Impact of Excipient and Cell Concentration on the Viability, Proliferation, and Adhesion of Mesenchymal Stem Cells: Future Relevance for the Development of a New Advanced Therapy Medicinal Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Cell Culture
2.2. Study Group Formulation
2.3. Cell Viability Assessment at Study Time Points
2.4. Cell Proliferation Assessment—Ki67 Immunohistochemistry
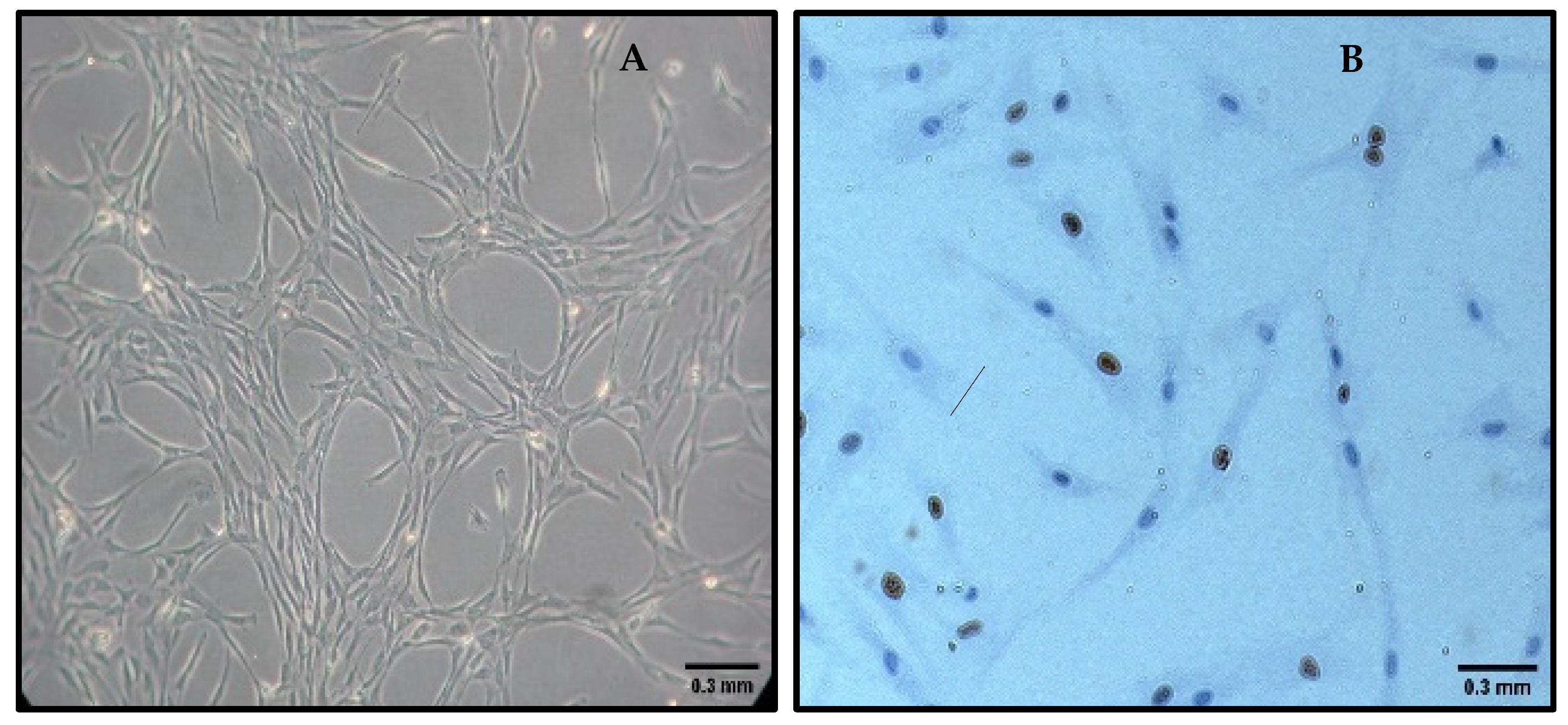
2.5. Cell Adhesion Assessment
2.6. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. Cell Adhesion
3.3. Ki67 Marker Expression
4. Discussion
Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ten Ham, R.M.T.; Hoekman, J.; Hövels, A.M.; Broekmans, A.W.; Leufkens, H.G.M.; Klungel, O.H. Challenges in advanced therapy medicinal product development: A survey among companies in Europe. Mol. Ther. Methods Clin. Dev. 2018, 11, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Johanna, I.; Daudeij, A.; Devina, F.; Nijenhuis, C.; Nuijen, B.; Romberg, B.; de Haar, C.; Haanen, J.; Dolstra, H.; Bremer, E.; et al. Basics of advanced therapy medicinal product development in academic pharma and the role of a GMP simulation unit. Immuno-Oncol. Technol. 2023, 20, 100411. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Clinical grade adult stem cell banking. Organogenesis 2009, 5, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Gudleviciene, Z.; Kundrotas, G.; Liudkeviciene, R.; Rascon, J.; Jurga, M. Quick and effective method of bone marrow mesenchymal stem cell extraction. Open Med. 2014, 10, 44–49. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy 2016, 18, 160–171. [Google Scholar] [CrossRef]
- Tekkatte, C.; Gunasingh, G.P.; Cherian, K.M.; Sankaranarayanan, K. “Humanized” stem cell culture techniques: The animal serum controversy. Stem Cells Int. 2011, 2011, 504723. [Google Scholar] [CrossRef]
- Kinzebach, S.; Bieback, K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. Adv. Biochem. Eng. Biotechnol. 2013, 129, 33–57. [Google Scholar] [CrossRef]
- Freitas-Ribeiro, S.; Carvalho, A.F.; Costa, M.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Pirraco, R.P. Correction: Strategies for the hypothermic preservation of cell sheets of human adipose stem cells. PLoS ONE 2021, 16, e0259406. [Google Scholar] [CrossRef]
- Petrenko, Y.; Chudickova, M.; Vackova, I.; Groh, T.; Kosnarova, E.; Cejkova, J.; Turnovcova, K.; Petrenko, A.; Sykova, E.; Kubinova, S. Clinically relevant solution for the hypothermic storage and transportation of human multipotent mesenchymal stromal cells. Stem Cells Int. 2019, 2019, 5909524. [Google Scholar] [CrossRef]
- Aabling, R.R.; Alstrup, T.; Kjær, E.M.; Poulsen, K.J.; Pedersen, J.O.; Revenfeld, A.L.; Møller, B.K.; Eijken, M. Reconstitution and post-thaw storage of cryopreserved human mesenchymal stromal cells: Pitfalls and optimizations for clinically compatible formulants. Regen. Ther. 2023, 23, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Zhang, F.; Zeng, X.; He, F.; Shang, G.; Guo, T.; Wang, Q.; Wu, J.; Li, T.; Zhong, Z.Z.; et al. A GMP-compliant manufacturing method for Wharton’s jelly-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2024, 15, 131. [Google Scholar] [CrossRef]
- Shanskii, Y.D.; Sergeeva, N.S.; Sviridova, I.K.; Kirakozov, M.S.; Kirsanova, V.A.; Akhmedova, S.A.; Antokhin, A.I.; Chissov, V.I. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull. Exp. Biol. Med. 2013, 156, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Chao, F.C.; Stiles, C.D.; Antoniades, H.N.; Scher, C.D. Platelet alpha granules contain a growth factor for fibroblasts. Blood 1979, 53, 1043–1052. [Google Scholar] [CrossRef]
- Zimmermann, R.; Jakubietz, R.; Jakubietz, M.; Strasser, E.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 2001, 41, 1217–1224. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gu, K.; Bode, D.C.; Van Buskirk, R.G. Hypothermic storage of isolated human hepatocytes: A comparison between University of Wisconsin solution and a hypothermosol platform. Arch. Toxicol. 2009, 83, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Eichelberger, H.; Robert, S.; Rauch, J.; Baust, J.G.; Taylor, M.J.; Buskirk, R.G. Cold-storage of synthetic human epidermis in HypoThermosol. Tissue Eng. 1995, 1, 361–377. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxidative Med. Cell. Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef]
- Pal, R.; Hanwate, M.; Totey, S.M. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J. Tissue Eng. Regen. Med. 2008, 2, 436–444. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Xue, G.; Zhao, J.; Li, R.-K.; Liu, Z.; Niu, B. Effects of storage solutions on the viability of human umbilical cord mesenchymal stem cells for transplantation. Cell Transplant. 2013, 22, 1075–1086. [Google Scholar] [CrossRef]
- Mirzaeian, L.; Ramezani, M.; Feizi, Z.; Ghorbanian, M.T.; Mozdziak, P.; Kiani, N. Ki67 protein: Important proliferative marker for cultured mesenchymal stem cells evaluation. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 1033–1039. [Google Scholar] [CrossRef]
- Chan, L.L.; Rice, W.L.; Qiu, J. Observation and quantification of the morphological effect of trypan blue rupturing dead or dying cells. PLoS ONE 2020, 15, e0227950. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Park, J.K.; Park, S.J.; Jeong, B. Hypothermic stem cell storage using a polypeptide thermogel. Biomacromolecules 2021, 22, 5390–5399. [Google Scholar] [CrossRef] [PubMed]


| Excipient | Concentration | Mean Viability (24 h) | [95% Conf. Interval] | Mean Viability (48 h) | [95% Conf. Interval] | Change over Time | p-Value |
|---|---|---|---|---|---|---|---|
| 100% HPL | 0.1 × 106 MSC/μL | 88.84 ± 1.16 | [86.57–91.11] | 84.80 ± 1.16 | [82.53–87.04] | −2.10 | <0.001 |
| 0.008 × 106 MSC/μL | 93.11 ± 1.16 | [90.84–95.38] | 89.07 ± 1.16 | [86.80–91.34] | −3.07 | <0.001 | |
| 25% HPL/75% HYP | 0.1 × 106 MSC/μL | 88.72 ± 1.16 | [86.45–90.99] | 84.68 ± 1.16 | [82.41–86.96] | −2.14 | <0.001 |
| 0.008 × 106 MSC/μL | 95.43 ± 1.16 | [93.16–97.70] | 91.39 ± 1.16 | [89.12–93.67] | −3.14 | <0.001 | |
| 50% HPL/50% HYP | 0.1 × 106 MSC/μL | 93.32 ± 1.16 | [91.05–95.59] | 89.28 ± 1.16 | [87.01–91.56] | −2.85 | <0.001 |
| 0.008 × 106 MSC/μL | 94.96 ± 1.16 | [92.69–97.23] | 90.92 ± 1.16 | [88.65–93.20] | −3.27 | <0.001 | |
| 75% HPL/25% HYP | 0.1 × 106 MSC/μL | 93.34 ± 1.16 | [91.07–95.62] | 89.31 ± 1.16 | [87.04–91.58] | −2.83 | <0.001 |
| 0.008 × 106 MSC/μL | 95.69 ± 1.16 | [93.42–97.96] | 91.65 ± 1.16 | [89.38–93.92] | −3.32 | <0.001 | |
| 100% HYP | 0.1 × 106 MSC/μL | 92.12 ± 1.16 | [89.85–94.39] | 88.08 ± 1.16 | [85.81–90.36] | −3.33 | <0.001 |
| 0.008 × 106 MSC/μL | 93.21 ± 1.16 | [90.93–95.48] | 89.17 ± 1.16 | [86.90–91.44] | −3.36 | <0.001 |
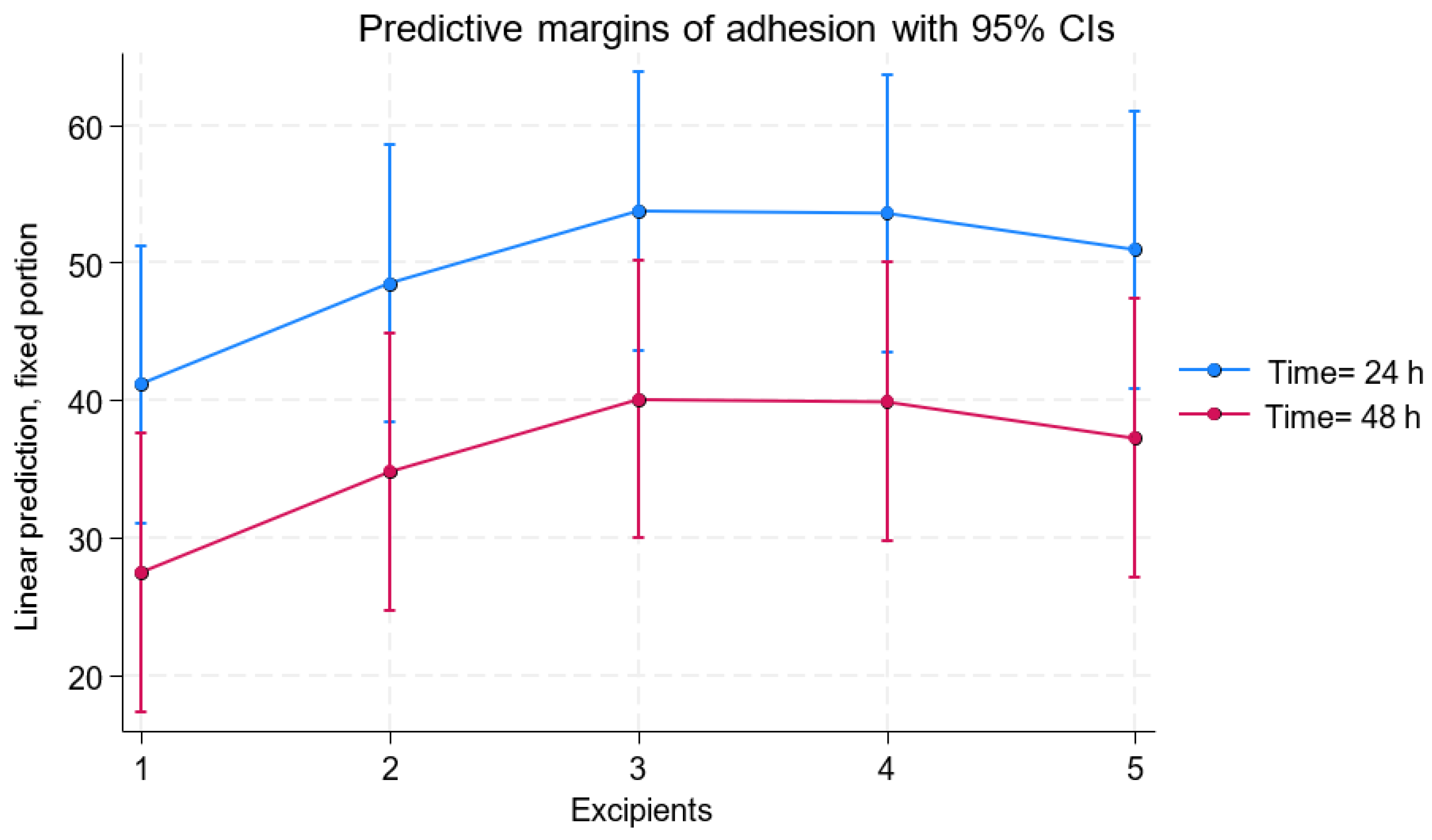
| Excipient | Adhesion at 24 h (Mean ± SD) | [95% Conf. Interval] | Adhesion at 48 h (Mean ± SD) | [95% Conf. Interval] | p-Value |
|---|---|---|---|---|---|
| 100% HPL | 41.15 ± 5.15 | [31.04–51.24] | 27.44 ± 5.15 | [17.34–37.55] | <0.001 |
| 25% HPL/75% HYP | 48.48 ± 5.15 | [38.38–58.58] | 34.78 ± 5.15 | [24.68–44.88] | <0.001 |
| 50% HPL/50% HYP | 53.75 ± 5.15 | [43.65–63.86] | 40.06 ± 5.15 | [29.95–50,16] | <0.001 |
| 75% HPL/25% HYP | 53.59 ± 5.15 | [43.49–63.70] | 39.90 ± 5.15 | [29.79–50.00] | <0.001 |
| 100% HYP | 50.93 ± 5.15 | [40.83–61.04] | 37.24 ± 5.15 | [27.13–47.34] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moñivas, E.; Aguayo, C.; Rodera, B.; Zurita, M. Impact of Excipient and Cell Concentration on the Viability, Proliferation, and Adhesion of Mesenchymal Stem Cells: Future Relevance for the Development of a New Advanced Therapy Medicinal Product. Pharmaceutics 2025, 17, 642. https://doi.org/10.3390/pharmaceutics17050642
Moñivas E, Aguayo C, Rodera B, Zurita M. Impact of Excipient and Cell Concentration on the Viability, Proliferation, and Adhesion of Mesenchymal Stem Cells: Future Relevance for the Development of a New Advanced Therapy Medicinal Product. Pharmaceutics. 2025; 17(5):642. https://doi.org/10.3390/pharmaceutics17050642
Chicago/Turabian StyleMoñivas, Ester, Concepción Aguayo, Beatriz Rodera, and Mercedes Zurita. 2025. "Impact of Excipient and Cell Concentration on the Viability, Proliferation, and Adhesion of Mesenchymal Stem Cells: Future Relevance for the Development of a New Advanced Therapy Medicinal Product" Pharmaceutics 17, no. 5: 642. https://doi.org/10.3390/pharmaceutics17050642
APA StyleMoñivas, E., Aguayo, C., Rodera, B., & Zurita, M. (2025). Impact of Excipient and Cell Concentration on the Viability, Proliferation, and Adhesion of Mesenchymal Stem Cells: Future Relevance for the Development of a New Advanced Therapy Medicinal Product. Pharmaceutics, 17(5), 642. https://doi.org/10.3390/pharmaceutics17050642







