Construction of pH-Sensitive Multifunctional Hydrogel with Synergistic Anti-Inflammatory Effect for Treatment of Diabetic Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized Dextran (ODex)
2.3. Preparation and Characterization of the Hydrogels
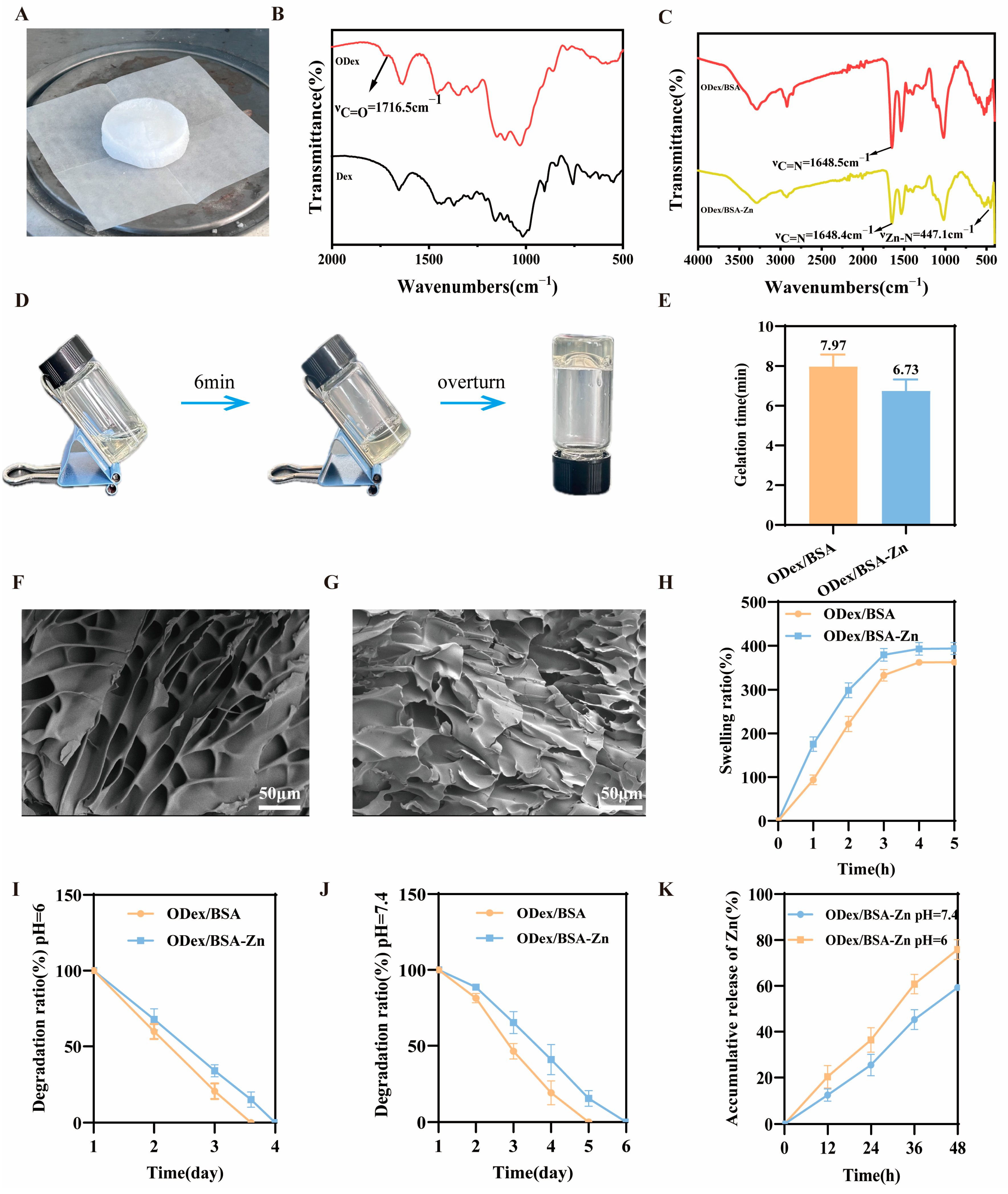
2.4. Swelling and Degradationcharacteristics of the Hydrogels
2.5. Release Profile of Zn2+
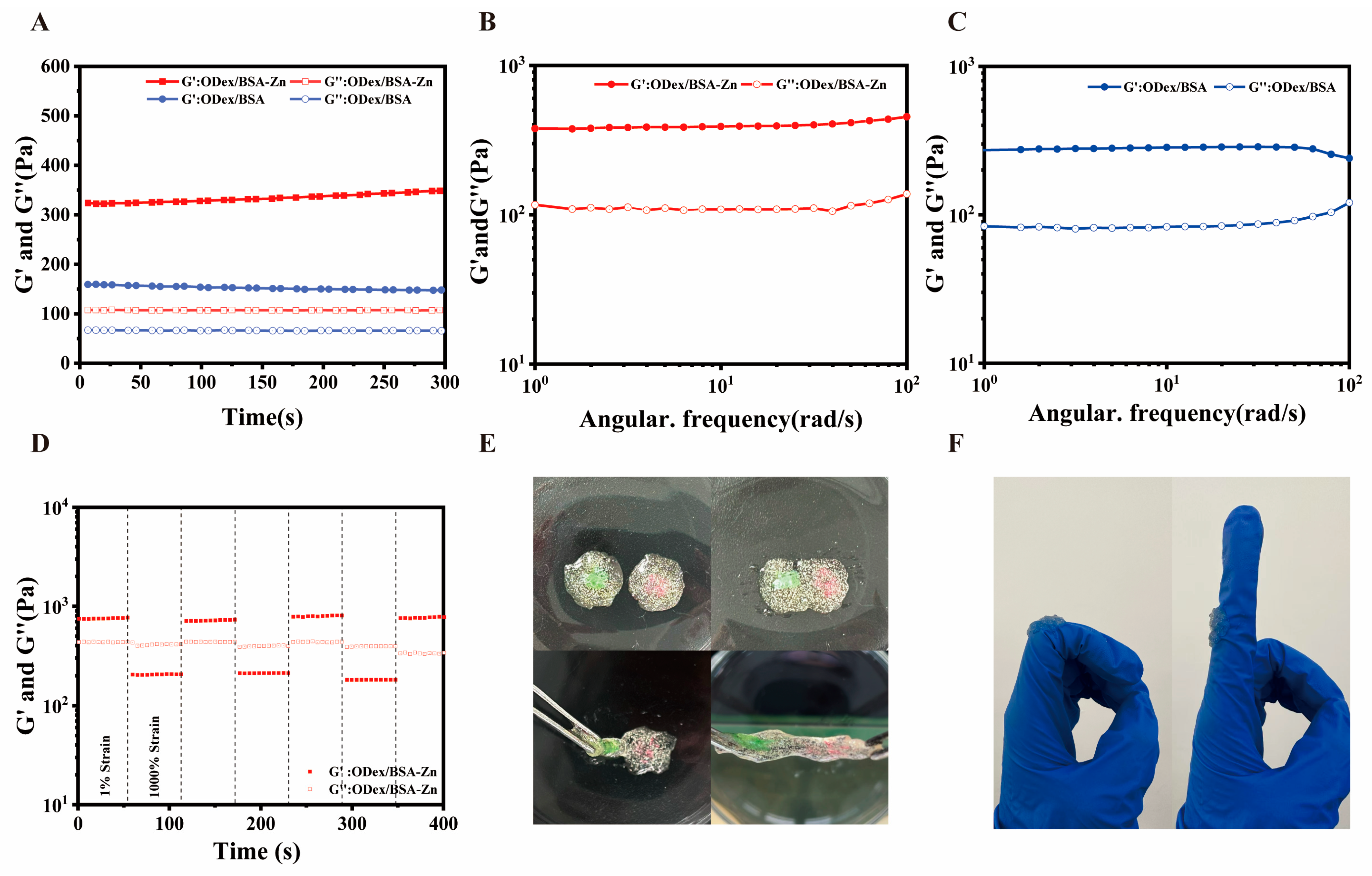
2.6. Rheological Characterization of the Hydrogels
2.7. Cytotoxicity Evaluation of the Hydrogels
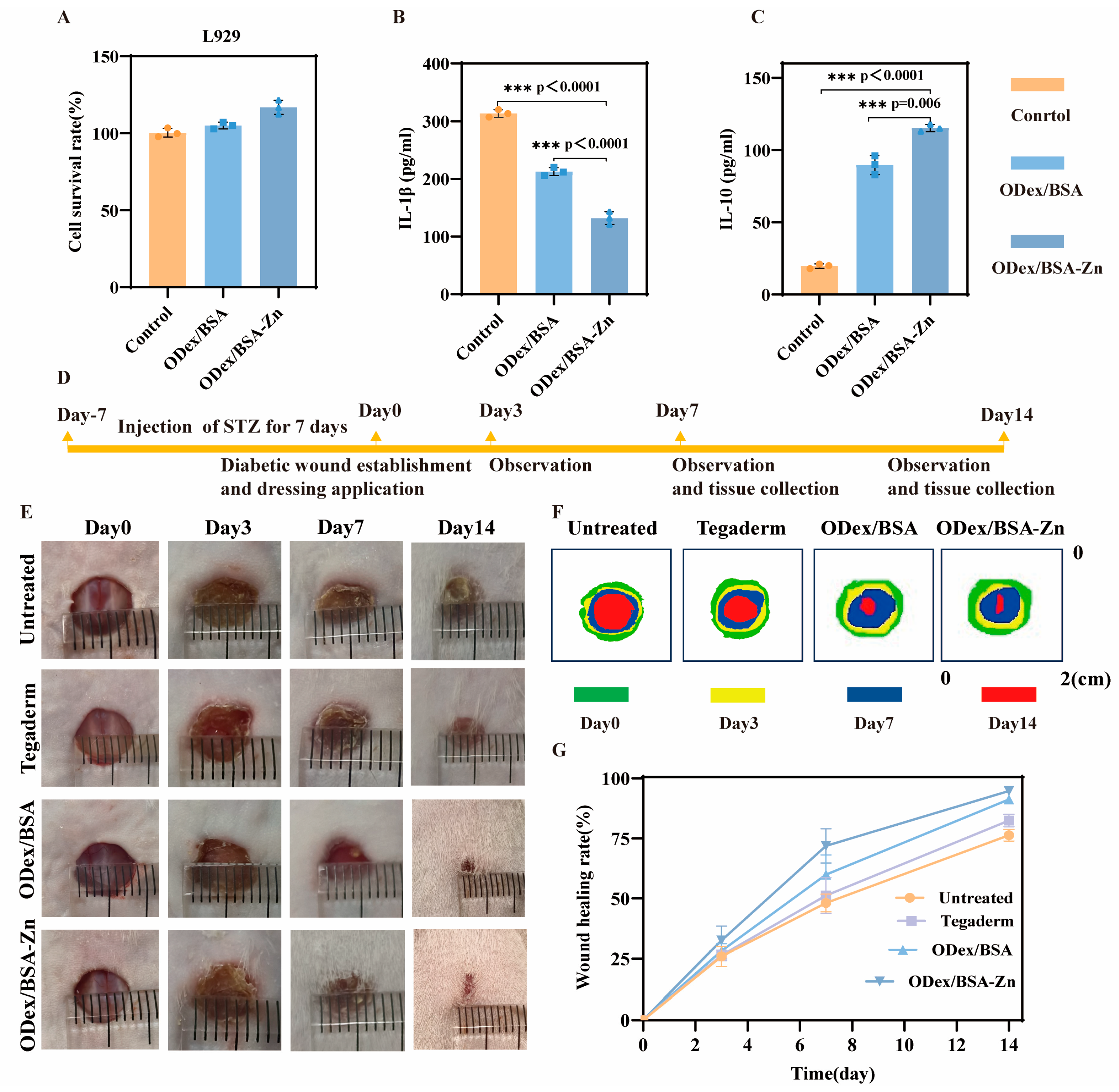
2.8. In Vitro Anti-Inflammatory Activity Evaluation
2.9. In Vivo Evaluation of Diabetic Wound Healing
2.10. Histology and Immunofluorescence Staining Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Oxidized Dextran
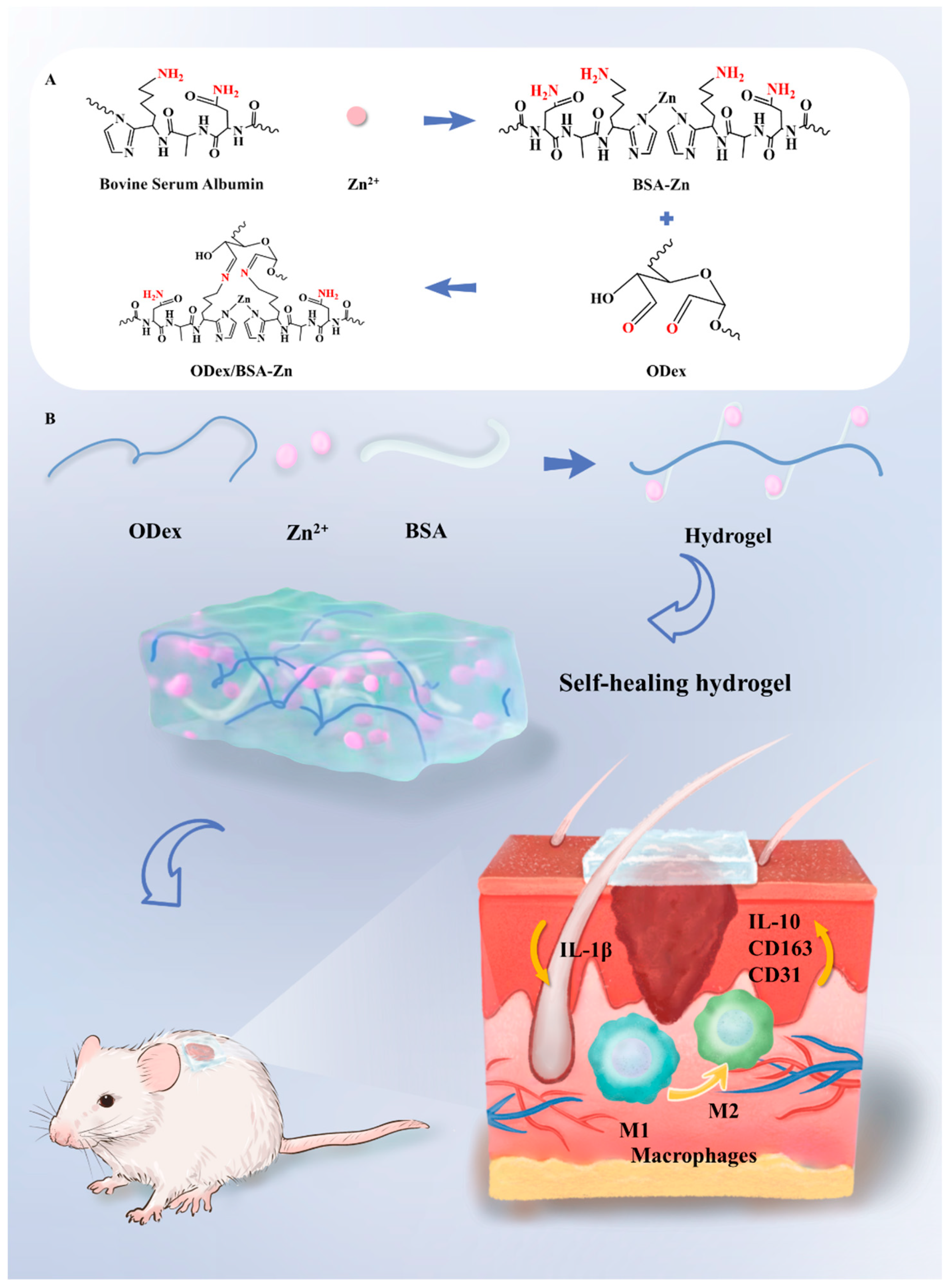
3.2. Fabrication and Characterization of the ODex/BSA-Zn Hydrogel
3.3. Rheological Characterization of ODex/BSA-Zn Hydrogel
3.4. In Vitro Cytotoxicity and Anti-Inflammatory Activity of ODex/BSA-Zn Hydrogel
3.5. In Vivo Wound Healing of ODex/BSA-Zn Hydrogel
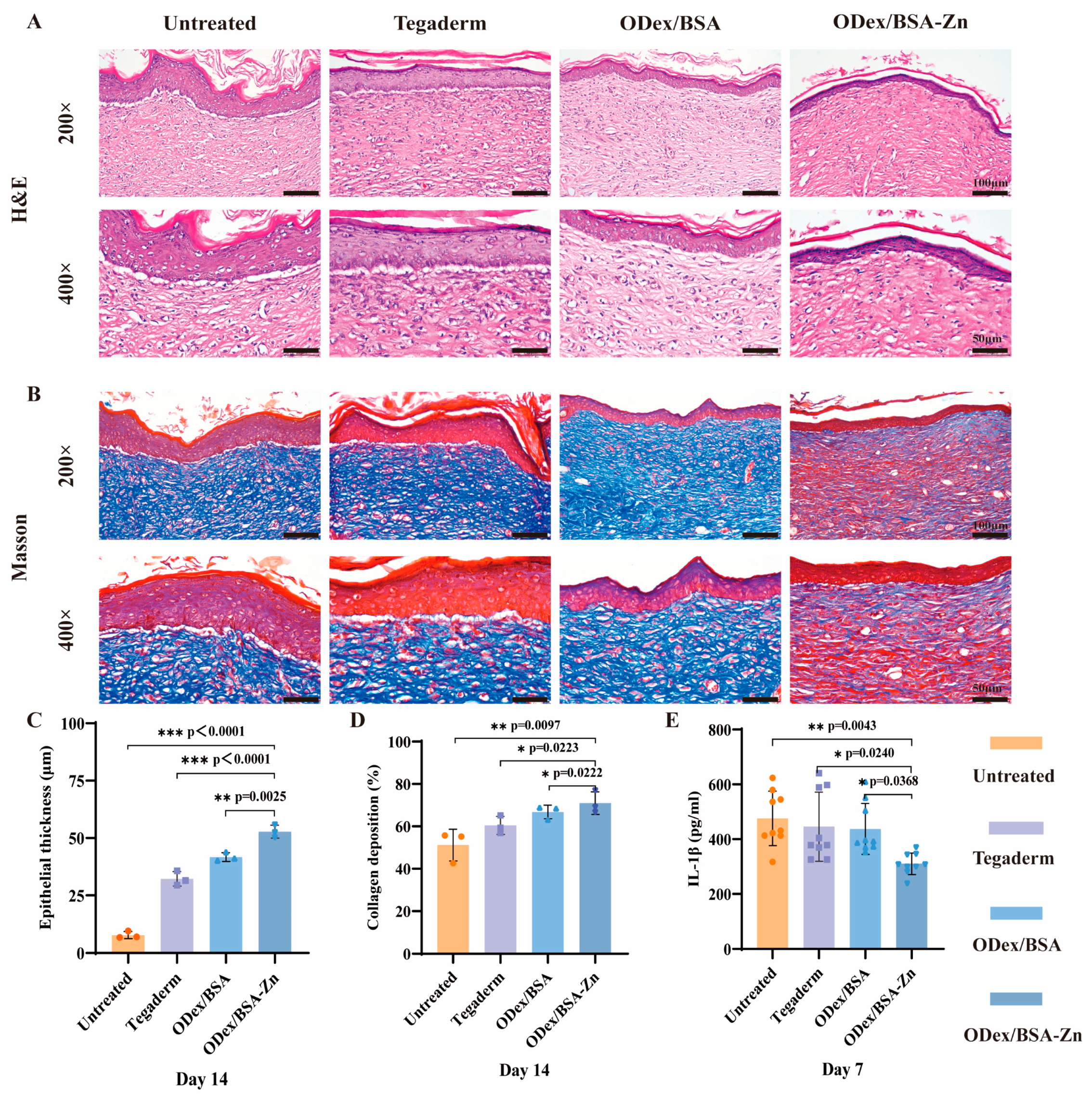
3.6. In Vivo Histological Assessment of Wound Healing
3.7. Immunofluorescence Staining Analysis
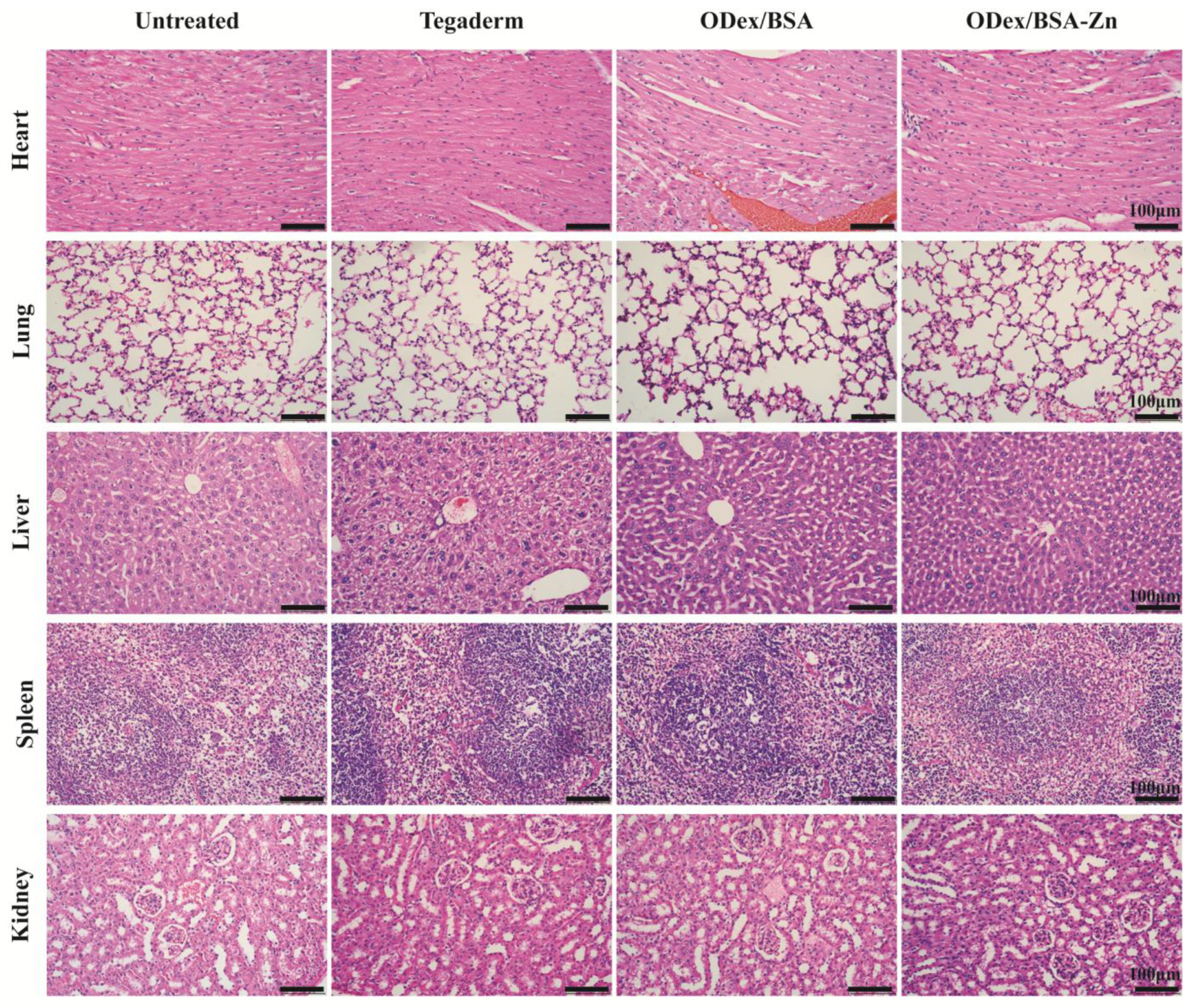
3.8. In Vivo Assessment of Systemic Safety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ODex | Oxidized dextran |
| BSA | Bovine serum albumin |
| LPS | Lipopolysaccharide |
| STZ | Streptozotocin |
References
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Filipová, A.; Garza-Veloz, I.; Martinez-Fierro, M.L. A Beginner’s Introduction to Skin Stem Cells and Wound Healing. Int. J. Mol. Sci. 2021, 22, 11030. [Google Scholar] [CrossRef]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Xu, B.; Liu, H.; Chang, Q. Microenvironmental dynamics of diabetic wounds and insights for hydrogel-based therapeutics. J. Tissue Eng. 2024, 15, 20417314241253290. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; You, S.; Gao, T.; Lan, Y.; Deng, H.; Li, Z.; Hu, R.; Shen, J. All-in-one: Harnessing multifunctional injectable natural hydrogels for ordered therapy of bacteria-infected diabetic wounds. Chem. Eng. J. 2022, 439, 135691. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Wang, J.; Yao, M.; Peng, Y.; Liu, T.; Li, Z.; Luo, G.; Deng, J. Engineering Bacteria-Activated Multifunctionalized Hydrogel for Promoting Diabetic Wound Healing. Adv. Funct. Mater. 2021, 31, 2105749. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Q.; Wei, Z.; Ou, Y.; Cao, Y.; Zhou, H.; Wang, M.; Yu, K.; Liang, B. Advanced polymer hydrogels that promote diabetic ulcer healing: Mechanisms, classifications, and medical applications. Biomater. Res. 2023, 27, 36. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, Y.; Jin, J.; Wu, X.; Xu, K.; Dai, M.; Niu, Q.; Zheng, H.; He, X.; Shen, J. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv. Mater. 2022, 34, 2200521. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Xu, X.; Hu, J.; Xue, H.; Hu, Y.; Liu, Y.-n.; Lin, G.; Liu, L.; Xu, R.-A. Applications of human and bovine serum albumins in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 253, 126914. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 2938–2953. [Google Scholar] [CrossRef]
- Mamta; Chaudhary, A. Synthesis, DFT calculation, molecular docking studies and biological evaluation of a novel series of Schiff base tetradentate macrocyclic ligands and their Zn(II) complexes as antimicrobial, anti-inflammatory and anticancer agents. Res. Chem. Intermed. 2023, 49, 4671–4712. [Google Scholar] [CrossRef]
- Yuting, C.; Jinhong, C.; Dachang, L.; Shuhan, L.; Doudou, L.; Li, Z.; Qingjun, W.; Ming, G. Zinc-based metal organic framework with antibacterial and anti-inflammatory properties for promoting wound healing. Regen. Biomater. 2022, 9, rbac019. [Google Scholar]
- Chunxu, L.; Fengbo, S.; Jingjing, T.; Jiahao, L.; Haidan, S.; Yong, Z.; Shigong, G.; Yuanhua, L.; Xiaodan, S.; Yu, Z. Continuously released Zn2+ in 3D-printed PLGA/β-TCP/Zn scaffolds for bone defect repair by improving osteoinductive and anti-inflammatory properties. Bioact. Mater. 2023, 24, 361–375. [Google Scholar]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Liu, Z.; Liu, J.; Lu, J.; Zhu, W.; Pei, D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: Preparation, characterization and evaluation. Drug Deliv. 2019, 26, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Z.; Wang, F.; Liu, L.; Wei, R.; Zhang, M. Preparation of self-regulating/anti-adhesive hydrogels and their ability to promote healing in burn wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1471–1482. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.-Q.; Li, Z.-B.; Wu, Y.-L.; Li, D.-W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, X.; Xiu, J.; Liu, M.; Liu, S.; Zhang, B.; Miao, Q.; Cun, D.; Yang, C.; Li, K.; et al. pH-Responsive Injectable Multifunctional Pluronic F127/Gelatin-Based Hydrogels with Hydrogen Production for Treating Diabetic Wounds. ACS Appl. Mater. Interfaces 2023, 15, 55392–55408. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-Regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Liu, Q.; Huang, H.; Wang, X.; Shi, Z.; Li, Y.; Liu, S.; Xue, L.; Lei, Y. A smart hydrogel system for visual detection of glucose. Biosens. Bioelectron. 2019, 142, 111547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Awasthi, A.; Gulati, M.; Kumar, B.; Kaur, J.; Vishwas, S.; Khursheed, R.; Porwal, O.; Alam, A.; Kr, A.; Corrie, L.; et al. Recent Progress in Development of Dressings Used for Diabetic Wounds with Special Emphasis on Scaffolds. BioMed Res. Int. 2022, 2022, 1659338. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 2100025. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Li, X.; Zhang, X.; Zhang, D.; Wang, L.-N.; Lee, C.-S. A double-crosslinked self-healing antibacterial hydrogel with enhanced mechanical performance for wound treatment. Acta Biomater. 2021, 124, 139–152. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; He, X.; Wang, X.; Wen, Y.; Zeng, L.; Yu, W.; Hu, P.; Chen, H. A multifunctional hydrogel based on nature polysaccharide fabricated by Schiff base reaction. Eur. Polym. J. 2023, 197, 112330. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Guo, M.; Mao, J.; Li, Y.-T.; Wu, Y.-P.; Guo, H.; Xiang, D.; Li, H. Self-healing, Stretchable, Temperature-Sensitive and Strain-Sensitive Hydrogel-based Flexible Sensors. Chin. J. Polym. Sci. 2022, 41, 334–344. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Ortiz, C.; Rueda, N.; Berenguer-Murcia, Á.; Acosta, N.; Aranaz, I.; Civera, C.; Fernandez-Lafuente, R.; Alcántara, A.R. Dextran Aldehyde in Biocatalysis: More Than a Mere Immobilization System. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020, 69, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.J.; Simões, P.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Berillo, D.; Elowsson, L.; Kirsebom, H. Oxidized Dextran as Crosslinker for Chitosan Cryogel Scaffolds and Formation of Polyelectrolyte Complexes between Chitosan and Gelatin. Macromol. Biosci. 2012, 12, 1090–1099. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Tatikonda, R.; Kalenius, E.; Haukka, M. Synthesis and characterization of Zwitterionic Zn(II) and Cu(II) coordination compounds with ring-substituted 2,2’-biimidazole derivatives. Inorganica Chim. Acta 2016, 453, 298–304. [Google Scholar] [CrossRef][Green Version]
- Shi, T.; Lu, H.; Zhu, J.; Zhou, X.; He, C.; Li, F.; Yang, G. Naturally derived dual dynamic crosslinked multifunctional hydrogel for diabetic wound healing. Compos. Part B Eng. 2023, 257, 110687. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Chen, L.; Mei, W.; Song, J.; Chen, K.; Ni, W.; Wang, L.; Li, Z.; Ge, X.; Su, L.; Jiang, C.; et al. CD163 protein inhibits lipopolysaccharide-induced macrophage transformation from M2 to M1 involved in disruption of the TWEAK–Fn14 interaction. Heliyon 2024, 10, e23223. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, Y.; Wang, H.; Meng, Y.; Dai, X.; Du, L.; Li, L. Construction of pH-Sensitive Multifunctional Hydrogel with Synergistic Anti-Inflammatory Effect for Treatment of Diabetic Wounds. Pharmaceutics 2025, 17, 644. https://doi.org/10.3390/pharmaceutics17050644
Sun X, Li Y, Wang H, Meng Y, Dai X, Du L, Li L. Construction of pH-Sensitive Multifunctional Hydrogel with Synergistic Anti-Inflammatory Effect for Treatment of Diabetic Wounds. Pharmaceutics. 2025; 17(5):644. https://doi.org/10.3390/pharmaceutics17050644
Chicago/Turabian StyleSun, Xiaoyan, Yan Li, Haifeng Wang, Yanqiu Meng, Xu Dai, Lina Du, and Lei Li. 2025. "Construction of pH-Sensitive Multifunctional Hydrogel with Synergistic Anti-Inflammatory Effect for Treatment of Diabetic Wounds" Pharmaceutics 17, no. 5: 644. https://doi.org/10.3390/pharmaceutics17050644
APA StyleSun, X., Li, Y., Wang, H., Meng, Y., Dai, X., Du, L., & Li, L. (2025). Construction of pH-Sensitive Multifunctional Hydrogel with Synergistic Anti-Inflammatory Effect for Treatment of Diabetic Wounds. Pharmaceutics, 17(5), 644. https://doi.org/10.3390/pharmaceutics17050644




