The Impact of Substance Use Disorder and Drug Transfer into Breast Milk: Implications for Maternal and Infant Health
Abstract
1. Why Is Milk Important?
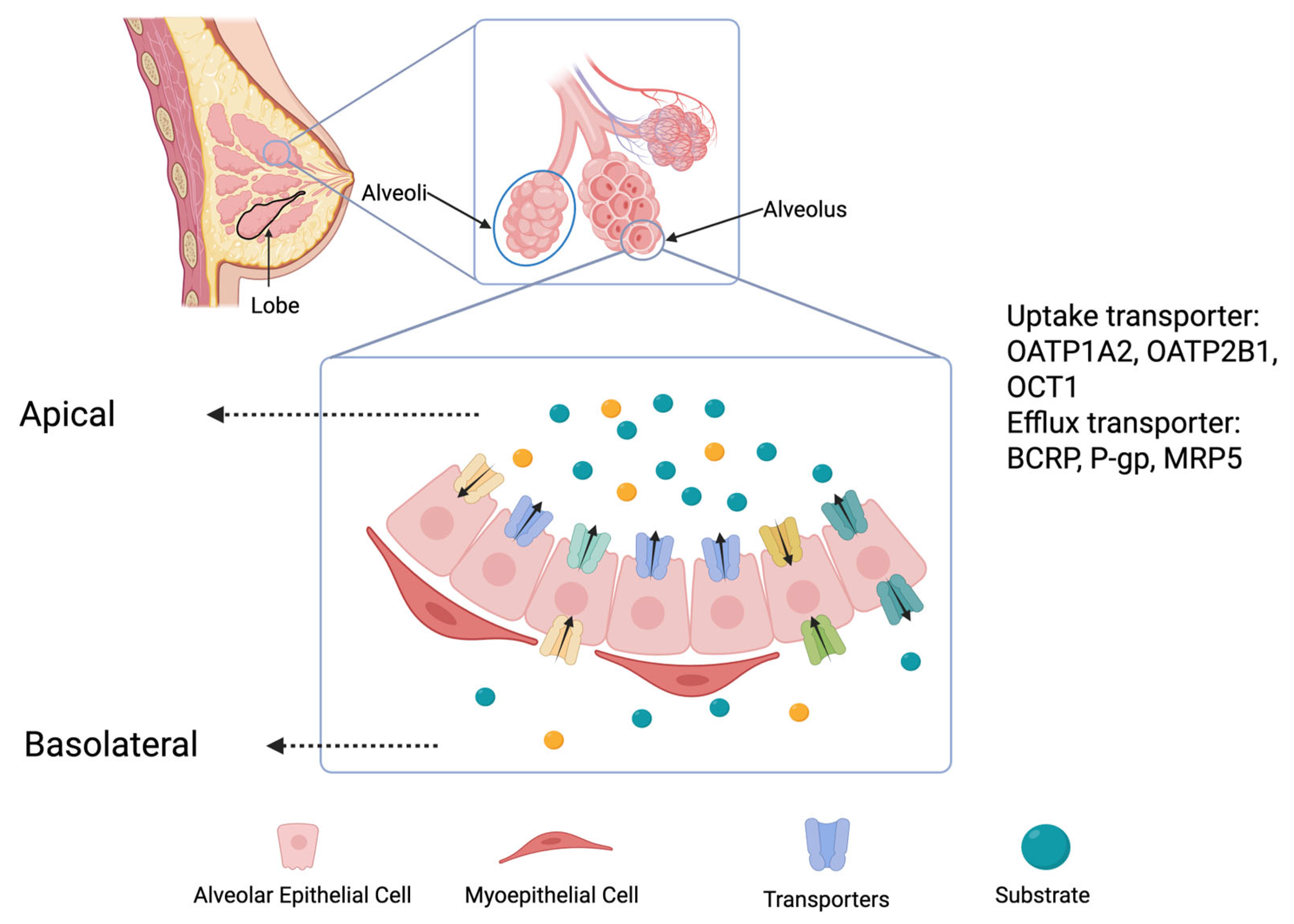
2. Mechanism of Drug Secretion into Breast Milk
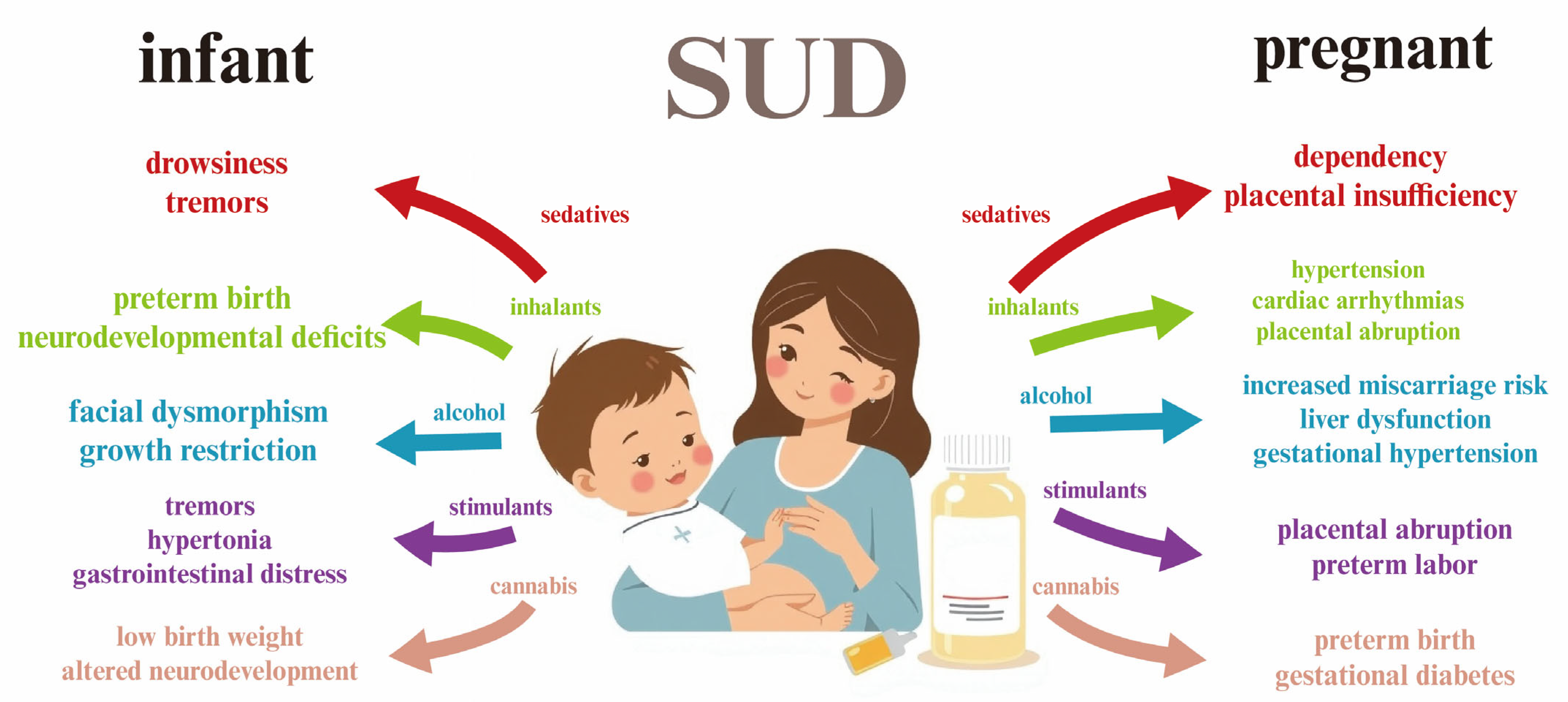
3. Substance Use Disorder (SUD) in Pregnancy
4. Sedatives in Pregnancy and Breastfeeding
4.1. Diazepam
4.2. Phenobarbital
4.3. Haloperidol
4.4. Dichloralphenazone
4.5. Fospropofol
4.6. Alprazolam
4.7. Propofol
4.8. Carisoprodol
4.9. Lorazepam
5. Tobacco in Pregnancy and Breastfeeding
Nicotine
6. Stimulants in Pregnancy and Breastfeeding
6.1. Sulpiride
6.2. Castor Oil
6.3. Caffeine
6.4. Methamphetamine
6.5. Cocaine
6.6. Alcohol
7. Cannabis in Breastfeeding
7.1. Tetrahydrocannabinol
7.2. Cannabidiol
8. Pharmacokinetics: Relative Infant Dose (RID) and Milk-To-Plasma (MP) Ratio
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estorninos, E.; Lawenko, R.B.; Palestroque, E.; Sprenger, N.; Benyacoub, J.; Kortman, G.A.; Boekhorst, J.; Bettler, J.; Cercamondi, C.I.; Berger, B. Term infant formula supplemented with milk-derived oligosaccharides shifts the gut microbiota closer to that of human milk-fed infants and improves intestinal immune defense: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H. The impact of breastmilk on infant and child health. Breastfeed. Rev. 2002, 10, 5–18. [Google Scholar]
- Pediatrics, A.A.o. Policy Statement Breast feeding and the use of human milk. Pediatrics 2012, 129, e829. [Google Scholar]
- Kovar, M.G.; Serdula, M.K.; Marks, J.S.; Fraser, D.W. Review of the epidemiologic evidence for an association between infant feeding and infant health. Pediatrics 1984, 74, 615–638. [Google Scholar] [CrossRef]
- Goldman, A.S. The immune system in human milk and the developing infant. Breastfeed. Med. 2007, 2, 195–204. [Google Scholar] [CrossRef]
- Newburg, D.S.; Walker, W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007, 61, 2–8. [Google Scholar] [CrossRef]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The impact of human milk on necrotizing enterocolitis: A systematic review and meta-analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef]
- Cacho, N.T.; Parker, L.A.; Neu, J. Necrotizing enterocolitis and human milk feeding: A systematic review. Clin. Perinatol. 2017, 44, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast milk feeding, brain development, and neurocognitive outcomes: A 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J. Pediatr. 2016, 177, 133-139.e1. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Lewis, E.D.; Field, C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016, 41, 461–475. [Google Scholar] [CrossRef]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Knight, C.; Peaker, M. Development of the mammary gland. Reproduction 1982, 65, 521–536. [Google Scholar] [CrossRef]
- Linzell, J.; Peaker, M. Mechanism of milk secretion. Physiol. Rev. 1971, 51, 564–597. [Google Scholar] [CrossRef]
- Al-Azemi, M.; Kyrou, D.; Kolibianakis, E.; Humaidan, P.; Van Vaerenbergh, I.; Devroey, P.; Fatemi, H. Elevated progesterone during ovarian stimulation for IVF. Reprod. Biomed. Online 2012, 24, 381–388. [Google Scholar] [CrossRef]
- Fleishaker, J.C. Models and methods for predicting drug transfer into human milk. Adv. Drug Deliv. Rev. 2003, 55, 643–652. [Google Scholar] [CrossRef]
- Brodin, B.; Steffansen, B.; Nielsen, C.U. Passive diffusion of drug substances: The concepts of flux and permeability. In Molecular Biopharmaceutics; Pharmaceutical Press: London, UK, 2010; pp. 135–152. [Google Scholar]
- Özdemir, Z.; Traş, B. Behaviours of drugs in the milk—A review. Atatürk Üniversitesi Vet. Bilim. Derg. 2018, 13, 364–372. [Google Scholar] [CrossRef]
- García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the mammary gland—Contribution to presence of nutrients and drugs into milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef]
- Ito, N.; Ito, K.; Ikebuchi, Y.; Toyoda, Y.; Takada, T.; Hisaka, A.; Oka, A.; Suzuki, H. Prediction of drug transfer into milk considering breast cancer resistance protein (BCRP)-mediated transport. Pharm. Res. 2015, 32, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Bertagnolli, L.N.; Boulton, D.W.; Coppola, P. A literature review of drug transport mechanisms during lactation. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zou, P.; Fang, Y.; Li, Y. Physiologically based pharmacokinetic model to predict drug concentrations of breast cancer resistance protein substrates in milk. Biopharm. Drug Dispos. 2022, 43, 221–232. [Google Scholar] [CrossRef]
- Real, R.; Egido, E.; Pérez, M.; Gonzalez-Lobato, L.; Barrera, B.; Prieto, J.; Alvarez, A.; Merino, G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: Interaction with ivermectin. J. Vet. Pharmacol. Ther. 2011, 34, 313–321. [Google Scholar] [CrossRef]
- Merino, G.; Alvarez, A.I.; Pulido, M.M.; Molina, A.J.; Schinkel, A.H.; Prieto, J.G. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 2006, 34, 690–695. [Google Scholar] [CrossRef]
- Uguz, F. A new safety scoring system for the use of psychotropic drugs during lactation. Am. J. Ther. 2021, 28, e118–e126. [Google Scholar] [CrossRef]
- Merikangas, K.R.; McClair, V.L. Epidemiology of substance use disorders. Hum. Genet. 2012, 131, 779–789. [Google Scholar] [CrossRef]
- Hasin, D.S.; O’brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef]
- Brady, K.T.; Sinha, R. Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. Am. J. Psychiatry 2005, 162, 1483–1493. [Google Scholar] [CrossRef]
- Ferré, S. Mechanisms of the psychostimulant effects of caffeine: Implications for substance use disorders. Psychopharmacology 2016, 233, 1963–1979. [Google Scholar] [CrossRef]
- Tohen, M.; Greenfield, S.F.; Weiss, R.D.; Zarate, C.A., Jr.; Vagge, L.M. The effect of comorbid substance use disorders on the course of bipolar disorder: A review. Harv. Rev. Psychiatry 1998, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vanyukov, M.M.; Tarter, R.E.; Kirisci, L.; Kirillova, G.P.; Maher, B.S.; Clark, D.B. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci. Biobehav. Rev. 2003, 27, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K.; Pfund, R.A.; Tucker, J.A. Mechanisms of behavior change in substance use disorder with and without formal treatment. Annu. Rev. Clin. Psychol. 2022, 18, 497–525. [Google Scholar] [CrossRef]
- Najavits, L.M.; Weiss, R.D. Variations in therapist effectiveness in the treatment of patients with substance use disorders: An empirical review. Addiction 1994, 89, 679–688. [Google Scholar] [CrossRef]
- Forray, A. Substance use during pregnancy. F1000Research 2016, 5, 887. [Google Scholar] [CrossRef]
- Frazer, Z.; McConnell, K.; Jansson, L.M. Treatment for substance use disorders in pregnant women: Motivators and barriers. Drug Alcohol Depend. 2019, 205, 107652. [Google Scholar] [CrossRef]
- Rayburn, W.F. Maternal and fetal effects from substance use. Clin. Perinatol. 2007, 34, 559–571. [Google Scholar] [CrossRef]
- Ecker, J.; Abuhamad, A.; Hill, W.; Bailit, J.; Bateman, B.T.; Berghella, V.; Blake-Lamb, T.; Guille, C.; Landau, R.; Minkoff, H. Substance use disorders in pregnancy: Clinical, ethical, and research imperatives of the opioid epidemic: A report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of Addiction Medicine. Am. J. Obstet. Gynecol. 2019, 221, B5–B28. [Google Scholar]
- Mennella, J.A.; Beauchamp, G.K. The transfer of alcohol to human milk: Effects on flavor and the infant’s behavior. N. Engl. J. Med. 1991, 325, 981–985. [Google Scholar] [CrossRef]
- Ito, S. Opioids in breast milk: Pharmacokinetic principles and clinical implications. J. Clin. Pharmacol. 2018, 58, S151–S163. [Google Scholar] [CrossRef]
- Salameh, T.N.; Hall, L.A.; Crawford, T.N.; Staten, R.R.; Hall, M.T. Racial/ethnic differences in mental health treatment among a national sample of pregnant women with mental health and/or substance use disorders in the United States. J. Psychosom. Res. 2019, 121, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Jones, W.; Winkley, E.; Kinsella, S. Guideline on anaesthesia and sedation in breastfeeding women 2020: Guideline from the Association of Anaesthetists. Anaesthesia 2020, 75, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, R.; Ohkubo, T.; Sugawara, K. Characteristics of interaction between barbiturate derivatives and various sorbents on liquid chromatography and determination of phenobarbital in Japanese human breast milk. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 587–599. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Rosani, A.; Saadabadi, A. Diazepam. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Tani, N.; Ikeda, T.; Ishikawa, T. Relationship between clock gene expression and CYP2C19 and CYP3A4 with benzodiazepines. Hum. Exp. Toxicol. 2023, 42, 09603271231171643. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Benzodiazepine metabolism: An analytical perspective. Curr. Drug Metabolism 2008, 9, 827–844. [Google Scholar] [CrossRef]
- Erkkola, R.; Kanto, J. Diazepam and breast-feeding. Lancet 1972, 1, 1235–1236. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.; Stillwell, W.; Nowlin, J.; Lertratanangkoon, K.; Stillwell, R.; Hill, R. Identification and quantification of drugs and drug metabolites in human breast milk using GC-MS-COM methods. Mod. Probl. Paediatr. 1975, 15, 73–79. [Google Scholar]
- Brandt, R. Passage of diazepam and desmethyldiazepam into breast milk. Arzneim.-Forsch. 1976, 26, 454–457. [Google Scholar]
- Wesson, D.R.; Camber, S.; Harkey, M.; Smith, D.E. Diazepam and desmethyldiazepam in breast milk. J. Psychoact. Drugs 1985, 17, 55–56. [Google Scholar] [CrossRef]
- Dusci, L.; Good, S.; Hall, R.; Ilett, K. Excretion of diazepam and its metabolites in human milk during withdrawal from combination high dose diazepam and oxazepam. Br. J. Clin. Pharmacol. 1990, 29, 123–126. [Google Scholar] [CrossRef]
- Borgatta, L.; Jenny, R.W.; Gruss, L.; Ong, C.; Barad, D. Clinical significance of methohexital, meperidine, and diazepam in breast milk. J. Clin. Pharmacol. 1997, 37, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.; Tilstone, W.; Reavey, P. Diazepam and breast-feeding. Lancet 1972, 1, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.E.; Poon, S.; Madadi, P.; Koren, G. Neonatal benzodiazepines exposure during breastfeeding. J. Pediatr. 2012, 161, 448–451. [Google Scholar] [CrossRef]
- Duman, N.C.; Gulcebi, M.I.; Ayhan, G.B.; Ozkula, S.; Asık, Z.T.; Gulhan, R.; Onat, F.; Goren, M.Z.; Karaalp, A. Assessment of colchicine use during pregnancy and breastfeeding in a University Hospital. Reprod. Toxicol. 2016, 60, 179. [Google Scholar] [CrossRef]
- Gilder, M.E.; Tun, N.W.; Carter, A.; Tan, F.F.S.L.; Min, A.M.; Eh, H.; Aye, P.; Carrara, V.I.; Angkurawaranon, C.; McGready, R. Outcomes for 298 breastfed neonates whose mothers received ketamine and diazepam for postpartum tubal ligation in a resource-limited setting. BMC Pregnancy Childbirth 2021, 21, 121. [Google Scholar] [CrossRef]
- Michelucci, R.; Pasini, E. Phenobarbital, primidone and other barbiturates. In The Treatment of Epilepsy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 555–573. [Google Scholar] [CrossRef]
- Gotoh, S.; Ohno, M.; Yoshinari, K.; Negishi, M.; Kawajiri, K. Nuclear receptor-mediated regulation of cytochrome P450 genes. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 787–812. [Google Scholar]
- Besag, F.M.; Berry, D. Interactions between antiepileptic and antipsychotic drugs. Drug Safety 2006, 29, 95–118. [Google Scholar] [CrossRef]
- Westerink, D.; Glerum, J. Separation and microdetermination of phenobarbital and phenytoin in human milk. Pharm. Weekbl. 1965, 100, 577–583. [Google Scholar]
- Granstrom, M.; Bardy, A.; Hiilesmaa, V. Prolonged feeding difficulties of infants of primidone mothers during neonatal period. Preliminary results from the helsinki study. In Epilepsy, Pregnancy and the Child; Janz, D., Bossi, L., Dam, M., Eds.; Raven Press: New York, NY, USA, 1982; pp. 357–358. [Google Scholar]
- Gomita, Y.; Furuno, K.; Araki, Y.; Yamatogi, Y.; Ohtahara, S. Phenobarbital in sera of epileptic mothers and their infants. Am. J. Ther. 1995, 2, 968–971. [Google Scholar] [CrossRef]
- Tyson, R.M.; Shrader, E.A.; Perlman, H.H. Drugs transmitted through breast milk: II. Barbiturates. J. Pediatr. 1938, 13, 86–90. [Google Scholar] [CrossRef]
- Finch, E.; Lorber, J. Methaemoglobinaemia in the newborn probably due to phenytoin excreted in human milk. J. Obstet. Gynaecol. Br. Emp. 1954, 61, 833–834. [Google Scholar] [CrossRef]
- Juul, S. Barbiturate poisoning via breast milk? Ugeskr. Laeger 1969, 131, 2257–2258. [Google Scholar] [PubMed]
- Gopfert-Geyer, I.; Koch, S.; Rating, D.; Jager-Roman, E.; Hartmann, A.; Jacob, S.; Offermann, G.; Hedge, H. Delivery, gestation, data at birth, and neonatal period in children of epileptic mothers. In Epilepsy, Pregnancy and the Child; Raven Press: New York, NY, USA, 1982; pp. 297–298. [Google Scholar]
- Davanzo, R.; Dal Bo, S.; Bua, J.; Copertino, M.; Zanelli, E.; Matarazzo, L. Antiepileptic drugs and breastfeeding. Ital. J. Pediatr. 2013, 39, 50. [Google Scholar] [CrossRef] [PubMed]
- Knott, C.; Reynolds, F.; Clayden, G. Infantile spasms on weaning from breast milk containing anticonvulsants. Lancet 1987, 2, 272–273. [Google Scholar] [CrossRef]
- Tolledo, C. CYP2D Is Functional in the Brain and Alters Haloperidol-Induced Side Effects. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2020. [Google Scholar]
- Chavan, A. Haloperidol In Vitro Glucuronidation and Oxidation in Human Liver Microsomes; Massachusetts College of Pharmacy and Health Sciences: Boston, MA, USA, 2007. [Google Scholar]
- Yoshida, K.; Smith, B.; Craggs, M.; Kumar, R. Neuroleptic drugs in breast-milk: A study of pharmacokinetics and of possible adverse effects in breast-fed infants. Psychol. Med. 1998, 28, 81–91. [Google Scholar] [CrossRef]
- Stewart, R.; Karas, B.; Springer, P. Haloperidol excretion in human milk. Am. J. Psychiatry 1981, 137, 849–850. [Google Scholar]
- Whalley, L.; Blain, P.; Prime, J. Haloperidol secreted in breast milk. Br. Med. J. Clin. Res. Ed. 1981, 282, 1746–1747. [Google Scholar] [CrossRef]
- Uguz, F. Adverse events in a breastfed infant exposed to risperidone and haloperidol. Breastfeed. Med. 2019, 14, 683–684. [Google Scholar] [CrossRef]
- Ziman, M.R. A Comparison of the Effects of Xenobiotics on Hepatic Haem Metabolism. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1983. [Google Scholar]
- Becker, W.J. Acute migraine treatment. Contin. Lifelong Learn. Neurol. 2015, 21, 953–972. [Google Scholar] [CrossRef]
- Lacey, J. Dichloralphenazone and breast milk. Br. Med. J. 1971, 4, 684. [Google Scholar] [CrossRef]
- Berlin, C.M., Jr.; Vesell, E.S. Antipyrine disposition in milk and saliva of lactating women. Clin. Pharmacol. Ther. 1982, 31, 38–44. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolic profiles of propofol and fospropofol: Clinical and forensic interpretative aspects. BioMed Res. Int. 2018, 2018, 6852857. [Google Scholar] [CrossRef]
- Schmitt, J.; Schwoerer, D.; Diemunsch, P.; Gauthier-Lafaye, J. Passage du propofol dans le colostrum. Données préliminaires. In Annales Françaises d’Anesthésie et de Réanimation; Elsevier BV: Amsterdam, The Netherlands, 1987; pp. 267–268. [Google Scholar]
- Dailland, P.; Cockshott, I.D.; Lirzin, J.D.; Jacquinot, P.; Jorrot, J.C.; Devery, J.; Harmey, J.-L.; Conseiller, C. Intravenous propofol during cesarean section: Placental transfer, concentrations in breast milk, and neonatal effects. A preliminary study. Anesthesiology 1989, 71, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Nitsun, M.; Szokol, J.W.; Saleh, H.J.; Murphy, G.S.; Vender, J.S.; Luong, L.; Raikoff, K.; Avram, M.J. Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin. Pharmacol. Ther. 2006, 79, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Angeli, M. Neonatal Abstinence Syndrome: Comparing the Hepatic Metabolism of Addictive Medicines In Vitro in the Fetus, Neonate and Adult Female. Master’s Thesis, Tufts University Graduate School of Biomedical Sciences, Boston, MA, USA, 2018. [Google Scholar]
- Oo, C.; Kuhn, R.; Desai, N.; Wright, C.; McNamara, P. Pharmacokinetics in lactating women: Prediction of alprazolam transfer into milk. Br. J. Clin. Pharmacol. 1995, 40, 231–236. [Google Scholar] [CrossRef]
- Furugen, A.; Nishimura, A.; Kobayashi, M.; Umazume, T.; Narumi, K.; Iseki, K. Quantification of eight benzodiazepines in human breastmilk and plasma by liquid-liquid extraction and liquid-chromatography tandem mass spectrometry: Application to evaluation of alprazolam transfer into breastmilk. J. Pharm. Biomed. Anal. 2019, 168, 83–93. [Google Scholar] [CrossRef]
- Anderson, P.O.; Mcguire, G.G. Neonatal alprazolam withdrawal—Possible effects of breast feeding. DICP 1989, 23, 614. [Google Scholar] [CrossRef]
- Ito, S.; Blajchman, A.; Stephenson, M.; Eliopoulos, C.; Koren, G. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am. J. Obstet. Gynecol. 1993, 168, 1393–1399. [Google Scholar] [CrossRef]
- Petrić, D.; Vučić Peitl, M.; Peitl, V. High doses alprazolam induced amenorrhoea and galactorrhoea. Psychiatr. Danub. 2011, 23, 123–124. [Google Scholar]
- Stuttmann, R.; Schäfer, C.; Hilbert, P.; Meyer, M.R.; Maurer, H.H. The breast feeding mother and xenon anaesthesia: Four case reports. Breast feeding and xenon anaesthesia. BMC Anesthesiol. 2010, 10, 1. [Google Scholar] [CrossRef]
- Kutlucan, L.; Seker, İ.S.; Demiraran, Y.; Ersoy, Ö.; Karagöz, İ.; Sezen, G.; Köse, S.A. Effects of different anesthesia protocols on lactation in the postpartum period. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 233. [Google Scholar] [CrossRef]
- Birkholz, T.; Eckardt, G.; Renner, S.; Irouschek, A.; Schmidt, J. Green breast milk after propofol administration. Anesthesiology 2009, 111, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Rainone, A.; Delucilla, L.; Elofer, S.; Bensimon, L.; Abittan, G. Propofol-induced green breast milk: A case report. Can. J. Hosp. Pharm. 2018, 71, 389. [Google Scholar] [CrossRef]
- Bulut, O.; Ovali, F. Green breast milk: A rare side effect of propofol. J. Paediatr. Child. Health 2021, 57, 153–154. [Google Scholar] [CrossRef]
- Kumar, M.; Dillon, G.H. Carisoprodol: Update on abuse potential and mechanism of action. Mol. Cell Pharmacol. 2015, 7, 1–10. [Google Scholar]
- Nordeng, H.; Zahlsen, K.; Spigset, O. Transfer of carisoprodol to breast milk. Ther. Drug Monit. 2001, 23, 298–300. [Google Scholar] [CrossRef]
- Bailey, D.N.; Briggs, J.R. Carisoprodol: An unrecognized drug of abuse. Am. J. Clin. Pathol. 2002, 117, 396–400. [Google Scholar] [CrossRef]
- Briggs, G.G.; Ambrose, P.J.; Nageotte, M.P.; Padilla, G. High-dose carisoprodol during pregnancy and lactation. Ann. Pharmacother. 2008, 42, 898–901. [Google Scholar] [CrossRef]
- Yang, G.; Ge, S.; Singh, R.; Basu, S.; Shatzer, K.; Zen, M.; Liu, J.; Tu, Y.; Zhang, C.; Wei, J. Glucuronidation: Driving factors and their impact on glucuronide disposition. Drug Metab. Rev. 2017, 49, 105–138. [Google Scholar] [CrossRef]
- Sommerfield, R.; Nielsen, M. Excretion of lorazepam into breast milk. BJA Br. J. Anaesth. 1985, 57, 1042–1043. [Google Scholar] [CrossRef]
- Whitelaw, A.; Cummings, A.; McFadyen, I. Effect of maternal lorazepam on the neonate. Br. Med. J. Clin. Res. Ed. 1981, 282, 1106–1108. [Google Scholar] [CrossRef]
- Lemmer, P.; Schneider, S.; Mühe, A.; Wennig, R. Quantification of lorazepam and lormetazepam in human breast milk using GC-MS in the negative chemical ionization mode. J. Anal. Toxicol. 2007, 31, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Furugen, A.; Umazume, T.; Kitamura, S.; Soma, M.; Noshiro, K.; Takekuma, Y.; Sugawara, M.; Iseki, K.; Kobayashi, M. Benzodiazepine concentrations in the breast milk and plasma of nursing mothers: Estimation of relative infant dose. Breastfeed. Med. 2021, 16, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Kishore, M.T.; Thippeswamy, H.; Kommu, J.V.S.; Chandra, P.S. Adverse effects and short-term developmental outcomes of infants exposed to atypical antipsychotics during breastfeeding. Indian J. Psychiatry 2021, 63, 52–57. [Google Scholar] [CrossRef]
- Somogyi, A.; Beck, H. Nurturing and breast-feeding: Exposure to chemicals in breast milk. Environ. Health Perspect. 1993, 101, 45–52. [Google Scholar]
- Napierala, M.; Mazela, J.; Merritt, T.A.; Florek, E. Tobacco smoking and breastfeeding: Effect on the lactation process, breast milk composition and infant development. A critical review. Environ. Res. 2016, 151, 321–338. [Google Scholar] [CrossRef]
- Cone, E.J.; Fant, R.V.; Henningfield, J.E. Nicotine and tobacco. In Handbook of Drug Interactions: A Clinical and Forensic Guide; Springer: Berlin/Heidelberg, Germany, 2011; pp. 587–623. [Google Scholar]
- Zhu, A.Z.; Tyndale, R.F. Nicotine metabolism and its implications. In Metabolism of Drugs and Other Xenobiotics; Wiley: Hoboken, NJ, USA, 2012; pp. 465–492. [Google Scholar]
- Brodribb, W. ABM Clinical Protocol #9: Use of Galactogogues in Initiating or Augmenting Maternal Milk Production, Second Revision 2018. Breastfeed. Med. 2018, 13, 307–314. [Google Scholar] [CrossRef]
- Breastfeeding Challenges: ACOG Committee Opinion, Number 820. Obstet. Gynecol. 2021, 137, e42–e53. [CrossRef]
- Organizers. Abstracts of the IV World Conference on Clinical Pharmacology & Therapeutics. Eur. J. Clin. Pharmacol. 1989, 36, A1–A341. [Google Scholar] [CrossRef]
- Ylikorkala, O.; Kauppila, A.; Kivinen, S.; Viinikka, L. Treatment of inadequate lactation with oral sulpiride and buccal oxytocin. Obstet. Gynecol. 1984, 63, 57–60. [Google Scholar]
- Ylikorkala, O.; Kauppila, A.; Kivinen, S.; Viinikka, L. Sulpiride improves inadequate lactation. Br. Med. J. Clin. Res. Ed. 1982, 285, 249–251. [Google Scholar] [CrossRef]
- Aono, T.; Shioji, T.; Aki, T.; Hirota, K.; Nomura, A.; Kurachi, K. Augmentation of puerperal lactation by oral administration of sulpiride. J. Clin. Endocrinol. Metab. 1979, 48, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Tachibana, Y.; Wada, Y.S.; Yakuwa, N.; Kawasaki, H.; Suzuki, T.; Sago, H.; Yamatani, A.; Murashima, A. Transfer of brotizolam, periciazine, and sulpiride in cord blood and breast milk, and alprazolam in breast milk: A case report. J. Pharm. Health Care Sci. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Barguño, J.M.; del Pozo, E.; Cruz, M.; Figueras, J. Failure of maintained hyperprolactinemia to improve lactational performance in late puerperium. J. Clin. Endocrinol. Metab. 1988, 66, 876–879. [Google Scholar] [CrossRef]
- Polatti, F. Sulpiride isomers and milk secretion in puerperium. Clin. Exp. Obstet. Gynecol. 1982, 9, 144–147. [Google Scholar]
- McMurdo, M.E.; Howie, P.W.; Lewis, M.; Marnie, M.; McEwen, J.; McNeilly, A.S. Prolactin response to low dose sulpiride. Br. J. Clin. Pharmacol. 1987, 24, 133–137. [Google Scholar] [CrossRef]
- Hanew, K.; Utsumi, A.; Sugawara, A.; Shimizu, Y.; Yoshinaga, K. Simultaneous administration of TRH and sulpiride caused additive but not synergistic PRL responses in normal subjects. Endocrinol. Jpn. 1992, 39, 465–468. [Google Scholar] [CrossRef]
- Kropp, S.; Ziegenbein, M.; Grohmann, R.; Engel, R.R.; Degner, D. Galactorrhea due to psychotropic drugs. Pharmacopsychiatry 2004, 37 (Suppl. 1), S84–S88. [Google Scholar] [CrossRef]
- Lu, M.L.; Shen, W.W.; Chen, C.H. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1978–1981. [Google Scholar] [CrossRef]
- Polatti, F.; Brambilla, A.; Mandelli, B.; Forgione, A. Can pharmacologic hyperprolactinemia and breast-suction induce lactation in women with normal menstrual cycles? Clin. Exp. Obstet. Gynecol. 1984, 11, 123–125. [Google Scholar]
- Aono, T.; Aki, T.; Koike, K.; Kurachi, K. Effect of sulpiride on poor puerperal lactation. Am. J. Obstet. Gynecol. 1982, 143, 927–932. [Google Scholar] [CrossRef]
- Eglash, A. Treatment of maternal hypergalactia. Breastfeed. Med. 2014, 9, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Winterfeld, U.; Meyer, Y.; Panchaud, A.; Einarson, A. Management of deficient lactation in Switzerland and Canada: A survey of midwives’ current practices. Breastfeed. Med. 2012, 7, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, E. Botanical Medicine in Pregnancy and Lactation. Altern. Complement. Ther. 1997, 3, 93–100. [Google Scholar] [CrossRef]
- Hardy, M.L. Herbs of special interest to women. J. Am. Pharm. Assoc. 2000, 40, 234–242. [Google Scholar] [CrossRef]
- Benakappa, D.G.; Raju, M.; Shivananda; Benakappa, A.D. Breast-feeding practices in rural Karnataka (India) with special reference to lactation failure. Acta Paediatr. Jpn. 1989, 31, 391–398. [Google Scholar] [CrossRef]
- Jayaprakash, D.G.; Raghu Raman, T.S.; Singh, D.; Raja, L.N. Laxative induced hypoalbuminemia. Indian Pediatr. 1995, 32, 1037–1038. [Google Scholar]
- Ryu, J.E. Effect of maternal caffeine consumption on heart rate and sleep time of breast-fed infants. Dev. Pharmacol. Ther. 1985, 8, 355–363. [Google Scholar] [CrossRef]
- McNamara, P.J.; Abbassi, M. Neonatal exposure to drugs in breast milk. Pharm. Res. 2004, 21, 555–566. [Google Scholar] [CrossRef]
- Oo, C.Y.; Burgio, D.E.; Kuhn, R.C.; Desai, N.; McNamara, P.J. Pharmacokinetics of caffeine and its demethylated metabolites in lactation: Predictions of milk to serum concentration ratios. Pharm. Res. 1995, 12, 313–316. [Google Scholar] [CrossRef]
- Muñoz, L.M.; Lönnerdal, B.; Keen, C.L.; Dewey, K.G. Coffee consumption as a factor in iron deficiency anemia among pregnant women and their infants in Costa Rica. Am. J. Clin. Nutr. 1988, 48, 645–651. [Google Scholar] [CrossRef]
- Knutti, R.; Rothweiler, H.; Schlatter, C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur. J. Clin. Pharmacol. 1981, 21, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.M., Jr.; Denson, H.M.; Daniel, C.H.; Ward, R.M. Disposition of dietary caffeine in milk, saliva, and plasma of lactating women. Pediatrics 1984, 73, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.W.; DeAngelis, R.L.; Kearney, M.F.; Welch, R.M.; Findlay, J.M. Analgesic drugs in breast milk and plasma. Clin. Pharmacol. Ther. 1981, 29, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Stavchansky, S.; Combs, A.; Sagraves, R.; Delgado, M.; Joshi, A. Pharmacokinetics of caffeine in breast milk and plasma after single oral administration of caffeine to lactating mothers. Biopharm. Drug Dispos. 1988, 9, 285–299. [Google Scholar] [CrossRef]
- Tyrala, E.E.; Dodson, W.E. Caffeine secretion into breast milk. Arch. Dis. Child. 1979, 54, 787–789. [Google Scholar] [CrossRef]
- Ryu, J.E. Caffeine in human milk and in serum of breast-fed infants. Dev. Pharmacol. Ther. 1985, 8, 329–337. [Google Scholar] [CrossRef]
- Calvaresi, V.; Escuder, D.; Minutillo, A.; Bastons-Compta, A.; García-Algar, O.; Pallás Alonso, C.R.; Pacifici, R.; Pichini, S. Transfer of Nicotine, Cotinine and Caffeine into Breast Milk in a Smoker Mother Consuming Caffeinated Drinks. J. Anal. Toxicol. 2016, 40, 473–477. [Google Scholar] [CrossRef]
- Rivera-Calimlim, L. Drugs in breast milk. Drug Ther. 1977, 7, 59–63. [Google Scholar]
- Bailey, D.N.; Weibert, R.T.; Naylor, A.J.; Shaw, R.F. A study of salicylate and caffeine excretion in the breast milk of two nursing mothers. J. Anal. Toxicol. 1982, 6, 64–68. [Google Scholar] [CrossRef]
- Meyer, M.R.; Maurer, H.H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics 2011, 12, 215–233. [Google Scholar] [CrossRef]
- Cook, C.E.; Jeffcoat, A.R.; Sadler, B.M.; Hill, J.M.; Voyksner, R.D.; Pugh, D.E.; White, W.R.; Perez-Reyes, M. Pharmacokinetics of oral methamphetamine and effects of repeated daily dosing in humans. Drug Metab. Dispos. 1992, 20, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Bartu, A.; Dusci, L.J.; Ilett, K.F. Transfer of methylamphetamine and amphetamine into breast milk following recreational use of methylamphetamine. Br. J. Clin. Pharmacol. 2009, 67, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Chomchai, C.; Chomchai, S.; Kitsommart, R. Transfer of methamphetamine (MA) into breast milk and urine of postpartum women who smoked MA tablets during pregnancy: Implications for initiation of breastfeeding. J. Hum. Lact. 2016, 32, 333–339. [Google Scholar] [CrossRef]
- Piskáčková, H.B.; Nemeškalová, A.; Kučera, R.; Pedersen-Bjergaard, S.; Najmanová, V.; Štěrbová-Kovaříková, P.; Kuchař, M.; Sýkora, D. Advanced microextraction techniques for the analysis of amphetamines in human breast milk and their comparison with conventional methods. J. Pharm. Biomed. Anal. 2022, 210, 114549. [Google Scholar]
- Ariagno, R.; Karch, S.B.; Middleberg, R.; Stephens, B.G.; Valdès-Dapena, M. Methamphetamine ingestion by a breast-feeding mother and her infant’s death: People v Henderson. JAMA 1995, 274, 215. [Google Scholar]
- Kenneally, M.; Byard, R.W. Increasing methamphetamine detection in cases of early childhood fatalities. J. Forensic Sci. 2020, 65, 1376–1378. [Google Scholar] [CrossRef]
- Gouzoulis-Mayfrank, E.; Thelen, B.; Habermeyer, E.; Kunert, H.; Kovar, K.-A.; Lindenblatt, H.; Hermle, L.; Spitzer, M.; Sass, H. Psychopathological, neuroendocrine and autonomic effects of 3, 4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers Results of an experimental double-blind placebo-controlled study: Results of an experimental double-blind placebo-controlled study. Psychopharmacology 1999, 142, 41–50. [Google Scholar]
- DeLeo, V.; Cella, S.; Camanni, F.; Genazzani, A.; Müller, E. Prolactin lowering effect of amphetamine in normoprolactinemic subjects and in physiological and pathological hyperprolactinemia. Horm. Metab. Res. 1983, 15, 439–443. [Google Scholar] [CrossRef]
- Petraglia, F.; De Leo, V.; Sardelli, S.; Mazzullo, G.; Gioffre, W.; Genazzani, A.; D’Antona, N. Prolactin changes after administration of agonist and antagonist dopaminergic drugs in puerperal women. Gynecol. Obstet. Investig. 1987, 23, 103–109. [Google Scholar] [CrossRef]
- Shah, R.; Diaz, S.D.; Arria, A.; LaGasse, L.L.; Derauf, C.; Newman, E.; Smith, L.M.; Huestis, M.A.; Haning, W.; Strauss, A. Prenatal methamphetamine exposure and short-term maternal and infant medical outcomes. Am. J. Perinatol. 2012, 29, 391–400. [Google Scholar] [CrossRef]
- Cressman, A.M.; Koren, G.; Pupco, A.; Kim, E.; Ito, S.; Bozzo, P. Maternal cocaine use during breastfeeding. Can. Fam. Physician 2012, 58, 1218–1219. [Google Scholar] [PubMed]
- Chasnoff, I.J.; Lewis, D.E.; Squires, L. Cocaine intoxication in a breast-fed infant. Pediatrics 1987, 80, 836–838. [Google Scholar] [PubMed]
- D’Avila, F.B.; Pereira, A.G.; Salazar, F.R.; Ferreira, P.L.; Salazar, C.R.; Limberger, R.P.; Fröehlich, P.E. Determination of cocaine/crack biomarkers in colostrum by LC-MS following protein precipitation. J. Pharm. Biomed. Anal. 2015, 103, 67–72. [Google Scholar] [CrossRef]
- Winecker, R.E.; Goldberger, B.A.; Tebbett, I.R.; Behnke, M.; Eyler, F.D.; Karlix, J.L.; Wobie, K.; Conlon, M.; Phillips, D.; Bertholf, R.L. Detection of cocaine and its metabolites in breast milk. J. Forensic Sci. 2001, 46, 1221–1223. [Google Scholar] [CrossRef]
- Silveira Gde, O.; Belitsky, Í.T.; Loddi, S.; Rodrigues de Oliveira, C.D.; Zucoloto, A.D.; Fruchtengarten, L.V.; Yonamine, M. Development of a method for the determination of cocaine, cocaethylene and norcocaine in human breast milk using liquid phase microextraction and gas chromatography-mass spectrometry. Forensic Sci. Int. 2016, 265, 22–28. [Google Scholar] [CrossRef]
- Chaney, N.E.; Franke, J.; Wadlington, W.B. Cocaine convulsions in a breast-feeding baby. J. Pediatr. 1988, 112, 134–135. [Google Scholar] [CrossRef]
- Dos Santos, R.R.; Nunes Paiva, M.J.; Veloso, J.C.; Serp, P.; Lourdes Cardeal, Z.; Menezes, H.C. Efficient extraction method using magnetic carbon nanotubes to analyze cocaine and benzoylecgonine in breast milk by GC/MS. Bioanalysis 2017, 9, 1655–1666. [Google Scholar] [CrossRef]
- da-Silva, V.A.; Malheiros, L.R.; Moraes-Santos, A.R.; Barzano, M.A.; McLean, A.E. Ethanol pharmacokinetics in lactating women. Braz. J. Med. Biol. Res. 1993, 26, 1097–1103. [Google Scholar]
- Kesäniemi, Y.A. Ethanol and acetaldehyde in the milk and peripheral blood of lactating women after ethanol administration. J. Obstet. Gynaecol. Br. Commonw. 1974, 81, 84–86. [Google Scholar] [CrossRef]
- Cobo, E.; Quintero, C.A. Milk-ejecting and antidiuretic activities under neurohypophyseal inhibition with alcohol and water overload. Am. J. Obstet. Gynecol. 1969, 105, 877–887. [Google Scholar] [CrossRef]
- Cobo, E. Effect of different doses of ethanol on the milk-ejecting reflex in lactating women. Am. J. Obstet. Gynecol. 1973, 115, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Coiro, V.; Alboni, A.; Gramellini, D.; Cigarini, C.; Bianconi, L.; Pignatti, D.; Volpi, R.; Chiodera, P. Inhibition by ethanol of the oxytocin response to breast stimulation in normal women and the role of endogenous opioids. Acta Endocrinol. 1992, 126, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A. Short-term effects of maternal alcohol consumption on lactational performance. Alcohol. Clin. Exp. Res. 1998, 22, 1389–1392. [Google Scholar] [CrossRef]
- Schuetze, P.; Eiden, R.D.; Chan, A.W.K. The Effects of Alcohol in Breast Milk on Infant Behavioral State and Mother-Infant Feeding Interactions. Infancy 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Little, R.E.; Anderson, K.W.; Ervin, C.H.; Worthington-Roberts, B.; Clarren, S.K. Maternal alcohol use during breast-feeding and infant mental and motor development at one year. N. Engl. J. Med. 1989, 321, 425–430. [Google Scholar] [CrossRef]
- Little, R.E.; Northstone, K.; Golding, J. Alcohol, breastfeeding, and development at 18 months. Pediatrics 2002, 109, e72. [Google Scholar] [CrossRef]
- Wilson, J.; Tay, R.Y.; McCormack, C.; Allsop, S.; Najman, J.; Burns, L.; Olsson, C.A.; Elliott, E.; Jacobs, S.; Mattick, R.P.; et al. Alcohol consumption by breastfeeding mothers: Frequency, correlates and infant outcomes. Drug Alcohol. Rev. 2017, 36, 667–676. [Google Scholar] [CrossRef]
- Lex, B.W.; Ellingboe, J.E.; Teoh, S.K.; Mendelson, J.H.; Rhoades, E. Prolactin and cortisol levels following acute alcohol challenges in women with and without a family history of alcoholism. Alcohol 1991, 8, 383–387. [Google Scholar] [CrossRef]
- Ho, E.; Collantes, A.; Kapur, B.M.; Moretti, M.; Koren, G. Alcohol and breast feeding: Calculation of time to zero level in milk. Biol. Neonate 2001, 80, 219–222. [Google Scholar] [CrossRef]
- Chien, Y.C.; Huang, Y.J.; Hsu, C.S.; Chao, J.C.; Liu, J.F. Maternal lactation characteristics after consumption of an alcoholic soup during the postpartum ‘doing-the-month’ ritual. Public Health Nutr. 2009, 12, 382–388. [Google Scholar] [CrossRef]
- Argote-Espinosa, R.M.; Flores-Huerta, S.; Hernández-Montes, H.; Villalpando-Hernández, S. Plasma clearance of ethanol and its excretion in the milk of rural women who consume pulque. Rev. Investig. Clin. 1992, 44, 31–36. [Google Scholar]
- Mennella, J.A.; Garcia-Gomez, P.L. Sleep disturbances after acute exposure to alcohol in mothers’ milk. Alcohol 2001, 25, 153–158. [Google Scholar] [CrossRef]
- Binkiewicz, A.; Robinson, M.J.; Senior, B. Pseudo-Cushing syndrome caused by alcohol in breast milk. J. Pediatr. 1978, 93, 965–967. [Google Scholar] [CrossRef]
- JAMA. Current Medical Literature. J. Am. Med. Assoc. 1937, 109, 163–178. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, A.K.; Cheung, E.; Lee, B.; Tsang, M.M.; Torres, A.R. An overview of exposure to ethanol-containing substances and ethanol intoxication in children based on three illustrated cases. Drugs Context 2018, 7, 212512. [Google Scholar] [CrossRef]
- Flores-Huerta, S.; Hernández-Montes, H.; Argote, R.M.; Villalpando, S. Effects of ethanol consumption during pregnancy and lactation on the outcome and postnatal growth of the offspring. Ann. Nutr. Metab. 1992, 36, 121–128. [Google Scholar] [CrossRef]
- Backstrand, J.R.; Goodman, A.H.; Allen, L.H.; Pelto, G.H. Pulque intake during pregnancy and lactation in rural Mexico: Alcohol and child growth from 1 to 57 months. Eur. J. Clin. Nutr. 2004, 58, 1626–1634. [Google Scholar] [CrossRef]
- Tadesse, A.W.; Ayano, G.; Dachew, B.A.; Betts, K.; Alati, R. Exposure to maternal cannabis use disorder and risk of autism spectrum disorder in offspring: A data linkage cohort study. Psychiatry Res. 2024, 337, 115971. [Google Scholar] [CrossRef]
- Castro-Navarro, I.; McGuire, M.A.; Williams, J.E.; Holdsworth, E.A.; Meehan, C.L.; McGuire, M.K. Maternal Cannabis Use during Lactation and Potential Effects on Human Milk Composition and Production: A Narrative Review. Adv. Nutr. 2024, 15, 100196. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Gómez, M.; Hernández, M.; de Miguel, R.; Ramos, J.A. Cannabinoids and gene expression during brain development. Neurotox. Res. 2004, 6, 389–401. [Google Scholar] [CrossRef]
- Coy, K.C.; Haight, S.C.; Anstey, E.; Grant, A.M.; Ruffo, N.; Ko, J.Y. Postpartum Marijuana Use, Perceptions of Safety, and Breastfeeding Initiation and Duration: An Analysis of PRAMS Data from Seven States, 2017. J. Hum. Lact. 2021, 37, 803–812. [Google Scholar] [CrossRef]
- Astley, S.J.; Little, R.E. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol. Teratol. 1990, 12, 161–168. [Google Scholar] [CrossRef]
- Tennes, K.; Avitable, N.; Blackard, C.; Boyles, C.; Hassoun, B.; Holmes, L.; Kreye, M. Marijuana: Prenatal and postnatal exposure in the human. NIDA Res. Monogr. 1985, 59, 48–60. [Google Scholar]
- Narayanan, P.; Bertrand, K.; Waalen, J.; Chambers, C.; Ferran, K.; Bandoli, G. The Effect of Cannabis Consumption During Lactation on the Macronutrient Concentrations in Breast Milk. Breastfeed. Med. 2024, 20, 33–41. [Google Scholar] [CrossRef]
- Metz, T.D.; Borgelt, L.M. Marijuana Use in Pregnancy and While Breastfeeding. Obstet. Gynecol. 2018, 132, 1198–1210. [Google Scholar] [CrossRef]
- Josan, C.; Shiplo, S.; Fusch, G.; Raha, S.; Shea, A.K. Cannabis use during lactation may alter the composition of human breast milk. Pediatr. Res. 2023, 93, 1959–1968. [Google Scholar] [CrossRef]
- Harris, M.; Schiff, D.M.; Saia, K.; Muftu, S.; Standish, K.R.; Wachman, E.M. Academy of Breastfeeding Medicine Clinical Protocol #21: Breastfeeding in the Setting of Substance Use and Substance Use Disorder (Revised 2023). Breastfeed. Med. 2023, 18, 715–733. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Wall, M.E. Presence of delta9-tetrahydrocannabinol in human milk. N. Engl. J. Med. 1982, 307, 819–820. [Google Scholar] [CrossRef]
- Baker, T.; Datta, P.; Rewers-Felkins, K.; Thompson, H.; Kallem, R.R.; Hale, T.W. Transfer of Inhaled Cannabis into Human Breast Milk. Obstet. Gynecol. 2018, 131, 783–788. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Hanan, N.J.; Honerkamp-Smith, G.; Best, B.M.; Chambers, C.D. Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics 2018, 142, e20181076. [Google Scholar] [CrossRef]
- Moss, M.J.; Bushlin, I.; Kazmierczak, S.; Koop, D.; Hendrickson, R.G.; Zuckerman, K.E.; Grigsby, T.M. Cannabis use and measurement of cannabinoids in plasma and breast milk of breastfeeding mothers. Pediatr. Res. 2021, 90, 861–868. [Google Scholar] [CrossRef]
- Holdsworth, E.A.; Berim, A.; Gang, D.R.; Williams, J.E.; Smith, C.B.; Caffé, B.; Brooks, O.; Barbosa-Leiker, C.; McGuire, M.A.; McGuire, M.K.; et al. Human Milk Cannabinoid Concentrations and Associations with Maternal Factors: The Lactation and Cannabis (LAC) Study. Breastfeed. Med. 2024, 19, 515–524. [Google Scholar] [CrossRef]
- North American Congress of Clinical Toxicology (NACCT) 2022. Clin. Toxicol. 2022, 60, 1–162. [CrossRef]
- Wiley. Poster Abstracts. Fundam. Clin. Pharmacol. 2023, 37, 92–209. [Google Scholar] [CrossRef]
- Shenkoya, B.; Yellepeddi, V.; Mark, K.; Gopalakrishnan, M. Predicting Maternal and Infant Tetrahydrocannabinol Exposure in Lactating Cannabis Users: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2023, 15, 2467. [Google Scholar] [CrossRef]
- Silveira, G.d.O.; Loddi, S.; de Oliveira, C.D.R.; Zucoloto, A.D.; Fruchtengarten, L.V.G.; Yonamine, M. Headspace solid-phase microextraction and gas chromatography−mass spectrometry for determination of cannabinoids in human breast milk. Forensic Toxicol. 2017, 35, 125–132. [Google Scholar] [CrossRef]
- Yeung, C.H.T.; Bertrand, K.A.; Best, B.M.; Capparelli, E.; Chambers, C.D.; Hajducek, D.M.; Hamadeh, A.; Ito, S.; Momper, J.D.; Edginton, A.N. Cannabidiol Exposure Through Maternal Marijuana Use: Predictions in Breastfed Infants. Clin. Pharmacokinet. 2023, 62, 1611–1619. [Google Scholar] [CrossRef]
- Larsen, L.A.; Ito, S.; Koren, G. Prediction of milk/plasma concentration ratio of drugs. Ann. Pharmacother. 2003, 37, 1299–1306. [Google Scholar] [CrossRef]
- Anderson, P.O. Drugs in lactation. Pharm. Res. 2018, 35, 45. [Google Scholar] [CrossRef]
| Type | Name | Metabolites | Maternal Weight-Adjusted Dosage (%) |
|---|---|---|---|
| Sedative | Diazepam | nordiazepam, temazepam, and oxazepam | 3 |
| Phenobarbital | p-hydroxyphenobarbital | 72.5 | |
| Haloperidol | glucuronidation | None | |
| Dichloralphenazone | chloral hydrate, trichloroethanol and trichloroacetic acid | 0.59 | |
| Fospropofol | propofol | 0.2 | |
| Alprazolam | alpha-hydroxyalprazolam | 3 | |
| Propofol | propofol-glucuronide and sulfo- and glucuro-conjugation | 0.2 | |
| Carisoprodol | meprobamate | 6–6.9 | |
| Lorazepam | glucuronide conjugate | 8.5 | |
| Inhalant | Methamphetamine | amphetamine | None |
| Tobacco | Nicotine | cotinine | 1.9 |
| Alcohol | Alcohol | acetaldehyde and acetate | 0.5–3.3 |
| Stimulant | Sulpiride | desmethylsulpiride | 2.0–18.0 |
| Castor oil | ricinoleic acid and glycerol | None | |
| Caffeine | paraxanthine and theobromine | 10.0–18.0 | |
| Cannabis | Cannabis | 11-OH-THC and glucuronide conjugates | 0.4–8.7 |
| Cannabidiol | 7-OH-CBD and glucuronide conjugates of CBD metabolites | None | |
| Cocaine | benzoylecgonine, ecgonine methyl ester, and cocaethylene | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yi, B.; Zhang, T. The Impact of Substance Use Disorder and Drug Transfer into Breast Milk: Implications for Maternal and Infant Health. Pharmaceutics 2025, 17, 719. https://doi.org/10.3390/pharmaceutics17060719
Yang Y, Yi B, Zhang T. The Impact of Substance Use Disorder and Drug Transfer into Breast Milk: Implications for Maternal and Infant Health. Pharmaceutics. 2025; 17(6):719. https://doi.org/10.3390/pharmaceutics17060719
Chicago/Turabian StyleYang, Yongzong, Bofang Yi, and Tao Zhang. 2025. "The Impact of Substance Use Disorder and Drug Transfer into Breast Milk: Implications for Maternal and Infant Health" Pharmaceutics 17, no. 6: 719. https://doi.org/10.3390/pharmaceutics17060719
APA StyleYang, Y., Yi, B., & Zhang, T. (2025). The Impact of Substance Use Disorder and Drug Transfer into Breast Milk: Implications for Maternal and Infant Health. Pharmaceutics, 17(6), 719. https://doi.org/10.3390/pharmaceutics17060719







