Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives
Abstract
1. Introduction
2. Delivery System Functionalization Strategies
2.1. Chemical and Biological Functionalization
2.1.1. Functionalization with Carbohydrate Ligands
2.1.2. Functionalization with Peptides and Proteins
2.1.3. Functionalization with Lipids, Nucleic Acids and Polymers
2.2. Physical Properties of Vaccine Carriers and Their Immunomodulatory Effects
2.2.1. Size
2.2.2. Shape
2.2.3. Surface Charge
3. Functionalized Delivery Systems in Development
3.1. Lipid and Polymeric Nanoparticles
3.2. Biomimetic Systems
3.2.1. Extracellular Vesicles
3.2.2. Cell Membranes
3.2.3. Metal–Organic Frameworks and Metallic Nanoparticles
3.3. Microorganism-Based
3.3.1. Bacteria as Functionalized Delivery Vehicles
Bacterial Ghosts and Bacterial Membrane Vesicles
Spores as Delivery Vehicles
3.3.2. Phages, Virosomes and Archaeosomes
3.3.3. Yeasts as Functionalized Delivery Vehicles
4. Routes of Vaccine Administration: Traditional Methods and Innovations
5. Clinical Applications and Translational Challenges
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADM | allyl-α-D-mannopyranoside |
| ALC-0315 | ionizable lipid used in Pfizer-BioNTech’s COVID-19 vaccine |
| AMA-1 | Apical Membrane Antigen-1 |
| APC | antigen-presenting cell |
| BCG | Bacille Calmette-Guérin |
| BG | bacterial ghost |
| BMV | bacterial membrane vesicle |
| cGAMP | cyclic GMP-AMP (STING agonist) |
| CLR | C-type Lectin Receptor |
| COE | neutralizing epitope of porcine epidemic diarrhea virus (PEDV) |
| CPP | cell-penetrating peptide |
| CTL | cytotoxic T lymphocyte |
| DC | dendritic cell |
| DC-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3 |
| Dex | Dendritic Cell-derived Exosomes |
| DOPE | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium-propane |
| DOTMA | N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride |
| EP | electroporation |
| FMDV | foot-and-mouth disease virus |
| GAS | Group A streptococcus |
| GP | glucan particle |
| GPI | glycosyl-phosphatidylinositol |
| GRAS | generally recognized as safe |
| HA | hemagglutinin |
| HLA | Human Leukocyte Antigen |
| ID | intradermal |
| IM | intramuscular |
| LNP | lipid nanoparticle |
| LSCExo | lung cell-derived exosome |
| MHC | major histocompatibility complex |
| MR | mannose receptor |
| mRNA | Messenger RNA |
| MeNPs | metallic nanoparticles |
| MOFs | metal–organic frameworks |
| MUC1 | Mucin 1 |
| PAMP | pathogen-associated molecular pattern |
| PBAE | poly(β-amino ester) |
| PEDV | porcine epidemic diarrhea virus |
| PEG | polyethylene glycol |
| PEI | polyethylenimine |
| PLGA | poly(lactic-co-glycolic acid) |
| PLPs | phage-like particles |
| PNP | polymeric nanoparticle |
| PRR | pattern recognition receptor |
| RABV | rabies virus |
| RBD | receptor-binding domain |
| RGD | arginine–glycine–aspartic acid (integrin-binding peptide) |
| SC | subcutaneous |
| siRNA | Small Interfering RNA |
| SM-102 | ionizable lipid used in Moderna’s COVID-19 vaccine |
| STING | Stimulator of Interferon Genes |
| TLR | Toll-like receptor |
| TNBC | triple-negative breast cancer |
| WYV | whole yeast vaccine |
| YSD | yeast surface display |
| YS | yeast shell |
References
- Bennett, N.R.; Zwick, D.B.; Courtney, A.H.; Kiessling, L.L. Multivalent Antigens for Promoting B and T Cell Activation. ACS Chem. Biol. 2015, 10, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Majhen, D.; van der Ley, P.; Thakur, A.; Summerfield, A.; Berisio, R.; Nativi, C.; Fernández-Tejada, A.; Alvarez-Dominguez, C.; Gizurarson, S.; et al. Rational Vaccine Design in Times of Emerging Diseases: The Critical Choices of Immunological Correlates of Protection, Vaccine Antigen and Immunomodulation. Pharmaceutics 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA Vaccines for Cancer Immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Sig. Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Hamid, F.A.; Marker, C.L.; Raleigh, M.D.; Khaimraj, A.; Winston, S.; Pentel, P.R.; Pravetoni, M. Pre-Clinical Safety and Toxicology Profile of a Candidate Vaccine to Treat Oxycodone Use Disorder. Vaccine 2022, 40, 3244–3252. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Zheng, X.; Tong, R.; Sun, X. Advanced Subunit Vaccine Delivery Technologies: From Vaccine Cascade Obstacles to Design Strategies. Acta Pharm. Sin. B 2023, 13, 3321–3338. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The Next-Generation DNA Vaccine Platforms and Delivery Systems: Advances, Challenges and Prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Rando, H.M.; Lordan, R.; Kolla, L.; Sell, E.; Lee, A.J.; Wellhausen, N.; Naik, A.; Kamil, J.P.; COVID-19 Review Consortium; Gitter, A.; et al. The Coming of Age of Nucleic Acid Vaccines during COVID-19. mSystems 2023, 8, e00928-22. [Google Scholar] [CrossRef]
- Black, M.; Trent, A.; Tirrell, M.; Olive, C. Advances in the Design and Delivery of Peptide Subunit Vaccines with a Focus on Toll-like Receptor Agonists. Expert Rev. Vaccines 2010, 9, 157–173. [Google Scholar] [CrossRef]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the Point: Targeted mRNA-Delivery to Immune Cells for Improved Vaccine Design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
- De Moura, I.A.; Silva, A.J.D.; De Macêdo, L.S.; Invenção, M.D.C.V.; De Sousa, M.M.G.; De Freitas, A.C. Enhancing the Effect of Nucleic Acid Vaccines in the Treatment of HPV-Related Cancers: An Overview of Delivery Systems. Pathogens 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Tan, D.; Li, G.; Fu, W.; Lei, C. Exosomes: The next Frontier in Vaccine Development and Delivery. Front. Immunol. 2024, 15, 1435426. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a Promising Delivery Platform for DNA and RNA Vaccines. FEMS Yeast Res. 2021, 21, foab018. [Google Scholar] [CrossRef]
- Hosseini-Kharat, M.; Bremmell, K.E.; Grubor-Bauk, B.; Prestidge, C.A. Enhancing Non-Viral DNA Delivery Systems: Recent Advances in Improving Efficiency and Target Specificity. J. Control. Release 2025, 378, 170–194. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.-H.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.G.; Swartz, J.R. Surface Functionalization of Virus-Like Particles by Direct Conjugation Using Azide−Alkyne Click Chemistry. Bioconjugate Chem. 2011, 22, 376–387. [Google Scholar] [CrossRef]
- Petrini, M.; Lokerse, W.J.; Mach, A.; Hossann, M.; Merkel, O.M.; Lindner, L.H. Effects of Surface Charge, PEGylation and Functionalization with Dipalmitoylphosphatidyldiglycerol on Liposome–Cell Interactions and Local Drug Delivery to Solid Tumors via Thermosensitive Liposomes. Indian J. Nephrol. 2021, 16, 4045–4061. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Steffens, R.C.; Wagner, E. Directing the Way—Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 47–76. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M. The Use of RNA-Based Treatments in the Field of Cancer Immunotherapy. Mol. Cancer 2023, 22, 106. [Google Scholar] [CrossRef]
- Seidu, T.A.; Kutoka, P.T.; Asante, D.O.; Farooq, M.A.; Alolga, R.N.; Bo, W. Functionalization of Nanoparticulate Drug Delivery Systems and Its Influence in Cancer Therapy. Pharmaceutics 2022, 14, 1113. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J. Surface Functionalization of PLGA Nanoparticles for Potential Oral Vaccine Delivery Targeting Intestinal Immune Cells. Colloids Surf. B Biointerfaces 2023, 222, 113121. [Google Scholar] [CrossRef]
- Eras, A.; Castillo, D.; Suárez, M.; Vispo, N.S.; Albericio, F.; Rodriguez, H. Chemical Conjugation in Drug Delivery Systems. Front. Chem. 2022, 10, 889083. [Google Scholar] [CrossRef]
- Friedman, A.; Claypool, S.; Liu, R. The Smart Targeting of Nanoparticles. CPD 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed]
- Mustafa Khidir, A.; Saeed, A.A. Ligand-Targeted Liposomes. Health Prim. Care 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-W.; Zhang, F.-D.; Li, J.-H.; Lv, J.-L.; Zhang, L.-P.; Zhai, K.-G.; Wang, Y.-L.; Guo, H.-C.; Liu, X.-S.; et al. Double Synergic Chitosan-Coated Poly (Lactic-Co-Glycolic) Acid Nanospheres Loaded with Nucleic Acids as an Intranasally Administered Vaccine Delivery System to Control the Infection of Foot-and-Mouth Disease Virus. Antivir. Res. 2024, 226, 105900. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Ceglia, V.; Zurawski, S.; Montes, M.; Kroll, M.; Bouteau, A.; Wang, Z.; Ellis, J.; Igyártó, B.Z.; Lévy, Y.; Zurawski, G. Anti-CD40 Antibody Fused to CD40 Ligand Is a Superagonist Platform for Adjuvant Intrinsic DC-Targeting Vaccines. Front. Immunol. 2022, 12, 786144. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Aryal, S. Surface Functionalization Strategies of Extracellular Vesicles. J. Mater. Chem. B 2020, 8, 4552–4569. [Google Scholar] [CrossRef]
- Ung, T.; Rutledge, N.S.; Weiss, A.M.; Esser-Kahn, A.P.; Deak, P. Cell-Targeted Vaccines: Implications for Adaptive Immunity. Front. Immunol. 2023, 14, 1221008. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Deng, J.; Wang, Y.; Wu, C.-Q.; Li, X.; Dai, H.-W. Hybrid Cell Membrane-Coated Nanoparticles: A Multifunctional Biomimetic Platform for Cancer Diagnosis and Therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef]
- Dai, Z.; Cai, R.; Zeng, H.; Zhu, H.; Dou, Y.; Sun, S. Exosome May Be the next Generation of Promising Cell-Free Vaccines. Hum. Vaccines Immunother. 2024, 20, 2345940. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Nurunnabi, M. Potential Application of Exosomes in Vaccine Development and Delivery. Pharm. Res. 2022, 39, 2635–2671. [Google Scholar] [CrossRef]
- Alter, C.L.; Lotter, C.; Puligilla, R.D.; Bolten, J.S.; Sedzicki, J.; Marchese, J.; Schittny, V.; Rucci, F.; Beverly, M.; Palivan, C.G.; et al. Nano Plasma Membrane Vesicle-Lipid Nanoparticle Hybrids for Enhanced Gene Delivery and Expression. Adv Healthc. Mater. 2025, 14, 2401888. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics 2020, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Ki, C.S. Modular Formation of Hyaluronic Acid/β-Glucan Hybrid Nanogels for Topical Dermal Delivery Targeting Skin Dendritic Cells. Carbohydr. Polym. 2021, 252, 117132. [Google Scholar] [CrossRef]
- Hulbert, S.W.; Desai, P.; Jewett, M.C.; DeLisa, M.P.; Williams, A.J. Glycovaccinology: The Design and Engineering of Carbohydrate-Based Vaccine Components. Biotechnol. Adv. 2023, 68, 108234. [Google Scholar] [CrossRef]
- Nahar, U.J.; Toth, I.; Skwarczynski, M. Mannose in Vaccine Delivery. J. Control. Release 2022, 351, 284–300. [Google Scholar] [CrossRef]
- Vendele, I.; Willment, J.A.; Silva, L.M.; Palma, A.S.; Chai, W.; Liu, Y.; Feizi, T.; Spyrou, M.; Stappers, M.H.T.; Brown, G.D.; et al. Mannan Detecting C-Type Lectin Receptor Probes Recog-nise Immune Epitopes with Diverse Chemical, Spatial and Phylogenetic Heterogeneity in Fun-gal Cell Walls. PLoS Pathog. 2020, 16, e1007927. [Google Scholar] [CrossRef]
- Li, M.; Zhang, R.; Li, J.; Li, J. The Role of C-Type Lectin Receptor Signaling in the Intestinal Microbiota-Inflammation-Cancer Axis. Front. Immunol. 2022, 13, 894445. [Google Scholar] [CrossRef]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic Cells Use Macropinocytosis and the Mannose Receptor to Concentrate Macromolecules in the Major Histocompatibility Complex Class II Compartment: Downregulation by Cytokines and Bacterial Products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Colaço, M.; Cruz, M.T.; Almeida, L.P.D.; Borges, O. Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors. Pharmaceutics 2024, 16, 1308. [Google Scholar] [CrossRef]
- Keler, T.; Ramakrishna, V.; Fanger, M.W. Mannose Receptor-Targeted Vaccines. Expert Opin. Biol. Ther. 2004, 4, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Paurević, M.; Šrajer Gajdošik, M.; Ribić, R. Mannose Ligands for Mannose Receptor Targeting. Int. J. Mol. Sci. 2024, 25, 1370. [Google Scholar] [CrossRef]
- Glass, E.B.; Masjedi, S.; Dudzinski, S.O.; Wilson, A.J.; Duvall, C.L.; Yull, F.E.; Giorgio, T.D. Optimizing Mannose “Click” Conjugation to Polymeric Nanoparticles for Targeted siRNA Delivery to Human and Murine Macrophages. ACS Omega 2019, 4, 16756–16767. [Google Scholar] [CrossRef]
- Montiel, L.; Spada, F.; Bessonov, S.; Crisp, A.; Berger, C.; Wiedemann, S.; Carell, T.; Frischmuth, T. Synthesis and Validation of Clickable Multimeric Mannose Ligands for Dendritic Cell Targeting. J. Carbohydr. Chem. 2024, 43, 323–348. [Google Scholar] [CrossRef]
- Dalle Vedove, E.; Costabile, G.; Merkel, O.M. Mannose and Mannose-6-Phosphate Receptor–Targeted Drug Delivery Systems and Their Application in Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1701398. [Google Scholar] [CrossRef]
- Wallace, R.P.; Refvik, K.C.; Antane, J.T.; Brünggel, K.; Tremain, A.C.; Raczy, M.R.; Alpar, A.T.; Nguyen, M.; Solanki, A.; Slezak, A.J.; et al. Synthetically Mannosylated Antigens Induce Antigen-Specific Humoral Tolerance and Reduce Anti-Drug Antibody Responses to Immunogenic Biologics. Cell Rep. Med. 2024, 5, 101345. [Google Scholar] [CrossRef]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef]
- Serra, A.S.; Eusébio, D.; Neves, A.R.; Albuquerque, T.; Bhatt, H.; Biswas, S.; Costa, D.; Sousa, Â. Synthesis and Characterization of Mannosylated Formulations to Deliver a Minicircle DNA Vaccine. Pharmaceutics 2021, 13, 673. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Bartlett, S.; Hussein, W.M.; Lu, L.; Wright, Q.; Huang, W.; Nahar, U.J.; Yang, J.; Khongkow, M.; Veitch, M.; et al. Liposomal Formulations of a Polyleucine–Antigen Conjugate as Therapeutic Vaccines against Cervical Cancer. Pharmaceutics 2023, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, Z.; Wang, L.; Wu, L.; Zhang, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Circular RNA Vaccines with Long-Term Lymph Node-Targeting Delivery Stability after Lyophilization Induce Potent and Persistent Immune Responses. mBio 2024, 15, e0177523. [Google Scholar] [CrossRef] [PubMed]
- Puigmal, N.; Ramos, V.; Artzi, N.; Borrós, S. Poly(β-Amino Ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics 2023, 15, 1262. [Google Scholar] [CrossRef]
- Yu, W.; Shen, L.; Qi, J.; Hu, T. Conjugation with Loxoribine and Mannan Improves the Immunogenicity of Mycobacterium Tuberculosis CFP10-TB10.4 Fusion Protein. Eur. J. Pharm. Biopharm. 2022, 172, 193–202. [Google Scholar] [CrossRef]
- Gaglio, S.C.; Perduca, M.; Zipeto, D.; Bardi, G. Efficiency of Chitosan Nanocarriers in Vaccinology for Mucosal Immunization. Vaccines 2023, 11, 1333. [Google Scholar] [CrossRef]
- Liang, X.; Mu, M.; Fan, R.; Zou, B.; Guo, G. Functionalized Chitosan as a Promising Platform for Cancer Immunotherapy: A Review. Carbohydr. Polym. 2022, 290, 119452. [Google Scholar] [CrossRef]
- Bottens, R.A.; Yamada, T. Cell-Penetrating Peptides (CPPs) as Therapeutic and Diagnostic Agents for Cancer. Cancers 2022, 14, 5546. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Q.; Alves, P.M.; Roldão, A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics 2021, 13, 1621. [Google Scholar] [CrossRef]
- Ouyang, J.; Sheng, Y.; Wang, W. Recent Advances of Studies on Cell-Penetrating Peptides Based on Molecular Dynamics Simulations. Cells 2022, 11, 4016. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Davoodi, S.; Bolhassani, A.; Namazi, F. In Vivo Delivery of a Multiepitope Peptide and Nef Protein Using Novel Cell-Penetrating Peptides for Development of HIV-1 Vaccine Candidate. Biotechnol. Lett. 2021, 43, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Dussouillez, C.; Lointier, M.; Sebane, M.; Fournel, S.; Bechinger, B.; Kichler, A. N-terminal Modification of an LAH4-derived Peptide Increases mRNA Delivery in the Presence of Serum. J. Pept. Sci. 2024, 30, e3597. [Google Scholar] [CrossRef] [PubMed]
- Feyzyab, H.; Milani, A.; Agi, E.; Hashemi, M.; Bolhassani, A. Investigation of the Potency of KALA and REV Cell-Penetrating Peptides for In Vitro/In Vivo Delivery of an HPV Multiepitope DNA Construct. J. Pept. Sci. 2025, 31, e70000. [Google Scholar] [CrossRef]
- Gross, D.A.; Leborgne, C.; Chappert, P.; Masurier, C.; Leboeuf, M.; Monteilhet, V.; Boutin, S.; Lemonnier, F.A.; Davoust, J.; Kichler, A. Induction of Tumor-Specific CTL Responses Using the C-Terminal Fragment of Viral Protein R as Cell Penetrating Peptide. Sci. Rep. 2019, 9, 3937. [Google Scholar] [CrossRef]
- Li, D.; Quan, Z.; Ni, J.; Li, H.; Qing, H. The Many Faces of the Zinc Finger Protein 335 in Brain Development and Immune System. Biomed. Pharmacother. 2023, 165, 115257. [Google Scholar] [CrossRef]
- Yang, J.; Firdaus, F.; Azuar, A.; Khalil, Z.G.; Marasini, N.; Capon, R.J.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Cell-Penetrating Peptides-Based Liposomal Delivery System Enhanced Immunogenicity of Peptide-Based Vaccine against Group A Streptococcus. Vaccines 2021, 9, 499. [Google Scholar] [CrossRef]
- Coolen, A.-L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.-Y.; Verrier, B. Poly(Lactic Acid) Nanoparticles and Cell-Penetrating Peptide Potentiate mRNA-Based Vaccine Expression in Dendritic Cells Triggering Their Activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef]
- Davoodi, S.; Bolhassani, A.; Sadat, S.M.; Irani, S. Design and in Vitro Delivery of HIV-1 Multi-Epitope DNA and Peptide Constructs Using Novel Cell-Penetrating Peptides. Biotechnol. Lett. 2019, 41, 1283–1298. [Google Scholar] [CrossRef]
- Li, Y.; Ma, W.; Su, W.; Yan, Z.; Jia, L.; Deng, J.; Zhu, A.; Xie, Y.; Li, X.; Shao, W.; et al. Synthesis of Cell Penetrating Peptide Sterol Coupler and Its Liposome Study on S-mRNA. Eur. J. Med. Chem. 2023, 261, 115822. [Google Scholar] [CrossRef]
- Jiang, S.; Zu, C.; Wang, B.; Zhong, Y. Enhancing DNA Vaccine Delivery Through Stearyl-Modified Cell-Penetrating Peptides: Improved Antigen Expression and Immune Response In Vitro and In Vivo. Vaccines 2025, 13, 94. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 803304. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.K.; Yong, C.Y.; Ho, K.L.; Omar, A.R.; Alitheen, N.B.; Tan, W.S. Targeted Delivery of Cell Penetrating Peptide Virus-like Nanoparticles to Skin Cancer Cells. Sci. Rep. 2018, 8, 8499. [Google Scholar] [CrossRef] [PubMed]
- Garinot, M.; Fiévez, V.; Pourcelle, V.; Stoffelbach, F.; Des Rieux, A.; Plapied, L.; Theate, I.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; et al. PEGylated PLGA-Based Nanoparticles Targeting M Cells for Oral Vaccination. J. Control. Release 2007, 120, 195–204. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Ni, B.; Jing, T.; Tang, J. Tumor Targeting Nanoparticle E749-57 -HSP110-RGD Elicits Potent Anti-Tumor Immune Response in a CD8-Dependent Manner in Cervical Cancer-Bearing Mouse Model. Hum. Vaccines Immunother. 2021, 17, 3529–3538. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Jiang, K.; Zhang, Y.; Zhan, C.; Ying, M.; Zhang, M.; Lu, L.; Wang, R.; Wang, S.; et al. Liposomes with Cyclic RGD Peptide Motif Triggers Acute Immune Response in Mice. J. Control. Release 2019, 293, 201–214. [Google Scholar] [CrossRef]
- Hatlem, D.; Trunk, T.; Linke, D.; Leo, J.C. Catching a SPY: Using the SpyCatcher-SpyTag and Related Systems for Labeling and Localizing Bacterial Proteins. Int. J. Mol. Sci. 2019, 20, 2129. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin Nanoparticle-Based SpyTag/SpyCatcher-Enabled Click Vaccine for Tumor Immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Huisman, B.D.; Balivada, P.A.; Birnbaum, M.E. Yeast Display Platform with Expression of Linear Peptide Epitopes for High-Throughput Assessment of Peptide-MHC-II Binding. J. Biol. Chem. 2023, 299, 102913. [Google Scholar] [CrossRef]
- Silva, V.A.R.; Pauna, H.F.; Lavinsky, J.; Hyppolito, M.A.; Vianna, M.F.; Leal, M.; Massuda, E.T.; Hamerschmidt, R.; Bahmad Jr, F.; Cal, R.V.; et al. Task Force Guideline of Brazilian Society of Otology—Hearing Loss in Children—Part I—Evaluation. Braz. J. Otorhinolaryngol. 2023, 89, 159–189. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Xiang, X.; Pang, X.; Chen, S.; Zhang, Y.; Ren, E.; Zhang, L.; Liu, X.; Lv, P.; et al. A Nanovaccine for Antigen Self-Presentation and Immunosuppression Reversal as a Personalized Cancer Immunotherapy Strategy. Nat. Nanotechnol. 2022, 17, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Haftcheshmeh, S.; Zamani, P.; Mashreghi, M.; Nikpoor, A.R.; Tavakkol-Afshari, J.; Jaafari, M.R. Immunoliposomes Bearing Lymphocyte Activation Gene 3 Fusion Protein and P5 Peptide: A Novel Vaccine for Breast Cancer. Biotechnol. Prog. 2021, 37, e3095. [Google Scholar] [CrossRef]
- Wang, W.; Zou, C.; Liu, X.; He, L.; Cao, Z.; Zhu, M.; Wu, Y.; Liu, X.; Ma, J.; Wang, Y.; et al. Biomimetic Dendritic Cell-Based Nanovaccines for Reprogramming the Immune Microenvironment to Boost Tumor Immunotherapy. ACS Nano 2024, 18, 34063–34076. [Google Scholar] [CrossRef] [PubMed]
- Lamrayah, M.; Charriaud, F.; Desmares, M.; Coiffier, C.; Megy, S.; Colomb, E.; Terreux, R.; Lucifora, J.; Durantel, D.; Verrier, B. Induction of a Strong and Long-Lasting Neutralizing Immune Response by dPreS1-TLR2 Agonist Nanovaccine against Hepatitis B Virus. Antivir. Res. 2023, 209, 105483. [Google Scholar] [CrossRef]
- Boldyrev, I.A.; Shendrikov, V.P.; Vostrova, A.G.; Vodovozova, E.L. A Route to Synthesize Ionizable Lipid ALC-0315, a Key Component of the mRNA Vaccine Lipid Matrix. Russ. J. Bioorg. Chem. 2023, 49, 412–415. [Google Scholar] [CrossRef]
- Meulewaeter, S.; Aernout, I.; Deprez, J.; Engelen, Y.; De Velder, M.; Franceschini, L.; Breckpot, K.; Van Calenbergh, S.; Asselman, C.; Boucher, K.; et al. Alpha-Galactosylceramide Improves the Potency of mRNA LNP Vaccines against Cancer and Intracellular Bacteria. J. Control. Release 2024, 370, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Alameh, M.-G.; Butowska, K.; Knox, J.J.; Lundgreen, K.; Ghattas, M.; Gong, N.; Xue, L.; Xu, Y.; Lavertu, M.; et al. Adjuvant Lipidoid-Substituted Lipid Nanoparticles Augment the Immunogenicity of SARS-CoV-2 mRNA Vaccines. Nat. Nanotechnol. 2023, 18, 1105–1114. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Pro-Inflammatory Concerns with Lipid Nanoparticles. Mol. Ther. 2022, 30, 2109–2110. [Google Scholar] [CrossRef]
- Zhang, W.; Pfeifle, A.; Lansdell, C.; Frahm, G.; Cecillon, J.; Tamming, L.; Gravel, C.; Gao, J.; Thulasi Raman, S.N.; Wang, L.; et al. The Expression Kinetics and Immunogenicity of Lipid Nanoparticles Delivering Plasmid DNA and mRNA in Mice. Vaccines 2023, 11, 1580. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Lamparelli, E.P.; Ciaglia, E.; Ciardulli, M.C.; Lopardo, V.; Montella, F.; Puca, A.A.; Della Porta, G. Optimizing mRNA Delivery: A Microfluidic Exploration of DOTMA vs. DOTAP Lipid Nanoparticles for GFP Expression on Human PBMCs and THP-1 Cell Line. Int. J. Pharm. 2025, 672, 125324. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Khalil, I.A.; Elewa, Y.H.A.; Harashima, H. Novel Lipid Combination for Delivery of Plasmid DNA to Immune Cells in the Spleen. J. Control. Release 2021, 330, 753–764. [Google Scholar] [CrossRef]
- Kimura, S.; Khalil, I.A.; Elewa, Y.H.A.; Harashima, H. Spleen Selective Enhancement of Transfection Activities of Plasmid DNA Driven by Octaarginine and an Ionizable Lipid and Its Implications for Cancer Immunization. J. Control. Release 2019, 313, 70–79. [Google Scholar] [CrossRef]
- Sun, D.; Lu, Z.-R. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Hashida, M. Cell-Specific Delivery of Genes with Glycosylated Carriers. Adv. Drug Deliv. Rev. 2001, 52, 187–196. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, G.P.; Chakraborty, H. Cholesterol: A Key Player in Membrane Fusion That Modulates the Efficacy of Fusion Inhibitor Peptides. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2021; Volume 117, pp. 133–155. ISBN 978-0-323-90731-6. [Google Scholar]
- Cabanillas, B.; Novak, N.; Akdis, C.A. The Form of PEG Matters: PEG Conjugated with Lipids and Not PEG Alone Could Be the Specific Form Involved in Allergic Reactions to COVID-19 Vaccines. Allergy 2022, 77, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jing, Q.; Xu, Z.; Zhang, D.; Zheng, W.; Ren, F. Corosolic Acid-Modified Lipid Nanoparticles as Delivery Carriers for DNA Vaccines against Avian Influenza. Int. J. Pharm. 2023, 638, 122914. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, M.; Bernard, M.-C.; Vaure, C.; Bazin, E.; Commandeur, S.; Perkov, V.; Lemdani, K.; Nicolaï, M.-C.; Bonifassi, P.; Kichler, A.; et al. An Imidazole Modified Lipid Confers Enhanced mRNA-LNP Stability and Strong Immunization Properties in Mice and Non-Human Primates. Biomaterials 2022, 286, 121570. [Google Scholar] [CrossRef]
- Tada, R.; Honjo, E.; Muto, S.; Takayama, N.; Kiyono, H.; Kunisawa, J.; Negishi, Y. Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes. Membranes 2022, 12, 635. [Google Scholar] [CrossRef]
- Pant, S.; Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Basturk, O.; VanWyk, H.; et al. Lymph-Node-Targeted, mKRAS-Specific Amphiphile Vaccine in Pancreatic and Colorectal Cancer: The Phase 1 AMPLIFY-201 Trial. Nat. Med. 2024, 30, 531–542. [Google Scholar] [CrossRef]
- Bayyurt Kocabas, B.; Almacioglu, K.; Bulut, E.A.; Gucluler, G.; Tincer, G.; Bayik, D.; Gursel, M.; Gursel, I. Dual-Adjuvant Effect of pH-Sensitive Liposomes Loaded with STING and TLR9 Agonists Regress Tumor Development by Enhancing Th1 Immune Response. J. Control. Release 2020, 328, 587–595. [Google Scholar] [CrossRef]
- Tandel, N.; Patel, D.; Thakkar, M.; Shah, J.; Tyagi, R.K.; Dalai, S.K. Poly(I:C) and R848 Ligands Show Better Adjuvanticity to Induce B and T Cell Responses against the Antigen(s). Heliyon 2024, 10, e26887. [Google Scholar] [CrossRef]
- Renu, S.; Feliciano-Ruiz, N.; Lu, F.; Ghimire, S.; Han, Y.; Schrock, J.; Dhakal, S.; Patil, V.; Krakowka, S.; HogenEsch, H.; et al. A Nanoparticle-Poly(I:C) Combination Adjuvant Enhances the Breadth of the Immune Response to Inactivated Influenza Virus Vaccine in Pigs. Vaccines 2020, 8, 229. [Google Scholar] [CrossRef]
- Alikhani, Z.; Kazemi-Pour, N.; Shafieeardestani, M.; Noofeli, M. Development and Characteristics of Novel PLGA-Chitosan as a New Nanocarrier for Pentavalent Vaccine Delivery. Chem. Pharm. Bull. 2021, 22, 1641–1653. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Li, X.; Guo, Z.; Liu, Y.; Ma, X.; Zheng, S. PEI-Modified Macrophage Cell Membrane-Coated PLGA Nanoparticles Encapsulating Dendrobium Polysaccharides as a Vaccine Delivery System for Ovalbumin to Improve Immune Responses. Int. J. Biol. Macromol. 2020, 165, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Sakurai, N.; Okamoto, A.; Koide, H.; Oku, N.; Dewa, T.; Asai, T. Design of a Novel PEGylated Liposomal Vector for Systemic Delivery of siRNA to Solid Tumors. Biol. Pharm. Bull. 2019, 42, 996–1003. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of Lipid-Based Nanoparticles for Delivery of Therapeutic Nucleic Acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the Potency of siRNA Lipid Nanoparticles for Hepatic Gene Silencing In Vivo**. Angew. Chem. Int. Ed. 2012, 51, 8529–8533. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; Van Der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. An Ionizable Lipid Toolbox for RNA Delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Patel, S.K.; Billingsley, M.M.; Frazee, C.; Han, X.; Swingle, K.L.; Qin, J.; Alameh, M.-G.; Wang, K.; Weissman, D.; Mitchell, M.J. Hydroxycholesterol Substitution in Ionizable Lipid Nanoparticles for mRNA Delivery to T Cells. J. Control. Release 2022, 347, 521–532. [Google Scholar] [CrossRef]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic Lipids for Gene Delivery: Many Players, One Goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef]
- Chen, H.; Ren, X.; Xu, S.; Zhang, D.; Han, T. Optimization of Lipid Nanoformulations for Effective mRNA Delivery. Indian J. Nephrol. 2022, 17, 2893–2905. [Google Scholar] [CrossRef]
- Anfray, C.; Varela, C.F.; Ummarino, A.; Maeda, A.; Sironi, M.; Gandoy, S.; Brea, J.; Loza, M.I.; León, S.; Calvo, A.; et al. Polymeric Nanocapsules Loaded with Poly(I:C) and Resiquimod to Reprogram Tumor-Associated Macrophages for the Treatment of Solid Tumors. Front. Immunol. 2023, 14, 1334800. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric Nanoparticle Vaccines to Combat Emerging and Pandemic Threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Wang, S.; Zhang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-Coated PLGA Nanoparticles-Encapsulated Angelica Sinensis Polysaccharide as an Adjuvant to Enhance Immune Responses. Carbohydr. Polym. 2019, 223, 115128. [Google Scholar] [CrossRef]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; El-Bassossy, H.M.; El-Sayed, H.M.; El-Hay, S.S.A. Rp-HPLC Determination of Quercetin in a Novel D-α-Tocopherol Polyethylene Glycol 1000 Succinate Based SNEDDS Formulation: Pharmacokinetics in Rat Plasma. Molecules 2021, 26, 1435. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle Vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, Y.-G.; Sun, T. Engineering Optimal Vaccination Strategies: Effects of Physical Properties of the Delivery System on Functions. Biomater. Sci. 2022, 10, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, W.; Liu, K.; Lu, Y.; Wu, W.; Qi, J.; Chen, Z. Effects on Immunization of the Physicochemical Parameters of Particles as Vaccine Carriers. Drug Discov. Today 2021, 26, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Jiskoot, W. Sizing the Optimal Dimensions of a Vaccine Delivery System: A Particulate Matter. Expert Opin. Drug Deliv. 2016, 13, 167–170. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef]
- Pacheco, P.; White, D.; Sulchek, T. Effects of Microparticle Size and Fc Density on Macrophage Phagocytosis. PLoS ONE 2013, 8, e60989. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef]
- Ke, X.; Howard, G.P.; Tang, H.; Cheng, B.; Saung, M.T.; Santos, J.L.; Mao, H.-Q. Physical and Chemical Profiles of Nanoparticles for Lymphatic Targeting. Adv. Drug Deliv. Rev. 2019, 151–152, 72–93. [Google Scholar] [CrossRef]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer Particle Shape Independently Influences Binding and Internalization by Macrophages. J. Control. Release 2010, 147, 408–412. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Wei, W.; You, Z.; Yang, Q.; Yue, H.; Su, Z.; Ma, G. Drug Delivery: Iron Oxide Nanotubes for Magnetically Guided Delivery and pH-Activated Release of Insoluble Anticancer Drugs. Adv. Funct. Mater. 2011, 21, 3397. [Google Scholar] [CrossRef]
- Yue, H.; Ma, G. Polymeric Micro/Nanoparticles: Particle Design and Potential Vaccine Delivery Applications. Vaccine 2015, 33, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Wibroe, P.P.; Anselmo, A.C.; Nilsson, P.H.; Sarode, A.; Gupta, V.; Urbanics, R.; Szebeni, J.; Hunter, A.C.; Mitragotri, S.; Mollnes, T.E.; et al. Bypassing Adverse Injection Reactions to Nanoparticles through Shape Modification and Attachment to Erythrocytes. Nat. Nanotech. 2017, 12, 589–594. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Sun, X. Lymph Node Targeting Strategies to Improve Vaccination Efficacy. J. Control. Release 2017, 267, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gupta, V.; Ahsan, F. Influence of Surface Charge of PLGA Particles of Recombinant Hepatitis B Surface Antigen in Enhancing Systemic and Mucosal Immune Responses. Int. J. Pharm. 2009, 379, 41–50. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; He, G.; Guo, C.; Dong, J.; Wu, L. Development of mRNA Lipid Nanoparticles: Targeting and Therapeutic Aspects. Int. J. Mol. Sci. 2024, 25, 10166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, Y.; Zhu, L.; Chu, H.; Shao, X.; Asakiya, C.; Huang, K.; Xu, W. Insights into Nucleic Acid-Based Self-Assembling Nanocarriers for Targeted Drug Delivery and Controlled Drug Release. J. Control. Release 2022, 341, 869–891. [Google Scholar] [CrossRef]
- Zhang, L.; More, K.R.; Ojha, A.; Jackson, C.B.; Quinlan, B.D.; Li, H.; He, W.; Farzan, M.; Pardi, N.; Choe, H. Effect of mRNA-LNP Components of Two Globally-Marketed COVID-19 Vaccines on Efficacy and Stability. npj Vaccines 2023, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Nahar, U.J.; Wang, J.; Shalash, A.O.; Lu, L.; Islam, M.T.; Alharbi, N.; Koirala, P.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; et al. Self-Assembled Monovalent Lipidated Mannose Ligand as a Standalone Nanoadjuvant. Vaccine 2024, 42, 126060. [Google Scholar] [CrossRef]
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; et al. Development of Mannosylated Lipid Nanoparticles for mRNA Cancer Vaccine with High Antigen Presentation Efficiency and Immunomodulatory Capability. Angew. Chem. Int. Ed. 2024, 63, e202318515. [Google Scholar] [CrossRef]
- Rizwan, S.B.; McBurney, W.T.; Young, K.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Cubosomes Containing the Adjuvants Imiquimod and Monophosphoryl Lipid A Stimulate Robust Cellular and Humoral Immune Responses. J. Control. Release 2013, 165, 16–21. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Lin, S.-Y.; Wu, Y.-N.; Shen, C.-Y.; Sheu, M.-T.; Ho, H.-O. Glycosylation of OVA Antigen-Loaded PLGA Nanoparticles Enhances DC-Targeting for Cancer Vaccination. J. Control. Release 2022, 351, 970–988. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kageyama, S.; Miyahara, Y.; Okayama, T.; Kokura, S.; Wang, L.; Sato, E.; Yagita, H.; Itoh, Y.; Shiku, H. Safety and Antibody Immune Response of CHP-NY-ESO-1 Vaccine Combined with Poly-ICLC in Advanced or Recurrent Esophageal Cancer Patients. Cancer Immunol. Immunother. 2021, 70, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Kokate, R.A.; Thamake, S.I.; Chaudhary, P.; Mott, B.; Raut, S.; Vishwanatha, J.K.; Jones, H.P. Enhancement of Anti-Tumor Effect of Particulate Vaccine Delivery System by ‘Bacteriomimetic’ CpG Functionalization of Poly-Lactic-Co-Glycolic Acid Nanoparticles. Nanomedicine 2015, 10, 915–929. [Google Scholar] [CrossRef]
- Zhang, W.; An, M.; Xi, J.; Liu, H. Targeting CpG Adjuvant to Lymph Node via Dextran Conjugate Enhances Antitumor Immunotherapy. Bioconjugate Chem. 2017, 28, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.-L.; Hsieh, P.C.-H. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef]
- Desai, D.N.; Mahal, A.; Varshney, R.; Obaidullah, A.J.; Gupta, B.; Mohanty, P.; Pattnaik, P.; Mohapatra, N.C.; Mishra, S.; Kandi, V.; et al. Nanoadjuvants: Promising Bioinspired and Biomimetic Approaches in Vaccine Innovation. ACS Omega 2023, 8, 27953–27968. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, C.; Yang, Q.; Zheng, K.; Wang, Z.; Zhang, W. Biomimetic Nano-Drug Delivery System: An Emerging Platform for Promoting Tumor Treatment. Indian J. Nephrol. 2024, 19, 571–608. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Li, T.; Yi, Q.; Thakur, A.; Zhang, K.; Ma, X.; Qin, J.-J.; Yan, Y. Application of Biomimetic Nanovaccines in Cancer Immunotherapy: A Useful Strategy to Help Combat Immunotherapy Resistance. Drug Resist. Updates 2024, 75, 101098. [Google Scholar] [CrossRef]
- Marques Neto, L.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Tousian, B.; Khosravi, A.R.; Ghasemi, M.H.; Kadkhodaie, M. Biomimetic Functionalized Metal Organic Frameworks as Multifunctional Agents: Paving the Way for Cancer Vaccine Advances. Mater. Today Bio 2024, 27, 101134. [Google Scholar] [CrossRef]
- Wang, X.; Sun, C.; Huang, X.; Li, J.; Fu, Z.; Li, W.; Yin, Y. The Advancing Roles of Exosomes in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 731062. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Sun, M.; Cui, Y.; Xing, J.; Teng, L.; Xi, Z.; Yang, Z. Exosomes as Smart Drug Delivery Vehicles for Cancer Immunotherapy. Front. Immunol. 2023, 13, 1093607. [Google Scholar] [CrossRef]
- Torres Quintas, S.; Canha-Borges, A.; Oliveira, M.J.; Sarmento, B.; Castro, F. Special Issue: Nanotherapeutics in Women’s Health Emerging Nanotechnologies for Triple-Negative Breast Cancer Treatment. Small 2024, 20, 2300666. [Google Scholar] [CrossRef] [PubMed]
- Picon, M.A.; Wang, L.; Da Fonseca Ferreira, A.; Dong, C.; Marzouka, G.R. Extracellular Vesicles as Delivery Systems in Disease Therapy. Int. J. Mol. Sci. 2023, 24, 17134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Popowski, K.D.; Zhu, D.; De Juan Abad, B.L.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N.; et al. Exosomes Decorated with a Recombinant SARS-CoV-2 Receptor-Binding Domain as an Inhalable COVID-19 Vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef]
- Ferrero-Andrés, A.; Closa, D.; Roselló-Catafau, J.; Folch-Puy, E. Polyethylene Glycol 35 (PEG35) Modulates Exosomal Uptake and Function. Polymers 2020, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.-R.; Jin, X.-X.; Tang, T.-T.; Ding, Y.; Liu, X.-T.; Zhong, X.; Wu, Y.-D.; Han, X.-L.; Zhao, G.-Y.; Shen, C.-L.; et al. Exosomal Vaccine Loading T Cell Epitope Peptides of SARS-CoV-2 Induces Robust CD8+ T Cell Response in HLA-A Transgenic Mice. Indian J. Nephrol. 2022, 17, 3325–3341. [Google Scholar] [CrossRef]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered Exosomes as an in Situ DC-Primed Vaccine to Boost Antitumor Immunity in Breast Cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Yildirim, M.; Yildirim, T.C.; Turay, N.; Bildik, T.; Ibibik, B.; Evcili, I.; Ersan, P.G.; Tokat, U.M.; Sahin, O.; Gursel, I. TLR Ligand Loaded Exosome Mediated Immunotherapy of Established Mammary Tumor in Mice. Immunol. Lett. 2021, 239, 32–41. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Kashanchi, F.; Jafari, R. Dexosomes as a Cell-Free Vaccine for Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 258. [Google Scholar] [CrossRef]
- Ruan, S.; Greenberg, Z.; Pan, X.; Zhuang, P.; Erwin, N.; He, M. Extracellular Vesicles as an Advanced Delivery Biomaterial for Precision Cancer Immunotherapy. Adv. Healthc. Mater. 2022, 11, 2100650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, K.; Wang, Z.; Wang, D.; Yin, X.; Liu, Y.; Yu, F.; Zhao, W. An Efficient and Safe MUC1-Dendritic Cell-Derived Exosome Conjugate Vaccine Elicits Potent Cellular and Humoral Immunity and Tumor Inhibition in Vivo. Acta Biomater. 2022, 138, 491–504. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-Inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, J.; Lu, L.; Deng, D.; Huang, J.; Tang, Z.; Shi, X.; Lo, P.; Lovell, J.F.; Zheng, Y.; et al. Tumor Cell Membrane-based Vaccines: A Potential Boost for Cancer Immunotherapy. Exploration 2024, 4, 20230171. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Santos, A.C.; Conde, J.; Hoskins, C.; Concheiro, A.; Alvarez-Lorenzo, C.; Veiga, F. Biomimetic Cancer Cell Membrane-Coated Nanosystems as next-Generation Cancer Therapies. Expert Opin. Drug Deliv. 2020, 17, 1515–1518. [Google Scholar] [CrossRef]
- Liu, W.-L.; Zou, M.-Z.; Liu, T.; Zeng, J.-Y.; Li, X.; Yu, W.-Y.; Li, C.-X.; Ye, J.-J.; Song, W.; Feng, J.; et al. Cytomembrane Nanovaccines Show Therapeutic Effects by Mimicking Tumor Cells and Antigen Presenting Cells. Nat. Commun. 2019, 10, 3199. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, Y.; Yang, Y.; Miao, Z.; Zhu, J.; Zhong, M.; Feng, C.; Tang, W.; Zhou, J.; Wang, L.; et al. Biomimetic Cytomembrane Nanovaccines Prevent Breast Cancer Development in the Long Term. Nanoscale 2021, 13, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, M.; Chauhan, G.; Shin, M.D.; Hoskins, C.; Madou, M.J.; Martinez-Chapa, S.O.; Steinmetz, N.F.; Veiga, F.; Paiva-Santos, A.C. Unleashing the Potential of Cell Membrane-Based Nanoparticles for COVID-19 Treatment and Vaccination. Expert Opin. Drug Deliv. 2021, 18, 1395–1414. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Hao, Y.; Li, R.; Pan, W.; Ma, X.; Weng, J.; Min, Y. Red Blood Cell-Based Vaccines for Ameliorating Cancer Chemoimmunotherapy. Acta Biomater. 2022, 154, 401–411. [Google Scholar] [CrossRef]
- Guo, Z.; Kubiatowicz, L.J.; Fang, R.H.; Zhang, L. Nanotoxoids: Biomimetic Nanoparticle Vaccines against Infections. Adv. Ther. 2021, 4, 2100072. [Google Scholar] [CrossRef]
- Zhou, J.; Ventura, C.J.; Yu, Y.; Gao, W.; Fang, R.H.; Zhang, L. Biomimetic Neutrophil Nanotoxoids Elicit Potent Immunity against Acinetobacter Baumannii in Multiple Models of Infection. Nano Lett. 2022, 22, 7057–7065. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ran, D.; Campeau, A.; Xiao, C.; Zhou, J.; Dehaini, D.; Jiang, Y.; Kroll, A.V.; Zhang, Q.; Gao, W.; et al. Multiantigenic Nanotoxoids for Antivirulence Vaccination against Antibiotic-Resistant Gram-Negative Bacteria. Nano Lett. 2019, 19, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-G.; Shi, M.; Yu, E.-D.; Xu, Y.-L.; Zhang, Y.-Y.; Geng, X.-L.; Liu, F.; Li, J.-D.; Chen, Z.; Yu, J.; et al. Metal–Organic Framework–Based Delivery Systems as Nanovaccine for Enhancing Immunity against Porcine Circovirus Type 2. Mater. Today Bio 2025, 32, 101712. [Google Scholar] [CrossRef] [PubMed]
- Linnane, E.; Haddad, S.; Melle, F.; Mei, Z.; Fairen-Jimenez, D. The Uptake of Metal–Organic Frameworks: A Journey into the Cell. Chem. Soc. Rev. 2022, 51, 6065–6086. [Google Scholar] [CrossRef]
- Gao, J.; Chu, W.; Ding, X.; Ding, L.; Guo, Q.; Fu, Y. Degradation Kinetic Studies of BSA@ZIF-8 Nanoparticles with Various Zinc Precursors, Metal-to-Ligand Ratios, and pH Conditions. ACS Omega 2023, 8, 44601–44610. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Nadeef, S.; Daristotle, J.L.; Rothwell, W.; Du, B.; Garcia, J.; Zhang, L.; Sarmadi, M.; Forster, T.A.; Menon, N.; et al. Zeolitic Imidazolate Frameworks Activate Endosomal Toll-like Receptors and Potentiate Immunogenicity of SARS-CoV-2 Spike Protein Trimer. Sci. Adv. 2024, 10, eadj6380. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold Nanoparticles for Preparation of Antibodies and Vaccines against Infectious Diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold Nanoparticles as Carriers for A Synthetic Streptococcus pneumoniae Type 14 Conjugate Vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef]
- Rodriguez-Del Rio, E.; Marradi, M.; Calderon-Gonzalez, R.; Frande-Cabanes, E.; Penadés, S.; Petrovsky, N.; Alvarez-Dominguez, C. A Gold Glyco-Nanoparticle Carrying a Listeriolysin O Peptide and Formulated with AdvaxTM Delta Inulin Adjuvant Induces Robust T-Cell Protection against Listeria Infection. Vaccine 2015, 33, 1465–1473. [Google Scholar] [CrossRef]
- Tao, W.; Ziemer, K.S.; Gill, H.S. Gold Nanoparticle–M2E Conjugate Coformulated with Cpg Induces Protective Immunity Against Influenza A Virus. Nanomedicine 2014, 9, 237–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Shi, Y.; Peng, S.; Cai, Y.; Zhan, X.; Song, N.; Liu, Y.; Wang, Z.; Yu, Y.; et al. A New Cancer Immunotherapy via Simultaneous DC-mobilization and DC-targeted IDO Gene Silencing Using an Immune-stimulatory Nanosystem. Int. J. Cancer 2018, 143, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Gulla, S.K.; Kotcherlakota, R.; Nimushakavi, S.; Nimmu, N.V.; Khalid, S.; Patra, C.R.; Chaudhuri, A. Au-CGKRK Nanoconjugates for Combating Cancer through T-Cell-Driven Therapeutic RNA Interference. ACS Omega 2018, 3, 8663–8676. [Google Scholar] [CrossRef] [PubMed]
- Climent, N.; García, I.; Marradi, M.; Chiodo, F.; Miralles, L.; Maleno, M.J.; Gatell, J.M.; García, F.; Penadés, S.; Plana, M. Loading Dendritic Cells with Gold Nanoparticles (GNPs) Bearing HIV-Peptides and Mannosides Enhance HIV-Specific T Cell Responses. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 339–351. [Google Scholar] [CrossRef]
- Farjadian, F.; Moghoofei, M.; Mirkiani, S.; Ghasemi, A.; Rabiee, N.; Hadifar, S.; Beyzavi, A.; Karimi, M.; Hamblin, M.R. Bacterial Components as Naturally Inspired Nano-Carriers for Drug/Gene Delivery and Immunization: Set the Bugs to Work? Biotechnol. Adv. 2018, 36, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gao, Y.; Chen, R.; Zhang, Z.; Li, Q.; Jia, T.; Zhang, T.; Xu, R.; Shi, W.; Chen, L.; et al. Development of a Novel Multi-Epitope Oral DNA Vaccine for Rabies Based on a Food-Borne Microbial Vector. Int. J. Biol. Macromol. 2024, 255, 128085. [Google Scholar] [CrossRef]
- Xie, T.-Q.; Yan, X.; Qin, Y.-T.; Zhang, C.; Jin, X.-K.; Li, Q.-R.; Rao, Z.-Y.; Zhou, H.; Chen, W.-H.; Zhang, X.-Z. Lactate/Cysteine Dual-Consuming Probiotic–Nanomedicine Biohybrid System for Enhanced Cancer Chemo-Immunotherapy. Nano Lett. 2024, 24, 16132–16142. [Google Scholar] [CrossRef]
- He, C.; Yang, J.; Zhao, H.; Liu, M.; Wu, D.; Liu, B.; He, S.; Chen, Z. Vaccination with a Brucella Ghost Developed through a Double Inactivation Strategy Provides Protection in Guinea Pigs and Cattle. Microb. Pathog. 2022, 162, 105363. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Zhang, J.; Xi, L.; Cui, Y.; Zhang, W.; Zhang, J.; Zhang, H. A Safe Non-Toxic Brucella Abortus Ghosts Induce Immune Responses and Confer Protection in BALB/c Mice. Mol. Immunol. 2020, 124, 117–124. [Google Scholar] [CrossRef]
- Ji, S.; Gong, Q.; Zhang, W.; Zheng, J.; Peng, B.; Yang, M. Recombinant Vibrio Parahaemolyticus Ghosts Protect Zebrafish against Infection by Vibrio Species. Fish Shellfish Immunol. 2020, 107, 64–72. [Google Scholar] [CrossRef]
- Hoseini Shahidi, R.; Hashemi Tabar, G.; Bassami, M.R.; Jamshidi, A.; Dehghani, H. The Design and Application of a Bacterial Ghost Vaccine to Evaluate Immune Response and Defense against Avian Pathogenic Escherichia Coli O2:K1 Serotype. Res. Vet. Sci. 2019, 125, 153–161. [Google Scholar] [CrossRef]
- Miri, M.R.; Behzad-Behbahani, A.; Fardaei, M.; Farhadi, A.; Talebkhan, Y.; Mohammadi, M.; Tayebinia, M.; Farokhinejad, F.; Alavi, P.; Fanian, M.; et al. Construction of Bacterial Ghosts for Transfer and Expression of a Chimeric Hepatitis C Virus Gene in Macrophages. J. Microbiol. Methods 2015, 119, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskienė, N.; Pašukonienė, V.; Darinskas, A.; Kraśko, J.A.; Žilionytė, K.; Mlynska, A.; Gudlevičienė, Ž.; Mišeikytė-Kaubrienė, E.; Schijns, V.; Lubitz, W.; et al. Tumor Lysate-Loaded Bacterial Ghosts as a Tool for Optimized Production of Therapeutic Dendritic Cell-Based Cancer Vaccines. Vaccine 2018, 36, 4171–4180. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Li, Q.; Huang, Q.; Qin, Y.; Meng, D.; Liang, J.; Zhang, X. Amplifying Synergistic Effects of Cuproptosis and Bacterial Membrane Vesicles-Mediated Photothermal Therapy by Multifunctional Nano-Biohybrid for Anti-Tumor Immunotherapy. Adv. Funct. Mater. 2025, 35, 2413255. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.; Liu, F.; Wu, P.; Wu, F.; Xiao, H.; Kang, Q.; Li, Z.; Yang, S.; Wu, G.; et al. Bioorthogonal Engineering of Bacterial Outer Membrane Vesicles for NIR-II Fluorescence Imaging-Guided Synergistic Enhanced Immunotherapy. Anal. Chem. 2024, 96, 19585–19596. [Google Scholar] [CrossRef]
- Liu, X.; Sun, M.; Pu, F.; Ren, J.; Qu, X. Transforming Intratumor Bacteria into Immunopotentiators to Reverse Cold Tumors for Enhanced Immuno-Chemodynamic Therapy of Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2023, 145, 26296–26307. [Google Scholar] [CrossRef]
- Jia, X.; Yuan, B.; Wang, W.; Wang, K.; Ling, D.; Wei, M.; Hu, Y.; Guo, W.; Chen, Z.; Du, L.; et al. Gene Editing Tool-Loaded Biomimetic Cationic Vesicles with Highly Efficient Bacterial Internalization for in Vivo Eradication of Pathogens. J. Nanobiotechnol. 2024, 22, 787. [Google Scholar] [CrossRef]
- Heap, J.T.; Theys, J.; Ehsaan, M.; Kubiak, A.M.; Dubois, L.; Paesmans, K.; Van Mellaert, L.; Knox, R.; Kuehne, S.A.; Lambin, P.; et al. Spores of Clostridium Engineered for Clinical Efficacy and Safety Cause Regression and Cure of Tumors in Vivo. Oncotarget 2014, 5, 1761–1769. [Google Scholar] [CrossRef]
- Mauriello, E.M.F.; Duc, L.H.; Isticato, R.; Cangiano, G.; Hong, H.A.; Felice, M.D.; Ricca, E.; Cutting, S.M. Display of Heterologous Antigens on the Bacillus Subtilis Spore Coat Using CotC as a Fusion Partner. Vaccine 2004, 22, 1177–1187. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Sun, J.; Gu, J.; Xu, X.; Cai, X. Surface Display of the COE Antigen of Porcine Epidemic Diarrhoea Virus on Bacillus Subtilis Spores. Microb. Biotechnol. 2024, 17, e14518. [Google Scholar] [CrossRef]
- Min, H.; Cho, H.-S.; Lee, H.-S.; Park, Y.-T.; Lee, H.-J.; Park, H.-S. Oral Bacillus Subtilis Spores-Based Vaccine for Mass Vaccination against Porcine Reproductive and Respiratory Syndrome. Sci. Rep. 2024, 14, 27742. [Google Scholar] [CrossRef]
- Sibley, L.; Reljic, R.; Radford, D.S.; Huang, J.-M.; Hong, H.A.; Cranenburgh, R.M.; Cutting, S.M. Recombinant Bacillus Subtilis Spores Expressing MPT64 Evaluated as a Vaccine against Tuberculosis in the Murine Model. FEMS Microbiol. Lett. 2014, 358, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Thomas, T.; Garnica, O.; Dhandayuthapani, S. Recombinant Bacillus Subtilis Spores for the Delivery of Mycobacterium Tuberculosis Ag85B-CFP10 Secretory Antigens. Tuberculosis 2016, 101, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Huh, H.; Wong, S.; Jean, J.S.; Slavcev, R. Bacteriophage Interactions with Mammalian Tissue: Therapeutic Applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef]
- Mohammad Hasani, S.; Ghafouri, E.; Kouhpayeh, S.; Amerizadeh, F.; Rahimmanesh, I.; Amirkhani, Z.; Khanahmad, H. Phage Based Vaccine: A Novel Strategy in Prevention and Treatment. Heliyon 2023, 9, e19925. [Google Scholar] [CrossRef] [PubMed]
- Emencheta, S.C.; Onugwu, A.L.; Kalu, C.F.; Ezinkwo, P.N.; Eze, O.C.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A.; Onuigbo, E.B. Bacteriophages as Nanocarriers for Targeted Drug Delivery and Enhanced Therapeutic Effects. Mater. Adv. 2024, 5, 986–1016. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, Y.; Chen, Y.; Zhang, W.; Liu, T.; Chen, G.; Wang, K. Phage-Based Delivery Systems: Engineering, Applications, and Challenges in Nanomedicines. J. Nanobiotechnol. 2024, 22, 365. [Google Scholar] [CrossRef]
- Islam, M.S.; Fan, J.; Pan, F. The Power of Phages: Revolutionizing Cancer Treatment. Front. Oncol. 2023, 13, 1290296. [Google Scholar] [CrossRef]
- Ul Haq, I.; Krukiewicz, K.; Yahya, G.; Haq, M.U.; Maryam, S.; Mosbah, R.A.; Saber, S.; Alrouji, M. The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine. Int. J. Mol. Sci. 2023, 24, 1536. [Google Scholar] [CrossRef]
- Aksyuk, A.A.; Rossmann, M.G. Bacteriophage Assembly. Viruses 2011, 3, 172–203. [Google Scholar] [CrossRef]
- Palma, M. Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef]
- Hayes, S. Bacterial Virus Lambda Gpd-Fusions to Cathelicidins, α- and β-Defensins, and Disease-Specific Epitopes Evaluated for Antimicrobial Toxicity and Ability to Support Phage Display. Viruses 2019, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.T.; Cragnolini, J.J.; Popp, M.W.; Allen, M.A.; Dougan, S.K.; Spooner, E.; Ploegh, H.L.; Belcher, A.M.; Guimaraes, C.P. M13 Bacteriophage Display Framework That Allows Sortase-Mediated Modification of Surface-Accessible Phage Proteins. Bioconjugate Chem. 2012, 23, 1478–1487. [Google Scholar] [CrossRef]
- Lander, G.C.; Evilevitch, A.; Jeembaeva, M.; Potter, C.S.; Carragher, B.; Johnson, J.E. Bacteriophage Lambda Stabilization by Auxiliary Protein gpD: Timing, Location, and Mechanism of Attachment Determined by Cryo-EM. Structure 2008, 16, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.B.; Black, L.W. Structure and Assembly of Bacteriophage T4 Head. Virol. J. 2010, 7, 356. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; Rao, M.; Maclean, D.M.; Birx, D.L.; Alving, C.R.; Rao, V.B. Assembly of Human Immunodeficiency Virus (HIV) Antigens on Bacteriophage T4: A Novel In Vitro Approach To Construct Multicomponent HIV Vaccines. J. Virol. 2006, 80, 7688–7698. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tu, C.; Yu, X.; Zhang, M.; Zhang, N.; Zhao, M.; Nie, W.; Ren, Z. Bacteriophage T4 Nanoparticle Capsid Surface SOC and HOC Bipartite Display with Enhanced Classical Swine Fever Virus Immunogenicity: A Powerful Immunological Approach. J. Virol. Methods 2007, 139, 50–60. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Kirtley, M.L.; Van Lier, C.J.; Sha, J.; Yeager, L.A.; Chopra, A.K.; Rao, V.B. Mutated and Bacteriophage T4 Nanoparticle Arrayed F1-V Immunogens from Yersinia Pestis as Next Generation Plague Vaccines. PLoS Pathog. 2013, 9, e1003495. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Sha, J.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; Rao, V.B. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio 2018, 9, 01926-18. [Google Scholar] [CrossRef]
- Ooi, V.Y.; Yeh, T.-Y. Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 9938. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chang, Y.-C.; Hu, C.-W.; Kao, C.-Y.; Yu, Y.-A.; Lim, S.-K.; Mou, K.Y. Development of a Novel Cytokine Vehicle Using Filamentous Phage Display for Colorectal Cancer Treatment. ACS Synth. Biol. 2021, 10, 2087–2095. [Google Scholar] [CrossRef]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef]
- Yata, T.; Lee, K.-Y.; Dharakul, T.; Songsivilai, S.; Bismarck, A.; Mintz, P.J.; Hajitou, A. Hybrid Nanomaterial Complexes for Advanced Phage-Guided Gene Delivery. Mol. Ther.—Nucleic Acids 2014, 3, e185. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 Bacteriophage-Based Vaccine Platform for Personalized Cancer Immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef] [PubMed]
- Davenport, B.J.; Catala, A.; Weston, S.M.; Johnson, R.M.; Ardanuy, J.; Hammond, H.L.; Dillen, C.; Frieman, M.B.; Catalano, C.E.; Morrison, T.E. Phage-like Particle Vaccines Are Highly Immunogenic and Protect against Pathogenic Coronavirus Infection and Disease. npj Vaccines 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahrani, M.; Asavarut, P.; Waramit, S.; Suwan, K.; Hajitou, A. Transmorphic Phage-guided Systemic Delivery of TNFα Gene for the Treatment of Human Pediatric Medulloblastoma. FASEB J. 2023, 37, e23038. [Google Scholar] [CrossRef]
- Przystal, J.M.; Umukoro, E.; Stoneham, C.A.; Yata, T.; O’Neill, K.; Syed, N.; Hajitou, A. Proteasome Inhibition in Cancer Is Associated with Enhanced Tumor Targeting by the Adeno-associated Virus/Phage. Mol. Oncol. 2013, 7, 55–66. [Google Scholar] [CrossRef]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-Associated Virus (AAV) Vectors in Cancer Gene Therapy. J. Control. Release 2016, 240, 287–301. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J. A Novel Delivery Platform Based on Bacteriophage MS2 Virus-like Particles. Virus Res. 2016, 211, 9–16. [Google Scholar] [CrossRef]
- Kim, K.; Cha, J.; Jang, E.; Klumpp, J.; Hagens, S.; Hardt, W.; Lee, K.; Loessner, M.J. PEGylation of Bacteriophages Increases Blood Circulation Time and Reduces T-helper Type 1 Immune Response. Microb. Biotechnol. 2008, 1, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Batra, H.; Ananthaswamy, N.; Mahalingam, M.; Tao, P.; Wu, X.; Guo, W.; Fokine, A.; Rao, V.B. Design of Bacteriophage T4-Based Artificial Viral Vectors for Human Genome Remodeling. Nat. Commun. 2023, 14, 2928. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage Therapy: What Factors Shape Phage Pharmacokinetics and Bioavailability? Systematic and Critical Review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Edwards, D.C.; Brand, C.; Heath, T. FORMATION OF VIROSOMES FROM INFLUENZA SUBUNITS AND LIPOSOMES. Lancet 1975, 306, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Akbar, M.; Iqbal, B.; Ali, F.; Sharma, N.K.; Kumar, N.; Najmi, A.; Albratty, M.; Alhazmi, H.A.; Madkhali, O.A.; et al. Virosome: An Engineered Virus for Vaccine Delivery. Saudi Pharm. J. 2023, 31, 752–764. [Google Scholar] [CrossRef]
- Bovier, P.A. Epaxal®: A Virosomal Vaccine to Prevent Hepatitis A Infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- Mischler, R.; Metcalfe, I.C. Inflexal® V a Trivalent Virosome Subunit Influenza Vaccine: Production. Vaccine 2002, 20, B17–B23. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Wang, L.; Cao, Q.; Kim, J.-H. Nanovaccines: An Effe ctive Therapeutic Approach for Cancer Therapy. Biomed. Pharmacother. 2024, 170, 115992. [Google Scholar] [CrossRef]
- Saga, K.; Kaneda, Y. Virosome Presents Multimodel Cancer Therapy without Viral Replication. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Adamiak, N.; Krawczyk, K.T.; Locht, C.; Kowalewicz-Kulbat, M. Archaeosomes and Gas Vesicles as Tools for Vaccine Development. Front. Immunol. 2021, 12, 746235. [Google Scholar] [CrossRef]
- Krishnan, L.; Sprott, G.D. Archaeosome Adjuvants: Immunological Capabilities and Mechanism(s) of Action. Vaccine 2008, 26, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Deschatelets, L.; Krishnan, L. Sulfated Archaeal Glycolipid Archaeosomes as a Safe and Effective Vaccine Adjuvant for Induction of Cell-Mediated Immunity. Hum. Vaccines Immunother. 2017, 13, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, I.; Kornmueller, K.; Fiedler, D.; Khinast, J.; Fröhlich, E.; Leitinger, G.; Horn, C.; Quehenberger, J.; Spadiut, O.; Prassl, R. Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations. Pharmaceutics 2024, 16, 694. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, L.; Li, K.; Lou, B.; Liu, Y.; Liu, Z. Yeast as Carrier for Drug Delivery and Vaccine Construction. J. Control. Release 2022, 346, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E. Yeasts in Nanotechnology-Enabled Oral Vaccine and Gene Delivery. Bioengineered 2021, 12, 8325–8335. [Google Scholar] [CrossRef]
- Bernardi, B.; Wendland, J. Homologous Recombination: A GRAS Yeast Genome Editing Tool. Fermentation 2020, 6, 57. [Google Scholar] [CrossRef]
- Çelik, E.; Çalık, P. Production of Recombinant Proteins by Yeast Cells. Biotechnol. Adv. 2012, 30, 1108–1118. [Google Scholar] [CrossRef]
- Silva, A.J.D.; De Sousa, M.M.G.; De Macêdo, L.S.; De França Neto, P.L.; De Moura, I.A.; Espinoza, B.C.F.; Invenção, M.D.C.V.; De Pinho, S.S.; Da Gama, M.A.T.M.; De Freitas, A.C. RNA Vaccines: Yeast as a Novel Antigen Vehicle. Vaccines 2023, 11, 1334. [Google Scholar] [CrossRef]
- Walch, B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Delivery of Functional DNA and Messenger RNA to Mammalian Phagocytic Cells by Recombinant Yeast. Gene Ther. 2012, 19, 237–245. [Google Scholar] [CrossRef]
- Cabib, E.; Arroyo, J. How Carbohydrates Sculpt Cells: Chemical Control of Morphogenesis in the Yeast Cell Wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef]
- Angrand, G.; Quillévéré, A.; Loaëc, N.; Daskalogianni, C.; Granzhan, A.; Teulade-Fichou, M.-P.; Fahraeus, R.; Prado Martins, R.; Blondel, M. Sneaking Out for Happy Hour: Yeast-Based Approaches to Explore and Modulate Immune Response and Immune Evasion. Genes 2019, 10, 667. [Google Scholar] [CrossRef]
- De Smet, R.; Allais, L.; Cuvelier, C.A. Recent Advances in Oral Vaccine Development: Yeast-Derived β-Glucan Particles. Hum. Vaccines Immunother. 2014, 10, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, Preparation, Modification, and Bioactivities of β-Glucan and Mannan from Yeast Cell Wall: A Review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Steger, M.; Bermejo-Jambrina, M.; Yordanov, T.; Wagener, J.; Brakhage, A.A.; Pittl, V.; Huber, L.A.; Haas, H.; Lass-Flörl, C.; Posch, W.; et al. β-1,3-Glucan-Lacking Aspergillus Fumigatus Mediates an Efficient Antifungal Immune Response by Activating Complement and Dendritic Cells. Virulence 2019, 10, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, W.; Gu, X.; Chang, C.; Wu, J. Repression of Deoxynivalenol-Triggered Cytotoxicity and Apoptosis by Mannan/β-Glucans from Yeast Cell Wall: Involvement of Autophagy and PI3K-AKT-mTOR Signaling Pathway. Int. J. Biol. Macromol. 2020, 164, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.Ø.; Lagos, L.; Lei, P.; Reveco-Urzua, F.E.; Morales-Lange, B.; Hansen, L.D.; Schiavone, M.; Mydland, L.T.; Arntzen, M.Ø.; Mercado, L.; et al. Down-Stream Processing of Baker’s Yeast (Saccharomyces Cerevisiae)—Effect on Nutrient Digestibility and Immune Response in Atlantic Salmon (Salmo Salar). Aquaculture 2021, 530, 735707. [Google Scholar] [CrossRef]
- Roohvand, F.; Shokri, M.; Abdollahpour-Alitappeh, M.; Ehsani, P. Biomedical Applications of Yeast- a Patent View, Part One: Yeasts as Workhorses for the Production of Therapeutics and Vaccines. Expert Opin. Ther. Pat. 2017, 27, 929–951. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Wang, L.; Shao, S.; Chen, Z.; Zhang, Z. In Vivo Targeted Delivery of CD40 shRNA to Mouse Intestinal Dendritic Cells by Oral Administration of Recombinant Sacchromyces Cerevisiae. Gene Ther. 2014, 21, 709–714. [Google Scholar] [CrossRef]
- Kumar, R. Investigating the Long-Term Stability of Protein Immunogen(s) for Whole Recombinant Yeast-Based Vaccines. FEMS Yeast Res. 2018, 18, foy071. [Google Scholar] [CrossRef]
- Jacob, D.; Ruffie, C.; Dubois, M.; Combredet, C.; Amino, R.; Formaglio, P.; Gorgette, O.; Pehau-Arnaudet, G.; Guery, C.; Puijalon, O.; et al. Whole Pichia Pastoris Yeast Expressing Measles Virus Nucleoprotein as a Production and Delivery System to Multimerize Plasmodium Antigens. PLoS ONE 2014, 9, e86658. [Google Scholar] [CrossRef][Green Version]
- Xv, Z.; Lv, J.; Jiang, J.; Wang, W.; Feng, F.; Zhang, L.; Xue, X.; Li, W. Effective Neutralizing Antibody Produced in Mice Directly Immunized with Integrated Pichia Pastoris Expressing HPV16L1 Protein. Viral Immunol. 2019, 32, 308–317. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Brooks, S.M.; Nguyen, A.W.; Lin, W.-L.; Johnston, T.G.; Maynard, J.A.; Nelson, A.; Alper, H.S. Bioproduced Proteins On Demand (Bio-POD) in Hydrogels Using Pichia Pastoris. Bioact. Mater. 2021, 6, 2390–2399. [Google Scholar] [CrossRef]
- Grover, A.; McLean, J.L.; Troudt, J.M.; Foster, C.; Izzo, L.; Creissen, E.; MacDonald, E.; Troy, A.; Izzo, A.A. Heat Killed Saccharomyces Cerevisiae as an Adjuvant for the Induction of Vaccine-Mediated Immunity against Infection with Mycobacterium Tuberculosis. Vaccine 2016, 34, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Thera, M.A.; Coulibaly, D.; Kone, A.K.; Guindo, A.B.; Traore, K.; Sall, A.H.; Diarra, I.; Daou, M.; Traore, I.M.; Tolo, Y.; et al. Phase 1 Randomized Controlled Trial to Evaluate the Safety and Immunogenicity of Recombinant Pichia Pastoris-Expressed Plasmodium Falciparum Apical Membrane Antigen 1 (PfAMA1-FVO (25-545)) in Healthy Malian Adults in Bandiagara. Malar. J. 2016, 15, 442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Assis-Marques, M.A.; Oliveira, A.F.; Ruas, L.P.; Reis, T.F.D.; Roque-Barreira, M.C.; Coelho, P.S.R. Saccharomyces Cerevisiae Expressing Gp43 Protects Mice against Paracoccidioides Brasiliensis Infection. PLoS ONE 2015, 10, e0120201. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Kolakowski, J.; Holder, A.; Pfuhl, M.; Ngugi, D.; Ballingall, K.; Tombacz, K.; Werling, D. Development of a Potential Yeast-Based Vaccine Platform for Theileria Parva Infection in Cattle. Front. Immunol. 2021, 12, 674484. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Lu, X.; Li, S.; Ren, Y. High Immune Efficacy against Different Avian Influenza H5N1 Viruses Due to Oral Administration of a Saccharomyces Cerevisiae-Based Vaccine in Chickens. Sci. Rep. 2021, 11, 8977. [Google Scholar] [CrossRef]
- Goyal, G.; Tsai, S.-L.; Madan, B.; DaSilva, N.A.; Chen, W. Simultaneous Cell Growth and Ethanol Production from Cellulose by an Engineered Yeast Consortium Displaying a Functional Mini-Cellulosome. Microb. Cell. Fact. 2011, 10, 89. [Google Scholar] [CrossRef]
- Marshall, C.J.; Agarwal, N.; Kalia, J.; Grosskopf, V.A.; McGrath, N.A.; Abbott, N.L.; Raines, R.T.; Shusta, E.V. Facile Chemical Functionalization of Proteins through Intein-Linked Yeast Display. Bioconjugate Chem. 2013, 24, 1634–1644. [Google Scholar] [CrossRef][Green Version]
- Pinho, S.S.D.; Invenção, M.D.C.V.; Silva, A.J.D.; Macêdo, L.S.D.; Espinoza, B.C.F.; Leal, L.R.S.; Da Gama, M.A.T.M.; Moura, I.A.D.; Silva, M.E.D.S.; Souza, D.V.S.D.; et al. Pichia Pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines. Vaccines 2024, 12, 1428. [Google Scholar] [CrossRef]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-d-Glucan-Based Drug Delivery System and Its Potential Application in Targeting Tumor Associated Macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-Glucan Particles as Vaccine Adjuvant Carriers. In Vaccines for Invasive Fungal Infections; Kalkum, M., Semis, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1625, pp. 143–157. ISBN 978-1-4939-7103-9. [Google Scholar]
- Soto, E.R.; Specht, C.A.; Rus, F.; Lee, C.K.; Abraham, A.; Levitz, S.M.; Ostroff, G.R. An Efficient (Nano) Silica—In Glucan Particles Protein Encapsulation Approach for Improved Thermal Stability. J. Control. Release 2023, 357, 175–184. [Google Scholar] [CrossRef]
- Soto, E.R.; Specht, C.A.; Lee, C.K.; Levitz, S.M.; Ostroff, G.R. One Step Purification—Vaccine Delivery System. Pharmaceutics 2023, 15, 1390. [Google Scholar] [CrossRef]
- Liu, D.; Lu, S.; Zhang, L.; Zhang, L.; Ji, M.; Liu, X.-G.; Yu, Z.; Liu, R. A Biomimetic Yeast Shell Vaccine Coated with Layered Double Hydroxides Induces a Robust Humoral and Cellular Immune Response against Tumors. Nanoscale Adv. 2020, 2, 3494–3506. [Google Scholar] [CrossRef]
- Cohen, J.L.; Shen, Y.; Aouadi, M.; Vangala, P.; Tencerova, M.; Amano, S.U.; Nicoloro, S.M.; Yawe, J.C.; Czech, M.P. Peptide- and Amine-Modified Glucan Particles for the Delivery of Therapeutic siRNA. Mol. Pharm. 2016, 13, 964–978. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally Delivered siRNA Targeting Macrophage Map4k4 Suppresses Systemic Inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Feng, M.; Zhang, W.; Li, Y. Yeast Microcapsule-Mediated Oral Delivery of IL-1β shRNA for Post-Traumatic Osteoarthritis Therapy. Mol. Ther.—Nucleic Acids 2021, 23, 336–346. [Google Scholar] [CrossRef]
- Cheng, T.; Yan, T.; Wu, J.; Wang, Q.; Zhang, H. Yeast β-D-Glucan Functionalized Graphene Oxide for Macrophage-Targeted Delivery of CpG Oligodeoxynucleotides and Synergistically Enhanced Antitumor Immunity. Int. J. Biol. Macromol. 2023, 234, 123432. [Google Scholar] [CrossRef]
- Sabu, C.; Raghav, D.; Jijith, U.S.; Mufeedha, P.; Naseef, P.P.; Rathinasamy, K.; Pramod, K. Bioinspired Oral Insulin Delivery System Using Yeast Microcapsules. Mater. Sci. Eng. C 2019, 103, 109753. [Google Scholar] [CrossRef]
- Wang, M.; Yang, R.; Zhang, L.; Meng, X.; Fei, C.; Zhang, K.; Wang, X.; Zheng, W.; Xiao, S.; Zhang, S.; et al. Sulfated Glucan Can Improve the Immune Efficacy of Newcastle Disease Vaccine in Chicken. Int. J. Biol. Macromol. 2014, 70, 193–198. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.H. Vaccine Delivery Systems and Administration Routes: Advanced Biotechnological Techniques to Improve the Immunization Efficacy. Vaccine X 2024, 19, 100500. [Google Scholar] [CrossRef]
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Karlsson Hedestam, G.B.; Wyatt, R.T.; et al. Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell. Rep. 2020, 30, 3964–3971. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Rodriguez Pozo, A.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A.; et al. Vaccine Inoculation Route Modulates Early Immunity and Consequently Antigen-Specific Immune Response. Front. Immunol. 2021, 12, 645210. [Google Scholar] [CrossRef]
- Beirne, P.V.; Hennessy, S.; Cadogan, S.L.; Shiely, F.; Fitzgerald, T.; MacLeod, F. Needle Size for Vaccination Procedures in Children and Adolescents. Cochrane Database Syst. Rev. 2018, 8, CD010720. [Google Scholar] [CrossRef]
- Cook, I.F. Best Vaccination Practice and Medically Attended Injection Site Events Following Deltoid Intramuscular Injection. Hum. Vaccin. Immunother. 2015, 11, 1184–1191. [Google Scholar] [CrossRef]
- Davenne, T.; McShane, H. Why Don’t We Have an Effective Tuberculosis Vaccine Yet? Expert Rev. Vaccines 2016, 15, 1009–1013. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst. Rev. 2021, 2021, CD004407. [Google Scholar] [CrossRef]
- Cook, I.F. Subcutaneous Vaccine Administration—An Outmoded Practice. Hum. Vaccin. Immunother. 2021, 17, 1329–1341. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The How’s and What’s of Vaccine Reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting Cells in the Skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef]
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving Microneedles: Applications and Growing Therapeutic Potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Chandbadshah, S.B.; Mannayee, G. Structural Analysis and Simulation of Solid Microneedle Array for Vaccine Delivery Applications. Mater. Today Proc. 2022, 65, 3774–3779. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, J.; Seok, J.H.; Park, H.; Cha, H.-R.; Ko, S.H.; Lee, J.M.; Park, M.-S.; Park, J.-H. Development of the H3N2 Influenza Microneedle Vaccine for Cross-Protection against Antigenic Variants. Sci. Rep. 2022, 12, 12189. [Google Scholar] [CrossRef]
- Leboux, R.J.T.; Schipper, P.; van Capel, T.M.M.; Kong, L.; van der Maaden, K.; Kros, A.; Jiskoot, W.; de Jong, E.C.; Bouwstra, J.A. Antigen Uptake After Intradermal Microinjection Depends on Antigen Nature and Formulation, but Not on Injection Depth. Front. Allergy 2021, 2, 642788. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal Delivery of Vaccine Nanoparticles Using Hollow Microneedle Array Generates Enhanced and Balanced Immune Response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Zuo, W.; Li, J.; Jiang, W.; Zhang, M.; Ma, Y.; Gu, Q.; Wang, X.; Cai, L.; Shi, L.; Sun, M. Dose-Sparing Intradermal DTaP-sIPV Immunization with a Hollow Microneedle Leads to Superior Immune Responses. Front. Microbiol. 2021, 12, 757375. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Hickling, J.; Jayashi Flores, C.M.; Griffin, P.; Anderson, C.D.; Skinner, S.R.; Davies, C.; Witham, K.; Pryor, M.; Bodle, J.; et al. Safety, Tolerability, Acceptability and Immunogenicity of an Influenza Vaccine Delivered to Human Skin by a Novel High-Density Microprojection Array Patch (NanopatchTM). Vaccine 2018, 36, 3779–3788. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Martin, M.D.; Zarnitsyn, V.G.; Sullivan, S.P.; Compans, R.W.; Prausnitz, M.R.; Skountzou, I. Transdermal Influenza Immunization with Vaccine-Coated Microneedle Arrays. PLoS ONE 2009, 4, e4773. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Henricson, J.; Baker, S.B.; Togö, T.; Jayashi Flores, C.M.; Lemaire, P.A.; Forster, A.; Anderson, C.D. Innate Local Response and Tissue Recovery Following Application of High Density Microarray Patches to Human Skin. Sci. Rep. 2020, 10, 18468. [Google Scholar] [CrossRef]
- Leone, M.; Priester, M.I.; Romeijn, S.; Nejadnik, M.R.; Mönkäre, J.; O’Mahony, C.; Jiskoot, W.; Kersten, G.; Bouwstra, J.A. Hyaluronan-Based Dissolving Microneedles with High Antigen Content for Intradermal Vaccination: Formulation, Physicochemical Characterization and Immunogenicity Assessment. Eur. J. Pharm. Biopharm. 2019, 134, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The Safety, Immunogenicity, and Acceptability of Inactivated Influenza Vaccine Delivered by Microneedle Patch (TIV-MNP 2015): A Randomised, Partly Blinded, Placebo-Controlled, Phase 1 Trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Wang, Y.; Zhang, C.; He, P.; Lin, Z.; Hu, Z.; Lu, W. Liposome-Loaded Dissolvable Microneedle Patches for More Efficient Intradermal Antigen Delivery of Hepatitis B Vaccine. Int. J. Pharm. 2025, 669, 125023. [Google Scholar] [CrossRef]
- Al-Kasasbeh, R.; Brady, A.J.; Courtenay, A.J.; Larrañeta, E.; McCrudden, M.T.C.; O’Kane, D.; Liggett, S.; Donnelly, R.F. Evaluation of the Clinical Impact of Repeat Application of Hydrogel-Forming Microneedle Array Patches. Drug Deliv. Transl. Res. 2020, 10, 690–705. [Google Scholar] [CrossRef]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019, 16, 118–127. [Google Scholar] [CrossRef]
- Fu, W.; Li, Q.; Sheng, J.; Wu, H.; Ma, M.; Zhang, Y. Whole-Cell Vaccine Preparation Through Prussian Blue Nanoparticles-Elicited Immunogenic Cell Death and Loading in Gel Microneedles Patches. Gels 2024, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, J.; Xiong, C.; Chen, B.; Xie, K.; Wang, M.; Liu, Z.; Yue, Z.; Liang, Z.; Wang, F.; et al. Transdermal Microarrayed Electroporation for Enhanced Cancer Immunotherapy Based on DNA Vaccination. Proc. Natl. Acad. Sci. USA 2024, 121, e2322264121. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.H.; Laurent, P.E. Intradermal Vaccine Delivery: Will New Delivery Systems Transform Vaccine Administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef]
- Moodie, Z.; Walsh, S.R.; Laher, F.; Maganga, L.; Herce, M.E.; Naidoo, S.; Hosseinipour, M.C.; Innes, C.; Bekker, L.-G.; Grunenberg, N.; et al. Antibody and Cellular Responses to HIV Vaccine Regimens with DNA Plasmid as Compared with ALVAC Priming: An Analysis of Two Randomized Controlled Trials. PLoS Med. 2020, 17, e1003117. [Google Scholar] [CrossRef]
- Paulsen, G.C.; Frenck, R.; Tomashek, K.M.; Alarcon, R.M.; Hensel, E.; Lowe, A.; Brocato, R.L.; Kwilas, S.A.; Josleyn, M.D.; Hooper, J.W. Safety and Immunogenicity of an Andes Virus DNA Vaccine by Needle-Free Injection: A Randomized, Controlled Phase 1 Study. J. Infect. Dis. 2024, 229, 30–38. [Google Scholar] [CrossRef]
- Peng, S.; Tu, H.-F.; Cheng, M.; Hu, M.-H.; Tsai, H.-L.; Tsai, Y.-C.; Koenig, C.; Brayton, C.; Wang, H.; Chang, Y.-N.; et al. Immune Responses, Therapeutic Anti-Tumor Effects, and Tolerability upon Therapeutic HPV16/18 E6/E7 DNA Vaccination via Needle-Free Biojector. mBio 2023, 14, e0212123. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Mach, O.; Sein, C.; Molodecky, N.; Jarrahian, C.; Saganic, L.; Zehrung, D.; Fonseca, M.; Diaz, M.; et al. Needle-Free Jet Injector Intradermal Delivery of Fractional Dose Inactivated Poliovirus Vaccine: Association between Injection Quality and Immunogenicity. Vaccine 2015, 33, 5873–5877. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, E.F.; Josleyn, M.; Ullman, D.; Fisher, D.; Dalrymple, L.; Sellers-Myers, K.; Loudon, P.; Rusnak, J.; Rivard, R.; Schmaljohn, C.; et al. A Phase 1 Clinical Trial of Hantaan Virus and Puumala Virus M-Segment DNA Vaccines for Hemorrhagic Fever with Renal Syndrome. Vaccine 2012, 30, 1951–1958. [Google Scholar] [CrossRef]
- Ginsberg, B.A.; Gallardo, H.F.; Rasalan, T.S.; Adamow, M.; Mu, Z.; Tandon, S.; Bewkes, B.B.; Roman, R.-A.; Chapman, P.B.; Schwartz, G.K.; et al. Immunologic Response to Xenogeneic Gp100 DNA in Melanoma Patients: Comparison of Particle-Mediated Epidermal Delivery with Intramuscular Injection. Clin. Cancer Res. 2010, 16, 4057–4065. [Google Scholar] [CrossRef]
- Martinez-Perez, A.G.; Garza-Morales, R.; Loera-Arias, M.D.; Villa-Cedillo, S.A.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Flores-Maldonado, O.E.; Valdes, J.; Perez-Trujillo, J.J.; Saucedo-Cardenas, O. Long-Term Antigen-Specific Immune Response by an Oncolytic Adenovirus Encoding SP-SA-E7-4-1BBL in HPV-16 Cancer Model. Mol. Biol. Rep. 2024, 51, 408. [Google Scholar] [CrossRef]
- Dewitte, H.; Van Lint, S.; Heirman, C.; Thielemans, K.; De Smedt, S.C.; Breckpot, K.; Lentacker, I. The Potential of Antigen and TriMix Sonoporation Using mRNA-Loaded Microbubbles for Ultrasound-Triggered Cancer Immunotherapy. J. Control. Release 2014, 194, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Weng, W.; Chen, M.; Huang, H.; Chen, X.; Peng, Y.; Hu, Y. Improving DNA Vaccination Performance through a New Microbubble Design and an Optimized Sonoporation Protocol. Ultrason. Sonochem. 2023, 101, 106685. [Google Scholar] [CrossRef]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic Potential of DNA Vaccine Candidate, ZyCoV-D against SARS-CoV-2 in Animal Models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, Safety, and Immunogenicity of the DNA SARS-CoV-2 Vaccine (ZyCoV-D): The Interim Efficacy Results of a Phase 3, Randomised, Double-Blind, Placebo-Controlled Study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Yadav, P.D.; Kumar, S.; Agarwal, K.; Jain, M.; Patil, D.R.; Maithal, K.; Mathapati, B.; Giri, S.; Mohandas, S.; Shete, A.; et al. Needle-Free Injection System Delivery of ZyCoV-D DNA Vaccine Demonstrated Improved Immunogenicity and Protective Efficacy in Rhesus Macaques against SARS-CoV-2. J. Med. Virol. 2023, 95, e28484. [Google Scholar] [CrossRef]
- Generotti, A.; Contreras, R.; Zounes, B.; Schade, E.; Kemme, A.; Rane, Y.; Liu, X.; Elwood, D.; Schultheis, K.; Marston, J.; et al. Intradermal DNA Vaccine Delivery Using Vacuum-Controlled, Needle-Free Electroporation. Mol. Ther. Nucleic Acids 2023, 34, 102070. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, H.; Yang, X.; Cheng, T.; Yuan, L.; Xia, N. Novel Vaccine Strategies to Induce Respiratory Mucosal Immunity: Advances and Implications. MedComm 2025, 6, e70056. [Google Scholar] [CrossRef] [PubMed]
- Buddy Creech, C.; Jimenez-Truque, N.; Kown, N.; Sokolow, K.; Brady, E.J.; Yoder, S.; Solovay, K.; Rubin, K.; Noviello, S.; Hensel, E.; et al. Safety and Immunogenicity of Live, Attenuated Intranasal Bordetella Pertussis Vaccine (BPZE1) in Healthy Adults. Vaccine 2022, 40, 6740–6746. [Google Scholar] [CrossRef] [PubMed]
- Eiden, J.; Fierro, C.; Schwartz, H.; Adams, M.; Ellis, K.J.; Aitchison, R.; Herber, R.; Hatta, Y.; Marshall, D.; Moser, M.J.; et al. Intranasal M2SR (M2-Deficient Single Replication) H3N2 Influenza Vaccine Provides Enhanced Mucosal and Serum Antibodies in Adults. J. Infect. Dis. 2022, 227, 103–112. [Google Scholar] [CrossRef]
- Eiden, J.; Fierro, C.; White, A.; Davis, M.; Rhee, M.; Turner, M.; Murray, B.; Herber, R.; Aitchison, R.; Marshall, D.; et al. Safety and Immunogenicity of the Intranasal H3N2 M2-Deficient Single-Replication Influenza Vaccine Alone or Coadministered with an Inactivated Influenza Vaccine (Fluzone High-Dose Quadrivalent) in Adults Aged 65-85 Years in the USA: A Multicentre, Randomised, Double-Blind, Double-Dummy, Phase 1b Trial. Lancet Infect. Dis. 2024, 24, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Sande, C.J.; Scarselli, E.; Capone, S.; Vitelli, A.; Nicosia, A.; Silva-Reyes, L.; Thompson, A.J.; de Lara, C.M.; Taylor, K.S.; et al. Novel Genetically-Modified Chimpanzee Adenovirus and MVA-Vectored Respiratory Syncytial Virus Vaccine Safely Boosts Humoral and Cellular Immunity in Healthy Older Adults. J. Infect. 2019, 78, 382–392. [Google Scholar] [CrossRef]
- Jackson, L.A.; Holmes, S.J.; Mendelman, P.M.; Huggins, L.; Cho, I.; Rhorer, J. Safety of a Trivalent Live Attenuated Intranasal Influenza Vaccine, FluMist, Administered in Addition to Parenteral Trivalent Inactivated Influenza Vaccine to Seniors with Chronic Medical Conditions. Vaccine 1999, 17, 1905–1909. [Google Scholar] [CrossRef]
- Lambkin-Williams, R.; Gelder, C.; Broughton, R.; Mallett, C.P.; Gilbert, A.S.; Mann, A.; He, D.; Oxford, J.S.; Burt, D. An Intranasal Proteosome-Adjuvanted Trivalent Influenza Vaccine Is Safe, Immunogenic & Efficacious in the Human Viral Influenza Challenge Model. Serum IgG & Mucosal IgA Are Important Correlates of Protection against Illness Associated with Infection. PLoS ONE 2016, 11, e0163089. [Google Scholar] [CrossRef]
- Canelli, E.; Ferrari, L.; Borghetti, P.; Candela, F.; Abiakam, N.S.; Bianchera, A.; Buttini, F.; Magi, G.E.; Sonvico, F.; Martelli, P.; et al. Nano-Adjuvanted Dry Powder Vaccine for the Mucosal Immunization against Airways Pathogens. Front. Vet. Sci. 2023, 10, 1116722. [Google Scholar] [CrossRef]
- Gomez, M.; Ahmed, M.; Das, S.; McCollum, J.; Mellett, L.; Swanson, R.; Gupta, A.; Carrigy, N.B.; Wang, H.; Barona, D.; et al. Development and Testing of a Spray-Dried Tuberculosis Vaccine Candidate in a Mouse Model. Front. Pharmacol. 2021, 12, 799034. [Google Scholar] [CrossRef]
- Thakkar, S.G.; Warnken, Z.N.; Alzhrani, R.F.; Valdes, S.A.; Aldayel, A.M.; Xu, H.; Williams, R.O.; Cui, Z. Intranasal Immunization with Aluminum Salt-Adjuvanted Dry Powder Vaccine. J. Control. Release 2018, 292, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Torikai, Y.; Sasaki, Y.; Sasaki, K.; Kyuno, A.; Haruta, S.; Tanimoto, A. Evaluation of Systemic and Mucosal Immune Responses Induced by a Nasal Powder Delivery System in Conjunction with an OVA Antigen in Cynomolgus Monkeys. J. Pharm. Sci. 2021, 110, 2038–2046. [Google Scholar] [CrossRef]
- Wang, S.H.; Kirwan, S.M.; Abraham, S.N.; Staats, H.F.; Hickey, A.J. Stable Dry Powder Formulation for Nasal Delivery of Anthrax Vaccine. J. Pharm. Sci. 2012, 101, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Huang, Z.; Jing, B.; Zhu, L.; Feng, Y.; Jiang, Q.; Xu, Z.; Wan, H.; Zhao, X. Mucoadhesive Chitosan-Catechol as an Efficient Vaccine Delivery System for Intranasal Immunization. Int. J. Biol. Macromol. 2024, 273, 133008. [Google Scholar] [CrossRef] [PubMed]
- Han, J.P.; Nam, Y.R.; Chung, H.Y.; Lee, H.; Yeom, S.C. Polyphenol-Enabled 2D Nanopatch for Enhanced Nasal Mucoadhesion and Immune Activation. Nano Lett. 2024, 24, 10380–10387. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ainai, A.; Suzuki, T.; Harada, N.; Ami, Y.; Yuki, Y.; Takeyama, H.; Kiyono, H.; Tsukada, H.; Hasegawa, H. The Effect of Mucoadhesive Excipient on the Nasal Retention Time of and the Antibody Responses Induced by an Intranasal Influenza Vaccine. Vaccine 2016, 34, 1201–1207. [Google Scholar] [CrossRef]
- Springer, M.J.; Ni, Y.; Finger-Baker, I.; Ball, J.P.; Hahn, J.; DiMarco, A.V.; Kobs, D.; Horne, B.; Talton, J.D.; Cobb, R.R. Preclinical Dose-Ranging Studies of a Novel Dry Powder Norovirus Vaccine Formulation. Vaccine 2016, 34, 1452–1458. [Google Scholar] [CrossRef]
- Yu, Y.-S.; AboulFotouh, K.; Xu, H.; Williams, G.; Suman, J.; Cano, C.; Warnken, Z.N.; Wu, K.C.-W.; Williams, R.O.; Cui, Z. Feasibility of Intranasal Delivery of Thin-Film Freeze-Dried, Mucoadhesive Vaccine Powders. Int. J. Pharm. 2023, 640, 122990. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Luke, C.; Coelingh, K. Current Status of Live Attenuated Influenza Vaccine in the United States for Seasonal and Pandemic Influenza. Influenza Other Respir. Viruses 2008, 2, 193–202. [Google Scholar] [CrossRef]
- Commissioner, O. Of the FDA Approves Nasal Spray Influenza Vaccine for Self—Or Caregiver-Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-nasal-spray-influenza-vaccine-self-or-caregiver-administration (accessed on 25 February 2025).
- Broderick, K.E.; Humeau, L.M. Enhanced Delivery of DNA or RNA Vaccines by Electroporation. Methods Mol. Biol. 2017, 1499, 193–200. [Google Scholar] [CrossRef]
- Campana, L.G.; Kis, E.; Bottyán, K.; Orlando, A.; de Terlizzi, F.; Mitsala, G.; Careri, R.; Curatolo, P.; Snoj, M.; Sersa, G.; et al. Electrochemotherapy for Advanced Cutaneous Angiosarcoma: A European Register-Based Cohort Study from the International Network for Sharing Practices of Electrochemotherapy (InspECT). Int. J. Surg. 2019, 72, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Clover, A.J.P.; Salwa, S.P.; Bourke, M.G.; McKiernan, J.; Forde, P.F.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M. Electrochemotherapy for the Treatment of Primary Basal Cell Carcinoma; A Randomised Control Trial Comparing Electrochemotherapy and Surgery with Five Year Follow Up. Eur. J. Surg. Oncol. 2020, 46, 847–854. [Google Scholar] [CrossRef]
- Hadzialjevic, B.; Omerzel, M.; Trotovsek, B.; Cemazar, M.; Jesenko, T.; Sersa, G.; Djokic, M. Electrochemotherapy Combined with Immunotherapy—A Promising Potential in the Treatment of Cancer. Front. Immunol. 2024, 14, 1336866. [Google Scholar] [CrossRef] [PubMed]
- Pohl-Guimarães, F.; Hoang-Minh, L.B.; Mitchell, D.A. RNA-Electroporated T Cells for Cancer Immunotherapy. Oncoimmunology 2020, 9, 1792625. [Google Scholar] [CrossRef] [PubMed]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical Potential of Electroporation for Gene Therapy and DNA Vaccine Delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Tchitchek, N.; Todorova, B.; Rosenbaum, P.; Joly, C.; Poux, C.; Chapon, C.; Spetz, A.-L.; Ustav, M.; Le Grand, R.; et al. Innate Molecular and Cellular Signature in the Skin Preceding Long-Lasting T Cell Responses after Electroporated DNA Vaccination. J. Immunol. 2020, 204, 3375–3388. [Google Scholar] [CrossRef]
- Amante, D.H.; Smith, T.R.F.; Mendoza, J.M.; Schultheis, K.; McCoy, J.R.; Khan, A.S.; Sardesai, N.Y.; Broderick, K.E. Skin Transfection Patterns and Expression Kinetics of Electroporation-Enhanced Plasmid Delivery Using the CELLECTRA-3P, a Portable Next-Generation Dermal Electroporation Device. Hum. Gene Ther. Methods 2015, 26, 134–146. [Google Scholar] [CrossRef]
- Dupuy, L.C.; Richards, M.J.; Ellefsen, B.; Chau, L.; Luxembourg, A.; Hannaman, D.; Livingston, B.D.; Schmaljohn, C.S. A DNA Vaccine for Venezuelan Equine Encephalitis Virus Delivered by Intramuscular Electroporation Elicits High Levels of Neutralizing Antibodies in Multiple Animal Models and Provides Protective Immunity to Mice and Nonhuman Primates. Clin. Vaccine Immunol. 2011, 18, 707–716. [Google Scholar] [CrossRef]
- Gary, E.N.; Weiner, D.B. DNA Vaccines: Prime Time Is Now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef]
- Eusébio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, Â. Methods to Improve the Immunogenicity of Plasmid DNA Vaccines. Drug Discov. Today 2021, 26, 2575–2592. [Google Scholar] [CrossRef]
- Pagliari, S.; Dema, B.; Sanchez-Martinez, A.; Montalvo Zurbia-Flores, G.; Rollier, C.S. DNA Vaccines: History, Molecular Mechanisms and Future Perspectives. J. Mol. Biol. 2023, 435, 168297. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine. N. Engl. J. Med. 2021, 385, e35. [Google Scholar] [CrossRef]
- Tebas, P.; Kraynyak, K.A.; Patel, A.; Maslow, J.N.; Morrow, M.P.; Sylvester, A.J.; Knoblock, D.; Gillespie, E.; Amante, D.; Racine, T.; et al. Intradermal SynCon® Ebola GP DNA Vaccine Is Temperature Stable and Safely Demonstrates Cellular and Humoral Immunogenicity Advantages in Healthy Volunteers. J. Infect. Dis. 2019, 220, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, S.; C De Rosa, S.; Elizaga, M.; Lu, Y.; Han, X.; Huang, Y.; Swann, E.; Polakowski, L.; A Kalams, S.; Keefer, M.; et al. Intramuscular and Intradermal Electroporation of HIV-1 PENNVAX-GP® DNA Vaccine and IL-12 Is Safe, Tolerable, Acceptable in Healthy Adults. Vaccines 2020, 8, 741. [Google Scholar] [CrossRef]
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and Tolerability of HIV-1 Multiantigen pDNA Vaccine given with IL-12 Plasmid DNA via Electroporation, Boosted with a Recombinant Vesicular Stomatitis Virus HIV Gag Vaccine in Healthy Volunteers in a Randomized, Controlled Clinical Trial. PLoS ONE 2018, 13, e0202753. [Google Scholar] [CrossRef] [PubMed]
- Kalams, S.A.; Parker, S.D.; Elizaga, M.; Metch, B.; Edupuganti, S.; Hural, J.; De Rosa, S.; Carter, D.K.; Rybczyk, K.; Frank, I.; et al. Safety and Comparative Immunogenicity of an HIV-1 DNA Vaccine in Combination with Plasmid Interleukin 12 and Impact of Intramuscular Electroporation for Delivery. J. Infect. Dis. 2013, 208, 818–829. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA Vaccine Candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and Immunogenicity of INO-4800 DNA Vaccine against SARS-CoV-2: A Preliminary Report of an Open-Label, Phase 1 Clinical Trial. EClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]
- Andrade, V.M.; Christensen-Quick, A.; Agnes, J.; Tur, J.; Reed, C.; Kalia, R.; Marrero, I.; Elwood, D.; Schultheis, K.; Purwar, M.; et al. INO-4800 DNA Vaccine Induces Neutralizing Antibodies and T Cell Activity against Global SARS-CoV-2 Variants. NPJ Vaccines 2021, 6, 121. [Google Scholar] [CrossRef]
- Kraynyak, K.A.; Blackwood, E.; Agnes, J.; Tebas, P.; Giffear, M.; Amante, D.; Reuschel, E.L.; Purwar, M.; Christensen-Quick, A.; Liu, N.; et al. SARS-CoV-2 DNA Vaccine INO-4800 Induces Durable Immune Responses Capable of Being Boosted in a Phase 1 Open-Label Trial. J. Infect. Dis. 2022, 225, 1923–1932. [Google Scholar] [CrossRef]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Immunotherapy against HPV16/18 Generates Potent TH1 and Cytotoxic Cellular Immune Responses. Sci. Transl. Med. 2012, 4, 155ra138. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Shen, X.; Amante, D.; Sakata, L.; Parker, L.; Yan, J.; Boyer, J.; Roh, C.; et al. Augmentation of Cellular and Humoral Immune Responses to HPV16 and HPV18 E6 and E7 Antigens by VGX-3100. Mol. Ther. Oncolytics 2016, 3, 16025. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, P.K.; Dallas, M.; Kraynyak, K.; Herring, T.; Morrow, M.; Boyer, J.; Duff, S.; Kim, J.; Weiner, D.B. Durability of Response to VGX-3100 Treatment of HPV16/18 Positive Cervical HSIL. Hum. Vaccines Immunother. 2021, 17, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Lallow, E.O.; Jhumur, N.C.; Ahmed, I.; Kudchodkar, S.B.; Roberts, C.C.; Jeong, M.; Melnik, J.M.; Park, S.H.; Muthumani, K.; Shan, J.W.; et al. Novel Suction-Based in Vivo Cutaneous DNA Transfection Platform. Sci. Adv. 2021, 7, eabj0611. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Rohilla, P.; Felner, E.I.; Byagathvalli, G.; Azizoglu, E.; Bhamla, M.S.; Prausnitz, M.R. Tolerability of a Piezoelectric Microneedle Electroporator in Human Subjects. Bioeng. Transl. Med. 2024, 9, e10662. [Google Scholar] [CrossRef]
- Xia, D.; Jin, R.; Byagathvalli, G.; Yu, H.; Ye, L.; Lu, C.-Y.; Bhamla, M.S.; Yang, C.; Prausnitz, M.R. An Ultra-Low-Cost Electroporator with Microneedle Electrodes (ePatch) for SARS-CoV-2 Vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2110817118. [Google Scholar] [CrossRef]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef]
- Stripecke, R.; Münz, C.; Schuringa, J.J.; Bissig, K.; Soper, B.; Meeham, T.; Yao, L.; Di Santo, J.P.; Brehm, M.; Rodriguez, E.; et al. Innovations, Challenges, and Minimal Information for Standardization of Humanized Mice. EMBO Mol. Med. 2020, 12, e8662. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges, and Prospects. Sig. Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.; Chae, J.; Hong, J.; Park, J.; Jeong, E.; Kim, H.; Tanaka, M.; Okochi, M.; Choi, J. Surface Glycan Targeting for Cancer Nano-Immunotherapy. J. Control. Release 2022, 342, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, H.; Idoni, B.; Oppenheimer, S.B. A Glycobiology Review: Carbohydrates, Lectins and Implications in Cancer Therapeutics. Acta Histochem. 2011, 113, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Mantuano, N.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-Associated Carbohydrates and Immunomodulatory Lectins as Targets for Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef] [PubMed]
- Henson, T.R.; Richards, K.A.; Gandhapudi, S.K.; Woodward, J.G.; Sant, A.J. R-DOTAP Cationic Lipid Nanoparticles Outperform Squalene-Based Adjuvant Systems in Elicitation of CD4 T Cells after Recombinant Influenza Hemagglutinin Vaccination. Viruses 2023, 15, 538. [Google Scholar] [CrossRef]
- Hasannejad-Asl, B.; Pooresmaeil, F.; Takamoli, S.; Dabiri, M.; Bolhassani, A. Cell Penetrating Peptide: A Potent Delivery System in Vaccine Development. Front. Pharmacol. 2022, 13, 1072685. [Google Scholar] [CrossRef]
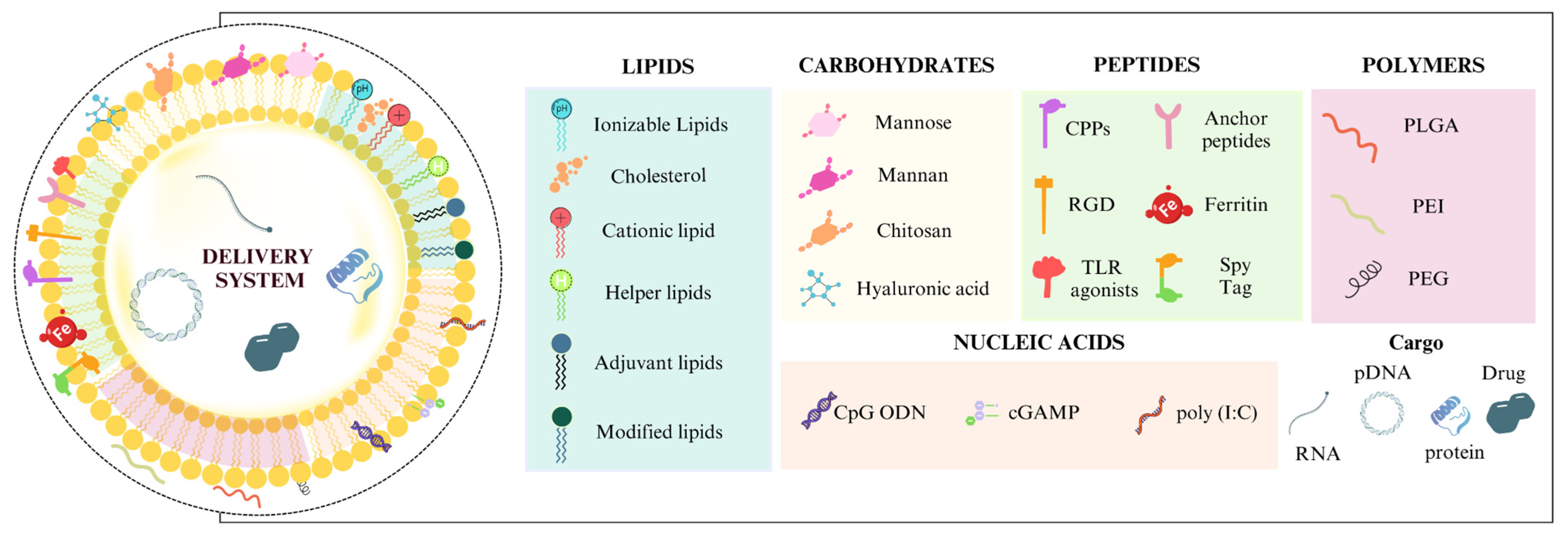
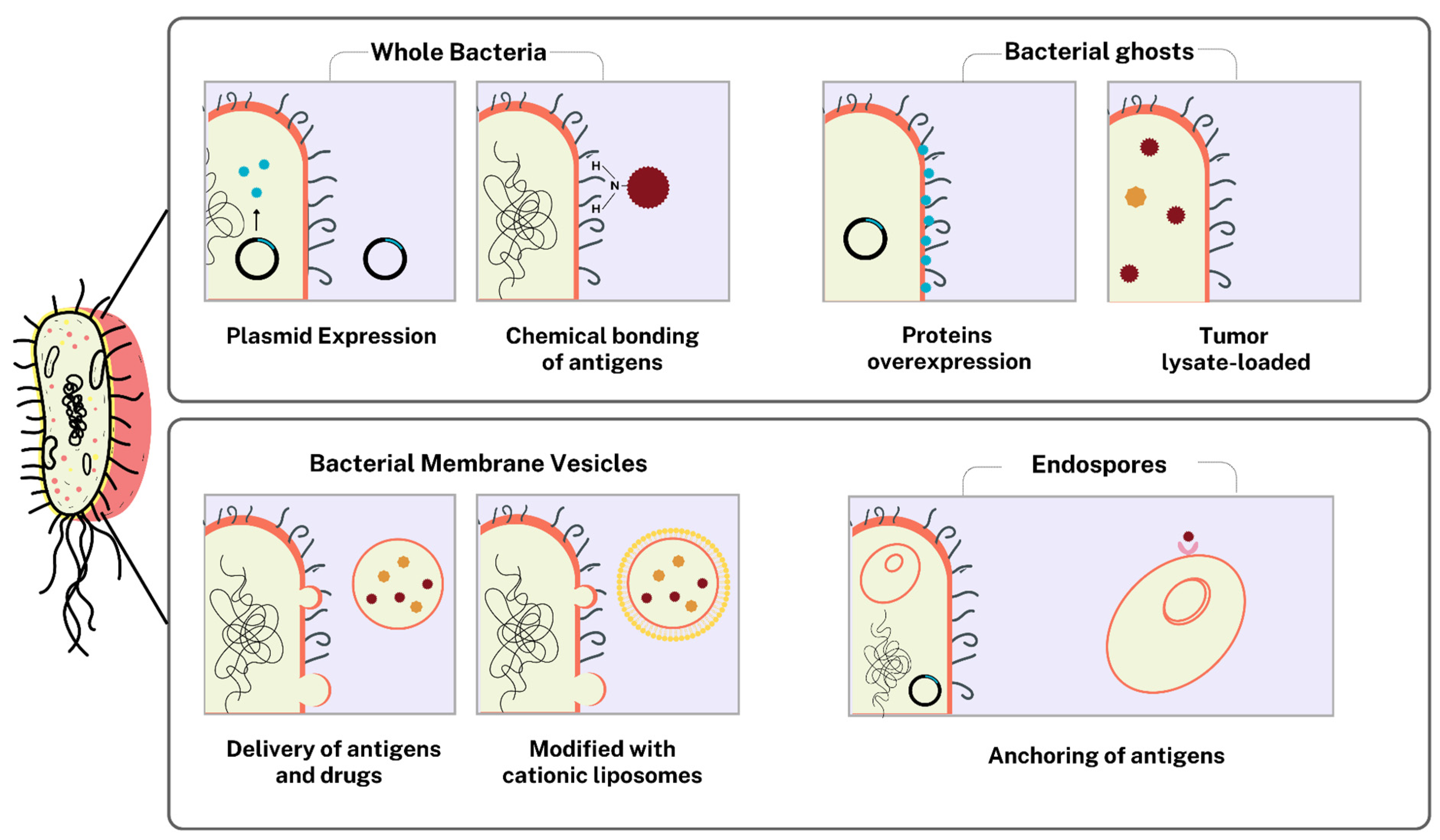
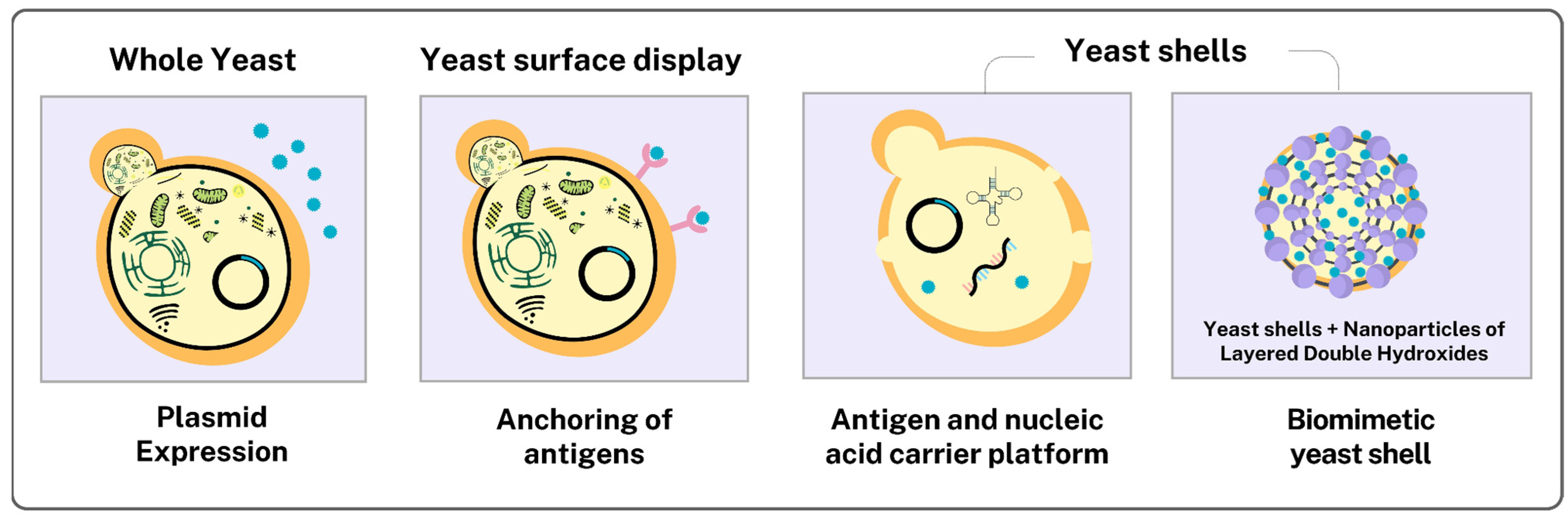

| Class of Biomolecule | Ligand | Example | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Lipids | Ionizable lipids | DLin-MC3-DMA | High delivery efficiency of siRNA/mRNA; stability at physiological pH. | Potential toxicity due to the slow degradation of the lipid tail. | [95,96] |
| SM-102 | Used in mRNA vaccines (Moderna); high delivery efficacy and long-term stability. | Pro-inflammatory nature and reactogenicity. | [97,98] | ||
| ALC-0315 | Component of the Pfizer/BioNTech vaccine; proven efficacy in mRNA vaccines. | Temperature-sensitive; requires ultra-cold storage. | [95,99] | ||
| C12-200 | High transfection efficiency; used in mRNA formulations. | It may be less stable under physiological conditions. | [96] | ||
| Cationic Lipids | DOTMA | High transfection efficiency; used in gene therapy. | Potential cellular toxicity at high concentrations. | [100,101] | |
| DOTAP | Widely used in cationic liposomes; stability. | It can induce inflammatory responses. | [102,103,104] | ||
| DODAC | Efficient in DNA delivery; stability in formulations. | Less studied compared to DOTAP and DOTMA. | [105] | ||
| Helper Lipids | DSPC | Stabilizes the outer layer of LNPs; improves nanoparticle formation. | It can reduce delivery efficiency in some formulations. | [100,106,107] | |
| DOPE | Facilitates membrane fusion; improves endosomal release. | It may be less stable in complex formulations. | [108,109] | ||
| Cholesterol | Increases the stability of LNPs; improves cargo delivery. | In excess, it can reduce transfection efficiency. | [110,111,112] | ||
| DMG-PEG2000 | Reduces particle aggregation; prolongs circulation time. | It can induce the production of anti-PEG antibodies after repeated doses. | [104,113] | ||
| Adjuvant lipids | C12-TLRa | Activation of TLR7/8; increase in the immunogenicity of mRNA vaccines. | May require dosage optimization to avoid excessive inflammatory reactions. | [97] | |
| Cholesterol substitutes | Corosolic acid (CA) | Improves cellular penetration; stabilizes nanoparticles. | Fewer clinical studies compared to traditional cholesterol. | [114] | |
| Modified lipids | DOG-IM4 | Greater thermal stability; efficacy in mRNA vaccines. | Complex synthesis; high cost. | [115] | |
| Nucleic Acids | TLR agonists | CpG ODN (TLR9) | Robust activation of the innate immune response; synergy with other adjuvants. | It can induce excessive inflammatory responses at high doses. | [116,117] |
| STING agonists | cGAMP | Activation of the STING pathway; potent antitumor responses. | It can be unstable in certain formulations. | [118] | |
| Synthetic RNA analog | Poly (I:C) | Potent immune activator; mimics viral RNA, stimulating innate immunity. | Can cause excessive immune activation, leading to toxicity or autoimmune responses. | [119,120] | |
| Polymers | PLGA | PLGA-chitosan | Biocompatibility and controlled release of antigens. | It can be quickly eliminated by the immune system. | [121] |
| Polyethylenimine (PEI) | PEI-MM-PLGA-DP/OVA | High efficiency of cellular internalization and improvement in the immune response. | Potential toxicity at high concentrations. | [122] | |
| Polyethylene glycol (PEG) | PEG-DMG | Increases circulation time; reduces particle aggregation. | It can induce the production of anti-PEG antibodies after repeated doses. | [113,123] |
| Functionalization | Purpose | Status | ID |
|---|---|---|---|
| DOTAP | Novel RNA-nanoparticle vaccine for the treatment of early melanoma recurrence | Phase I (Suspended) | NCT05264974 |
| DOTAP | Escalating dose study to evaluate the safety, tolerability, and pharmacodynamics of a therapeutic vaccine against HPV-positive cervical intraepithelial neoplasia | Phase I (Completed) | NCT02065973 |
| DOTAP | Study of an approach with combination immunotherapy in subjects with advanced HPV-associated malignancies | Phase I/II (Active, not recruiting) | NCT04287868 |
| DOTAP | Demonstration of the manufacturing feasibility and safety and determination of the maximum tolerated dose of RNA-LP vaccines for pediatric high-grade gliomas and adult glioblastoma | Phase I/II (Active, not recruiting) | NCT04573140 |
| Ferritin | Evaluation of the safety of and immune response of a vaccine against EBV | Phase I (Active, not recruiting) | NCT04645147 |
| Ferritin | Evaluation of the safety, reactogenicity, and immune response of a vaccine for COVID-19 | Unknown status | NCT04784767 |
| Ferritin | Dose setting and evaluation of the safety, tolerability, and immunogenicity of an influenza vaccine | Phase I (Completed) | NCT03814720; NCT03186781 |
| Matrix-MTM | Assessment of the safety and effectiveness of experimental malaria vaccines | Phase I/IIa (Active, not recruiting) | NCT05978037 |
| PEG-oxime linker | Evaluation of the safety and immunogenicity of a vaccine against respiratory syncytial virus | Phase I (Completed) | NCT04519073 |
| PLGA | Determination of therapeutic vaccine against melanoma | Phase I (Active, not recruiting) | NCT01753089 |
| Mannose | Dosage studies of a mannose receptor-targeted hCG-β vaccine in patients with incurable locally advanced or metastatic breast, colorectal, pancreatic, bladder, and ovarian cancer | Phase I (Completed) | NCT00648102 |
| Mannose | Evaluation of the safety of HIV-1 gp120 C4-V3 hybrid polyvalent peptide immunogen formulated in mineral oil containing mannose mono-oleate in HIV-1 uninfected volunteers | Phase I (Completed) | NCT00000886 |
| β-Glucan | Testing the potential of the allogeneic cellular vaccine 1650-G vaccine to enhance immune recognition of tumor cells in patients with lung cancer | Phase II (Completed) | NCT01829373 |
| β-Glucan | Investigation of the combination of a bivalent vaccine, β-glucan, and GM-CSF as an effective treatment for people with high-risk neuroblastoma | Phase II (Recruiting) | NCT04936529 |
| β-Glucan | Study about the efficacy and tolerability of β1,3/1,6-glucan with inactivated Saccharomyces cerevisiae rich in selenium and zinc in volunteers receiving influenza or COVID-19 vaccine | Completed | NCT04798677 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; Melo, K.M.T.B.d.; Leal, L.R.S.; Espinoza, B.C.F.; Invenção, M.d.C.V.; Pinho, S.S.d.; Freitas, A.C.d. Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives. Pharmaceutics 2025, 17, 640. https://doi.org/10.3390/pharmaceutics17050640
de Moura IA, Silva AJD, de Macêdo LS, Melo KMTBd, Leal LRS, Espinoza BCF, Invenção MdCV, Pinho SSd, Freitas ACd. Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives. Pharmaceutics. 2025; 17(5):640. https://doi.org/10.3390/pharmaceutics17050640
Chicago/Turabian Stylede Moura, Ingrid Andrêssa, Anna Jéssica Duarte Silva, Larissa Silva de Macêdo, Karina Mayumi Tani Bezerra de Melo, Lígia Rosa Sales Leal, Benigno Cristofer Flores Espinoza, Maria da Conceição Viana Invenção, Samara Sousa de Pinho, and Antonio Carlos de Freitas. 2025. "Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives" Pharmaceutics 17, no. 5: 640. https://doi.org/10.3390/pharmaceutics17050640
APA Stylede Moura, I. A., Silva, A. J. D., de Macêdo, L. S., Melo, K. M. T. B. d., Leal, L. R. S., Espinoza, B. C. F., Invenção, M. d. C. V., Pinho, S. S. d., & Freitas, A. C. d. (2025). Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives. Pharmaceutics, 17(5), 640. https://doi.org/10.3390/pharmaceutics17050640










