Molecular Design of Novel Protein-Degrading Therapeutics Agents Currently in Clinical Trial
Abstract
1. Introduction
2. Main Text
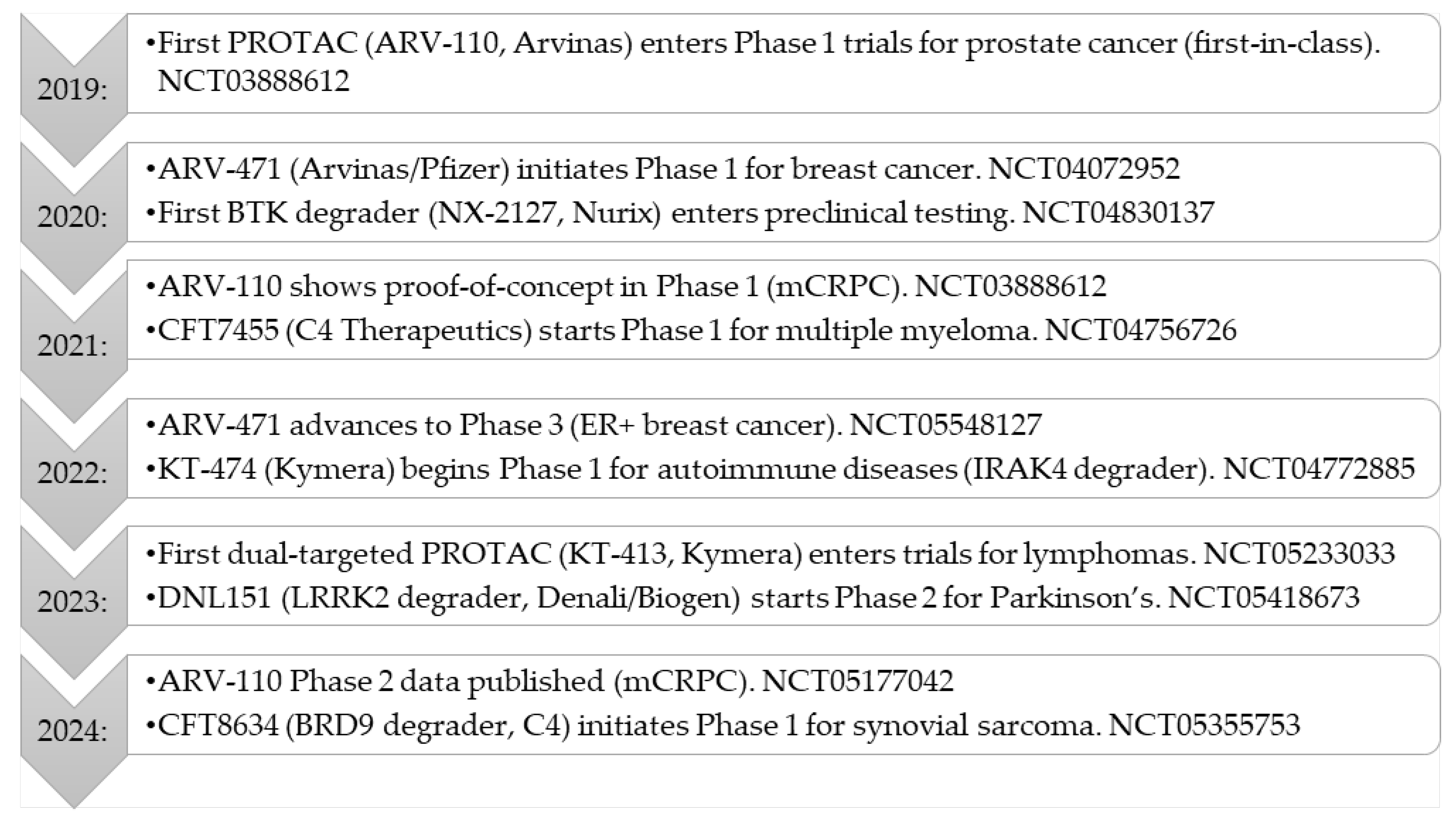
2.1. Current Status of Clinical Trials Against PROTACs
2.2. Molecular Design and Action Mechanism of PROTACs Currently in Clinical Trials
2.3. Current Clinical Trials
3. Discussion
3.1. Expansion of PROTACs Beyond Oncology and Neurological Diseases
3.2. Challenges in PROTAC Development
4. Conclusions
4.1. PROTACs Clinical Landscape
4.2. Comparison of PROTACs with Other Degrader Approaches
4.3. Final Considerations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug. Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. A new path to targeted protein degradation? Nat. Rev. Drug. Discov. 2022, 21, 706–707. [Google Scholar] [PubMed]
- Gough, S.M.; Flanagan, J.J.; The, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clin. Cancer Res. 2024, 30, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Biochempeg. Comprehensive Overview of PROTACs in Development. Available online: https://www.biochempeg.com/article/289.html (accessed on 15 May 2025).
- CAS. Insights on PROTAC Drug Development: Current Progress and Future Directions. Available online: https://www.cas.org/resources/cas-insights/protac-drug-development (accessed on 15 May 2025).
- Ackerman, L.; Acloque, G.; Bacchelli, S.; Schwartz, H.; Feinstein, B.J.; La Stella, P.; Alavi, A.; Gollerkeri, A.; Davis, J.; Campbell, V.; et al. IRAK4 degrader in hidradenitis suppurativa and atopic dermatitis: A phase 1 trial. Nat. Med. 2023, 29, 3127–3136. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Noviski, M.; Lu, H.; Thompson, M.C.; Chirino, A.; Jahn, J.; Sondhi, A.K.; Gajewski, S.; Tan, Y.S.M.; et al. Kinase-impaired BTK mutations are susceptible to clinical-stage BTK and IKZF1/3 degrader NX-2127. Science 2024, 383, eadi5798. [Google Scholar] [CrossRef]
- Weiss, M.M.; Zheng, X.; Ji, N.; Browne, C.M.; Campbell, V.; Chen, D.; Enerson, B.; Fei, X.; Huang, X.; Klaus, C.R.; et al. Discovery of KT-413, a Targeted Protein Degrader of IRAK4 and IMiD Substrates Targeting MYD88 Mutant Diffuse Large B-Cell Lymphoma. J. Med. Chem. 2024, 67, 10548–10566. [Google Scholar] [CrossRef]
- Jackson, K.L.; Agafonov, R.V.; Carlson, M.W.; Chaturvedi, P.; Cocozziello, D.; Cole, K.; Deibler, R.; Scott, J.; Good, E.A.; Hart, A.A.; et al. Abstract ND09: The discovery and characterization of CFT8634: A potent and selective degrader of BRD9 for the treatment of SMARCB1-perturbed cancers. Cancer Res. 2022, 82, ND09. [Google Scholar] [CrossRef]
- Lv, D.; Pal, P.; Liu, X.; Jia, Y.; Thummuri, D.; Zhang, P.; Hu, W.; Pei, J.; Zhang, Q.; Zhou, S.; et al. Development of a BCL-xL and BCL-2 dual degrader with improved anti-leukemic activity. Nat. Commun. 2021, 12, 6896. [Google Scholar] [CrossRef]
- Guedeney, N.; Cornu, M.; Schwalen, F.; Kieffer, C.; Voisin-Chiret, A.S. PROTAC technology: A new drug design for chemical biology with many challenges in drug discovery. Drug. Discov. Today 2023, 28, 103395. [Google Scholar] [CrossRef]
- Biochempeg. Summary of PROTAC Degraders in Clinical Trials. Available online: https://www.biochempeg.com/article/282.html (accessed on 15 May 2025).
- Hyun, S.; Shin, D. Chemical-Mediated Targeted Protein Degradation in Neurodegenerative Diseases. Life 2021, 11, 7. [Google Scholar] [CrossRef]
- Zhu, Y.; Dai, Y.; Tian, Y. The Peptide PROTAC Modality: A New Strategy for Drug Discovery. MedComm 2025, 6, e70133. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Alves Avelar, L.A.; Murray, L.; Kurz, T. Designing HDAC-PROTACs: Lessons learned so far. Future Med. Chem. 2022, 14, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bolinger, A.A.; Zhou, J. Novel approaches to targeted protein degradation technologies in drug discovery. Expert Opin. Drug Discov. 2023, 18, 467–483. [Google Scholar] [CrossRef]
- Schade, M.; Scott, J.S.; Hayhow, T.G.; Pike, A.; Terstiege, I.; Ahlqvist, M.; Johansson, J.R.; Diene, C.R.; Fallan, C.; Balazs, A.Y.S.; et al. Structural and Physicochemical Features of Oral PROTACs. J. Med. Chem. 2024, 67, 13106–13116. [Google Scholar] [CrossRef]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. 2021, 26, 484–502. [Google Scholar] [CrossRef]
- Stevens, R.; Shrives, H.J.; Cryan, J.; Klimaszewska, D.; Stacey, P.; Burley, G.A.; Harling, J.D.; Battersby, D.J.; Miah, A.H. Expanding the reaction toolbox for nanoscale direct-to-biology PROTAC synthesis and biological evaluation. RSC Med. Chem. 2025, 16, 1141–1150. [Google Scholar] [CrossRef]
- Rambacher, K.M.; Calabrese, M.F.; Yamaguchi, M. Perspectives on the development of first-in-class protein degraders. Future Med. Chem. 2021, 13, 1203–1226. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Dong, J.; Qin, J.J. An updated review on the role of small molecules in mediating protein degradation. Eur. J. Med. Chem. 2025, 287, 117370. [Google Scholar] [CrossRef]
- Tashima, T. Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier. Antibodies 2023, 12, 43. [Google Scholar] [CrossRef]
- Rovers, E.; Schapira, M. Methods for computer-assisted PROTAC design. Methods Enzym. 2023, 690, 311–340. [Google Scholar]
- VanDyke, D.; Taylor, J.D.; Kaeo, K.J.; Hunt, J.; Spangler, J.B. Biologics-based degraders—An expanding toolkit for targeted-protein degradation. Curr. Opin. Biotechnol. 2022, 78, 102807. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.M.; Nowak, R.P.; Ebert, B.L.; Fischer, E.S. Targeted protein degradation: From mechanisms to clinic. Nat. Rev. Mol. Cell Biol. 2024, 25, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Huang, W.; Zheng, X.; Cheng, L.; Zhang, Z.; Wang, J.; Shen, Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges. Eur. J. Med. Chem. 2021, 210, 112981. [Google Scholar] [CrossRef]
- Ming, H.; Li, B.; Jiang, J.; Qin, S.; Nice, E.C.; He, W.; Lang, T.; Huang, C. Protein degradation: Expanding the toolbox to restrain cancer drug resistance. J. Hematol. Oncol. 2023, 16, 6. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Liu, J.; Zhang, H.; Li, J.; Wang, Z.; Shang, G. PROTACs: Novel tools for improving immunotherapy in cancer. Cancer Lett. 2023, 560, 216128. [Google Scholar] [CrossRef]
- Li, K.; Crews, C.M. PROTACs: Past, present and future. Chem. Soc. Rev. 2022, 51, 5214–5236. [Google Scholar] [CrossRef]
- Espinoza-Chávez, R.M.; Salerno, A.; Liuzzi, A.; Ilari, A.; Milelli, A.; Uliassi, E.; Bolognesi, M.L. Targeted Protein Degradation for Infectious Diseases: From Basic Biology to Drug Discovery. ACS Bio Med. Chem. Au. 2022, 3, 32–45. [Google Scholar] [CrossRef]
- Poirson, J.; Cho, H.; Dhillon, A.; Haider, S.; Imrit, A.Z.; Lam, M.H.Y.; Alerasool, N.; Lacoste, J.; Mizan, L.; Wong, C.; et al. Proteome-scale discovery of protein degradation and stabilization effectors. Nature 2024, 628, 878–886. [Google Scholar] [CrossRef]
- Petrilli, W.L.; Adam, G.C.; Erdmann, R.S.; Abeywickrema, P.; Agnani, V.; Ai, X.; Baysarowich, J.; Byrne, N.; Caldwell, J.P.; Chang, W.; et al. From Screening to Targeted Degradation: Strategies for the Discovery and Optimization of Small Molecule Ligands for PCSK9. Cell Chem. Biol. 2020, 27, 32–40. [Google Scholar] [CrossRef]
- Venkatesan, J.; Murugan, D.; Lakshminarayanan, K.; Smith, A.R.; Vasanthakumari Thirumalaiswamy, H.; Kandhasamy, H.; Zender, B.; Zheng, G.; Rangasamy, L. Powering up targeted protein degradation through active and passive tumour-targeting strategies: Current and future scopes. Pharmacol. Ther. 2024, 263, 108725. [Google Scholar] [CrossRef] [PubMed]
- Bhole, R.P.; Labhade, S.; Gurav, S.S. Conquering PROTAC molecular design and drugability. Bioanalysis 2025, 17, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Kim, J.M. Targeted Protein Degradation to Overcome Resistance in Cancer Therapies: PROTAC and N-Degron Pathway. Biomedicines 2022, 10, 2100. [Google Scholar] [CrossRef] [PubMed]
- Arvinas. Phase 2 Data for ARV-110 in Prostate Cancer. ASCO Annual Meeting Abstracts. [Abstract #4505]. Journal Article Corresponding to a Meeting Abstract. 2024. Available online: https://meetings.asco.org/abstracts-presentations/169992 (accessed on 15 May 2025).
- Ramadas, B.; Kumar Pain, P.; Manna, D. LYTACs: An Emerging Tool for the Degradation of Non-Cytosolic Proteins. Chem. Med. Chem. 2021, 16, 2951–2953. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Churcher, I. Protac-induced protein degradation in drug discovery: Breaking the rules or just making new ones? J. Med. Chem. 2018, 61, 444–452. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef]
- Ahn, G.; Banik, S.M.; Miller, C.L.; Riley, N.M.; Cochran, J.R.; Bertozzi, C.R. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 2021, 17, 937–946. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Schreiber, S.L. The rise of molecular glues. Cell 2021, 184, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P.P.; Hamann, L.G. Development of targeted protein degradation therapeutics. Nat. Chem. Biol. 2019, 15, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Moriyama, J.; Nakamura, T.; Miki, E.; Takahashi, E.; Sato, A.; Akaike, T.; Itto-Nakama, K.; Arimoto, H. AUTACs: Cargo-specific degraders using selective autophagy. Mol. Cell 2019, 76, 797–810.e10. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.H.; Lee, M.J.; Kim, H.Y.; Heo, A.J.; Park, D.Y.; Kim, Y.K.; Kim, B.Y.; Kwon, Y.T. Targeted protein degradation via the autophagy-lysosome system: AUTOTAC (AUTOphagy-TArgeting Chimera). Autophagy 2022, 18, 2259–2262. [Google Scholar] [CrossRef]
- Danishuddin; Jamal, M.S.; Song, K.S.; Lee, K.W.; Kim, J.J.; Park, Y.M. Revolutionizing Drug Targeting Strategies: Integrating Artificial Intelligence and Structure-Based Methods in PROTAC Development. Pharmaceuticals 2023, 16, 1649. [Google Scholar] [CrossRef]
- Abbas, A.; Ye, F. Computational methods and key considerations for in silico design of proteolysis targeting chimera (PROTACs). Int. J. Biol. Macromol. 2024, 277, 134293. [Google Scholar] [CrossRef]
- Ge, J.; Hsieh, C.Y.; Fang, M.; Sun, H.; Hou, T. Development of PROTACs using computational approaches. Trends Pharmacol. Sci. 2024, 45, 1162–1174. [Google Scholar] [CrossRef]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great opportunities for academia and industry. Signal Transduct. Target. Ther. 2019, 4, 64. [Google Scholar] [CrossRef]
- Olson, C.M.; Jiang, B.; Erb, M.A.; Liang, Y.; Doctor, Z.M.; Zhang, Z.; Zhang, T.; Kwiatkowski, N.; Boukhali, M.; Green, J.L.; et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat. Chem. Biol. 2018, 14, 163–170. [Google Scholar] [CrossRef]
- Han, X.; Wang, C.; Qin, C.; Xiang, W.; Fernandez-Salas, E.; Yang, C.Y.; Wang, M.; Zhao, L.; Xu, T.; Chinnaswamy, K.; et al. Discovery of ARD-69 as a highly potent PROTAC degrader of androgen receptor for prostate cancer. J. Med. Chem. 2020, 63, 6649–6670. [Google Scholar]


| Drug Name | Target | Indication | Company | Clinical Trial Phase |
|---|---|---|---|---|
| ARV-471 | Estrogen receptor (ER) | Metastatic breast cancer | Arvinas/Pfizer | Phase 3, NCT05548127, NCT04072952 |
| ARV-110 | Androgen receptor (AR) | Prostate cancer | Arvinas | Phase 2, NCT03888612, NCT05177042 |
| ARV-766 | Androgen receptor (AR) | Prostate cancer | Arvinas | Phase 1/2, NCT05067140 |
| CFT7455 | IKZF1/3 | Multiple myeloma | C4 Therapeutics | Phase 1, NCT04756726 |
| KT-474 | IRAK4 | Autoimmune diseases | Kymera Therapeutics | Phase 1, NCT04772885 |
| CC-90009 | GSPT1 | Acute myeloid leukemia (AML) | Celgene (Bristol-Myers Squibb) | Phase 1, NCT02848001 |
| BCL-XL degrader | BCL-XL | Solid tumors | Not specified | Preclinical |
| NX-2127 | Bruton’s Tyrosine Kinase (BTK) | B-cell malignancies | Nurix Therapeutics | Phase 1, NCT04830137 |
| ACBI-001 | BCL6 | Non-Hodgkin lymphoma | Accent Therapeutics | Phase 1, NCT05508943 |
| DT2216 | BCL-XL | Hematologic malignancies | Dialectic Therapeutics | Phase 1, NCT05233033 |
| KT-413 | IRAK4/IMiD substrates | Lymphomas | Kymera Therapeutics | Phase 1, NCT05355753 |
| CFT8634 | BRD9 | Synovial sarcoma | C4 Therapeutics | Phase 1, NCT05355753 |
| Modality | Mechanism of Action | Clinical Status | References |
|---|---|---|---|
| PROTACs | Recruit E3 ubiquitin ligase to target protein, leading to ubiquitination and proteasomal degradation | Multiple agents in Phase 1–3 clinical trials (e.g., ARV-471, ARV-110, CFT7455, and KT-474) | [39,40,41] |
| LYTACs | Recruit lysosome-targeting receptors to mediate lysosomal degradation of extracellular and membrane proteins | Preclinical, early clinical evaluation | [42,43] |
| Molecular glues | Induce or stabilize protein–protein interactions between E3 ligase and target, leading to ubiquitination and degradation | Some agents (e.g., lenalidomide and CC-885) approved or in clinical trials | [44,45,46] |
| AUTACs | Tag target proteins with autophagy-targeting motifs for selective autophagic degradation | Preclinical | [47,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacin, E.; Sewduth, R.N. Molecular Design of Novel Protein-Degrading Therapeutics Agents Currently in Clinical Trial. Pharmaceutics 2025, 17, 744. https://doi.org/10.3390/pharmaceutics17060744
Kacin E, Sewduth RN. Molecular Design of Novel Protein-Degrading Therapeutics Agents Currently in Clinical Trial. Pharmaceutics. 2025; 17(6):744. https://doi.org/10.3390/pharmaceutics17060744
Chicago/Turabian StyleKacin, Ela, and Raj Nayan Sewduth. 2025. "Molecular Design of Novel Protein-Degrading Therapeutics Agents Currently in Clinical Trial" Pharmaceutics 17, no. 6: 744. https://doi.org/10.3390/pharmaceutics17060744
APA StyleKacin, E., & Sewduth, R. N. (2025). Molecular Design of Novel Protein-Degrading Therapeutics Agents Currently in Clinical Trial. Pharmaceutics, 17(6), 744. https://doi.org/10.3390/pharmaceutics17060744







