, C.; Zacharis, C.K.; Fatouros, D.G.; Markopoulou, C.K. Partial Least Square Model (PLS) as a Tool to Predict the Diffusion of Steroids across Artificial Membranes. Molecules 2020, 25, 1387. [Google Scholar] [CrossRef]
- Thorsten, V.; Cristiana, C.; Chéry, C.C.; Ruth, F.; Timothy, G.; Nomalie, J.; Bassam, N.; Jeremy, S.; Jason, S.; Jette, W.; et al. Analytical Quality by Design, Life Cycle Management, and Method Control. AAPS PharmSciTech 2022, 24, 34. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Review Article Understanding Pharmaceutical Quality by Design. Am. Assoc. Pharm. Sci. 2014, 16, 771–783. [Google Scholar]
- Chiarentin, L.; Gonçalves, C.; Augusto, C.; Miranda, M.; Cardoso, C.; Vitorino, C. Drilling into “Quality by Design” Approach for Analytical Methods. Crit. Rev. Anal. Chem. 2023, 54, 3478–3519. [Google Scholar] [CrossRef]
- EMA. Analytical Procedure Development—ICH (Q14); EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- United States Pharmacopeia. Analytical Procedure Lifecycle, General Chapter <1220>; United States Pharmacopeia: Rockville, MD, USA, 2022; pp. 1–18. [Google Scholar]
- Santos, J.R.; Sarraguça, M.C.; Rangel, A.O.S.S.; Lopes, J.A. Evaluation of Green Coffee Beans Quality Using near Infrared Spectroscopy: A Quantitative Approach. Food Chem. 2012, 135, 1828–1835. [Google Scholar] [CrossRef]
- Alam, M.A.; Liu, Y.A.; Dolph, S.; Pawliczek, M.; Peeters, E.; Palm, A. Benchtop NIR Method Development for Continuous Manufacturing Scale to Enable Efficient PAT Application for Solid Oral Dosage Form. Int. J. Pharm. 2021, 601, 120581. [Google Scholar] [CrossRef] [PubMed]
- JMP. Statistical Details for Partial Least Squares. Available online: https://www.jmp.com/support/help/en/18.1/index.shtml#page/jmp/statistical-details-for-partial-least-squares.shtml%23 (accessed on 30 December 2024).
- International Council Harmonization (ICH). Quality Risk Management Q9; International Council Harmonization (ICH): Geneva, Switzerland, 2023. [Google Scholar]
- Yun, Y.H.; Li, H.D.; Deng, B.C.; Cao, D.S. An Overview of Variable Selection Methods in Multivariate Analysis of Near-Infrared Spectra. TrAC-Trends Anal. Chem. 2019, 113, 102–115. [Google Scholar] [CrossRef]
- Sarragua, M.C.; Soares, S.O.; Lopes, J.A. A Near-Infrared Spectroscopy Method to Determine Aminoglycosides in Pharmaceutical Formulations. Vib. Spectrosc. 2011, 56, 184–192. [Google Scholar] [CrossRef]
- Felício, C.C.; Brás, L.P.; Lopes, J.A.; Cabrita, L.; Menezes, J.C. Comparison of PLS Algorithms in Gasoline and Gas Oil Parameter Monitoring with MIR and NIR. Chemom. Intell. Lab. Syst. 2005, 78, 74–80. [Google Scholar] [CrossRef]
- Validation in JMP Modeling. Available online: https://www.jmp.com/support/help/en/18.1/index.shtml#page/jmp/validation-in-jmp-modeling.shtml%23 (accessed on 4 January 2025).

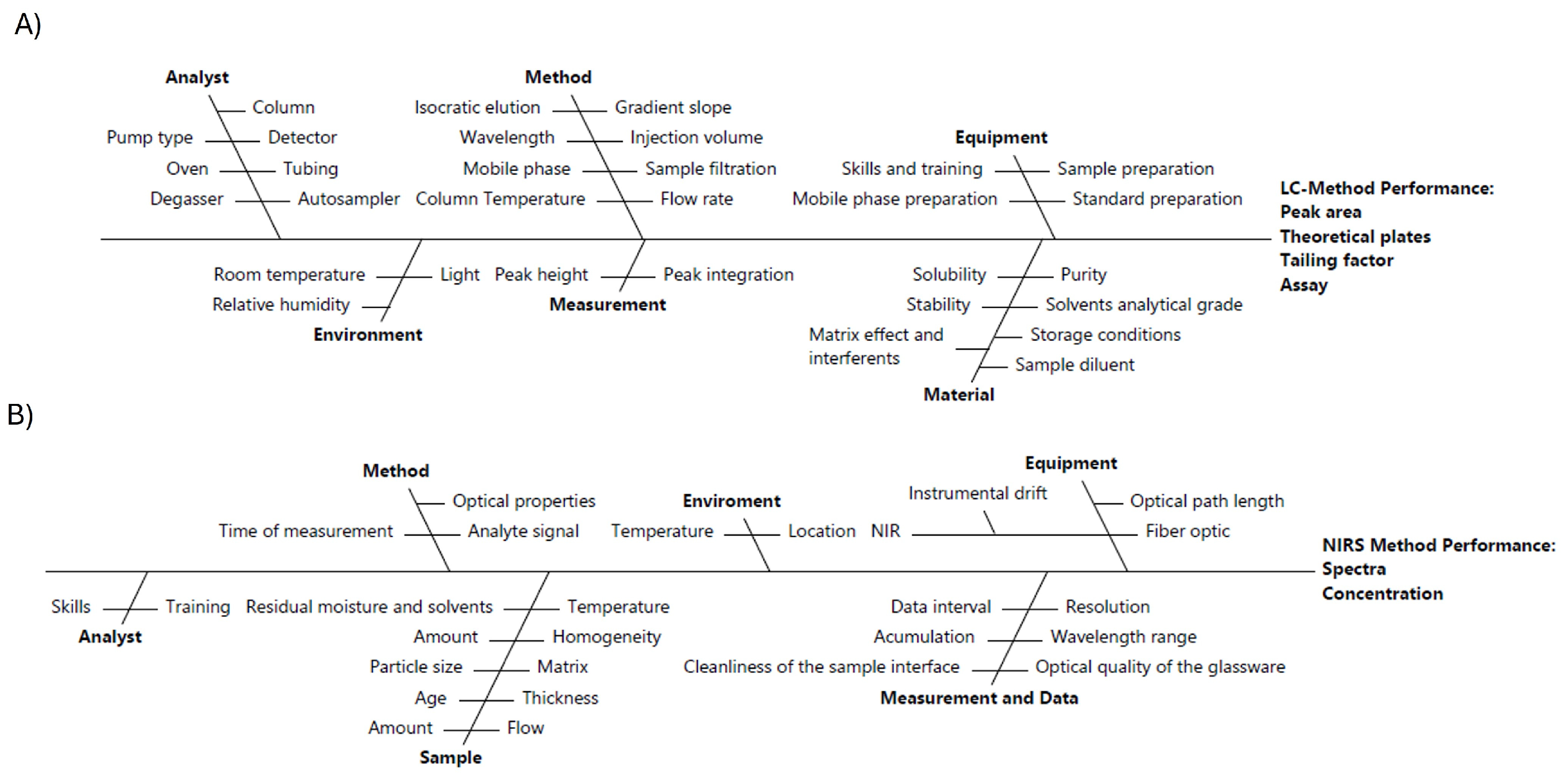

| ATP | Target | Justification | Specification |
|---|---|---|---|
| Analyte | Bifonazole (10 mg/g)–cream | Development of RP-HPLC and NIR methods to assist bifonazole assay and permeation kinetics in the pharmaceutical formulation | N.A. |
| Sample type (what/where should be measured?) | Bifonazole (10 mg/g)–cream-semisolid dosage form | Method development for quantification of bifonazole in the cream pharmaceutical formulation | N.A. |
| Product type (when should be measured?) | Development product | Measuring spectra, provide indispensable qualitative data to assess the feasibility of manufacturing process or the final effectiveness of formulation | N.A. |
| Method application | Quantification of bifonazole in semisolid dosage form | The spectra profile of cream dosage form needs to be inspected, as they may influence drug delivery as well as impact patient adherence to treatment | N.A. |
| Analytical method | RP-HPLC analysis | Perform the measurement of the semisolid dosage form by means chromatography | N.A. |
| NIR analysis | Perform the measurement of the semisolid dosage form by means spectroscopy | N.A. | |
| Equipment | HPLC equipped with quaternary pump system and UV-Vis detector | The use of a quaternary pump allows a precise mixing of mobile-phase solvents | N.A. |
| Spectrum 400 FT-IR and FT-NIR spectrometer, fitted with an InGaAs detector and an optic fiber probe with 1 mm path length | NIR is applicable to both organic and inorganic compounds, enabling its use in diverse areas | N.A. | |
| HPLC CAAs | Number of theoretical plates, retention time, tailing factor | These attributes should meet their formal or commonly acceptable quality criteria | Number of theoretical plates: >2000; retention time: ~5.0 min; tailing factor: <2 |
| NIR CAAs | Spectra and concentrations | These CAAs should reflect the maximization of the spectral signal | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarentin, L.; Moura, V.; Pais, A.A.C.C.; Vitorino, C. AQbD Approach Applied to NIR in a Complex Topical Formulation: Bifonazole as Case Study. Pharmaceutics 2025, 17, 835. https://doi.org/10.3390/pharmaceutics17070835
Chiarentin L, Moura V, Pais AACC, Vitorino C. AQbD Approach Applied to NIR in a Complex Topical Formulation: Bifonazole as Case Study. Pharmaceutics. 2025; 17(7):835. https://doi.org/10.3390/pharmaceutics17070835
Chicago/Turabian StyleChiarentin, Lucas, Vera Moura, Alberto A. C. C. Pais, and Carla Vitorino. 2025. "AQbD Approach Applied to NIR in a Complex Topical Formulation: Bifonazole as Case Study" Pharmaceutics 17, no. 7: 835. https://doi.org/10.3390/pharmaceutics17070835
APA StyleChiarentin, L., Moura, V., Pais, A. A. C. C., & Vitorino, C. (2025). AQbD Approach Applied to NIR in a Complex Topical Formulation: Bifonazole as Case Study. Pharmaceutics, 17(7), 835. https://doi.org/10.3390/pharmaceutics17070835








