Interleukin-15Rα-Sushi-Fc Fusion Protein Co-Hitchhikes Interleukin-15 and Pheophorbide A for Cancer Photoimmunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Animals
2.3. Cloning, Expression, and Characterization of Recombinant Proteins
2.4. Affinity Measurement
2.5. Preparation and Characterization of Nanoparticles
2.6. Pharmacokinetic Analysis
2.7. Biodistribution Analysis
2.8. Flow Cytometry Analysis of Cellular Uptake
2.9. Intravital Fluorescence Microscopic Imaging
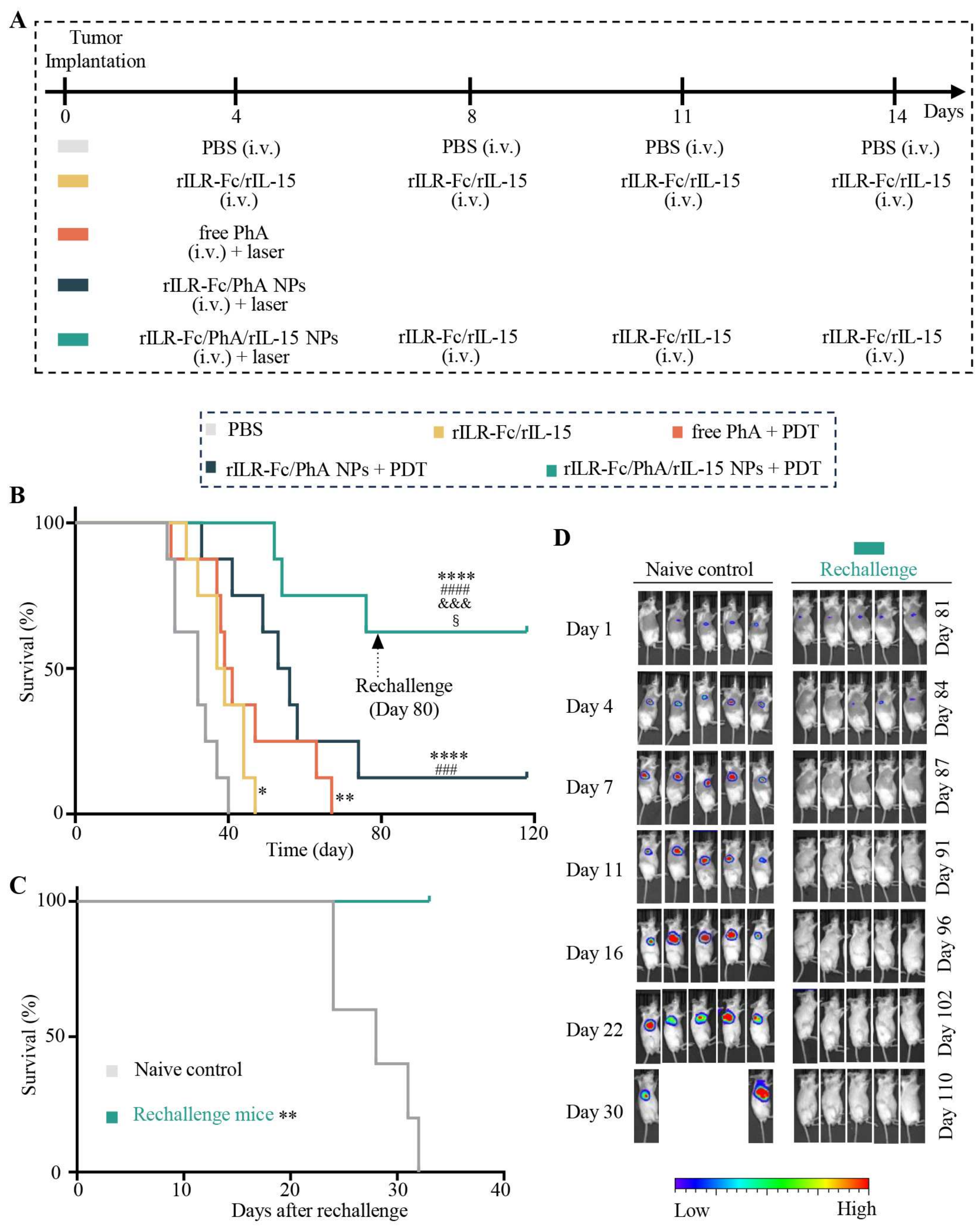
2.10. Tumor Treatment and Assessment of Immune Response
2.11. Toxicity Assessment
2.12. Statistical Analysis
3. Results and Discussion
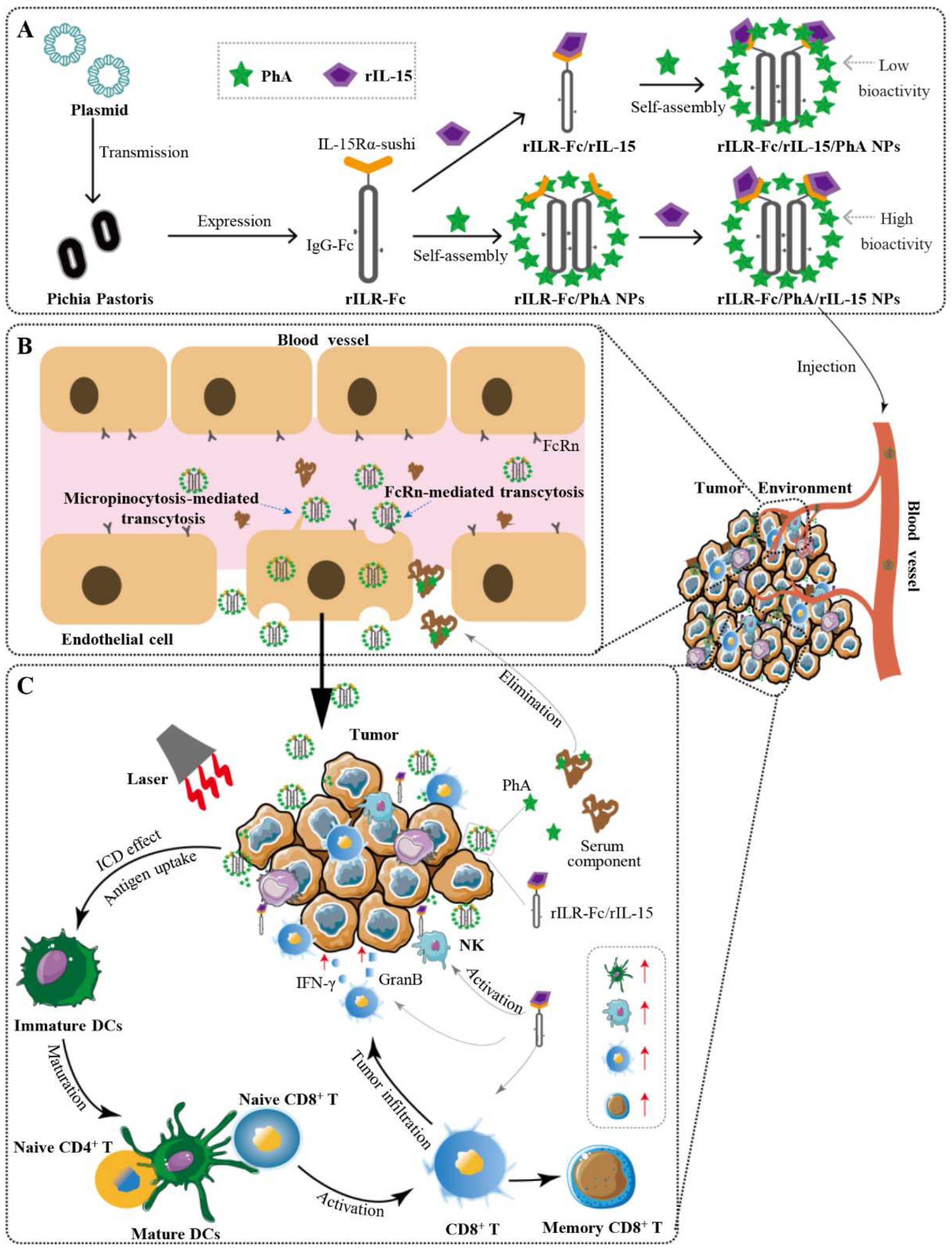
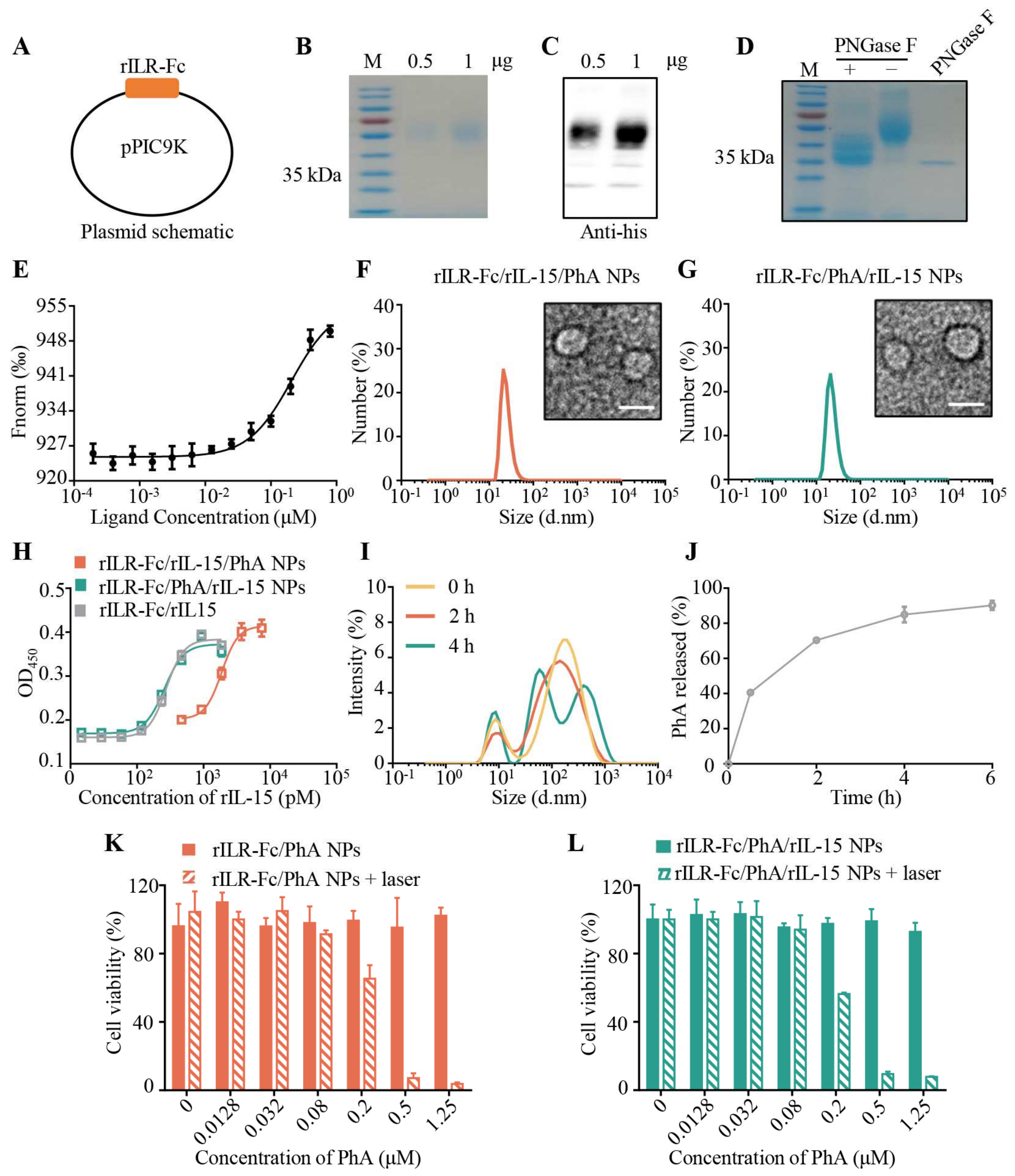
3.1. Preparation and Characterization of rILR-Fc/PhA/rIL-15 NPs
3.2. Pharmacokinetics of rILR-Fc/PhA/rIL-15 NPs in Mice
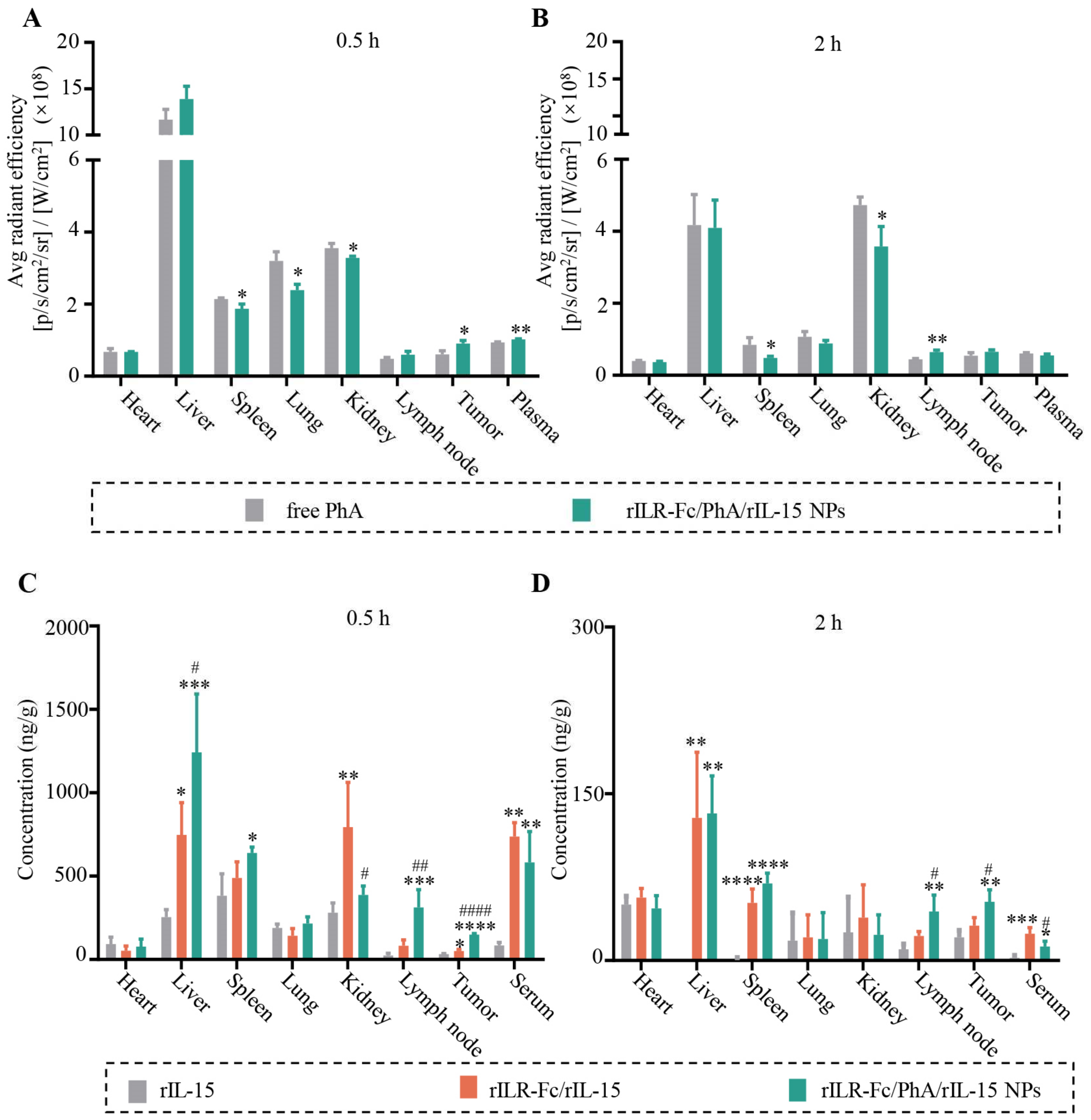
3.3. Enhanced Tumor Distribution of PhA and rIL-15 by rILR-Fc/PhA/rIL-15 NPs
3.4. The FcRn-Mediated Transcytosis of rILR-Fc/PhA/rIL-15 NPs
3.5. Enhanced Antitumor Immune Response of PDT Plus rILR-Fc/PhA/rIL-15 NPs
3.6. Enhanced Antitumor Effects of PDT with rILR-Fc/PhA/rIL-15 NPs
3.7. Biocompatible rILR-Fc/PhA/rIL-15 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Furusawa, A.; Kato, T.; Wakiyama, H.; Takao, S.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Intratumoral IL15 Improves efficacy of near-infrared photoimmunotherapy. Mol. Cancer Ther. 2023, 22, 1215–1227. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Interleukin-15 after near-Infrared photoimmunotherapy (NIR-PIT) enhances T cell response against syngeneic mouse tumors. Cancers 2020, 12, 2575. [Google Scholar] [CrossRef]
- Fiore, P.F.; Di Matteo, S.; Tumino, N.; Mariotti, F.R.; Pietra, G.; Ottonello, S.; Negrini, S.; Bottazzi, B.; Moretta, L.; Mortier, E.; et al. Interleukin-15 and cancer: Some solved and many unsolved questions. J. Immunother. Cancer 2020, 8, e001428. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the combination immunotherapy of cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Martomo, S.A.; Lu, D.; Polonskaya, Z.; Luna, X.; Zhang, Z.; Feldstein, S.; Lumban-Tobing, R.; Almstead, D.K.; Miyara, F.; Patel, J. Single-Dose Anti-PD-L1/IL-15 fusion protein KD033 generates synergistic antitumor immunity with robust tumor-immune gene signatures and memory responses. Mol. Cancer Ther. 2021, 20, 347–356. [Google Scholar] [CrossRef]
- Zhu, X.; Marcus, W.D.; Xu, W.; Lee, H.I.; Han, K.; Egan, J.O.; Yovandich, J.L.; Rhode, P.R.; Wong, H.C. Novel human interleukin-15 agonists. J. Immunol. 2009, 183, 3598–3607. [Google Scholar] [CrossRef]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Han, K.P.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.A.; Romero, C.A.; et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011, 56, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Desbois, M.; Béal, C.; Charrier, M.; Besse, B.; Meurice, G.; Cagnard, N.; Jacques, Y.; Béchard, D.; Cassard, L.; Chaput, N. IL-15 superagonist RLI has potent immunostimulatory properties on NK cells: Implications for antimetastatic treatment. J. Immunother. Cancer 2020, 8, e000632. [Google Scholar] [CrossRef]
- Dubois, S.; Patel, H.J.; Zhang, M.; Waldmann, T.A.; Müller, J.R. Preassociation of IL-15 with IL-15Rα-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008, 180, 2099–2106. [Google Scholar] [CrossRef]
- Stoklasek, T.A.; Schluns, K.S.; Lefrancois, L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006, 177, 6072–6080. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Stravokefalou, V.; Stellas, D.; Karaliota, S.; Felber, B.K.; Pavlakis, G.N. Heterodimeric IL-15 in cancer immunotherapy. Cancers 2021, 13, 837. [Google Scholar] [CrossRef]
- Neri, D. Antibody-cytokine fusions: Versatile products for the modulation of anticancer immunity. Cancer Immunol. Res. 2019, 7, 348–354. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xing, J.; Li, Y.; Liu, J.; Wang, Z. A novel multifunctional anti-CEA-IL15 molecule displays potent antitumor activities. Drug Des. Dev. Ther. 2018, 12, 2645–2654. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Xue, D.; Shen, J.; Cai, Y.; Zhu, J.; Fu, Y.X.; Peng, H. Tumor-conditional IL-15 pro-cytokine reactivates anti-tumor immunity with limited toxicity. Cell Res. 2021, 31, 1190–1198. [Google Scholar] [CrossRef]
- Shi, W.; Liu, N.; Liu, Z.; Yang, Y.; Zeng, Q.; Wang, Y.; Song, L.; Hu, F.; Fu, J.; Chen, J.; et al. Next-generation anti-PD-L1/IL-15 immunocytokine elicits superior antitumor immunity in cold tumors with minimal toxicity. Cell Rep. Med. 2024, 5, 101531. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Amorim, C.A. Nanoemulsion applications in photodynamic therapy. J. Control. Release 2022, 351, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C 2020, 115, 111099. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.Y.; Ke, M.R.; Zhang, Y.; Huang, J.D.; Yoon, J. A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal In vivo skin phototoxicity. Theranostics 2017, 7, 2746–2756. [Google Scholar] [CrossRef]
- Xu, J.; Yu, S.; Wang, X.; Qian, Y.; Wu, W.; Zhang, S.; Zheng, B.; Wei, G.; Gao, S.; Cao, Z.; et al. High affinity of chlorin e6 to immunoglobulin G for intraoperative fluorescence image-guided cancer photodynamic and checkpoint blockade therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef]
- Lin, H.; Tong, Q.; Xu, J.; Li, T.; Yang, A.; Sun, J.; Lu, W. An “IgG-hitchhiking” approach for rapid tumor accumulation and clearance of photosensitizers. J. Control. Release 2023, 356, 242–255. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, L.T.; Jiang, Y.; Ba, D.; Cui, L.; He, W. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell. Mol. Immunol. 2008, 5, 189–196. [Google Scholar] [CrossRef]
- Stellas, D.; Karaliota, S.; Stravokefalou, V.; Angel, M.; Nagy, B.A.; Goldfarbmuren, K.C.; Bergamaschi, C.; Felber, B.K.; Pavlakis, G.N. Tumor eradication by hetIL-15 locoregional therapy correlates with an induced intratumoral CD103intCD11b+ dendritic cell population. Cell Rep. 2023, 42, 112501. [Google Scholar] [CrossRef]
- Umetsu, M.; Tsumoto, K.; Hara, M.; Ashish, K.; Goda, S.; Adschiri, T.; Kumagai, I. How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system. J. Biol. Chem. 2003, 278, 8979–8987. [Google Scholar] [CrossRef]
- Wu, A.; Yang, A.; Tong, Q.; Wei, G.; Zhang, S.; Yu, S.; Zhang, C.; Xu, J.; Lu, W. A rationally designed cancer vaccine based on NIR-II fluorescence image-guided light-triggered remote control of antigen cross-presentation and autophagy. Acta Pharm. Sin. B 2023, 13, 3121–3136. [Google Scholar] [CrossRef]
- Goto, B.; Iriuchishima, T.; Horaguchi, T.; Tokuhashi, Y.; Nagai, Y.; Harada, T.; Saito, A.; Aizawa, S. Therapeutic effect of photodynamic therapy using Na-pheophorbide a on osteomyelitis models in rats. Photomed. Laser Surg 2011, 29, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, Y.; Nakamura, S.; Sakaguchi, S. New method of photosensitizer accumulation for photodynamic therapy in an experimental liver tumor. Lasers Surg. Med. 1989, 9, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z.; et al. Albumin tailoring fluorescence and photothermal conversion effect of near-infrared-II fluorophore with aggregation-induced emission characteristics. Nat. Commun. 2019, 10, 2206. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-amplified drug delivery with light-Inducible nanocargoes to enhance cancer immunotherapy. Adv Mater 2019, 31, e1902960. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, S.; Yu, S.; Lu, W. Intravital microscopy reveals endothelial transcytosis contributing to significant tumor accumulation of albumin nanoparticles. Pharmaceutics 2023, 15, 519. [Google Scholar] [CrossRef]
- Zheng, B.; Xu, J.; Chen, G.; Zhang, S.; Xiao, Z.; Lu, W. Bacterium-Mimicking vector with enhanced adjuvanticity for cancer immunotherapy and minimized toxicity. Adv. Funct. Mater. 2019, 29, 1901437. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Y.; Xian, X.J.; Zhang, J.; Liu, Z.F. Sorting out semiconducting single-walled carbon nanotube arrays by preferential destruction of metallic tubes using xenon-lamp irradiation. J. Phys. Chem. C 2008, 112, 3849–3856. [Google Scholar] [CrossRef]
- Zhu, M.; Zhuang, J.; Li, Z.; Liu, Q.; Zhao, R.; Gao, Z.; Midgley, A.C.; Qi, T.; Tian, J.; Zhang, Z.; et al. Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat. Nanotechnol. 2023, 18, 657–666. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, C.; Fan, W.; Jiang, H.; Xiang, J.; Qiu, N.; Piao, Y.; Xie, T.; Luo, Y.; Li, Z.; et al. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials 2020, 240, 119902. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, Y.; Fan, W.; Xiang, J.; Wang, G.; Zhou, Q.; Wang, J.; Geng, Y.; Sun, R.; Zhang, Z.; et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion–drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 2021, 5, 1019–1037. [Google Scholar] [CrossRef]
- Fan, W.; Xiang, J.; Wei, Q.; Tang, Y.; Piao, Y.; Shao, S.; Zhou, Z.; Tang, J.; Li, Z.C.; Shen, Y. Role of micelle size in cell transcytosis-based tumor extravasation, infiltration, and treatment efficacy. Nano Lett. 2023, 23, 3904–3912. [Google Scholar] [CrossRef]
- Deissler, H.L.; Lang, G.K.; Lang, G.E. Neonatal Fc receptor FcRn is involved in intracellular transport of the Fc fusion protein aflibercept and its transition through retinal endothelial cells. Exp. Eye Res. 2017, 154, 39–46. [Google Scholar] [CrossRef]
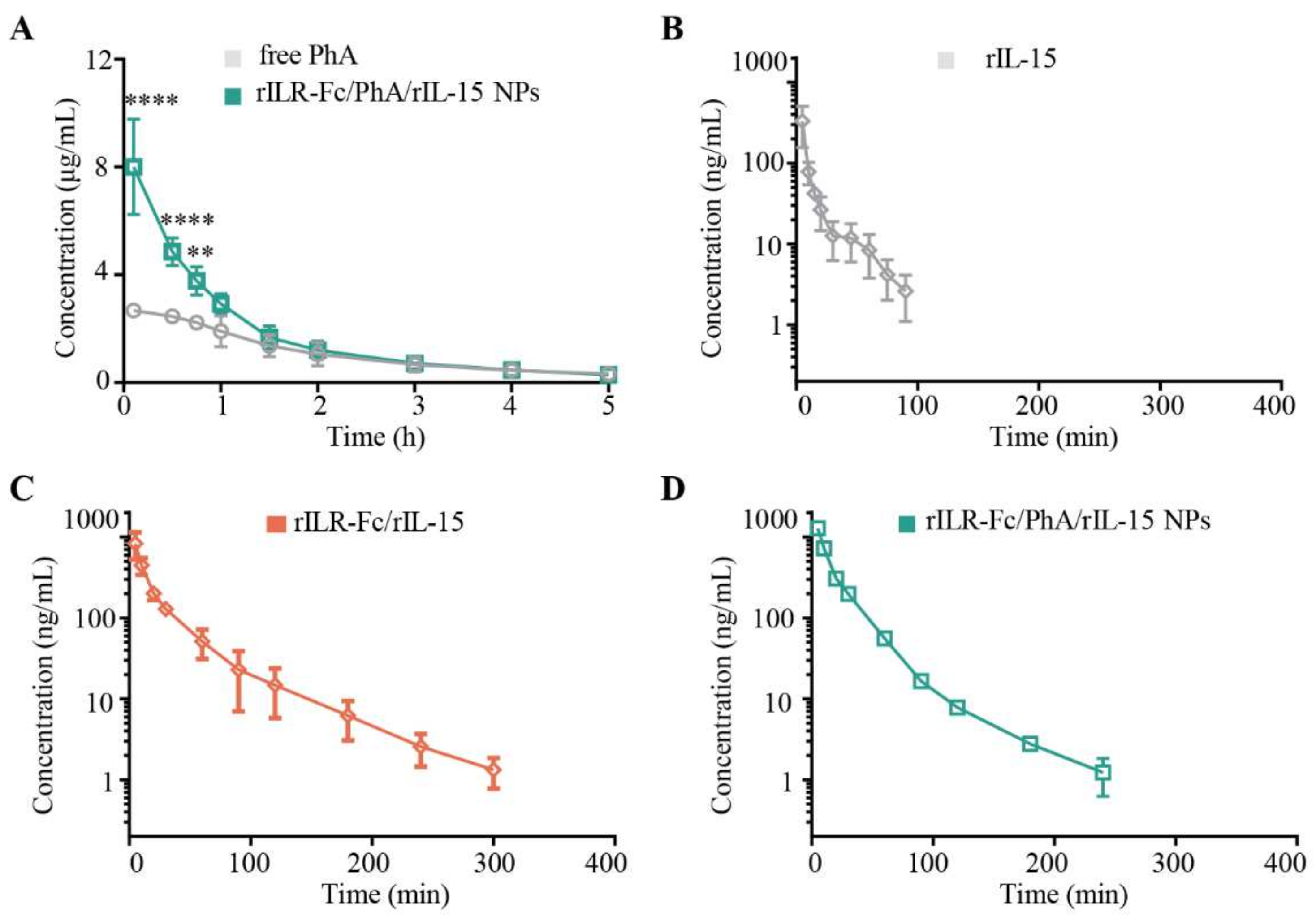



| Parameter | Free PhA | rILR-Fc/PhA/rIL-15 NPs |
|---|---|---|
| t1/2 (h) | 1.764 ± 0.1639 | 1.402 ± 0.1458 |
| AUC0→n (μg/mL·h) | 6.489 ± 1.009 | 9.745 ± 0.4210 * |
| AUMC (μg/mL·h2) | 15.97 ± 4.162 | 14.18 ± 2.267 |
| MRT (h) | 2.349 ± 0.3148 | 1.454 ± 0.1853 |
| Cl ((μg)/(μg/mL)/h) | 8.129 ± 1.527 | 5.192 ± 0.2163 |
| Vss ((μg)/(μg/mL)) | 18.20 ± 1.111 | 7.472 ± 0.7034 ** |
| Parameter | rIL-15 | rILR-Fc/rIL-15 | rILR-Fc/PhA/rIL-15 NPs |
|---|---|---|---|
| t1/2 (min) | 20.24 ± 1.170 | 58.33 ± 3.180 *** | 39.44 ± 4.584 *,§ |
| AUC0→n (ng/mL·min) (×10) | 664.4 ± 196.1 | 2227 ± 229.9 ** | 2732 ± 129.8 *** |
| AUMC (ng/mL·min2) (×102) | 544.5 ± 73.17 | 5644 ± 1084 **** | 5102 ± 1417 **** |
| MRT (min) | 9.913 ± 2.956 | 29.21 ± 6.852 | 18.80 ± 1.391 |
| Cl ((μg)/(ng/mL)/min) (×10−4) | 5.766 ± 2.519 | 2.635 ± 0.2533 | 1.875 ± 0.08779 |
| Vss ((μg)/(ng/mL)) (×10−3) | 6.351 ± 3.348 | 7.851 ± 2.311 | 3.550 ± 4.229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, J.; Lin, H.; Yu, S.; Sun, J.; Zhang, C.; Zhang, S.; Li, T.; Yang, A.; Lu, W. Interleukin-15Rα-Sushi-Fc Fusion Protein Co-Hitchhikes Interleukin-15 and Pheophorbide A for Cancer Photoimmunotherapy. Pharmaceutics 2025, 17, 615. https://doi.org/10.3390/pharmaceutics17050615
Li Z, Xu J, Lin H, Yu S, Sun J, Zhang C, Zhang S, Li T, Yang A, Lu W. Interleukin-15Rα-Sushi-Fc Fusion Protein Co-Hitchhikes Interleukin-15 and Pheophorbide A for Cancer Photoimmunotherapy. Pharmaceutics. 2025; 17(5):615. https://doi.org/10.3390/pharmaceutics17050615
Chicago/Turabian StyleLi, Zhe, Jiaojiao Xu, Hongzheng Lin, Sheng Yu, Jingwen Sun, Chen Zhang, Sihang Zhang, Tingting Li, Afeng Yang, and Wei Lu. 2025. "Interleukin-15Rα-Sushi-Fc Fusion Protein Co-Hitchhikes Interleukin-15 and Pheophorbide A for Cancer Photoimmunotherapy" Pharmaceutics 17, no. 5: 615. https://doi.org/10.3390/pharmaceutics17050615
APA StyleLi, Z., Xu, J., Lin, H., Yu, S., Sun, J., Zhang, C., Zhang, S., Li, T., Yang, A., & Lu, W. (2025). Interleukin-15Rα-Sushi-Fc Fusion Protein Co-Hitchhikes Interleukin-15 and Pheophorbide A for Cancer Photoimmunotherapy. Pharmaceutics, 17(5), 615. https://doi.org/10.3390/pharmaceutics17050615







