Agents for Fluorescence-Guided Glioblastoma Surgery
Abstract
1. Introduction
2. 5-Aminolevulinic Acid (5-ALA)
3. Sodium Fluorescein (SF)
4. Indocyanine Green (ICG) and Second Window ICG (SWIG) in Fluorescence-Guided Surgery
5. Other Agents
5.1. Tozuleristide (BLZ-100)
5.2. Alkylphosphocholine (APC) Analogs
5.3. Tumor-Targeting Monoclonal Antibodies
5.4. Tumor-Targeting Peptides and Protease-Activated Probes
6. Conclusions
Funding
Conflicts of Interest
References
- Rock, K.; Mcardle, O.; Forde, P.; Dunne, M.; Fitzpatrick, D.; O’Neill, B.; Faul, C. A clinical review of treatment outcomes in glioblastoma multiforme—The validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br. J. Radiol. 2012, 85, e729–e733. [Google Scholar] [CrossRef]
- Liu, Y.; Shete, S.; Etzel, C.J.; Scheurer, M.; Alexiou, G.; Armstrong, G.; Tsavachidis, S.; Liang, F.W.; Gilbert, M.; Aldape, K.; et al. Polymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes involved in the double-strand break repair pathway predict glioblastoma survival. J. Clin. Oncol. 2010, 28, 2467–2474. [Google Scholar] [CrossRef]
- Koukoulithras, I.; Gkampenis, A.; Markopoulos, G.S.; Vartholomatos, G.; Siempis, T.; Voulgaris, S.; Alexiou, G.A. Intraoperative methods to maximize gliomas resection: A review of both established and novel techniques. Discov. Med. 2024, 1, 79. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Voulgaris, S. The role of the PTEN gene in malignant gliomas. Neurol. Neurochir. Pol. 2010, 44, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; Von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Revilla-Pacheco, F.; Rodríguez-Salgado, P.; Barrera-Ramírez, M.; Morales-Ruiz, M.P.; Loyo-Varela, M.; Rubalcava-Ortega, J.; Herrada-Pineda, T. Extent of resection and survival in patients with glioblastoma multiforme: Systematic review and meta-analysis. Medicine 2021, 100, e26432. [Google Scholar] [CrossRef]
- Murray, K.J. Improved surgical resection of human brain tumors: Part 1. A preliminary study. Surg. Neurol. 1982, 17, 316–319. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neuro-Oncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- Leroy, H.; Vermandel, M.; Lejeune, J.; Mordon, S.; Reyns, N. Fluorescence guided resection and glioblastoma in 2015: A review. Lasers Surg. Med. 2015, 47, 441–451. [Google Scholar] [CrossRef]
- Dellaretti, M.; Melo, M.T.D.; Faraj De Lima, F.B.; Guazzelli, S.; Costa, B.B.R.; Pereira, P.D.S.S.; Torres, R.E. Fluorescein-Guided Surgery for Malignant Gliomas. World Neurosurg. 2023, 174, 62. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Smith, E.J.; Barreau, A.; Nyaeme, M.; Cramer, S.W.; Najafali, D.; Krist, D.T.; Arnold, P.M.; Hassaneen, W. Comparison of fluorescein sodium, 5-ALA, and intraoperative MRI for resection of high-grade gliomas: A systematic review and network meta-analysis. J. Clin. Neurosci. 2022, 98, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neuro-Oncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Mazurek, M.; Szczepanek, D.; Orzyłowska, A.; Rola, R. Analysis of Factors Affecting 5-ALA Fluorescence Intensity in Visualizing Glial Tumor Cells—Literature Review. Int. J. Mol. Sci. 2022, 23, 926. [Google Scholar] [CrossRef]
- Broekx, S.; Weyns, F.; De Vleeschouwer, S. 5-Aminolevulinic acid for recurrent malignant gliomas: A systematic review. Clin. Neurol. Neurosurg. 2020, 195, 105913. [Google Scholar] [CrossRef]
- La Rocca, G.; Sabatino, G.; Menna, G.; Altieri, R.; Ius, T.; Marchese, E.; Olivi, A.; Barresi, V.; Della Pepa, G.M. 5-Aminolevulinic Acid False Positives in Cerebral Neuro-Oncology: Not All That Is Fluorescent Is Tumor. A Case-Based Update and Literature Review. World Neurosurg. 2020, 137, 187–193. [Google Scholar] [CrossRef]
- Kon, T.; Sato, Y.; Kobayashi, Y.; Shimizu, K.; Mizutani, T. Efficacy of 5-ALA brightness analysis for malignant brain tumor surgery. Chin. Clin. Oncol. 2024, 13 (Suppl. 1), AB025. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Peciu-Florianu, I.; Vannod-Michel, Q.; Vauleon, E.; Bonneterre, M.-E.; Reyns, N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: An update from the INDYGO trial (NCT03048240). J. Neuro-Oncol. 2024, 168, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, N.J.; Michael, A.P. 5-Aminolevulinic acid radiodynamic therapy for treatment of high-grade gliomas: A systematic review. Clin. Neurol. Neurosurg. 2021, 201, 106430. [Google Scholar] [CrossRef]
- Schipmann, S.; Müther, M.; Stögbauer, L.; Zimmer, S.; Brokinkel, B.; Holling, M.; Grauer, O.; Suero Molina, E.; Warneke, N.; Stummer, W. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: Case series on a promising dual strategy for local tumor control. J. Neurosurg. 2021, 134, 426–436. [Google Scholar] [CrossRef]
- Yamamoto, J. Radiosensitizing effect of 5-aminolevulinic acid-induced protoporphyrin IX in glioma cells in vitro. Oncol. Rep. 2012, 27, 1748–1752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pepper, N.B.; Eich, H.T.; Müther, M.; Oertel, M.; Rehn, S.; Spille, D.C.; Stummer, W. ALA-RDT in GBM: Protocol of the phase I/II dose escalation trial of radiodynamic therapy with 5-Aminolevulinic acid in patients with recurrent glioblastoma. Radiat. Oncol. 2024, 19, 11. [Google Scholar] [CrossRef]
- Schupper, A.J.; Hadjipanayis, C.G. Novel approaches to targeting gliomas at the leading/cutting edge. J. Neurosurg. 2023, 139, 760–768. [Google Scholar] [CrossRef]
- De Laurentis, C.; Del Bene, M.; Fociani, P.; Tonello, C.; Pollo, B.; DiMeco, F. 5-ALA Fluorescence in Case of Brain Abscess by Aggregatibacter Mimicking Glioblastoma. World Neurosurg. 2019, 125, 175–178. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chen, C.-C. 5-aminolevulinic enhanced brain lesions mimic glioblastoma: A case report and literature review. Medicine 2024, 103, e34518. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cavallo, C.; Boffano, C.; Cordella, R.; Cuppini, L.; Pollo, B.; Schiariti, M.; et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg. Focus 2014, 36, E5. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Cavallo, C.; Broggi, M.; Cordella, R.; Anghileri, E.; Eoli, M.; Schiariti, M.; Broggi, G.; Ferroli, P. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg. Rev. 2014, 37, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.-M.; De Laurentis, C.; Akçakaya, M.O.; Pedersen, C.B.; Brawanski, A.; Poulsen, F.R.; Kiris, T.; Cavallo, C.; Broggi, M.; et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg. 2019, 125, e158–e164. [Google Scholar] [CrossRef]
- Dilek, O.; Ihsan, A.; Tulay, H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J. Clin. Neurosci. 2011, 18, 430–431. [Google Scholar] [CrossRef]
- Pesaresi, A.; La Cava, P.; Bonada, M.; Zeppa, P.; Melcarne, A.; Cofano, F.; Fiaschi, P.; Garbossa, D.; Bianconi, A. Combined Fluorescence-Guided Surgery with 5-Aminolevulinic Acid and Fluorescein in Glioblastoma: Technical Description and Report of 100 Cases. Cancers 2024, 16, 2771. [Google Scholar] [CrossRef]
- Craandijk, A.; Van Beek, C.A. Indocyanine green fluorescence angiography of the choroid. Br. J. Ophthalmol. 1976, 60, 377–385. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Lee, J.Y.K.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- ClinicalTrialsgov. Tumor Glow Intraoperative Molecular Imaging (IMI); National Library of Medicine: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04723810 (accessed on 17 April 2025).
- ClinicalTrialsgov. Second Window ICG for All Nervous System Tumors; National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05746104 (accessed on 17 April 2025).
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Pept. Sci. 2016, 106, 25–36. [Google Scholar] [CrossRef]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef] [PubMed]
- Kittle, D.S.; Mamelak Md, A.; Parrish-Novak, J.E.; Hansen, S.; Patil, R.; Gangalum, P.R.; Ljubimova, J.; Black, K.L.; Butte, P. Fluorescence-guided Tumor Visualization Using the Tumor Paint BLZ-100. Cureus 2014, 6, e210. [Google Scholar] [CrossRef]
- Leary, S.; Blatt, J.E.; Cohen, A.R.; Cohen, K.J.; Cole, B.; Governale, L.; Gupta, N.; Hauptman, J.S.; Jackson, E.M.; Kebriaei, M.A.; et al. A phase II/III randomized, blinded study of tozuleristide for fluorescence imaging detection during neurosurgical resection of pediatric primary central nervous system (CNS) tumors: PNOC012 (Pacific Pediatric Neuro-oncology Consortium). J. Clin. Oncol. 2020, 38 (Suppl. 15), TPS2575. [Google Scholar] [CrossRef]
- Hall, L.T.; Titz, B.; Baidya, N.; Van Der Kolk, A.G.; Robins, H.I.; Otto, M.; Perlman, S.B.; Weichert, J.P.; Kuo, J.S. [124I]CLR1404 PET/CT in High-Grade Primary and Metastatic Brain Tumors. Mol. Imaging Biol. 2020, 22, 434–443. [Google Scholar] [CrossRef]
- Hall, L.T.; Titz, B.; Robins, H.I.; Bednarz, B.P.; Perlman, S.B.; Weichert, J.P.; Kuo, J.S. PET/CT imaging of the diapeutic alkylphosphocholine analog 124I-CLR1404 in high and low-grade brain tumors. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 157–166. [Google Scholar]
- Weichert, J.P.; Clark, P.A.; Kandela, I.K.; Vaccaro, A.M.; Clarke, W.; Longino, M.A.; Pinchuk, A.N.; Farhoud, M.; Swanson, K.I.; Floberg, J.M.; et al. Alkylphosphocholine Analogs for Broad-Spectrum Cancer Imaging and Therapy. Sci. Transl. Med. 2014, 6, 240ra75. [Google Scholar] [CrossRef]
- Swanson, K.I.; Clark, P.A.; Zhang, R.R.; Kandela, I.K.; Farhoud, M.; Weichert, J.P.; Kuo, J.S. Fluorescent Cancer-Selective Alkylphosphocholine Analogs for Intraoperative Glioma Detection. Neurosurgery 2015, 76, 115–124. [Google Scholar] [CrossRef]
- Warram, J.M.; De Boer, E.; Korb, M.; Hartman, Y.; Kovar, J.; Markert, J.M.; Gillespie, G.Y.; Rosenthal, E.L. Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. Br. J. Neurosurg. 2015, 29, 850–858. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; Van Den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neuro-Onology 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Multispectral Bimodal Fluorescence Guided Surgery of High-grade Glioma with Cetuximab-800CW and 5-ALA (5-Aminolevulinic Acid) (MIRROR). Identifier NCT05929456; National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/study/NCT05929456?term=IRDye800CW%20&page=2&rank=14 (accessed on 10 March 2025).
- Gao, R.W.; Teraphongphom, N.; De Boer, E.; Berg, N.S.V.D.; Divi, V.; Kaplan, M.J.; Oberhelman, N.J.; Hong, S.S.; Capes, E.; Colevas, A.D.; et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics 2018, 8, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Napier, T.S.; Udayakumar, N.; Jani, A.H.; Hartman, Y.E.; Houson, H.A.; Moore, L.; Amm, H.M.; Van Den Berg, N.S.; Sorace, A.G.; Warram, J.M. Comparison of Panitumumab-IRDye800CW and 5-Aminolevulinic Acid to Provide Optical Contrast in a Model of Glioblastoma Multiforme. Mol. Cancer Ther. 2020, 19, 1922–1929. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Panitumumab-IRDye800 in Diagnosing Participants with Malignant Glioma Undergoing Surgery. Identifier NCT03510208; National Library of Medicine: Bethesda, MD, USA, 2018. Available online: https://clinicaltrials.gov/study/NCT03510208?cond=Brain%20Tumor&intr=Panitumumab-IRDye800&rank=1 (accessed on 10 March 2025).
- National Library of Medicine (U.S.). Panitumumab-IRDye800 to Detect Pediatric Neoplasms During Neurosurgical Procedures. Identifier NCT0 04085887; National Library of Medicine: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT04085887?cond=Brain%20Tumor&intr=Panitumumab-IRDye800&rank=2 (accessed on 10 March 2025).
- Samkoe, K.S.; Gunn, J.R.; Marra, K.; Hull, S.M.; Moodie, K.L.; Feldwisch, J.; Strong, T.V.; Draney, D.R.; Hoopes, P.J.; Roberts, D.W.; et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: A Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use. Mol. Imaging Biol. 2017, 19, 512–521. [Google Scholar] [CrossRef]
- De Souza, A.L.R.; Marra, K.; Gunn, J.; Samkoe, K.S.; Hoopes, P.J.; Feldwisch, J.; Paulsen, K.D.; Pogue, B.W. Fluorescent Affibody Molecule Administered In Vivo at a Microdose Level Labels EGFR Expressing Glioma Tumor Regions. Mol. Imaging Biol. 2017, 19, 41–48. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Microdose Evaluation Study of ABY-029 in Recurrent Glioma (ABY-029). Identifier NCT02901925; National Library of Medicine: Bethesda, MD, USA, 2016. Available online: https://clinicaltrials.gov/study/NCT02901925?cond=Glioma&intr=ABY-029&rank=1 (accessed on 15 March 2025).
- Listik, E.; Toma, L. Glypican-1 in human glioblastoma: Implications in tumorigenesis and chemotherapy. Oncotarget 2020, 11, 828–845. [Google Scholar] [CrossRef] [PubMed]
- Polikarpov, D.M.; Campbell, D.H.; McRobb, L.S.; Wu, J.; Lund, M.E.; Lu, Y.; Deyev, S.M.; Davidson, A.S.; Walsh, B.J.; Zvyagin, A.V.; et al. Near-Infrared Molecular Imaging of Glioblastoma by Miltuximab®-IRDye800CW as a Potential Tool for Fluorescence-Guided Surgery. Cancers 2020, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- He, K.; Chi, C.; Li, D.; Zhang, J.; Niu, G.; Lv, F.; Wang, J.; Che, W.; Zhang, L.; Ji, N.; et al. Resection and survival data from a clinical trial of glioblastoma multiforme-specific IRDye800-BBN fluorescence-guided surgery. Bioeng. Transl. Med. 2021, 6, e10182. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Chi, C.; Che, W.; Dong, G.; Wang, J.; Du, Y.; Wang, R.; Zhu, Z.; Tian, J.; et al. Lower-grade gliomas surgery guided by GRPR-targeting PET/NIR dual-modality image probe: A prospective and single-arm clinical trial. Theranostics 2024, 14, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Cardona, D.M.; Lazarides, A.L.; Spasojevic, I.; Ferrer, J.M.; Cahill, J.; Lee, C.-L.; Snuderl, M.; Blazer, D.G.; Hwang, E.S.; et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 2016, 8, 320ra4. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-Guided Surgery in the Surgical Treatment of Gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Feasibility of the LUM Imaging System for Detection of Cancer to the Brain. Identifier NCT03717142; National Library of Medicine: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT03717142 (accessed on 15 March 2025).
- Kim, H.; Liu, M.; Choi, Y. Quenched Zwitterionic Cyclic Arg-Gly-Asp-Containing Pentapeptide Probe for Real-Time Brain Tumor Imaging. Pharmaceutics 2024, 16, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef]
- Kurbegovic, S.; Juhl, K.; Sørensen, K.K.; Leth, J.; Willemoe, G.L.; Christensen, A.; Adams, Y.; Jensen, A.R.; Von Buchwald, C.; Skjøth-Rasmussen, J.; et al. IRDye800CW labeled uPAR-targeting peptide for fluorescence-guided glioblastoma surgery: Preclinical studies in orthotopic xenografts. Theranostics 2021, 11, 7159–7174. [Google Scholar] [CrossRef]
- Konečná, D.; Výmola, P.; Ternerová, N.; Výmolová, B.; Garcia-Borja, E.; Mateu, R.; Šroubek, F.; Pankrác, J.; Widen, J.C.; Bogyo, M.; et al. Molecularly targeted protease-activated probes for visualization of glioblastoma: A comparison with 5-ALA. J. Neurosurg. 2024, 141, 762–772. [Google Scholar] [CrossRef]
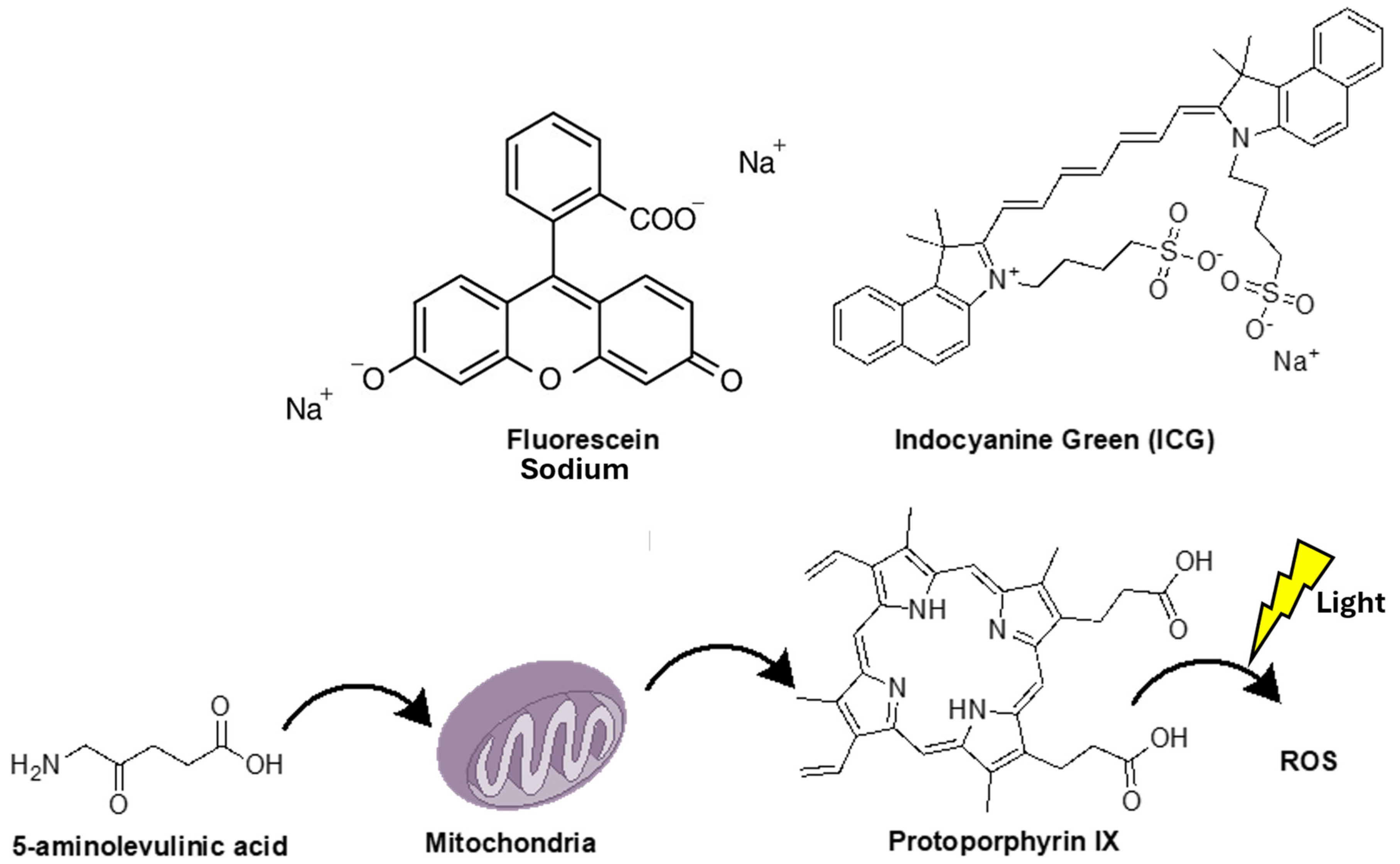
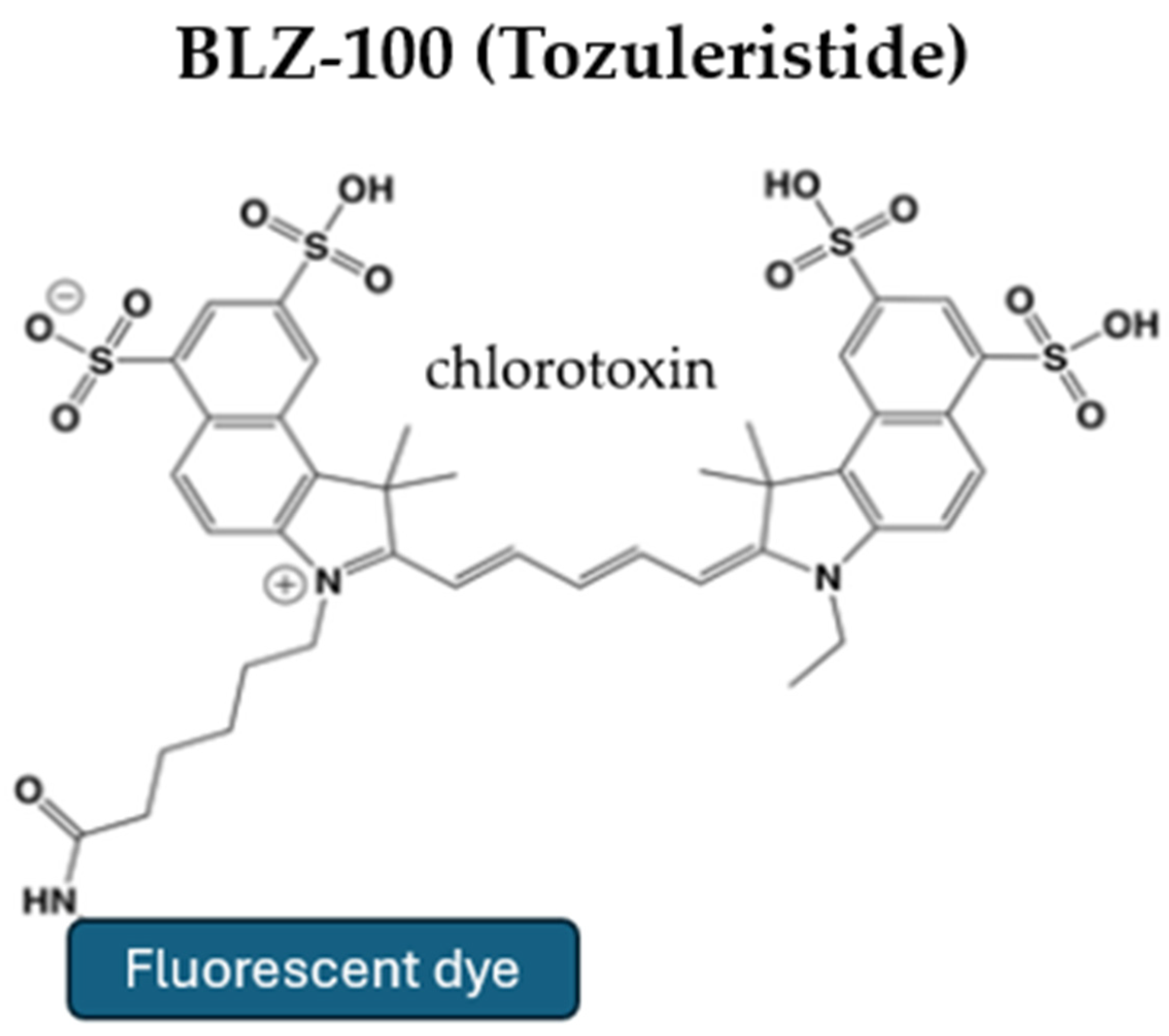
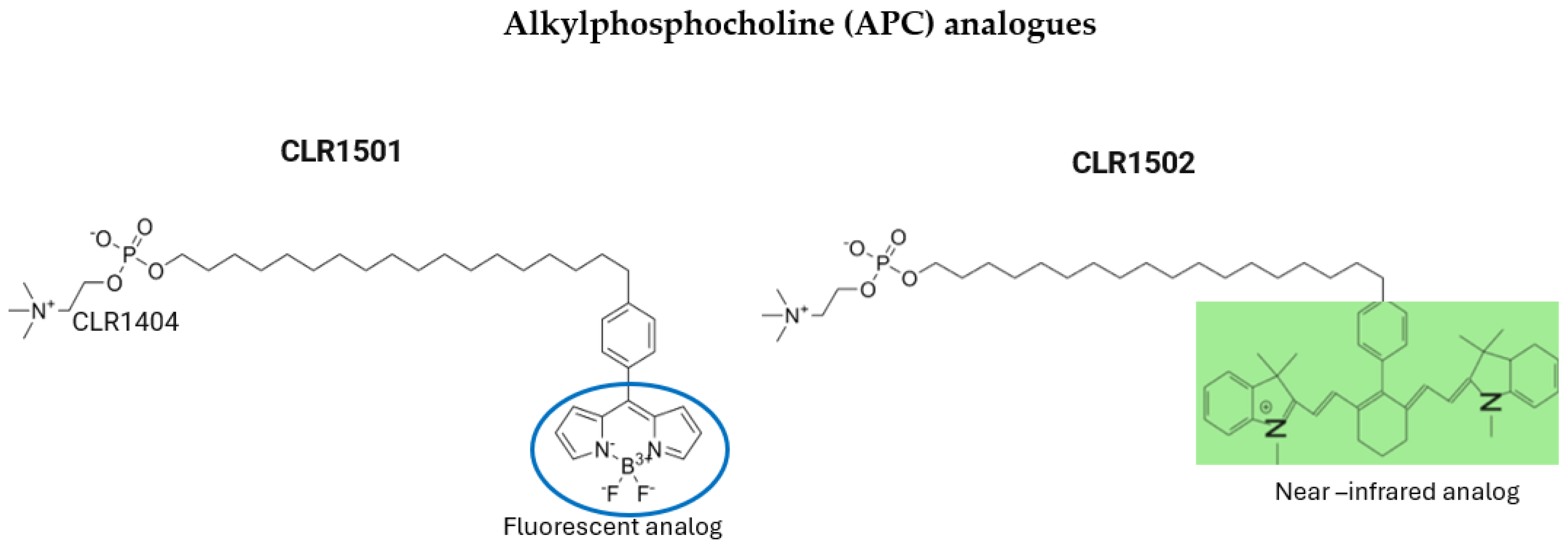
| Agent | Administration | Advantages | Disadvantages | Limitations | Comments |
|---|---|---|---|---|---|
| 5-ALA (PpIX) | Oral 3 h preoperation | Only FDA-approved dye for FGR of HGGs Enhance GTR and patient survival based on RCT | High cost Causes photosensitivity Limited in deep/periventricular tumors Cannot be used in LGGs It has been associated with side effects, such as transient hypotension and liver dysfunction | False positives with inflammation and necrosis Not ideal for recurrent or periventricular gliomas Needs a dark environment for 24 h | Can also be used in PTD and RTD therapy Sen.: 73.9–91.4% Sp.: 83.8–93.9% |
| Sodium fluorescein (SF) | IV, minutes before surgery | Cost effective Intravenous use at surgery star-useful for emergencies No skin photosensitivity Rapid visualization | Non-selective entry into areas with a disrupted BBB False positives in normal tissues (e.g., dura) Risk of anaphylaxis at high doses | Limited selectivity for tumor tissue SF may leak far from target Not recommended in end-stage renal failure (kidney excretion) | No RCT trial phase III to confirm its safety Can be combined with 5-ALA for improved GTR Sen.: 80.8% Sp.: 79.1% |
| Indocyanine green (ICG) | IV, 12–24 h preop | Visualizes tumors deeper than 1 cm Can show tumor before dura is opened Useful in various tumors Good for residual tumor detection | Requires NIR equipment Expensive Not usable in emergencies due to the need for 12–24 h preop administration | No large RCTs or long-term data Lower specificity (45%) No proven impact on GTR/survival yet—still in the clinical trial phase | ICG in brain tumors is referred as second window ICG (SWIG) Could be used in SBB Sen.: 98% Sp.: 45% |
| BLZ-100 | IV administration at least three hours prior to surgery | Tumor-specific binding Safe for human use with no observed toxicity in clinical trials Selective accumulation in high-grade gliomas | Requires NIR imaging devices Not suitable for emergency surgeries Limited clinical data available | Efficacy in low-grade gliomas is not fully established Delayed administration time | In doses > 9 mg, the serum half-life is 30 min, while the fluorophore remains in the tumor for over 24 h |
| APC analog CLR 1501 CLR 1502 | n/a | Tumor-specific targeting due to their interaction with lipid rafts Dual use: PET tracers and potential therapeutic agents Better tumor uptake and penetration compared to standard dyes, like 5-ALA | High affinity for lipoproteins accelerates clearance from circulation CLR1502 requires an NIR system, and both require specialized microscopes for tumor visualization No data about humans used for FGR | Bioavailability challenges Insufficient clinical data for safety and efficacy Specialized equipment needed | Only studies in human glioblastoma cells, glioblastoma stem-like cells, and rthotopic murine xenograf glioblastoma models |
| Cetuximab–IRDye 800CW | In early clinical trials IV 2 days before the surgery | Selectively targets EGFR, overexpressed in up to 70% of GBMs Proven safety and effectiveness in early clinical trials Enhances extent of GTR without adverse effects | Potential adverse effects based on dosage Limited to EGFR-expressing tumors | Ongoing Phase I trial to identify the optimal dose The sensitivity and specificity depend on the administration dose | Chimeric human–mouse antibody Phase I clinical trial to identify the optimal dose of cetuximab–IRDye 800CW for FGR is currently ongoing |
| Panitumumab–IRDye 800CW | In clinical trials IV 1–5 days before surgery | Better safety profile than cetuximab Up to 30% higher tumor-to-normal tissue uptake compared to 5-ALA Promising for FGR with improved accuracy in tumor margin determination | Still in the early stages of clinical trials Limited long-term data Limited to EGFR-expressing tumors | Requires further trials to establish optimal dosing Unsuitable for emergency surgery | Fully humanized antibody Phase I/II clinical trial for side effects and optimal dose for FGR in GBM still in recruitment status |
| ABY-029–IRDye 800CW | IV microdose 1–3 h prior to surgery in clinical trials | No toxicity observed even at high doses Safe in early clinical trials Promising for detecting tumor fluorescence 48 h after administration | Limited clinical data, particularly in humans Investigational phase with incomplete clinical results Limited to EGFR-expressing tumors | Needs further research for practical use | Synthetic antibody Preclinical studies show up to 16 times greater fluorescence in tumor areas compared to normal |
| 68Ga–IRDye800CW–BBN | IV in clinical trial 1 h before the surgery | Dual-modality (PET and NIR fluorescence) imaging High sensitivity (93.9%) and specificity (100%) for tumor localization Applicable in both LGGs and HGGs Effective for pre- and intraoperative imaging | Requires specialized imaging devices (PET/near-infrared fluorescence imaging) Relatively limited by patient availability and specific imaging setups | Requires high-tech imaging equipment Not suitable for all tumor types Due to the used of radiotracers it is not suitable for patient with renal or liver failure | More studies are required |
| LUM015 | n/a | Protease-activated, offering real-time tumor imaging Highly selective for cancer cells due to protease activation Promising for visualizing primary and metastatic tumors | Clinical trial suspended for protocol modifications Limited data on its full clinical efficacy | Requires further data on safety and effectiveness | No clinical trial for used in GBM FGR |
| Q-cRGD, NGR, isoNGR | n/a | Targets integrins (RGD) and aminopeptidase N (CD13) in tumors Shows potential for improved tumor-to-background ratio Potential for FGR of gliomas | Still early stages clinical trials. Not fully validated in human studies | Needs more clinical validation Primarily limited to preclinical data | No studies in humans. |
| IRDye 800CW-AE344 (uPAR-targeting) | n/a | Targets uPAR overexpressed in GBM Shows a high tumor-to-background ratio and favorable safety profile Crosses the blood–brain barrier in preclinical studies | Requires further investigation to confirm clinical applicability Limited human data | Needs more clinical trials to verify its full potential Limited research on its safety and long-term efficacy | More studies are required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, E.; Tzakos, A.G.; Crook, T.; Syed, N.; Voulgaris, S.; Alexiou, G.A. Agents for Fluorescence-Guided Glioblastoma Surgery. Pharmaceutics 2025, 17, 637. https://doi.org/10.3390/pharmaceutics17050637
Romeo E, Tzakos AG, Crook T, Syed N, Voulgaris S, Alexiou GA. Agents for Fluorescence-Guided Glioblastoma Surgery. Pharmaceutics. 2025; 17(5):637. https://doi.org/10.3390/pharmaceutics17050637
Chicago/Turabian StyleRomeo, Eleni, Andreas G. Tzakos, Timothy Crook, Nelofer Syed, Spyridon Voulgaris, and George A. Alexiou. 2025. "Agents for Fluorescence-Guided Glioblastoma Surgery" Pharmaceutics 17, no. 5: 637. https://doi.org/10.3390/pharmaceutics17050637
APA StyleRomeo, E., Tzakos, A. G., Crook, T., Syed, N., Voulgaris, S., & Alexiou, G. A. (2025). Agents for Fluorescence-Guided Glioblastoma Surgery. Pharmaceutics, 17(5), 637. https://doi.org/10.3390/pharmaceutics17050637









