A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Plant Extract
2.2. Optimized Synthesis of AgNPs
2.3. In Vitro Stability of AgNPs
2.4. Characterization of AgNPs
2.5. Phytotoxicity and Cytogenetic Analysis of AgNPs on Triticum aestivum
2.6. Antioxidant Activity
2.7. Photocatalytic Activity
2.8. Photoprotective Activity
3. Results
3.1. A. melanocarpa Berry Extract Characterization
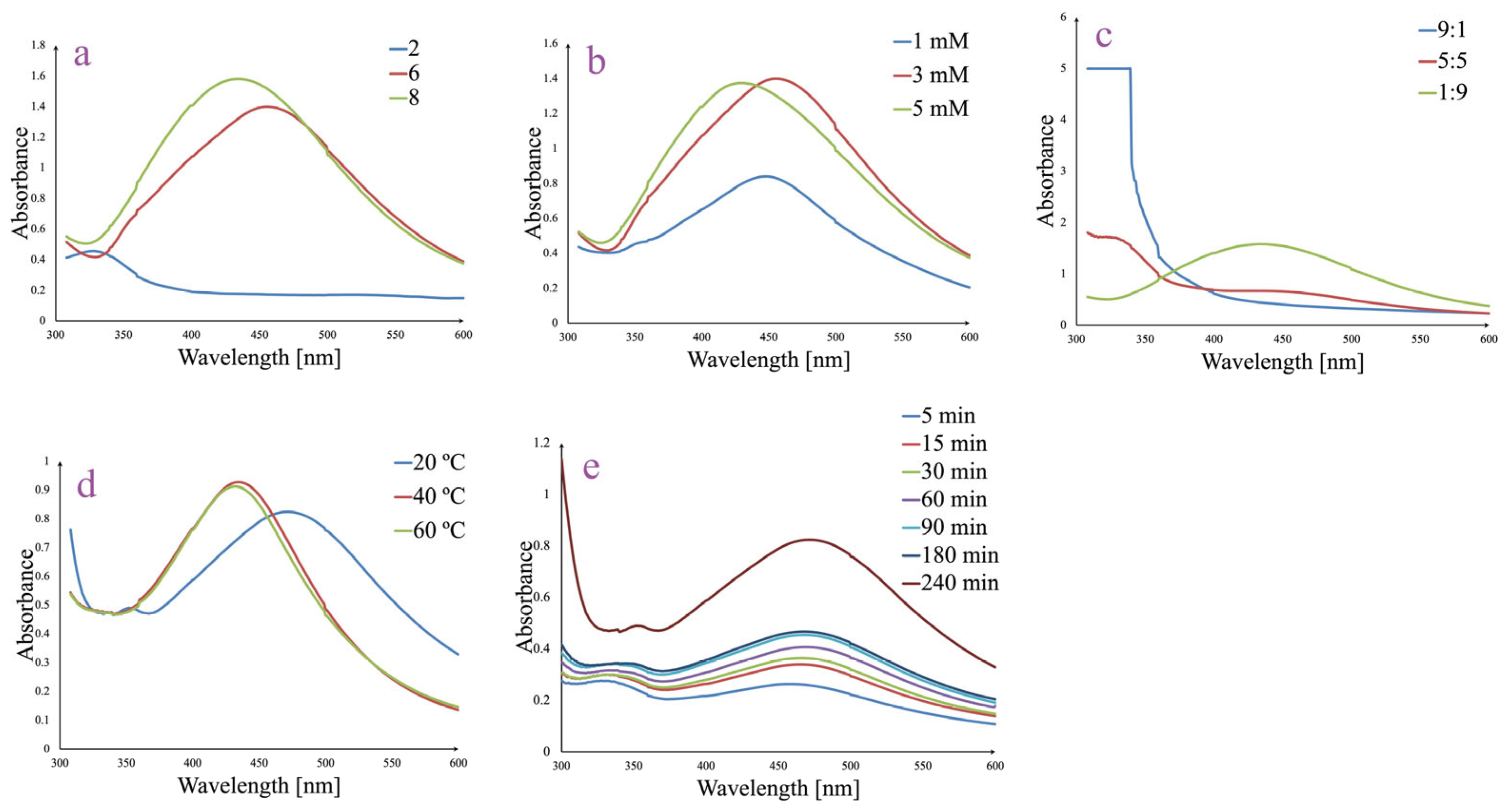
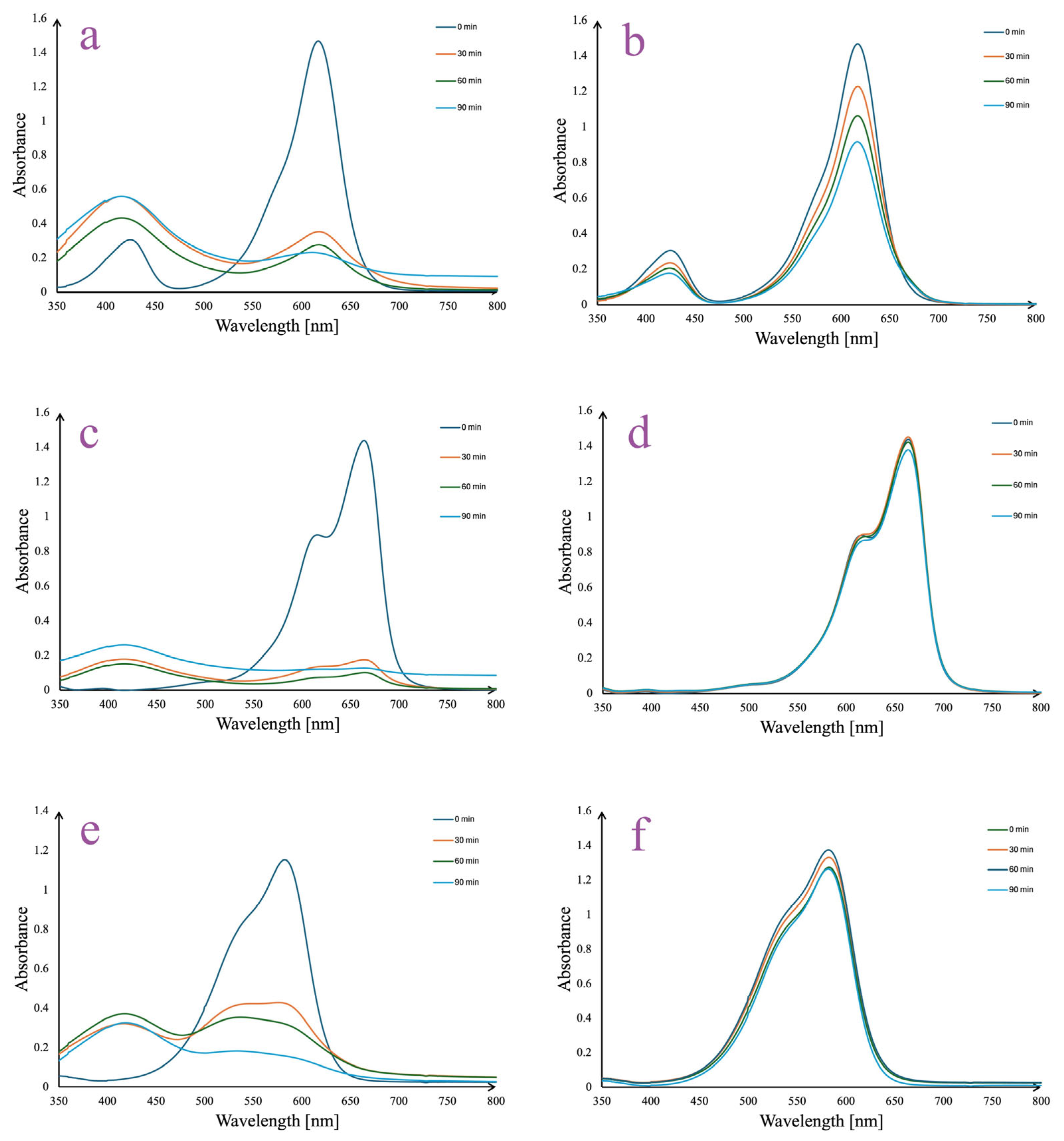
3.2. Optimized Synthesis and Generation of AgNPs
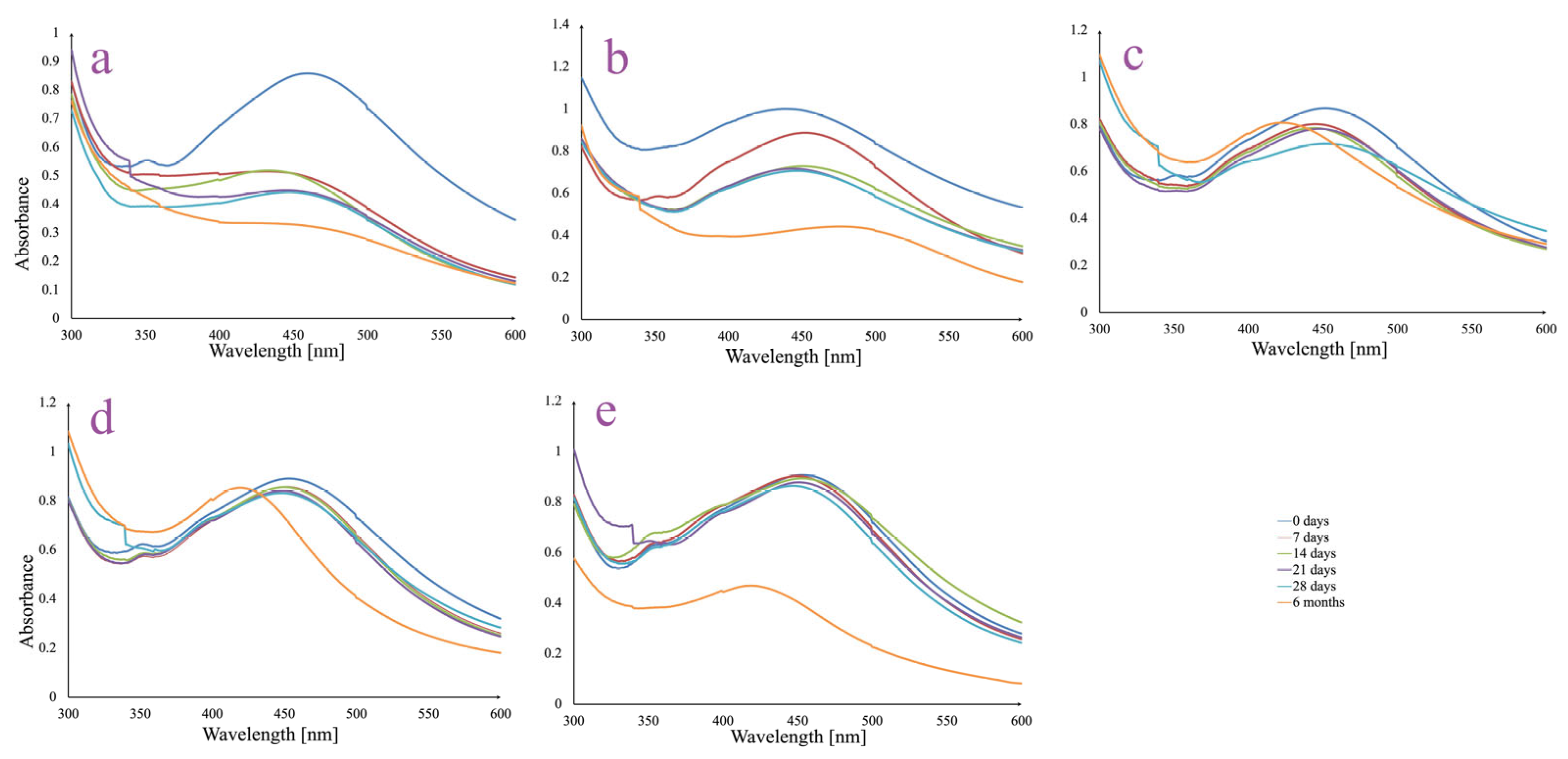
3.3. AgNPs In Vitro Stability
3.4. AgNPs Characterization
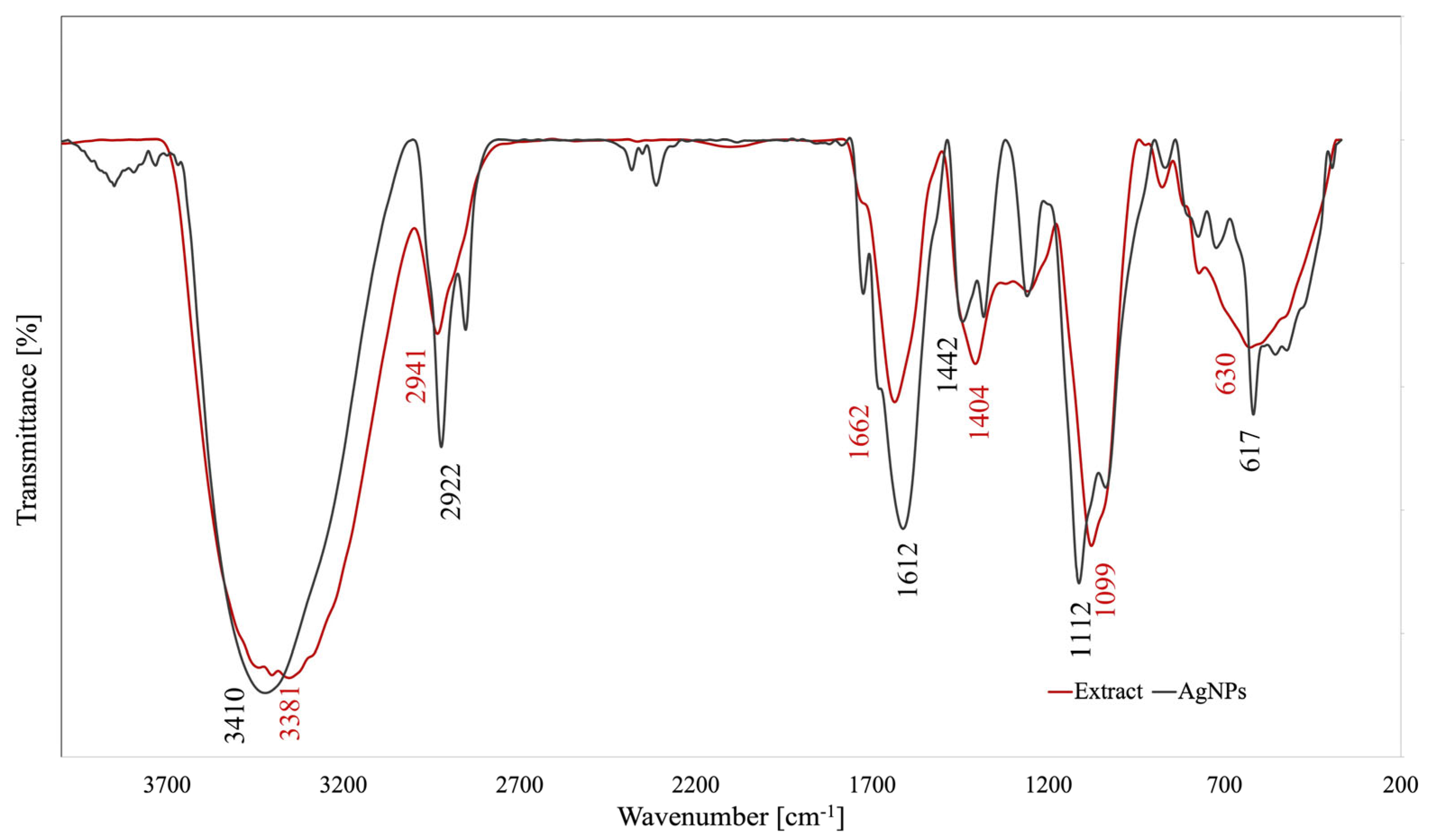
3.4.1. FTIR Analysis
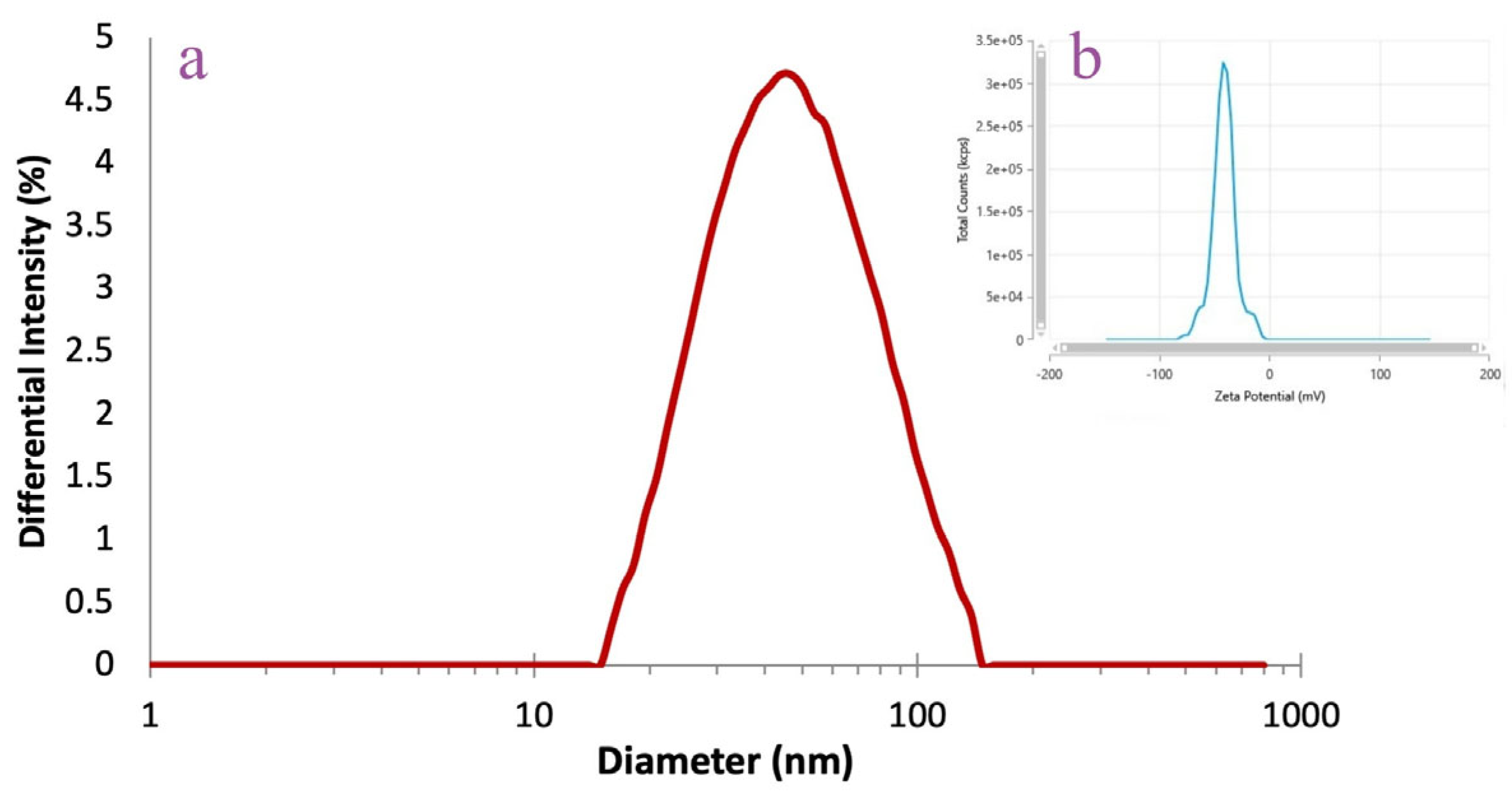
3.4.2. DLS Analysis
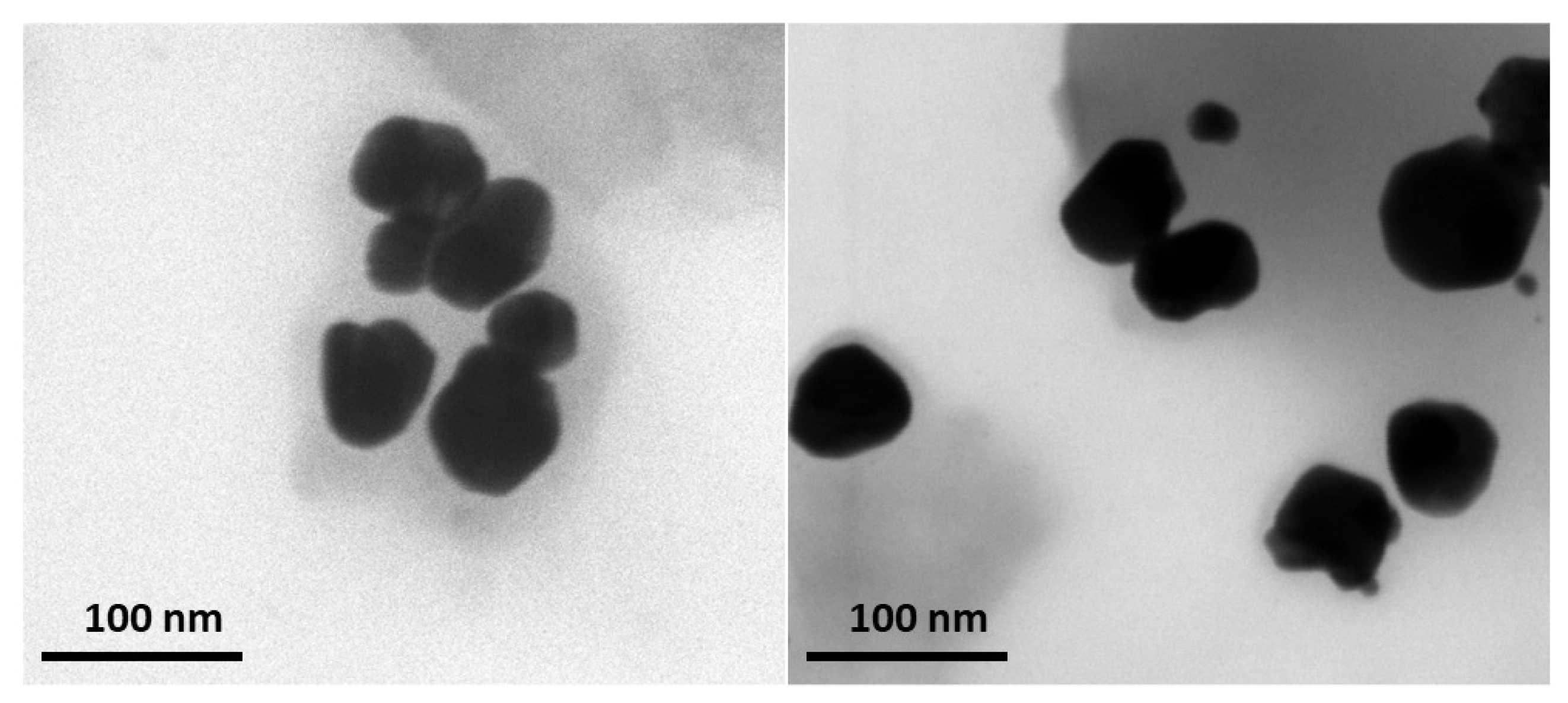
3.4.3. TEM Analysis
3.4.4. EDX Analysis
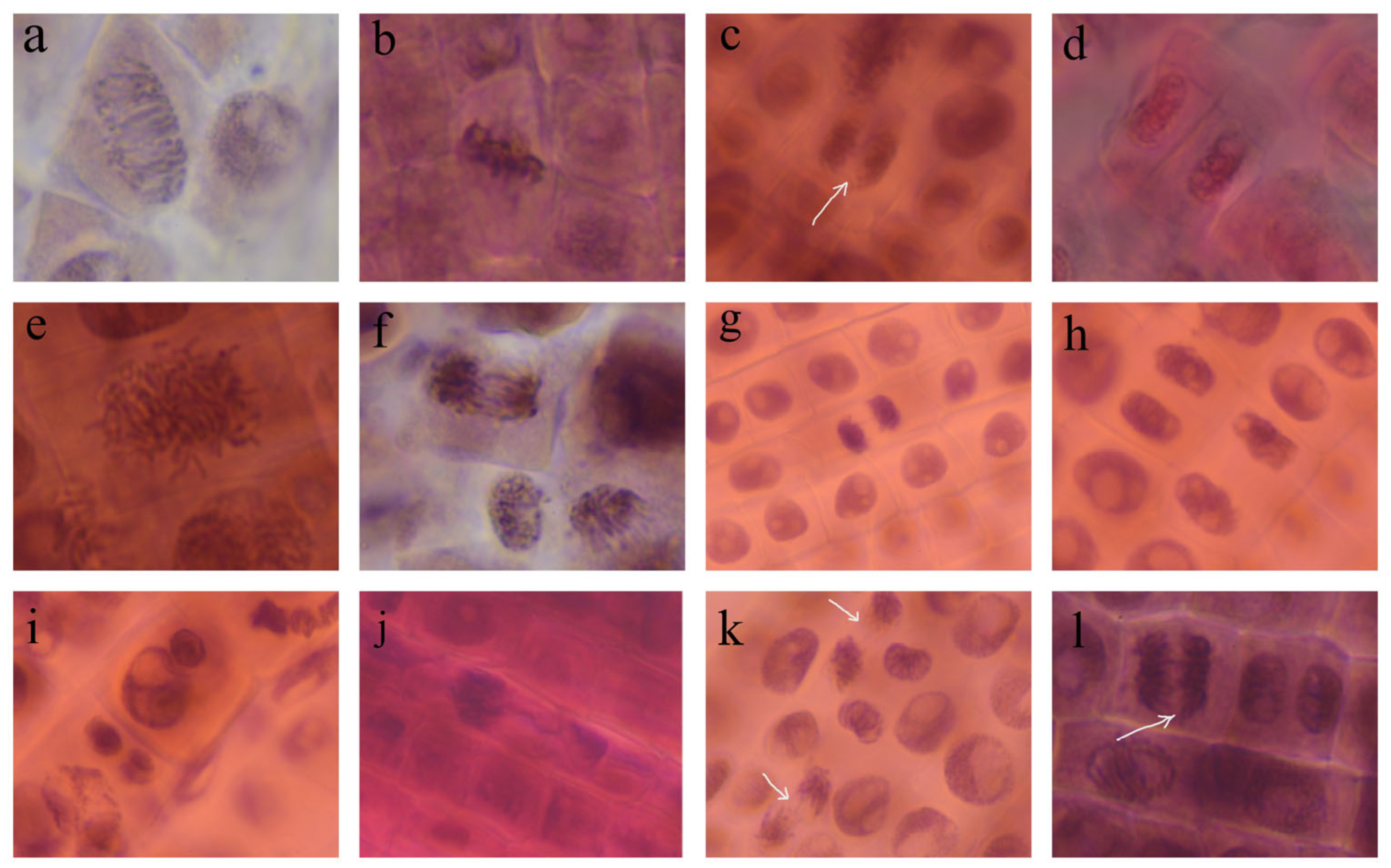
3.5. AgNPs Phytotoxicity and Cytogenetic Analysis
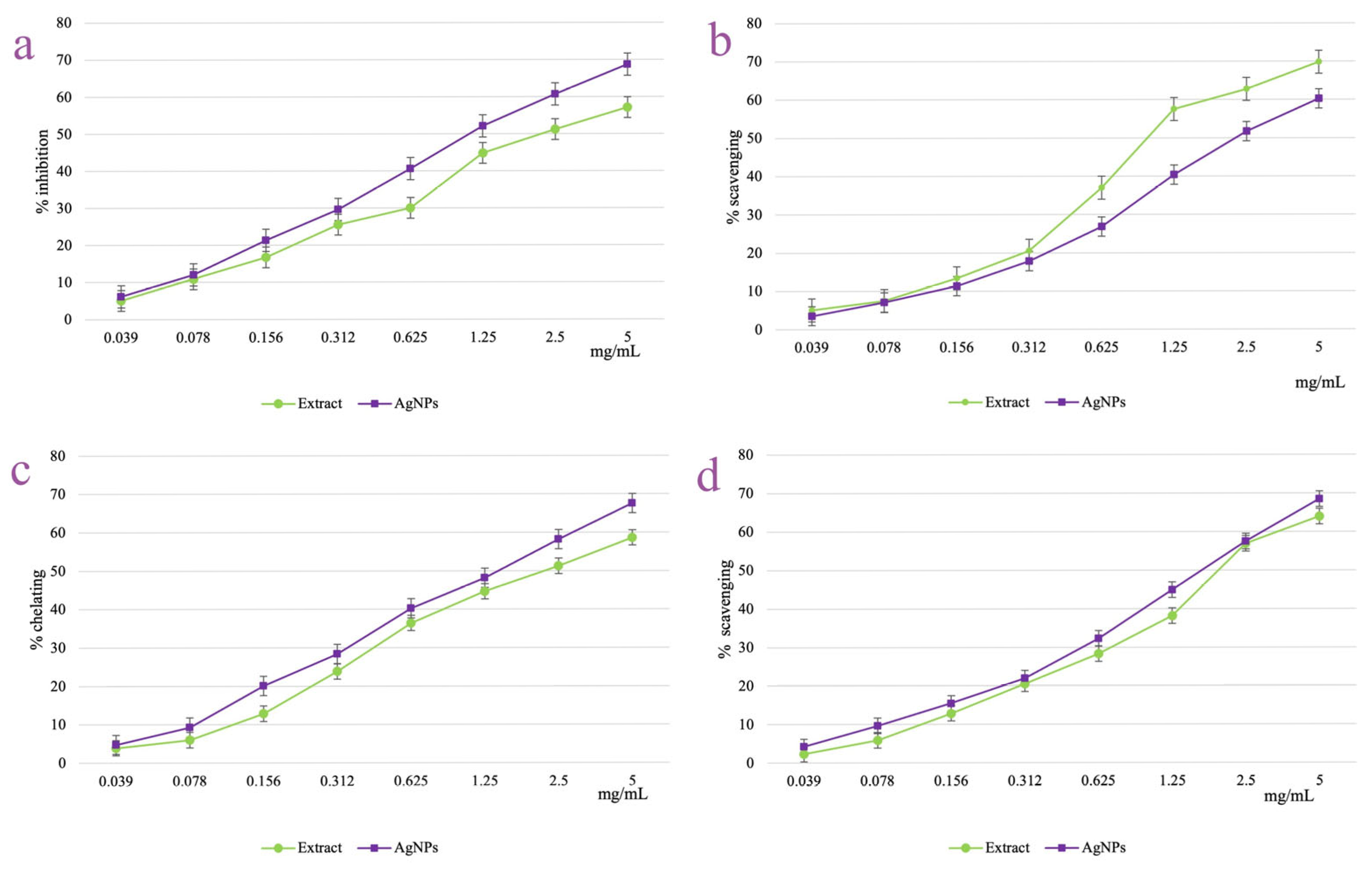
3.6. AgNPs Antioxidant Activity
3.7. AgNPs Photocatalytic Activity
3.8. AgNPs Photoprotective Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AgNPs | silver nanoparticles |
| AgNO3 | silver nitrate |
| LC-MS | liquid chromatography coupled to mass spectrometry |
| SPF | sun protection factor |
| UV-Vis | ultraviolet-visible spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| PDI | polydispersity index |
| DLS | dynamic light scattering |
| SEM | scanning electron microscopy |
| EDX | energy-dispersive X-ray analysis |
| TEM | transmission electron microscopy |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
References
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Khan, M.R.; Urmi, M.A.; Kamaraj, C.; Malafaia, G.; Ragavendran, C.; Rahman, M.M. Green Synthesis of Silver Nanoparticles with Its Bioactivity, Toxicity and Environmental Applications: A Comprehensive Literature Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100872. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-Mediated Synthesis of Nanoparticles and Their Applications: A Review. Mater. Res. Bull. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Jurendi’c, T.J.; Ščetar, M.; Voilley, A.; Kurek, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Corciovă, A.; Mircea, C.; Fifere, A.; Turin-Moleavin, I.A.; Roşca, I.; Macovei, I.; Ivănescu, B.; Vlase, A.M.; Hăncianu, M.; Burlec, A.F. Biogenic Synthesis of Silver Nanoparticles Mediated by Aronia melanocarpa and Their Biological Evaluation. Life 2024, 14, 1211. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Zheng, M.; Sheng, L.; Shi, D.; Song, K. A Comprehensive Review of the Functional Potential and Sustainable Applications of Aronia melanocarpa in the Food Industry. Plants 2024, 13, 3557. [Google Scholar] [CrossRef]
- Vlase, A.M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef]
- Pârvu, M.; Vlase, L.; Pârvu, A.E.; Rosca-Casian, O.; Gheldiu, A.M.; Pârvu, O. Phenolic Compounds and Antifungal Activity of Hedera helix L. (Ivy) Flowers and Fruits. Not. Bot. Horti Agrobot. Cluj. Napoca 2015, 43, 53–58. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants 2023, 12, 96. [Google Scholar] [CrossRef]
- Solcan, M.B.; Fizeșan, I.; Vlase, L.; Vlase, A.M.; Rusu, M.E.; Mateș, L.; Petru, A.E.; Creștin, I.V.; Tomuțǎ, I.; Popa, D.S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes Nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Gîrd, C.E.; Nencu, I.; Popescu, M.L.; Costea, T.; Duțu, L.E.; Balaci, T.D.; Olaru, T. Chemical, antioxidant and toxicity evaluation of rosemary leaves and its dry extract. Farmacia 2017, 65, 978–983. [Google Scholar]
- EDQM. European Pharmocopoeia 11th Edition 2022; EDQM: Strasbourg, France, 2022. [Google Scholar]
- Macovei, I.; Harabagiu, V.; Burlec, A.F.; Mircea, C.; Horhogea, C.E.; Rimbu, C.M.; Săcărescu, L.; Panainte, A.D.; Miron, A.; Hăncianu, M.; et al. Biosynthesis of Silver and Gold Nanoparticles Using Geum urbanum L. Rhizome Extracts and Their Biological Efficiency. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5831–5853. [Google Scholar] [CrossRef]
- Burlec, A.F.; Hăncianu, M.; Macovei, I.; Mircea, C.; Fifere, A.; Turin-Moleavin, I.A.; Tuchiluș, C.; Robu, S.; Corciovă, A. Eco-Friendly Synthesis and Comparative In Vitro Biological Evaluation of Silver Nanoparticles Using Tagetes erecta Flower Extracts. Appl. Sci. 2022, 12, 887. [Google Scholar] [CrossRef]
- Corciovă, A.; Mircea, C.; Burlec, A.F.; Fifere, A.; Moleavin, I.T.; Sarghi, A.; Tuchiluș, C.; Ivănescu, B.; Macovei, I. Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and In Vitro Exploration of Their Biological Activities. Life 2022, 12, 1643. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and Genotoxic Effects of Silver Nanoparticles Exposure on Germinating Wheat Seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity Effects of Silver Nanoparticles on Wheat (Triticum aestivum L.) Root Tip Cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2022, 27, 217. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-Lipoxygenase from Orange Peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 15, 61–169. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Chul Hong, S.; Jin Jeong, H. 3,4-Dihydroxybenzaldehyde Purified from the Barley Seeds (Hordeum vulgare) Inhibits Oxidative DNA Damage and Apoptosis via Its Antioxidant Activity. Phytomedicine 2009, 16, 85–94. [Google Scholar] [CrossRef]
- Ouettar, L.; Guechi, E.K.; Hamdaoui, O.; Fertikh, N.; Saoudi, F.; Alghyamah, A. Biosorption of Triphenyl Methane Dyes (Malachite Green and Crystal Violet) from Aqueous Media by Alfa (Stipa tenacissima L.) Leaf Powder. Molecules 2023, 28, 3313. [Google Scholar] [CrossRef] [PubMed]
- Corciovă, A.; Mircea, C.; Burlec, A.F.; Cioancă, O.; Tuchiluş, C.; Fifere, A.; Lungoci, A.L.; Marangoci, N.; Hăncianu, M. Antioxidant, Antimicrobial and Photocatalytic Activities of Silver Nanoparticles Obtained by Bee Propolis Extract Assisted Biosynthesis. Farmacia 2019, 67, 482–489. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ezhumalai, G.; Gnanadesigan, M. A Green Approach for the Synthesis of Silver Nanoparticles by Chlorella vulgaris and Its Application in Photocatalytic Dye Degradation Activity. Environ. Technol. Innov. 2021, 21, 101282. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Elhusseiny, A.F.; Hussein, S.M.; Sadik, W.A. Sustainable and Energy-Efficient Photocatalytic Degradation of Textile Dye Assisted by Ecofriendly Synthesized Silver Nanoparticles. Sci. Rep. 2023, 13, 2302. [Google Scholar] [CrossRef]
- Santos, E.P.; Freitas, Z.M.; Souza, K.R.; Garcia, S.; Vergnanini, A. In Vitro and in Vivo Determinations of Sun Protection Factors of Sunscreen Lotions with Octyl Methoxycinnamate. Int. J. Cosmet. Sci. 1999, 21, 1–5. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hani, U.; Alqarni, M.H.; Alam, A. Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics 2023, 15, 1434. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Horhogea, C.E.; Rimbu, C.M.; Sacarescu, L.; Vochita, G.; Gherghel, D.; Ivanescu, B.L.; Panainte, A.D.; et al. Silver Nanoparticles Synthesized from Abies alba and Pinus sylvestris Bark Extracts: Characterization, Antioxidant, Cytotoxic, and Antibacterial Effects. Antioxidants 2023, 12, 797. [Google Scholar] [CrossRef]
- Safarzaei, A.; Sarhadi, H.; Haddad Khodaparast, M.H.; Shahdadi, F.; Dashipour, A.R. Optimization of Aqueous and Alcoholic Extraction of Phenolic and Antioxidant Compounds From Caper (Capparis spinosa L.) Roots Assisted by Ultrasound Waves. Zahedan J. Res. Med. Sci. 2020, 22, 100747. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of Ethanolic and Aqueous Populus balsamifera l. Bud Extracts by Different Extraction Methods: Chemical Composition, Antioxidant and Antibacterial Activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef] [PubMed]
- Dhankas, R.; Verma, K.K.; Das, A.; Chandra, P. Phytochemical-Based Synthesis of Silver Nanoparticle: Mechanism and Potential Applications. Bionanoscience 2023, 13, 1359–1380. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Bhusal, M.; Pathak, I.; Bhadel, A.; Shrestha, D.K.; Sharma, K.R. Synthesis of Silver Nanoparticles Assisted by Aqueous Root and Leaf Extracts of Rhus chinensis Mill and Its Antibacterial Activity. Heliyon 2024, 10, e33603. [Google Scholar] [CrossRef]
- Nasiriboroumand, M.; Montazer, M.; Barani, H. Preparation and Characterization of Biocompatible Silver Nanoparticles Using Pomegranate Peel Extract. J. Photochem. Photobiol. B 2018, 179, 98–104. [Google Scholar] [CrossRef]
- Alam, M. Analyses of Biosynthesized Silver Nanoparticles Produced from Strawberry Fruit Pomace Extracts in Terms of Biocompatibility, Cytotoxicity, Antioxidant Ability, Photodegradation, and in-Silico Studies. J. King Saud. Univ. Sci. 2022, 34, 102327. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Watcharaporn, K.; Opaprakasit, M.; Pimpan, V. Effects of UV Radiation and PH of Tannic Acid Solution in the Synthesis of Silver Nanoparticles. Adv. Mater. Res. 2014, 911, 110–114. [Google Scholar] [CrossRef]
- Lee, C.; Zhang, P. Facile Synthesis of Gelatin-Protected Silver Nanoparticles for SERS Applications. J. Raman Spectrosc. 2013, 44, 823–826. [Google Scholar] [CrossRef]
- Ramli, N.A.; Jai, J.; Mohd Yusof, N.; Zamanhuri, N.A. Green Synthesis of Silver Nanoparticles Using Elaeis guineensis from Palm Leaves: Influence of PH in Reaction Kinetic. Adv. Mater. Res. 2015, 1113, 560–565. [Google Scholar] [CrossRef]
- Daublyte, E.; Kalnaityte, M.; Klimovich, A.; Drabavičius, A.; Charkova, T. Synthesis of Silver Nanoparticles with Polyols under Reflux and Microwave Irradiation Conditions. Chemija 2023, 34, 113–122. [Google Scholar] [CrossRef]
- Thi, P.P.N.; Nguyen, M.T.; Nguyen, D.H. Role of Collagen Concentration in Stability of Star-Shaped Silver@Gold Nanoparticles. J. Nano Res. 2016, 40, 113–119. [Google Scholar] [CrossRef]
- Tripathi, S.; Mahra, S.; Sharma, S.; Mathew, S.; Sharma, S. Interaction of Silver Nanoparticles with Plants: A Focus on the Phytotoxicity, Underlying Mechanism, and Alleviation Strategies. Plant Nano Biol. 2024, 9, 100082. [Google Scholar] [CrossRef]
- Yanık, F.; Vardar, F. Assessment of Silver Nanoparticle-Induced Morphological, Biochemical and Physiological Alterations in Wheat Roots. Ann. Di Bot. 2019, 9, 83–94. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Yanık, F.; Aytürk, Ö.; Vardar, F. Programmed Cell Death Evidence in Wheat (Triticum aestivum L.) Roots Induced by Aluminum Oxide (Al2O3) Nanoparticles. Caryologia 2017, 70, 112–119. [Google Scholar] [CrossRef]
- Garcia, E.B.; Alms, C.; Hinman, A.W.; Kelly, C.; Smith, A.; Vance, M.; Loncarek, J.; Marr, L.C.; Cimini, D. Single-Cell Analysis Reveals That Chronic Silver Nanoparticle Exposure Induces Cell Division Defects in Human Epithelial Cells. Int. J. Environ. Res. Public. Health 2019, 16, 2061. [Google Scholar] [CrossRef]
- Goldblatt, E.M.; Lee, E.; Lee, W.H. Mitotic Regulator Hec1 as a Potential Target for Cancer Therapy. In Recent Advances in Cancer Research and Therapy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 97–111. ISBN 9780123978332. [Google Scholar]
- Debnath, P.; Mondal, A.; Hajra, A.; Das, C.; Mondal, N.K. Cytogenetic Effects of Silver and Gold Nanoparticles on Allium cepa Roots. J. Genet. Eng. Biotechnol. 2018, 16, 519–526. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles. Int. J. Environ. Res. Public. Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Namita, K.; Sharma, S. Allium cepa Root Chromosomal Aberration Assay: A Review. Indian J. Pharm. Biol. Res. 2013, 1, 105–119. [Google Scholar]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef] [PubMed]
- Dorneanu, R.; Cioancă, O.; Chifiriuc, O.; Albu, E.; Tuchiluş, C.; Mircea, C.; Salamon, I.; Hăncianu, M. Synergic benefits of Aronia melanocarpa anthocyanin-rich extracts and antibiotics used for urinary tract infections. Farmacia 2017, 65, 778–783. [Google Scholar]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating Activity of Aronia melanocarpa Polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, Proanthocyanidins and Total Phenolics in Four Cultivars of Aronia: Antioxidant and Enzyme Inhibitory Effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Aronia melanocarpa Flavonol Extract—Antiradical and Immunomodulating Activities Analysis. Plants 2023, 12, 2976. [Google Scholar] [CrossRef]
- van der Vlag, R.; Guo, H.; Hapko, U.; Eleftheriadis, N.; Monjas, L.; Dekker, F.J.; Hirsch, A.K.H. A Combinatorial Approach for the Discovery of Drug-like Inhibitors of 15-Lipoxygenase-1. Eur. J. Med. Chem. 2019, 174, 45–55. [Google Scholar] [CrossRef]
- Tsai, W.C.; Gilbert, N.C.; Ohler, A.; Armstrong, M.; Perry, S.; Kalyanaraman, C.; Yasgar, A.; Rai, G.; Simeonov, A.; Jadhav, A.; et al. Kinetic and Structural Investigations of Novel Inhibitors of Human Epithelial 15-Lipoxygenase-2. Bioorg. Med. Chem. 2021, 46, 116349. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, X. Aronia melanocarpa Anthocyanin Extracts Improve Hepatic Structure and Function in High-Fat Diet-/Streptozotocin-Induced T2DM Mice. J. Agric. Food Chem. 2022, 70, 11531–11543. [Google Scholar] [CrossRef] [PubMed]
- Neculai-Văleanu, A.-S.; Mirela, A.; Mădescu, B.-M.; Porosnicu, I.; Ariton, A.M. Effect of various environmentally friendly reducing agents on the antioxidant activity of green synthesized silver nanoparticles. LSSD J. 2022, 3, 49–53. [Google Scholar] [CrossRef]
- Kumar, A.; Haque, A. Assessment of Antioxidant Activity of Silver Nanoparticles Synthesized Using Aqueous Extract of Schizophyllum commune InTriazophos-Induced Oxidative Stress in Albino Rats. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Y.; Cao, X.; Zangeneh, A.; Zangeneh, M.M.; Liu, S.; Li, J. Application of a Novel Lung Protective Drug Formulated by Silver Nanoparticles Containing Curcuma longa L Leaf Aqueous Extract on α-Guttiferin-Induced DNA Fragmentation and Apoptosis in Lung HEL 299, MRC-5, IMR-90, CCD-19Lu, WI-38, and BEAS-2B Cell Lines. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Code of Federal Regulations 2025, 21. Available online: https://www.govinfo.gov/app/collection/CFR (accessed on 14 March 2025).
- European Union. Commission Recommendation of 22 September 2006 on the Efficacy of Sunscreen Products and the Claims Made Relating Thereto. EUR-Lex 2025. Available online: https://eur-lex.europa.eu/eli/reco/2006/647/oj/eng (accessed on 28 March 2025).
- Jain, A.; Kongkham, B.; Puttaswamy, H.; Butola, B.S.; Malik, H.K.; Malik, A. Development of Wash-Durable Antimicrobial Cotton Fabrics by In Situ Green Synthesis of Silver Nanoparticles and Investigation of Their Antimicrobial Efficacy against Drug-Resistant Bacteria. Antibiotics 2022, 11, 864. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Channabasava; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of Silver Nanoparticles Using Extract of Endophytic Fungi, Penicillium Species of Glycosmis Mauritiana, and Its Antioxidant, Antimicrobial, Anti-Inflammatory and Tyrokinase Inhibitory Activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Photocatalytic, Antioxidant, Antibacterial and Anti-Inflammatory Activity of Silver Nanoparticles Synthesised Using Forest and Edible Mushroom. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045012. [Google Scholar] [CrossRef]
- Gamage McEvoy, J.; Zhang, Z. Antimicrobial and Photocatalytic Disinfection Mechanisms in Silver-Modified Photocatalysts under Dark and Light Conditions. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 62–75. [Google Scholar] [CrossRef]
- Gungure, A.S.; Jule, L.T.; Nagaprasad, N.; Ramaswamy, K. Studying the Properties of Green Synthesized Silver Oxide Nanoparticles in the Application of Organic Dye Degradation under Visible Light. Sci. Rep. 2024, 14, 26967. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, J.; Zhao, D.; Han, H.; Li, C. A Novel Sr2CuInO3S P-Type Semiconductor Photocatalyst for Hydrogen Production under Visible Light Irradiation. J. Energy Chem. 2014, 23, 420–426. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Plasmon-Induced Photocatalytic Degradation of Methylene Blue Dye Using Biosynthesized Silver Nanoparticles as Photocatalyst. Environ. Technol. 2020, 41, 1520–1534. [Google Scholar] [CrossRef]
- Ali, S.; Ijaz, H.; Ahmad, M.U.; Rukhma; Ullah, N.; Sarwar, A.; Khan, M.J.; Aziz, T.; Shami, A.; Al-Asmari, F. Photocatalytic Removal of Textile Wastewater-Originated Methylene Blue and Malachite Green Dyes Using Spent Black Tea Extract-Coated Silver Nanoparticles. Sci. Rep. 2025, 15, 1851. [Google Scholar] [CrossRef]
- Seerangaraj, V.; Sathiyavimal, S.; Shankar, S.N.; Nandagopal, J.G.T.; Balashanmugam, P.; Al-Misned, F.A.; Shanmugavel, M.; Senthilkumar, P.; Pugazhendhi, A. Cytotoxic Effects of Silver Nanoparticles on Ruellia Tuberosa: Photocatalytic Degradation Properties against Crystal Violet and Coomassie Brilliant Blue. J. Environ. Chem. Eng. 2021, 9, 105088. [Google Scholar] [CrossRef]



| Compounds | Extract | Supernatant |
|---|---|---|
| Gentisic acid | 0.608 ± 0.042 | <LOQ |
| Chlorogenic acid | 81.406 ± 0.814 | 2.289 ± 0.045 |
| 4-O-caffeoylquinic acid | 22.902 ± 2.061 | 2.468 ± 0.296 |
| Hyperoside | 9.422 ± 0.942 | 0.611 ± 0.079 |
| Isoquercitrin | 8.981 ± 0.449 | 0.658 ± 0.066 |
| Rutoside | 5.607 ± 0.112 | 1.302 ± 0.156 |
| Quercitrin | <LOQ | ND |
| Quercetin | 1.054 ± 0.063 | ND |
| Sample/Antioxidant Test | Lipoxygenase Inhibition | DPPH Radical Scavenging | Metal Ion Chelating | Hydroxyl Radical Scavenging |
|---|---|---|---|---|
| Extract | 36.44 ± 3.88 | 80.45 ± 0.52 | 438.34 ± 1.97 | 181.17 ± 0.39 |
| AgNPs | 18.29 ± 0.52 | 224.74 ± 5.27 | 284.11 ± 1.44 | 155.05 ± 0.63 |
| Sample and Concentration (g %) | SPF Value |
|---|---|
| Extract 0.01 | 2.09 |
| Extract 0.02 | 2.84 |
| AgNPs 0.005 | 2.78 |
| AgNPs 0.01 | 4.50 |
| AgNPs 0.02 | 8.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corciova, A.; Mircea, C.; Fifere, A.; Turin Moleavin, I.-A.; Burlec, A.F.; Ivanescu, B.; Vlase, A.-M.; Hancianu, M.; Macovei, I. A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa. Pharmaceutics 2025, 17, 669. https://doi.org/10.3390/pharmaceutics17050669
Corciova A, Mircea C, Fifere A, Turin Moleavin I-A, Burlec AF, Ivanescu B, Vlase A-M, Hancianu M, Macovei I. A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa. Pharmaceutics. 2025; 17(5):669. https://doi.org/10.3390/pharmaceutics17050669
Chicago/Turabian StyleCorciova, Andreia, Cornelia Mircea, Adrian Fifere, Ioana-Andreea Turin Moleavin, Ana Flavia Burlec, Bianca Ivanescu, Ana-Maria Vlase, Monica Hancianu, and Irina Macovei. 2025. "A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa" Pharmaceutics 17, no. 5: 669. https://doi.org/10.3390/pharmaceutics17050669
APA StyleCorciova, A., Mircea, C., Fifere, A., Turin Moleavin, I.-A., Burlec, A. F., Ivanescu, B., Vlase, A.-M., Hancianu, M., & Macovei, I. (2025). A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa. Pharmaceutics, 17(5), 669. https://doi.org/10.3390/pharmaceutics17050669








