Bioadhesive Eudragit RL®100 Nanocapsules for Melanoma Therapy: A Repurposing Strategy for Propranolol
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Analytical Method
2.3. Nanocapsule Preparation
2.4. Physicochemical Characterization
2.4.1. pH Determination
2.4.2. Mean Particle Size, Polydispersity Index, and Zeta Potential
2.4.3. Drug Content and Encapsulation Efficiency (EE)
2.5. In Vitro Drug Release
2.6. Antioxidant Activity
2.7. Bioadhesiveness
2.8. Hemolysis Assay
2.9. Cell Viability Assay
2.10. Scratch Wound Assay
2.11. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characterization
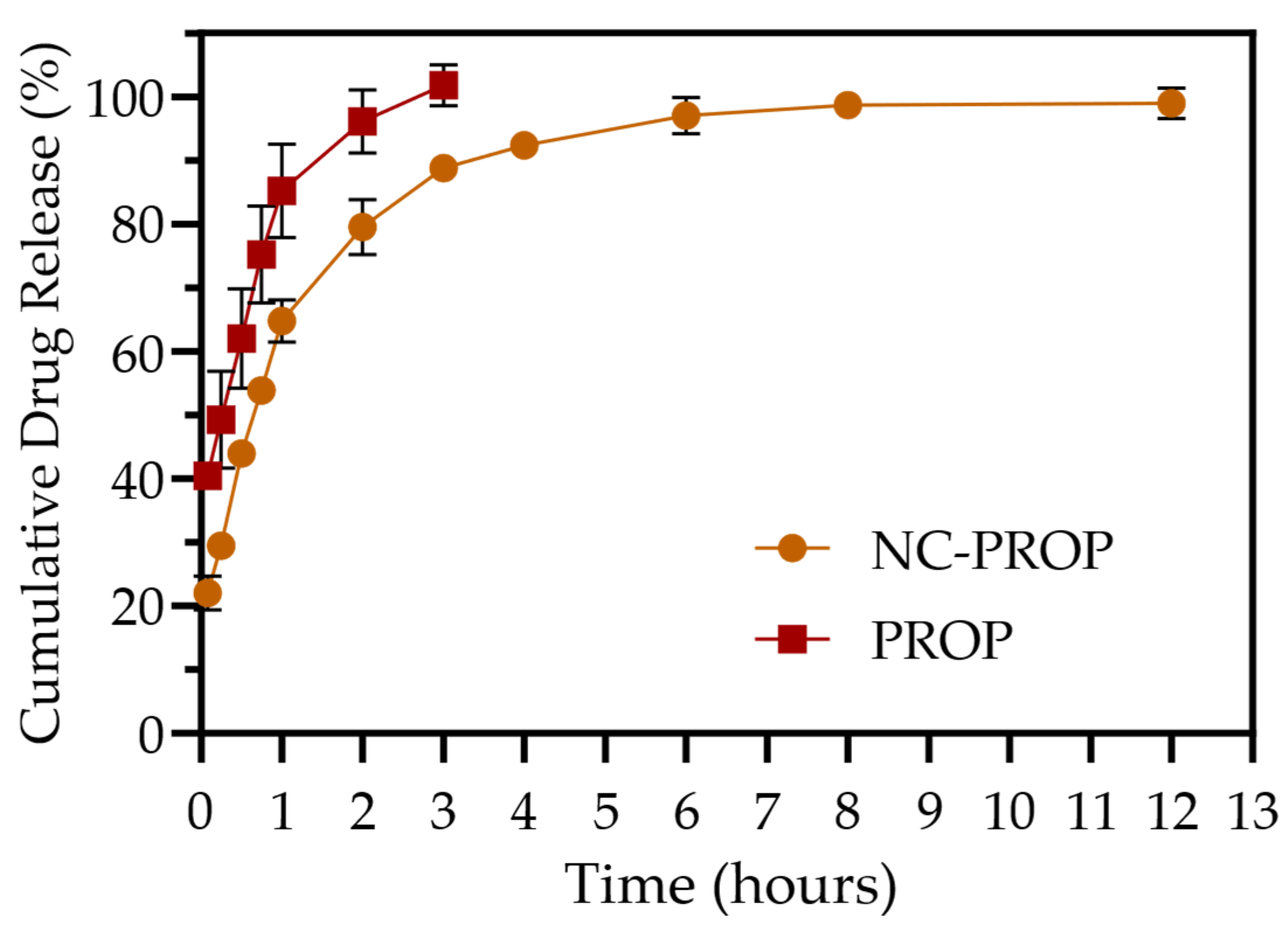
3.2. In Vitro Drug Release
3.3. Antioxidant Activity
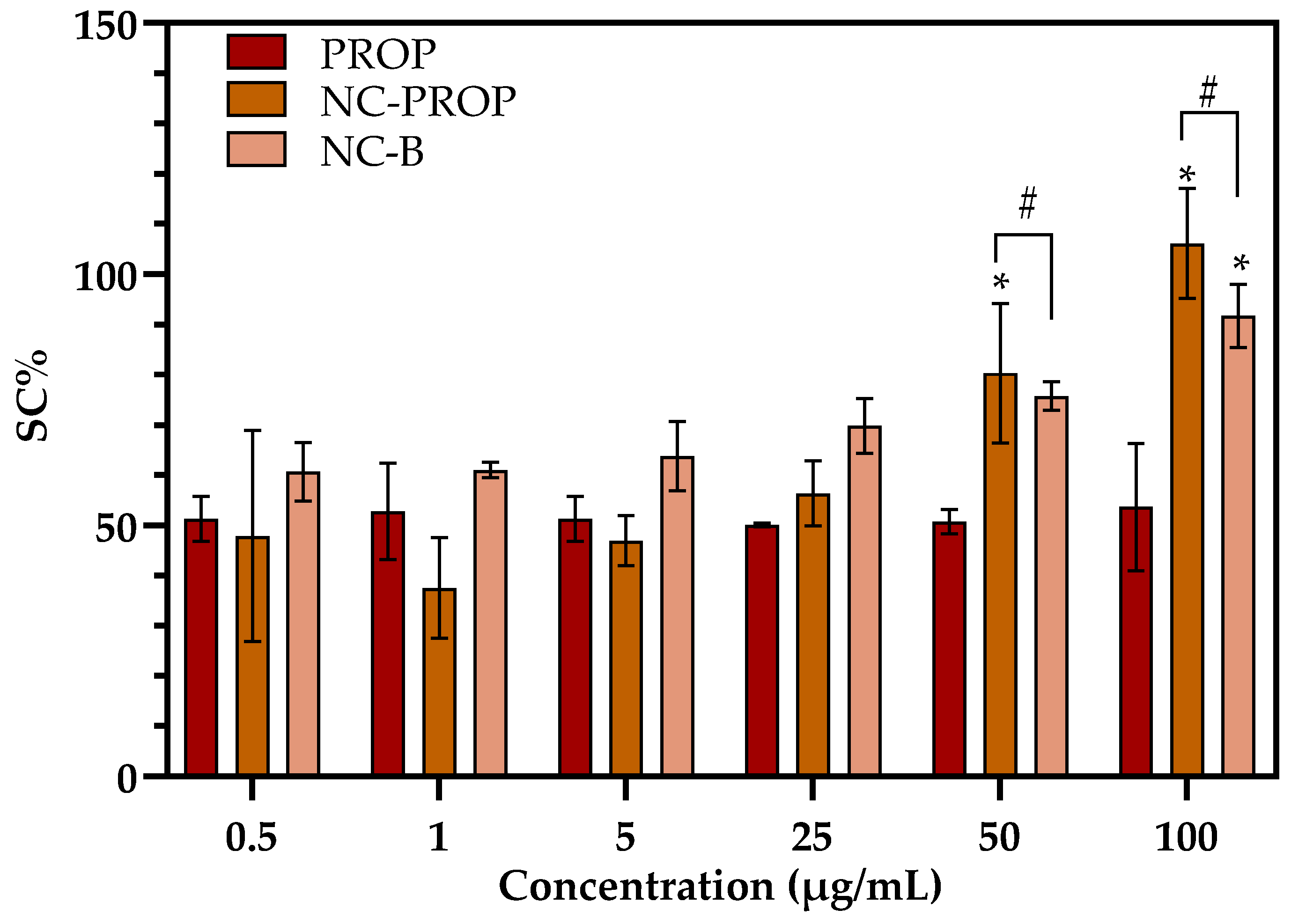
3.4. Bioadhesiveness
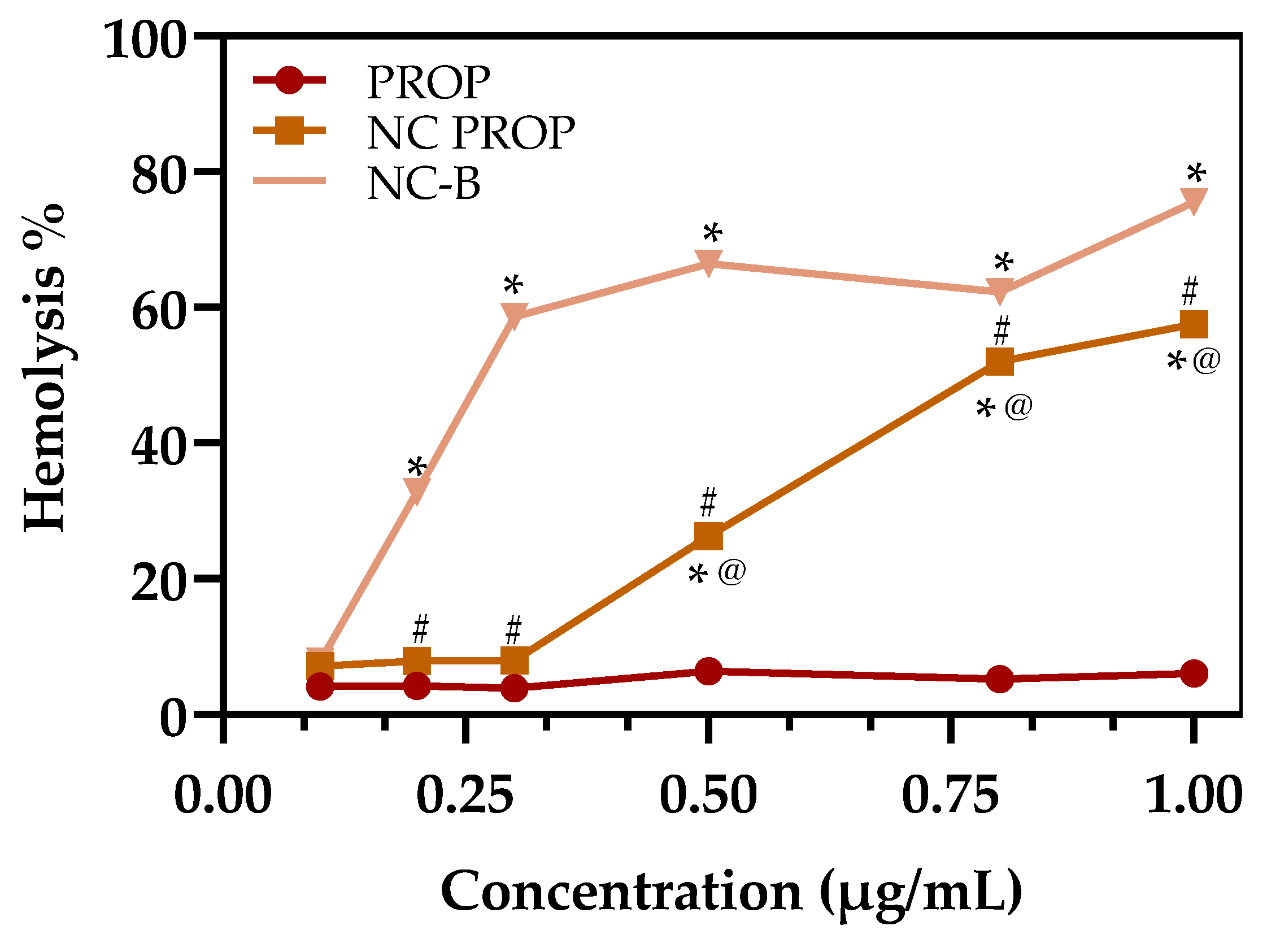
3.5. Hemolysis Assay
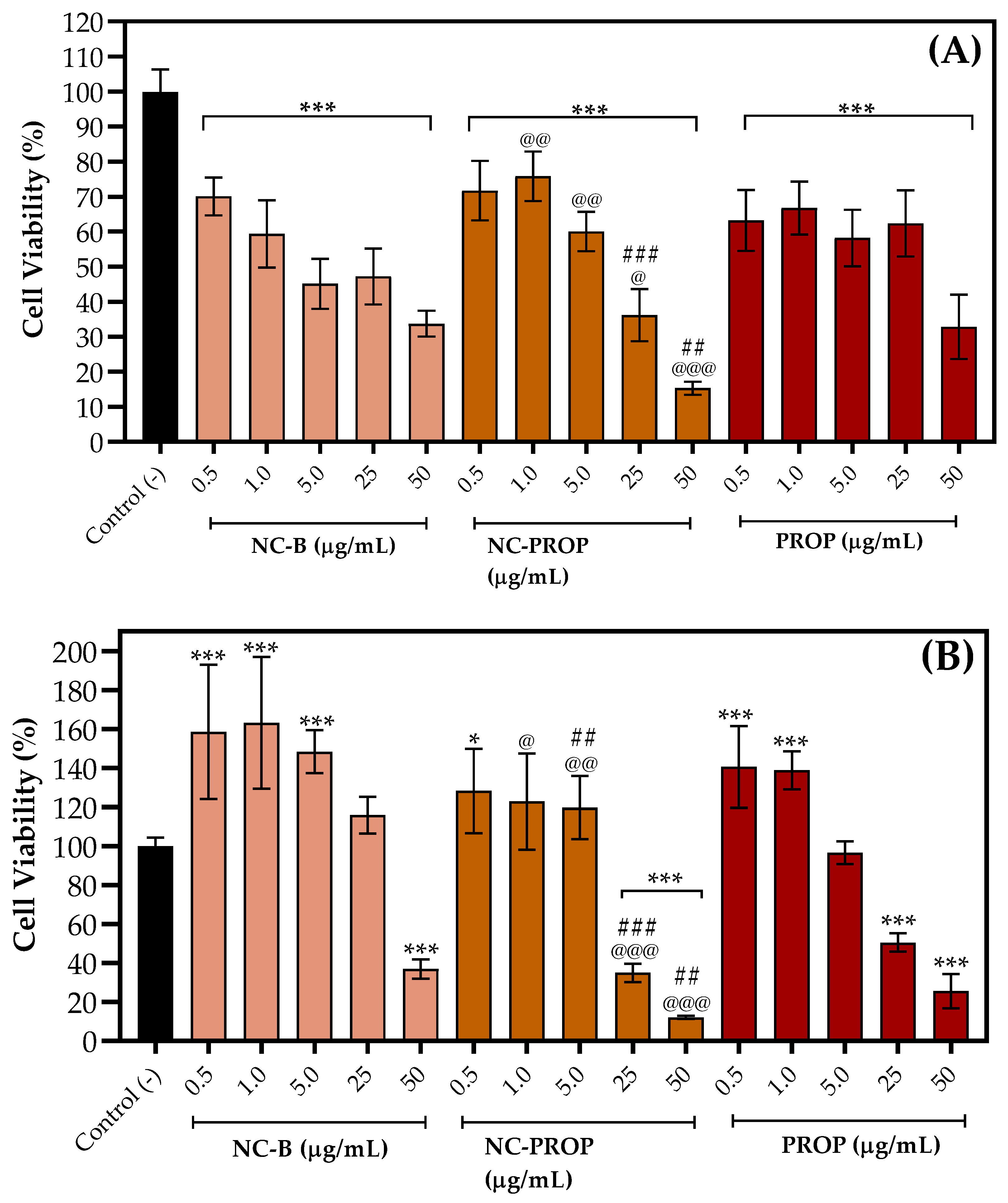
3.6. Cell Viability Assay
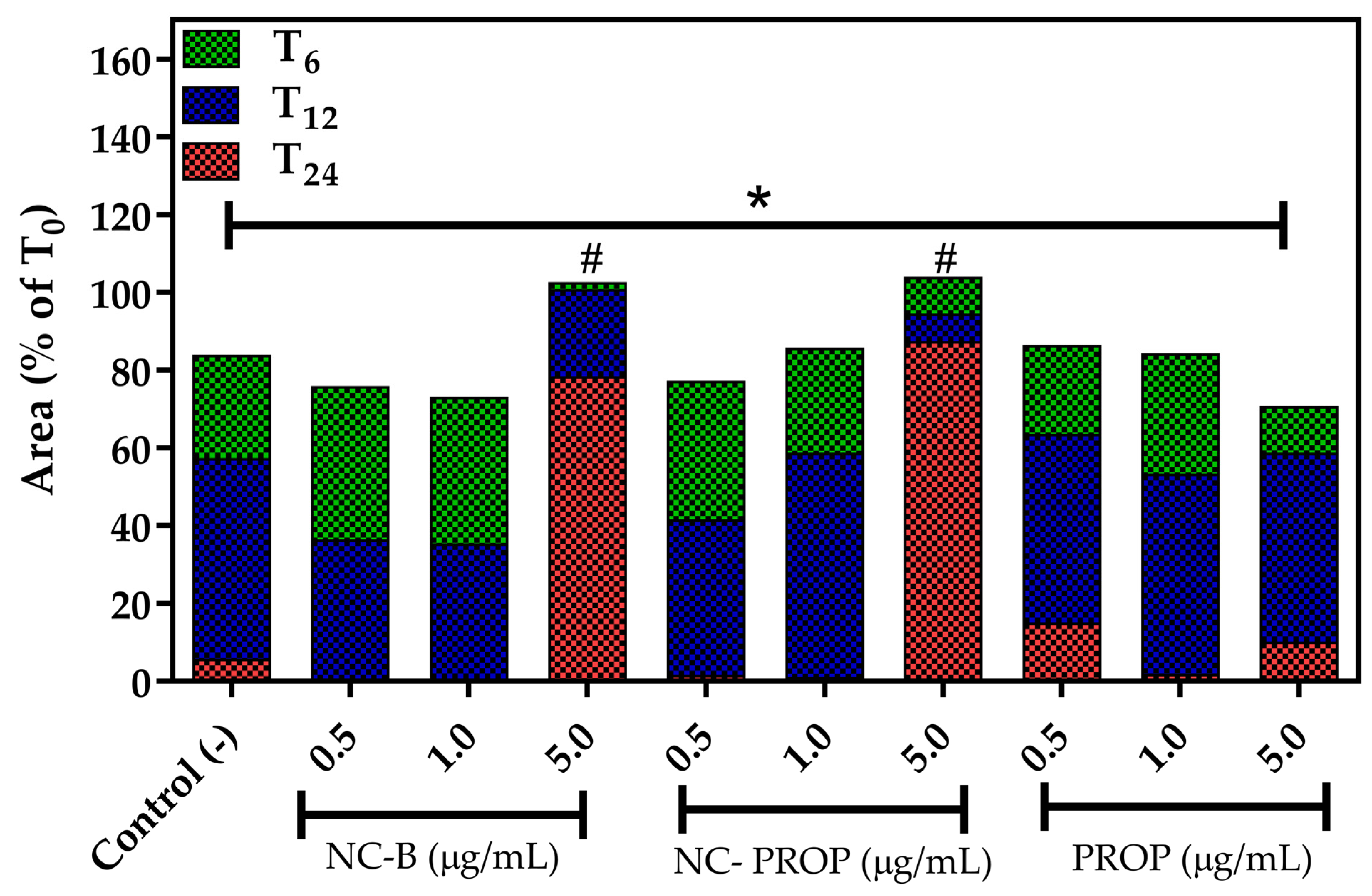
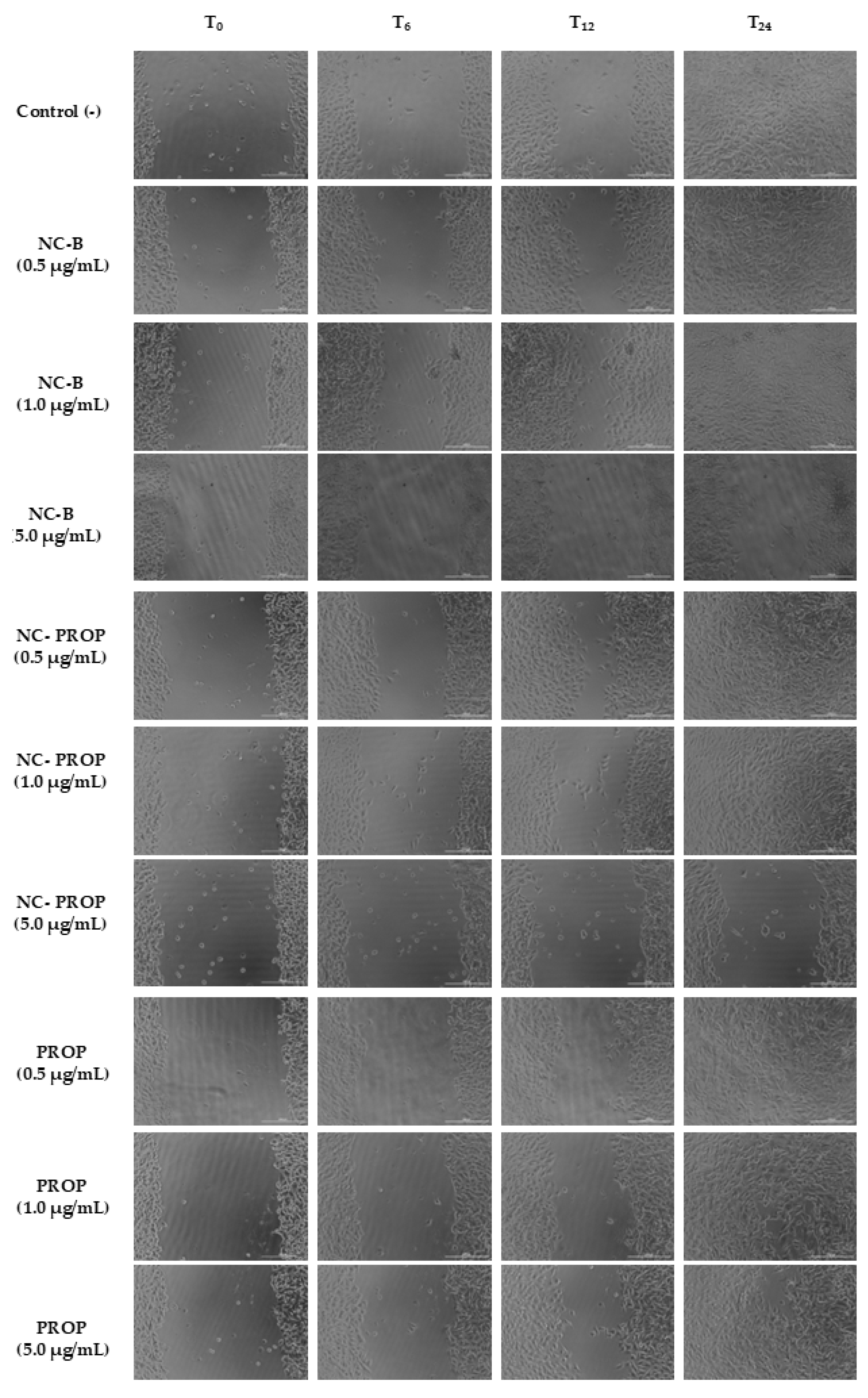
3.7. Scratch Wound Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, B.; Latorre, A.; Posch, C.; Somoza, Á. Recent Advances in Uveal Melanoma Treatment. Med. Res. Rev. 2017, 37, 1350–1372. [Google Scholar] [CrossRef] [PubMed]
- Maria LUCCHESE, A.; Nocchi KALIL, A.; Diniz, A.L.; Oldhafer, K.J.; Pawlik, T.M.; Adam, R.; Soubrane, O.; Ignez Braghiroli, M.; Lemos Cotta-Pereira, R.; de Biase Silva-Neto, W.; et al. Melanomas, sarcomas, and renal metastases in the liver: How to treat? ABCD Arq. Bras. Cir. Dig. 2025, 37, e1866. [Google Scholar] [CrossRef]
- Lavine, J.A.; Farnoodian, M.; Wang, S.; Darjatmoko, S.R.; Wright, L.S.; Gamm, D.M.; Ip, M.S.; Sorenson, C.M.; Sheibani, N. Β2–Adrenergic Receptor Antagonism Attenuates CNV Through Inhibition of VEGF and IL-6 Expression. Investig. Ophthalmol. Vis. Sci. 2017, 58, 299–308. [Google Scholar] [CrossRef]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. Beta Blockers and Melanoma. Open Access Maced. J. Med. Sci. 2019, 7, 2961–2963. [Google Scholar] [CrossRef]
- Bustamante, P.; Miyamoto, D.; Goyeneche, A.; de Alba Graue, P.G.; Jin, E.; Tsering, T.; Dias, A.B.; Burnier, M.N.; Burnier, J.V. Beta-blockers Exert Potent Anti-tumor Effects in Cutaneous and Uveal Melanoma. Cancer Med. 2019, 8, 7265. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Distler, J.H.; Hirth, A.; Kurowska-Stolarska, M.; Gay, R.E. Angiogenic and Angiostatic Factors in the Molecular Control of Angiogenesis. Q. J. Nucl. Med. Mol. 2003, 47, 149–161. [Google Scholar]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef]
- Modi, S.; Lowder, D.M. Medications for migraine prophylaxis. Am. Fam. Physician 2006, 73, 72–78, Erratum in Am. Fam. Physician 2006, 74, 1685. [Google Scholar] [PubMed]
- Cheema, S.A.; Ahmed, U.T.; Nasir, H.; Dogar, S.R.; Mustafa, Z. Effects of Propranolol in Accelerating Wound Healing and Attenuation of Hypermetabolism in Adult Burn Patients. J. Coll. Physicians Surg. Pak. 2020, 30, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.N.; Khanh, P.Q.; An, N.H. The Use of Propranolol in Adult Burn Patients: Safety and Outcome Influence. Burns 2022, 48, 767–773. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Botteri, E.; Pennacchioli, E.; Geppetti, P.; Gandini, S. Propranolol for Off-Label Treatment of Patients with Melanoma: Results from a Cohort Study. JAMA Oncol. 2018, 4, e172908. [Google Scholar] [CrossRef]
- Chaharband, F.; Varshochian, R.; Dinarvand, R.; Sabbaghi, H.; Rezaei Kanavi, M.; Daftarian, N.; Nourinia, R. Polymeric Propranolol Nanoparticles for Intraocular Delivery: Formulation, Characterization, and Vitreous Pharmacokinetics. J. Ophthalmic Vis. Res. 2024, 19, 41–50. [Google Scholar] [CrossRef]
- Ågesen, F.N.; Weeke, P.E.; Tfelt-Hansen, P.; Tfelt-Hansen, J. Pharmacokinetic Variability of Beta-adrenergic Blocking Agents Used in Cardiology. Pharmacol. Res. Perspect. 2019, 7, e00496. [Google Scholar] [CrossRef]
- DailyMed-Propranolol Hydrochloride Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee09b4db-4d28-4748-a471-0f7b8c491f54 (accessed on 21 April 2025).
- Léaute-Labrèze, C.; Boccara, O.; Degrugillier-Chopinet, C.; Mazereeuw-Hautier, J.; Prey, S.; Lebbé, G.; Gautier, S.; Ortis, V.; Lafon, M.; Montagne, A.; et al. Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review. Pediatrics 2016, 138, e20160353. [Google Scholar] [CrossRef]
- Droitcourt, C.; Kerbrat, S.; Rault, C.; Botrel, M.A.; Happe, A.; Garlantezec, R.; Guillot, B.; Schleich, J.M.; Oger, E.; Dupuy, A. Safety of Oral Propranolol for Infantile Hemangioma. Pediatrics 2018, 141, e20173783. [Google Scholar] [CrossRef]
- Wood, A.; Carr, K.; Vestal, R.; Belcher, S.; Wilkinson, G.; Shand, D. Direct Measurement of Propranolol Bioavailability during Accumulation to Steady-state. Br. J. Clin. Pharmacol. 1978, 6, 345–350. [Google Scholar] [CrossRef]
- Nace, G.S.; Wood, A.J.J. Pharmacokinetics of Long Acting Propranolol Implications for Therapeutic Use. Clin. Pharmacokinet. 1987, 13, 51–64. [Google Scholar] [CrossRef]
- Siahi-Shadbad, M.R.; Asare-Addo, K.; Azizian, K.; Hassanzadeh, D.; Nokhodchi, A. Release Behaviour of Propranolol HCl from Hydrophilic Matrix Tablets Containing Psyllium Powder in Combination with Hydrophilic Polymers. AAPS PharmSciTech 2011, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Perez-Marcos, B.; Ford, J.L.; Armstrong, D.J.; Elliott, P.N.C.; Rostron, C.; Hogan, J.E. Influence of PH on the Release of Propranolol Hydrochloride from Matrices Containing Hydroxypropylmethylcellulose K4M and Carbopol 974. J. Pharm. Sci. 1996, 85, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Padula, C.; Nicoli, S.; Pescina, S.; Santi, P. The Influence of Formulation and Excipients on Propranolol Skin Permeation and Retention. Biomed. Res. Int. 2018, 2018, 1281673. [Google Scholar] [CrossRef]
- Uwai, K.; Tani, M.; Ohtake, Y.; Abe, S.; Maruko, A.; Chiba, T.; Hamaya, Y.; Ohkubo, Y.; Takeshita, M. Photodegradation Products of Propranolol: The Structures and Pharmacological Studies. Life Sci. 2005, 78, 357–365. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-Based Nanocapsules for Drug Delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Ourique, A.F.; Gomes, P. Cationic Nanocapsules Containing Eudragit RS100® and Its Potential for Application in Nanomedicine. Discip. Sci.|Nat. E Tecnológicas 2018, 18, 545–566. [Google Scholar]
- Reolon, J.B.; Merg, C.D.; de Oliveira, D.B.; Cabral, F.L.; Osmari, B.F.; Sari, M.H.M.; Brucker, N.; Leal, D.B.R.; Cruz, L. Pomegranate Oil-Based Nanocapsules Enhance 3,3’-Diindolylmethane Action against Melanoma Cells. Braz. J. Pharm. Sci. 2024, 60, e23931. [Google Scholar] [CrossRef]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Schäfer-Korting, M. Skin Penetration and Dermal Tolerability of Acrylic Nanocapsules: Influence of the Surface Charge and a Chitosan Gel Used as Vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Cardoso, A.M.L.; Oliveira, E.E.; Machado, B.A.S.; Marcelino, H.R. Eudragit®-Based Nanoparticles for Controlled Release through Topical Use. J. Nanoparticle Res. 2023, 25, 32. [Google Scholar] [CrossRef]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Beber, T.C.; De Andrade, D.F.; Dos Santos Chaves, P.; Pohlmann, A.R.; Guterres, S.S.; Ruver Beck, R.C. Cationic Polymeric Nanocapsules as a Strategy to Target Dexamethasone to Viable Epidermis: Skin Penetration and Permeation Studies. J. Nanosci. Nanotechnol. 2016, 16, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Kutz, A.; Rabia, S.H.; Ilan, E.B.; Goldberg, I.; Sprecher, E.; Harel, A. Assessment of the Effectiveness of Topical Propranolol 4% Gel for Infantile Hemangiomas. Int. J. Dermatol. 2017, 56, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagura, Y.; Hakamata, A.; Shirai, M.; Endoh, A.; Tanaka, S.; Inui, N.; Watanabe, H.; Namiki, N.; Uchida, S. Topical Formulations of Propranolol for Infantile Hemangiomas: Characteristics of Formulations and Three Cases of Infants Administered Topical Propranolol Cream. Chem. Pharm. Bull. 2022, 70, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Garjani, A.; Barzegar-Jalali, M.; Osouli-Bostanabad, K.; Ranjbar, H.; Adibkia, K. Morphological and Physicochemical Evaluation of the Propranolol HCl–Eudragit® RS100 Electrosprayed Nanoformulations. Artif. Cells Nanomed. Biotechnol. 2018, 46, 749–756. [Google Scholar] [CrossRef]
- BRASIL. Agência Nacional de Vigilância Sanitária. In Farmacopeia Brasileira, 7th ed.; Anvisa: Brasília, Brazil, 2024.
- Sari, M.H.M.; da Cruz Weber Fulco, B.; Ferreira, L.M.; Pegoraro, N.S.; da Silva Brum, E.; Casola, K.K.; Marchiori, M.C.L.; de Oliveira, S.M.; Nogueira, C.W.; Cruz, L. Nanoencapsulation Potentiates the Cutaneous Anti-Inflammatory Effect of p,P′-Methoxyl-Diphenyl Diselenide: Design, Permeation, and in Vivo Studies of a Nanotechnological-Based Carrageenan Gum Hydrogel. Eur. J. Pharm. Sci. 2020, 153, 105500. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Takeuchi, H.; Thongborisute, J.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Novel Mucoadhesion Tests for Polymers and Polymer-Coated Particles to Design Optimal Mucoadhesive Drug Delivery Systems. Adv. Drug Deliv. Rev. 2005, 57, 1583–1594. [Google Scholar] [CrossRef]
- F756 Standard Practice for Assessment of Hemolytic Properties of Materials. Available online: https://store.astm.org/f0756-17.html (accessed on 21 April 2025).
- Bürk, R.R. A Factor from a Transformed Cell Line That Affects Cell Migration. Proc. Natl. Acad. Sci. USA 1973, 70, 369–372. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle Formulations of Cisplatin for Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef]
- Younis, M.K.; Elakkad, Y.E.; Fakhr Eldeen, R.R.; Ali, I.H.; Khalil, I.A. Propranolol-Loaded Trehalosome as Antiproliferative Agent for Treating Skin Cancer: Optimization, Cytotoxicity, and In Silico Studies. Pharmaceutics 2023, 15, 2033. [Google Scholar] [CrossRef]
- Maeda, H. The Enhanced Permeability and Retention (EPR) Effect in Tumor Vasculature: The Key Role of Tumor-Selective Macromolecular Drug Targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Yang, L.; Yao, D.; Shen, Y. Combinative Effects of β-Elemene and Propranolol on the Proliferation, Migration, and Angiogenesis of Hemangioma. PeerJ 2023, 11, e15643. [Google Scholar] [CrossRef] [PubMed]
- Zanela da Silva Marques, T.; Santos-Oliveira, R.; de Oliveira de Siqueira L., B.; Da Silva Cardoso, V.; De Freitas, Z.M.F.; Da Silva Ascenção Barros, R.D.C.; Villa, A.L.V.; de Souza de Bustamante Monteiro, M.S.; Dos Santos, E.P.; Ricci-Junior, E. Development and Characterization of a Nanoemulsion Containing Propranolol for Topical Delivery. Int. J. Nanomed. 2018, 13, 2827. [Google Scholar] [CrossRef]
- Kunzi-Rapp, K. Topical Propranolol Therapy for Infantile Hemangiomas. Pediatr. Dermatol. 2012, 29, 154–159. [Google Scholar] [CrossRef]
- Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines 2022, 13, 1623. [Google Scholar] [CrossRef]
- Khizar, S.; Alrushaid, N.; Alam Khan, F.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers Based Novel and Effective Drug Delivery System. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Ali, I.H.; Khalil, I.A.; El-Sherbiny, I.M. Single-Dose Electrospun Nanoparticles-in-Nanofibers Wound Dressings with Enhanced Epithelialization, Collagen Deposition, and Granulation Properties. ACS Appl. Mater. Interfaces 2016, 8, 14453–14469. [Google Scholar] [CrossRef]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-Loaded Nanocapsules Inhibit Murine Melanoma Tumor Growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Goodman & Gilman’s: The Pharmacolological Basis of Therapeutics, 14th ed.; AccessMedicine; McGraw Hill Medical: Chicago, IL, USA, 2023; Available online: https://accessmedicine.mhmedical.com/book.aspx?bookID=3191 (accessed on 21 April 2025).
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ourique, A.F.; Melero, A.; de Bona da Silva, C.; Schaefer, U.F.; Pohlmann, A.R.; Guterres, S.S.; Lehr, C.M.; Kostka, K.H.; Beck, R.C.R. Improved Photostability and Reduced Skin Permeation of Tretinoin: Development of a Semisolid Nanomedicine. Eur. J. Pharm. Biopharm. 2011, 79, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Taprantzi, D.; Zisimopoulos, D.; Thomopoulos, K.C.; Spiliopoulou, I.; Georgiou, C.D.; Tsiaoussis, G.; Triantos, C.; Gogos, C.A.; Labropoulou-Karatza, C.; Assimakopoulos, S.F. Propranolol Reduces Systemic Oxidative Stress and Endotoxemia in Cirrhotic Patients with Esophageal Varices. Ann. Gastroenterol. 2018, 31, 1–7. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, D.; Lima, J.L.F.C.; Fernandes, E. Antioxidant Activity of β-Blockers: An Effect Mediated by Scavenging Reactive Oxygen and Nitrogen Species? Bioorganic Med. Chem. 2006, 14, 4568–4577. [Google Scholar] [CrossRef]
- Lauko, K.K.; Nesterowicz, M.; Trocka, D.; Dańkowska, K.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Novel Properties of Old Propranolol─Assessment of Antiglycation Activity through In Vitro and In Silico Approaches. ACS Omega 2024, 9, 27559–27577. [Google Scholar] [CrossRef]
- Englert, A.V.; Verdi, C.M.; Santos, R.C.V.; Cruz, L.; Sari, M.H.M. Diphenyl Diselenide and Clotrimazole Co-Loaded into Eudragit® RS 100 Nanocapsules Formulation Has Superior Antioxidant Potential and Promising Anti-Candida Activity. Braz. Arch. Biol. Technol. 2020, 63, e20200087. [Google Scholar] [CrossRef]
- Sanzo, M.; Colucci, R.; Arunachalam, M.; Berti, S.; Moretti, S. Stress as a Possible Mechanism in Melanoma Progression. Dermatol. Res. Pract. 2010, 2010, 483493. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Vizzotto, B.S.; Bochi, G.V.; Guarda, N.S.; Nascimento, K.; Sagrillo, M.R.; Moresco, R.N.; Schuch, A.P.; Ourique, A.F.; Gomes, P. Nanoencapsulation of the Flavonoid Dihydromyricetin Protects against the Genotoxicity and Cytotoxicity Induced by Cationic Nanocapsules. Colloids Surf. B Biointerfaces 2019, 173, 798–805. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Nagy, Y.I.; Shehabeldine, A.M.; Okba, M.M. Thymol-Loaded Eudragit RS30D Cationic Nanoparticles-Based Hydrogels for Topical Application in Wounds: In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Osmari, B.F.; Medeiros, G.A.; Reolon, J.B.; Prado, V.C.; Brucker, N.; Cruz, L. Cationic Nanocapsule Suspension as an Alternative to the Sublingual Delivery of Nifedipine. Pharm. Dev. Technol. 2023, 28, 403–413. [Google Scholar] [CrossRef]
- Almuqbil, R.M.; Aldhubiab, B. Bioadhesive Nanoparticles in Topical Drug Delivery: Advances, Applications, and Potential for Skin Disorder Treatments. Pharmaceutics 2025, 17, 229. [Google Scholar] [CrossRef]
- Pautu, V.; Lepeltier, E.; Mellinger, A.; Riou, J.; Debuigne, A.; Jerome, C.; Clere, N.; Passirani, C. PH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers 2021, 13, 2028. [Google Scholar] [CrossRef]
- Hu, J.K.; Suh, H.W.; Qureshi, M.; Lewis, J.M.; Yaqoob, S.; Moscato, Z.M.; Griff, S.; Lee, A.K.; Yin, E.S.; Mark Saltzman, W.; et al. Nonsurgical Treatment of Skin Cancer with Local Delivery of Bioadhesive Nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2020575118. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Mahjub, R.; Haddadi, R.; Vafaei, S.Y. Design and Optimization of Cationic Nanocapsules for Topical Delivery of Tretinoin: Application of the Box-Behnken Design, In Vitro Evaluation, and Ex Vivo Skin Deposition Study. Biomed. Res. Int. 2021, 2021, 4603545. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Prado, V.C.; Nadal, J.M.; Azambuja, J.H.; da Silveira, E.F.; Nogueira, C.W.; Farago, P.V.; Braganhol, E.; et al. Design of Pegylated-Nanocapsules to Diphenyl Diselenide Administration: In Vitro Evidence of Hemocompatible and Selective Antiglioma Formulation. AAPS PharmSciTech 2020, 21, 307. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Gehrcke, M.; Ferrari Cervi, V.; Eliete Rodrigues Bitencourt, P.; Ferreira da Silveira, E.; Hofstatter Azambuja, J.; Prates Ramos, A.; Nascimento, K.; Beatriz Moretto, M.; Braganhol, E.; et al. Pomegranate Seed Oil Nanoemulsions with Selective Antiglioma Activity: Optimization and Evaluation of Cytotoxicity, Genotoxicity and Oxidative Effects on Mononuclear Cells. Pharm. Biol. 2016, 54, 2968–2977. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cervi, V.F.; Gehrcke, M.; da Silveira, E.F.; Azambuja, J.H.; Braganhol, E.; Sari, M.H.M.; Zborowski, V.A.; Nogueira, C.W.; Cruz, L. Ketoprofen-Loaded Pomegranate Seed Oil Nanoemulsion Stabilized by Pullulan: Selective Antiglioma Formulation for Intravenous Administration. Colloids Surf. B Biointerfaces 2015, 130, 272–277. [Google Scholar] [CrossRef]
- Katara, R.; Majumdar, D.K. Eudragit RL 100-Based Nanoparticulate System of Aceclofenac for Ocular Delivery. Colloids Surf. B Biointerfaces 2013, 103, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Mathes, D.; Bueno Macedo, L.; Baldissera Pieta, T.; Costa Maia, B.; Bueno Rolim, C.M.; Rubert Nogueira-Librelotto, D. The Role of Polymer Type and Surfactant Composition on the Toxicological Profile of Nanoparticles: An in Vitro Comparative Study. J. Biomater. Sci. Polym. Ed. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent Effect of Polymeric Nanoparticle Zeta Potential/Surface Charge, on Their Cytotoxicity and Affinity to Cells. Cell Prolif. 2015, 48, 465. [Google Scholar] [CrossRef]
- Yedgar, S.; Barshtein, G.; Gural, A. Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility. Micromachines 2022, 13, 2091. [Google Scholar] [CrossRef]
- Rahnama, S.; Movaffagh, J.; Shahroodi, A.; Jirofti, N.; Fazly Bazzaz, B.S.; Beyraghdari, M.; Hashemi, M.; Kalalinia, F. Development and Characterization of the Electrospun Melittin-Loaded Chitosan Nanofibers for Treatment of Acne Vulgaris in Animal Model. J. Ind. Text. 2022, 52, 1–24. [Google Scholar] [CrossRef]
- Tan, X.; Guo, S.; Wang, C. Propranolol in the Treatment of Infantile Hemangiomas. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1155–1163. [Google Scholar] [CrossRef]
- Shibuya, C.M.; Tjioe, K.C.; Oliveira, S.H.P.; Bernabé, D.G. Propranolol Inhibits Cell Viability and Expression of the Pro-Tumorigenic Proteins Akt, NF-ĸB, and VEGF in Oral Squamous Cell Carcinoma. Arch. Oral Biol. 2022, 136, 105383. [Google Scholar] [CrossRef]
- Yusuf Terzi, M. Propranolol Treatment Reduces A549-Derived Lung Cancer Spheroids via Intrinsic Apoptosis. J. Clin. Pract. Res. 2023, 45, 614–623. [Google Scholar] [CrossRef]
- Koh, M.; Takahashi, T.; Kurokawa, Y.; Kobayashi, T.; Saito, T.; Ishida, T.; Serada, S.; Fujimoto, M.; Naka, T.; Wada, N.; et al. Propranolol Suppresses Gastric Cancer Cell Growth by Regulating Proliferation and Apoptosis. Gastric Cancer 2021, 24, 1037–1049. [Google Scholar] [CrossRef]
- Terzi, M.Y.; Urhan-Kucuk, M. Anti-Proliferative Effects of Beta-Blocker Propranolol on Human Lung Cancer and Noncancer Cells. Bratisl. Med. J. 2023, 124, 292–303. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, G.; Cui, H.; Yao, Z.; Shao, C.; Yuan, W.; Chen, B. Cyclosporin-A Reduced the Cytotoxicity of Propranolol in HUVECs via P38 MAPK Signaling. Medicine 2022, 101, E28329. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, R.; Wang, J.; Yang, X.; Tu, J.; Xie, L.; Li, C.; Lao, Q.; Sun, C. Component-Based Biocompatibility and Safety Evaluation of Polysorbate 80. RSC Adv. 2017, 7, 15127–15138. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S.; Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, Y.; Bhatt, A.N. Anti-cancer drug-mediated increase in mitochondrial mass limits the application of metabolic viability-based MTT assay in cytotoxicity screening. Cytotechnology 2024, 76, 301–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Trindade, G.A.d.M.; Alves, L.A.; Lazo, R.E.L.; Dallabrida, K.G.; Reolon, J.B.; Bonini, J.S.; Nunes, K.C.; Garcia, F.P.; Nakamura, C.V.; Rego, F.G.d.M.; et al. Polysaccharide-Stabilized Semisolid Emulsion with Vegetable Oils for Skin Wound Healing: Impact of Composition on Physicochemical and Biological Properties. Pharmaceutics 2024, 16, 1426. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The Mtt Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- An, J.; Yao, W.; Tang, W.; Jiang, J.; Shang, Y. Hormesis Effect of Methyl Triclosan on Cell Proliferation and Migration in Human Hepatocyte L02 Cells. ACS Omega 2021, 6, 18904–18913. [Google Scholar] [CrossRef]
- Maccari, S.; Buoncervello, M.; Rampin, A.; Spada, M.; Macchia, D.; Giordani, L.; Stati, T.; Bearzi, C.; Catalano, L.; Rizzi, R.; et al. Biphasic Effects of Propranolol on Tumour Growth in B16F10 Melanoma-Bearing Mice. Br. J. Pharmacol. 2017, 174, 139–149. [Google Scholar] [CrossRef]
- Aoishi, Y.; Yoshimasu, T.; Oura, S.; Kawago, M.; Hirai, Y.; Miyasaka, M.; Ohashi, T.; Nishimura, Y. Quantitative Evaluation of Hormesis in Breast Cancer Using Histoculture Drug Response Assay. Dose-Response 2019, 17, 1559325819896183. [Google Scholar] [CrossRef]
- Rashkov, P.; Barrett, I.P.; Beardmore, R.E.; Bendtsen, C.; Gudelj, I. Kinase Inhibition Leads to Hormesis in a Dual Phosphorylation-Dephosphorylation Cycle. PLoS Comput. Biol. 2016, 12, e1005216. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Leso, V.; Calabrese, E.J. Hormetic Dose-Responses in Nanotechnology Studies. Sci. Total Environ. 2014, 487, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Feng, Z.Z.; Iavicoli, I.; Calabrese, E.J. The Two Faces of Nanomaterials: A Quantification of Hormesis in Algae and Plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, L.J.; Le Gal, F.A. Inhibition of Human Melanoma Growth by a Non-Cardioselective β-Blocker. J. Investig. Dermatol. 2015, 135, 525–531. [Google Scholar] [CrossRef]
- Fu, Q.T.; Zhong, X.Q.; Chen, M.Y.; Gu, J.Y.; Zhao, J.; Yu, D.H.; Tan, F. Luteolin-Loaded Nanoparticles for the Treatment of Melanoma. Int. J. Nanomed. 2023, 18, 2053–2068. [Google Scholar] [CrossRef]
- Trombino, S.; Malivindi, R.; Barbarossa, G.; Sole, R.; Curcio, F.; Cassano, R. Solid Lipid Nanoparticles Hydroquinone-Based for the Treatment of Melanoma: Efficacy and Safety Studies. Pharmaceutics 2023, 15, 1375. [Google Scholar] [CrossRef]
- Zare, M.; Shaverdi, H.; Kalaee, S.E.V. Anti-Cancer Effects of Pomegranate Seed Oil on Esophageal Cancer Cell Line (KYSE-30). Gene Cell Tissue 2021, 8, e108995. [Google Scholar] [CrossRef]

| Scheme | Particle Size | PDI | Zeta Potential | pH |
|---|---|---|---|---|
| NC-PROP | 151 ± 7.86 | 0.134 ± 0.01 | 25.84 ± 4.89 | 3.85 ± 0.117 |
| NC-B | 150 ± 1.74 | 0.166 ± 0.03 | 34.13 ± 8.70 | 3.95 ± 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieres, N.G.; Simião, S.d.O.; Cruz, L.S.; Melo, R.C.d.; Khalil, N.M.; Bonini, J.S.; Rego, F.G.d.M.; Sari, M.H.M.; Pontarolo, R.; Lazo, R.E.L.; et al. Bioadhesive Eudragit RL®100 Nanocapsules for Melanoma Therapy: A Repurposing Strategy for Propranolol. Pharmaceutics 2025, 17, 718. https://doi.org/10.3390/pharmaceutics17060718
Mieres NG, Simião SdO, Cruz LS, Melo RCd, Khalil NM, Bonini JS, Rego FGdM, Sari MHM, Pontarolo R, Lazo REL, et al. Bioadhesive Eudragit RL®100 Nanocapsules for Melanoma Therapy: A Repurposing Strategy for Propranolol. Pharmaceutics. 2025; 17(6):718. https://doi.org/10.3390/pharmaceutics17060718
Chicago/Turabian StyleMieres, Naomi Gerzvolf, Soraia de Oliveira Simião, Luiza Stolz Cruz, Rafaela Cirillo de Melo, Najeh Maissar Khalil, Juliana Sartori Bonini, Fabiane Gomes de Moraes Rego, Marcel Henrique Marcondes Sari, Roberto Pontarolo, Raul Edison Luna Lazo, and et al. 2025. "Bioadhesive Eudragit RL®100 Nanocapsules for Melanoma Therapy: A Repurposing Strategy for Propranolol" Pharmaceutics 17, no. 6: 718. https://doi.org/10.3390/pharmaceutics17060718
APA StyleMieres, N. G., Simião, S. d. O., Cruz, L. S., Melo, R. C. d., Khalil, N. M., Bonini, J. S., Rego, F. G. d. M., Sari, M. H. M., Pontarolo, R., Lazo, R. E. L., Reolon, J. B., & Ferreira, L. M. (2025). Bioadhesive Eudragit RL®100 Nanocapsules for Melanoma Therapy: A Repurposing Strategy for Propranolol. Pharmaceutics, 17(6), 718. https://doi.org/10.3390/pharmaceutics17060718












