Effervescent Tablet Preparation by Twin-Screw Melt Granulation with Sorbitol as a Melt Binder †
Abstract
1. Introduction
2. Materials and Methods
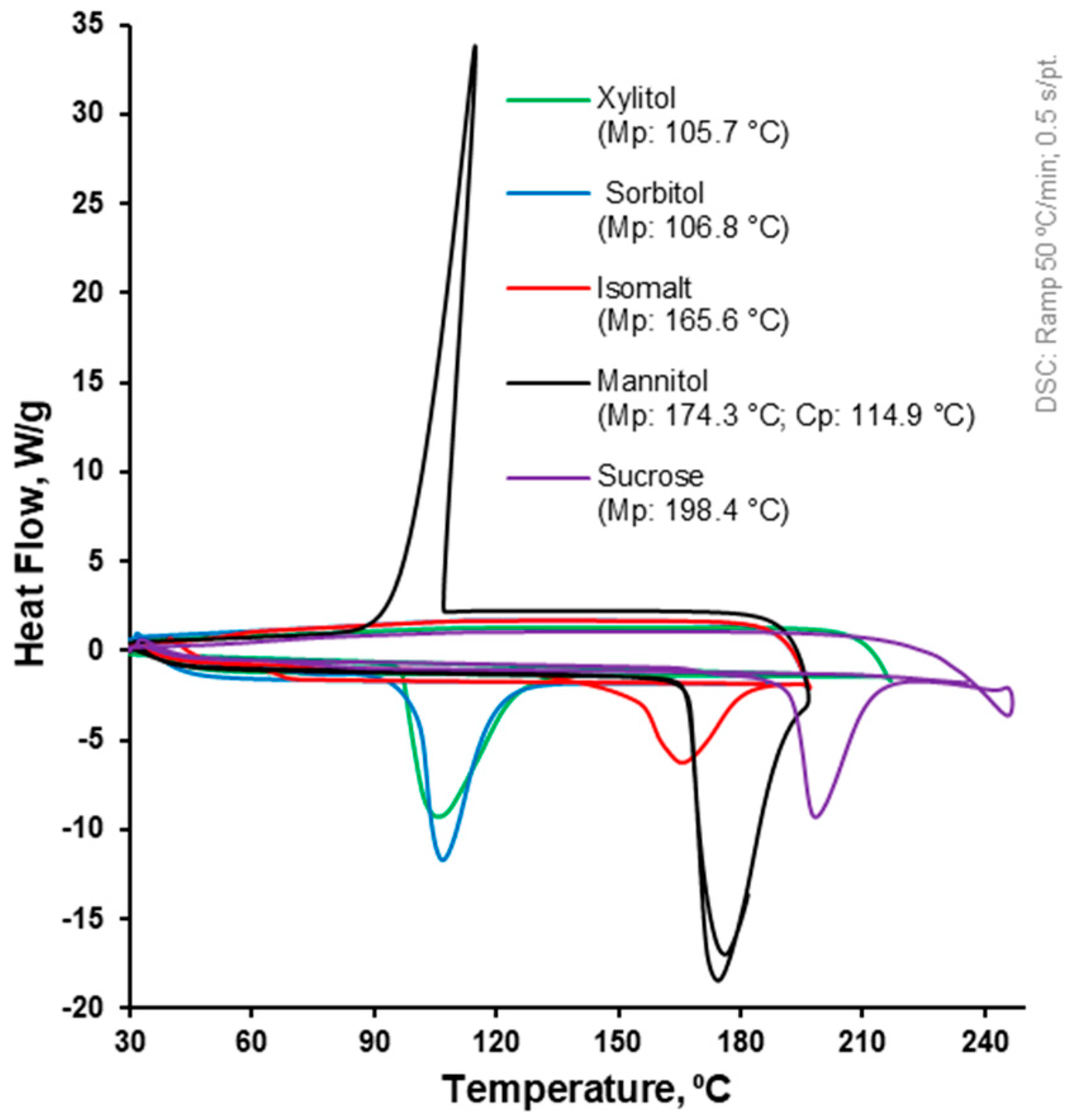
2.1. Differential Scanning Calorimetry (DSC)
2.2. Sample Preparation
2.3. Moisture Content Measurement
2.4. Optical Microscopy
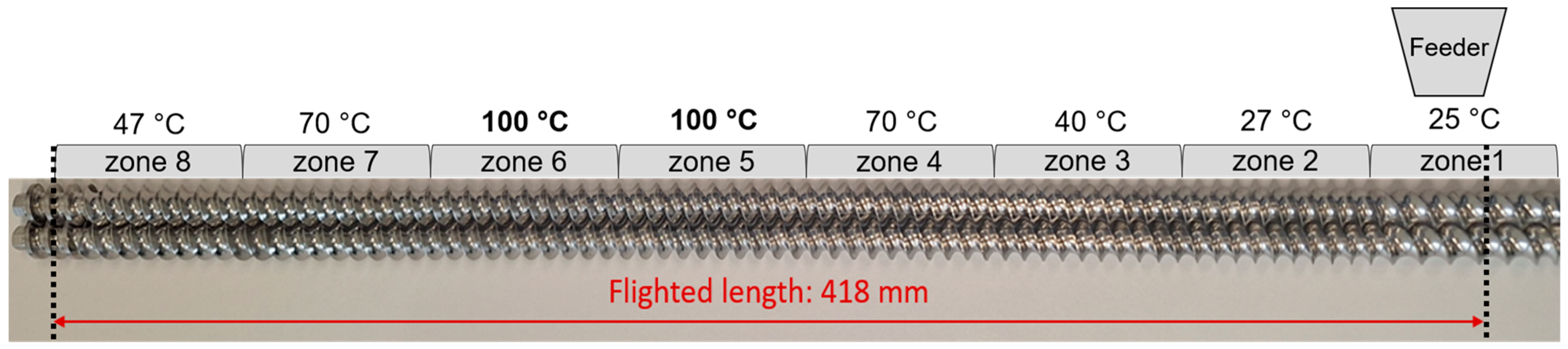
2.5. Twin-Screw Melt Granulation
2.6. Calculation of TS-MG Free Volume and Fill Level
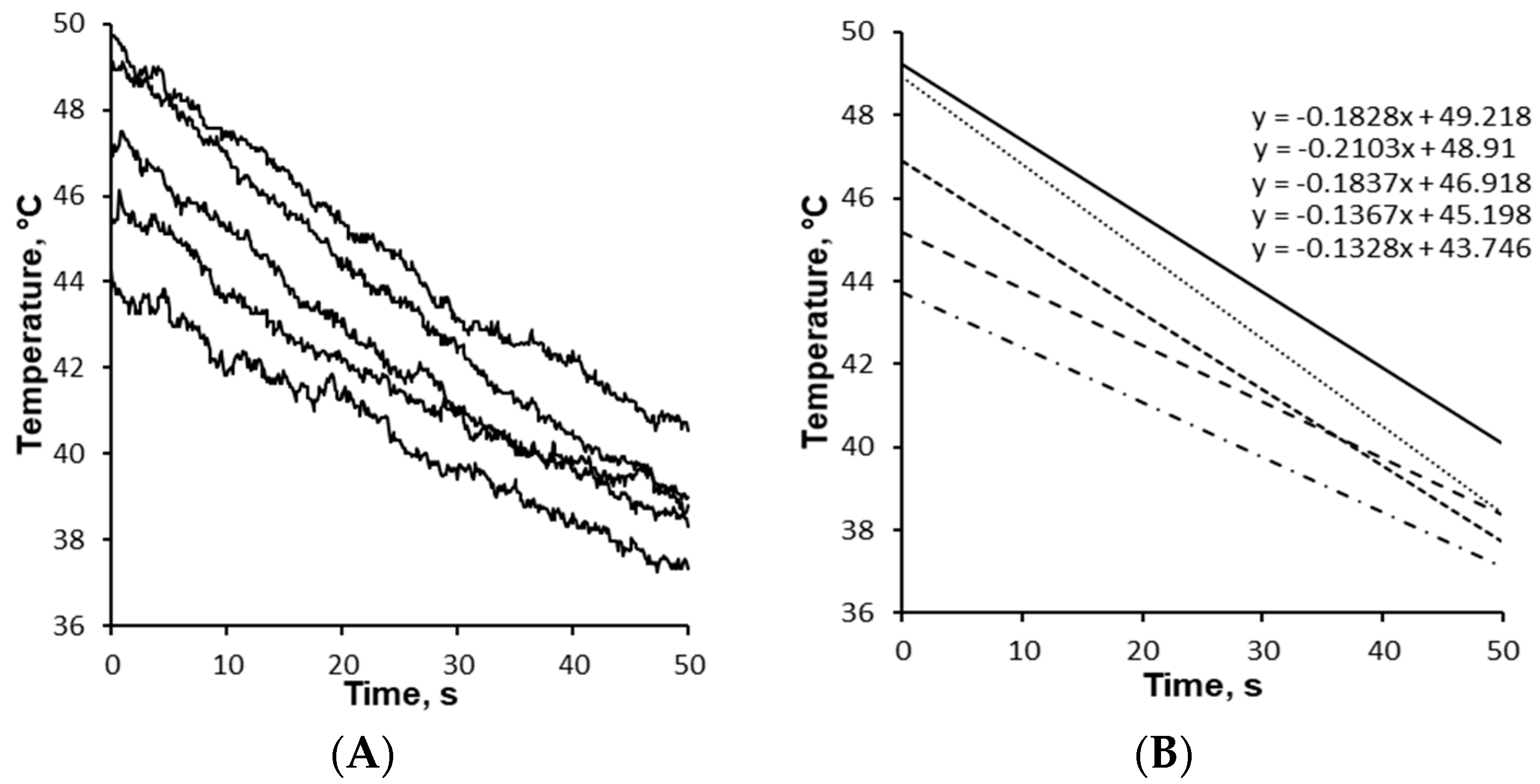
2.7. Cooling Rate of Granules
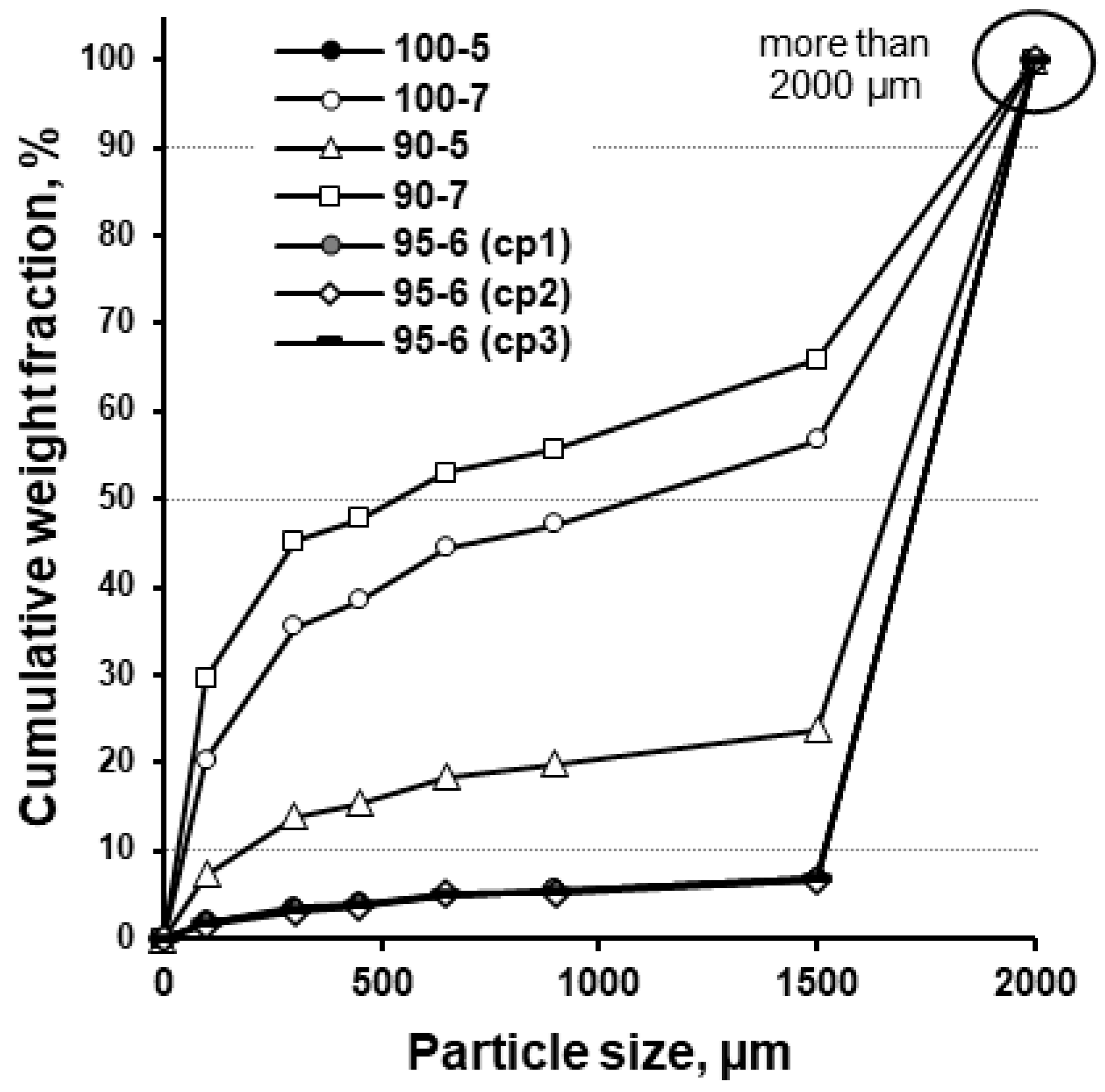
2.8. Sieve Analysis of Effervescent Granules
2.9. Bulk and Tapped Density Measurement
2.10. Preparation of Effervescent Tablets
2.11. Tablet Hardness Measurement and Tensile Strength Calculation
2.12. Tablet Disintegration Measurements
2.13. Tablet Homogeneity Measurements
2.14. Statistical Analysis
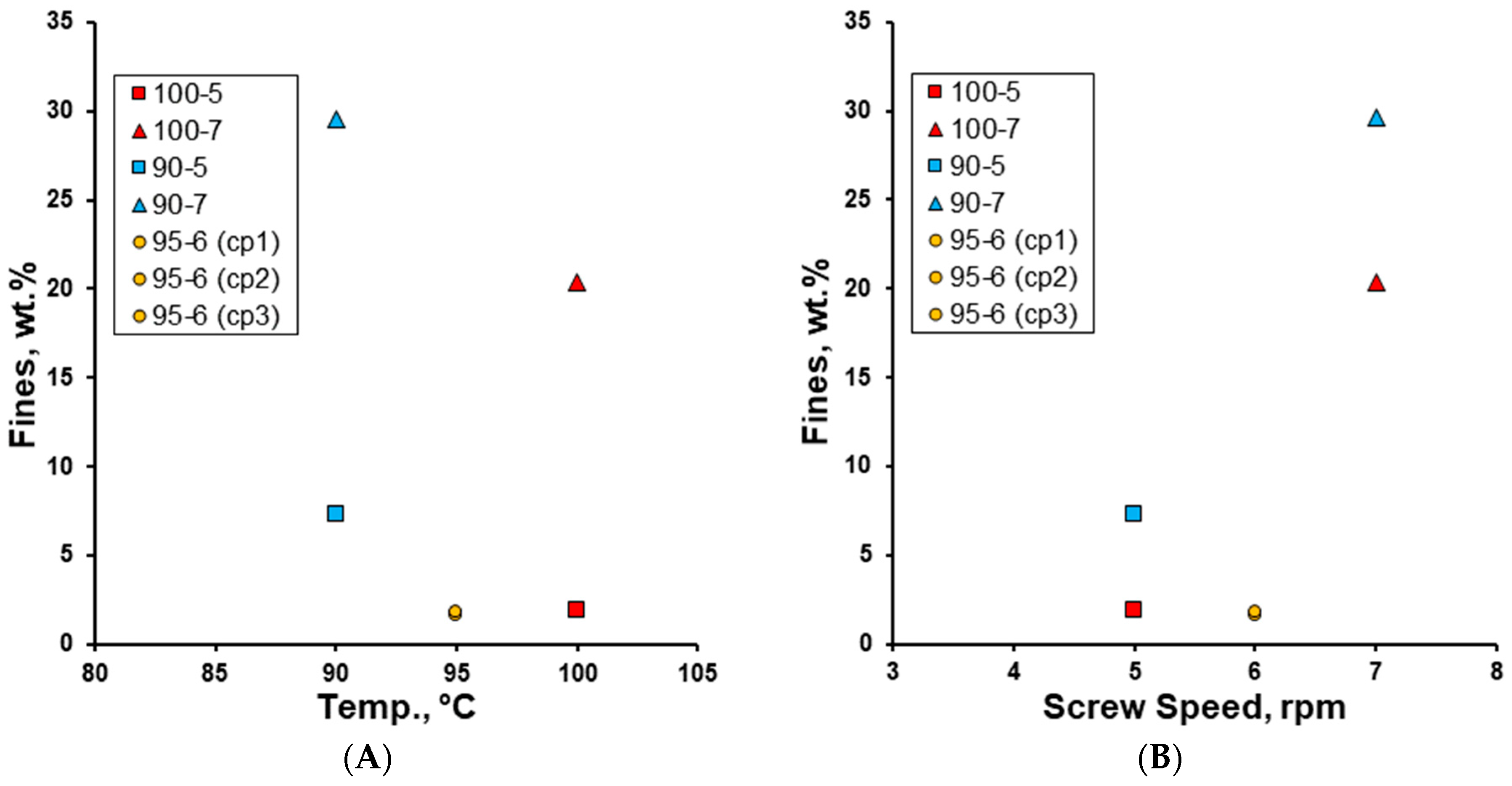
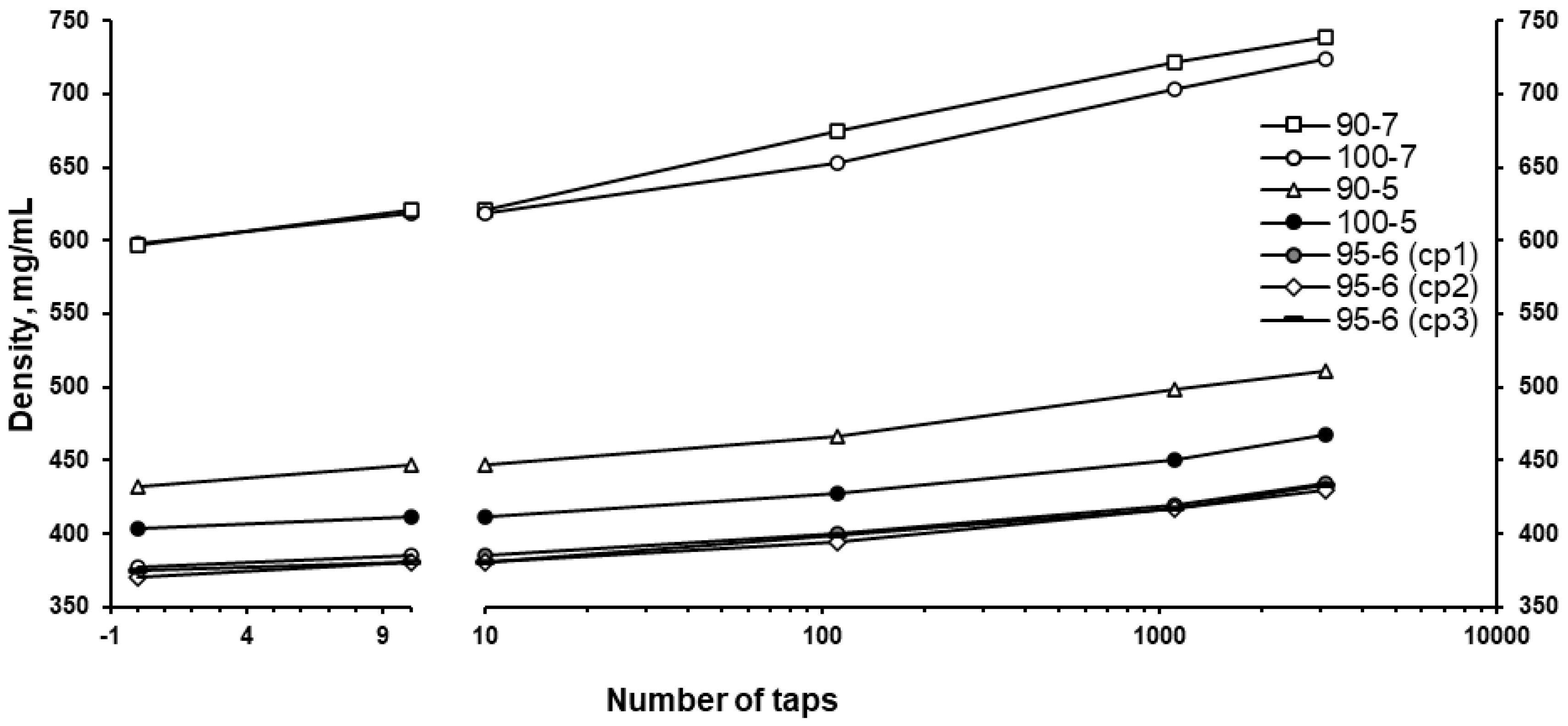
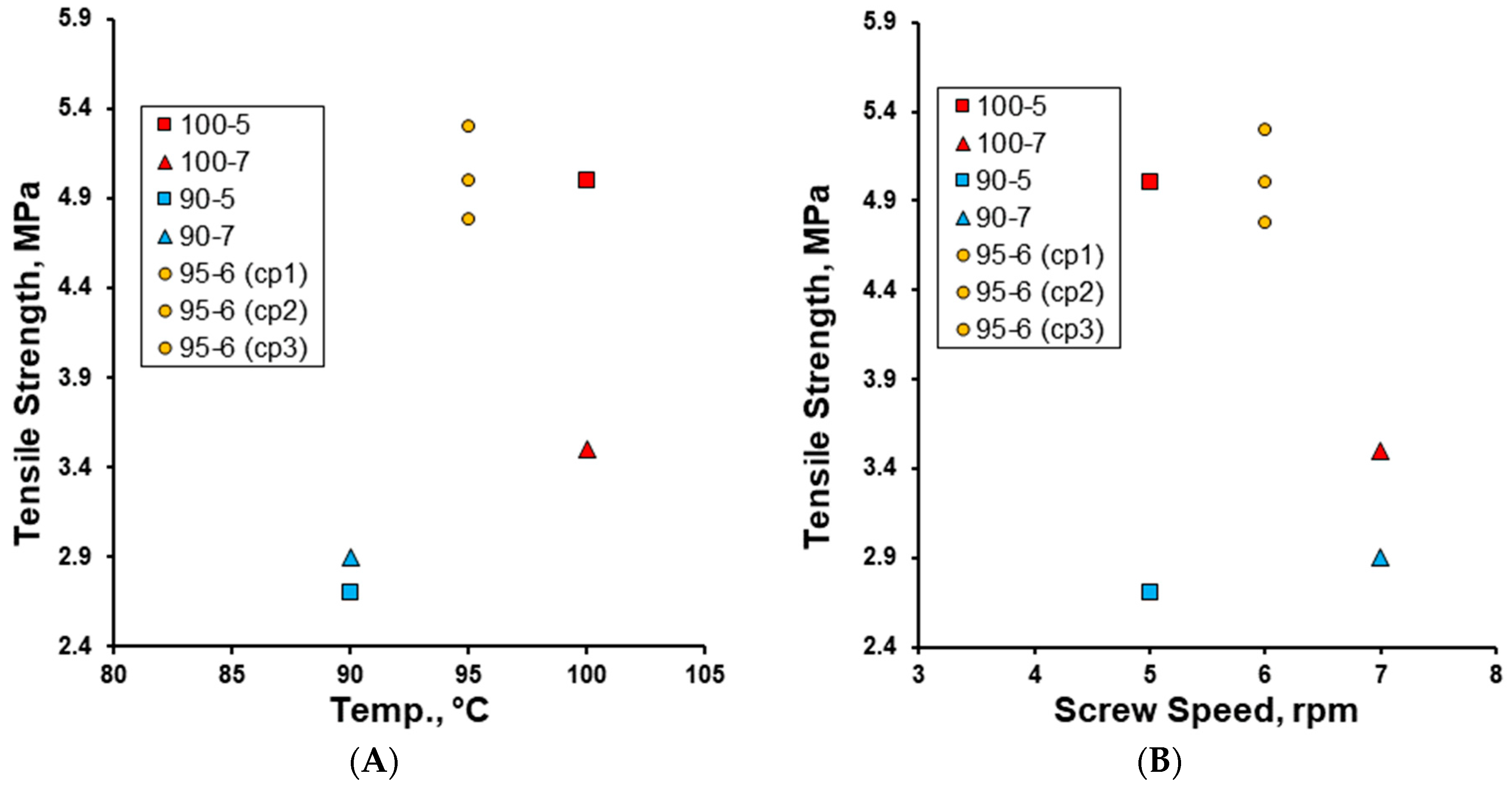
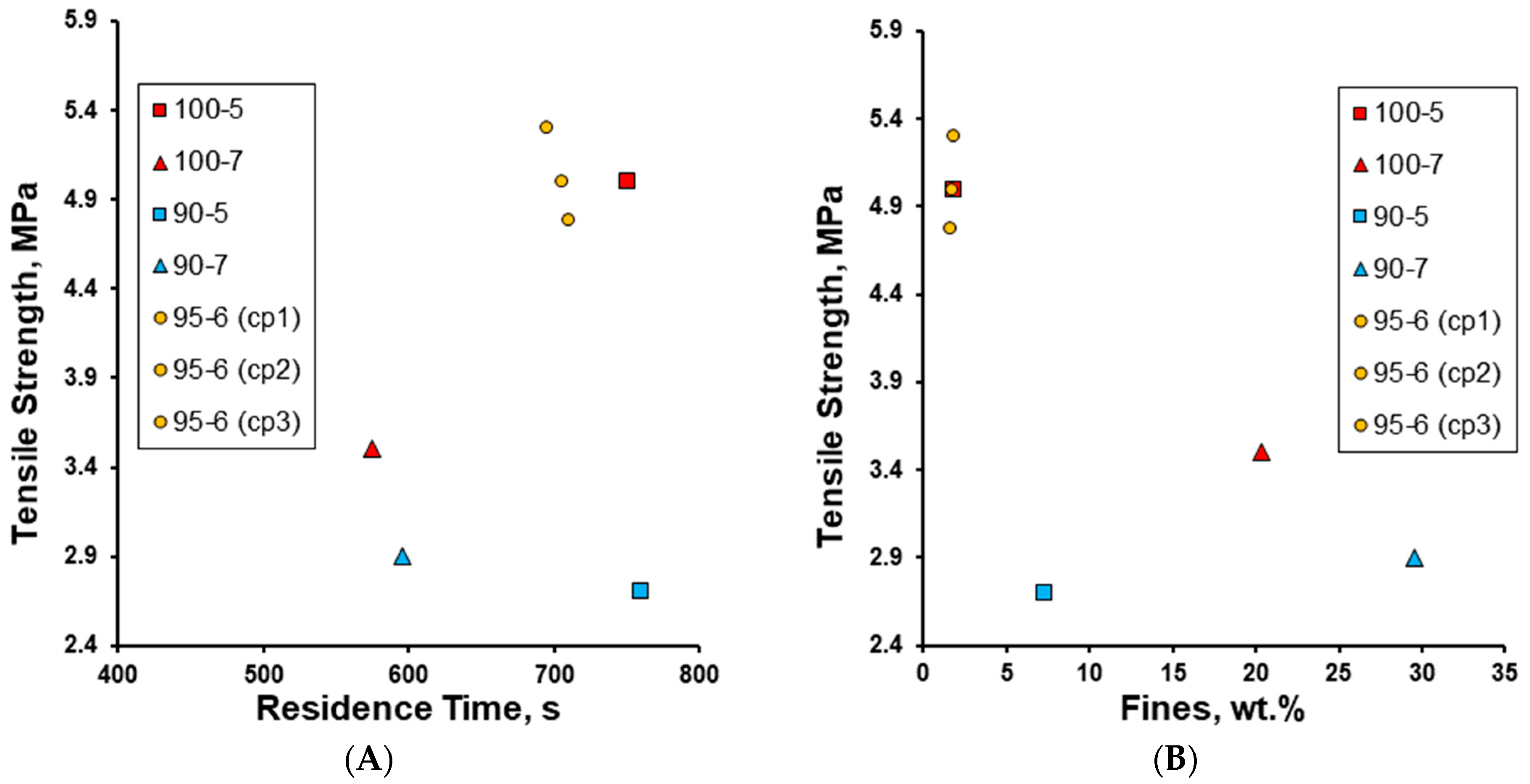
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasok, C. Joseph Priestly (1733–1804). In Philosophy of Chemistry; Woody, A.I., Hendry, R.F., Needham, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 55–62. [Google Scholar] [CrossRef]
- Chatzidopavlaki, P.; Triantafyllopoulou, E.; Pippa, N.; Valsami, G.; Dallas, P.P. Recent advances in the technology of effervescent tablets: Lessons learned and future perspectives. RSC Pharm. 2025, 2, 8–18. [Google Scholar] [CrossRef]
- Freitag, F.; Kleinebudde, P. How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials? Comparison of four magnesium carbonates. Eur. J. Pharm. Sci. 2003, 19, 281–289. [Google Scholar] [CrossRef]
- Khan, A. Optimization of the process variables of roller compaction, on the basis of granules characteristics (flow, mechanical strength, and disintegration behavior): An application of SeDeM-ODT expert system. Drug Dev. Ind. Pharm. 2019, 45, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Pratama, R.; Melinda, K.; Muhsinin, S. Viability of Lactobacillus acidophilus in effervescent granules prepared via wet granulation method: In vitro study. Sci. Pharm. 2023, 2, 22–36. [Google Scholar] [CrossRef]
- Aslani, A.; Fattahi, F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. Adv. Pharm. Bull. 2013, 3, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Parajuli-Baral, K. Formulation and Evaluation of Quality Parameters of Effervescent Granules from the Potent Antioxidant between Two Variants of the Adaptogenic Herb Ocimum tenuiflorum L. Sci. World J. 2023, 2023, 2050846. [Google Scholar] [CrossRef]
- Yanze, F.M.; Duru, C.; Jacob, M. A process to produce effervescent tablets: Fluidized bed dryer melt granulation. Drug Dev. Ind. Pharm. 2000, 26, 1167–1176. [Google Scholar] [CrossRef]
- Lima, A.L.; Pinho, L.A.G.; Chaker, J.A.; Sa-Barreto, L.L.; Marreto, R.N.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Hot-Melt Extrusion as an Advantageous Technology to Obtain Effervescent Drug Products. Pharmaceutics 2020, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Passerini, N.; Calogera, G.; Albertini, B.; Rodriguez, L. Melt granulation of pharmaceutical powders: A comparison of high-shear mixer and fluidised bed processes. Int. J. Pharm. 2010, 391, 177–186. [Google Scholar] [CrossRef]
- Nordstrom, J.; Alderborn, G. The granule porosity controls the loss of compactibility for both dry- and wet-processed cellulose granules but at different rate. J. Pharm. Sci. 2015, 104, 2029–2039. [Google Scholar] [CrossRef]
- Dash, A.K. Chapter 9—Solid Dosage Forms. In Pharmaceutics, 2nd ed.; Dash, A.K., Singh, S., Eds.; Academic Press: New York, NY, USA, 2024; pp. 239–269. [Google Scholar] [CrossRef]
- Monaco, D.; Omar, C.; Reynolds, G.K.; Tajarobi, P.; Litster, J.D.; Salman, A.D. Drying in a continuous wet granulation line: Investigation of different end of drying control methods. Powder Technol. 2021, 392, 157–166. [Google Scholar] [CrossRef]
- Horváth, Z.M.; Petersone, L.; Mohylyuk, V. Twin-screw melt granulation with PEG 8000: Effect of binder particle size and processing temperature on the granule and tablet properties. Adv. Powder Technol. 2024, 35, 104585. [Google Scholar] [CrossRef]
- Mangal, H.; Kirsolak, M.; Kleinebudde, P. Roll compaction/dry granulation: Suitability of different binders. Int. J. Pharm. 2016, 503, 213–219. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Mullarney, M.P.; Hancock, B.C. Simulation of roller compaction using a laboratory scale compaction simulator. Int. J. Pharm. 2004, 269, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Farber, L.; Hapgood, K.P.; Michaels, J.N.; Fu, X.Y.; Meyer, R.; Johnson, M.A.; Li, F. Unified compaction curve model for tensile strength of tablets made by roller compaction and direct compression. Int. J. Pharm. 2008, 346, 17–24. [Google Scholar] [CrossRef]
- Parikh, D.M. Handbook of Pharmaceutical Granulation Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Suzuki, H.; Takaishi, Y.; Yoshimura, M.; Hoshina, W.; Shinogi, Y.; Hisazumi, J.; Yamada, A.; Sugie, Y.; Aikawa, S.; Yamada, R.; et al. Application of manufacturing Classification System to manufacturing method selection for oral solid dosage forms in Japan. Adv. Powder Technol. 2024, 35, 104470. [Google Scholar] [CrossRef]
- Liu, T.; Paul, S.; Beeson, B.T.; Alexander, J.; Yang, F.; Bi, V.; Durig, T.; Sun, C.C.; Zhang, F. Effect of Hydroxypropyl Cellulose Level on Twin-Screw Melt Granulation of Acetaminophen. AAPS PharmSciTech 2020, 21, 240. [Google Scholar] [CrossRef]
- Shirazian, S.; Zeglinski, J.; Darwish, S.; Kuhs, M.; Albadarin, A.B.; Croker, D.M.; Walker, G.M. Continuous twin screw wet granulation: The combined effect of process parameters on residence time, particle size, and granule morphology. J. Drug Deliv. Sci. Technol. 2018, 48, 319–327. [Google Scholar] [CrossRef]
- Sadik, T.; Pillon, C.; Carrot, C.; Reglero-Ruiz, J.A. Dsc studies on the decomposition of chemical blowing agents based on citric acid and sodium bicarbonate. Thermochim. Acta 2018, 659, 74–81. [Google Scholar] [CrossRef]
- Batra, A.; Yang, F.; Kogan, M.; Sosnowik, A.; Usher, C.; Oldham, E.W.; Chen, N.; Lawal, K.; Bi, Y.; Dürig, T. Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties. Macromolecules 2021, 2, 1–19. [Google Scholar] [CrossRef]
- Liu, T.; Kittikunakorn, N.; Zhang, Y.; Zhang, F. Mechanisms of twin screw melt granulation. J. Drug Deliv. Sci. Technol. 2021, 61, 102150. [Google Scholar] [CrossRef]
- Batra, A.; Thongsukmak, A.; Desai, D.; Serajuddin, A.T.M. The Effect of Process Variables and Binder Concentration on Tabletability of Metformin Hydrochloride and Acetaminophen Granules Produced by Twin Screw Melt Granulation with Different Polymeric Binders. AAPS PharmSciTech 2021, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Poka, M.S.; Milne, M.; Wessels, A.; Aucamp, M. Sugars and Polyols of Natural Origin as Carriers for Solubility and Dissolution Enhancement. Pharmaceutics 2023, 15, 2557. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Hattori, Y.; Otsuka, M. Characterization of ternary amorphous solid dispersion containing hypromellose phthalate and erythritol prepared by hot melt extrusion using melting point depression. J. Drug Deliv. Sci. Technol. 2020, 58, 101797. [Google Scholar] [CrossRef]
- Wdowiak, K.; Tajber, L.; Miklaszewski, A.; Cielecka-Piontek, J. Sweeteners Show a Plasticizing Effect on PVP K30-A Solution for the Hot-Melt Extrusion of Fixed-Dose Amorphous Curcumin-Hesperetin Solid Dispersions. Pharmaceutics 2024, 16, 659. [Google Scholar] [CrossRef]
- Karnik, I.; Youssef, A.A.A.; Joshi, P.; Munnangi, S.R.; Narala, S.; Varner, C.; Vemula, S.K.; Majumdar, S.; Repka, M. Formulation development and characterization of dual drug loaded hot-melt extruded inserts for better ocular therapeutic outcomes: Sulfacetamide/prednisolone. J. Drug Deliv. Sci. Technol. 2023, 84, 104558. [Google Scholar] [CrossRef]
- Pawar, J.N.; Fule, R.A.; Maniruzzaman, M.; Amin, P.D. Solid crystal suspension of Efavirenz using hot melt extrusion: Exploring the role of crystalline polyols in improving solubility and dissolution rate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Narala, S.; Komanduri, N.; Nyavanandi, D.; Youssef, A.A.A.; Mandati, P.; Alzahrani, A.; Kolimi, P.; Narala, N.; Repka, M.A. Hard Gelatin Capsules Containing Hot Melt Extruded Solid Crystal Suspension of Carbamazepine for improving dissolution: Preparation and In vitro Evaluation. J. Drug Deliv. Sci. Technol. 2023, 82, 104384. [Google Scholar] [CrossRef]
- Pradhan, A.; Costello, M.; Yang, F.; Bi, V.; Durig, T.; Zhang, F. Using twin-screw melt granulation to co-process mannitol and hydroxypropylcellulose. J. Drug Deliv. Sci. Technol. 2022, 77, 103880. [Google Scholar] [CrossRef]
- Forster, S.P.; Dippold, E.; Chiang, T. Twin-Screw Melt Granulation for Oral Solid Pharmaceutical Products. Pharmaceutics 2021, 13, 665. [Google Scholar] [CrossRef]
- Paulausks, A.; Kolisnyk, T.; Mohylyuk, V. The Increase in the Plasticity of Microcrystalline Cellulose Spheres’ When Loaded with a Plasticizer. Pharmaceutics 2024, 16, 945. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.M.; Grundsteins, K.; Radzins, O.; Kons, A.; Berzins, A.; Viter, R.; Lamprou, D.A.; Mohylyuk, V. FDM 3D-printed oral dosage form of prednisolone—Improvement of printability and influencing drug release. Int. J. Pharm. 2025, 673, 125391. [Google Scholar] [CrossRef]
- CHRONIFER M-15 KL Data Sheet. Available online: https://www.kleinmetals.ch/en/produkt/chronifer-m-15-kl/ (accessed on 6 May 2025).
- Apelblat, A. Citric Acid; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 88th ed.; Taylor & Francis: Abingdon, UK, 2007. [Google Scholar]
- Mohylyuk, V.; Bandere, D. High-Speed Tableting of High Drug-Loaded Tablets Prepared from Fluid-Bed Granulated Isoniazid. Pharmaceutics 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, S.; Mohylyuk, V.; Jones, D.S.; Andrews, G.P. 3D printing of pharmaceutical oral solid dosage forms by fused deposition: The enhancement of printability using plasticised HPMCAS. Int. J. Pharm. 2022, 616, 121553. [Google Scholar] [CrossRef]
- United States Pharmacopeia (USP). Revision Bulletin <701> Disintegration; United States Pharmacopeia (USP): Frederick, MD, USA, 2020. [Google Scholar]
- Kukuls, K.; Frolova, A.J.; Horváth, Z.M.; Gniazdowska, E.M.; Mohylyuk, V. Dataset: Particle Size Distribution (PSD) Profiles of Polyols, 1st ed.; Riga Stradins University: Riga, Latvia, 2025. [Google Scholar] [CrossRef]
- Sudarshan, M.; Baharat, T.; Mohan, K. Formulation and Optimization of Effervescent Tablets by Design of Experiments. Int. J. Sci. Res. Technol. 2025, 2, 218–235. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, J.A.; Kausar, T.N.; Mahnashi, M.H.; Alasiri, A.; Alqahtani, A.A.; Alqahtani, T.S.; Walbi, I.A.; Alshehri, O.M.; Elnoubi, O.A.; et al. Preparation, Characterization and Evaluation of Flavonolignan Silymarin Effervescent Floating Matrix Tablets for Enhanced Oral Bioavailability. Molecules 2023, 28, 2606. [Google Scholar] [CrossRef]
- Sun, C.C. Decoding Powder Tabletability: Roles of Particle Adhesion and Plasticity. J. Adhes. Sci. Technol. 2012, 25, 483–499. [Google Scholar] [CrossRef]
- Polak, P.; Sinka, I.C.; Reynolds, G.K.; Roberts, R.J. Successful Formulation Window for the design of pharmaceutical tablets with required mechanical properties. Int. J. Pharm. 2024, 650, 123705. [Google Scholar] [CrossRef]
- Al-Gousous, J.; Langguth, P. Oral Solid Dosage Form Disintegration Testing—The Forgotten Test. J. Pharm. Sci. 2015, 104, 2664–2675. [Google Scholar] [CrossRef]
- Horváth, Z.M.; Kukuls, K.; Frolova, A.J.; Žogota, M.; Buczkowska, E.M.; Pētersone, L.; Mohylyuk, V. Sorbitol as a melt binder in twin-screw melt granulation for effervescent tablets preparation. In Proceedings of the 16th Global Drug Delivery and Formulation (DDF) Summit, Berlin, Germany, 2–4 June 2025. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, Z.M.; Kukuls, K.; Frolova, A.J.; Žogota, M.; Buczkowska, E.M.; Pētersone, L.; Mohylyuk, V. Effervescent Tablet Preparation by Twin-Screw Melt Granulation with Sorbitol as a Melt Binder. Pharmaceutics 2025, 17, 676. https://doi.org/10.3390/pharmaceutics17050676
Horváth ZM, Kukuls K, Frolova AJ, Žogota M, Buczkowska EM, Pētersone L, Mohylyuk V. Effervescent Tablet Preparation by Twin-Screw Melt Granulation with Sorbitol as a Melt Binder. Pharmaceutics. 2025; 17(5):676. https://doi.org/10.3390/pharmaceutics17050676
Chicago/Turabian StyleHorváth, Zoltán Márk, Kirils Kukuls, Alīna Jaroslava Frolova, Marta Žogota, Elżbieta Maria Buczkowska, Līga Pētersone, and Valentyn Mohylyuk. 2025. "Effervescent Tablet Preparation by Twin-Screw Melt Granulation with Sorbitol as a Melt Binder" Pharmaceutics 17, no. 5: 676. https://doi.org/10.3390/pharmaceutics17050676
APA StyleHorváth, Z. M., Kukuls, K., Frolova, A. J., Žogota, M., Buczkowska, E. M., Pētersone, L., & Mohylyuk, V. (2025). Effervescent Tablet Preparation by Twin-Screw Melt Granulation with Sorbitol as a Melt Binder. Pharmaceutics, 17(5), 676. https://doi.org/10.3390/pharmaceutics17050676






