1. Introduction
Physiologically based biopharmaceutics modelling (PBBM) is an evolving tool that is being increasingly used to inform and facilitate decision-making in the drug development process as well as gaining increasing acceptance by regulatory agencies as part of the product approval process [
1]. This subset of physiologically based pharmacokinetic (PBPK) modelling integrates physicochemical drug properties, dissolution profiles and specific characteristics of the gastrointestinal (GI) tract to predict in vivo exposures and bioavailability in both fasted and fed states. PBPK models are constructed using a bottom-up approach, whereby the actual physiological characteristics are integrated to characterise pharmacokinetic (PK) behaviours in both the preclinical and clinical environment [
2]. In contrast to empirical compartmental PK modelling methods, PBBM models enable the prediction of food effects [
3] and the assessment of formulation performance [
4] in in vivo drug absorption. Hence, such approaches provide a robust scientific basis for understanding the PK of drugs, for which there is increasing regulatory support.
This study used PBBM to facilitate a better understanding of the impact of food on the absorption and clinical outcomes of two widely used NSAIDs (nonsteroidal anti-inflammatory drugs), diclofenac and ibuprofen, when formulated as ultra-rapid-release tablets using the Surge Dose
® technology [
5]. Both drugs are available in a variety of commercial products ideally taken after food to reduce the risk of GI side-effects which can also be minimised by using the lowest effective dose of these drugs [
6]. Like many other drugs, diclofenac and ibuprofen demonstrate variable absorption in the fasted state [
7,
8], attributable to the impact of the migrating motor complex (MMC) on gastric emptying (GE), which is an important determinant of drug dissolution and absorption in the fasted state [
9]. In the fed state, absorption is typically delayed and is more variable, being heavily influenced by the fed-state GE processes, especially for weak acids. Rapid, consistent absorption and onset of action for any analgesic are important, so any delays will potentially compromise clinical efficacy. Therefore, tablet formulations that provide fast and consistent drug absorption in both fasted and fed states, independent of the MMC and GE, are needed to provide optimal clinical outcomes.
Consistent with recent publications outlining the importance of the biopharmaceutics risk assessment [
10,
11] for optimised clinical drug product performance, the Surge Dose
® tablets have been designed to achieve ultra-rapid activated dissolution of a drug in co-administered liquid and gastric contents. This will then allow for the resultant solution to empty rapidly from the stomach independent of the MMC. This results in faster and more consistent absorption, as demonstrated previously for lornoxicam, diclofenac and paracetamol formulated as Surge Dose
® tablets in fasted PK studies in human volunteers [
12]. More rapid absorption produces earlier and higher maximum plasma concentrations (C
max), with earlier onset of analgesia as well as better overall and longer lasting analgesia. PK-PD modelling predicted faster and greater analgesia for Surge Dose
® paracetamol tablets within 30 min post-dosing compared with other commercial products [
13]. Given the consumer need for fast and consistent onset of action from analgesic tablets, whether taken in the fasted state or after food, PBBM was identified as a computational modelling strategy to predict how Surge Dose
® formulations would be absorbed in the fed state without the need for lengthy and expensive biostudies.
4. Discussion
This paper shows how a PBBM approach can be used to provide greater insights into the in vivo performance of new tablet formulations, particularly in the fed state, based on biorelevant in vitro dissolution characteristics and fasted PK data. This can significantly reduce development time and costs, minimising extensive PK studies in humans.
The results demonstrate that diclofenac and ibuprofen delivered as ultra-rapid-release Surge Dose
® tablets are absorbed like liquids in the fasted state, which allows similarly rapid absorption in the fed state. This is an important finding for drugs taken for acute indications such as pain, particularly NSAIDs, which are frequently taken after a meal to minimise the potential for GI side-effects and the risk of subsequent delayed and increased variability of absorption dependent on GE. Since rapid absorption of ibuprofen leads to an earlier and higher C
max, associated with earlier onset of action combined with enhanced overall and prolonged analgesia [
26], the ideal analgesic formulation needs to provide fast and consistent onset of action whether the dose is taken with or without food.
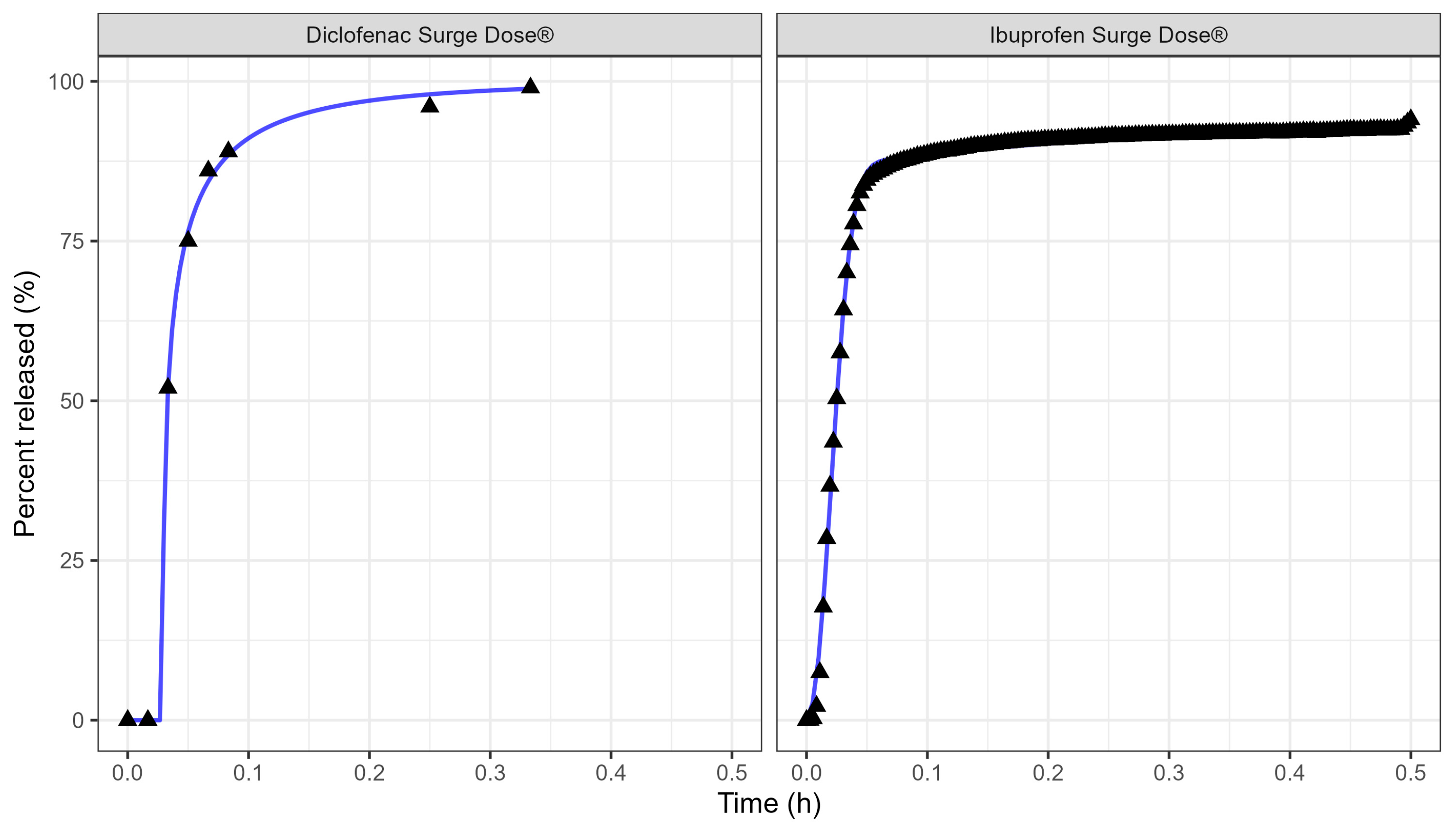
Ultra-rapid-release Surge Dose® tablet formulations have been developed to release at least 50% of the drug under highly discriminating and biorelevant, non-sink in vitro test conditions within the first 5 min. These dissolution conditions use 900 mL of 0.0033 M hydrochloric acid at 37 °C with low stirring speeds as a development tool to achieve maximal dissolution under minimal agitation. Tablets of lornoxicam, diclofenac and paracetamol that meet this release specification have been shown to achieve significantly faster and more consistent absorption in fasted subjects compared with conventional solid oral dosage forms, which typically achieve less than 5% dissolution after 20 min under these biorelevant dissolution conditions. The employment of PBBM has allowed prediction of the absorption kinetics from these ultra-rapid-release tablets in the fed state, avoiding the need for expensive and time-consuming studies. Furthermore, it has enabled the prediction of the absorption of other drugs from such tablets in both fasted and fed states based on in vitro dissolution data collected under these biorelevant test conditions.
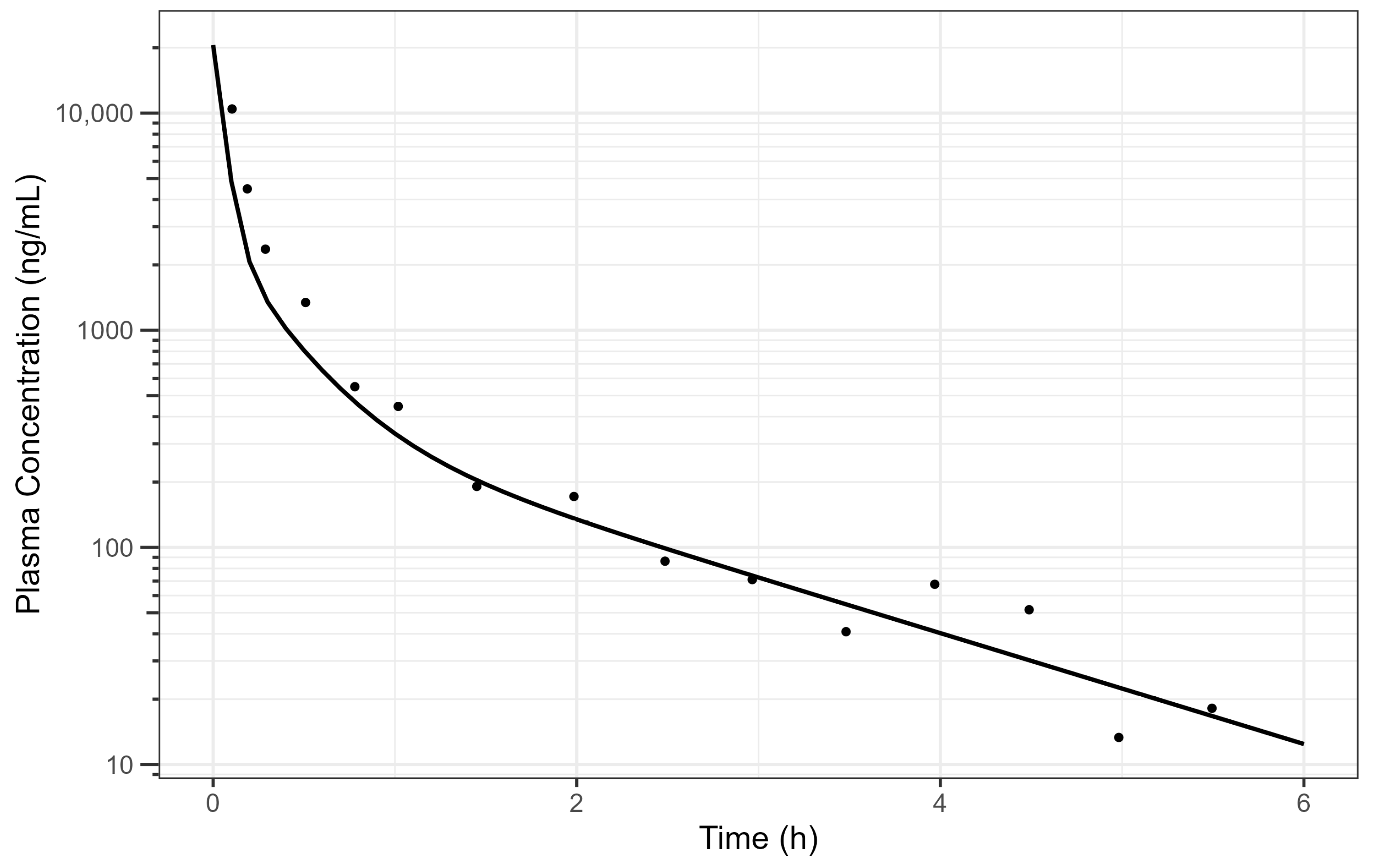
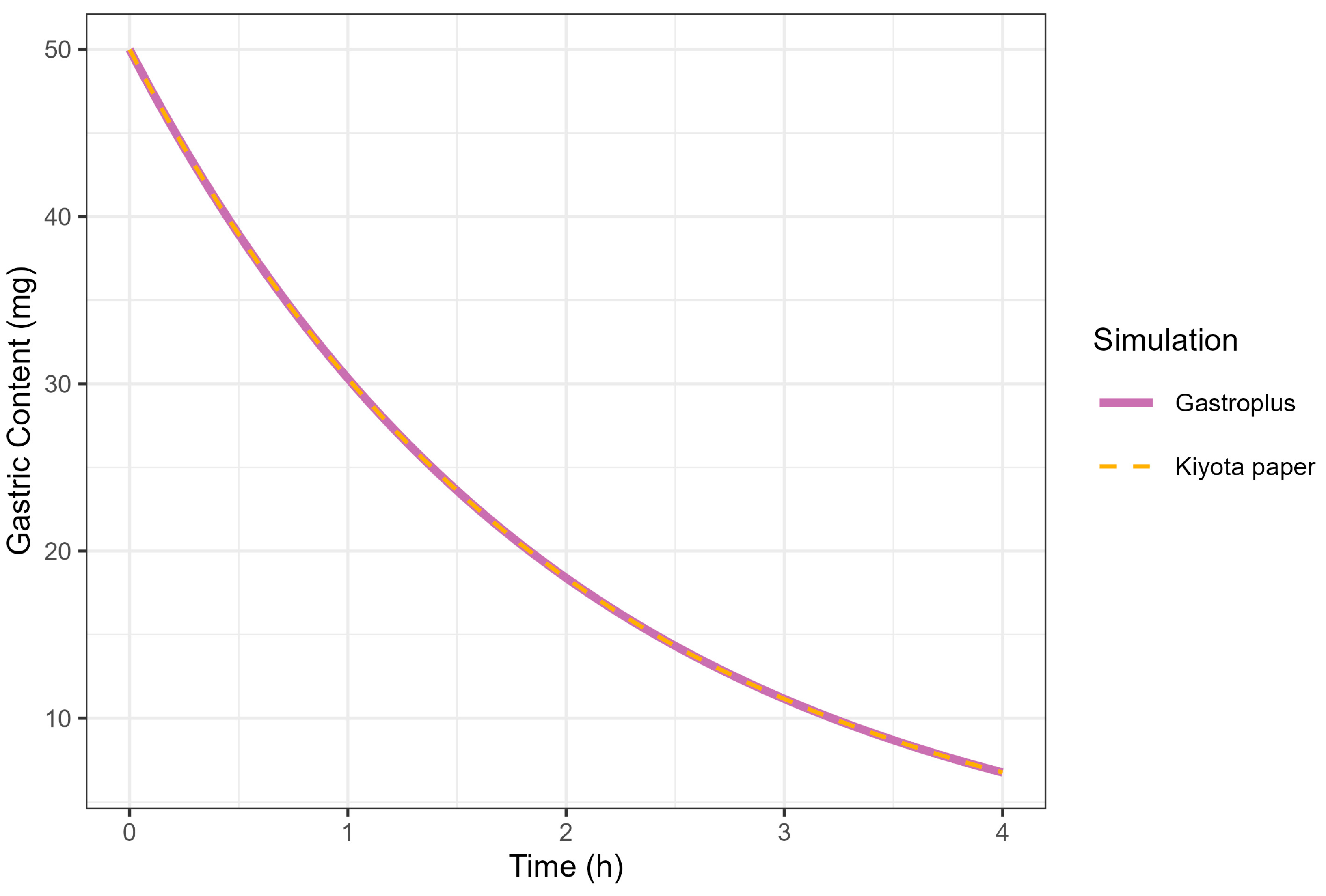
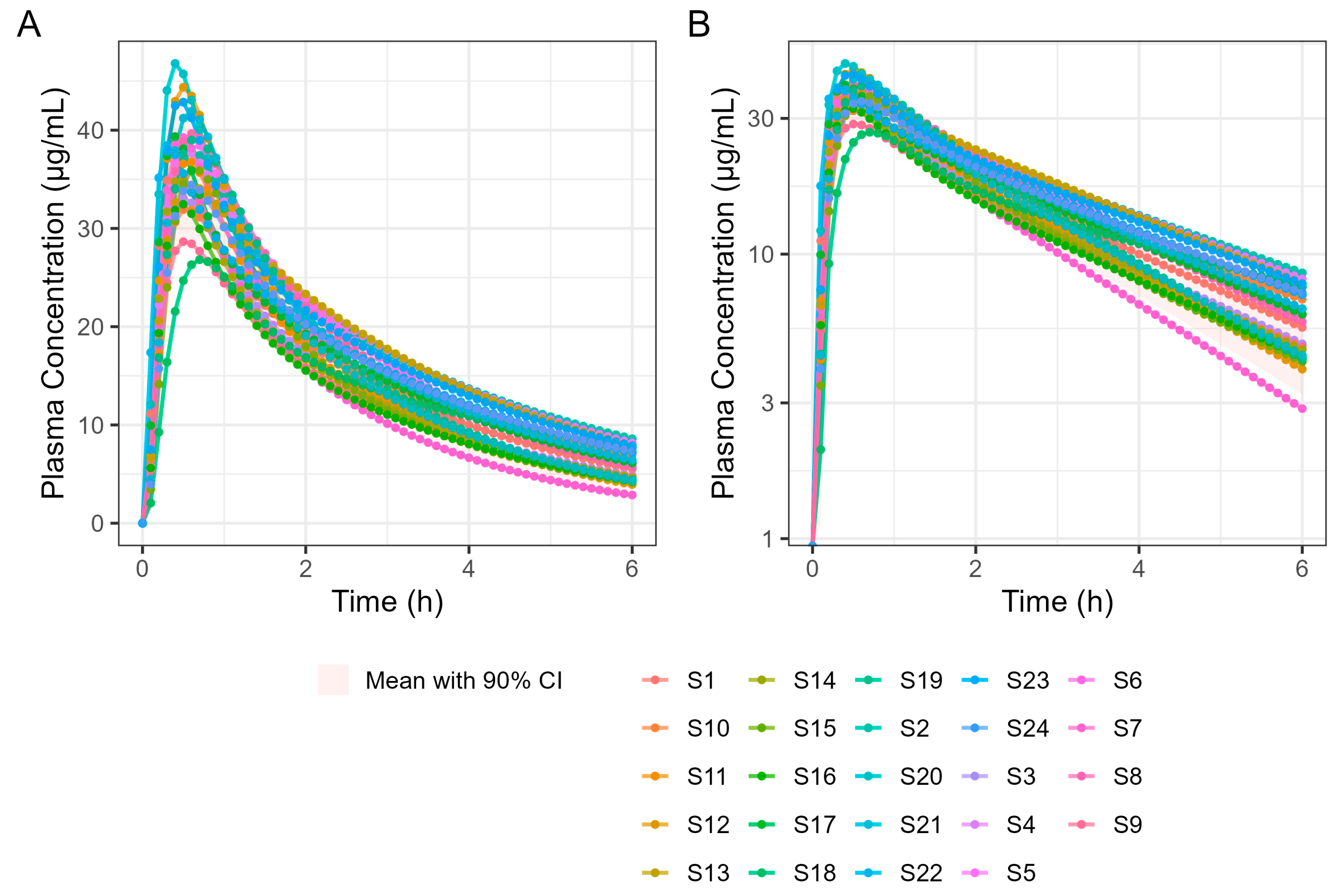
Fitting fasted diclofenac PK data to an established PBBM model has provided valuable insights into the absorption kinetics for these ultra-rapid-release tablets in the fasted state. An excellent fit for the human PK data with a Tmax value of 20 min was achieved using GastroPlus® standard settings by reducing the gastric mean transit time to 0.1 h. This finding provides compelling evidence that the rapid disintegration and dissolution of the drug from the Surge Dose® tablet in vivo provide absorption kinetics similar to an aqueous solution where disintegration and dissolution are not rate limiting for absorption. Rapid dissolution of the drug in the co-administered water and gastric contents provides an optimal tablet formulation that behaves like a solution, facilitating earlier and more consistent onset of analgesia.
PPSA assessment of the fasted diclofenac model has shown that the gastric MTT of 0.1 h for the Surge Dose® tablet formulations is actually a conservative value and could in fact be even faster to match the observed rate of absorption in the clinic. However, values faster than 0.1 h would be more rapid than the default value in GastroPlus® for solutions, which suggests further characterisation of the gastric emptying of liquids and the subsequent impact on PK may be valuable, especially for highly permeable drugs with a short half-life (where Cmax/Tmax is very sensitive to gastric emptying). Additional PPSA around the MPT has confirmed that, because of the unique performance of Surge Dose® formulations, precipitation does not occur in the stomach and is also not relevant for a highly permeable weak acid in the small intestine. As a consequence, there were no scientific barriers to increasing the MPT from the GastroPlus® default of 900 s.
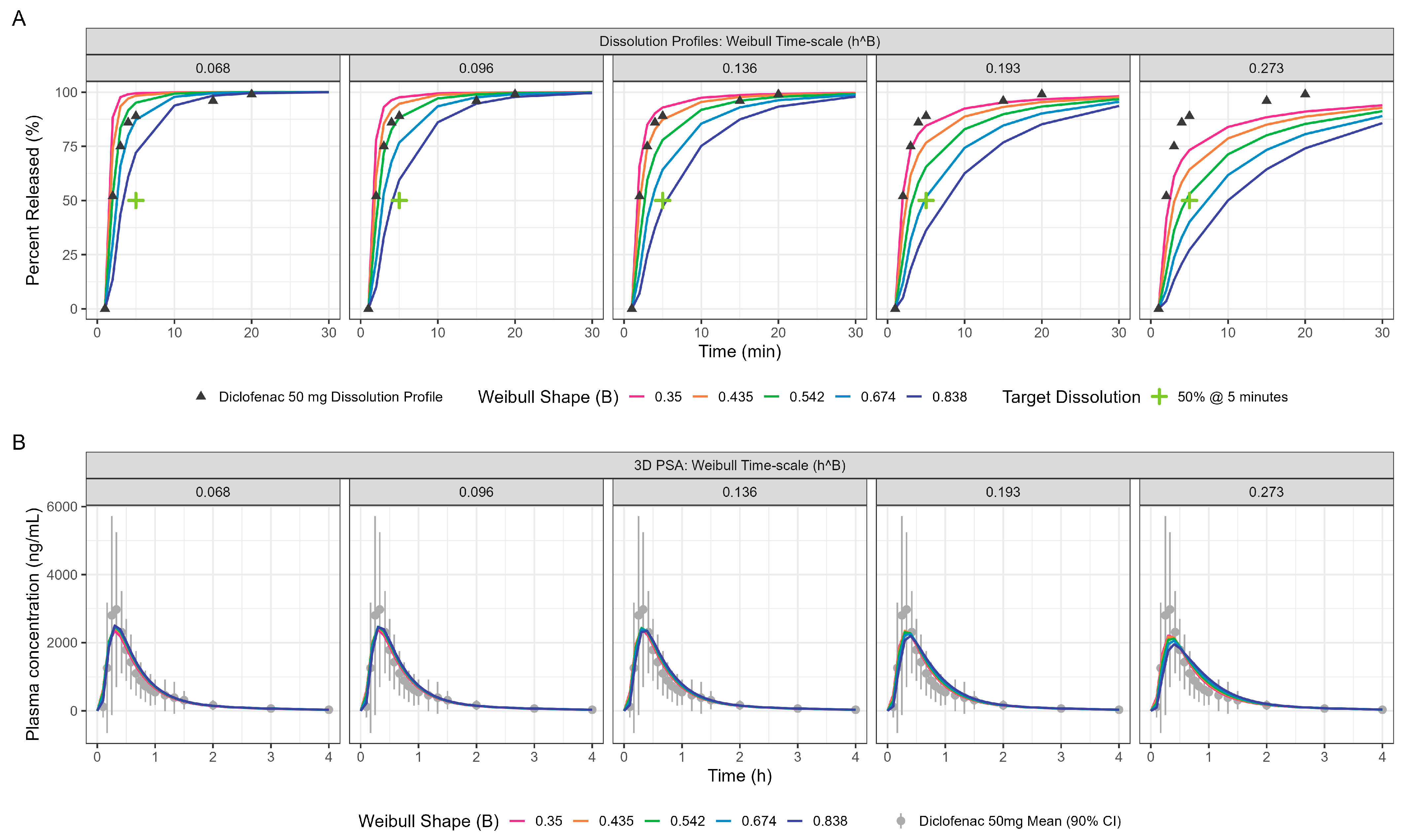
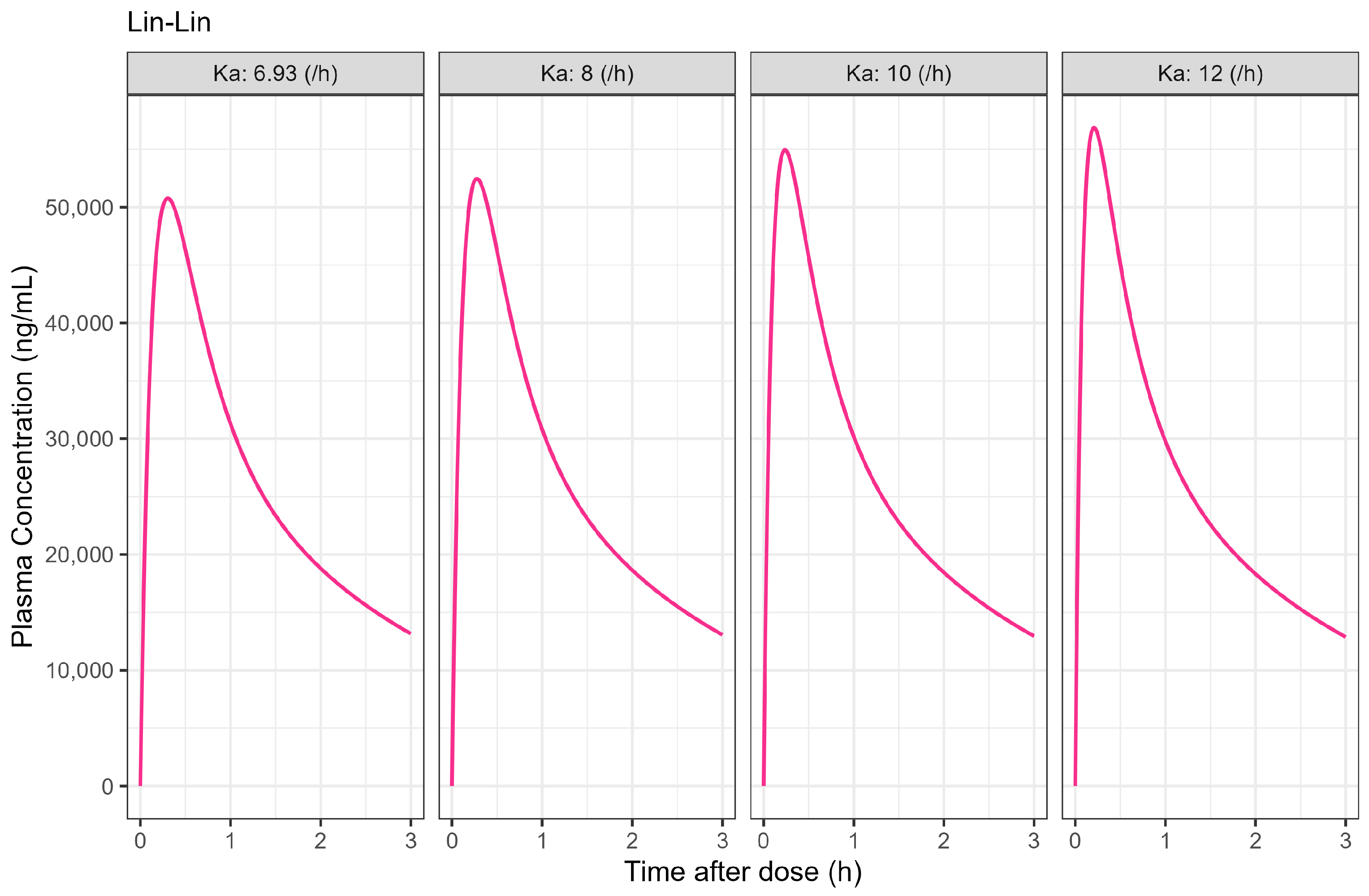
PPSA was also used to investigate the impact of changes in dissolution rate outside the target of >50% in 5 min used in development of the Surge Dose
® formulations. The results presented in
Figure 5 and
Table 4 clearly demonstrate that the slowing of tablet dissolution for the utilised mean gastric MTT had a consequential impact on C
max with a subtle delaying of T
max. These findings will be extended in due course to the establishment of safe space dissolution for the Surge Dose
® products.
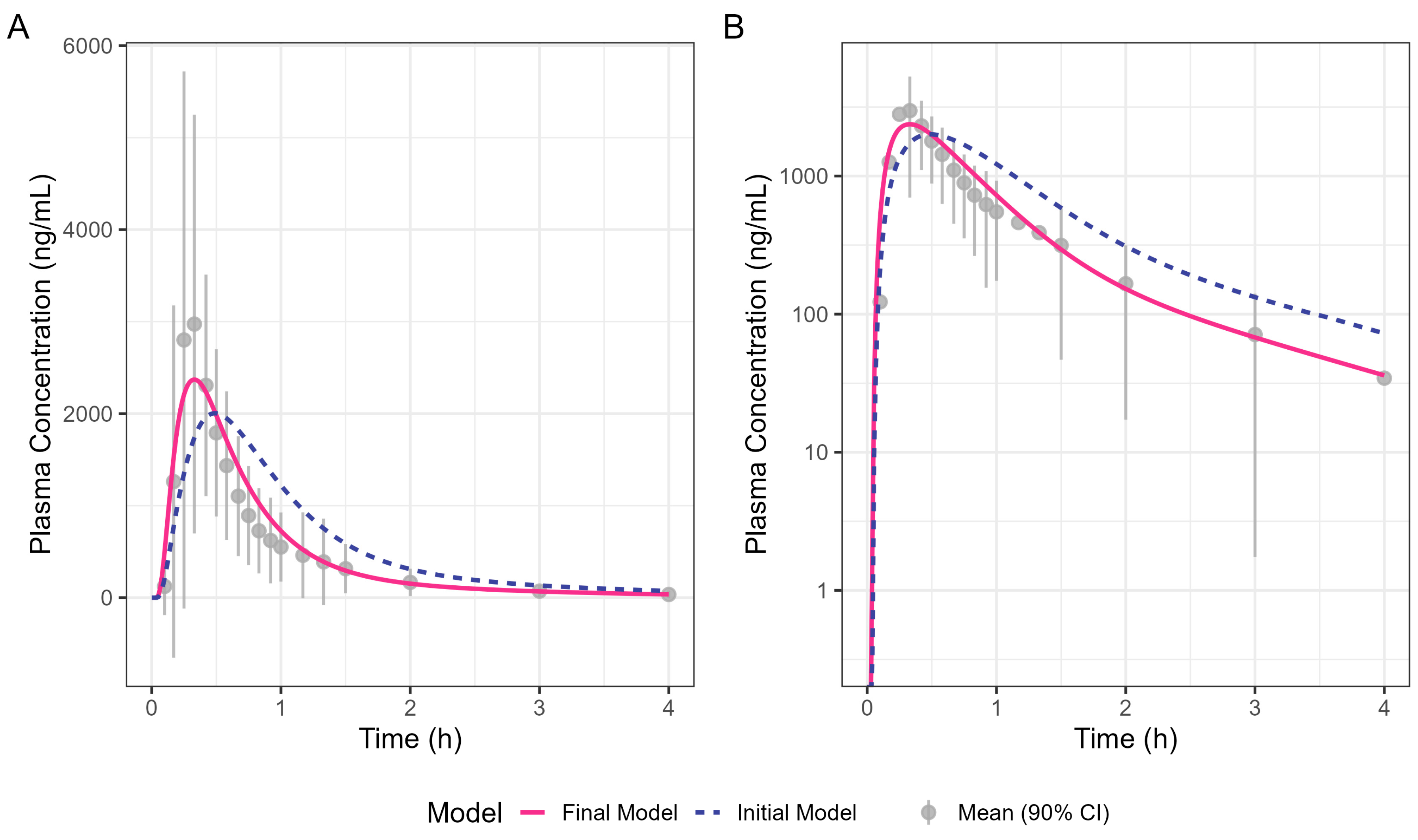
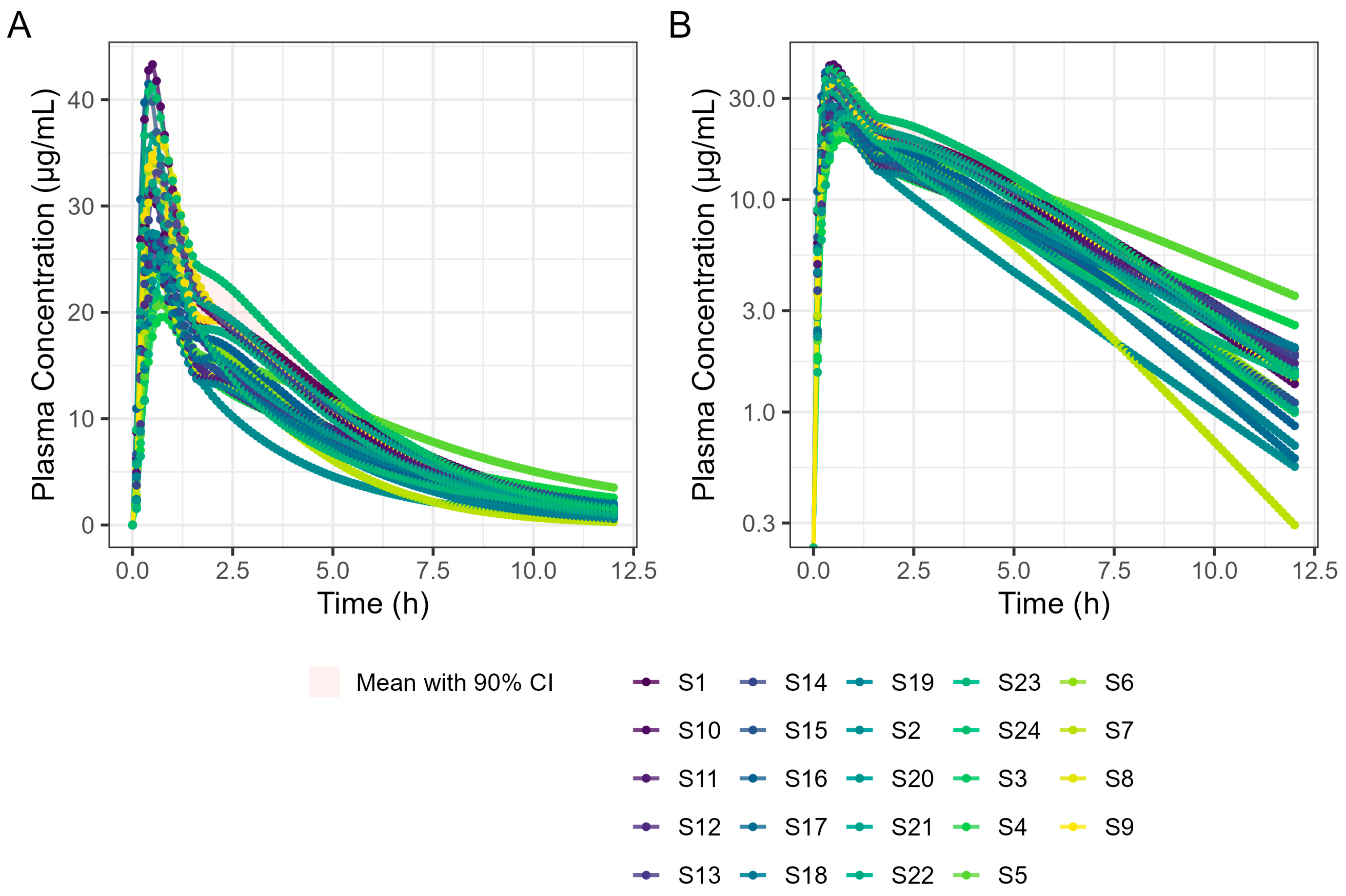
By incorporating systemic PK parameters for ibuprofen with biorelevant dissolution data on a prototype Surge Dose
® tablet into the PBBM model that fitted the fasted diclofenac PK data, rapid and consistent absorption for the ultra-rapid-release ibuprofen tablet, again comparable to a solution, was predicted in the fasted state. A single-subject simulation for an ibuprofen solution and a Surge Dose
® tablet predicted T
max values of 26.4 min and 28.8 min, respectively. In the virtual trial of 24 subjects, T
max values ranged from 18.0 to 42.0 min for Surge Dose
® ibuprofen with mean and median T
max values of 31.8 min and 30.0 min, respectively. Similar mean and median T
max values are indicative of a normal distribution of absorption profiles with few slow or multiple peak profiles, attributable to GE being rate limiting for absorption. This compares with the shortest published mean T
max value of 32.52 min for ibuprofen lysine tablets [
24].
Of interest were the different Tmax values for diclofenac and ibuprofen, even for a solution of the drug, with Tmax for ibuprofen being longer at 26–30 min than for diclofenac (around 20 min). This reflects that, although Tmax and Cmax are generally used as indicators of absorption rate, they are also dependent on the systemic PK for each drug. When comparing different drugs with different systemic PK characteristics, the post-absorption processes may have a greater influence on Tmax than the dissolution characteristics of the formulation. In this case, the PBBM reveals that even the fastest dissolving ibuprofen tablet will not have as short a Tmax as the fastest-dissolving diclofenac tablet.
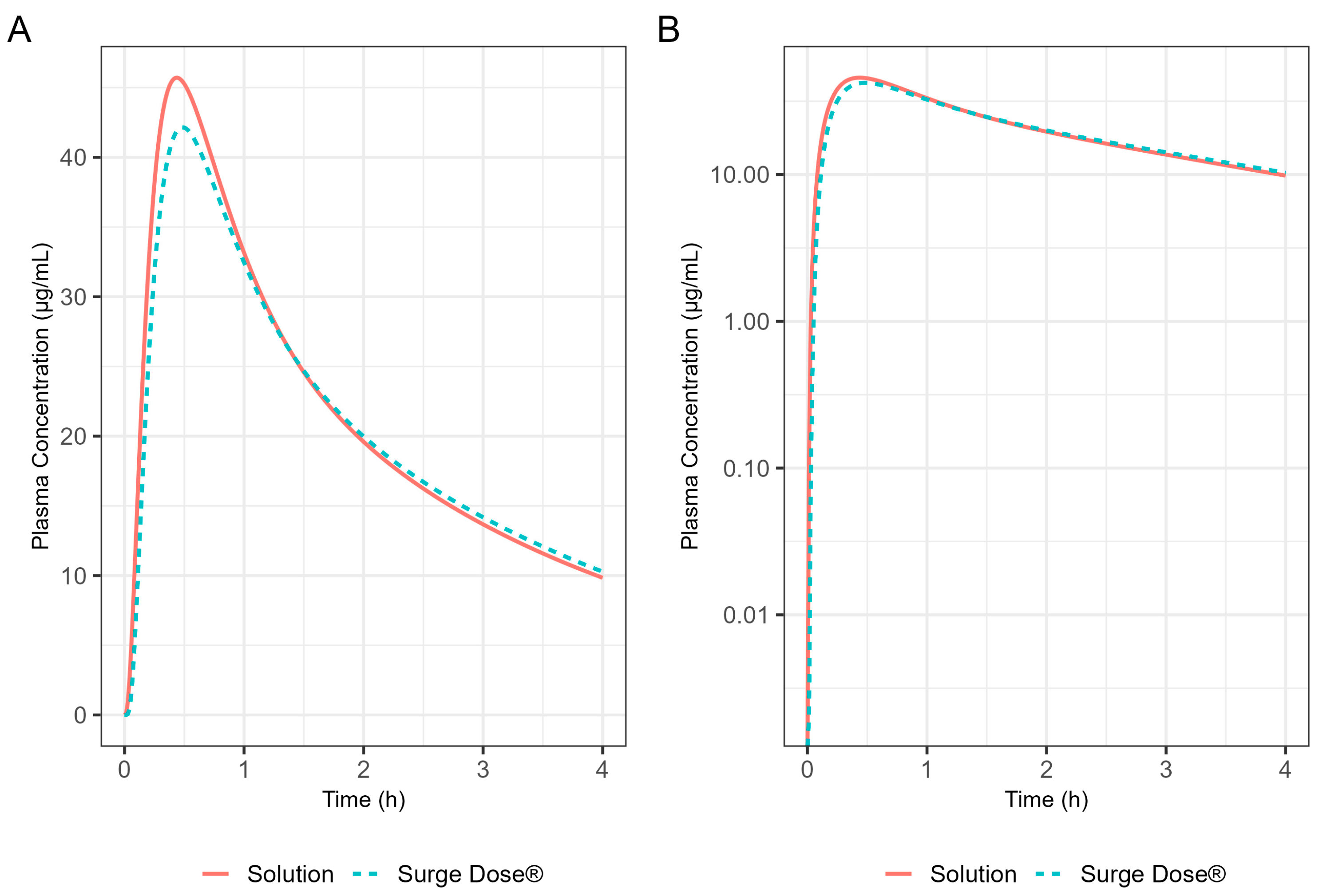
In the fed state, rapid dissolution of the drug from the Surge Dose® tablets in the co-administered water takes advantage of the early-phase gastric Magenstraße, producing a solution such that 75% of the dose will reach the small intestine for absorption in the first 90 min post-dosing. The remaining 25% will mix with stomach contents and reach the small intestine because of late-phase emptying. The fed GastroPlus® PBBM diclofenac model suggests that there would be little impact of food on the absorption of this drug from an ultra-rapid-release tablet. The predicted fed-state Tmax was 21.0 min compared with 19.8 min in the fasted state. This has important clinical implications as, when such a formulation is taken with food, it will avoid the delayed and variable absorption and associated variable onset of action observed with conventional tablets. A significant predicted benefit to the patient with this Surge Dose® tablet formulation that behaves like a solution is that food will have little effect on absorption and onset of action, which is an important clinical benefit for such formulations.
Application of the diclofenac PBBM to ibuprofen predicted little effect of food on T
max values for Surge Dose
® ibuprofen tablets, consistent with the ultra-rapid dissolution of the drug in vivo where the tablet behaves like a solution. While the median T
max value of 30 min was the same for fasted and fed states, the predicted mean fed T
max value was only slightly longer, 35.4 min compared with 31.8 min for fasted subjects. Predicted mean AUC
(0–12 h) values were similar for fasted and fed subjects, confirming that the presence of food did not reduce the overall exposure to the drug. In contrast, absorption from conventional ibuprofen acid tablet was delayed from a T
max of 53 min to a T
max of 93 min by food, and even a fast-acting ibuprofen lysine tablet showed a significant food delay, from a T
max of 33.0 min in the fasted state to a T
max of 70.8 min after food [
27].
Of particular note is that the predicted fasted and fed T
max values for Surge Dose
® ibuprofen tablets are faster and more consistent than the T
max values reported in the literature for conventional tablet formulations (
Table 11).
Reported mean or median Tmax values for ibuprofen lysine tablets in the fasted state are in the range of 30–45 min, typically with high variability, as demonstrated by CVs of 30–40% and ranges of 25 to 90 min. The simulated Surge Dose® ibuprofen Tmax values in the fasted state predicted more consistent absorption with similar mean and median values for Tmax of 31 and 30 min, respectively, but with significantly lower variability (17% CV) and a narrow range of 18 to 42 min. Published Tmax data on ibuprofen lysine tablets in the fed state show a significant delay in absorption, with median values of 70 to 90 min and an extended range from 30 to 180 min. In contrast, the fed simulated data for Surge Dose® ibuprofen predict faster and more consistent absorption, with mean and median Tmax values around 30 min with a narrower range of 24 to 54 min.
Similar simulated Surge Dose® ibuprofen Tmax data for both fasted and fed states suggest that such formulations will offer rapid absorption and consistent clinical outcomes irrespective of whether the dose is taken on an empty stomach or not. This rapid in vivo dissolution and absorption also has clinical benefits in terms of reducing the potential for GI damage with ibuprofen and other NSAIDs, improving their GI tolerability. Rapid dissolution in the stomach converts the drug to the ionised species, which is less absorbed by the gastric mucosa and avoids direct local damage from undissolved drug in contact with the mucosa. The short gastric residence time of the dissolved drug predicted by the model for these rapidly disintegrating tablets has the potential to also reduce the time over which gastric damage can occur.